Abstract
Mycobacterium celatum is a slow-growing, non-tuberculous mycobacterium (NTM) and a rare cause of infection in humans. Infection occurs primarily by inhalation or direct inoculation from environmental sources, and this pathogen has been reported to cause localized infections in the lungs and lymph nodes of both immunocompetent and immunocompromised patients, and disseminated disease in immunocompromised patients. Here, we present a case of pulmonary infection with M. celatum in an immunocompetent 68-year-old male with clinical features similar to tuberculosis. The patient initially developed palpitations, worsening fatigue, night sweats, dyspnea, productive cough, and weight loss. Computed tomography angiogram of the chest revealed a right upper lobe pulmonary artery embolus and extensive biapical fibronodular cavitary densities. Two separate sputum samples were positive for acid-fast bacilli (AFB) and sputum cultures were positive for M. celatum. The patient responded well to treatment with clarithromycin, ciprofloxacin, and ethambutol. We advise physicians to consider M. celatum infection in the differential diagnosis of patients with symptoms and radiographic and microbiologic evidence suggestive of NTM pulmonary infection.
Keywords: Mycobacterium celatum, Non-tuberculous mycobacteria, Atypical mycobacteria
Introduction
M. celatum was first identified in 1993 from clinical isolates of mycobacteria collected from human patients infected with immunodeficiency virus (HIV) [1]. M. celatum is an acid-fast, non-photochromogenic (i.e., unpigmented), slow-growing organism that biochemically resembles M. xenopi [2]. M. celatum is one of the clinically important NTM, which are ubiquitous in nature and can be isolated from groundwater, soil, house dust, domestic and wild animals, and birds [3]. The NTM are not spread from person to person, rather infections, including nosocomial infections, are the result of inhalation or direct inoculation from environmental sources [[4], [5], [6]]. M. celatum has been reported to cause localized infections in the lungs and lymph nodes, and disseminated disease in immunocompromised patients [7]. The species name, celatum, Latin for secret, was chosen to reflect the concealed or hidden nature of the organism among the characterized mycobacteria. Here, we report a case of a HIV-negative patient with a suspected Mycobacterium tuberculosis, atypical mycobacteria, or fungal pulmonary infection, who was subsequently diagnosed with M. celatum infection and responded well to treatment with clarithromycin, ciprofloxacin, and ethambutol.
Case presentation
A 68-year-old Caucasian male presented for an infectious disease consult with intermittent fever, night sweats, atrial flutter, and a productive cough of 6-month duration. His sputum was clear/white in the morning and improved during the day although he reported that his sputum sometimes became gray during the day. The patient’s weight decreased from 210 to 130 pounds over the last year, which he described as intentional. Past medical history included a 55-year history of cigarette smoking (2 ppd), a 50-year history of marijuana use, chronic obstructive pulmonary disease, peripheral vascular disease, peripheral artery disease, and basal and squamous cell skin cancer. The patient reported no recent sick contacts and no one in his family had similar symptoms.
The patient was born and raised in Illinois and currently lives in a rural area of Michigan with his wife, children, dogs, a parrot, and canaries. The birds are kept in cages, which he cleans without wearing a mask. He served in Vietnam from 1966 to 1968 and denied exposure to M. tuberculosis during that time. He was employed as a nurse with his most recent employment in a prison in Allegan, Michigan where he worked for 2 years before retiring 6 years ago. His PPD (purified protein derivative) tests were negative during his employment, and no inmates had a positive PPD while he worked at the prison. He indicated a remote history of close exposure to tuberculosis while working as a nurse in the 1970s when he performed CPR without a barrier on a patient with active tuberculosis. Serial chest x-rays and four PPD skin tests in the following year were negative.
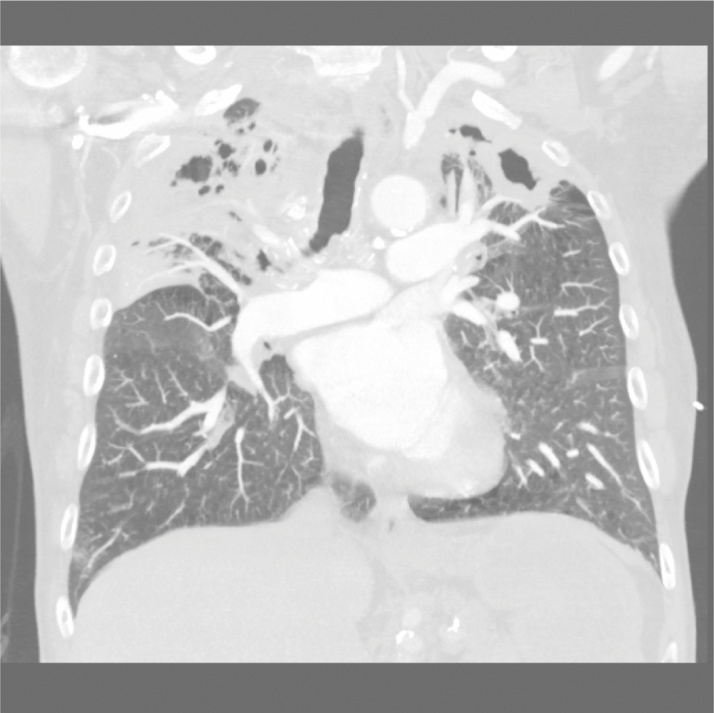
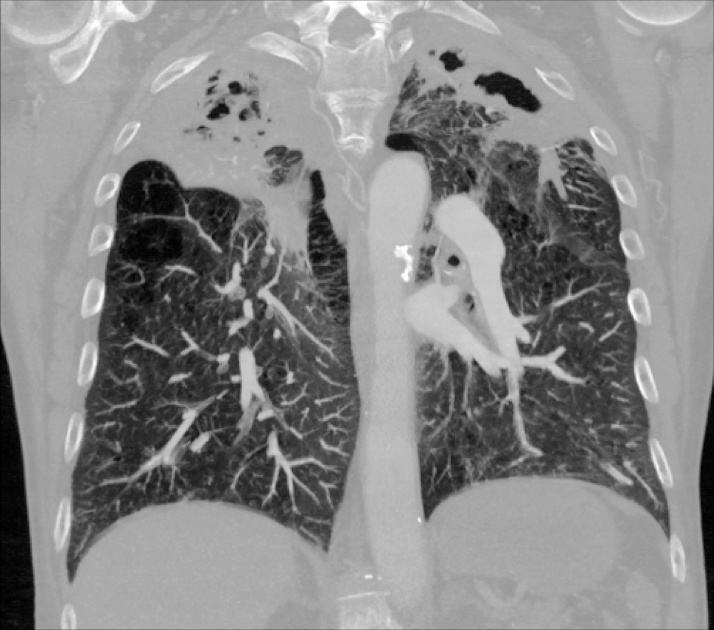
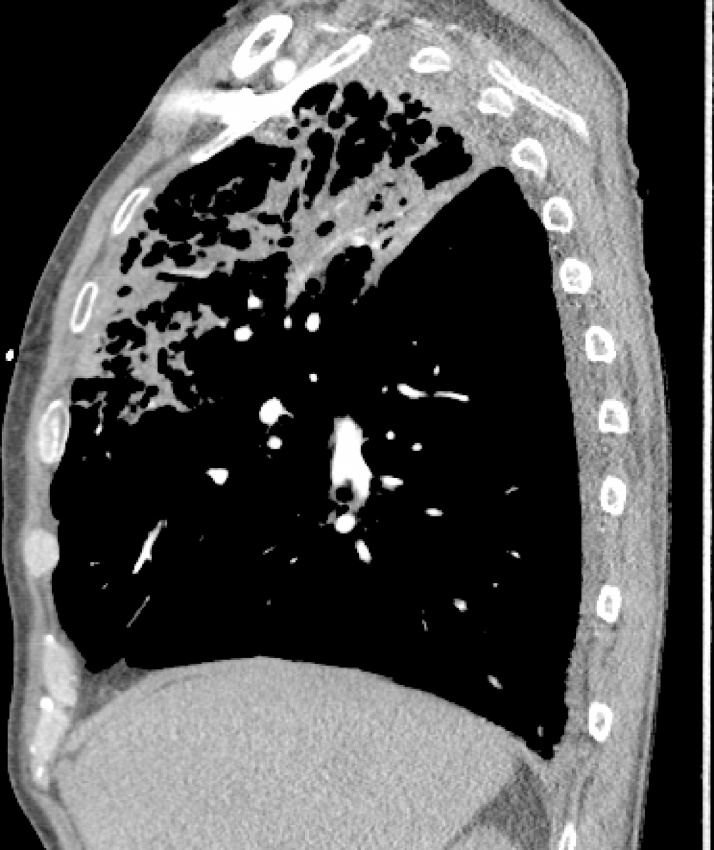
On examination, he appeared alert and well-oriented with decreased breath sounds bilaterally on auscultation. Chest x-rays revealed bilateral upper lobe caviation with coarse interstitial markings, parenchymal distortion, hilar retraction, and pleural thickening consistent with possible infection with M. tuberculosis, atypical mycobacteria, or a fungus. Computed tomography angiography of the chest demonstrated a single small embolus in the right upper lobe artery and severe emphysematous changes bilaterally, with extensive cavitation in each upper lobe consistent with a mycobacterial infection (Fig. 1, Fig. 2, Fig. 3). The patient was admitted with a presumptive diagnosis of reactivation pulmonary tuberculosis based on his history of employment as a nurse in several hospitals and a prison, and his history of direct contact with a patient with active tuberculosis. Quadruple anti-tuberculosis therapy with isoniazid, rifampin, pyrazinamide, and ethambutol was initiated. Sputum samples were collected for microscopic analysis and culture, and blood was collected for labs and testing for HIV and hepatitis B and C.
Fig. 1.
Coronal view (anterior chest). Severe emphysematous changes bilaterally with extensive cavitation in each upper lobe.
Fig. 2.
Coronal view (posterior chest). Severe emphysematous changes bilaterally with extensive cavitation in each upper lobe.
Fig. 3.
Sagittal view (right lung). Severe emphysematous changes bilaterally with extensive cavitation in each upper lobe.
Lab results for HIV, hepatitis B and hepatitis C were negative, however two separate acid-fast bacilli smears of sputum demonstrated numerous acid-fast bacilli. During the second week of admission, the quadruple anti-tuberculosis therapy was discontinued due to elevations in alanine aminotransferase (305 U/L) and aspartate aminotransferase (185 U/L) levels, and a negative interferon gamma release assay (Quantiferon-TB Gold) for tuberculosis. Despite discontinuing the anti-tuberculosis therapy, the patient improved and was released from the hospital. The discharge plan was thoroughly discussed with the patient and a follow-up visit was scheduled.
During initial outpatient follow-up two weeks later, the patient was clinically improved and reported feeling “much better.” However, repeat acid-fast bacilli stains continued to demonstrate numerous acid-fast bacilli and the patient’s chest x-ray remained unchanged. Sputum cultures were negative when examined with a direct M. tuberculosis complex gene probe (AccuProbe, Hologic), and broth cultures (Middlebrook 7H9 broth) were sent to the Michigan Department of Community Health Bureau of Laboratories and subsequently identified as M. celatum by high performance liquid chromatography (HPLC) and chemistries. The patient was started on clarithromycin, ciprofloxacin, and ethambutol. Four weeks later, the patient reported no fever, no sweats, diminished cough, and a sputum with decreased viscosity and coloration. The patient has continued follow up in the outpatient setting, and six months after discharge, both acid-fast bacilli smears and cultures for M. celatum have continued to be negative.
Discussion
M. celatum was first identified in 1993 by Butler et al., from unusual strains not considered typical of the previously described Mycobacterium species [1]. These strains were isolated from patients residing in diverse geographic locations in the United States, including California, New York, Ohio, Michigan, Illinois, Texas, Pennsylvania, Tennessee, and Virginia. M. celatum was isolated from a variety of clinical specimens from these patients, including bronchial washes, stools, sputum, and vertebral bone. M. celatum is a non-photochromogenic (Runyon classification group III), slow-growing, acid-fast, NTM that does not form cords or branches (cord factor negative), and morphologically appears as a slender rod although occasional coccoidal forms may also be observed [1]. M. celatum biocehmcially resembles M. xenopi, but is differentiated from M. xenopi by its inability to grow consistently at 45 °C, resistance to most anti-tuberculosis drugs, and a distinctive mycolic acid composition containing alpha-, keto-, and methoxy-mycolic acids [8]. While unique, the M. celatum 16S rRNA gene sequence clearly links this species to the genus Mycobacterium, and to date three types of M. celatum (types 1–3) have been identified. Patients encounter M. celatum in environmental sources, and although M. celatum has been found in ferrets [9], white-tailed trogon [10], and domestic pigs and roe deer [11], there is currently limited epidemiologic evidence to suggest that M. celatum infection is a zoonotic disease.
Shortly after the identification of M. celatum, cases began to appear in the literature. In 1993, for example, a HIV-positive 28-year-old woman presented with fever, malaise, severe anemia, and splenomegaly. Her past medical history was remarkable for repeated admission to the hospital with Pneumocystis carinii (now P. jiroveci) pneumonia and cytomegalovirus retinitis. Following isolation of the organism from the patient’s blood, sequencing of the 16S rRNA gene confirmed M. celatum as the cause of the patient’s disseminated infection [12]. Following this report, M. celatum was identified as the cause of cervical lymphadenitis in an immunocompetent 15-month-old boy. Acid-fast bacilli were identified in the pus obtained by incision of one lymph node from this patient, and histology showed lymphadenitis with granulomatous inflammation, caseating necrosis, and acid-fast bacilli. A diagnosis of M. celatum was confirmed by sequencing the 16S rRNA gene [13]. Additional reports continued to identify M. celatum infection in HIV-positive patients [[14], [15]], and M. celatum has also been shown to cause fatal disease in immunocompetent patients [[16], [17]].
Infection with M. celatum or M. tuberculosis produce similar symptoms, including cough, weight loss, lung infiltrates, and cavitary lung lesions [19], likely reflecting similar pathogenic mechanisms. Complicating diagnosis further is the finding that M. celatum types 1 and 3 cross-react with gene probes used to detect M. tuberculosis, which has made this pathogen a challenge to diagnose [18]. Thus, M. tuberculosis complex and M. avium complex DNA is currently detected by real-time polymerase chain reaction (PCR), and negative cultures are further characterized using matrix-assisted later desorption/ionization-time of flight mass spectrometry (MALDI-TOF). Newer techniques include nucleic acid amplification and sequencing of the 16S rRNA gene, which is both highly sensitive and fast [20].
Standardized therapy for M. celatum has not been firmly established, however, results of in vitro susceptibility testing are useful in guiding therapy selection [[21], [22]]. It is noteworthy, however, that discrepancy between resistance results in NTM isolates and clinical response in patients has been observed [23]. M. celatum isolates have shown variable susceptibility to antimicrobial agents, with most isolates showing susceptibility to azithromycin and clarithromycin, variable susceptibility/resistance to ciprofloxacin and isoniazid, and resistance to rifampin. Thus, treatment regimens have included various combinations of clarithromycin, ciprofloxacin, pyrazinamide, ethambutol, rifabutin, clofazimine, and amikacin [[7], [24], [25]]. In one report, a patient with primary skin involvement as well as extensive pulmonary treatment was treated initially with clarithromycin, isoniazid, and ethambutol for 15 months. After new infiltrates on both lungs and new enlarged lymph nodes were observed, therapy was changed to azithromycin, ciprofloxacin, and pyrazinamide resulting in rapid improvement and discontinuation of treatment after 24 months [26].
Conclusion
M. celatum should be suspected when acid-fast bacilli that are biochemically similar to and behave like M. avium-intracellulare or M. xenopi are identified in patients that do not respond to an appropriate treatment regimen. Continued advancement of techniques for rapid identification of the NTM beyond the current use of HPLC, chemistry, and MALDI-TOF, are expected to facilitate rapid selection of appropriate therapeutic treatments and thus improve patient outcomes [[27], [28], [29]].
Conflicts of interest statement
The authors declare no conflicts of interest. L.L. is the Editor-in-Chief or ID Cases and one of the physicians of the patient described in this case.
Sources of funding
None declared.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available by the Editor-in-Chief of this journal on request.
References
- 1.Butler W.R., O'Connor S.P., Yakrus M.A., Smithwick R.W., Plikaytis B.B., Moss C.W. Mycobacterium celatum sp. nov. Int J Syst Bacteriol. 1993;43(3):539–548. doi: 10.1099/00207713-43-3-539. [DOI] [PubMed] [Google Scholar]
- 2.Tortoli E., Piersimoni C., Bacosi D., Bartoloni A., Betti F., Bono L. Isolation of the newly described species Mycobacterium celatum from AIDS patients. J Clin Microbiol. 1995;33(1):137–140. doi: 10.1128/jcm.33.1.137-140.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Primm T.P., Lucero C.A., Falkinham J.O. Health impacts of environmental mycobacteria. Clin Microbiol Rev. 2004;17(1):98–106. doi: 10.1128/CMR.17.1.98-106.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffith D.E., Aksamit T., Brown-Elliott B.A., Catanzaro A., Daley C., Gordin F. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 5.Phillips M.S., von Reyn C.F. Nosocomial infections due to nontuberculous mycobacteria. Clin Infect Dis. 2001;33(8):1363–1374. doi: 10.1086/323126. [DOI] [PubMed] [Google Scholar]
- 6.Piersimoni C., Scarparo C. Extrapulmonary infections associated with nontuberculous mycobacteria in immunocompetent persons. Emerg Infect Dis. 2009;15(9):1351–1358. doi: 10.3201/eid1509.081259. quiz 1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christiansen D.C., Roberts G.D., Patel R. Mycobacterium celatum, an emerging pathogen and cause of false positive amplified Mycobacterium tuberculosis direct test. Diagn Microbiol Infect Dis. 2004;49(1):19–24. doi: 10.1016/j.diagmicrobio.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Butler W.R., Thibert L., Kilburn J.O. Identification of Mycobacterium avium complex strains and some similar species by high-performance liquid chromatography. J Clin Microbiol. 1992;30(10):2698–2704. doi: 10.1128/jcm.30.10.2698-2704.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valheim M., Djonne B., Heiene R., Caugant D.A. Disseminated Mycobacterium celatum (type 3) infection in a domestic ferret (Mustela putorius furo) Vet Pathol. 2001;38(4):460–463. doi: 10.1354/vp.38-4-460. [DOI] [PubMed] [Google Scholar]
- 10.Bertelsen M.F., Grondahl C., Giese S.B. Disseminated Mycobacterium celatum infection in a white-tailed trogon (Trogon viridis) Avian Pathol. 2006;35(4):316–319. doi: 10.1080/03079450600821133. [DOI] [PubMed] [Google Scholar]
- 11.Pate M., Zolnir-Dovc M., Kusar D., Krt B., Spicic S., Cvetnic Z. The first report of Mycobacterium celatum isolation from domestic pig (Sus scrofa domestica) and Roe deer (Capreolus capreolus) and an overview of human infections in Slovenia. Vet Med Int. 2011;2011:432954. doi: 10.4061/2011/432954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piersimoni C., Tortoli E., De Sio G. Disseminated infection due to Mycobacterium celatum in patient with AIDS. Lancet. 1994;344(8918):332. doi: 10.1016/s0140-6736(94)91369-2. [DOI] [PubMed] [Google Scholar]
- 13.Haase G., Skopnik H., Batge S., Bottger E.C. Cervical lymphadenitis caused by Mycobacterium celatum. Lancet. 1994;344(8928):1020–1021. doi: 10.1016/s0140-6736(94)91680-2. [DOI] [PubMed] [Google Scholar]
- 14.Bonomo R.A., Briggs J.M., Gross W., Hassan M., Graham R.C., Butler W.R. Mycobacterium celatum infection in a patient with AIDS. Clin Infect Dis. 1998;26(1):243–245. doi: 10.1086/517040. [DOI] [PubMed] [Google Scholar]
- 15.Bell H.C., Heath C.H., French M.A. Pulmonary Mycobacterium celatum immune restoration disease: immunopathology and response to corticosteroid therapy. AIDS. 2005;19(17):2047–2049. doi: 10.1097/01.aids.0000191228.36797.5b. [DOI] [PubMed] [Google Scholar]
- 16.Jun H.J., Lee N.Y., Kim J., Koh W.J. Successful treatment of Mycobacterium celatum pulmonary disease in an immunocompetent patient using antimicobacterial chemotherapy and combined pulmonary resection. Yonsei Med J. 2010;51(6):980–983. doi: 10.3349/ymj.2010.51.6.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piersimoni C., Zitti P.G., Nista D., Bornigia S. Mycobacterium celatum pulmonary infection in the immunocompetent: case report and review. Emerg Infect Dis. 2003;9(3):399–402. doi: 10.3201/eid0903.020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gildeh E., Abdel-Rahman Z., Sengupta R., Johnson L. A case of false-positive Mycobacterium tuberculosis caused by Mycobacterium celatum. Case Rep Infect Dis. 2016;2016:1761923. doi: 10.1155/2016/1761923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butler W.R., O'Connor S.P., Yakrus M.A., Gross W.M. Cross-reactivity of genetic probe for detection of Mycobacterium tuberculosis with newly described species Mycobacterium celatum. J Clin Microbiol. 1994;32(2):536–538. doi: 10.1128/jcm.32.2.536-538.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greco S., Girardi E.G., Navarra A., Saltini C. Current evidence on diagnostic accuracy of commercially based nucleic acid amplification tests for the diagnosis of pulmonary tuberculosis. Thorax. 2006;61(9):783–790. doi: 10.1136/thx.2005.054908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown-Elliott B.A., Nash K.A., Wallace R.J. Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin Microbiol Rev. 2012;25(3):545–582. doi: 10.1128/CMR.05030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Ingen J., van der Laan T., Dekhuijzen R., Boeree M., van Soolingen D. In vitro drug susceptibility of 2275 clinical non-tuberculous Mycobacterium isolates of 49 species in The Netherlands. Int J Antimicrob Agents. 2010;35(2):169–173. doi: 10.1016/j.ijantimicag.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Petrini B. Non-tuberculous mycobacterial infections. Scand J Infect Dis. 2006;38(4):246–255. doi: 10.1080/00365540500444652. [DOI] [PubMed] [Google Scholar]
- 24.Piersimoni C., Tortoli E., de Lalla F., Nista D., Donato D., Bornigia S. Isolation of Mycobacterium celatum from patients infected with human immunodeficiency virus. Clin Infect Dis. 1997;24(2):144–147. doi: 10.1093/clinids/24.2.144. [DOI] [PubMed] [Google Scholar]
- 25.Tan C.K., Lai C.C., Chou C.H., Hsueh P.R. Mycobacterium celatum pulmonary infection mimicking pulmonary tuberculosis in a patient with ankylosing spondylitis. Int J Infect Dis. 2009;13(6):e459–e462. doi: 10.1016/j.ijid.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Patsche C.B., Svensson E., Wejse C. Disseminated Mycobacterium celatum disease with prolonged pulmonary involvement. Int J Infect Dis. 2014;26:88–90. doi: 10.1016/j.ijid.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 27.Tortoli E. Microbiological features and clinical relevance of new species of the genus Mycobacterium. Clin Microbiol Rev. 2014;27(4):727–752. doi: 10.1128/CMR.00035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson E.T., Samson D., Banaei N. Rapid identification of Mycobacterium tuberculosis and nontuberculous mycobacteria by multiplex, real-time PCR. J Clin Microbiol. 2009;47(5):1497–1502. doi: 10.1128/JCM.01868-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin J.H., Cho E.J., Lee J.Y., Yu J.Y., Kang Y.H. Novel diagnostic algorithm using tuf gene amplification and restriction fragment length polymorphism is promising tool for identification of nontuberculous mycobacteria. J Microbiol Biotechnol. 2009;19(3):323–330. doi: 10.4014/jmb.0804.267. [DOI] [PubMed] [Google Scholar]