Abstract
Purpose
To describe a case of orbital apex syndrome as a result of isolated bacterial sinusitis.
Observations
A 63-year-old woman presented with an orbital apex syndrome from isolated bacterial sinusitis with rapidly declining visual acuity to no light perception. We compared our case with 6 similar cases of severe vision loss from isolated bacterial sinusitis. In contrast to previously published cases, our patient presented with good vision yet deteriorated to no light perception despite appropriate treatment.
Conclusions and importance
Orbital apex syndrome can present as a constellation of cranial neuropathies including optic neuropathy from conditions affecting the orbital apex. Although vision loss remained permanent, prompt initiation of broad-spectrum antibiotics and antifungals and surgical intervention prevented further extension of infection into intracranial structures.
Keywords: Orbital apex syndrome, Optic neuropathy
1. Introduction
Orbital apex syndrome consists of multiple cranial neuropathies most commonly secondary to invasive fungal sinusitis or orbital cellulitis involving the orbital apex. Rarely, it has been reported to originate from isolated bacterial sinusitis.
2. Case report
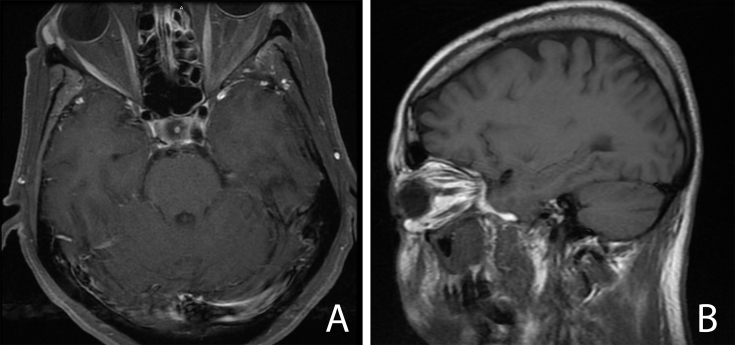
A 63-year-old Hispanic woman with a history of diabetes mellitus and metastatic invasive ductal carcinoma of the breast on hormonal therapy presented to the emergency department (ED) with a 2-day history of left-sided facial numbness and binocular horizontal diplopia. Magnetic resonance imaging (MRI) of the brain was obtained by the ED to rule out a stroke. The MRI demonstrated microvascular disease with periorbital soft tissue swelling and an inflammatory process involving the pterygopalatine fossa (Fig. 1). No cavernous sinus involvement or intraorbital involvement was identified.
Fig. 1.
Magnetic resonance imaging. Axial (A) and sagittal (B) T1 magnetic resonance imaging of the orbits with contrast showing periorbital soft tissue swelling with no intraorbital involvement and maxillary sinusitis. Image quality was limited due to motion artifact.
Ophthalmology was then consulted for further evaluation. On examination, best-corrected visual acuity was 20/30 OD and 20/40 OS. Pupils were symmetric without relative afferent pupillary defect (RAPD). She had a complete abduction deficit OS but otherwise full extraocular motility. External examination showed extensive left-sided facial and periorbital edema and hypoesthesia in the distribution of the ophthalmic and maxillary divisions of the left fifth cranial nerve. No proptosis or resistance to retropulsion was seen. The rest of her anterior segment exam was normal. Fundoscopic exam was unremarkable in both eyes without disc edema. Complete blood count with differential showed a normal white blood cell count of 7700/μL with 84% neutrophils. She was started on intravenous vancomycin by the primary team. Given concern for invasive fungal sinusitis, the otolaryngology service performed a nasal endoscopy with maxillary antrostomy. Significant pus was seen in the maxillary sinus, but biopsies showed no evidence of invasive fungal sinusitis or carcinoma. Antibiotics were broadened postoperatively to include cefepime and metronidazole.
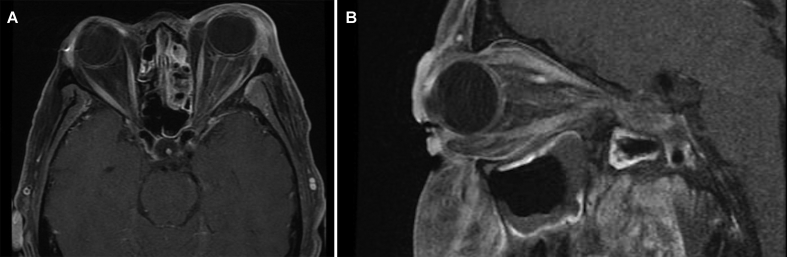
On postoperative day 1, her visual acuity deteriorated to count fingers OS, and she was noted to have a left RAPD. Motility exam revealed limitations of supraduction and infraduction on the left. Funduscopic examination was again normal OS. She was started on amphotericin B given high suspicion for invasive fungal disease in the orbital apex. A dedicated MRI of the orbits with contrast showed new enhancement of the left optic nerve and periosteum of the left lateral orbital wall near the orbital apex and pansinusitis involving the maxillary, ethmoid, and sphenoid sinuses (Fig. 2). Again, no involvement of the cavernous sinus was identified. However, there was an area of enhancement of the dura in the medial aspect of the left medial cranial fossa. Maxillofacial computed tomography (CT) scan also done postoperatively identified an area of osteomyelitis with adjacent necrotic mucosa in the maxillary sinus/pterygopalatine fossa area. No masses were present. Due to rapidly declining visual acuity, she underwent emergent endoscopic sinus surgery. Her vision deteriorated further overnight to no light perception with stable motility deficits. Blood and intraoperative cultures were positive for methicillin-sensitive Staphylococcus aureus (MSSA), and antimicrobial therapy was changed to intravenous nafcillin. She was discharged after completing a 14-day course of intravenous antibiotics and prescribed oral cephalexin for the continuation of antibiotic treatment after discharge. Neither her visual acuity nor motility deficits improved at 1-month follow-up.
Fig. 2.
Magnetic resonance imaging. Axial (A) and sagittal (B) T1 magnetic resonance imaging of the orbits with contrast showing enhancement of the left optic nerve and periosteum of the left lateral orbital wall near the orbital apex and post-surgical improvement of maxillary and sphenoid sinusitis.
3. Discussion
Orbital apex syndrome consists of multiple cranial neuropathies secondary to infiltration of the orbital apex typically by infectious, inflammatory, or neoplastic processes. The rapid progression of our patient's symptoms points best towards an infectious process. The abduction deficit and hypoesthesia present on admission are indicative of the involvement of the fifth and sixth cranial nerves, and corresponding involvement of the maxillary sinus and pterygopalatine fossa was seen on the initial MRI. The development of decreased acuity, RAPD, and worsening ophthalmoplegia indicates the involvement of the second and third cranial nerves and expansion of the infection to involve the orbital apex.
There are 6 case reports in the literature of bacterial sinusitis without concurrent orbital cellulitis causing orbital apex syndrome, yet ours is the only case that presented with good visual acuity without involvement of the optic nerve on presentation.1, 2, 3, 4, 5, 6 However, despite appropriate antibiotics and surgical intervention, her visual acuity rapidly deteriorated, and her deficits remained permanent. In previous studies, one patient presented with an acute decline in visual acuity to hand motion, and 5 presented with no light perception. Only one patient recovered some vision, improving minimally from no light perception to hand motion.3
Invasive fungal sinusitis is the most immediate life-threatening cause of orbital apex syndrome, with an 86% mortality in 1 series of 14 patients with biopsy-proven invasive fungal sinusitis and orbital apex syndrome.7 Clinical suspicion must, therefore, remain high when patients present with the rapid development of multiple cranial neuropathies. Our patient had neuroimaging with significant sinus disease, but multiple tissue biopsies showed no fungal elements and MSSA only. We, therefore, attribute her orbital apex syndrome and compressive optic neuropathy to isolated bacterial sinusitis without orbital cellulitis. She was immunocompromised, although never neutropenic, by both cancer and diabetes mellitus, and these factors presumably contributed to the rapid progression of her sinusitis to involve the sphenoid wing periosteum and orbital apex despite appropriate therapy.
This case reinforces the possibility of aggressive progression of bacterial sinusitis to orbital apex syndrome. Although we typically associate invasive fungal disease with acute orbital apex syndrome, clinicians must recognize that bacterial infection may present with a similarly aggressive presentation and rapid clinical deterioration. While the severity of vision loss and other cranial nerve damage in both our case and those similar in the literature were not improved or reversed by tailored antimicrobial therapy, broad-spectrum antibiotics and antifungals should still be initiated promptly in similar presentations. In our case and the literature, the use of early surgical intervention and broad antibiotic coverage were successful in preventing further extension of infection into intracranial structures. We recommend urgent endoscopic surgical intervention in order to decompress the orbital apex when optic neuropathy is detected, although this did not prevent further vision loss in our patient. There may be a role for corticosteroids initiated one to 2 days after intravenous antibiotic therapy. Ultimately, we believe that our treatment was appropriate for a complicated and unique case.
4. Patient consent
This report does not contain any personal information that could lead to the identification of the patient.
5. Acknowledgments and disclosures
Funding
Supported in part by National Eye Institute Vision Core Grant P30EY010608, a Challenge Grant from Research to Prevent Blindness to the McGovern Medical School, and the Hermann Eye Fund. The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Conflict of interest
No authors declare conflicts of interest.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgments
None.
References
- 1.Tarazi A.E., Shikani A.H. Irreversible unilateral visual loss due to acute sinusitis. Arch Otolaryngol Head Neck Surg. 1991;117:1400–1401. doi: 10.1001/archotol.1991.01870240092015. [DOI] [PubMed] [Google Scholar]
- 2.Kusunoki T., Kase K., Ikeda K. A case of orbital apex syndrome due to Pseudomonas aeruginosa infection. Clin Pract. 2011;1 doi: 10.4081/cp.2011.e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slavin M.L., Glaser J.S. Acute severe irreversible visual loss with sphenoethmoiditis-'posterior' orbital cellulitis. Arch Ophthalmol. 1987;105:345–348. doi: 10.1001/archopht.1987.01060030065027. [DOI] [PubMed] [Google Scholar]
- 4.Colson A.E., Daily J.P. Orbital apex syndrome and cavernous sinus thrombosis due to infection with Staphylococcus aureus and Pseudomonas aeruginosa. Clin Infect Dis. 1999;29:701–702. doi: 10.1086/598670. [DOI] [PubMed] [Google Scholar]
- 5.Nayar R.C., Mathur R.P., Gulati A., Mann S.B. Orbital apex syndrome due to rhinoscleroma. A case report. J Laryngol Otol. 1985;99:597–599. doi: 10.1017/s0022215100097310. [DOI] [PubMed] [Google Scholar]
- 6.Velasco D.V., Lapena J.F. Alternating orbital symptoms in cavernous sinus syndrome due to isolated sphenoid sinusitis. Kulak Burun Bogaz Ihtis Derg. 2010;20:142–145. [PubMed] [Google Scholar]
- 7.Thurtell M.J., Chiu A.L., Goold L.A. Neuro-ophthalmology of invasive fungal sinusitis: 14 consecutive patients and a review of the literature. Clin Experiment Ophthalmol. 2013;41:567–576. doi: 10.1111/ceo.12055. [DOI] [PubMed] [Google Scholar]