Following up on our previous work demonstrating the involvement of the transcription factor, forkhead box M1 (FOXM1), in the biology and outcome of a high-risk subset of newly diagnosed multiple myeloma (nMM)1, we sought to determine whether upregulation of FOXM1 may also be a feature of relapsed myeloma (rMM). To that end, we analyzed the total therapy 2 (TT2) dataset (GSE2658) from the University of Arkansas for Medical Science, which contains microarray-based gene expression and clinical outcome data on 88 patients with known FOXM1 mRNA levels at baseline and relapse. Statistical comparison demonstrated a significant increase (p = 10−4) in mean (by ~70%) and median (~2.9-fold) FOXM1 expression upon relapse (Fig. 1a, left). Just like in nMM1, the distinction between high and low FOXM1 message at relapse was of prognostic value: FOXM1High patients (n = 11, 12.5%) exhibited markedly reduced event-free survival and overall survival compared to FOXM1Low patients (n = 77, Fig. 1a, right). To confirm these results with the help of an independent dataset that is more recent than TT2, we took advantage of myeloma patients from the University of Heidelberg, Germany, which were uniformly treated upfront using HDT/ASCT and new myeloma drugs. In this cohort of 692 patients with nMM, FOXM1High status was as negative a predictor of survival (Fig. 1b) as in the original study1. Furthermore, relapsed myelomas from the Heidelberg cohort (n = 55) also demonstrated significant elevations of FOXM1 message compared to nMM (Fig. 1c, left) and FOXM1High status at relapse—found in 8 of 60 (13%) patients—led to an even more dramatic reduction in survival than seen in the TT2 cohort (Fig. 1c, right). These findings led us to conclude that upregulation of FOXM1 is a molecular marker of a subset of relapsed myelomas that results in inferior outcome in patients with peak expression levels.
Fig. 1. Expression of FOXM1, a proliferation gene in normal and neoplastic plasma cells, is elevated in relapsed myeloma and predicts poor outcome in the subset of patients with peak expression levels.
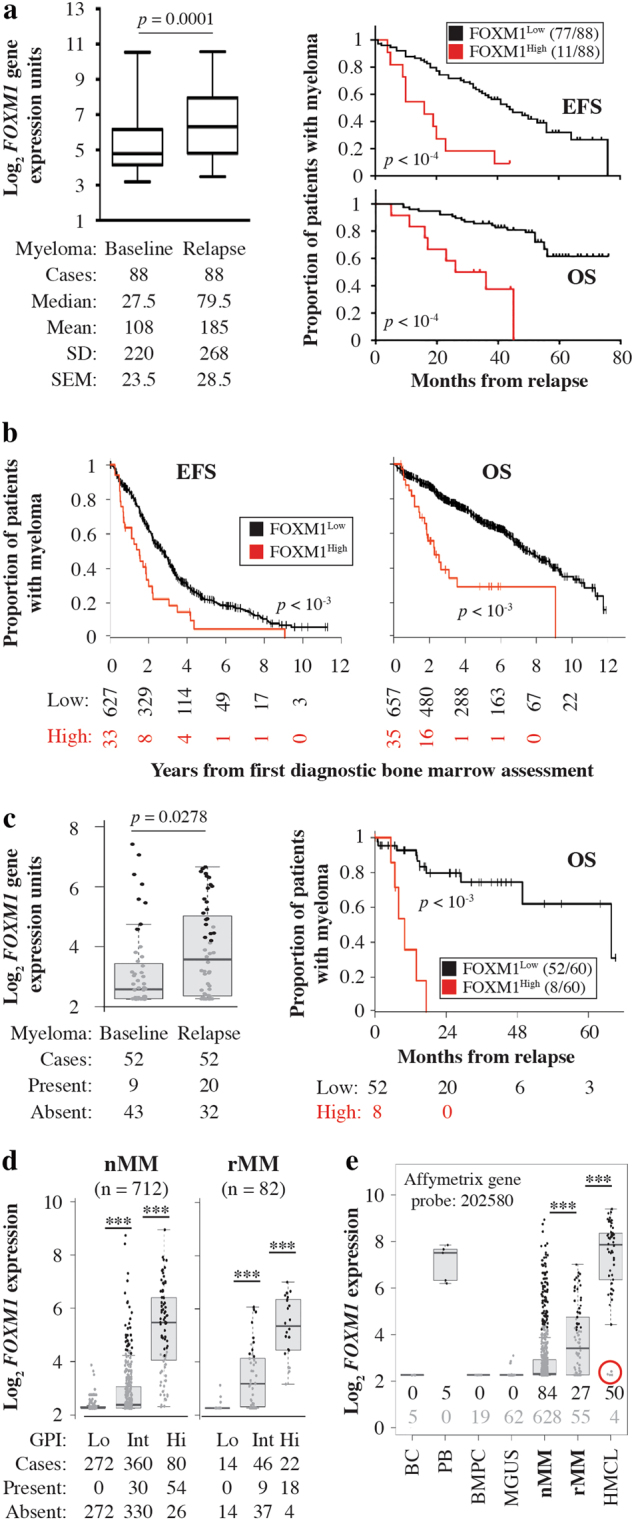
a Shown on the left is a box plot of FOXM1 mRNA levels determined by microarray analysis (gene probe set 202580_x_at) of magnetic bead-sorted CD138+ myeloma cells collected from 88 patients of the UAMS Total Therapy 2 (TT2) trial at baseline (nMM) and relapse (rMM). Median FOXM1 expression is indicated by a black horizontal line within the box. The 25th and 75th percentiles of gene expression are indicated by lower and upper horizontal lines, respectively. Mean FOXM1 expression, standard deviation of the mean (SD) and standard error of the mean (SEM) are indicated below the plot. The increase in FOXM1 message was driven by 61 of 88 (69%) patients; in 20 patients FOXM1 mRNA was down at relapse compared to baseline and in 7 patients there was no change. Hence, upregulation of FOXM1 at relapse is a feature of the majority of cases, but it is not a universal phenomenon. Presented on the right is a Kaplan-Meier analysis of event-free survival (EFS) and overall survival (OS) of rMM patients stratified into cases containing FOXM1 message above the median (red curve, FOXM1High) or below the median (black curve, FOXM1Low). b High FOXM1 expression prognosticates poor survival of patients with nMM in the University of Heidelberg cohort. Myeloma patients were assigned to 2 groups distinguished by low FOXM1 mRNA levels (FOXM1Low) and high FOXM1 mRNA levels (FOXM1High) based on microarray measurements. The decline in EFS (left) and OS (right) after the first diagnostic bone marrow assessment is plotted. Survival was determined using Cox’s proportional hazard model. EFS and OS are significantly reduced in FOXM1High patients compared to FOXM1Low patients (p < 10−3). c FOXM1 expression in 52 paired nMM vs. rMM samples from the University of Heidelberg cohort is depicted to the left. Just like in the TT2 sample presented in (a), FOXM1 at relapse was up in the majority of patients (35/56, 63%), but down in 17 (30%) patients and unchanged in 4 patients. Shown to the right is a plot of OS, determined using Cox’s proportional hazard model, of patients with FOXM1High relapse (n = 8) and FOXM1Low relapse (n = 52). Maximally selected rank statistics was used to stratify patients with rMM using 6 as cutoff. The proportion of FOXM1High relapse (13.3%) is similar to that in panel a (12.5%). d Box plots comparing FOXM1 mRNA levels in CD138+ fractionated myeloma cells obtained from 712 patients with newly diagnosed disease (nMM) and 82 patients with relapsed disease (rMM) from the Heidelberg cohort. Based on the GPI score, patients were stratified into 3 distinct groups of low, intermediate and high proliferation. FOXM1 expression was different in these groups at p < 10−3 (indicated by 3 asterisks). The proportion of cases in which FOXM1 was present (expressed) or absent (not expressed), based on a cutoff determined by PANP, is indicated for all three groups below the plots. While FOXM1 was not present in the low proliferation group of both new and relapsed disease, it was more frequently present in rMM compared to nMM the intermediate (9/46 or 20% vs. 30/360 or 8%) and high (18/22 or 82% vs. 54/80 or 68%) proliferation groups. e Box plot depicting median FOXM1 expression in B cells (BC, n = 5), plasmablasts (PB, n = 5) and bone marrow plasma cells (BMPC, n = 19) from healthy individuals, or in CD138+ BMPCs from patients with monoclonal gammopathy of undetermined significance (MGUS, n = 62), newly diagnosed myeloma (nMM, n = 712) and relapsed myeloma (rMM, n = 54) from the Heidelberg cohort. A panel of human myeloma cell lines (HMCL, n = 54), which represent the terminal disease stage of plasma cell leukemia, is included for comparison. Presence and absence of FOXM1 is indicated for all groups using black and grey numbers, respectively. The circumstance that in 4 of 54 (7%) HMCLs FOXM1 was absent (indicated by red circle) suggests that malignant plasma cells may switch to a FOXM1-independent proliferation program, perhaps following secondary genetic change during serial in vitro propagation of cells
Because (1) FOXM1 is best known as positive regulator of cell cycle progression2, (2) tumor cell proliferation is an important, independent, adverse determinant of myeloma patient survival3 and (3) myeloma relapse from chemotherapy ultimately results in selection of cell clones with increased proliferation rates4, we decided to evaluate whether increased FOXM1 expression at relapse may be associated with increased myeloma proliferation. For this purpose, we relied on a validated gene expression-based proliferation index (GPI) of myeloma3 to stratify 712 patients with nMM and 82 patients with rMM from the Heidelberg cohort into three different groups defined by low, intermediate or high proliferation rates of tumor cells. Fig. 1d shows that FOXM1 expression paralleled the GPI score in both nMM (left panel) and rMM (right panel). High FOXM1 message levels, which even surpassed those measured in GPIHigh myeloma, were also observed in normal plasmablasts derived from cytokine-induced peripheral blood B-lymphocytes of healthy donors and in rapidly dividing HMCLs (human myeloma cell lines) propagated in vitro (Fig. 1e). These results were consistent with the contention that FOXM1 regulates cell cycle progression in both normal and neoplastic plasma cells.
Recurrent cancer including relapsed myeloma is frequently associated with therapy resistance acquired by changes in genetic pathways wherein FOXM1 has been repeatedly implicated2. With this in mind, we asked whether upregulation of FOXM1 may decrease the sensitivity of myeloma cells to the widely used myeloma drugs, bortezomib (Bz) and doxorubicin (Dox). We chose two HMCLs, XG1 and CAG – engineered to overexpress (OE) lentivirus-encoded FOXM1 (FOXM1OE myeloma) or containing normal (N) levels of the transcription factor due to transduction with “empty” virus (FOXM1N myeloma)—as experimental model system. We found that FOXM1OE cells exhibited partial resistance to both drugs. Specifically, the half-maximal inhibitory concentration (IC50) of Bz was 5.6-fold (CAG) and 1.9-fold (XG1) higher in FOXM1OE than FOXM1N cells, whereas the corresponding difference upon treatment with Dox was 2.9-fold (CAG) and 1.5-fold (XG1). Flow cytometric measurements of annexin V, a marker of apoptosis, were in agreement with these results, as FOXM1OE cells invariably experienced less drug-induced death than FOXM1N cells. Additionally, FOXM1OE cells demonstrated enhanced clonogenic growth in soft agar and increased ABC-transporter drug efflux-pump activity by eFluxx-ID Gold MDR analysis. These results—not shown here but to be presented in greater depth in a future publication—indicated that FOXM1 promotes drug resistance in myeloma. Our findings further suggested that suppression of FOXM1 may re-sensitize myeloma to killing by genotoxic drugs, as seen in other types of cancer2.
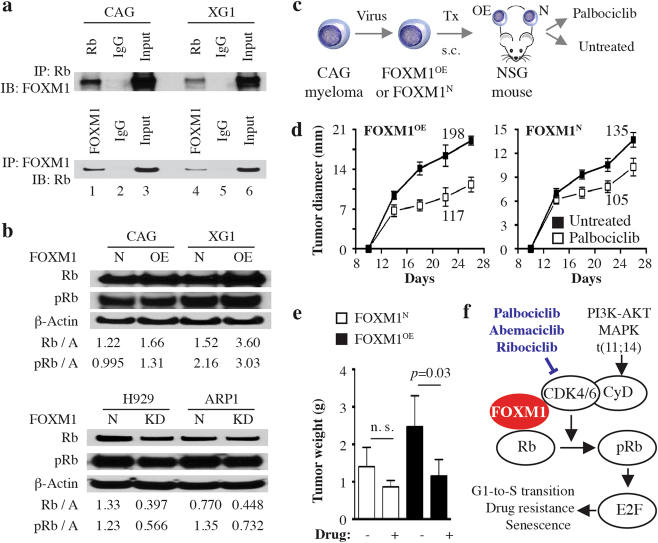
Taking into account that (1) the mechanism by which FOXM1 promotes drug resistance in myeloma may involve increased proliferation of myeloma cells, (2) our previous work implicated the G1-to-S cell cycle regulator, cyclin-dependent kinase 6 (CDK6), in the genetic network of FOXM1 in myeloma1, (3) new findings indicate that CDK6 and FOXM1 are co-expressed in myeloma and, when used as 2-gene mini-signature, predictive of patient survival (Supplemental Fig. 1) and, finally, (4) CDK6 is a key upstream activator of the retinoblastoma Rb/E2F (E2F transcription factor 1) axis, a key regulator of the cell cycle and pathways of senecscence and drug resistance—we decided to determine whether FOXM1 interacts physically with Rb in myeloma. Co-IP analysis showed that antibody to Rb (bait) pulled down FOXM1 in myeloma cells (Fig. 2a, top), whereas antibody to FOXM1 (bait) pulled down Rb (Fig. 2a, bottom). Western analysis of paired FOXM1OE and FOXM1N samples demonstrated that total and phosphorylated Rb were increased in FOXM1OE cells, with higher levels seen in XG1 than CAG cells (Fig. 2b, top). Conversely, Rb was lower in myeloma in which FOXM1 had been “knocked down” (FOXM1KD) by RNA interference (Fig. 2b, bottom). These results supported the contention that FOXM1 regulates, in part, the Rb/E2F axis in myeloma. Bearing in mind that FOXM1-targeted inhibitors suitable for clinical application are not available at this juncture, the results further suggested that pharmacological targeting CDK6 may afford an indirect, alternative approach to inhibiting FOXM1High myeloma.
Fig. 2. FOXM1 interacts with the cyclin D-CDK4/6-Rb-E2F pathway in myeloma.
a FOXM1 binds Rb (official gene symbol, RB1) in myeloma cells. Co-immunoprecipitation (Co-IP) result indicating physical interaction of Rb and FOXM1 in CAG and XG1 myeloma cells. Immunoblots using a specific IgG antibody (Ab) to FOXM1 (following IP with Ab to Rb) or Rb (following IP with Ab to FOXM1) are shown on top of each other. An IgG isotype control is labeled “IgG.” Whole cell lysates not subjected to Co-IP (Input) were included as additional control. b Shown on top is a Western blot of total Rb and its activated, phosphorylated form (pRb) in FOXM1OE and FOXM1N CAG and XG1 myeloma cells. A similar blot containing samples of FOXM1KD and FOXM1N H929 and ARP1 myeloma cells is presented at bottom. The abundance of Rb and pRb, relative to β-actin, was determined using densitometry. The ratios are indicated below the blots. c Study scheme for assessing the efficacy with which the small-drug CDK4/6 inhibitor, palbociclib, inhibits human myeloma growth in immunodeficient mice. FOXM1OE and FOXM1N CAG cells were xenografted s.c. into the right and left flank of NSG mice (n = 10), respectively. Beginning on day 7 after myeloma cell transfer, five mice were treated with daily i.p. injections of the CDK4/6 inhibitor, PD0332991, until study end on day 26. Five mice were left untreated. Tumor diameters were measured on days 14, 18, 22, and 26 after xenografting, using a caliper. d Time course of tumor growth in hosts. Mean tumor diameters (squares) and standard deviations of the mean (short vertical lines with error bars) are plotted. The area under the curve (AUC), ranging from 105 to 198, is indicated for all study groups. e Mean tumor weight at study end. FOXM1OE xenografts treated with PD (1.14 ± 0.456 g) were significantly smaller than their untreated counterparts (2.46 ± 0.833 g) using Mann–Whitney’s t test (p = 0.032). FOXM1N xenografts also showed a difference (0.862 ± 0.172 g vs. 1.40 ± 0.515 g) but missed the 5% threshold of statistical significance (p = 0.095). Median tumor weights in the four groups were significantly different by Kruskall-Wallis analysis (p = 0.0178). f Working model on the interaction of FOXM1 with CDK4/6 and Rb in myeloma. FOXM1 is a proliferation-associated transcription factor that interacts with the cyclin D-CDK4/6-Rb-E2F pathway, a key regulator of the G1-to-S cell cycle transition. Upstream signals activating this pathway include those emanating from MAPK-ERK and PI3K-AKT-mTOR signaling and, in a subset of myeloma, deregulated cyclin D expression following from chromosomal translocation. In addition to cell cycle progression, the Rb/E2F-dependent transcriptional program regulates cellular senescence and response to drug treatment. Small-molecule CDK4/6 inhibitors, such as palbocicblib, abemacicblib and ribocicblib, that have the targeting of the ATP-binding pocket of CDK4 and CDK6 in common, may lend themselves to new treatment approaches to FOXM1High myeloma. Palbocicblib and the CDK5-targeted inhibitor dinacicblib (not shown) are currently undergoing clinical testing in myeloma
To follow up on that, we determined the efficacy with which the selective, potent, orally administered, small-molecule inhibitor of CDK6 and CDK45, palbociclib (PD0332991), hampers myeloma growth in vivo. Since an accurate mouse model of relapsed human myeloma is not available at this juncture, we assessed palbociclib with the assistance of a surrogate model, HMCL-in-mouse xenografting (Fig. 2c). Although the drug hindered the growth of both FOXM1OE and FOXM1N xenografts (Fig. 2d), the former were more sensitive than the latter. Using the area under the growth curve as metric of drug efficacy, palbociclib inhibited FOXM1OE tumors by ~41% (117 vs. 198) and FOXM1N tumors by ~22% (105 vs. 135). Similarly, at study endpoint, drug-dependent growth inhibition of FOXM1OE xenografts (1.14 ± 0.456 g mean tumor weight) amounted to 54% (relative to untreated controls: 2.46 ± 0.833 g), whereas FOXM1N xenografts were inhibited by 38% (0.862 ± 0.172 g vs. 1.40 ± 0.515 g; Fig. 2e). These results provided preclinical support for the potential benefit of a CDK4/6-targeted therapy for FOXM1High myeloma. This is in line with studies by other investigators who demonstrated that palbociclib arrested cell cycle progression of patient-derived tumor cells at G1 in vitro6 and, in combination with Bz, inhibited myeloma-like 5T33 mouse tumors in vivo7. Fig. 2f depicts a working model on the interaction of FOXM1 with the CDK4/6-Rb-E2F pathway in myeloma. Included are 3 small-molecule inhibitors of CDK4/6 that are in clinical use or advanced stages of clinical testing8.
The results presented in Fig. 2 are in line with encouraging evidence on the efficacy of CDK4/6 inhibitors in solid cancers9 and the ongoing clinical assessment of these drugs in blood cancers including MM. In regards to myeloma, the outcome of the recently completed phase 1/2 clinical trial of palbociclib in rMM (NCT00555906)8—and interim results of new trials that are currently recruiting (NCT02030483, NCT02693535)—are eagerly awaited. Of great additional import is the finding of the Mayo Phase 2 Consortium that dinaciclib, a potent inhibitor of CDK5 involved in acquired resistance to proteasome inhibition in myeloma10, exhibits single-agent activity in rMM11. Because dinaciclib blocks cell division at later stages of the mitotic cycle (late G1, S, and G2) than palbociclib does (early G1), therapeutic synergism of these inhibitors is possible. To better put the promise of CDK-targeted treatments for FOXM1High myeloma into perspective, it is helpful to recognize that FOXM1 is critically involved in the natural history and outcome of other B-cell neoplasms, such as acute lymphoblastic leukemia12, diffuse large cell lymphoma13 and follicular lymphoma14. The broad significance of FOXM1 for B-lineage malignancies agrees with its pleiotropic function in cancer biology, impacting—in addition to cell cycle progression—DNA damage repair, self-renewal of stem cell-like cancer cells, and response to both cytostatic and targeted treatments2. Finally, FOXM1 is a crucial determinant of cancer outcome; e.g., a recent pan-cancer meta-analysis of ~18,000 gene expression signatures identified the FOXM1 regulatory network as a major predictor of adverse outcomes across 39 solid and hematologic malignancies, including MM15.
Electronic supplementary material
Acknowledgements
This work was supported by National Natural Science Foundation of China grants 81600177 (to CG) and 81670200 (to YY); Jiangsu Natural Science Foundation grants BK20160048 (to YY) and BK20161041 (to CG); a Key Project of Chinese National Programs for Research and Development grant (2016YFC0905900) and start-up funding from The Priority Academic Program Development of Jiangsu Higher Education Institutions for Chinese Medicine (both to YY). This work was also supported by NIH R01CA152105 and grants from the Multiple Myeloma Research Foundation, International Myeloma Foundation and American Society of Hematology (ASH) Bridge Funding Program (all to FZ). Additional assistance was provided by NCI Core Grant P30CA086862 in support of The University of Iowa Holden Comprehensive Cancer Center. Investigators in Germany were supported by grant CAMPSIMM (01ES1103) from the Federal Ministry of Education (BMBF), BMBF’s e:Med Systems Medicine CLIOMMICS program (01ZX1309) and grants from the Deutsche Forschungsgemeinschaft (SFB/TRR79) and 7th EU framework program (OverMyR). Institutional start-up funds from the Department of Internal Medicine, Carver College of Medicine (to FZ and GT) as well as support from ASH’s 7th Round Bridge Grant Program and NIH grants R21CA187388 and R01CA151354 (all to SJ) are gratefully acknowledged.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Y.Y. and S.J. are co-senior authors.
C.G. and C.H. are co-first authors.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41408-018-0060-0).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ye Yang, Phone: +(86) -25-85811276, Email: yangye876@sina.com.
Siegfried Janz, Phone: +(319) 384-2869, Email: siegfried-janz@uiowa.edu.
References
- 1.Gu C, et al. FOXM1 is a therapeutic target for high-risk multiple myeloma. Leukemia. 2016;30:873–882. doi: 10.1038/leu.2015.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nandi, D., Cheema, P. S., Jaiswal, N., Nag, A. FoxM1: repurposing an oncogene as a biomarker. Semin. Cancer Biol. (2017) Pii: S1044-579X(17)30061-5. [DOI] [PubMed]
- 3.Hose D, et al. Proliferation is a central independent prognostic factor and target for personalized and risk-adapted treatment in multiple myeloma. Haematologica. 2011;96:87–95. doi: 10.3324/haematol.2010.030296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinhold N, et al. Clonal selection and double hit events involving tumor suppressor genes underlie relapse from chemotherapy: myeloma as a model. Blood. 2016;128:1735–1744. doi: 10.1182/blood-2016-06-723007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fry DW, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol. Cancer Ther. 2004;3:1427–1438. [PubMed] [Google Scholar]
- 6.Baughn LB, et al. A novel orally active small molecule potently induces G1 arrest in primary myeloma cells and prevents tumor growth by specific inhibition of cyclin-dependent kinase 4/6. Cancer Res. 2006;66:7661–7667. doi: 10.1158/0008-5472.CAN-06-1098. [DOI] [PubMed] [Google Scholar]
- 7.Menu E, et al. A novel therapeutic combination using PD 0332991 and bortezomib: study in the 5T33MM myeloma model. Cancer Res. 2008;68:5519–5523. doi: 10.1158/0008-5472.CAN-07-6404. [DOI] [PubMed] [Google Scholar]
- 8.Niesvizky R, et al. Phase 1/2 study of cyclin-dependent kinase (CDK)4/6 inhibitor palbociclib (PD-0332991) with bortezomib and dexamethasone in relapsed/refractory multiple myeloma. Leuk. & Lymphoma. 2015;56:3320–3328. doi: 10.3109/10428194.2015.1030641. [DOI] [PubMed] [Google Scholar]
- 9.Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: From Discovery to Therapy. Cancer Discov. 2016;6:353–367. doi: 10.1158/2159-8290.CD-15-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu YX, et al. RNAi screen of the druggable genome identifies modulators of proteasome inhibitor sensitivity in myeloma including CDK5. Blood. 2011;117:3847–3857. doi: 10.1182/blood-2010-08-304022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar SK, et al. Dinaciclib, a novel CDK inhibitor, demonstrates encouraging single-agent activity in patients with relapsed multiple myeloma. Blood. 2015;125:443–448. doi: 10.1182/blood-2014-05-573741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchner M, et al. Identification of FOXM1 as a therapeutic target in B-cell lineage acute lymphoblastic leukaemia. Nat. Commun. 2015;6:6471. doi: 10.1038/ncomms7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uddin S, et al. Overexpression of FoxM1 offers a promising therapeutic target in diffuse large B-cell lymphoma. Haematologica. 2012;97:1092–1100. doi: 10.3324/haematol.2011.053421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bisikirska B, et al. Elucidation and pharmacological targeting of novel molecular drivers of follicular lymphoma progression. Cancer Res. 2016;76:664–674. doi: 10.1158/0008-5472.CAN-15-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gentles AJ, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.