Abstract
Objective
The goal of the study was to perform a systematic review with meta-analysis in order to determine the mean change in the 22-item SinoNasal Outcomes Test (SNOT-22) across patients who have had endoscopic sinus surgery (ESS) for chronic rhinosinusitis (CRS) in the literature.
Methods
A literature search was performed to identify studies that assessed SNOT-22 scores before and after ESS in adult patients with CRS. A random effects model with inverse variance weighting was used to generate the mean change after surgery, along with the forest plot and 95% confidence interval (CI). The impact of patient-specific factors across studies was assessed using a mixed-effects meta-regression.
Results
The final study list included 40 unique patient cohorts published from 2008–2016. All studies showed a statistically significant change in mean SNOT-22 scores between baseline and post-operative time points (p<0.001), ranging from 12.7 to 44.8 at an average follow-up of 10.6 months. The summary change in mean SNOT-22 across all studies was 24.4 (95% CI: 22.0–26.8). After forward, step-wise multivariate modelling, studies with higher mean preoperative SNOT-22 score and higher asthma prevalence were associated with greater changes in SNOT-22 score after ESS, whereas studies with longer mean follow-up had smaller changes in SNOT-22 score.
Conclusion
Studies evaluating QOL outcomes after sinus surgery using the SNOT-22 instrument universally show significant improvement after ESS. Across the published literature, the magnitude of change is quite variable and appears to be influenced by a number of factors including baseline SNOT-22 score, asthma prevalence, and length of follow-up.
Keywords: chronic rhinosinusitis, meta-analysis, quality of life, SNOT-22, quality improvement
Introduction
Improving the quality of healthcare delivery remains a major priority for all stakeholders across the healthcare landscape. This renewed emphasis can be seen at nearly every level, ranging from the solo provider who reviews their individual practice with quality improvement in mind, to the third party payer who institutes a program that links payment to quality outcomes. Implicit in any effort that focuses on quality improvement is the understanding that quality can actually be measured and that references exist which allow one to determine if an individual or entity is under-performing, over-performing, or performing at an expected level as compared to that reference [1]. A recent review of quality measurement for chronic rhinosinusitis (CRS) highlighted that all currently collected quality measures were process metrics and specifically lacked assessment of patient outcomes [2]. Process metrics measure adherence to guidelines or best-practice standards, such as imaging or antibiotic utilization, but do not actually measure health outcomes. Outcome metrics, on the other hand, assess the change in health state after a health care activity is performed and typically focus on health states that are important to the patient.
The measurement of sinus-specific quality-of-life (QOL) is perhaps the most commonly utilized outcome measure for CRS. Although sinus-specific QOL measures are routinely employed in studies examining efficacy of treatment for CRS, their utility in quality improvement initiatives is less clear-cut. Currently, no regulatory bodies recommend assessing the change in sinus-specific QOL before and after an intervention for CRS. On one hand, this is surprising, because validated sinus-specific QOL measures are readily available, easy to implement, and quantify outcomes in a patient-centered fashion. However, one could argue that the biggest hurdle to incorporating an outcome metric in any quality improvement program is the lack of available reference standards. In order for any individual or entity to implement a change in QOL as an outcome metric, one must have a clear sense for the expected change in that instrument after a given intervention, along with the various factors that might influence the degree of change.
Roughly 300,000 endoscopic sinus surgery (ESS) procedures are performed yearly in the United States, primarily for the purpose of improving patient-reported QOL and optimizing daily productivity [3]. Studies from several countries have reported geographic variation in the rate of ESS[4–6], as well as differences in outcomes across centers[7]. At present, the 22-item SinoNasal Outcomes Test (SNOT-22) is the most commonly utilized and highest quality sinus-specific QOL instrument available[8, 9]. The SNOT-22 containing 22 questions each scored 0–5 (total score range 0–110), with higher scores representing worse QOL. The minimal clinically important difference (MCID) is commonly considered to be a change of 8.9 points. Individual studies have consistently shown that SNOT-22 scores improve after ESS in adults with CRS. However, in order to utilize SNOT-22 scores as an outcome metric in quality improvement initiatives one must have some reference for the expected range of improvement. Implicit in development of this reference would be an understanding of the typical improvement experienced after ESS and underlying factors intrinsic to the patient that might influence expected outcomes. In this way, an individual physician or entity could compare their SNOT-22 outcomes to a standard reference, adjusting for factors that might be unique to their patient population.
With the above concepts in mind, we sought to perform a systematic review of all studies that examined the change in SNOT-22 score after ESS for adult patients with CRS. Our primary goal was to perform a meta-analysis to determine mean change in SNOT-22 across all patients who have had ESS for CRS in the literature. Our secondary goal was to assess variation in mean change across studies and to identify patient-specific factors that might influence this variation. Lastly, we sought to explore the feasibility of developing a reference standard for expected SNOT-22 change after ESS and a model that would allow adjustment for patient-specific factors that might influence that change.
Methods
Literature search
A literature search was performed by two authors in parallel to identify studies that assessed SNOT-22 scores before and after ESS in adult patients with CRS. Reviewers queried a number of databases including Pubmed, Embase, Cochrane database of systematic review, Scopus, Ebscohost, and the York center for reviews and dissemination. The strategy combined terms for chronic sinusitis [“chronic” AND “sinusitis”] and various terms for surgery and SNOT-22 to develop an inclusive list of possible studies. After searching all databases, duplicates were removed and abstracts reviewed. In order to be included, all patients in the study must have been ≥16 years and considered to have CRS, or data from patients with CRS must have been presented separate and distinct from data from patients without CRS. Furthermore, included studies must have examined patients undergoing surgical intervention for CRS and must have utilized the SNOT-22 before and after surgery. Studies that enrolled patients with acute rhinosinusitis or recurrent acute rhinosinusitis were excluded, as were studies that utilized other outcome metrics but not the SNOT-22. Full text articles were then reviewed to confirm they met eligibility requirements. References of all included studies were reviewed to identify any additional articles that may have been missed. Studies from the same institution were then examined to determine whether they are reporting data from the same cohort. If more than one study from an institution had overlapping enrollment dates, such that they were likely to report duplicate data, the study with the largest sample size and/or complete data was chosen. The reviewers then combined lists and any discrepancies were resolved through discussion with all authors.
Data extraction and quality review
Data from included studies was then extracted by two reviewers working independently and in parallel. This included the diagnostic criteria utilized by original study authors to determine CRS for any given study. Average patient characteristics for each study were determined, including demographics (age and sex) and frequency of medical comorbidities (asthma, allergic rhinitis, depression, and current tobacco use). Disease-specific measures were also recorded, including the frequency of polyps or prior sinus surgery for each study. If available, the mean Lund-Mackay computed tomography (CT) score and Lund-Kennedy endoscopy score were recorded for each study [10, 11]. Although other CT and endoscopic scoring systems exist, these were specifically chosen as they are the most commonly reported. Lastly, the pre-operative (baseline) and post-operative SNOT-22 score was recorded, including means and standard deviations (SD). For any given study, we used the last available SNOT-22 score and recorded the length of follow-up in months. If not specifically provided, mean and/or SD was calculated from provided data whenever possible. Studies were excluded if data was unable to be obtained or had missing values that could not be otherwise calculated. A quality assessment was then performed using the Quality Assessment Tool for Before-After (Pre-Post) Studies with No Control Group provided by the National Heart, Lung, and Blood Institute [12].
Meta-analysis, study variation, and model building
A meta-analysis was then performed determining the mean change in SNOT-22 across all studies using Comprehensive Meta Analysis Version 3.0. Given expected heterogeneity, a random effects model with inverse variance weighting was used to generate the mean change after surgery, along with the forest plot and 95% confidence interval (CI). Studies were then arranged from highest to lowest change and examined to determine how they compare to “average.” The review was structured following the “Preferred reporting items for systematic reviews and meta-analyses” (PRISMA) guidelines [13].
We next wanted to explore how patient-specific factors might influence outcomes, focusing on the demographic, medical comorbidity, and disease-specific factors relevant to CRS. This was done in two ways. First, individual studies were queried as to whether they specifically studied the impact of this factor on SNOT-22 outcomes using patient-level data. If the factor was studied, its impact was then recorded (ie effect size and significance) for that individual study. However, no attempt was made to combine this data across studies as this would require patient-level data that was neither available nor feasible. Next, we investigated the impact of these factors across studies using a mixed-effects meta-regression. In the meta-regression, the mean (SD) or frequency of each variable in each study was placed into a model to determine whether it influenced outcomes across studies, ie using study-level data. The sensitivity of regression findings to any single study was then explored for positive findings.
Lastly, we sought to build a multivariate model that would provide the reference change in SNOT-22 after surgery for any specific population, based on published literature to date. This was done using forward, step-wise selection for any variable with a p-value <0.10 on univariate meta-regression and robust to sensitivity analysis. This model would potentially serve as an available reference at present, and as proof of concept and starting point for future models that might better inform quality improvement initiatives in an unbiased fashion.
Results
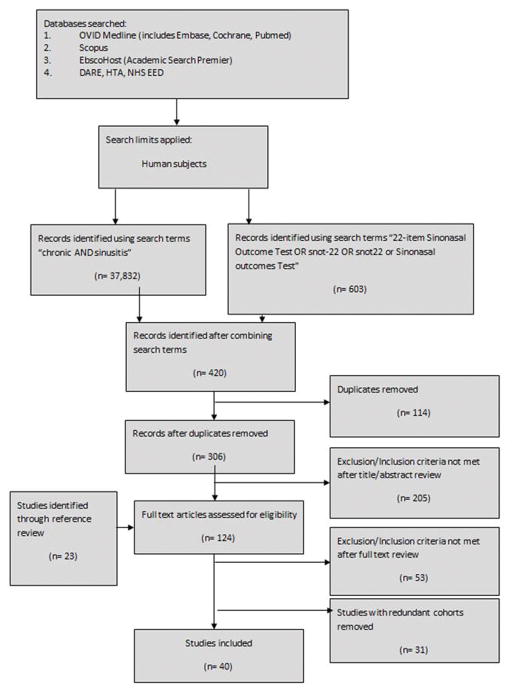
Initial literature search identified 420 study abstracts, of which 101 remained after eliminating duplicates and applying inclusion/exclusion criteria (Figure 1) [13]. Full-length texts were then reviewed and an additional 23 studies identified from references. A total of 31 studies were considered to be duplicates from same cohort [8, 14–44] and an additional 53 failed to meet inclusion/exclusion criteria. The final study list included 40 unique patient cohorts published from 2008–2016 and representing institutions from North America (n=14), Europe (n=12), Middle East (n=6), Australia (n=3), Asia (n=3), and South America (n=2) [27, 45–83]. The majority of studies were prospective observational cohorts (n=23), with the remainder being surgical arms from randomized clinical trials (n=7), retrospective cohorts (n=7), or case control studies (n=3). Data represented outcomes from 5,547 patients, with individual study sizes ranging from 6 to 1,459. Details for individual studies can be found in Table 1. With regard to quality, 29 of 40 studies utilized established diagnostic criteria for CRS, with the remainder describing alternate criteria. Quality assessment findings are presented in Supplementary Table 1. Five of the studies were rated as “Good” quality with the remainder “Fair.” Most studies failed to document whether all possible participants that met criteria were actually enrolled and failed to comment on sufficient sample size.
Figure 1.
PRISMA Flow Diagram.
Table 1.
Summary Table of Included Studies
| First Author | Year | Study Design | Number of patients | Country | Diagnosis of CRS | Surgical Intervention | Length of Post-operative Follow-Up (mos) | Select Population | Polyps (%) |
|---|---|---|---|---|---|---|---|---|---|
| Ye [45] | 2008 | Prospective cohort | 113 | China | Guidelines1 | Revision ESS | 12 | - | 100 |
| Hopkins [46] | 2009 | Prospective cohort | 1459 | UK | Guidelines1 | Primary and Revision ESS | 3–60 | - | 71 |
| Proimos [47] | 2010 | Prospective cohort | 86 | Greece | Guidelines1 | ESS | 6–12 | Asthma | 100 |
| Schaberg [48] | 2010 | Retrospective cohort | 9 | US | NR | Revision ESS | 1.5 | Hyperostotic Sinusitis | - |
| Hoseini [49] | 2011 | Prospective cohort | 100 | Iran | NR | Primary and Revision ESS | 24 | CF, Diabetes | 100 |
| Kosugi [50] | 2011 | Prospective cohort | 89 | Brazil | Guidelines1 | ESS | 3 | - | 49 |
| Conger [51] | 2012 | Prospective cohort | 9 | US | NR | Draf III procedure | 3–12 | - | - |
| Sadeghi [52] | 2012 | Case-control | 50 | Iran | Other | ESS +/− Rhinoplasty | 12 | - | - |
| Snidvongs [53] | 2012 | Prospective cohort | 104 | Australia | Guidelines1 | Primary and Revision ESS | 13.975 | - | 56 |
| Vaitkus [54] | 2012 | Case-Control | 36 | Lithuania | Guidelines1 | ESS | 3 | - | - |
| Virgin [55] | 2012 | Prospective cohort | 22 | US | Guidelines2 | Primary and Revision ESS | 1–12 | Cystic Fibrosis | - |
| Garetier [56] | 2013 | Retrospective cohort | 33 | US | Guidelines3 | Primary ESS | 3–12 | - | - |
| Mascarenhas [57] | 2013 | Prospective cohort | 38 | Brazil | Guidelines4 | ESS | 3–24 | - | 45 |
| Sacks [58] | 2013 | Prospective cohort | 53 | Australia | Guidelines1 | Primary ESS | 12 | - | 44 |
| Bizaki [59] | 2014 | RCT | 21 | Finland | Guidelines4 | Primary ESS | 3 | - | 0 |
| Cantone [60] | 2014 | RCT | 122 | Italy | Guidelines4 | Primary ESS | 1 | - | 100 |
| Cho [61] | 2014 | Prospective cohort | 21 | South Korea | Guidelines5 | Primary and Revision ESS | 0.25–1 | AERD | 100 |
| Dautremont [62] | 2014 | RCT | 36 | Canada | Guidelines6 | ESS with Steroid-eluting spacer | 2 | - | 100 |
| DeConde [27] | 2014 | Prospective cohort | 229 | US | Guidelines3,6 | Primary and Revision ESS | 15 | Migraine | 50 |
| ElBadawey [63] | 2014 | Prospective cohort | 6 | UK | Other | Frontal Recess Clearance | 12 | - | 33 |
| Hashemian [64] | 2014 | RCT | 53 | Iran | Guidelines6 | ESS | 2 | - | 41 |
| Lachanas [65] | 2014 | Prospective cohort | 32 | Greece | Guidelines4 | ESS | 3 | - | - |
| Saedi [66] | 2014 | Prospective cohort | 47 | Iran | Other | Primary and Revision ESS | 12 | - | 100 |
| Savastano [67] | 2014 | Prospective cohort | 33 | Italy | Guidelines1 | ESS | 6–24 | Cystic Fibrosis | 100 |
| Zhang [68] | 2014 | Retrospective cohort | 187 | US | Guidelines2 | Primary and Revision ESS | 1–6 | Asthma | 60 |
| Amali [69] | 2015 | RCT | 66 | Iran | Guidelines6 | Primary ESS | 3 | - | 42 |
| Divekar [70] | 2015 | Retrospective cohort | 97 | US | Guidelines6 | ESS | 0.75–3 | - | 85 |
| Lee [71] | 2015 | Prospective cohort | 35 | Canada | Guidelines6 | Primary and Revision ESS | 1–6 | - | 85 |
| Lind [72] | 2015 | Prospective cohort | 88 | Denmark | Guidelines4 | Primary and Revision ESS | 1–6 | - | 77 |
| Ottaviano [73] | 2015 | RCT | 34 | Italy | Guidelines4 | ESS | 0.5–3 | - | 59 |
| Rotenberg [74] | 2015 | Retrospective cohort | 53 | Canada | Guidelines6 | Primary and Revision ESS | 6 | - | 0 |
| Shapira Galitz [75] | 2015 | Prospective cohort | 28 | Israel | Guidelines4 | ESS | 7.59 | - | - |
| Adappa [76] | 2016 | Prospective cohort | 123 | US | Guidelines4 | Primary and Revision ESS | 6 | - | 69 |
| Bizaki [77] | 2016 | RCT | 16 | Finland | Guidelines4 | Primary Uncinectomy | 6 | - | 0 |
| Do [78] | 2016 | Case-control | 110 | Australia | Guidelines4 | Primary and Revision ESS | 12 | - | 50 |
| Lal [79] | 2016 | Retrospective cohort | 88 | US | Guidelines4 | Primary and Revision ESS | 3–24 | - | 43 |
| Masterson [80] | 2016 | Retrospective cohort | 78 | UK | Guidelines4 | Primary and Revision ESS | 1–12 | - | 62 |
| Meier [81] | 2016 | Prospective cohort | 309 | US | Guidelines6 | Primary and Revision ESS | 12 | - | 46 |
| Soler [82] | 2016 | Prospective cohort | 160 | US | Guidelines4 | Primary and Revision ESS | 6–18 | - | 37 |
| Wang [83] | 2016 | Prospective cohort | 79 | China | Guidelines7 | ESS | 6 | Bronchiectasis | 45 |
RCT = Randomized control trial; UK = United Kingdom; US = United States; ESS = endoscopic sinus surgery; CF = cystic fibrosis; AERD = aspirin exacerbated respiratory disease. NR = Not Reported.
Guidelines1 = European Position Paper on Rhinosinusitis and Nasal Polyps 2007 [86]
Guidelines2= Sinus & Allergy Health Partnership Criteria 2004 [87]
Guidelines3= Rhinosinusitis Task Force 2003 [88]
Guidelines4= European Position Paper on Rhinosinusitis and Nasal Polyps 2012 [89]
Guidelines5= Rhinosinusitis Task Force 1997 [90]
Guidelines6=American Academy of Otolaryngology-Head and Neck Surgery Guidelines 2007 [91]
Guidelines7= Chinese Guidelines 2012 [92]
Other= Diagnosis made by history, physical exam, and imaging
Meta-analysis
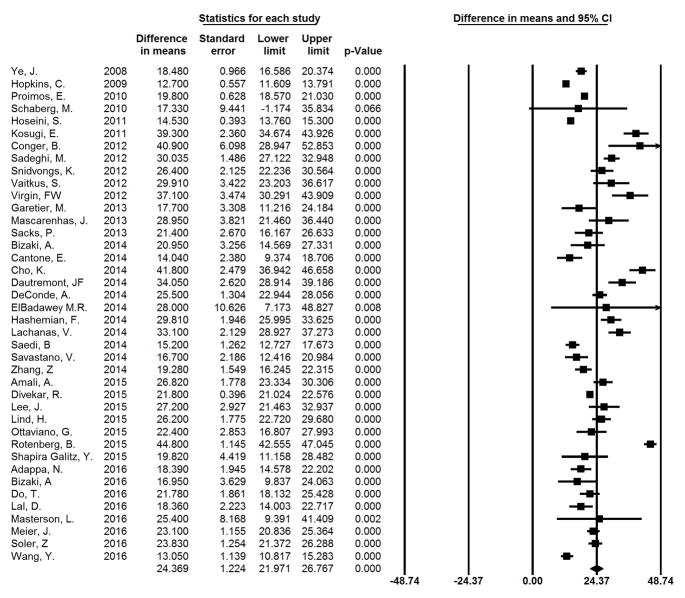
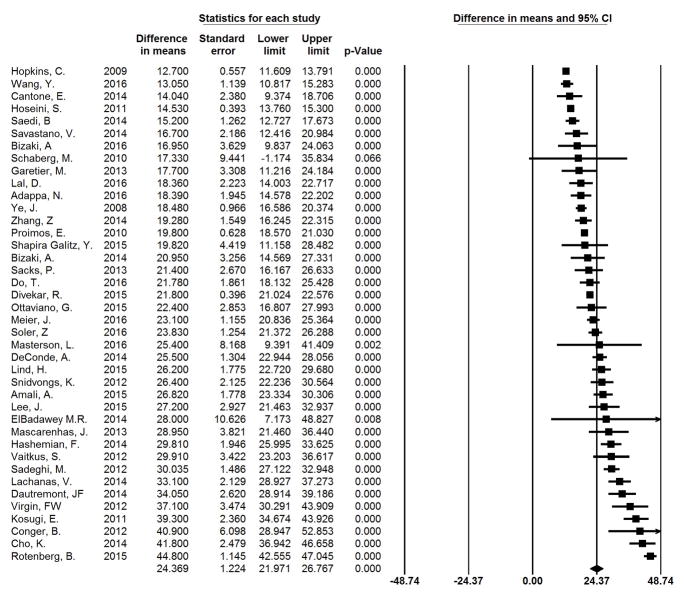
All studies included in this cohort had an individual, statistically significant change in mean SNOT-22 scores between baseline and post-operative time points (p<0.001), ranging from 12.7 to 44.8 at an average follow-up of 10.6 months. The summary change in mean SNOT-22 across all studies was 24.4 (95% CI: 22.0–26.8; I2=13.5%). Individual study findings and the forest plot for summary measures are shown in Figure 2 arranged by year of publication. Figure 3 shows studies arranged by mean change in SNOT-22 from least to greatest. It can be seen that 19 of 40 studies have a 95% CI that crosses the mean summary change of 24.4 and thus had outcomes that might be called “average”. Nine studies had a mean change in SNOT-22 and 95% CI greater than 24.4, whereas in 12 studies the mean change and 95% CI was below 24.4. No significant difference was seen between studies rated as “Good” versus those considered “Fair.” Additionally, no significant difference was seen between those studies which utilized established diagnostic criteria for CRS and those which used alternate criteria.
Figure 2.
Meta-analysis Results by Year of Publication. The difference in means represents the mean change in SNOT-22 from pre-operative to post-operative time points.
Figure 3.
Meta-analysis Results by Mean Change SNOT-22. The difference in means represents the mean change in SNOT-22 from pre-operative to post-operative time points.
Impact of patient-specific factors
Within study findings
Few studies explicitly described the impact of patient-specific factors on SNOT-22 change after ESS (Table 2). Only four studies evaluated the impact of polyp status on SNOT-22 change scores within their cohorts. One study found greater improvement in SNOT-22 in patients with polyps compared to those without [50]. One study found lower staged polyps had greater improvement than higher staged polyps [66]. One study found patients with polyps have greater improvement, but only when combined with asthma [68]. The fourth study found no significant correlation between polyp status and SNOT-22 improvement [57]. Three studies examined the association of preoperative CT scores with SNOT-22 change scores, with one finding a positive correlation [72] and the other two showing no impact [56, 67]. Sex [69, 76, 79] and age [69, 76, 82] were each evaluated by 3 studies with none showing any association. Other factors of interest included allergy, asthma, revision surgery, depression, tobacco use, pre-operative SNOT-22 score and length of follow-up. These factors were not evaluated specifically or described in only a single study, thus none of these factors was amenable to quantitative meta-analysis.
Table 2.
Within-Study Findings: Polyps, CT Score, Gender, and Age
| Study | Type | Findings |
|---|---|---|
| Polyps | ||
| Kosugi 2011 [50] | Prospective Cohort | Greater SNOT-22 improvement in those with polyps |
| Mascarenhas 2013 [57] | Prospective Cohort | No difference in SNOT-22 improvement between polyp and non-polyp patients. |
| Saedi 2014 [66] | Prospective Cohort | Greater SNOT-22 improvement in those with lower-staged polyps |
| Zhang 2014 [68] | Retrospective Cohort | Greater SNOT-22 improvement only in those with polyps and asthma |
| CT Score | ||
| Garetier 2013 [56] | Retrospective Cohort | No correlation between SNOT-22 and pre-/post-operative CT score change |
| Savastano 2014 [67] | Retrospective Cohort | No statistical correlation between SNOT-22 and CT scores |
| Lind 2015 [72] | Prospective Cohort | Higher pre-operative CT scores correlated with greater post-operative improvement in SNOT-22 |
| Sex | ||
| Amali 2015 [69] | Randomized-Control Trial | No difference observed in SNOT-22 scores between genders |
| Adappa 2016 [76] | Prospective Cohort | No difference in SNOT-22 change between genders |
| Lal 2016 [79] | Retrospective Cohort | No difference in trend or magnitude of SNOT-22 improvement between genders |
| Age | ||
| Amali 2015 [69] | Randomized-Control Trial | No significant correlation between SNOT-22 scores and patient age |
| Adappa 2016 [76] | Prospective Cohort | No significant correlation between mean SNOT-22 change and patients aged < 50 years vs. patients aged > 50 years |
| Soler 2016 [82] | Prospective Cohort | SNOT-22 improvement not significantly correlated with age |
SNOT-22 = 22-item Sino-nasal Outcome Test; CT= computed tomography
Across study findings (meta-regression)
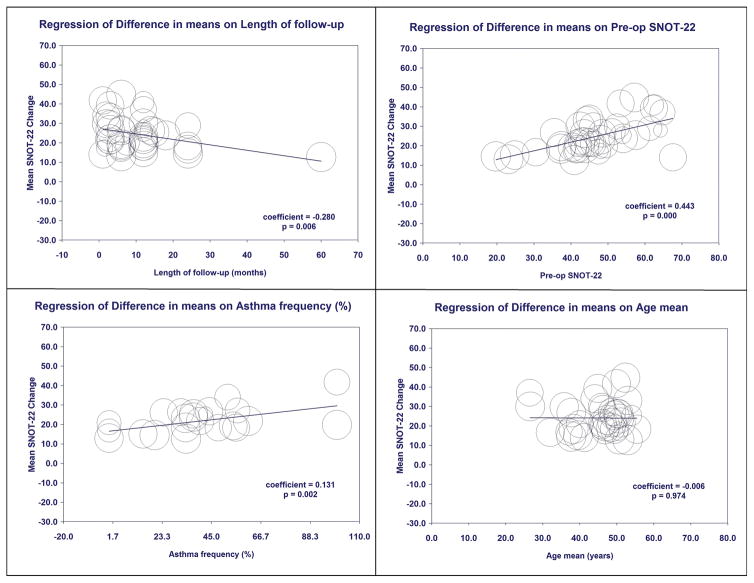
Univariate meta-regression was used to explore the association of patient-specific factors across studies on mean change in SNOT-22 scores after ESS (Table 3, Figure 4). No significant impact was found for patient age, sex, endoscopy score, CT score, polyp status, tobacco use, depression, or allergic rhinitis. The presence of asthma, prior sinus surgery, and higher pre-operative SNOT-22 scores were associated with greater mean changes in SNOT-22 scores. Greater length of follow-up was associated with less change in SNOT-22 scores. Aspirin intolerance was associated with greater change in SNOT-22 but this association was dependent on a single study and therefore not robust to sensitivity analysis. After forward, step-wise multivariate modelling, preoperative SNOT-22 score, asthma prevalence, and length of follow-up remained significant (Table 3). Studies with higher mean preoperative SNOT-22 score and higher asthma prevalence were associated with greater changes in SNOT-22 score after ESS, whereas studies with longer mean follow-up had smaller changes in SNOT-22 score.
Table 3.
Univariate Meta Regression Results
| Variable | Number of Studies (n) | Coefficient | SE | 95% CI | p-value |
|---|---|---|---|---|---|
| Year | 40 | 0.057 | 0.558 | [−1.05 – 1.15] | 0.919 |
| Age | 37 | −0.006 | 0.186 | [−0.37 – 0.36] | 0.974 |
| Gender (% female) | 37 | −0.019 | 0.123 | [−0.26 – 0.22] | 0.880 |
| Allergy (%) | 17 | 0.147 | 0.086 | [−0.02 – 0.32] | 0.090 |
| Asthma (%) | 23 | 0.131 | 0.041 | [0.05 – 0.21] | 0.002 |
| Polyps (%) | 32 | −0.067 | 0.039 | [−0.14 – 0.01] | 0.081 |
| Prior Sinus Surgery (%) | 27 | 0.109 | 0.054 | [0.00 – 0.22] | 0.045 |
| ASA Intolerance (%) | 20 | 0.220 | 0.056 | [0.11 – 0.33] | 0.001 |
| Depression (%) | 7 | −0.203 | 0.670 | [−1.52 – 1.11] | 0.762 |
| Current Tobacco Use (%) | 15 | −0.183 | 0.215 | [−0.60 – 0.24] | 0.395 |
| Baseline CT Score | 24 | 0.574 | 0.321 | [−0.05 – 1.20] | 0.074 |
| Baseline Endoscopy Score | 12 | 1.628 | 1.451 | [−1.22 – 4.47] | 0.262 |
| Length of Follow-up (mos) | 40 | −0.280 | 0.102 | [−0.48 – 0.08] | 0.006 |
| Pre-op SNOT22 Score | 40 | 0.443 | 0.092 | [0.26 – 0.62] | 0.001 |
SE = standard error; CI = confidence interval; ASA = acetylsalicylic acid; CT = computed tomography; Pre-op = preoperative; SNOT-22 = 22-item Sino-nasal Outcome Test
Figure 4.
Univariate Meta-Regression Bubble Plots for Length of Follow-Up (months), Pre-op SNOT-22, Asthma prevalence (%), and Age (years).
Discussion
Currently, individual surgeons or group practices that wish to evaluate their patient outcomes are able to collect SNOT-22 data on their CRS patients before and after surgery, along with basic patient-specific data. However, if they wish to compare their outcomes to a benchmark in order to understand if they are achieving expected improvements, they would have to choose a reference point. Selecting reference points based upon Individual studies is problematic due to wide variability in over 40 published series. Thus, a summary measure for expected SNOT-22 change generated across all published studies would be an improved reference point as opposed to any individual study. Data from this study will allow providers to compare their individual data with the summary mean and 95% CI. Outcomes that fall within the expected range would provide confidence whereas those falling below this range might prompt further investigation and perhaps quality improvement initiatives. If desired, one could go a step further and use the regression model to adjust for baseline differences in that individual surgeon’s practice such as preoperative SNOT-22, asthma prevalence, or length of follow-up which appear to impact published outcomes across studies.
Summary measures, such as this, provide a starting point based on current available data, but should not be misconstrued as the ideal. Data generated by combining all of the published studies has the potential to be biased in a number of ways. It is possible that published studies come from centers that are more experienced and higher performing than might be expected across all providers. Studies are likely to come from academic centers and thus study populations may not reflect what is typical in community practices. Alternatively, centers with poorer results may be less inclined to publish their findings, biasing the results further. Overall, there was high heterogeneity (I2=84.4%) seen on meta-regression and visual inspection of the overall funnel plot did have some asymmetry with Egger’s Test of the Intercept being significant (p=0.003) (Supplementary Figure 1). These findings suggests there may be some degree of publication bias. Certainly, expected results should shift over time as techniques are refined, indications change, and post-operative medications improve and this may not be reflected in published literature. Although this review only included patients with CRS undergoing ESS, there certainly is variability within this population with regard to underlying pathophysiology, extent and technique of surgery, adjuvant procedures and post-operative medical management that is not reflected in the variables commonly reported across studies and used in this analysis. There is also variability in study quality, with some data coming from prospective clinical trials and others representing retrospective reviews, but none providing robust controlled comparisons to non-surgical interventions. Lastly, meta-regression is a “study-level” analysis, which should best be thought of as hypothesis-generating [84]. Some associations seen across studies may not be replicated on an individual patient level. For example, individual studies have reported that changes in QOL are lower in patients undergoing revision ESS compared to primary ESS [85]; however, in this analysis, studies that reported a higher percentage of revision surgeries had greater improvement in SNOT-22 scores. This apparent discrepancy could be explained if one hypothesized that surgeons who perform more revision surgery are more experienced and thus on average have better outcomes across their entire patient population, even if patients undergoing revision surgery have worse outcomes compared to those undergoing primary. Discerning these relationships requires individual, patient-level data.
One might theorize how to develop an ideal reference point for quality improvement programs interested in expected outcomes after ESS for patients with CRS. This would require establishment of a registry and care taken to eliminate potential biases. Ideally, all patients undergoing ESS would be enrolled, as opposed to a select group, and relevant patient-specific factors would be recorded. This would allow patient-level data to be evaluated as opposed to study-level data as reported in the above meta-regressions. Outcomes from all patients would be queried, as opposed to just those who do well, eliminating problems with follow-up bias and reporting bias. Lastly, care would have to be taken that SNOT-22 values are not influenced for the purposes of artificially enhancing results. Ways in which a surgeon could “game” the results would be to ask patients to “give them good scores”, as is often done in the service industry when customer surveys are tied to reimbursement. Another way would be to increase medical treatment (ie burst of oral steroids) just prior to post-operative SNOT-22, giving an artificially improved snap-shot rather than a more accurate representation of their long-term condition. It is only with unbiased, patient-level data across a large range of patients, providers, healthcare systems, and countries that an ideal reference and accurate estimate of variability could be established and used to guide quality improvement initiatives.
Conclusion
Studies evaluating QOL outcomes after sinus surgery using the SNOT-22 instrument universally show significant improvement after ESS. Across the published literature, the magnitude of change is quite variable and appears to be influenced by a number of factors including baseline SNOT-22 score, asthma prevalence, and length of follow-up. Findings from this study provide a point estimate and range of expected changes after surgery that could inform quality improvement initiatives. Future efforts to report unbiased data and patient level metrics across a wide spectrum of patients and providers will allow future analyses to improve the accuracy and precision of this estimate and range.
Supplementary Material
Supplementary Figure 1. Funnel plot of standard error by difference in means. Y-axis=standard error; X-axis=difference in means.
Supplementary Table 1. Quality Assessment of Included Studies
Table 4.
Multivariate Meta-Regression Model
| Multivariate Meta-Regression Results | ||||||
|---|---|---|---|---|---|---|
| Variable | Univariate Regression p-Value | Number of Studies (n) | Coefficient | SE | 95% CI | p-value |
| Pre-Op SNOT-22 | 0.001 | 40 | 0.324 | 0.077 | [0.17 – 0.47] | 0.001 |
| Asthma (%) | 0.002 | 23 | 0.064 | 0.031 | [0.004 – 0.124] | 0.036 |
| Length of Follow-Up (mos) | 0.006 | 23 | −0.191 | 0.055 | [−0.30 – (−0.08)] | 0.001 |
I2=84.41%; SE= standard error; CI = confidence interval; SNOT-22 = 22-item Sino-nasal Outcome Test; mos= months
Acknowledgments
Funding: This work was supported by grants from the National Institute on Deafness and Other Communication Disorders (NIDCD), one of the National Institutes of Health, Bethesda, MD (R03 DC013651-01 and 3R01-DC005805).
Footnotes
Level of Evidence: NA
Authors’ financial disclosures and conflict of interest statements: ZS is a consultant for Olympus and 480 Biomedical. RS is consultant for Olympus and Arrinex and has received grant support from Entellus and Intersect. LR is a consultant for BioInspire and 480 Biomedical.
References
- 1.Vila PM, et al. Understanding Quality Measures in Otolaryngology-Head and Neck Surgery. JAMA Otolaryngol Head Neck Surg. 2016;142(1):86–90. doi: 10.1001/jamaoto.2015.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudmik LMJ, Schneider J, et al. Quality Measurement for Rhinosinusitis: A review from the Quality Improvement Committee of the American Rhinologic Society. Int Forum Allergy Rhinol. doi: 10.1002/alr.21983. (under review) [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharyya N. Ambulatory sinus and nasal surgery in the United States: demographics and perioperative outcomes. Laryngoscope. 2010;120(3):635–8. doi: 10.1002/lary.20777. [DOI] [PubMed] [Google Scholar]
- 4.Rudmik L, et al. Geographic Variation of Endoscopic Sinus Surgery in Canada: An Alberta-Based Small Area Variation Analysis. Otolaryngol Head Neck Surg. 2015;153(5):865–74. doi: 10.1177/0194599815602679. [DOI] [PubMed] [Google Scholar]
- 5.Rudmik L, Holy CE, Smith TL. Geographic variation of endoscopic sinus surgery in the United States. Laryngoscope. 2015;125(8):1772–8. doi: 10.1002/lary.25314. [DOI] [PubMed] [Google Scholar]
- 6.Venkatraman G, et al. Small area variation in endoscopic sinus surgery rates among the Medicare population. Arch Otolaryngol Head Neck Surg. 2011;137(3):253–7. doi: 10.1001/archoto.2011.17. [DOI] [PubMed] [Google Scholar]
- 7.Smith TL, et al. Comparing surgeon outcomes in endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope. 2017;127(1):14–21. doi: 10.1002/lary.26095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hopkins C, et al. Psychometric validity of the 22-item Sinonasal Outcome Test. Clinical Otolaryngology. 2009;34(5):447–454. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 9.Rudmik L, et al. Patient-reported outcome measures for adult chronic rhinosinusitis: A systematic review and quality assessment. Journal of Allergy and Clinical Immunology. 2015;136(6):1532–1540e2. doi: 10.1016/j.jaci.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngology - Head and Neck Surgery. 1997;117(3 II SUPPL):S35–S40. doi: 10.1016/S0194-59989770005-6. [DOI] [PubMed] [Google Scholar]
- 11.Lund VJ, I, Mackay S. Staging in rhinosinusitus. Rhinology. 1993;31(4):183–184. [PubMed] [Google Scholar]
- 12.National Heart, L., and Blood Institute. Quality Assessment Tool for Before-After (Pre-Post) Studies With No Control Group. 2014 Mar;2014 cited 2016; Available from: https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/before-after. [Google Scholar]
- 13.Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hopkins C, et al. The national comparative audit of surgery for nasal polyposis and chronic rhinosinusitis. Clinical Otolaryngology. 2006;31(5):390–398. doi: 10.1111/j.1749-4486.2006.01275.x. [DOI] [PubMed] [Google Scholar]
- 15.Browne JP, et al. Health-related quality of life after polypectomy with and without additional surgery. Laryngoscope. 2006;116(2):297–302. doi: 10.1097/01.mlg.0000198338.05826.18. [DOI] [PubMed] [Google Scholar]
- 16.Hopkins C, et al. The Lund-Mackay staging system for chronic rhinosinusitis: How is it used and what does it predict? Otolaryngology - Head and Neck Surgery. 2007;137(4):555–561. doi: 10.1016/j.otohns.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Hopkins C, Rudmik L, Lund VJ. The predictive value of the preoperative Sinonasal outcome test-22 score in patients undergoing endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope. 2015;125(8):1779–1784. doi: 10.1002/lary.25318. [DOI] [PubMed] [Google Scholar]
- 18.Abdalla S, Alreefy H, Hopkins C. Prevalence of sinonasal outcome test (SNOT-22) symptoms in patients undergoing surgery for chronic rhinosinusitis in the England and Wales National prospective audit. Clinical Otolaryngology. 2012;37(4):276–282. doi: 10.1111/j.1749-4486.2012.02527.x. [DOI] [PubMed] [Google Scholar]
- 19.Hopkins C, Andrews P, Holy CE. Does time to endoscopic sinus surgery impact outcomes in chronic rhinosinusitis? Retrospective analysis using the UK clinical practice research data. Rhinology. 2015;53(1):18–24. doi: 10.4193/Rhino14.077. [DOI] [PubMed] [Google Scholar]
- 20.Hopkins C, et al. Balloon sinuplasty: our first year. The Journal of laryngology and otology. 2011;125(1):43–52. doi: 10.1017/S0022215110001520. [DOI] [PubMed] [Google Scholar]
- 21.Remenschneider AK, et al. Long-term outcomes in sinus surgery: A new tool for measuring health-related quality of life. Otolaryngology - Head and Neck Surgery (United States) 2014;151(1):164–170. doi: 10.1177/0194599814529536. [DOI] [PubMed] [Google Scholar]
- 22.Sedaghat AR, Metson R, Gray ST. Impact of day of week on outcomes of endoscopic sinus surgery for chronic rhinosinusitis. American journal of rhinology & allergy. 2015;29(5):378–82. doi: 10.2500/ajra.2015.29.4228. [DOI] [PubMed] [Google Scholar]
- 23.Remenschneider AK, et al. The EQ-5D: a new tool for studying clinical outcomes in chronic rhinosinusitis. The Laryngoscope. 2015;125(1):7–15. doi: 10.1002/lary.24715. [DOI] [PubMed] [Google Scholar]
- 24.Abuzeid WM, et al. Outcomes of chronic frontal sinusitis treated with ethmoidectomy: a prospective study. International Forum of Allergy and Rhinology. 2016;6(6):597–604. doi: 10.1002/alr.21726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlosser RJ, et al. Impact of postoperative endoscopy upon clinical outcomes after endoscopic sinus surgery. International Forum of Allergy and Rhinology. 2016;6(2):115–123. doi: 10.1002/alr.21651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlosser RJ, et al. Depression-specific outcomes after treatment of chronic rhinosinusitis. JAMA Otolaryngology - Head and Neck Surgery. 2016;142(4):370–376. doi: 10.1001/jamaoto.2015.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deconde AS, Mace JC, Smith TL. The impact of comorbid gastroesophageal reflux disease on endoscopic sinus surgery quality-of-life outcomes. International Forum of Allergy and Rhinology. 2014;4(8):663–669. doi: 10.1002/alr.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeConde AS, et al. Response shift in quality of life after endoscopic sinus surgery for chronic rhinosinusitis. JAMA otolaryngology-- head & neck surgery. 2014;140(8):712–9. doi: 10.1001/jamaoto.2014.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steele TO, et al. Does comorbid obesity impact quality of life outcomes in patients undergoing endoscopic sinus surgery? International Forum of Allergy and Rhinology. 2015;5(12):1085–1094. doi: 10.1002/alr.21599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeConde AS, et al. Comparative effectiveness of medical and surgical therapy on olfaction in chronic rhinosinusitis: a prospective, multi-institutional study. International forum of allergy & rhinology. 2014;4(9):725–33. doi: 10.1002/alr.21350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alt JA, et al. Quality of life in patients with chronic rhinosinusitis and sleep dysfunction undergoing endoscopic sinus surgery: A pilot investigation of comorbid obstructive sleep apnea. JAMA Otolaryngology - Head and Neck Surgery. 2015;141(10):873–881. doi: 10.1001/jamaoto.2015.1673. [DOI] [PubMed] [Google Scholar]
- 32.Steele TO, Mace JC, Smith TL. Does comorbid anxiety predict quality of life outcomes in patients with chronic rhinosinusitis following endoscopic sinus surgery? International forum of allergy & rhinology. 2015;5(9):829–38. doi: 10.1002/alr.21543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hajjij A, et al. The impact of diabetes mellitus on outcomes of endoscopic sinus surgery: a nested case-control study. International forum of allergy & rhinology. 2015;5(6):533–40. doi: 10.1002/alr.21495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Rassi E, et al. Improvements in sleep-related symptoms after endoscopic sinus surgery in patients with chronic rhinosinusitis. International Forum of Allergy and Rhinology. 2016;6(4):414–422. doi: 10.1002/alr.21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deconde AS, et al. Can sinus anatomy predict quality of life outcomes and operative times of endoscopic frontal sinus surgery? American Journal of Otolaryngology - Head and Neck Medicine and Surgery. 2015;36(1):13–19. doi: 10.1016/j.amjoto.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deconde AS, et al. SNOT-22 quality of life domains differentially predict treatment modality selection in chronic rhinosinusitis. International Forum of Allergy and Rhinology. 2014;4(12):972–979. doi: 10.1002/alr.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeConde AS, et al. Longitudinal improvement and stability of the SNOT-22 survey in the evaluation of surgical management for chronic rhinosinusitis. International forum of allergy & rhinology. 2015;5(3):233–9. doi: 10.1002/alr.21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steele TO, et al. Productivity outcomes following endoscopic sinus surgery for recurrent acute rhinosinusitis. Laryngoscope. 2016;126(5):1046–1053. doi: 10.1002/lary.25705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alt JA, et al. Endoscopic sinus surgery improves cognitive dysfunction in patients with chronic rhinosinusitis. International Forum of Allergy and Rhinology. 2016 doi: 10.1002/alr.21820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deconde AS, et al. Outcomes of complete vs targeted approaches to endoscopic sinus surgery. International Forum of Allergy and Rhinology. 2015;5(8):691–700. doi: 10.1002/alr.21541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lal D, Rounds AB, Divekar R. Gender-specific differences in chronic rhinosinusitis patients electing endoscopic sinus surgery. International Forum of Allergy and Rhinology. 2016;6(3):278–286. doi: 10.1002/alr.21667. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z, et al. Culture-inappropriate antibiotic therapy decreases quality of life improvement after sinus surgery. International Forum of Allergy and Rhinology. 2014;4(5):403–410. doi: 10.1002/alr.21277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Z, et al. The effect of diabetes mellitus on chronic rhinosinusitis and sinus surgery outcome. International forum of allergy & rhinology. 2014;4(4):315–20. doi: 10.1002/alr.21269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Z, et al. Biofilm-forming bacteria and quality of life improvement after sinus surgery. International forum of allergy & rhinology. 2015;5(7):643–9. doi: 10.1002/alr.21505. [DOI] [PubMed] [Google Scholar]
- 45.Ye J, et al. Technique and Results of the Anterior-to-Posterior-to-Anterior Approach in Revision Endoscopic Sinus Surgery. ORL. 2009;71(5):257–262. doi: 10.1159/000240648. [DOI] [PubMed] [Google Scholar]
- 46.Hopkins C, et al. Long-term outcomes from the english national comparative audit of surgery for nasal polyposis and chronic rhinosinusitis. Laryngoscope. 2009;119(12):2459–2465. doi: 10.1002/lary.20653. [DOI] [PubMed] [Google Scholar]
- 47.Proimos E, et al. The effect of functional endoscopic sinus surgery on patients with asthma and CRS with nasal polyps. Rhinology. 2010;48(3):331–338. doi: 10.4193/Rhino09.123. [DOI] [PubMed] [Google Scholar]
- 48.Schaberg MR, V, Anand K, Singh A. Hyperostotic chronic sinusitis as an indication for outpatient intravenous antibiotics. The Laryngoscope. 2010;120(Suppl 4):S245. doi: 10.1002/lary.21712. [DOI] [PubMed] [Google Scholar]
- 49.Hoseini SMS, Saedi B, Aghazadeh K. Meticulous endoscopic sinus surgery to prevent recurrence of massive nasal polyposis. Journal of Laryngology and Otology. 2012;126(8):789–794. doi: 10.1017/S0022215112001193. [DOI] [PubMed] [Google Scholar]
- 50.Kosugi EM, et al. Translation, cross-cultural adaptation and validation of sinonasal outcome test (SNOT)-22 to Brazilian Portuguese. Brazilian Journal of Otorhinolaryngology. 2011;77(5):663–669. doi: 10.1590/S1808-86942011000500021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conger BT, Riley K, Woodworth BA. The draf III mucosal grafting technique: A prospective study. Otolaryngology - Head and Neck Surgery (United States) 2012;146(4):664–668. doi: 10.1177/0194599811432423. [DOI] [PubMed] [Google Scholar]
- 52.Sadeghi M, et al. Outcomes of Concurrent Endoscopic Sinus Surgery and Rhinoplasty: A Case Control Study. Acta Medica Iranica. 2013;51(11):765–770. [PubMed] [Google Scholar]
- 53.Snidvongs K, et al. Corticosteroid nasal irrigations after endoscopic sinus surgery in the management of chronic rhinosinusitis. International Forum of Allergy and Rhinology. 2012;2(5):415–421. doi: 10.1002/alr.21047. [DOI] [PubMed] [Google Scholar]
- 54.Vaitkus S, et al. Translation, cross-cultural adaptation, and validation of the sino-nasal outcome test (SNOT)-22 for Lithuanian patients. European Archives of Oto-Rhino-Laryngology. 2013;270(6):1843–1848. doi: 10.1007/s00405-012-2282-2. [DOI] [PubMed] [Google Scholar]
- 55.Virgin FW, et al. Extensive surgical and comprehensive postoperative medical management for cystic fibrosis chronic rhinosinusitis. American Journal of Rhinology and Allergy. 2012;26(1):70–75. doi: 10.2500/ajra.2012.26.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garetier M, et al. Clinical-radiological correlation after functional endoscopic sinus surgery in patients with chronic rhinosinusitis: Interest of a sinonasal aerial volumetry. Rhinology. 2013;51(2):162–170. doi: 10.4193/Rhino12.131. [DOI] [PubMed] [Google Scholar]
- 57.Mascarenhas JG, et al. Long-term outcomes of endoscopic sinus surgery for chronic rhinosinusitis with and without nasal polyps. Brazilian Journal of Otorhinolaryngology. 2013;79(3):306–311. doi: 10.5935/1808-8694.20130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sacks PL, et al. The impact of neo-osteogenesis on disease control in chronic rhinosinusitis after primary surgery. International Forum of Allergy and Rhinology. 2013;3(10):823–827. doi: 10.1002/alr.21192. [DOI] [PubMed] [Google Scholar]
- 59.Bizaki AJ, et al. Quality of life after endoscopic sinus surgery or balloon sinuplasty: A randomized clinical study. Rhinology. 2014;52(4):300–305. doi: 10.4193/Rhino12.198. [DOI] [PubMed] [Google Scholar]
- 60.Cantone E, et al. Impact of intranasal sodium hyaluronate on the short-term quality of life of patients undergoing functional endoscopic sinus surgery for chronic rhinosinusitis. International Forum of Allergy and Rhinology. 2014;4(6):484–487. doi: 10.1002/alr.21310. [DOI] [PubMed] [Google Scholar]
- 61.Cho KS, et al. Long-term sinonasal outcomes of aspirin desensitization in aspirin exacerbated respiratory disease. Otolaryngology - Head and Neck Surgery (United States) 2014;151(4):575–581. doi: 10.1177/0194599814545750. [DOI] [PubMed] [Google Scholar]
- 62.Dautremont JF, Mechor B, Rudmik L. The role of immediate postoperative systemic corticosteroids when utilizing a steroid-eluting spacer following sinus surgery. Otolaryngology - Head and Neck Surgery (United States) 2014;150(4):689–695. doi: 10.1177/0194599814521373. [DOI] [PubMed] [Google Scholar]
- 63.ElBadawey MR, et al. Quality of life benefit after endoscopic frontal sinus surgery. American Journal of Rhinology and Allergy. 2014;28(5):428–432. doi: 10.2500/ajra.2014.28.4063. [DOI] [PubMed] [Google Scholar]
- 64.Hashemian F, et al. The effect of thyme honey nasal spray on chronic rhinosinusitis: a double-blind randomized controlled clinical trial. European Archives of Oto-Rhino-Laryngology. 2015;272(6):1429–1435. doi: 10.1007/s00405-014-3233-x. [DOI] [PubMed] [Google Scholar]
- 65.Lachanas VA, et al. The sino-nasal outcome test (SNOT)-22: validation for Greek patients. European archives of oto-rhino-laryngology: official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS): affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2014;271(10):2723–2728. doi: 10.1007/s00405-014-2969-7. [DOI] [PubMed] [Google Scholar]
- 66.Saedi B, et al. Impact of endoscopic sinus surgery on the quality of life of patients with nasal polyposis. B-ENT. 2014;10(1):59–65. [PubMed] [Google Scholar]
- 67.Savastano V, et al. Evaluation of chronic rhinosinusitis management sing the SNOT-22 in adult cystic fibrosis patients. European Review for Medical and Pharmacological Sciences. 2014;18(14):1985–1989. [PubMed] [Google Scholar]
- 68.Zhang Z, et al. Quality of life improvement from sinus surgery in chronic rhinosinusitis patients with asthma and nasal polyps. International Forum of Allergy and Rhinology. 2014;4(11):885–892. doi: 10.1002/alr.21406. [DOI] [PubMed] [Google Scholar]
- 69.Amali A, et al. Long-term postoperative azithromycin in patients with chronic rhinosinusitis: A randomized clinical trial. American Journal of Rhinology and Allergy. 2015;29(6):421–424. doi: 10.2500/ajra.2015.29.4244. [DOI] [PubMed] [Google Scholar]
- 70.Divekar R, et al. Symptom-Based Clustering in Chronic Rhinosinusitis Relates to History of Aspirin Sensitivity and Postsurgical Outcomes. Journal of Allergy and Clinical Immunology: In Practice. 2015;3(6):934–940.e3. doi: 10.1016/j.jaip.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee JM, et al. Nasal nitric oxide as a marker of sinus mucosal health in patients with nasal polyposis. International Forum of Allergy and Rhinology. 2015;5(10):894–899. doi: 10.1002/alr.21598. [DOI] [PubMed] [Google Scholar]
- 72.Lind H, et al. Efficacy of ESS in chronic rhinosinusitis with and without nasal polyposis: a Danish cohort study. European Archives of Oto-Rhino-Laryngology. 2016;273(4):911–919. doi: 10.1007/s00405-015-3667-9. [DOI] [PubMed] [Google Scholar]
- 73.Ottaviano G, et al. Silver sucrose octasulfate nasal applications and wound healing after endoscopic sinus surgery: a prospective, randomized, double-blind, placebo-controlled study. American Journal of Otolaryngology - Head and Neck Medicine and Surgery. 2015;36(5):625–631. doi: 10.1016/j.amjoto.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 74.Rotenberg BW, Pang KP. The impact of sinus surgery on sleep outcomes. International Forum of Allergy and Rhinology. 2015;5(4):329–332. doi: 10.1002/alr.21488. [DOI] [PubMed] [Google Scholar]
- 75.Shapira Galitz Y, et al. Sino-Nasal Outcome Test-22: Translation, Cross-cultural Adaptation, and Validation in Hebrew-Speaking Patients. Otolaryngology - Head and Neck Surgery (United States) 2016;154(5):951–956. doi: 10.1177/0194599816629378. [DOI] [PubMed] [Google Scholar]
- 76.Adappa ND, et al. TAS2R38 genotype predicts surgical outcome in nonpolypoid chronic rhinosinusitis. International Forum of Allergy and Rhinology. 2016;6(1):25–33. doi: 10.1002/alr.21666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bizaki AJ, et al. A Controlled, Randomized Clinical Study on the Impact of Treatment on Antral Mucociliary Clearance. Annals of Otology, Rhinology & Laryngology. 2016;125(5):408–414. doi: 10.1177/0003489415618676. [DOI] [PubMed] [Google Scholar]
- 78.Do TQ, et al. Clinical implications of mucosal remodeling from chronic rhinosinusitis. International Forum of Allergy & Rhinology. 2016;6(8):835–840. doi: 10.1002/alr.21754. [DOI] [PubMed] [Google Scholar]
- 79.Lal D, et al. Gender-specific analysis of outcomes from endoscopic sinus surgery for chronic rhinosinusitis. International Forum of Allergy & Rhinology. 2016;6(9):896–905. doi: 10.1002/alr.21773. [DOI] [PubMed] [Google Scholar]
- 80.Masterson L, et al. Quality-of-life outcomes after sinus surgery in allergic fungal rhinosinusitis versus nonfungal chronic rhinosinusitis. American Journal of Rhinology and Allergy. 2016;30(2):e30–e35. doi: 10.2500/ajra.2016.30.4280. [DOI] [PubMed] [Google Scholar]
- 81.Meier JC, et al. The impact of surgical trainee participation on sinus surgery outcomes. Laryngoscope. 2016;126(2):316–321. doi: 10.1002/lary.25504. [DOI] [PubMed] [Google Scholar]
- 82.Soler ZM, et al. Cluster analysis and prediction of treatment outcomes for chronic rhinosinusitis. The Journal of allergy and clinical immunology. 2016;137(4):1054–62. doi: 10.1016/j.jaci.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang YingYang, Hai-Bo Effects of functional endoscopic sinus surgery on the treatment of bronchiectasis combined with chronic rhino-sinusitis. Acta Oto-Laryngologica. 2016;136(8):860–863. doi: 10.3109/00016489.2016.1157730. [DOI] [PubMed] [Google Scholar]
- 84.Chakraborty A, et al. Exploring Multivariate Data with the Forward Search. JSTOR; 2004. [Google Scholar]
- 85.Rudmik L, et al. Productivity costs decrease after endoscopic sinus surgery for refractory chronic rhinosinusitis. Laryngoscope. 2016;126(3):570–574. doi: 10.1002/lary.25656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fokkens W, Lund V, Mullol J. European position paper on rhinosinusitis and nasal polyps 2007. Rhinology. Supplement. 2007;(20):1–136. [PubMed] [Google Scholar]
- 87.Meltzer EO, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114(6 Suppl):155–212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Benninger MS, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129(3 Suppl):S1–32. doi: 10.1016/s0194-5998(03)01397-4. [DOI] [PubMed] [Google Scholar]
- 89.Fokkens WJ, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinology. Supplement. 2012;(23):3. 1–298. p preceding table of contents. [PubMed] [Google Scholar]
- 90.Lanza DC, Kennedy DW. Adult rhinosinusitis defined. Otolaryngol Head Neck Surg. 1997;117(3 Pt 2):S1–7. doi: 10.1016/S0194-59989770001-9. [DOI] [PubMed] [Google Scholar]
- 91.Rosenfeld RM. Clinical practice guideline on adult sinusitis. Otolaryngology - Head and Neck Surgery. 2007;137(3):365–377. doi: 10.1016/j.otohns.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 92.Guidelines for diagnosis and treatment of chronic rhinosinusitis (2012, Kunming) Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013;48(2):92–4. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Funnel plot of standard error by difference in means. Y-axis=standard error; X-axis=difference in means.
Supplementary Table 1. Quality Assessment of Included Studies