Abstract
Four heteropod lizard trackways discovered in the Hasandong Formation (Aptian-early Albian), South Korea assigned to Sauripes hadongensis, n. ichnogen., n. ichnosp., which represents the oldest lizard tracks in the world. Most tracks are pes tracks (N = 25) that are very small, average 22.29 mm long and 12.46 mm wide. The pes tracks show “typical” lizard morphology as having curved digit imprints that progressively increase in length from digits I to IV, a smaller digit V that is separated from the other digits by a large interdigital angle. The manus track is 19.18 mm long and 19.23 mm wide, and shows a different morphology from the pes. The predominant pes tracks, the long stride length of pes, narrow trackway width, digitigrade manus and pes prints, and anteriorly oriented long axis of the fourth pedal digit indicate that these trackways were made by lizards running bipedally, suggesting that bipedality was possible early in lizard evolution.
Introduction
Although lizards are the most successful of modern reptiles in terms of the number of species (more than 5,800 extant species) and their wide geographical distribution1, their fossil record is relatively poor with respect to both skeletons and tracks. It is, in general, because their small bodies require a suitable depositional environment for preservation. Crown-group Squamata originated between the Late Triassic and the Early Jurassic (213~176 Ma) based on molecules and fossils2. Some skeletal materials of Iguania, Gekkota, Scincoidea, Lacertoidea, and Anguimorpha have been reported in Asia, Europe, and North America by the Early Cretaceous3. Unfortunately, fossil footprint records attributable to lizards are even rarer than those of body fossils because of the general light body weight of lizards and their preferred range of habitats4. There are thus far only three previous reports of fossil lizard tracks. Two were attributed to a lizard without a description from the Eocene Green River Formation, Utah: one is a lizard trackway5,6 and the other is one isolated track7. A new lizard trackway, Neosauroides koreaensis, was recently named from the Haman Formation (Late Albian~Early Cenomanian) of South Korea8.
Here we describe the oldest crown-group lizard trackways known anywhere in the world which show bipedal locomotion. Tracks come from the Hasandong Formation (Aptian~early Albian)9, South Korea which are extraordinarily well-preserved and allow identification of detailed foot anatomy. Associated with a complete manus imprint, most tracks are very small (less than 26 mm) and appear as ectaxonic (i.e., longer fourth digit) and asymmetrical imprints which are frequently recognized as a diagnostic feature of the “typical” lizard pes10. Many modern lizards can bipedally run on the land and even on the surface of water (e.g., the “Jesus lizard”, Basiliscus basiliscus)11. However, it has been unclear as to when lizards developed a capability for bipedal locomotion, though bipedal locomotion has been inferred in some fossil lizards based on the relationship between forelimb and hind limb lengths (e.g., Tijubina pontei)12,13. Therefore, the discovery described in this report is highly significant because it is the first direct evidence of bipedal locomotion in fossil lizards, suggesting that lizard bipedality is deeply rooted in the phylogeny of lizard evolution.
Results
Systematic ichnology
Order Squamata Oppel, 1811
Sauripes hadongensis ichnogen. et ichnosp. nov.
Etymology
Ichnogenus named from ancient Greek “sauros” (lizard) and “pes” (foot). Ichnospecies named after Hadong County that yielded the holotype.
Holotype
Manus and pes prints on a mudstone slab (70 × 30 cm) (KIGAM VP 201501: Korea Institute of Geoscience and Mineral Resources, Vertebrate Paleontology).
Type locality and horizon
Hasandong Formation, Lower Cretaceous (Aptian-early Albian)9, an abandoned quarry next to Hadong power plant, Hadong County, South Gyeongsang Province, South Korea (Supplementary Information Fig. S1).
Diagnosis
Quadrupedal tracks; manus prints are medial to the pes prints; the pes prints are larger than the manus prints; plantigrade and pentadactyl pes prints are longer than wide; the digit length progressively increasing from digits I to IV (ectaxonic); digit V is oriented more laterally and offset from other digits; digit imprint IV is more than twice the length of the metatarsal impression; plantigrade and pentadactyl manus print has similar length and width dimensions; digits II and IV are shorter than digit III (mesaxonic); the interdigital angle between digits I and V of the manus is larger than that of the pes.
Description
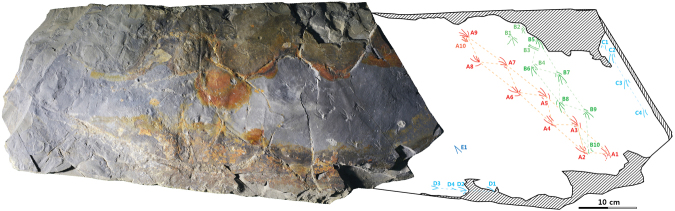
The slab contains 29 lizard tracks without any other vertebrate traces (Fig. 1). They are preserved as depressions and are not underprints. Although the tracks are shallowly depressed and very small in size, the quality of the impressions of the autopod anatomy in some tracks is good enough to provide detailed descriptions.
Figure 1.
Photograph and drawing of lizard trackways on the block.
Based on track morphology, two different types of tracks are observed on this slab. One type (N = 25, pes tracks) has curved digit imprints that progressively increase in length from digits I to IV, a smaller digit V that is distinctly separated from the other digits by a large interdigital angle, and digit V is oriented more laterally. The other type (N = 4, manus tracks) is mesaxonic, having a longer digit III compared to the others (digits I, II, IV, V). The average manus and pes length is 19.18 mm and 22.29 mm, respectively (heteropody). Although manus-pes sets are not regularly imprinted, the tracks visible on the slab clearly show locomotion patterns without tail trails.
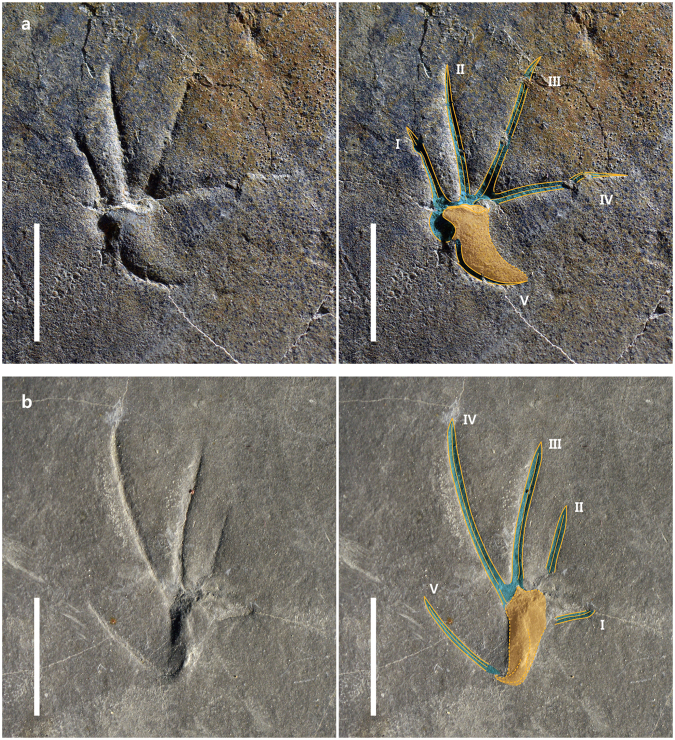
Of the four manus tracks, one in trackway B (B1, Fig. 2a) is better preserved than the other manus tracks in trackway A (A10) and trackway B (B3, B4). It has five digits which appear nearly straight except for digit V which is strongly curved medially. All digit impressions are very narrow (less than 1 mm). The digit I (7.58 mm long) and II (11.91 mm long) imprints are anteromedially oriented, whereas those of digit III (13.64 mm long) are oriented anterolaterally, digit IV (12.69 mm long) laterally, and digit V (6.84 mm long) posterolaterally. Therefore, the divarication of digit I and V impressions is very wide (134.42°). The distal ends of digit III and IV show slightly curved claw marks. The metacarpal depression is small and slightly raised compared to the digital impressions. There is no indication of webbing between the digits.
Figure 2.
Manus and pes tracks of Sauripes hadongensis, n. ichnogen., n. ichnosp. (a) Enlarged photograph and drawing of a manus imprint (B1). (b) A pes imprint (A6). Scale bars equal 1 cm.
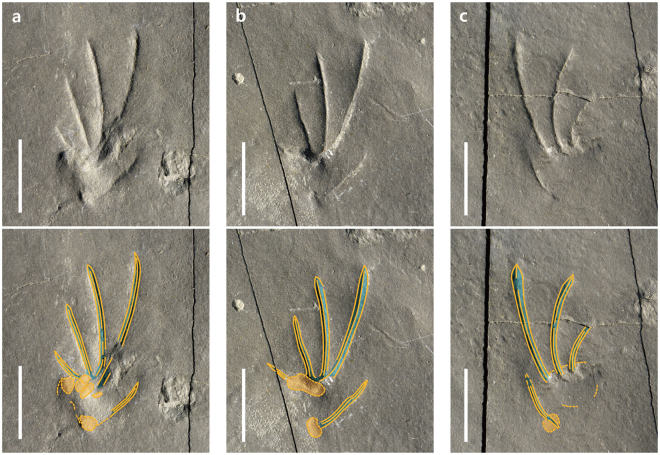
The pes prints are plantigrade, pentadactyl, and distinctly ectaxonic (Figs 2b, 3). Digit I imprint is the shortest (average 4.34 mm) and digit II, III, and IV imprints (7.07 mm, 12.84 mm, and 15.80 mm, respectively) increase progressively in length. Digit V (9.48 mm) is shorter than III and IV, but longer than I and II. Digit I imprint is oriented medially (A6, Fig. 2b) or anteromedially (A3, B8, Fig. 3a,b). Digit V imprint is distinctly separated from other imprints and is connected to the back of the heel trace. The divarication of digit I and V impressions is less than 90°. The metatarsal impression is elongated and located behind digits I to IV. It is slightly raised in relation to the digit imprints, with the metatarsophalangeal joint area the most deeply impressed, especially in digits II, III, and IV.
Figure 3.
Pes tracks of Sauripes hadongensis, n. ichnogen., n. ichnosp. (a) Enlarged photograph and drawing of a pes imprint (A3). (b) A pes imprint (B8). (c) A pes imprint (B9). Scale bars equal 1 cm.
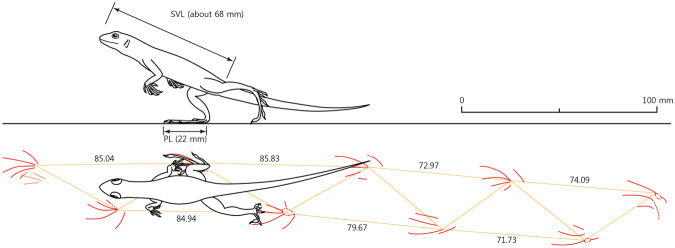
Twenty-eight tracks comprise four trackways, and the trackways have roughly two directions (Fig. 1). Trackways A and B slightly overlap in the opposite direction, indicating a short time interval between two formations. They consist of ten left and right tracks, respectively, while trackways C and D preserve four right tracks, respectively, on the slab (Supplementary Information Table S1). Pes prints are predominant in all trackways, so it is not easy to recognize a normal quadrupedal gait pattern, comprising manus and pes prints. The manus gait-width is narrower than the pes gait-width as seen in the trackways of extant lacertids14 and varanids15. Trackway A is the longest and best preserved among the four trackways, comprising one incomplete right manus and nine pes prints (4 left and 5 right). The average pes stride length is 79.18 mm and the average pace length is 47.82 mm with 112.88° as pace angulation. The trackway width becomes narrower as stride length increases in trackways A and B (Fig. 4). The snout-vent length (SVL) of the trackmaker was approximately 68 mm, based on the allometric plot for snout-vent length in relation to foot length of an iguanian Tropidurus torquatus16.
Figure 4.
A reconstruction of a lizard bipedal running on the substrate, based on trackway A. Abbreviations: SVL, snout-vent length; PL, pes length.
Discussion
Bipedality of Sauripeshadongensis
Many extant lizard species can run bipedally, but not as obligate bipedality. Lizards exhibit different gaits, from quadrupedal and bipedal species to terrestrial and arboreal specialists. Lizard locomotion is strongly influenced by body shape and length, as well as by differences in habitat17. Nevertheless, four forms of gait; a quadrupedal walk at low speeds, a quadrupedal fast gait, a diagonal run at high speeds, and the bipedal run (e.g., Basiliscus basiliscus) are recognized for locomotion in lizards18. Many lizards (more than 50 species) are known to have the capability for bipedal locomotion19. Although some lizards appear to run bipedally without acceleration19, bipedality usually occurs as a consequence of acceleration in a lizard with hind limbs that are significantly longer than the forelimbs, moving the center of mass, and the rotational force on the hip joints20,21. When lizards are moving at relatively slow speeds, they retain a sprawling limb posture with laterally oriented plantigrade feet22. With this locomotor pattern, the front feet are positioned under the body with the head up, increasing the chance of leaving manus imprints rather than pes ones as seen in Neosauroides koreanesis8. In contrast, Sauripes hadongensis shows pes-dominant trackways characterized by long strides, a large pace angulation, and digitigrade prints throughout the locomotion sequences. Snyder pointed out that long hind limbs, short forelimbs, a narrow pelvis and a long tail could aid bipedality in lizards, mostly through increased stride length23. Running lizards at high speeds frequently also leave digitigrade footprints rather than plantigrade ones24.
S. hadongensis has better defined impressions of the digits than of the sole pads, with distinctly deep metatarsophalangeal joint in digits II, III, and IV, behind which the sediment is slightly pushed up, indicating that they ran mainly on the digits, instead of touching the whole soles plantar surface on the substrate (Fig. 3). At fast speeds, the long axis of the fourth toe is nearly parallel to the direction of movement, generating a great proportion of the forces25, as is clearly shown in trackway A and B (Fig. 1). However, the extant and fossil lizard pes prints in walking trackways show strong outward rotation by outwardly rotated feet10,22. Two trackways show evidence of increasing speed based on the increasing stride length and pace angulation (Supplementary Information Table S1). The trackway width is getting narrow in the trackways A and B because the hind limbs are more fully straightened as they attain a bipedal posture and a higher hip position24,26 (Fig. 4).
In trackway B, three successive manus prints (B1, B3, and B4) are preserved before the transition to bipedal locomotion. The lizard is the only vertebrate animal that starts on all limb pairs, then transitions to the bipedal gait by acceleration23. B1 has the best preservation amongst the three, which shows a complete manus imprint including metacarpal depression. On the other hand, B3 and B4 have only two or three incomplete distal digit imprints. The first bipedal stride by acceleration in lizards increases trunk angle, hence increases forelimb clearance24. The locomotor behaviour of the lizards that left trackways in the Hasandong Formation is similar to the experimental observations for the bipedal running of Dipsosaurus dorsalis and Callisaurus draconoides27. Therefore, consideration of all the evidence above strongly suggests that S. hadongensis was made by lizards transitioning to their hind limbs during locomotion and becoming bipedal.
For the most part, bipedality has been related to fast locomotion and to predator avoidance17. Bipedality in lizards may be advantageous for enhanced environmental perception during locomotion by elevating the head and expanding the visual field during obstacle negotiation28. It is not certain whether S. hadongensis tracks were made when escaping from predators or not, but interestingly, the pterosaur track Pteraichnus koreanensis was reported from the same horizon at the same site29. Some pterosaurs likely foraged in diverse environments for small animals and carrion30. The occurrence of P. koreanensis and S. hadongensis tracks together may imply that these two trackmakers had a contemporary antagonistic relationship. If true, the threat of pterosaur predation might have caused these running lizards to leave the bipedal trackways found in the Hasandong Formation (Fig. 5).
Figure 5.
A reconstruction of a lizard running bipedally chased by the pterosaur Pteraichnus koreanensis, based on the trackway (Drawn by Chuang Zhao).
About the trackmaker
Bipedality can be observed in phylogenetically diverse extant lizard families such as Lacertoidea (teiids), Anguimorpha (varanids, bipedal posture), and Iguania (agamids, iguanids, crotaphytids, and phrynosomatids), particularly among the species that live in sandy, rocky or open environments18,21. The Gekkota was established in the Old World tropics by at least mid-Cretaceous31, and Late Jurassic basal gekkonomorphs (Eichstaettisaurus schroederi and Ardeosaurus digitatellus) already showed a capability for scansorial locomotion32. The Teiidae is native to the Americas33,34. In Asia, they first appeared in the Late Cretaceous in Mongolia and China35. Varanid lizards radiated from Mongolia during the Late Cretaceous to Early Cenozoic (80~50 Ma) and dispersed to almost all major fragments of Laurasia and Gondwana36.
Based on fossils and molecular data2,37–39, primitive iguanians (acrodontans and non-acrodontans) existed in Laurasia by the Aptian/Albian. Extant iguanians usually have well-developed, strong legs suitable for bipedality21. In addition, the extinct polyglyphanodonts are known from the Early Cretaceous in Asia40 and became abundant in the Upper Cretaceous of Mongolia and China35,41. They have strong hind limbs and a rather iguanian skeletal morphology. Therefore, based on the palaeobiogeographic distribution of facultative extant families, the lizard that produced S. hadongensis tracks could well have been a member of an extinct family or stem members of Iguania, which was present in the Early Cretaceous.
Methods
Geological Setting
The Hasandong Formation is inferred to be of Aptian to early Albian age based on a comprehensive paleomagnetic and radiometric data9. The overlying Jinju Formation and underlying Nakdong Formation have been dated to 109.9 ± 3.2 Ma and 127.67 ± 1.3 Ma, respectively42,43. The Hasandong Formation has yielded the most abundant vertebrate body fossils in the Gyeongsang Supergroup (Barremian~Campanian), part of the largest Mesozoic Gyeongsang Basin in the Korean Peninsula. Vertebrate fossils include turtles, pterosaurs, crocodilians, and dinosaurs44. Most bones occur as scattered, broken, and isolated pieces which had probably undergone long aerial exposure, transportation, and scattering on the floodplain before burial45. Previously described vertebrate ichnofossils from the Hasandong Formation include dinosaur tracks46 and pterosaur tracks of Pteraichnus koreanensis29.
The lizard track site is from an abandoned quarry next to the Hadong power plant, Hadong County where there is approximately 5,000 m2 of exposure, representing the middle part of the Hasandong Formation (Supplementary Information Fig. S1). The lizard trackways occur in the same horizon as the pterosaur ichnotaxon, Pteraichnus koreanensis, which comprises a dark grey mudstone layer in the middle part of the section. This layer also produces dinosaur tracks and plant fossils (Ptilphylum sp., Cladophlebis sp., Ruffordia sp.), presenting sediments thought to have been deposited in small swamps and/or marginal lakes associated with floodplains between channels.
Electronic supplementary material
Acknowledgements
For the excavation in 2004, we are indebted to Drs. Y.-S. Lee and B.-C. Kim in KIGAM. We thank Professor Susan E. Evans for providing careful comments on an earlier version. We also thank Dr. Tiago R. Simões and one anonymous reviewer for improving an earlier version of this manuscript with their comments. This work was supported by the Basic Research in Application and Development of Geological Samples and Geo-technology R&D Policy (grant number 17–3117–2) to H.-J. Lee and the Research Resettlement Fund (3345–20150015) for the new faculty of Seoul National University and the National Research Foundation of Korea (grant number 2016R1A2B2015012) to Y.-N. Lee.
Author Contributions
Y.-N.L. designed the project; H.-J.L., Y.-N.L. collected fossils and performed the research; H.-J.L. assembled figures; Y.-N.L. developed and wrote the manuscript with contributions from H.-J. L., A.R.F. and J.L. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-20809-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vitt, L. J. & Caldwell, J. P. Herpetology: an introductory biology of amphibians and reptiles. 1–776 (Academic Press, 2013).
- 2.Jones ME, et al. Integration of molecules and new fossils supports a Triassic origin for Lepidosauria (lizards, snakes, and tuatara) BMC Evol. Biol. 2013;13:1–1. doi: 10.1186/1471-2148-13-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans SE. At the feet of the dinosaurs: the early history and radiation of lizards. Biol. Rev. Camb. Philos. Soc. 2003;78:513–551. doi: 10.1017/S1464793103006134. [DOI] [PubMed] [Google Scholar]
- 4.Padian K, Olsen PE. Footprints of the Komodo monitor and the trackways of fossil reptiles. Copeia. 1984;1984:662–671. doi: 10.2307/1445147. [DOI] [Google Scholar]
- 5.Curry, H. D. In Guidebook to the Geology of the Uinta Basin: 8th Annual Field Conference (ed. Seal, O. G.) 42–47 (Intermountain Association of Petroleum Geologists, 1957).
- 6.Grande, L. Paleontology of the Green River Formation, with a review of the fish fauna. 63, 1–333 (Geological Survey of Wyoming Bulletin, 1984).
- 7.Lockley MG, Ritts BD, Leonardi G. Mammal track assemblages from the early Tertiary of China, Peru, Europe and North America. Palaios. 1999;14:398–404. doi: 10.2307/3515465. [DOI] [Google Scholar]
- 8.Kim KS, et al. First report of lacertiform (lizard) tracks from the Cretaceous of Asia. Cretaceous Res. 2017;69:62–70. doi: 10.1016/j.cretres.2016.08.013. [DOI] [Google Scholar]
- 9.Kang HC, Paik IS. Review on the geological ages of the formations in the Gyeongsang Basin, Korea. J. Geol. Soc. Korea. 2013;49:17–29. [Google Scholar]
- 10.Kubo T. Extant lizard tracks: variation and implications for Paleoichnology. Ichnos. 2010;17:187–196. doi: 10.1080/10420940.2010.502500. [DOI] [Google Scholar]
- 11.Snyder RC. Bipedal locomotion of the lizard Basiliscus basiliscus. Copeia. 1949;1949:129–137. doi: 10.2307/1438487. [DOI] [Google Scholar]
- 12.Bonfim-Júnior F, de C, Rocha-Barbosa O. The Paleoautoecology of Tijubina pontei Bonfim-Júnior & Marques, 1997 (Lepidosauria, Basal Squamata of Santana Formation, Aptian of Araripe basin, Lower Cretaceous of Northwest of Brazil) Anuário do Instituto de Geociências. 2006;29:54–65. [Google Scholar]
- 13.Simões TR, Caldwell MW, Weinschütz LC, Wilner E, Kellner AWA. Mesozoic lizards from Brazil and their role in early squamate evolution in South America. J. Herpetol. 2017;51:307–315. doi: 10.1670/16-007. [DOI] [Google Scholar]
- 14.Fichter J. Aktuopaläontologische Untersuchungen an den Fährten einheimischer Urodelen und Lacertilier. Teil I: Die morphologie der Fäherten in Abhängigkeit von der Sedimentbeschaffenheit. Mainz. nat. wiss. Arch. 1982;20:91–129. [Google Scholar]
- 15.Farlow JO, Pianka ER. Body form and trackway pattern in Australian desert monitors (Squamata: Varanidae): comparing zoological and ichnological diversity. Palaios. 2000;15:235–247. doi: 10.1669/0883-1351(2000)015<0235:BFATPI>2.0.CO;2. [DOI] [Google Scholar]
- 16.Kohlsdorf T, Garland T, Jr, Navas CA. Limb and tail lengths in relation to substrate usage in Tropidurus lizards. J. Morphol. 2001;248:151–164. doi: 10.1002/jmor.1026. [DOI] [PubMed] [Google Scholar]
- 17.Rocha-Barbosa O, Loguercio MFC, Velloso ALR, Bonates ACC. Bipedal locomotion in Tropidurus torquatus (Wied, 1820) and Liolaemus lutzae Mertens, 1938. Braz. J. Biol. 2008;68:649–655. doi: 10.1590/S1519-69842008000300024. [DOI] [PubMed] [Google Scholar]
- 18.Snyder RC. Quadrupedal and bipedal locomotion of lizards. Copeia. 1952;1952:64–70. doi: 10.2307/1438533. [DOI] [Google Scholar]
- 19.Aerts P, Damme RV, D’Aout K, Hooydonck BV. Bipedalism in lizards: whole-body modelling reveals a possible spandrel. Phil. Trans. R. Soc. Lond. B. 2003;358:1525–1533. doi: 10.1098/rstb.2003.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clemente CJ, Withers PC, Thompson G, Lloyd D. Why go bipedal? Locomotion and morphology in Australian agamid lizards. J. Exp. Biol. 2008;211:2058–2065. doi: 10.1242/jeb.018044. [DOI] [PubMed] [Google Scholar]
- 21.Clemente CJ. The evolution of bipedal running in lizards suggests a consequential origin may be exploited in later lineages. Evolution. 2014;68:2171–2183. doi: 10.1111/evo.12447. [DOI] [PubMed] [Google Scholar]
- 22.Brinkman DB. The hind limb step cycle of Iguana and primitive reptiles. J. Zool. 1981;181:91–103. [Google Scholar]
- 23.Snyder RC. Adaptations for bipedal locomotion of lizards. Integr. Comp. Biol. 1962;2:191–203. [Google Scholar]
- 24.Irschick DJ, Jayne BC. Comparative three-dimensional kinematics of the hindlimb for high-speed bipedal and quadrupedal locomotion of lizards. J. Exp. Biol. 1999;202:1047–1065. doi: 10.1242/jeb.202.9.1047. [DOI] [PubMed] [Google Scholar]
- 25.Fieler CL, Jayne BC. Effects of speed on the hindlimb kenematics of the lizard Dipsosaurus dorsalis. J. Exp. Biol. 1998;201:609–622. doi: 10.1242/jeb.201.4.609. [DOI] [PubMed] [Google Scholar]
- 26.Urban EK. Quantitative study of locomotion in teiid lizards. Anim. Behav. 1965;13:513–529. doi: 10.1016/0003-3472(65)90115-6. [DOI] [PubMed] [Google Scholar]
- 27.Irschick DJ, Jayne BC. A field study of the effects of incline on the escape locomotion of a bipedal lizard. Callisaurus draconoides. Physiol. Biochem. Zool. 1999;72:44–56. doi: 10.1086/316641. [DOI] [PubMed] [Google Scholar]
- 28.Kohlsdorf T, Biewener AA. Negotiating obstacles: running kinematics of the lizard Sceloporus malachiticus. J. Zool. 2006;270:359–371. doi: 10.1111/j.1469-7998.2006.00150.x. [DOI] [Google Scholar]
- 29.Lee Y-N, Lee H-J, Lu J, Kobayashi Y. New pterosaur tracks from the Hasandong Formation (Lower Cretaceous) of Hadong County, South Korea. Cretaceous Res. 2008;29:345–353. doi: 10.1016/j.cretres.2007.05.004. [DOI] [Google Scholar]
- 30.Witton MP, Naish D. A reappraisal of azhdarchid pterosaur functional morphology and paleoecology. PLoS ONE. 2008;3:e2271. doi: 10.1371/journal.pone.0002271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daza JD, Bauer AM, Snively ED. On the fossil record of the Gekkota. Anat. Rec. 2014;297:433–462. doi: 10.1002/ar.22856. [DOI] [PubMed] [Google Scholar]
- 32.Simões TR, Caldwell MW, Nydam RL, Jiménez-Huidobro P. Osteology, phylogeny, and functional morphology of two Jurassic lizard species and the early evolution of scansoriality in geckoes. Zool. J. Linn. Soc. 2017;180:216–241. [Google Scholar]
- 33.Winkler DA, Murry PA, Jacobs LL. Early Cretaceous (Comanchean) vertebrates of central Texas. J. Vert. Paleontol. 1990;10:95–116. doi: 10.1080/02724634.1990.10011794. [DOI] [Google Scholar]
- 34.Krause L. Fossil record of the family Teiidae. notes on paleobiogeography, current distribution, and habits of the Macroteiids. (Sauria, Scincomorpha, Teiidae) Stud. Neotrop. Fauna E. 1985;20:175–188. doi: 10.1080/01650528509360686. [DOI] [Google Scholar]
- 35.Gao K, Hou L. Systematics and taxonomic diversity of squamates from the Upper Cretaceous Djadochta Formation, Bayan Mandahu, Gobi Desert, People’s Republic of China. Can. J. Earth Sci. 1996;33:578–598. doi: 10.1139/e96-043. [DOI] [Google Scholar]
- 36.Estes, R. In Advances in herpetology and evolutionary biology: essays in honor of Ernest E. Williams (eds Rhodin, A. G. & Miyata, K.) 365–398 (Museum of Comparative Zoology, Harvard University, 1983).
- 37.Gao K, Nessov LA. Early Cretaceous squamates from the Kyzylkum Desert, Uzbekistan. Neues Jahrb. Geol. Palaontol. Abh. 1998;207:289–309. [Google Scholar]
- 38.Li P, Gao K, Hou L, Xu X. A gliding lizard from the Early Cretaceous of China. P. Natl. Acad. Sci. USA. 2007;104:5507–5509. doi: 10.1073/pnas.0609552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daza JD, Stanley EL, Wagner P, Bauer AM, Grimaldi DA. Mid-Cretaceous amber fossils illuminate the past diversity of tropical lizards. Sci. Adv. 2016;2:e1501080. doi: 10.1126/sciadv.1501080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans SE, Manabe M. An early herbivorous lizard from the Lower Cretaceous of Japan. Palaeontology. 2008;51:487–498. doi: 10.1111/j.1475-4983.2008.00759.x. [DOI] [Google Scholar]
- 41.Sulimski AM. and Polyglyphanodontidae (Sauria) from the Late Cretaceous of Mongolia. Palaeontol. Pol. 1975;33:25–102. [Google Scholar]
- 42.Lee TH, Park KH, Chun JH, Yi K. SHRIMP U-Pb zircon ages of the Jinju Formation and Silla Conglomerate, Gyeongsang Basin. J. Petrol. Soc. Korea. 2010;19:89–101. [Google Scholar]
- 43.Lee, T. H., Park, K. H. & Yi, K. SHRIMP U-Pb detrital zircon ages of the Nakdong Formation and the Ulleynsan Formation, Gyeongsang Basin. 2012 Fall Joint Annual Conference of the Geological Societies in Korea 134 (2012).
- 44.Lee Y-N, Yu K-M, Wood CB. A review of vertebrate faunas from the Gyeongsang Supergroup (Cretaceous) in South Korea. Palaeogeogr. Palaeocl. 2001;165:357–373. doi: 10.1016/S0031-0182(00)00171-1. [DOI] [Google Scholar]
- 45.Paik IS, et al. Palaeoenvironments and taphonomic preservation of dinosaur bone-bearing deposits in the Lower Cretaceous Hasandong Formation, Korea. Cretaceous Res. 2001;22:627–642. doi: 10.1006/cres.2001.0282. [DOI] [Google Scholar]
- 46.Lim, S. K., Yang, S.-Y., Baek, K.-S. & Kim, T.-W. Cretaceous dinosaur footprints found in the bottom surface of the Gawha River, South Gyeongsang Province. 13th Annual Meeting of Paleontological Society of Korea 16 (1997).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.