Abstract
The highest risk of opportunistic infections is from 1 to 6 months post-transplant. We report a rare case of Pneumocystis jirovecii pneumonia in a renal transplant recipient only on maintenance immunosuppression eleven years after transplant without concomitant CMV infection or recent episodes of graft rejection.
Keywords: Pneumocystis jirovecii, Immunosuppression, Opportunistic infection, Solid organ transplant, Opportunistic infection prophylaxis
1. Introduction
Pneumocystis jirovecii (formerly known as Pneumocystis carinii) is a yeast like fungus that was first identified as pathogen causing pneumonia around eight decades ago, during World War II. However, its incidence was largely sporadic with only a few reported cases in patients with malignancy until the dawn of the HIV pandemic. Immunosuppressed patients such as those with HIV and a CD4 cell count less than 200 per microliter, solid organ and hematopoietic stem cell transplant recipients, active hematologic malignancies and those who receive chronic glucocorticoid therapy among others are known to be at increased risk of developing Pneumocystis jirovecii pneumonia (PJP, formerly known as PCP). In solid organ transplant recipients, the highest risk of opportunistic infections including PJP is from 1 to 6 months post-transplant [1]. Its incidence later than the first year after transplant is rarely reported. We present one such case of PJP pneumonia in a patient occurring eleven years post-transplant.
2. Case
A 51-year-old immunosuppressed Caucasian male was admitted (Day 0) with worsening dyspnea for 1 week. His medical history included hypertension, type I diabetes mellitus (Hemoglobin-A1c 8.2) and his transplant history included simultaneous pancreatic and kidney transplant (unknown donor status due to unavailability of records) in 1992 (pancreas transplant failure in 1997), followed by a second kidney transplant (live related donor – sister, age of donor 39 years) in 2005 following chronic graft failure of his first kidney transplant. His immunosuppressive regimen on presentation consisted of tacrolimus, mycophenolate and prednisone 5 mg once daily. He had no medication adjustments made in the past several years leading up to his hospital admission. His last exposure to high dose corticosteroids was in November 2005, two months after his repeat kidney transplant. He was on a calcium channel blocker for hypertension and on an insulin pump for his diabetes. He denied smoking, alcohol or drug use. He had not travelled recently and had not started any new medications. He denied any occupational exposures to toxins and had not been to a hospital in over two years. He was compliant with his medication regimen and followed up regularly with his outpatient provider.
On arrival he was afebrile and his vital signs were stable except for hypoxia to a saturation of 83% at room air. He had bilateral crackles on his lung exam with a chest x ray showing bilateral mild interstitial and alveolar opacities. His arterial blood gas (ABG) on an FiO2 35% showed a pH of 7.41 and a pO2 of 64 mm Hg. He was initially started on treatment for community acquired pneumonia with ceftriaxone and azithromycin. However, on day two of hospital stay, in view of deteriorating clinical status and progressive pulmonary interstitial and airspace opacities on repeat imaging [Fig. 1], he was started on trimethoprim-sulfamethoxazole (TMP-SMX) and high dose prednisone. CMV serology was negative. A bronchoalveolar lavage done on day three of hospital stay, showed Pneumocystis jirovecii on methenamine silver staining [Fig. 2]. Despite receiving appropriate doses of trimethoprim-sulfamethoxazole and prednisone, the patient's clinical and radiological status continued to deteriorate and the patient required intubation for hypoxic respiratory failure on day 8. During the course of his ICU stay his TMP-SMX was changed to intravenous pentamidine due to persistent hyperkalemia. He was extubated on day 15 and transferred out to a general medical floor on day 17, where his clinical course worsened again. He developed aspiration pneumonia as well as E. coli and Klebsiella pneumoniae bacteremia which was treated with appropriate antimicrobials. He completed a total of 4 weeks of anti-pneumocystis therapy and was initiated on PJP prophylaxis with dapsone thereafter. He was discharged on day 29 to follow up with the infectious disease specialist as an outpatient and with the intention of continuing dapsone indefinitely.
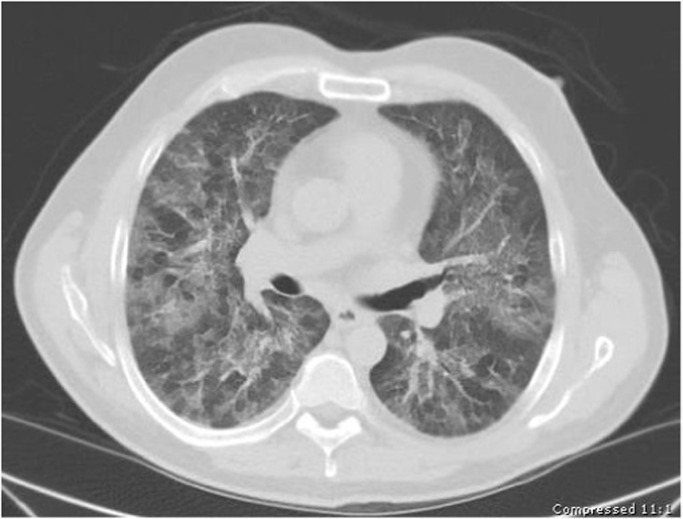
Fig. 1.
Non-contrast CT Chest showing bilateral symmetric patchy ground glass opacities with interstitial and septal thickening.
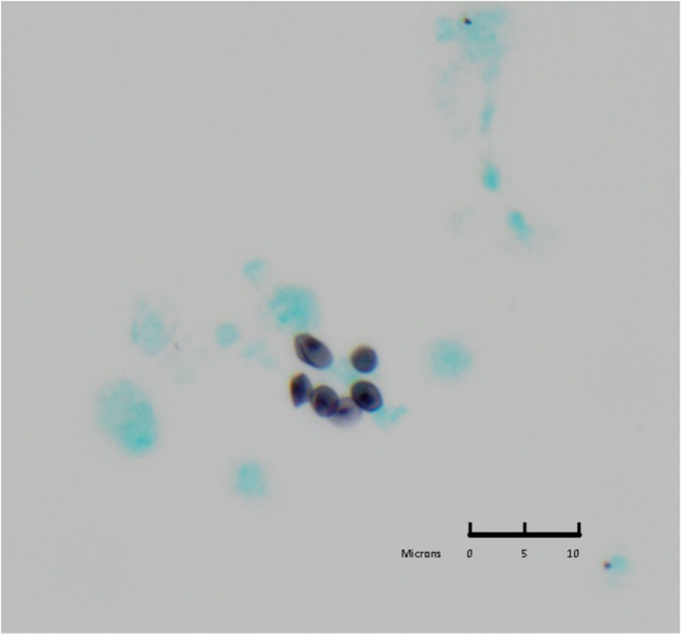
Fig. 2.
BAL specimen, Methenamine Silver staining, 60×: single cysts are visible containing the fungal element (dot within cyst). The cysts are cup-shaped, smoothly thin walled and occasionally show a crushed appearance.
3. Discussion
The duration of PJP prophylaxis in solid organ transplant (SOT) recipients is generally accepted to be around 6–12 months depending on the organ transplanted. For kidney transplant recipients, it is usually 6 months, with added prophylaxis for at least 6 weeks following each episode of acute cellular rejection thereafter 1, 2. This is because the risk of opportunistic infections peak around these time periods for reasons mentioned below [1]. Incidence of PJP greater than 1 year after SOT is rare. However, with effective prophylactic medications in the immediate 1-year post-transplant, the risk of PJP nowadays may be significant several years after transplant when prophylaxis has been discontinued. This is borne out by a recent case series showing that most PJP cases occur after a mean of approximately 3 years post-transplant [3]. After a thorough literature search, we found case reports that show PJP pneumonia occurring 4 years post-transplant [4] to as great as 13 years post-transplant (in association with CMV infection) [5]. There is an isolated case of PJP reported 9 years post cardiac transplantation [6]. Our case is unique in that PJP has never been reported in isolation 11 years following a kidney transplant.
The opportunistic infection “clock” is “reset” with each new transplant or episode of acute cellular rejection due to the need for increasing immunosuppression to achieve “induction” levels each time [ 1].
Our patient was unusual because his graft function and level of immunosuppression had been stable for more than 10 years without any episodes of rejection in the interim. Furthermore, he did not have a coexisting CMV infection, which is thought to increase risk of PJP infection because of its immunomodulatory effects 5, 7. A limitation in this case is the absence of immunofluorescence staining of the diagnostic specimen. Many patients are colonized with Pneumocystis jirovecii and immunofluorescence is used to identify the infective trophic forms of the organism. We did not feel this to be important to the management of this case, however, as this patient's clinical picture was typical for PJP.
This case serves as a reminder that transplant patients are at lifelong risk of PJP pneumonia and a high index of suspicion should be maintained when they present with hypoxia or respiratory distress. This assumes even greater important as advances in transplant medicine enable more patients to live longer with their transplanted organs. Our case adds to the building body of evidence of delayed presentation of PJP pneumonia in transplanted patients and at present, there is no clear evidence for or against continuing indefinite PJP prophylaxis following SOT. Further studies and more long term data are needed before considering longer durations of PJP prophylaxis in transplant recipients [8].
Conflict of interest
There are none.
References
- 1.Fishman J.A. Infection in solid-organ transplant recipients. New Engl. J. Med. 2007;357(25):2601–2604. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 2.Kasiske B.L., Zeier M.G., Chapman J.R., Craig J.C., Ekberg H., Garvey C.A., Green M.D., Jha V., Josephson M.A., Kiberd B.A., Kreis H.A. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. 2010;77(4):299–311. doi: 10.1038/ki.2009.377. [DOI] [PubMed] [Google Scholar]
- 3.McKinnell J.A., Cannella A.P., Kunz D.F., Moser S.A., Miller L.G., Baddley J.W., Pappas P.G. Pneumocystis pneumonia in hospitalized patients: a detailed examination of symptoms, management, and outcomes in human immunodeficiency virus (HIV)‐infected and HIV‐uninfected persons. Transplant Infect. Dis. 2012;14(5):510–518. doi: 10.1111/j.1399-3062.2012.00739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez‐Ordoño L., Hoyo I., Sanclemente G., Ricart M.J., Cofan F., Perez‐Villa F., Bellacasa J.P., Moreno A., Cervera C. Late‐onset Pneumocystis jirovecii pneumonia in solid organ transplant recipients. Transplant Infect. Dis. 2014;16(2):324–328. doi: 10.1111/tid.12184. [DOI] [PubMed] [Google Scholar]
- 5.Muhammad Iqbal A.H., Lim S.K., Ng K.P., Tan L.P., Chong Y.B., Keng T.C. Pneumocystis jirovecii pneumonia 13 years post renal transplant following a recurrent cytomegalovirus infection. Transplant Infect. Dis. 2012;14(4):E23–E26. doi: 10.1111/j.1399-3062.2012.00738.x. [DOI] [PubMed] [Google Scholar]
- 6.Craker L.R. Late presentation of Pneumocystis jiroveci pneumonia after cardiac transplantation. BMJ case Rep. 2010 doi: 10.1136/bcr.06.2010.3111. (2010:bcr0620103111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arend S.M., Westendorp R.G., Kroon F.P., Van't Wout J.W., Vandenbroucke J.P., Van Es L.A., Van Der Woude F.J. Rejection treatment and cytomegalovirus infection as risk factors for Pneumocystis carinii pneumonia in renal transplant recipients. Clin. Infect. Dis. 1996;22(6):920–925. doi: 10.1093/clinids/22.6.920. [DOI] [PubMed] [Google Scholar]
- 8.Gordon S.M., LaRosa S.P., Kalmadi S. Should prophylaxis for Pneumocystis carinii pneumonia in solid organ transplant recipients ever be discontinued? Clin. Infect. Dis. 1999;28(2):240–246. doi: 10.1086/515126. [DOI] [PubMed] [Google Scholar]