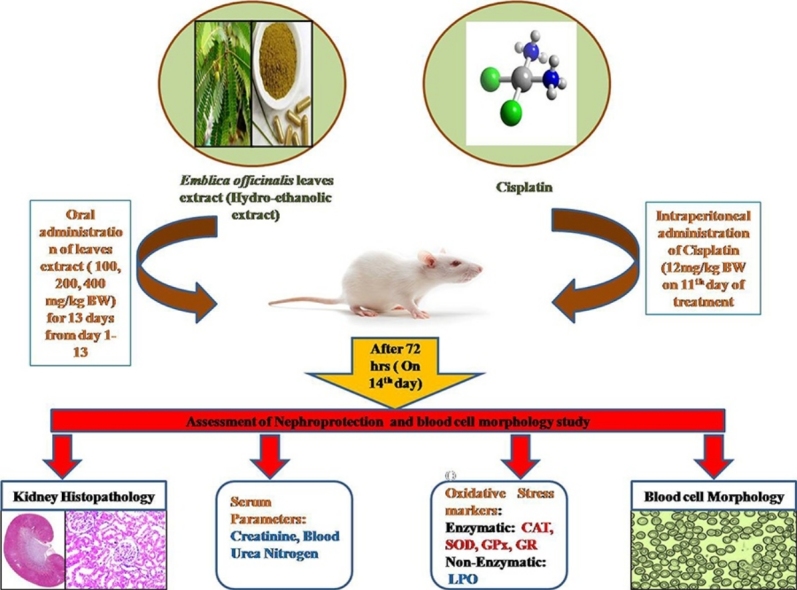
Graphical abstract
Abbreviations: BUN, blood urea nitrogen; BW, body weight; BSA, bovine serum albumin; CAT, catalase; DNA, deoxy-ribonucleic acid; DMSO, dimethyl sulfoxide; DTNB, 5,5-dithio-bis(2-nitrobenzoic acid); FDA, food and drug administration; GPx, glutathione peroxidase; GR, glutathione reductase; GAMT, guanidinoacetate methyltransferase; H&E, hematoxylene and eosin; MDA, malondialdehyde; NaCl, sodium chloride; NADPH, nicotinamide adenine dinucleotide phosphate; RBCs, red blood cells; ROS, reactive oxygen species; RNS, reactive nitrogen species; SOD, superoxide dismutase; SEM, standard error mean; TBA, 2-thiobarbituric acid; TCA, trichloroacetic acid
Keywords: Emblica officinalis, Cisplatin, Nephrotoxicity, Oxidative stress, Rats
Highlights
-
•
Serum analysis for renal toxicity showed E. officinalis leaf extract able to significantly reduce creatinine and BUN in cisplatin toxicity.
-
•
Biochemical observation showed E. officinalis leaf extract significantly increase the activity of CAT, SOD, GPx, and GR to normal values.
-
•
Histopathology and RBC morphology study revealed that E. officinalis leaf extract decrease the toxic effects of cisplatin in kidney and RBCs.
Abstract
Nephrotoxicity is a major limiting factor in cisplatin treatment. In the present study hydro-ethanolic leaf extract of Emblica officinalis was investigated for its protective role in cisplatin induced nephrotoxicity. The experiment was designed for 14 days and male Wistar rats were divided into 9 groups (n = 5). Group 1 served as control (with no treatment), group 2 served as a vehicle control and received 0.9% NaCl intraperitoneally (i.p.) on 11th day of the treatment, group 3 received a single dose of cisplatin on 11th day (12 mg/kg body weight, i.p.), group 4–6 received leaf extract only (100 mg/kg, 200 mg/kg and 400 mg/kg body weight, respectively) throughout the treatment, group 7–9 received leaf extract (100 mg/kg, 200 mg/kg and 400 mg/kg body weight, respectively) throughout the treatment and single dose of cisplatin on the 11th day of the leaf extract treatment. At the end of the experiment (i.e. on 14th day) blood samples were collected from all the groups and were sacrificed to study renal functional parameters. Treatment with above doses of E. officinalis leaf extract significantly (p ≤ 0.05) attenuates renal damage by decreasing serum creatinine and blood urea nitrogen (BUN), enhanced the activities of Catalase, SOD, GPx, GR and decreased the renal MDA level compared with the cisplatin treatment group. Furthermore the oral administration of Amla leaf extract improves histological damage and morphological changes in RBCs. Our results suggest that, leaf extract of E. officinalis may ameliorate renal damage caused by cisplatin.
1. Introduction
Cisplatin (Cis-diamminedichloro platinum II) also called as “The Penicillin of cancer” was the first big chemotherapy drug [1]. FDA in 1978, approved cisplatin as leading anti- cancer drug for several types of cancer, such as bladder cancer, cervical cancer, ovarian cancer, testicular cancer, non-small cell lung cancer, squamous cell carcinoma of the head & neck, with 90% cure rates in testicular cancer, either used alone or combined with other drugs. Even with the advancement of new therapies in the past decades, the use of cisplatin remains strong [[2], [3], [4]]. Although the profound effects of cisplatin in different types of the cancer, the patients experience severe side effects such as vomiting and nausea, myelosuppression, neurotoxicity, ototoxicity and nephrotoxicity that limit its use [5]. Nephrotoxicity is the major limitation in cisplatin treatment as Kidney is exposed to large amounts of parent and active metabolites of drugs. The toxic effects of cisplatin in kidney affects approximately 25–35% of patients treated with a single dose, thereby limiting its use in higher doses and compromising its chemotherapeutic efficacy [4]. In kidney the proximal tubules especially S3 segment is severely affected by the cisplatin treatment and is characterized by the loss of microvilli, cellular swelling and condensation of nuclear chromatin. Other functional characters of renal dysfunction are decrease in glomerular filtration, increased serum creatinine and BUN levels, increased lipid peroxidation, and decrease in enzymatic and non enzymatic antioxidants [[2], [4], [6]].
Cisplatin causes tubular cell death by either necrosis or apoptosis depending upon the concentration and duration of exposure of drug. Many of the pathways involved in tumor cell death involve mitochondrial dysfunction and oxidative stress. Many studies have focused on the use of natural and synthetic antioxidants or ROS scavengers for Reno-protection [7]. In vitro and in vivo studies have reported protective effect of several natural and synthetic antioxidants in cisplatin induced nephrotoxicity [[8], [9], [10]]. However, lack of data regarding their effects on the antitumor activity of cisplatin and lack of clinical trials limits the clinical application of compounds [[4], [11], [12]].
E. officinalis commonly called as Amla (Indian gooseberry) is one of the most studied plant. Plant parts of E. officinalis show antibacterial, antioxidant, antidiabetic, hypolipidemic, anticancer, hepatoprotective, gastroprotective, antiulcerogenic, nephroprotective and chemopreventive properties [[13], [14]]. Most important part of plant is fruit and has been explored more for its medicinal values. However, the leaves are explored substantially less. The active constituents of leaves include Apigenin-7-O-(6_-butyryl- _-glucopyranoside), flavanone glycosides, gallic acid, methyl gallate, luteolin-4_-O-neohesperidoside, 1,2,3,4,6-penta-O-galloylglucose, trihydroxysitosterol, 5 _, 6 _, 7 _-acetoxysitosterol, 5-hydroxymethylfurfural, 2-acetyl-5-methyl furan, pyrogallol, ellagic acid [[15], [16], [17], [18]]. The active constituents of leaves of E. officinalis show antibacterial and antioxidant properties [19], anti-inflammatory properties [[20], [21]]. Huang and Zhong [22] studied the anticancer mechanism of gallic acid isolated from Phyllanthus emblica against hepatocellular carcinoma cells. Nain et al. [23] reported antidiabetic and antioxidant properties of leaves against streptozotocin-induced type-2 diabetes mellitus (T2DM) rats by enhancing the activities of antioxidant enzymes Recently, Malik et al. [24] reported E. officinalis dried fruit extract ameliorates cisplatin induced renal damage through suppression of MAPK induced inflammation and apoptosis.
Based on the pharmacological and ethno botanical reports of E. officinalis, the present investigation was focused on studying antioxidant properties of plant and protective role of its hydro-ethanolic leaf extract in cisplatin induced nephrotoxicity. We measured enzymatic activities such as SOD, CAT, GPx, GR, along with the hematological and histopathological parameters.
2. Materials and method
2.1. Chemicals
Cisplatin was purchased from Thermo Fisher, Waltham, USA. Sulphanilamide, EDTA (Ethylenediamine- tetra acetic acid disodium salt dihydrate), NBT (Nitroblue tetrazolium chloride), Methionine, Riboflavin were procured from Himedia, Mumbai, India. Creatinine assay kit (Erba) was purchased from Transasia Bio-medicals Ltd., Solan, H.P. India, and Urea assay Kit (Auto span) was purchased from Arkray healthcare Pvt. Ltd., Gujrat, India, Hydrogen peroxide was obtained from Fisher Scientific Mumbai, India. All other chemicals were of analytical grade and were purchased from local vendors supplying scientific grade chemicals.
2.2. Preparation of leaf extract
The leaves of Emblica officinalis (Amla) were collected from areas near Bilaspur district. The leaves were identified and authenticated by qualified taxonomist from Guru Ghasidas University, Bilaspur, C.G. The collected leaves were washed with tap water to remove dirt and air dried at room temperature for 7 days. The dried leaves were grounded into powder form; 30 g of grounded leaves was extracted by 70% ethanolic solvent using soxhlet extractor for 6–8 h at 70 °C. After the extraction, the extracts were concentrated by evaporating the solvent until it got reduced to a solid mass.
2.3. Animals
Male Wistar rats weighing between 120 to 180gm were used in the experiment. Animals were maintained under controlled environment. Five animals per cage were placed at a temperature of 22 ± 2 °C with 12 h light and 12 h dark cycle. Rats were kept on bed of rice husk and fed on standard rodent diet and water ad libitium.
All animal experiments were performed subsequent to the approval of institutional animal ethical committee and by animal regulatory body of the government (Regd. No. 1321/PO/ReBi/10/CPCSEA).
2.4. Experimental design
Male Wistar rats were divided into nine groups each group containing five rats. Total duration of experiment was 14 days. Group 1 served as control (with no treatment), group 2 served as vehicle control and given vehicle only (0.9% saline) on 11th day of the treatment, group 3 received a single dose of cisplatin (12 mg/kg body weight) in 0.9% saline on 11th day, groups 4–6 were orally administered hydro ethanolic extract of Emblica officinalis leaves (100 mg/kg, 200 mg/kg and 400 mg/kg body weight, respectively) during entire treatment period, groups 7–9 received hydro ethanolic extract of Emblica officinalis leaves (100 mg/kg, 200 mg/kg and 400 mg/kg body weight, respectively) during entire treatment period and along with a single dose of cisplatin (12 mg/kg body weight) in 0.9% saline on 11th day.
Cisplatin was prepared in normal saline (0.9% NaCl) and administered intraperitoneally (i.p). Plant extracts were administered via oral gavage and prepared in normal water. Dose of Amla leave extract was selected on the basis of acute toxicity study in its leaves extract [23]. At the end of the experiment (i.e. 14th day of experiment) blood samples were collected for hematological study followed by sacrificing the animal. Kidneys were dissected bilaterally one part of the kidney was fixed in Bouin’s fixative for histopathological study and the other part was kept frozen in −80 °C for enzymatic and biochemical assays.
2.5. Collection of blood and isolation of serum
Blood was collected from animals by puncturing retro-orbital venous sinus and allowed to clot at 37 °C for 40 min and centrifuged at 3000 rpm for 20 min. The serum samples was taken in a clean mini centrifuge tubes and kept refrigerated until further used.
2.6. Preparation of tissue homogenate
72 h after cisplatin treatment all rats were weighed and sacrificed for isolation of kidney. After isolation, kidney samples were immediately weighed and frozen in dry ice and stored at −80 °C until further analysis. Kidney to body weight ratio was calculated. All the enzyme assays were performed on next day of kidney isolation. The Kidney were minced into small pieces and homogenized in ice cold phosphate buffer saline (0.05 M, pH 7.0) to obtain a 10% homogenate. The homogenate was centrifuged at 17000g for 60 min at 4 °C and the supernatant was used for assay of protein, Catalase, SOD, GPx, GR and MDA level.
2.7. Assessment of nephroprotective activity
2.7.1. Hematological analysis
The Renal function test was determined by assaying blood urea nitrogen (BUN) and serum creatinine, BUN and creatinine concentration were measured by Urea assay Kit and Creatinine assay kit respectively.
2.7.2. Estimation of free radical scavenging enzyme
The activities of superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT) and glutathione reductase (GR) in kidney tissue were assayed according to the methods reported by various groups (Masayasu and Hiroshi [25], Paglia and Valentine [26], Cohen et al. [27] and Carlberg and Mannervik [28] respectively).
2.7.3. Estimation of lipid per-oxidation (LPO)
The concentration of MDA (Malondialdehyde) in kidney tissue as an index of lipid peroxidation was determined by method of Ohkawa et al. [29] using extinction coefficient of 1.56 × 105 M−1 cm−1 and expressed as n moles of MDA/g of tissue.
2.8. Estimation of total protein
Total protein content in tissue was determined by the method of Lowry et al. [30] using bovine serum albumin (BSA) as standard.
2.9. Histological analysis
For histological assessment kidney from all the groups were fixed in Bouin’s fixative for 2 days, dehydrated in graded alcohol, cleared in xylene and embedded in paraffin wax. Fine sections (4–5 μm) were cut using a microtome (Leica RM 2125 RTS), mounted on glass slides and stained with eosin- hematoxylin and examined under light microscope for histological damage at 10× magnification, image was taken with a digital camera (Leica DM IL LED) attached to the microscope. Scale bars are equivalent of 50 μm.
2.10. Study of blood cell morphology
The effect of Cisplatin on the blood cell morphology was studied by simple staining of blood smear with gram stain. Blood smear was prepared by placing a single drop of blood on glass slide at a distance of 2 cm from one end and carefully extended to form a uniform smear. The smear was air dried and fixed in 95% ethanol for 10 min. Staining was done using freshly prepared mixture of Giemsa stain (HiMedia lab, Mumbai, India) (0.5 ml of commercial liquid stain diluted in 9.5 ml distilled water) for 30 min. The slides were carefully washed with distilled water, air dried and examined under light microscope equipped with a digital camera (Leica DM IL LED).
2.11. Statistical analysis
All the data was analyzed by one- way analysis of variance (ANOVA) using Minitab 17 software. Values P ≤ 0.05 were considered as statistically significant. Values are represented as Mean ± SEM, for four rats in each group.
3. Results
3.1. Effect of cisplatin and leaf extract on body weight and kidney to body weight ratio
A significant decrease (p ≤ 0.01) in body weight and a significant increase (p ≤ 0.05) in kidney/body weight ratio was observed in cisplatin treated group when compared to normal control group (Table 1). Groups administered various doses of E. officinalis hydro-ethanolic leaf extract along with cisplatin showed there is no significant increase in body weight and corresponding decrease (p > 0.05) in kidney/body weight ratio with dose 400 mg/kg body wt. However, a significant change (p ≤ 0.05) in above parameters was observed in the group treated with 100 mg/kg and 200 mg/kg leaf extract when compared to cisplatin alone treated group. Also, there are no significant changes in body weight and kidney/body wt ratio of groups that received only leaf extract and vehicle control compared to normal control. Our observation suggest that animals receiving cisplatin alone registered a significant decrease in body weight and an increase in kidney/body wt. ratio which was prevented in animals receiving cisplatin along with Amla leaf extract.
Table 1.
Effect of E. officinalis leaf extract on body weight, kidney to body weight ratio in normal, leaf extract and Cisplatin treated rats.
| Groups | % Change in body weight (g) | (Kidney/body weight ratio)× 1000 |
|---|---|---|
| Normal Control | +9.22 ± 0.32 | 9.28 ± 0.30 |
| Vehicle Control | +6.08 ± 1.50ns | 9.44 ± 0.13ns |
| Cisplatin | −3.59 ± 0.19** | 11.37 ± 0.02* |
| AET 100 mg/kg | +6.89 ± 0.90ns | 6.97 ± 0.02ns |
| AET 200 mg/kg | +7.16 ± 1.69ns | 7.45 ± 0.36ns |
| AET 400 mg/kg | +9.71 ± 1.68ns | 7.75 ± 0.11ns |
| AET 100 + Cis | −1.90 ± 0.42# | 9.23 ± 0.2# |
| AET 200 + Cis | −1.80 ± 0.30# | 9.03 ± 0.28# |
| AET 400 + Cis | −2.14 ± 0.40NS | 10.01 ± 0.61NS |
Values are expressed as mean ± SEM, n = 4. **P ≤ 0.01 vs normal control, *p ≤ 0.05 vs normal control, #p ≤ 0.05 vs cisplatin, ns = non significant vs normal control, NS = non significant vs cisplatin. AET = Amla ethanolic extract, Cis = Cisplatin.
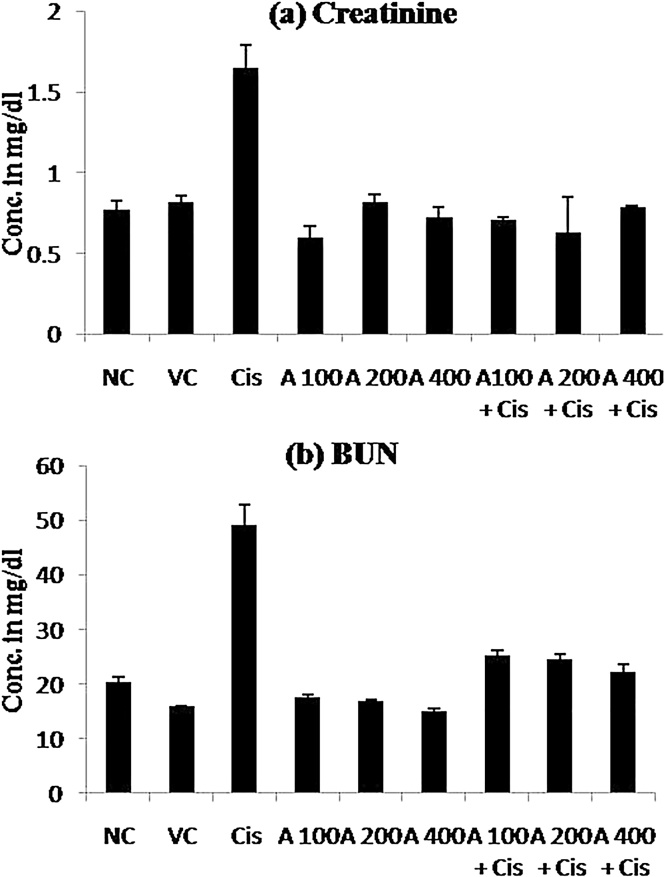
3.2. Effect of cisplatin and leaf extract on serum creatinine and blood urea nitrogen
There was no significant changes in both serum creatinine and BUN in vehicle control and groups treated with varying doses of leaf extract, however, both parameters was significantly (p ≤ 0.05) increased in cisplatin treated groups when compared to normal control group (Table 2; Fig. 1). Treatment with leaf extract at 100 mg/kg, 200 mg/kg and 400 mg/kg bodyweight significantly (p ≤ 0.05) prevented the renal dysfunctions, which is reflected by normal serum creatinine and BUN values when compared to cisplatin treated group.
Table 2.
Effect of E. officinalis leaf extract in cisplatin induced renal Creatinine and Blood Urea Nitrogen (BUN) level in normal, leaf extract and Cisplatin treated rats.
| Groups | Creatinine (mg/dl) | BUN (mg/dl) |
|---|---|---|
| Normal Control | 0.77 ± 0.06 | 20.29 ± 0.98 |
| Vehicle Control | 0.82 ± 0.04ns | 15.74 ± 0.06ns |
| Cisplatin | 1.65 ± 0.14* | 49.04 ± 3.81* |
| AET 100 mg/kg | 0.6 ± 0.07 ns | 17.40 ± 0.51ns |
| AET 200 mg/kg | 0.82 ± 0.04ns | 16.86 ± 0.18ns |
| AET 400 mg/kg | 0.72 ± 0.07ns | 14.87 ± 0.61ns |
| AET 100 + Cis | 0.71 ± 0.01# | 25.09 ± 0.98 # |
| AET 200 + Cis | 0.63 ± 0.22# | 24.37 ± 1.14# |
| AET 400 + Cis | 0.79 ± 0.01# | 22.14 ± 1.43# |
Values are expressed as mean ± SEM, n = 4. *p ≤ 0.05 vs normal control, #p ≤ 0.05 vs cisplatin, ns = non-significant vs normal control, NS = non significant vs cisplatin.AET = Amla ethanolic extract, Cis = Cisplatin.
Fig. 1.
Effect of E. officinalis leaf extract in cisplatin induced renal (a) Creatinine and (b) Blood Urea Nitrogen (BUN) level in normal, leaf extract and Cisplatin treated rats. NC = Normal Control, VC = Vehicle Control, Cis. = Cisplatin, A = E. officinalis hydro ethanolic leaf extract.
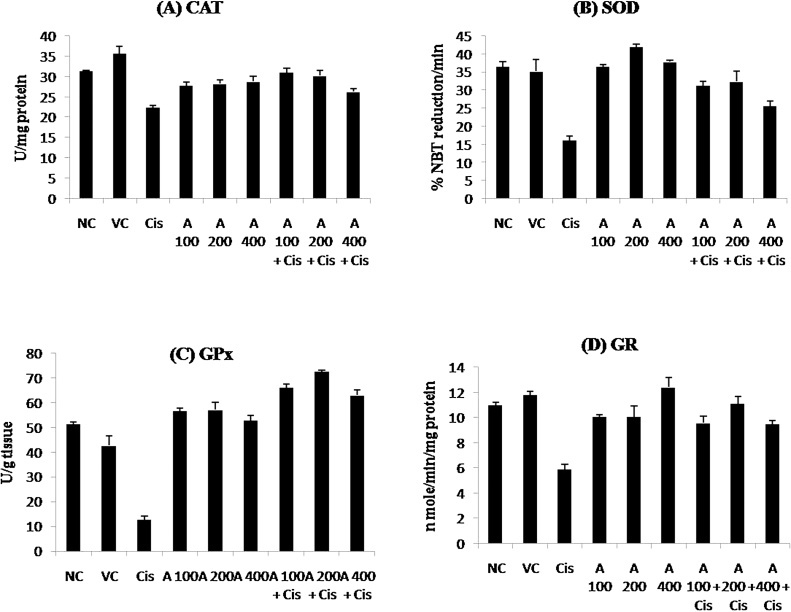
3.3. Effect of cisplatin and leaf extract on markers of oxidative stress
The renal toxicity of cisplatin is mainly associated with induction of oxidative stress, for this reason various enzymatic and non-enzymatic antioxidant markers were measured. As shown in Table 3; Fig. 2 cisplatin treatment significantly decrease (p ≤ 0.01) the activities of various antioxidant enzymes (i.e. CAT, SOD, GPx and GR) and enhanced the renal MDA level (Table 4; Fig. 3; p ≤ 0.01) as compared to normal control group. The effect of various doses of leaf extract (100 mg/kg, 200 mg/kg and 400 mg/kg body weight) on the above markers of oxidative stress was as follows:
Table 3.
Effect of E. officinalis leaf extract on antioxidant enzymes in normal, leaf extract and Cisplatin treated rats.
| Groups | Catalase (U/mg protein) | SOD (% NBT reduction/min) | GPx (U/g tissue) | GR (n mole/min/mg protein) |
|---|---|---|---|---|
| Normal Control | 31.41 ± 0.06 | 36.45 ± 1.36 | 51.28 ± 0.89 | 11.00 ± 0.21 |
| Vehicle Control | 35.72 ± 1.65ns | 35.18 ± 3.31ns | 42.65 ± 3.80ns | 11.79 ± 0.27ns |
| Cisplatin | 22.45 ± 0.47** | 16.13 ± 1.16** | 12.83 ± 1.37** | 05.90 ± 0.39** |
| AET 100 mg/kg | 27.84 ± 0.79ns | 36.46 ± 0.64ns | 56.69 ± 1.16ns | 10.04 ± 0.19ns |
| AET 200 mg/kg | 28.22 ± 0.99ns | 41.88 ± 0.92ns | 57.04 ± 3.07ns | 10.04 ± 0.86ns |
| AET 400 mg/kg | 28.75 ± 1.27ns | 37.82 ± 0.50ns | 52.73 ± 2.07ns | 12.41 ± 0.75ns |
| AET 100 + Cis | 31.00 ± 0.98# | 31.27 ± 1.21# | 66.11 ± 1.45## | 9.97 ± 0.54# |
| AET 200 + Cis | 30.21 ± 1.39# | 32.35 ± 2.85# | 72.63 ±0.50### | 11.11 ± 0.58# |
| AET 400 + Cis | 26.25 ± 0.78NS | 25.60 ± 1.47# | 63.03 ±2.25## | 09.49 ± 0.29# |
Values are expressed as mean ± SEM, n = 4. **P ≤ 0.01 vs normal control, ###p ≤ 0.001 vs cisplatin, ##p ≤ 0.01 vs cisplatin, #p ≤ 0.05 vs cisplatin, ns = non significant vs normal control, NS = non significant vs cisplatin. AET = Amla ethanolic extract, Cis = Cisplatin.
Fig. 2.
Effect of E. officinalis leaf extracts on antioxidant enzymes in normal, leaf extract and Cisplatin treated rats: (a) Catalase (CAT) (b) Superoxide dismutase (SOD) (c) Glutathione Peroxidase (GPx) (d) Glutathione Reductase (GR). NC = Normal Control, VC = Vehicle Control, Cis. = Cisplatin, A = E. officinalis hydro ethanolic leaf extract.
Table 4.
Effect of E. officinalis leaf extract on renal MDAlevel in normal, leaf extract and Cisplatin treated rats..
| Groups | MDA (n mole MDA/g tissue) |
|---|---|
| Normal Control | 20.95 ± 0.22 |
| Vehicle Control | 15.34 ± 1.74ns |
| Cisplatin | 28.06 ± 0.21** |
| AET 100 mg/kg | 17.02 ± 1.21ns |
| AET 200 mg/kg | 16.65 ± 1.17ns |
| AET 400 mg/kg | 16.53 ± 1.23ns |
| AET 100 + Cis | 20.23 ± 0.98# |
| AET 200 + Cis | 19.89 ± 1.38# |
| AET 400 + Cis | 23.35 ± 0.41# |
Values are expressed as mean ± SEM, n = 4. **P ≤ 0.01 vs normalcontrol, *p ≤ 0.05 vs normal control,#p ≤ 0.05 vs cisplatin, ns = non significant vs normal control, NS = non significant vs cisplatin. AET = Amla ethanolic extract, Cis = Cisplatin.
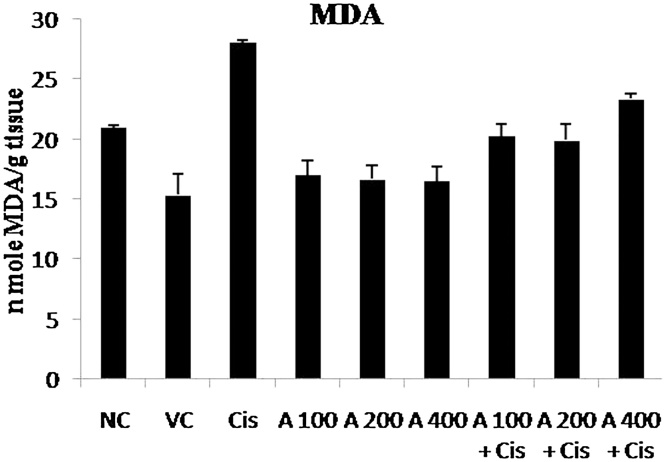
Fig. 3.
Effect of E. officinalis leaf extract on renal Malondialdehyde (MDA) level in normal, leaf extract and Cisplatin treated rats. NC = Normal Control, VC = Vehicle Control, Cis. = Cisplatin, A = E. officinalis hydro ethanolic leaf extract.
3.3.1. Effect of leaf extract on catalase (CAT) activity
The rats treated with 100 mg/kg and 200 mg/kg BW of leaf extract significantly (p ≤ 0.05) up regulated CAT activity when compared to the cisplatin treated group in which this was significantly decreased. Also, the groups receiving only leaf extracts supplementation did not show any difference in CAT activity when compared to normal control group (Table 3; Fig. 2A).
3.3.2. Effect of leaf extract on superoxide dismutase (SOD) activity
The activity of superoxide dismutase was significantly (p ≤ 0.05) high in all the groups receiving different doses of leaf extract when compared to cisplatin treated group in which activity of this enzyme was dramatically reduced. Also, the groups given leaf extracts alone did not showed any significant difference in SOD activity when compared to normal control group (Table 3; Fig. 2B). This data suggests that physiological levels of SOD were maintained in all the groups other than cisplatin treated group.
3.3.3. Effect of leaf extract on glutathione peroxidase (GPx) activity
The GPx activity was significantly elevated to the normal levels by all the above doses of leaf extract, however, a more pronounced effect (p ≤ 0.001) was noticed at the dose 200 mg/kg BW when compared to cisplatin treated group. Also, the groups receiving leaf extracts alone did not show any significant difference in GPx activity when compared to the normal control group (Table 3; Fig. 2C).
3.3.4. Effect of leaf extract on glutathione reductase (GR) activity
All the above doses of leaf extract were able to significantly (p ≤ 0.05) elevate the GR activity almost equivalent to the control group when compared with cisplatin treated group. Also, the rats receiving only leaf extracts did not show any significant difference in GR activity when compared to normal control group (Table 3; Fig. 2D).
3.3.5. Effect of leaf extract on renal MDA level
Oral administration of 100 mg/kg, 200 mg/kg and 400 mg/kg doses of leaf extract resulted in a significant (p ≤ 0.05) reduction in renal tissue MDA levels when compared to cisplatin treated group. Also, the groups receiving leaf extracts only did not show any significant difference in MDA level when compared to normal control group (Table 4; Fig. 3).
3.4. Histopathological study
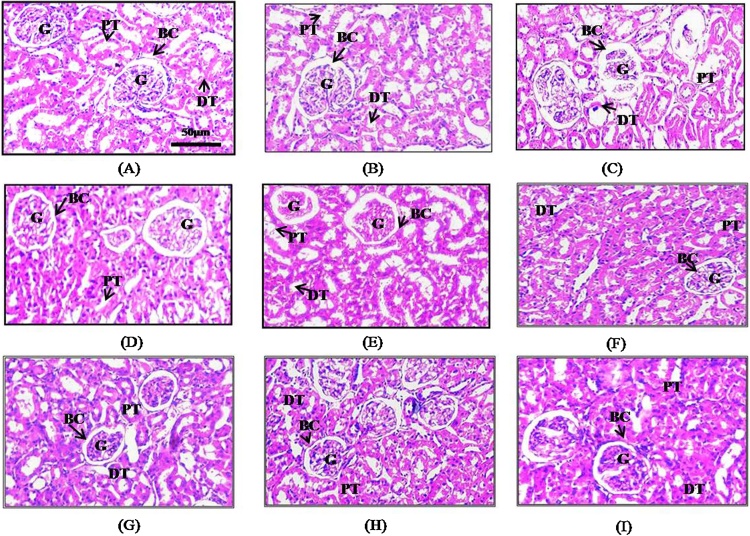
H&E staining of Kidney sections showed severe damage in cisplatin treated group (Fig. 4C), there is widespread necrosis, cellular swelling, sloughing of bowman’s capsule and degeneration of glomerulus, also in certain areas hemorrhage and polymorphic nuclei in renal tubular cells was observed, another noticeable feature is the dilated proximal convoluted tubule and distal convoluted tubule with sloughed off epithelium cells. In kidney sections of normal control, vehicle control and in groups receiving various dose of leaf extract (Fig. 4A, B, D, F, H) no pathological changes were observed. However, the groups receiving both cisplatin and various doses of leaf extract (Fig. 4E, G, I) showed slight necrosis and cellular swelling in renal tubules, sloughing of Bowman’s capsule and degeneration of glomerulus was considerably less when compared with cisplatin treated group. Among all the three groups receiving different doses of the leaf extract, tubular damage was less evident in group receiving 100 mg/kg and 200 mg/kg BW of leaf extract as compared 400 mg/kg BW suggesting the effect is dose dependent.
Fig. 4.
Histopathological Study: (A) Normal control (B) Vehicle control (C) Cisplatin (D) AET 100 mg/kg (E) AET 100 mg/kg + CIS (F) AET 200 mg/kg (G) AET 200 mg/kg + CIS (H) AET 400 mg/kg (I) AET 400 mg/kg + CIS. AET = Amla ethanolic extract, CIS = Cisplatin, BC = Bowman’s capsule, G = Glomerulus, PT = Proximal Tubule, DT = Distal Tubule.
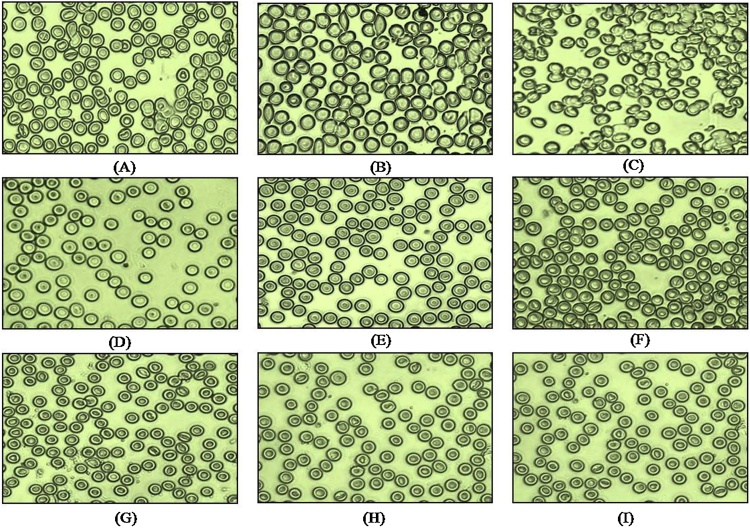
3.5. Effect of cisplatin and leaf extract on blood cell morphology
The effect of cisplatin on the morphology of blood cells of rats is shown in Fig. 5. Results suggest that the blood cells of normal control and vehicle control were similar in appearance, ellipsoidal in shape with intact cell wall. Contrary to this the blood cells of cisplatin treated rats appeared irregular in shape. The other visible effect that was evident in cisplatin treated group was lysis of red blood cells with disintegration of cell membrane and clumping of the nuclear material. Comparison of blood cells of the rats receiving leaf extract and the control rats showed a normal morphology with no symptoms of toxicity which is in contrast to the cisplatin treated group.
Fig. 5.
Study of blood cell morphology: (A) Normal control (B) Vehicle control (C) Cisplatin (D) AET 100 mg/kg (E) AET 100 mg/kg + CIS (F) AET 200 mg/kg (G) AET 200 mg/kg + CIS (H) AET 400 mg/kg (I) AET 400 mg/kg + CIS. AET = Amla ethanolic extract, CIS = Cisplatin.
4. Discussion
Despite of dose and duration dependent renal toxicity induced by cisplatin, it is still widely used in various cancer therapies. Among all the described pathways involved in nephrotoxicity, oxidative stress is most prominent. Hence, the agents which can guard against the oxidative stress in renal tissues with its exogenous antioxidants and additional properties like anti-inflammatory and cytoprotective should be used in combination with the chemotherapy drugs to reduce nephrotoxic and harmful effects of drugs like cisplatin. Although many agents have been reported to protect renal tissues via decreasing oxidative stress, still none of them has proved to be clinically effective [11].
In the present study, we have attempted to investigate the effect of hydro-ethanolic extracts of E. officinalis (Common name: Indian Gooseberry or Amla) leaf on CP- induced nephrotoxicity in rats. Research from other labs has confirmed that Phytoconsituents of Amla leaves have antioxidative, anti-carcinogenic, antidiabetic and anti-inflammatory potential [[19], [20], [21], [22]] and play a significant role in ameliorating the oxidative stress [23]. Based on the above phytochemical and pharmacological properties, we investigated the protective role of the crude hydro-ethanolic extract of E. officinalis leaves in cisplatin induced nephrotoxicity.
For this study we have used 12 mg/kg BW [31] dose of cisplatin which is slightly less than higher dose of cisplatin (16 mg/kg BW) which corresponds to the dose used in clinical practice [32].It is reported that Rats showed signs of nephrotoxicity at a dose >5 mg/kg BW of cisplatin [[33], [34]]. Evaluation of Nephroprotective activity by various agents can be done with higher doses of cisplatin [35]. Higher dose of cisplatin also induces other toxic effects and provides a better chance to evaluate the potential of the supplemental compound for its efficiency in regulating the toxic effects.
The results obtained from the present study clearly suggest that the hydro-ethanolic fractions of E. officinalis leaves supplementation suppressed the Cisplatin-induced damage to the kidney which is reflected by normal values of BUN and creatinine markers. The level of serum creatinine and BUN was enhanced significantly in cisplatin treated group compared to the normal groups. Increased creatinine and urea levels are the markers of renal dysfunction and enhanced in those taking nephrotoxic medications [[36], [37], [38], [39]]. Treatment with different doses of (100 mg/kg, 200 mg/kg, and 400 mg/kg BW) E. officinalis leave extract significantly reduced the serum creatinine and BUN to normal levels (Table 2, Fig. 1). These results are similar to the study in which E. Officinalis dried fruit extract was used with cisplatin [24]. Increased creatinine in cisplatin control group may be due to up regulation of Guanidinoacetate methyltransferase (GAMT) [[40], [41]].
Also a single dose of cisplatin resulted in significant loss in body weight and increase in relative kidney weight. In our results we reported that the treatment group receiving E. officinalis leave extract 100 mg/kg and 200 mg/kg BW showed a significant increase in body weight and decrease in relative kidney weight, however higher dose did not cause any significant change in kidney/body weight (Table 1). This results are in accordance with previous reports [[42], [43]]. This weight loss in cisplatin treated rats was may be due to gastrointestinal toxicity and reduced ingestion of food.
We also analyzed various antioxidant enzyme levels which get affected by severe nephrotoxicity caused by the administration of cisplatin. Our data suggests that a single dose of cisplatin resulted in a significant decrease in activity of different antioxidative enzymes (SOD, CAT, GPx, and GR) in cisplatin group compared to normal rats, accompanied by an increase in lipid peroxidation as evidenced by significant increase in MDA level in cisplatin group. This results supports observation of previous studies that provided evidence that cisplatin mediated nephrotoxicity mainly involve Reactive Oxygen Species (ROS) generation and decrease in activities of enzymatic and non-enzymatic antioxidative defense [[36], [43], [44]]. The oxidative damage induced by cisplatin has been associated with damage to mitochondrial respiratory chain, which causes an increase in the generation of ROS such as superoxide anions (O2.−) and H2O2 and subsequent depletion of non- enzymatic (GSH and NADPH) and the enzymatic antioxidant defense system (SOD, CAT, GPx, and GR) in rat kidneys. Superoxide anions might originate hydroxyl radicals by partial reduction catalyzed by transition metals, mainly iron. Hydroxyl radicals are very strong oxidants that react with any component of cell like lipids, proteins and DNA, leading to loss in cell integrity, enzyme function and genomic stability [4]. Administration of E. officinalis leaf extract significantly enhanced the activities of above enzymes thereby, ameliorating the toxic effect of cisplatin on renal cells. The lower doses of E. officinalis leave extract (100 mg/kg and 200 mg/kg) appear to be protective and elevated the enzymatic activity to counter the nephrotoxic effect of cisplatin. However, higher, doses of 400 mg/kg of leaf extract were not effective in up regulating the Catalase activity.
It is also evident from the findings that the leaf extract used in this study more significantly acted on the activity of GPx compared to other enzymes. Some reports suggest that CAT activity was more vulnerable than SOD activity or GPx activity in cisplatin induced nephrotoxicity [45]. The decrease in activity of SOD and CAT causes increased lipid peroxidation in cisplatin treated group as evidenced by an increase in concentration of the renal MDA level, which remained low in groups that received different doses of leaf extract (100 mg/kg, 200 mg/kg and 400 mg/kg BW) (Table 3, Table 4, Fig. 2, Fig. 3). We hypothesize that the protective role of E. officinalis leaf extract supplementation may be due to reduction in oxidative stress via enhancing activities of various enzymatic antioxidants. The results reported here are similar to the findings of other studies [[31], [46], [47], [48]].
Histopathological examination revealed intact glomerulus and normal renal tubules with no pathological changes in normal control, vehicle control groups and groups receiving leaf extracts (Fig. 4A, B, D, F, H). However, severe damage in glomerulus and renal tubules was noted in cisplatin treated groups, supporting the findings of previous studies [[1], [36], [46], [49]]. Treatment with different doses of E. officinalis leaf extract for 13 days not completely reduces the damage as evidenced by many degenerating tubules but the damage was significantly less when compared with the cisplatin treated group (Fig. 4E, G, I). Among all the three doses the damage was least in group receiving 100 mg/kg and 200 mg/kg leaf extract supporting the above biochemical observations.
One common side effect of cisplatin and other platinum compounds is anemia, especially after repeated infusions. The primary cause may be due to myelosuppression resulting in lower count of red cell precursors. Some authors reported hemolytic anemia caused by an antiglobulin antibody directed against red cell membrane bound cisplatin [50]. Kutwin et al. [51] reported structural damage of chicken red blood cells exposed to cisplatin, causing deformity and swelling of red blood cells as a result of membrane damage. However, other studies have not shown any harmful effect on human red blood cell morphology. In the present study, a single dose of 12 mg/kg BW of cisplatin resulted in morphological changes and hemolysis of red blood cells when compared to the control groups (Fig. 5). Treatment with different doses of E. officinalis leaf extract for 13 days prevents cisplatin induced RBC damage almost to the normal, thereby reducing the toxic manifestation of cisplatin on RBCs.
5. Conclusion
A single dose of cisplatin (12 mg/kg BW) leads to significant renal damage as evidenced by oxidative stress and histological damage. E. officinalis hydro-ethanolic leaf extract significantly reduces the oxidative stress. Our results also suggest that the hydro-ethanolic leaf extract of E. officinalis contains phytochemicals, which may be protecting nephrotoxicity induced by cisplatin by enhancing the activities of enzymatic and non-enzymatic antioxidative defense system. Further studies are warranted to identify the nature of the actual compounds responsible for nephroprotection present in the E. officinalis leaf extract and the possible mechanisms by which they work to rescue the oxidative damage to the kidney cells.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
Authors (R.P) acknowledge the funding from Rajiv Gandhi National Fellowship vide ref. no. RGNF-2015-17-SC-CHH-20147. Authors are also thankful to Prof. Amit Roy (Principal) and Dr. Trilochan Satpathy (Associate Professor) Columbia Institute of Pharmacy, Raipur, C.G., for providing animal study facilities.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.toxrep.2018.01.008.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Uppuluri S., Ali S.L., Nirmala T., Shanthi M., Sipay B., Uppuluri K.B. Nephroprotector activity of hydro alcoholic extract of Tinospora cordifolia roots on cisplatin induced nephrotoxicity in rats. Drug Invent. Today. 2013;5(4):281–287. [Google Scholar]
- 2.Pabla N., Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73(9):994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 3.Reedijk J., Lohman P.H.M. Cisplatin: synthesis, antitumour activity and mechanism of action. Pharm. World Sci. 1985;7(7):173–180. doi: 10.1007/BF02307573. [DOI] [PubMed] [Google Scholar]
- 4.dos Santos N.A.G., Rodrigues M.A.C., Martins N.M., dos Santos A.C. Cisplatin-induced nephrotoxicity and targets of nephroprotection: an update. Arch. Toxicol. 2012;86(8):1233–1250. doi: 10.1007/s00204-012-0821-7. [DOI] [PubMed] [Google Scholar]
- 5.Florea A.M., Busselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancer. 2011;3(1):1351–1371. doi: 10.3390/cancers3011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee H.S., Kim B.K., Nam Y., Sohn U.D., Park E.S., Hong S.A., Lee J.H., Chung Y.H., Jeong J.H. Protective role of phosphatidylcholine against cisplatin-induced renal toxicity and oxidative stress in rats. Food Chem. Toxicol. 2013;58:388–393. doi: 10.1016/j.fct.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Mamoulakis C., Tsarouhas K., Fragkiadoulaki I., Heretis I., Wilks M.F., Spandidos D.A., Christina T., Tsatsakis A. Contrast-induced nephropathy: basic concepts, pathophysiological implications and prevention strategies. Pharmacol. Ther. 2017;180:99–112. doi: 10.1016/j.pharmthera.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Longchar A., Prasad S.B. Biochemical changes associated with ascorbic acid?cisplatin combination therapeutic efficacy and protective effect on cisplatin-induced toxicity in tumor-bearing mice. Toxicol. Rep. 2015;2:489–503. doi: 10.1016/j.toxrep.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosic G., Srejovic I., Zivkovic V., Selakovic D., Joksimovic J., Jakovljevic V. The effects of N-acetylcysteine on cisplatin-induced cardiotoxicity on isolated rat hearts after short-term global ischemia. Toxicol. Rep. 2015;2:996–1006. doi: 10.1016/j.toxrep.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasr A.Y. Protective effect of aged garlic extract against the oxidative stress induced by cisplatin on blood cells parameters and hepatic antioxidant enzymes in rats. Toxicol. Rep. 2014;1:682–691. doi: 10.1016/j.toxrep.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller R.P., Tadagavadi R.K., Ramesh G., Reeves W.B. Mechanisms of cisplatin nephrotoxicity. Toxins. 2010;2(11):2490–2518. doi: 10.3390/toxins2112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chirino Y.I., Pedraza-Chaverri J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp. Toxicol. Pathol. 2009;61(3):223–242. doi: 10.1016/j.etp.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Baliga M.S., Dsouza J.J. Amla (Emblica officinalis Gaertn), a wonder berry in the treatment and prevention of cancer. Eur. J. Cancer Prev. 2011;20(3):225–239. doi: 10.1097/CEJ.0b013e32834473f4. [DOI] [PubMed] [Google Scholar]
- 14.Jain R., Pandey R., Mahant R.N., Rathore D.S. A Review on medicinal importance of Emblica officinalis. IJPSR. 2015;6(1):72–84. [Google Scholar]
- 15.El-Desouky S.K., Ryu S.Y., Kim Y.K. A new cytotoxic acylated apigenin glucoside from Phyllanthus emblica, L. Nat. Prod. Res. 2008;22(1):91–95. doi: 10.1080/14786410701590236. [DOI] [PubMed] [Google Scholar]
- 16.Qi W.Y., Li Y., Hua L., Wang K., Gao K. Cytotoxicity and structure activity relationships of phytosterol from Phyllanthus emblica. Fitoterapia. 2013;84:252–256. doi: 10.1016/j.fitote.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 17.Balasubramanian S., Ganesh D., Panchal P., Teimouri M., Surya Narayana V.V.S. GC–MS analysis of phytocomponents in the methanolic extract of Emblica officinalis Gaertn (Indian Gooseberry) J. Chem. Pharm. Res. 2014;6(6):843–845. [Google Scholar]
- 18.Chugh C.A., Bharti D. Chemical characterization of antifungal constituents of Emblica officinalis. Allelopath. J. 2014;34(2):155–178. [Google Scholar]
- 19.Nain P., Saini V., Sharma S. In-vitro antibacterial and antioxidant activity of Emblica officinalis leaves extract. Int. J. Pharm. Sci. 2012;4(1):385–389. [Google Scholar]
- 20.Asmawi M.Z., Kankaanranta H., Moilanen E., Vapaatalo H. Anti-inflammatory activities of Emblica officinalis Gaertn leaf extracts. J. Pharm. Pharmacol. 1993;45(6):581–584. doi: 10.1111/j.2042-7158.1993.tb05605.x. [DOI] [PubMed] [Google Scholar]
- 21.Ihantola-Vormisto A., Summanen J., Kankaanranta H., Vuorela H., Asmawi Z.M., Moilanen E. Anti-inflammatory activity of extracts from leaves of Phyllanthus emblica. Planta Med. 1997;63(06):518–524. doi: 10.1055/s-2006-957754. [DOI] [PubMed] [Google Scholar]
- 22.Huang J.L., Zhong Z.G. Study of gallic acid extracted from the leaves of Phyllanthus emblica on apoptotic mechanism of human hepatocellular carcinoma cells BEL-7404. Zhongyaocai. 2011;34(2):246–249. [PubMed] [Google Scholar]
- 23.Nain P., Saini V., Sharma S., Nain J. Antidiabetic and antioxidant potential of Emblica officinalis Gaertn. Leaves extract in streptozotocin-induced type-2 diabetes mellitus (T2DM) rats. J. Ethnopharmacol. 2012;142(1):65–71. doi: 10.1016/j.jep.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Malik S., Suchal K., Bhatia J., Khan S.I., Vasisth S., Tomar A., Goyal S., Kumar Ra., Arya D.S., Ojha S.K. Therapeutic potential and molecular mechanisms of Emblica officinalis Gaertn in countering Nephrotoxicity in rats induced by the chemotherapeutic agent Cisplatin. Front. Pharmacol. 2016;7:1–11. doi: 10.3389/fphar.2016.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masayasu M., Hiroshi Y. A simplified assay method of superoxide dismutase activity for clinical use. Clin. Chim. Acta. 1979;92(3):337–342. doi: 10.1016/0009-8981(79)90211-0. [DOI] [PubMed] [Google Scholar]
- 26.Paglia D.E., Valentine W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione Peroxidase. Transl. Res. 1967;70(1):158–169. [PubMed] [Google Scholar]
- 27.Cohen G., Dembiec D., Marcus J. Measurement of catalase activity in tissue extracts. Anal. Biochem. 1970;34(1):30–38. doi: 10.1016/0003-2697(70)90083-7. [DOI] [PubMed] [Google Scholar]
- 28.Carlberg I., Mannervik B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 1975;250(14):5475–5480. [PubMed] [Google Scholar]
- 29.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 30.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 31.Divysree S., Nair C.K.K. Amelioration of cisplatin-induced nephrotoxicity by extracts of Hemidesmus indicus and Acorus calamus. Pharm. Biol. 2010;48(3):290–295. doi: 10.3109/13880200903116048. [DOI] [PubMed] [Google Scholar]
- 32.Somani S.M., Husain K., Whitworth C. Dose dependent protection by lipoic acid against cisplatin induced nephrotoxicity in rats: antioxidant defense system. Pharmacol. Toxicol. 2000;86:234–241. doi: 10.1034/j.1600-0773.2000.d01-41.x. [DOI] [PubMed] [Google Scholar]
- 33.DeWoskin R.S., Riviere J.E. Cisplatin-induced loss of kidney copper and nephrotoxicity is ameliorated by single dose diethyldithiocarbamate, but not mesna. Toxicol. Appl. Pharmacol. 1992;112:182–189. doi: 10.1016/0041-008x(92)90186-v. [DOI] [PubMed] [Google Scholar]
- 34.Babu E., Gopal Krishnan V.K., Sriganth I.N.P., Gopal Krishnan R., Sakthisekaran D. Cisplatin induced nephrotoxicity and the modulating effect of glutathione ester. Mol. Cell. Biochem. 1995;144:7–11. doi: 10.1007/BF00926734. [DOI] [PubMed] [Google Scholar]
- 35.Fontanelli R., Spatti G., Raspagliesi F., Zunino F., Re F.D. A preoperative single course of high-dose cisplatin and bleomycin with glutathione protection in bulky stage IB/II carcinoma of the cervix. Ann. Oncol. 1992;3(2):117–121. doi: 10.1093/oxfordjournals.annonc.a058125. [DOI] [PubMed] [Google Scholar]
- 36.Pillai T.G., John M., Thomas G.S. Prevention of cisplatin induced nephrotoxicity by terpenes isolated from Ganoderma lucidum occurring in Southern Parts of India. Exp. Toxicol. Pathol. 2011;63(1):157–160. doi: 10.1016/j.etp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Atessahin A., Ceribasi A.O., Yuce A., Bulmus O., Cikim G. Role of ellagic acid against cisplatin-induced nephrotoxicity and oxidative stress in rats. Basic Clin. Pharmacol.Toxicol. 2007;100(2):121–126. doi: 10.1111/j.1742-7843.2006.00015.x. [DOI] [PubMed] [Google Scholar]
- 38.Yilmaz H.R., Iraz M., Sogut S., Ozyurt H., Yildirim Z., Akyol O., Gergerlioglu S. The effects of erdosteine on the activities of some metabolic enzymes during cisplatin-induced nephrotoxicity in rats. Pharmacol. Res. 2004;50(3):287–290. doi: 10.1016/j.phrs.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Shino Y., Itoh Y., Kubota T., Yano T., Sendo T., Oishi R. Role of poly (ADP-ribose) polymerase in cisplatin-induced injury in LLC-PK1 cells. Free Radic. Biol. Med. 2003;35(8):966–977. doi: 10.1016/s0891-5849(03)00470-2. [DOI] [PubMed] [Google Scholar]
- 40.Hung Y.C., Huang G.S., Lin L.W., Hong M.Y., Se P.S. Thea sinensis melanin prevents cisplatin-induced nephrotoxicity in mice. Food Chem. Toxicol. 2007;45(7):1123–1130. doi: 10.1016/j.fct.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 41.Sahu B.D., Rentam K.K.R., Putcha U.K., Kuncha M., Vegi G.M.N., Sistla R. Carnosic acid attenuates renal injury in an experimental model of rat cisplatin-induced nephrotoxicity. Food Chem. Toxicol. 2011;49(12):3090–3097. doi: 10.1016/j.fct.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 42.Sahu B.D., Kuncha M., Sindhura G.J., Sistla R. Hesperidin attenuates cisplatin-induced acute renal injury by decreasing oxidative stress, inflammation and DNA damage. Phytomedicine. 2013;20(5):453–460. doi: 10.1016/j.phymed.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Naghizadeh B., Mansouri S.M.T., Mashhadian N.V. Crocin attenuates cisplatin-induced renal oxidative stress in rats. Food Chem. Toxicol. 2010;48(10):2650–2655. doi: 10.1016/j.fct.2010.06.035. [DOI] [PubMed] [Google Scholar]
- 44.Naziroglu M., Karaoglu A., Aksoy A.O. Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology. 2004;195(2):221–230. doi: 10.1016/j.tox.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Lee H.S., Kim B.K., Nam Y., Sohn U.D., Park E.S., Hong S.A., Lee J.H., Chung Y.H., Jeong J.H. Protective role of phosphatidylcholine against cisplatin-induced renal toxicity and oxidative stress in rats. Food Chem. Toxicol. 2013;58:388–393. doi: 10.1016/j.fct.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Ahmed E.A.M., Othman M.S., Aref A.M. Azadirachta indica attenuates cisplatin-induced nephrotoxicity and oxidative stress. Bio. Med. Res. Int. 2014;2014:1–11. doi: 10.1155/2014/647131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saad S.Y., Al-Rikabi A.C. Protection effects of taurine supplementation against cisplatin-induced nephrotoxicity in rats. Chemotherapy. 2002;48(1):42–48. doi: 10.1159/000048587. [DOI] [PubMed] [Google Scholar]
- 48.Somani S.M., Husain K., Whitworth C., Trammell G.L., Malafa M., Rybak L.P. Dose-dependent protection by lipoic acid against cisplatin-induced nephrotoxicity in rats: antioxidant defense system. Basic Clin. Pharmacol. Toxicol. 2000;86(5):234–241. doi: 10.1034/j.1600-0773.2000.d01-41.x. [DOI] [PubMed] [Google Scholar]
- 49.Surendra K.S., Goyal N. Protective effect of Heliotropium eichwaldi against cisplatin-induced nephrotoxicity in mice. J. Chin. Integr. Med. 2012;10(5):555–560. doi: 10.3736/jcim20120511. [DOI] [PubMed] [Google Scholar]
- 50.Maloisel F., Kurtz J.E., Andres E., Gorodetsky C., Dufour P., Oberling F. Platin salts-induced hemolytic anemia: cisplatin-and the first case of carboplatin-induced hemolysis. Anticancer Drugs. 1995;6(2):324–326. [PubMed] [Google Scholar]
- 51.Kutwin M., Sawosz E., Jaworski S., Kurantowicz N., Strojny B., Chwalibog A. Structural damage of chicken red blood cells exposed to platinum nano-particles and cisplatin. Nanoscale Res. lett. 2014;9(1):1–6. doi: 10.1186/1556-276X-9-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.