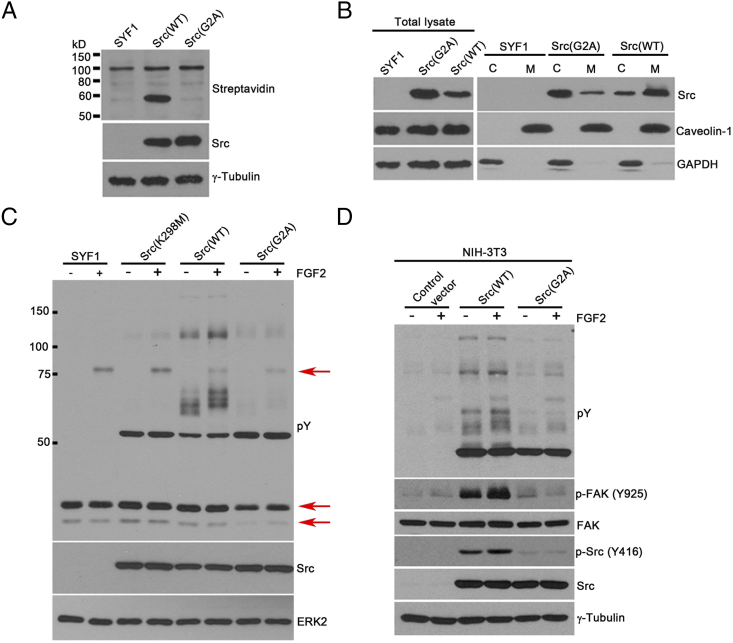
Figure 6.
Loss of myristoylation in Src kinase inhibits FGF2 induced signaling in vitro. (A) SYF1 (Src−/−Yes−/−Fyn−/−) cells expressing Src(WT) or Src(G2A) were grown in medium containing 50 μM myristic acid-azide for 24 hours. Myristoylated proteins were detected by Click chemistry. Expression levels of Src and tubulin in cell lysates were measured by immunoblotting. (B) SYF1 cells transduced with Src(WT) or Src(G2A) were fractionated into cytosol (C) and cell membrane (M) fractions. Levels of Src kinase were examined in both fractions by immunoblotting. Caveolin-1 and GAPDH were used as markers for total cell membrane fraction (M) and cytosolic fraction (C), respectively. Both markers were used as the loading control for the total lysate analysis. (C) SYF1(Src−/−Yes−/−Fyn−/−) parental cells and SYF1 cells transduced with Src(WT), Src(G2A), or Src(K298M) were grown with/without FGF2 (50 ng/ml) for 10 minutes. Protein lysates were analyzed for phosphorylated tyrosine, Src, and ERK2 levels by immunoblotting. Levels of phosphorylated tyrosine were detected by the anti-phosphotyrosine antibody (4G10). The phosphorylation of bands around 25 kDa and 75 kDa (as indicated by the red arrows) were inhibited in the Src(G2A) groups in comparison with Src(WT) and Src(K298M) group. (D) NIH-3T3 cells transduced with Src(WT), Src(G2A), or control vector were grown with/without FGF2 (50 ng/ml) for 10 minutes. Protein lysates were analyzed for phosphorylated tyrosine (detected by 4G10 antibody), Src, p-Src (Y416), FAK, p-FAK (Y925), and γ-tubulin levels by immunoblotting.