Abstract
Colorectal cancer is the second most common type of cancer both in Europe and Poland. During the last 30 years more than a 3-fold increase has been observed in Poland due to environmental and genetic factors. Almost all colorectal malignancies are related to the formation and malignant transformation of colorectal dysplasia and adenoma. Efforts aiming to decrease the number of colorectal cancer deaths are focused on the disease early detection. Genetic diagnosis for hereditary syndromes predisposing to colorectal cancer has been developed and is a part of the routine treatment. Most cancers are sporadic. They often develop from polyps in the colon. In addition to the genetic events described in the 1990s, showing the adenoma transformation into carcinoma that has been a prime example of malignant transformation for a long time, there are also other possibilities of neoplastic transformation. The recognition of colorectal cancer risk factors make sense as their nature is lifestyle- and diet-related. In this review paper those risk factors are presented and the prevention of colorectal cancer is discussed taking into account genetic factors.
Keywords: Colorectal adenomas, Colorectal cancer, Genetic risk factors
1. Introduction
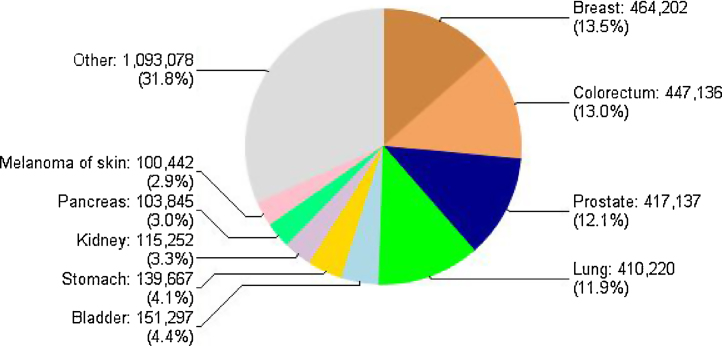
Colorectal cancer is the second most common cancer site both in Europe (Fig. 1) and Poland when analyzing cancer incidence.1 During the last 30 years, in Poland, more than a three-fold increase in incidence has been observed in women and nearly a four-fold increase in men.2
Fig. 1.
Cancer incidence in Europe by site, both sexes 2012.
Environmental and genetic factors are basic contributors to this increase. Almost all of the colorectal malignancies are related to the formation and malignant transformation of colorectal dysplasia and adenoma. Colorectal cancer develops through many different pathways, including the serrated pathway and the classical adenoma-carcinoma sequence.3 Adenomas are classified due to the histopathological structure as: Traditional and Sessile Serrated Adenomas, Tubular, Tubulovillous and Villous. Among the Serrated Polyps, only those having dysplasia (traditional) or sessile type (SSA), even without dysplasia, are classified as adenomas – polyps with potential for carcinogenesis. Among the polyposis syndromes, we identify also those with a predominance of, for example, hyperplastic polyps that do not belong to adenomas, but remarkably increases the risk of developing adenoma, dysplasia and cancer. Juvenille Polyposis,6 MAP (MUTYH Associated Polyposis)4 and Peutz Jeghers Syndrome5 belong to those syndromes. Efforts aiming to decrease the number of colorectal cancer deaths are focused on the disease early detection.6, 7, 8 A reduction of the colorectal cancer incidence can be achieved by proper prevention. Genetic diagnosis for hereditary syndromes predisposing to colorectal cancer has been developed and is a part of the routine treatment of colorectal cancer patients and members of their families. Most cancers are sporadic, they often develop from polyps in the colon. The sequence of genetic events described in the 90s showing the adenoma/carcinoma transformation has been a prime example of malignant transformation for a long time,9 but there are also other possibilities of neoplastic transformation.3 Knowledge of the colorectal cancer risk factors is important because of the nature of these modifiable lifestyle and diet-related factors.
In this paper we present the risk factors and prevention of colorectal cancer taking into account genetic factors.
2. Risk factors for adenomas
2.1. Environmental factors
The environmental factors are associated with sporadic cancer and are responsible for about 83% of all colorectal cancer cases. Among these patients, the sole influence of the environment is detected in 30%. In 28% of them, cancer is associated with epigenetic mutations such as in DNA repair genes, the remaining 25% have a positive family history. Transcriptional inactivation by promoter CpG island methylation in tumor suppressor genes is an important mechanism which can silence tumor suppressor genes. Epigenetic suppressor gene silencing has commonly been involved in colorectal cancer.10, 11 Epigenetic changes are usually associated with age. The aging process is associated with a wide variety of exposure to risk factors, so its effect is a kind of an accumulation of various processes. A concatenation between the methylation status of genes and a familial tendency to colorectal cancer have been described in several reports.1, 10, 11 Polish studies conducted under the National Colorectal Cancer Screening Program emphasize the role of the male sex, because of the lifestyle associated with higher exposure to risk factors that promotes the formation of high-risk adenomas (advanced neoplasia).12
Obesity contributes to an increased risk of adenomas and cancer, as does a diet rich in processed products and red meat. Underlying this risk is not only epigenetic disorders associated with abnormal methylation, but also the inflammatory processes.13 The Metabolic Syndrome is in close connection with these factors which is a consequence of incorrect diet and lifestyle, but also limitation of physical activity. Metabolic Syndrome is associated with weight gain, low levels of high density lipoprotein (HDL), hypertriglyceridemia and hypertension hyperglycemia.14, 15, 16 One of its liver manifestations may be nonalcoholic fatty liver disease (NAFLD) that is an independent colorectal cancer risk factor.17 All these factors lead to epigenetic changes which, in combination with positive family history, increase the risk of colorectal cancer.
2.2. Diet and red meat consumption
No correlation has been found between the total intake of protein and the risk of colorectal cancer development. The focus on the role of eating processed and red meat is increasing. Food preparation methods such as grilling meat causes the formation of carcinogenic polycyclic aromatic hydrocarbons and heterocyclic amines, but also generates the formation of DNA-damaging free radicals.13
Protective diet-related factors documented in recent years include the intake of vegetables, fruit and whole grains. The protective effect is related to the content of dietary fiber, which dilutes, absorbs, and removes the cocarcinogens, carcinogens and/or tumor promoters that are present in the gut.13
2.3. Smoking
According to literature data, smokers are at increased risk of developing colorectal cancer and the mortality rate due to colorectal cancer in this group is 25% higher compared to non-smokers. Advanced adenomas and neoplastic polyps occur much more frequently in those who smoke. The correlation between neoplastic adenomas and the number of cigarettes smoked daily, years of smoking addiction and an age at which the habit begun are observed.18, 19, 20
2.4. Alcohol intake
Multiply studies showed that chronic alcohol intake raises the risk of advanced adenoma and plays a role in colorectal carcinogenesis in both men and women. Some study results indicate a positive correlation between alcohol consumption with particular emphasis on excessive alcohol intake and the occurrence of advanced adenoma. Therefore, limiting alcohol intake from a young age might reduce colorectal cancer.21, 22, 23
2.5. Diabetes
Many studies have provided strong evidence that patients with diabetes mellitus are at a significantly higher risk of developing colorectal adenoma and colorectal cancer. Metformin has been recommended as the first line oral therapy for newly diagnosed type 2 diabetes. Some researchers have indicated that metformin can reduce colorectal adenomas and cancer risk. Some other studies have found no relation between metformin therapy and colorectal adenomas or colorectal cancer. Thus the results are still inconsistent.24, 25 Hu et al. found that the combination of H. pylori infection and elevated HbA1c is associated with an increased risk of colon adenoma.26
2.6. Physical activity
According to test results, physical activity is a protective factor decreasing the risk of developing adenoma and colorectal cancer.27 Probably, a synergistic effect is associated with lifestyle, reduction of energy supply in calories and lack of weight gain.28 In addition, a beneficial effect of avoiding overweight, not smoking and limited alcohol consumption was proven.29 Non-steroidal anti-inflammatory drugs should be mentioned among the factors with proven protective action.30, 31 They are not commonly used, however, because of their side effects,32, 33 and remain limited to selected groups of patients such as those with Hereditary Polyposis Syndromes, FAP or with previous colorectal cancer.30, 32, 34, 35, 36
2.7. Hormone replacement therapy
Another factor with a documented protective role in colorectal cancer is the use of hormone replacement therapy. Many years of prospective research have shown that HRT is related to a lower cancer development risk, especially when using the estrogen-alone (E-alone) therapy. E-alone therapy is associated with a lower risk of CDKN1A-nonexpressed colorectal cancer. CDKN1A is a key protein protecting the cell cycle and transition from G1 to S phase, and is responsible for the regulation of the growth arrest, cell proliferation and apoptosis. HRT is limited by its serious side effects, including cardiovascular complications and increased breast cancer risk.37
3. From adenoma to colon cancer
Almost half of the European population will have adenomas during their life. A small percentage of them may transform into cancer. Adenomas are divided into Tubular, Tubulovillous, Villous and Serrated. Conventional ones differ in the content of villous elements. Tubular type accounts for less than 25% of them and villous for more than 75%. The more villous elements the higher the risk of cancer. Polyps larger than 1 cm are mostly villous. Serrated Polyps are a heterogeneous group of changes having a serrated architecture of the epithelium.38 This includes irrelevant traditional serrated polyps, hyperplastic polyps, and Sessile Serrated Adenoma.39 Small (to 5 mm) hyperplastic polyps, located most commonly in the distal part of the intestine, are difficult to distinguish from adenomas.
Polyps are difficult to distinguish endoscopically. A variety of techniques are combined, e.g. chromoendoscopy with all kinds of dyes and zoom of the image, to make an accurate assessment of the structure of the Superficial and Subepithelial Polyps Vascular Grid. Models of mucous membrane covering the polyp appearance are created to distinguish the type of histopathological changes visible in endoscopy.40, 41, 42 An example is the pit pattern classification by Kudo using the model of intestinal crypt pit disorders.43 They are scored from a regular type I, with round intestinal crypt pits (normal) characteristic for normal mucosa and hyperplastic polyps, to the irregular type V (non-structural pits) which usually corresponds to adenocarcinoma.43, 44
Research is ongoing on the sensitivity of these methods, for example related with the coexistence of several models within a single lesion. In some cases, it is necessary to remove even the benign polyps to fulfill the diagnostic criteria for Hyperplastic Polyposis Syndrome. However, those are rare situations and in the nearest future, owing to constant improvements in endoscopic techniques, histological diagnosis can be done without removing the lesion, based only on the image obtained in the right band of light such as NBI (Narrow Band Imaging) system.45
All polyps seen during colonoscopy require histopathological verification or equivalent visual assessment. Poland is one of three countries in Europe (next to Germany and Italy)12, 46 where the national colorectal cancer early detection program is based on colonoscopy.12 Studies show that colonoscopy with possible polypectomy, performed once every 10 years, is the most effective method of colorectal cancer screening. According to estimates, it is adjusted to reduce the colorectal cancer incidence by 76–90% and the mortality by 69%.46
The most important thing is to find adenomas with dysplasia. The greatest risk of developing cancer is observed among these changes. High-grade dysplasia occurs most often focally and is synonymous with intraepithelial carcinoma or carcinoma in situ.47 For both low- and high-grade dysplasia endoscopic polypectomy is a sufficient procedure.46
Inflammatory Bowel Diseases are a known risk factor for colorectal cancer and dysplasia. The inflammation related changes are often flat and difficult to detect during endoscopy.
Approximately one in every 10 patients after colonoscopy have flat adenomas, and dysplasia associated lesion were identified in about half of the cases. Dysplasia Associated Lesions or Masses (DALM) may be removed the same way as adenomas, if there are not coexisting with flat dysplasia in the neighboring area.48
4. Genetics factors
4.1. Lynch syndrome
The Hereditary Nonpolyposis Colorectal Cancer (HNPCC) also known as Lynch Syndrome is the most common genetic syndrome associated with the presence of 2–4% of colorectal cancers. It refers to cases with mutations in DNA repair genes (MMR: MisMatchRepair).
HNPCC is a wider concept and also includes cases that meet the criteria for the syndrome but without mutations in MMR genes. The syndrome is not only related to increased colorectal cancer risk factor but also to other malignancies i.e. endometrial, stomach, small intestine, ovary, kidney and brain. It is characterized by a low diversity, mucous, undifferentiated medullary carcinomas that occur primarily in the right half of the large intestine among relatively young patients. Tumor surroundings are dominated by lymphocytic infiltrations and focal lesions similar to those found in Leśniowski–Crohn's disease. It is the result of mutations in embryonic DNA repair genes (PMS2, MSH2, MSH6, MLH1) and is an inherited autosomal dominant. Genetic mutations in short repetitive microsatellite sections of DNA, within which there is a deletion and shortening of DNA, leading to microsatellite instability, a characteristic feature of this syndrome. Microsatellite instability is a diagnostic marker.49
It is rare among sporadic cancers as their cause is usually not a mutation in the DNA repair genes but epigenetic changes such as hypermethylation of MLH1 promoter. Diagnosis is based on the Amsterdam II criteria and diagnostics of microsomal instability based on the revised Bethesda criteria. Lynch Syndrome is associated with relatively wide spectrum of cancers including colorectal, stomach, endometrial, pancreatic, ovarian, ureter, renal pelvis, biliary tract and brain (glioblastoma) tumors, keratoacanthomas, sebaceous gland adenomas and carcinoma of the small bowel.49
4.2. FAP syndrome
Much rarer syndrome (1% of colorectal cancers) is an autosomal dominant FAP Syndrome characterized by early formation of adenomatous polyps from 100 to over 1000 lesions and leading to colorectal cancer at the age of 35–40 years. In this syndrome there are polyps of the stomach fundus and small intestine, fortunately rarely undergoing neoplastic transformation as compared to sporadic cancers.
The syndrome diagnosis is based on the presence of more than 100 polyps, history of illness in the family, mutations in the APC gene originating from negative regulation of WNT signaling pathway. Depending on the mutation type in the APC gene, different phenotypes may emerge of acute to mild form of polyposis (Attenuated Familial Adenomatous Polyposis – AFAP). Changes such as congenital hypertrophy of the retinal epithelial whether the presence of fibroids also depend on the place of mutation. APC gene is an important regulator of the WNT pathway which, through morphogens, is responsible for the embryogenesis. Morphogen gradients result in developing organs and tissues but they function also in the adulthood – failures in morphogenic mechanism can possibly cause carcinogenesis. Mutations in the APC gene are also encountered in about 60–80% of sporadic colorectal cancers.50
4.2.1. Turcot syndrome
Turcot syndrome, a variant of FAP, is synonymous with gastrointestinal polyposis (FAP or Lynch syndrome) in patients who develop tumors of the central nervous system, most commonly medulloblastoma.50
4.2.2. Gardner syndrome
Gardner syndrome represents the next variant of FAP characterized by gastrointestinal findings of FAP along with fibromatosis and thyroid tumors. There are characteristic extraintestinal manifestations pathognomonic for diagnosis. These include mandible, skull and long bones osteomas, dental abnormalities and epidermoid cysts which are a common dermatologic manifestation, involving the scalp, face, and limbs.50
4.3. MAP syndrome
Even less common syndrome (0.3–1% of colorectal cancer cases) related to colorectal polyps is an autosomal recessive MAP Syndrome (MUTYH Associated Polyposis). MUTYH gene encodes an enzyme involved in DNA repair. Its mutation can also promote damage to the APC gene. In the event of failure of one allele, the risk of CRC increases moderately and cancer occurs at an older age but mutations involving both alleles of colorectal cancer are diagnosed in 30% of patients aged 40–50 years and 100% at the age of 60.4
4.4. Peutz–Jeghers syndrome
Peutz–Jeghers syndrome related to about 0.1% of colorectal cancer cases is an autosomal dominant syndrome characterized by the presence of pigmented lesions and Hamartomatous Polyps in the gastrointestinal tract, but also by increased risk of endometrial cancer of the gastrointestinal tract and non-gastrointestinal cancer. Diagnostic criteria include histologically confirmed presence of hyperplastic polyps, the presence of polyps in the small intestine, family history, pigmented macules, buccal mucosa of the lips and toes.
Hamartomatous polyps are made predominantly of non-neoplastic hamartomatous normal epithelium and smooth muscle. Rarely, the formation of polyps is accompanied by dysplasia. Tumors of the colon can occur independently on the substrate forming de novo adenomas. It is believed that the mechanism of carcinogenesis pathway is different from the above-mentioned sporadic cancer. Mutations in the K-RAS gene are rare. The average age of disease in this syndrome is less than 50 years. The most common tumors, besides the GI tract are ovarian, cervical and testicular cancer. The occurrence of bilateral breast cancer, Fallopian Tube Cancer and pancreatic cancer is genuinely associated. Mayo Familial Cancer program recommends a strict screening for breast cancer, breast and gynecological screening with pelvic ultrasound for women, regular testicular examination and periodic endoscopic examination of the upper/lower gastrointestinal combined with radiography of the small bowel.51
4.5. Juvenile polyposis syndrome
Juvenile Polyposis Syndrome occurs in less than 1/100,000 and, like Peutz–Jeghers syndrome, is related to the Hamartomatous Polyps occurrence. It involves mutations in the gene Smad4 and BMPR1A and is autosomal dominant inherited. The risk of developing colorectal cancer is similar to Peutz Jeghers syndrome (about 40%) but cancer develops on the basis of Hyperplastic Youth Polyps, in which dysplasia often appears. Polyps have a different structure than in Peutz Jeghers Syndrome. Histopathologically, it presents expanded cystic crypts accompanied by edematous and inflammed steep. It is very important to distinguish these polyps from Juvenile Polyps that occur sporadically. In the case of sporadic Juvenile Polyps, there is no increased risk of cancer.
That is why the diagnostic criteria taking into account the finding of 5 youth polyps in colorectal location, the finding of Juvenile Polyps anywhere in the gastrointestinal tract, and the finding of any number of Juvenile Polyps and a positive family history of juvenile polyposis are so important. Juvenile Polyps can occur regardless of age.6
4.6. Serrated polyposis
Serrated polyposis (formerly called hyperplastic polyposis) defined as the occurrence of more than 5 hyperplastic polyps proximal to the sigmoid colon, 2 of which are larger than 1 cm, or any number of Serrated Polyps in individuals who have first-degree relatives with serrated polyposis, or presentation of above 20 Serrated Polyps of the type SSA/Ps or hyperplastic of any size anywhere in the intestine. It is a newly described syndrome that occurs rarely.52
4.7. KRAS mutations
Mutations in the K-RAS gene are found in about 40% of colorectal cancers. The gene produces a protein with GTPase activity playing an important signaling role. It is located inside the cell membrane and is directly related to the epidermal growth factor receptor (EGFR) signaling pathway. It acts as a cell switch that normally promotes cell cycle but in response to cell stress it switches to an apoptotic pathway. It is a tumor suppressor gene which, when mutated, leads to deregulation of cellular pathways. KRAS mutations have also been linked with the ability to express metalloproteinase-2 which increase tumor cell invasiveness. In two trials, administration of cetuximab to patients with mutation at codon 13(G13DKRAS) was associated with a significantly better outcome than that seen in patients with other types of K-RAS mutations.45, 46 However, it appears that K-RAS may not be a useful prognosis/predictive biomarker in CRC patients. Meta-analysis carried out by Rui et al. showed no association between K-RAS mutations and survival rate in patients who received chemotherapy.55
4.8. Cytogenetic and epigenetic processes
Cytogenetic and epigenetic processes leading to carcinogenesis arise within chromosomes in DNA as well as in the mechanism of extragenic regulation, which epigenetic changes are now blamed for. One of the mechanisms is the methylation process. Linking methyl groups to the cytosine nucleotide is widespread outside the so-called CpG islands in the promoter sequence. Connection of a methyl group to cytosine nucleotides within the CpG islands promoter suppressor gene can inhibit the transcription and become the first step in carcinogenesis. Under the influence of the aforementioned mutations, a genomic instability arises in the cells of the colon mucosa, leading to the formation of carcinogenesis promoting phenotype. A classic example is the microsatellite instability which is a marker for disorders of DNA repair genes.56 It includes changes in microsatellite sequences of chromosomes resulting in the loss or multiplication of the number of repetitions in nucleotides. This may arise as a result of polymerase slippage during the copying of template DNA or abnormal recombination of DNA segments. Another factor conducive to cytogenetic carcinogenesis is the occurrence of chromosomal instability manifested by frequent mutations, chromosomal translocations, such as multiplication or loss of chromosomes or their fragments. This leads to the loss of suppressor genes and multiplies the number of copies of oncogenes. The probable cause are mutations of cell cycle control genes, such as APC or KRAS or wrong or abnormal centrosome function of telomeres.57, 58
4.9. Vitamin D
Many scientific reports have confirmed that the reduced level of VitD3 is related to an increased risk of developing colorectal, prostate and breast cancer. Hypothetical role in this process is played by genetic variations in genes that control the metabolism of the vitamin. The most important of them is a gene associated with Vit. D Receptor (VDR). VDR is expressed in various tissues in the bones and kidney, but also in the colon mucosa, where it is responsible for the regulation of cell proliferation, differentiation, and induction of apoptosis.
There have been many studies on the relationship of polymorphisms of the VDR gene in colorectal cancer to with results considered to be inconclusive. The strongest relationship was demonstrated for Fok I polymorphisms and Bsm I.59 These polymorphisms affect the binding of 1,25(OH)2D3 receptor, which removes an anti-proliferative effect of vitamin D and increases the colorectal cancer risk.59, 60, 61
RxRa protein is also suspected to be related to colorectal cancer.61 This protein is essential to form a heterodimer with VDR, which causes the release of co-repressors and activation of co-activators, leading to increased transcriptional activity of sequences containing VDR response element (VDRE) in target genes, including those involved in the regulation of vitamin D metabolism. Some studies show an increasing concentration of the vitamin D metabolite 1,25(OH)2D in the blood serum related to each new copy of the A allele of rs9409929 polymorphism.62
A particularly important process for all cells, including the intestinal mucosal cells, is calcium metabolism, a disorder of which can contribute to cancer formation. The characteristic features of tumor cells are the loss of apoptosis ability, self-sufficiency in the stimulation of growth, lack of reactivity with the cell growth inhibiting agents, the ability to invade and metastasis, no limit in the capacity of replication and initiation of angiogenesis. In all of these processes, calcium signaling pathway plays, directly or indirectly, an important role. Some of these processes appear in the tumor, e.g. uncontrolled calcium transport across the cell membrane, and may be a trigger or control key progression through the stimulation of proliferation, migration, excluding apoptosis. In many cancers, including colon cancer, associated disorders regulate the endoplasmatic reticulum Ca2+ level. Mutations in the gene SERCA3 (Sarco/endoplasmic reticulum Ca2+-ATPase) associated with overexpression affect the differentiation of colon cell lines and the gene is down-regulated in colorectal cancer.63 R674C mutation was observed in all types of cancer, and its location in the domain responsible for protein phosphorylation may indicate its potential role as a biomarker for cancer susceptibility.63 One of the biomarkers of colorectal cancer risk is APC/β-catenin score in the colon mucosa. In this process, the important role is played by E-cadheryn which might antagonize the β-catenin. It is known that malfunctions of the signaling pathway APC/β-catenin are an early pathogenic event in colorectal cancer and occur in about 90% of sporadic tumors. Ahearn et al. in a randomized clinical trial demonstrated a beneficial effect of supplementation with Vit D3 and calcium on the APC/β-catenin pathway. The trial proves chemopreventive effect of supplementation and indicates the assessment of the expression of APC/β-catenin and E-cadherin in colorectal mucosa as a modified colorectal cancer biomarker.63
4.10. Protective effect of NSAIDs
Based on clinical trials and population studies a protective effect of NSAIDs inhibiting cyclooxygenases COX-1 and COX-2 at the risk of colorectal cancer was found. Overexpression of cyclooxygenase-2 and its enzymatic product PGE2 (prostaglandin E2) is present in the majority of colorectal tumors and take an important part in carcinogenesis. It has been proved that PGE2 inhibits apoptosis by repression of proapoptotic BH3-only protein Bim in human colorectal adenoma cells, being an important colorectal cancer development factor.
4.11. Polymorphism
The presence of 28160A/G (rs4648298) polymorphism60 has been shown to be related to an increased risk of developing colorectal cancer, but its functionality is not known (localized in exon 10, in the untranslated region). In the polymorphism – 1195G/A case (rs6894669) – allele leads to an increased promoter activity and COX-2 is associated with an increased risk of cancer of the stomach and esophagus.
On the other hand, 765G/C (rs204179) polymorphism variant is responsible for the decrease of promoter activity by 30% and leads to a change of a recognition site for the transcription factor Sp1.54 It is also known that the CC genotype is related to reduced risk of the development of polyps in patients using NSAIDs.65 It has also been discovered that Val511Ala polymorphism (rs5273) reduces the risk of developing colorectal cancer in the African–American population66 but it is very rarely found in Caucasians.
Molecular genetic testing is the future of clinical genomic. Despite the genetic syndromes diagnosis based on the criteria described above, the most common detection tests in the colorectal cancer diagnosis are MSI, mutations in the BRAF and KRAS gene. Increasingly, a wider knowledge of the colorectal cancer formation paths allows to introduce personalized therapies based on pharmacogenetics. There is an increasing number of molecular and genetic tests for checking the effectiveness of drugs based on the tumor molecular characteristics. This limits the use of ineffective treatment and improves the optimal choice.67, 68, 69, 70
MSI tumors showing microsatellite instability constitute about 15% of colorectal cancers. They are fairly homogeneous in terms of pathology and clinical course characterizing, e.g. resistance to chemotherapy with 5-fluorouracil. Microsatellites are reproducible fragments of DNA sequence likely to cause errors in replication in the presence of abnormal DNA repair genes (MMR system). MSI define the changes in the length of microsatellite sequences.71 Literature data emphasizes that MSI test must be conducted in all patients with newly diagnosed colorectal cancer and a positive family history, since approximately 25% of Lynch syndrome does not meet the Amsterdam II and Bethesda criteria.71, 72 In order to decrease the risk of missing the diagnosis of Lynch Syndrome, it has recently been strongly recommended to perform MSI test regardless of patient's age and family history.48
Detection of KRAS gene mutations is important because of the resistance to medications used in the treatment of patients with hormone EGFR. Approximately 40% of colorectal cancers with KRAS mutation do not respond to treatment with antibodies against the EGFR. The test is required to initiate the therapy without diminished overall survival.73
BRAF mutation is also closely related to the ineffectiveness of anti-EGFR therapy. It is often present with the lack of KRAS mutations. All mutations have the same point of mutation V600E, which can be easily detected in a commercial PCR-based assays. An interesting fact is that these mutations are most frequently found in sporadic MSI-positive carcinomas with the serrated tumorigenic pathway. BRAF mutations have never been described in Lynch syndrome. Colorectal tumors with BRAF mutation have a large degree of epigenetic changes, i.e. MLH1 gene methylation and silencing. Diagnosis of BRAF mutation allows to distinguish the sporadic nature of the tumor from a syndromic one. This gene mutation also renders prognosis. Wild type BRAF gene with a high degree of MSI bode well in contrast to the mutant type of MSS.74
5. Conclusions
Genetic factors in the process of development of colorectal cancer seem to be better and better understood. However, we already know the mutations that inevitably lead to cancer, they refer to a small group of about 5% of patients. In other cases, genetic predisposition occurs in a low penetration genes. Only the appearance of subsequent environmental factors, such as those related to nutrition and lifestyle, may be relevant in combination with genetic factors in the occurrence and development of colorectal cancer.
Colorectal cancer screening and surveillance of the patients families allow to detect precancerous conditions.
Colorectal cancer is diverse in terms of formation pathways. Modern molecular diagnostics provide a critical information to choose the most effective method of treatment. Determining the molecular type of tumor allows the assessment of prognosis and assessment of disease risk in the family. Understanding the molecular mechanisms is the basis for the development of modern treatment methods, the discovery of new therapy targets allows the development of pharmacogenetics and, eventually, may become beneficial for patients and their families.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Malvezzi M., Bertuccio P., Levi F., La Vecchia C., Negri E. European cancer mortality predictions for the year 2014. Ann Oncol. 2014;23 doi: 10.1093/annonc/mdu138. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Didkowska J., Wojciechowska U., Zatoński W. National Cancer Registry of Poland, the Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology; Warsaw: 2013. Cancer in Poland in 2011. [Google Scholar]
- 3.Rex D.K., Ahnen D.J., Baron J.A. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315–1329. doi: 10.1038/ajg.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen M., Morreau H., Vasen H.F., Hes F.J. MUTYH-associated polyposis (MAP) Crit Rev Oncol Hematol. 2011;79:1–16. doi: 10.1016/j.critrevonc.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Latchford A.R., Neale K., Phillips R.K., Clark S.K. Peutz–Jeghers syndrome: intriguing suggestion of gastrointestinal cancer prevention from surveillance. Dis Colon Rectum. 2011;54:1547–1551. doi: 10.1097/DCR.0b013e318233a11f. [DOI] [PubMed] [Google Scholar]
- 6.Tam B., Salamon A., Bajtai A. The real face of juvenile polyposis syndrome. J Gastrointest Oncol. 2012;3:362–368. doi: 10.3978/j.issn.2078-6891.2012.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zauber A.G., Winawer S.J., O’Brien M.J. Colonoscopic polypectomy and long-term prevention of colorectal cancer deaths. N Engl J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramirez M., Schierling S., Papaconstantinou H.T. Management of the malignant polyp. Clin Colon Rectal Surg. 2008;21:286–290. doi: 10.1055/s-0028-1089944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahnen D.J. The American College of Gastroenterology Emily Couric Lecture – the adenoma-to-carcinoma sequence revisited: has the era of genetic tailoring finally arrived? Am J Gastroenterol. 2011;106:190–198. doi: 10.1038/ajg.2010.423. [DOI] [PubMed] [Google Scholar]
- 10.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 11.Frazier M.L., Xi L., Zong J. Association of the CpG island methylator phenotype with family history of cancer in patients with colorectal cancer. Cancer Res. 2003;63:4805–4808. [PubMed] [Google Scholar]
- 12.Regula J., Rupinski M., Kraszewska E. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med. 2006;355:1863–1872. doi: 10.1056/NEJMoa054967. [DOI] [PubMed] [Google Scholar]
- 13.Campos F.G., Logullo Waitzberg A.G., Kiss D.R., Waitzberg D.L., Habr-Gama A., Gama-Rodrigues J. Diet and colorectal cancer: current evidence for etiology and prevention. Nutr Hosp. 2005;20:18–25. [PubMed] [Google Scholar]
- 14.Okabayashi K., Ashrafian H., Hasegawa H. Body mass index category as a risk factor for colorectal adenomas: a systematic review and meta-analysis. Am J Gastroenterol. 2012;107:1175–1185. doi: 10.1038/ajg.2012.180. [DOI] [PubMed] [Google Scholar]
- 15.Liu J.J., Druta M., Shibata D. Metabolic syndrome and colorectal cancer: is hyperinsulinemia/insulin receptor-mediated angiogenesis a critical process? J Geriatr Oncol. 2014;5:40–48. doi: 10.1016/j.jgo.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esposito K., Chiodini P., Capuano A. Colorectal cancer association with metabolic syndrome and its components: a systematic review with meta-analysis. Endocrine. 2013;44:634–647. doi: 10.1007/s12020-013-9939-5. [DOI] [PubMed] [Google Scholar]
- 17.Lin X.F., Shi K.Q., You J. Increased risk of colorectal malignant neoplasm in patients with nonalcoholic fatty liver disease: a large study. Mol Biol Rep. 2014;41:2989–2997. doi: 10.1007/s11033-014-3157-y. [DOI] [PubMed] [Google Scholar]
- 18.Botteri E., Iodice S., Bagnardi V., Raimondi S., Lowenfels A.B., Maisonneuve P. Smoking and colorectal cancer. A meta-analysis. JAMA. 2008;300:2765–2778. doi: 10.1001/jama.2008.839. [DOI] [PubMed] [Google Scholar]
- 19.Cichoż-Lach H., Szumiło J., Celiński K. Results of screening in Lublin Province, Poland, for colorectal cancer and neoplastic polyps – the role of environmental factors. Ann Agric Environ Med. 2017;24:108–112. doi: 10.5604/12321966.1227648. [DOI] [PubMed] [Google Scholar]
- 20.Liang T.J., Wang H.X., Zheng Y.Y. APC hypermethylation for early diagnosis of colorectal cancer: a meta-analysis and literature review. Oncotarget. 2017 doi: 10.18632/oncotarget.17576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizukami T., Yokoyama A., Yokoyama T., Onuki S., Maruyama K. Screening by total colonoscopy following fecal immunochemical tests and determinants of colorectal neoplasia in Japanese men with alcohol dependence. Alcohol. 2017;52:131–137. doi: 10.1093/alcalc/agw071. [DOI] [PubMed] [Google Scholar]
- 22.Jayasekara H., MacInnis R.J., Williamson E.J. Lifetime alcohol intake is associated with an increased risk of KRAS+ and BRAF−/KRAS− but not BRAF+ colorectal cancer. Int J Cancer. 2017;140:1485–1493. doi: 10.1002/ijc.30568. [DOI] [PubMed] [Google Scholar]
- 23.Lee S.E., Jo H.B., Kwack W.G., Jeong Y.J., Yoon Y.J., Kang H.W. Characteristics of and risk factors for colorectal neoplasms in young adults in a screening population. World J Gastroenterol. 2016;22:2981–2992. doi: 10.3748/wjg.v22.i10.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu F., Yan L., Wang Z. Metformin therapy and risk of colorectal adenomas and colorectal cancer in type 2 diabetes mellitus patients: a systematic review and meta-analysis. Oncotarget. 2017;8:16017–16026. doi: 10.18632/oncotarget.13762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung Y.S., Park C.H., Eun C.S., Park D.I., Han D.S. Metformin use and the risk of colorectal adenoma: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2017;32:957–965. doi: 10.1111/jgh.13639. [DOI] [PubMed] [Google Scholar]
- 26.Hu K.C., Wu M.S., Chu C.H. Synergistic effect of hyperglycemia and Helicobacter pylori infection status on colorectal adenoma risk. J Clin Endocrinol Metab. 2017 doi: 10.1210/jc.2017-00257. [DOI] [PubMed] [Google Scholar]
- 27.Wolin K.Y., Yan Y., Colditz G.A. Physical activity and risk of colon adenoma: a meta-analysis. Br J Cancer. 2011;104:882–885. doi: 10.1038/sj.bjc.6606045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kushi L.H., Doyle C., McCullough M. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62:30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 29.Baron J.A., Cole B.F., Sandler R.S. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 30.Stolfi C., De Simone V., Pallone F., Monteleone G. Mechanisms of action of non-steroidal anti-inflammatory drugs (NSAIDs) and mesalazine in the chemoprevention of colorectal cancer. Int J Mol Sci. 2013;14:17972–17985. doi: 10.3390/ijms140917972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandler R.S., Halabi S., Baron J.A. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 32.Logan R.F., Grainge M.J., Shepherd V.C., Armitage N.C., Muir K.R. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology. 2008;134:29–38. doi: 10.1053/j.gastro.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 33.Rothwell P.M., Wilson M., Elwin C.E. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 34.Smith M.L., Hawcroft G., Hull M.A. The effect of non-steroidal anti-inflammatory drugs on human colorectal cancer cells: evidence of different mechanisms of action. Eur J Cancer. 2000;36:664–674. doi: 10.1016/s0959-8049(99)00333-0. [DOI] [PubMed] [Google Scholar]
- 35.Burn J., Bishop D.T., Mecklin J.P. Effect of aspirin or resistant starch on colorectal neoplasia in the Lynch syndrome. N Engl J Med. 2008;359:2567–2578. doi: 10.1056/NEJMoa0801297. [DOI] [PubMed] [Google Scholar]
- 36.Murff H.J., Shrubsole M.J., Smalley W.E. The interaction of age and hormone replacement therapy on colon adenoma risk. Cancer Detect Prev. 2007;31:161–165. doi: 10.1016/j.cdp.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jass J.R., Baker K., Zlobec I. Advanced colorectal polyps with the molecular and morphological features of serrated polyps and adenomas: concept of a ‘fusion’ pathway to colorectal cancer. Histopathology. 2006;49:121–131. doi: 10.1111/j.1365-2559.2006.02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vu H.T., Lopez R., Bennett A., Burke C.A. Individuals with sessile serrated polyps express an aggressive colorectal phenotype. Dis Colon Rectum. 2011;54:1216–1223. doi: 10.1097/DCR.0b013e318228f8a9. [DOI] [PubMed] [Google Scholar]
- 39.Butte J.M., Tang P., Gonen M. Rate of residual disease after complete endoscopic resection of malignant colonic polyp. Dis Colon Rectum. 2012;55:122–127. doi: 10.1097/DCR.0b013e3182336c38. [DOI] [PubMed] [Google Scholar]
- 40.Gross S., Trautwein C., Behrens A. Computer-based classification of small colorectal polyps by using narrow-band imaging with optical magnification. Gastrointest Endosc. 2011;74:1354–1359. doi: 10.1016/j.gie.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Hewett D.G., Kaltenbach T., Sano Y. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology. 2012;143:599–607. doi: 10.1053/j.gastro.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Kudo S., Tamura S., Nakajima T., Yamano H., Kusaka H., Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8–14. doi: 10.1016/s0016-5107(96)70222-5. [DOI] [PubMed] [Google Scholar]
- 43.Kudo S., Rubio C.A., Teixeira C.R., Kashida H., Kogure E. Pit pattern in colorectal neoplasia: endoscopic magnifying view. Endoscopy. 2001;33:367–373. doi: 10.1055/s-2004-826104. [DOI] [PubMed] [Google Scholar]
- 44.Hazewinkel Y., López-Cerón M., East J.E. Endoscopic features of sessile serrated adenomas: validation by international experts using high-resolution white-light endoscopy and narrow-band imaging. Gastrointest Endosc. 2013;77:916–924. doi: 10.1016/j.gie.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 45.Lieberman D.A., Rex D.K., Winawer S.J., Giardiello F.M., Johnson D.A., Levin T.R. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 46.Bujanda L., Cosme A., Gil I., Arenas-Mirave J.I. Malignant colorectal polyps. World J Gastroenterol. 2010;16:3103–3111. doi: 10.3748/wjg.v16.i25.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicolás-Pérez D., Parra-Blanco A., Gimeno-García A.Z. Risk factors associated with colorectal flat adenoma detection. Eur J Gastroenterol Hepatol. 2013;25:302–308. doi: 10.1097/MEG.0b013e32835b2d45. [DOI] [PubMed] [Google Scholar]
- 48.Weissman S.M., Burt R., Church J. Identification of individuals at risk for Lynch syndrome using targeted evaluations and genetic testing: National Society of Genetic Counsellors and the Collaborative Group of the Americas on Inherited Colorectal Cancer Joint Practice Guideline. J Genet Couns. 2012;21:484–493. doi: 10.1007/s10897-011-9465-7. [DOI] [PubMed] [Google Scholar]
- 49.Barrow P., Khan M., Lalloo F., Evans D.G., Hill J. Systematic review of the impact of registration and screening on colorectal cancer incidence and mortality in familial adenomatous polyposis and Lynch syndrome. Br J Surg. 2013;100:1719–1731. doi: 10.1002/bjs.9316. [DOI] [PubMed] [Google Scholar]
- 50.Waller A., Findeis S., Lee M.J. Familial adenomatous polyposis. J Pediatr Genet. 2016;5:78–83. doi: 10.1055/s-0036-1579760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brosens L.A., Langeveld D., van Hattem W.A., Giardiello F.M., Offerhaus G.J. Juvenile polyposis syndrome. World J Gastroenterol. 2011;17:4839–4844. doi: 10.3748/wjg.v17.i44.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Roock W., Jonker D.J., Di Nicolantonio F. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304:1812–1820. doi: 10.1001/jama.2010.1535. [DOI] [PubMed] [Google Scholar]
- 54.Rui Y.Y., Zhang D., Zhou Z.G. Can K-ras gene mutation be utilized as prognostic biomarker for colorectal cancer patients receiving chemotherapy? A meta-analysis and systematic review. PLOS ONE. 2013;10:e77901. doi: 10.1371/journal.pone.0077901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pancione M., Remo A., Colantuoni V. Genetic and epigenetic events generate multiple pathways in colorectal cancer progression. Patholog Res Int. 2012;2012:509348. doi: 10.1155/2012/509348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hughes L.A., Khalid-de Bakker C.A., Smits K.M. The CpG island methylator phenotype in colorectal cancer: progress and problems. Biochim Biophys Acta. 2012;1825:77–85. doi: 10.1016/j.bbcan.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 57.Kaneda A., Yagi K. Two groups of DNA methylation markers to classify colorectal cancer into three epigenotypes. Cancer Sci. 2011;102:18–24. doi: 10.1111/j.1349-7006.2010.01712.x. [DOI] [PubMed] [Google Scholar]
- 58.Köstner K., Denzer N., Müller C.S., Klein R., Tilgen W., Reichrath J. The relevance of vitamin D receptor (VDR) gene polymorphisms for cancer: a review of the literature. Anticancer Res. 2009;29:3511–3536. [PubMed] [Google Scholar]
- 59.Egan J.B., Thompson P.A., Ashbeck E.L. Genetic polymorphisms in vitamin D receptor VDR/RXRA influence the likelihood of colon adenoma recurrence. Cancer Res. 2010;70:1496–1504. doi: 10.1158/0008-5472.CAN-09-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jacobs E.T., Hibler E.A., Lance P., Sardo C.L., Jurutka P.W. Association between circulating concentrations of 25(OH)D and colorectal adenoma: a pooled analysis. Int J Cancer. 2013;133:2980–2988. doi: 10.1002/ijc.28316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hibler E.A., Jurutka P.W., Egan J.B. Association between polymorphic variation in VDR and RXRA and circulating levels of vitamin D metabolites. J Steroid Biochem Mol Biol. 2010;121:438–441. doi: 10.1016/j.jsbmb.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Korosec B., Glavac D., Volavsek M., Ravnik-Glavac M. ATP2A3 gene is involved in cancer susceptibility. Cancer Genet Cytogenet. 2009;188:88–94. doi: 10.1016/j.cancergencyto.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 63.Papafili A., Hill M.R., Brull D.J. Common promoter variant in cyclooxygenase-2 represses gene expression: evidence of role in acute-phase inflammatory response. Arterioscler Thromb Vasc Biol. 2002;22:1631–1636. doi: 10.1161/01.atv.0000030340.80207.c5. [DOI] [PubMed] [Google Scholar]
- 65.Sansbury L.B., Millikan R.C., Schroeder J.C., North K.E., Moorman P.G., Keku T.O. COX-2 polymorphism, use of nonsteroidal anti-inflammatory drugs, and risk of colon cancer in African Americans (United States) Cancer Causes Control. 2006;17:257–266. doi: 10.1007/s10552-005-0417-0. [DOI] [PubMed] [Google Scholar]
- 66.Carethers J.M. DNA testing and molecular screening for colon cancer. Clin Gastroenterol Hepatol. 2014;12:377–381. doi: 10.1016/j.cgh.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kloor M., Staffa L., Ahadova A., von Knebel Doeberitz M. Clinical significance of microsatellite instability in colorectal cancer. Langenbecks Arch Surg. 2014;399:23–31. doi: 10.1007/s00423-013-1112-3. [DOI] [PubMed] [Google Scholar]
- 68.Cerezo L., Ciria J.P., Arbea L. Current treatment of rectal cancer adapter to the individual patient. Rep Pract Oncol Rad. 2013;18:353–362. doi: 10.1016/j.rpor.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malicki J. The importance of accurate treatment planning, delivery, and dose verification. Rep Pract Oncolo Rad. 2012;17:63–65. doi: 10.1016/j.rpor.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dahlin A.M., Palmqvist R., Henriksson M.L. The role of the CpG island methylator phenotype in colorectal cancer prognosis depends on microsatellite instability screening status. Clin Cancer Res. 2010;16:1845–1855. doi: 10.1158/1078-0432.CCR-09-2594. [DOI] [PubMed] [Google Scholar]
- 71.Sinicrope F.A., Sargent D.J. Molecular pathways: microsatellite instability in colorectal cancer: prognostic, predictive, and therapeutic implications. Clin Cancer Res. 2012;18:1506–1512. doi: 10.1158/1078-0432.CCR-11-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Collura A., Lagrange A., Svrcek M. Patients with colorectal tumors with microsatellite instability and large deletions in HSP110 T17 have improved response to 5-fluorouracil-based chemotherapy. Gastroenterology. 2014;146:401–411. doi: 10.1053/j.gastro.2013.10.054. [DOI] [PubMed] [Google Scholar]
- 73.Feigelson H.S., Zeng C., Pawloski P.A. Does KRAS testing in metastatic colorectal cancer impact overall survival? A comparative effectiveness study in a population-based sample. PLOS ONE. 2014;9:e94977. doi: 10.1371/journal.pone.0094977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ogino S., Shima K., Meyerhardt J.A. Predictive and prognostic roles of BRAF mutation in stage III colon cancer: results from intergroup trial CALGB 89803. Clin Cancer Res. 2012;18:890–900. doi: 10.1158/1078-0432.CCR-11-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]