Abstract
This study aims to (1) investigate the neuropathology of mild to severe pediatric TBI and (2) elucidate the predictive value of conventional and innovative neuroimaging for functional outcome. Children aged 8–14 years with trauma control (TC) injury (n = 27) were compared to children with mild TBI and risk factors for complicated TBI (mildRF+, n = 20) or moderate/severe TBI (n = 17) at 2.8 years post-injury. Neuroimaging measures included: acute computed tomography (CT), volumetric analysis on post-acute conventional T1-weighted magnetic resonance imaging (MRI) and post-acute diffusion tensor imaging (DTI, analyzed using tract-based spatial statistics and voxel-wise regression). Functional outcome was measured using Common Data Elements for neurocognitive and behavioral functioning. The results show that intracranial pathology on acute CT-scans was more prevalent after moderate/severe TBI (65%) than after mildRF+ TBI (35%; p = .035), while both groups had decreased white matter volume on conventional MRI (ps ≤ .029, ds ≥ −0.74). The moderate/severe TBI group further showed decreased fractional anisotropy (FA) in a widespread cluster affecting all white matter tracts, in which regional associations with neurocognitive functioning were observed (FSIQ, Digit Span and RAVLT Encoding) that consistently involved the corpus callosum. FA had superior predictive value for functional outcome (i.e. intelligence, attention and working memory, encoding in verbal memory and internalizing problems) relative to acute CT-scanning (i.e. internalizing problems) and conventional MRI (no predictive value). We conclude that children with mildRF+ TBI and moderate/severe TBI are at risk of persistent white matter abnormality. Furthermore, DTI has superior predictive value for neurocognitive out-come relative to conventional neuroimaging.
Electronic supplementary material
The online version of this article (doi:10.1007/s11682-017-9673-3) contains supplementary material, which is available to authorized users.
Keywords: Diffusion tensor imaging, Tract-based spatial statistics, Pediatrics, Traumatic brain injury, Neurocognitive functioning, Behavior problems
Introduction
Traumatic brain injury (TBI) is the leading cause of disability in children and young adults (World Health Organization 2006). Children with moderate to severe TBI are typically at risk of poor functional outcome in terms of neurocognitive impairment and behavior problems. Neurocognitive impairments include deficits in attention and working memory, learning and memory, and executive functioning (Babikian and Asarnow 2009), whereas behavior problems include anxiety, depression and aggression (Li and Liu 2013). Recent evidence indicates that even after mild TBI, children with risk factors for intracranial pathology (i.e. mildRF+; e.g. skull fracture, persistent vomiting, focal neurological impairment) are at risk of poor neurocognitive and behavioral outcome (Königs et al. 2015a, b). Importantly, functional outcome of mild to severe TBI is characterized by a distinct inter-individual heterogeneity that remains poorly understood (Polinder et al. 2015), and therefore complicates reliable prognosis of neurocognitive and behavioral outcome. The complexity of TBI neuropathology is thought to represent a crucial source of heterogeneity in functional outcome (Bigler et al. 2013a). Therefore, a better understanding of the relations between both conventional and innovative neuroimaging and functional outcome may contribute to a more reliable prognosis for children with TBI.
While the neuropathology of mild TBI remains obscure (Bigler and Maxwell 2012), the literature shows that moderate to severe TBI involves complex interactions between primary and secondary injury mechanisms, involving focal injuries (i.e. contusions and hemorrhages), diffuse injuries (i.e. diffuse axonal injury) as well as subsequently raised intracranial pressure and neurotoxic biochemical cascades (Bigler et al. 2013). Recent evidence suggests that the widespread and persistent disruption of white matter integrity observed after pediatric TBI (Roberts et al. 2014) plays a pivotal role in functional outcome, through the disturbance of neural networks that give rise to neurocognitive and behavioral functioning (Sharp et al. 2014). Unfortunately, clinical neuroimaging modalities (i.e. computed tomography [CT] and conventional magnetic resonance imaging [MRI], e.g. T1-weighted imaging) have limited sensitivity for the diffuse impact of TBI on white matter integrity (Mittl et al. 1994; Sigmund et al. 2007). This suggests that clinical neuroimaging modalities may lack the potential to account for the complexity of TBI neuropathology in the prognosis for functional outcome. In line with this idea, multiple studies have questioned the prognostic value of CT-scans and conventional MRI for functional outcome of pediatric TBI (Blackman et al. 2003; Gerlach et al. 2009; Mendelsohn et al. 1992).
Diffusion tensor imaging (DTI) is an innovative MRI technique with superior sensitivity for detecting the impact of TBI on white matter integrity as compared to CT and conventional MRI (Mac Donald et al. 2011; Niogi and Mukherjee 2010). Meta-analytic evidence also supports the predictive value of DTI parameters for neurocognitive functioning in the chronic phase of recovery from pediatric TBI (Roberts et al. 2016). Indeed, a convincing body of recent studies consistently reported associations between DTI parameters and multiple aspects of neurocognitive functioning, including attention and working memory (Ewing-Cobbs et al. 2008; Levin et al. 2008; Treble et al. 2013; Van Beek et al. 2015; Wilde et al. 2011; Wozniak et al. 2007; Wu et al. 2010), learning and memory (Dennis et al. 2015; Mccauley et al. 2011) and executive functioning (Dennis et al. 2015; Wilde et al. 2006; Wozniak et al. 2007; Wu et al. 2010). Although the literature on behavioral functioning is sparse, there is also some evidence supporting the relation between DTI and behavior problems after pediatric TBI (Johnson et al. 2011; Max et al. 2012) and the predictive value of DTI for the development of post-injury psychiatric disorders (Max et al. 2012).
Despite convincing evidence supporting the relevance of DTI for functional outcome after TBI, only one study compared DTI to conventional neuroimaging in terms of its predictive value (Oni et al. 2010). This study reported that DTI has superior predictive value for global functional outcome (i.e. Glasgow Outcome Scale) as compared to CT and/or conventional MRI. A meta-analytic review further revealed that the available literature on the predictive value of DTI is limited to region-of-interest (ROI) analyses (Roberts et al. 2016). The selection of ROIs greatly differs between studies, is prone to investigator bias and may provide incomplete information about regional associations between white matter integrity and aspects of functional outcome (Poldrack 2007; Roberts et al. 2016). Furthermore, the available studies have used a wide range of heterogeneous instruments to measure neurocognitive functioning, complicating an integrative interpretation of results across the literature (Roberts et al. 2016).
The current study aims to (1) investigate the neuropathology of mildRF+ to severe pediatric TBI and (2) elucidate the predictive value of conventional and innovative neuroimaging (clinical evaluation of acute CT scans, volumetric analysis of post-acute T1-weighted MRI scans and tract-based spatial statistics [TBSS] on post-acute DTI scans) for functional outcome. Functional outcome was defined in terms of neurocognitive functioning (i.e. intelligence, attention and working memory, verbal learning and memory) and behavioral functioning (i.e. parent and teacher ratings of internalizing and externalizing problems) as measured using Common Data Elements to maximize utility of the results for clinical and research purposes (McCauley et al. 2012). TBSS is a data-driven, model-free alternative to the ROI approach in DTI analysis, which was used to identify the white matter tracts that are affected by TBI. Pearson correlations and voxel-wise regression were subsequently used to investigate the predictive value of neuroimaging parameters for neurocognitive and behavioral outcome. Based on the available evidence, we expected that DTI parameters would have superior predictive value for functional outcome after pediatric TBI as compared to neuroimaging parameters from conventional neuro-imaging. The results of our study may contribute to the prognosis of neurocognitive and behavioral functioning after pediatric TBI, facilitating early planning of rehabilitation services and management of family expectations in clinical practice.
Methods
Participants
This study compared a group of 37 children with TBI to a group of 27 children with trauma control (TC) injury not involving the head, to control for pre-injury risk factors of traumatic injury and psychological effects of hospitalization (Max et al. 1998). Data were collected as part of a follow-up on a consecutive cohort that was retrospectively recruited from three university-affiliated level I trauma centers and three rehabilitation centers in the Netherlands (Königs et al. 2015a). Inclusion criteria were: (1) age 8–14 years at time of follow-up; (2) proficient in the Dutch language; (3) children in the TBI group were required to have a history of hospital admission with a clinical diagnosis of either: (a) mild TBI (GCS = 15–13, loss of consciousness [LOC] duration ≤30 min, post-traumatic amnesia [PTA] duration ≤1 h) with at least one of the following risk factors for complicated TBI (mildRF+ TBI) according to the European Federation of Neurological Societies guidelines on mild TBI: impaired consciousness (GCS = 13–14), focal neurological deficits, persistent vomiting (≥3 episodes), post-injury epileptic seizure, progressive headache and abnormal head CT-scan (Vos and Battistin 2002); or (b) moderate/severe TBI (GCS = 12–3, LOC duration >30 min, PTA duration >1 h (Teasdale and Jennett 1976)); and (4) children in the TC group were required to have a history of hospital admission for traumatic injuries below the clavicles (American College of Surgeons 2004). Exclusion criteria were: (1) previous TBI; (2) visual disorder interfering with neurocognitive testing; or (3) current neurological condition with known effects on neurocognitive functioning, other than TBI, as documented in medical records or reported in a parent-questionnaire for premorbid functioning.
Background information
Data on gender, age, socio-economic status (SES) and diagnosed psychiatric or learning disorders were collected using a parental questionnaire. SES was defined as the average level of parental education ranging from 1 (no education) to 8 (postdoctoral education; Statistics Netherlands 2006).
Injury severity
Diagnosed injuries, the lowest score on the GCS on the day of admission, presence of the described risk factors for complicated mild TBI (Vos and Battistin 2002), and length of hospital stay were extracted from medical files, as was information on any executed surgical procedures.
CT
CT-scans were performed as part of the protocol for acute clinical treatment in the trauma center of admission. The radiological results as evaluated by a senior radiologist were extracted from the medical files.
MRI acquisition and pre-processing
MRI was performed at an average 2.8 years post-injury on a 3 Tesla whole-body unit (Discovery MR750, GE Healthcare, Milwaukee, Wisconsin) using an 8-channel head-coil. Three-dimensional T1-weighted images were acquired using a fast spoiled gradient-echo sequence (176 slices, acquisition matrix 256 × 256, voxel-size 1 × 1 × 1 mm, TR/TE/TI = 8.2/3.2/450 ms, flip angle 15°). Furthermore, two-dimensional echo-planar diffusion-tensor images were acquired in 5 volumes without diffusion weighting and 30 volumes with non-collinear diffusion gradients (b-value = 750 s/mm2) in 47 transverse/slightly oblique slices of 2.5 mm thickness (axially angulated parallel to the line connecting the pituitary to the fastigium of the fourth ventricle) covering the whole brain (TR/TE =5000/74 ms). The acquired in-plane resolution was 2.5 × 2.5 mm, reconstructed to 1 × 1 × 2.5 mm. Parallel imaging was applied with an acceleration factor of 2. All processing of MR images was performed using the Functional MRI of the Brain (FMRIB) Software Library (FSL) version 5.0.8 (Jenkinson et al. 2012).
Pre-processing of diffusion-tensor images included correction of artifacts caused by head motion and eddy currents. Diffusion tensor fitting was performed on brain-extracted images to create fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD; largest eigenvalue of diffusion tensor) and radial diffusivity (RD; mean of the two lowest eigenvalues of diffusion tensor) maps, while the quality of the tensor fit was visually inspected on the sum of squared error maps (Behrens et al. 2003). Volumes with motion distortion causing evident artifacts in the SSE maps were removed from the original diffusion-weighted dataset, leading to the removal of 38 volumes (1.7%) in a total of 19 subjects, with a maximum of 6 out of 35 volumes per subject. For these 19 subjects with one or more discarded volumes, pre-processing was repeated on the data without the volumes containing motion artifacts.
Volumetric analysis of T1-weighted MRI scans
Brain tissue volume was measured in T1-weighted images, which were visually inspected for scanning artifacts due to head motion. T1 scans of two children in the TC group and two children in the mildRF+ TBI group were discarded from volumetric analyses due to severe motion distortion. Volumes of white matter, grey matter and subcortical structures (thalamus, caudate nucleus, putamen, globus pallidum, hippocampus, amygdala and accumbens nuclei in both hemispheres, and the upper brainstem) were estimated using SIENAX (Zhang et al. 2001) and FIRST (Patenaude et al. 2011). Whole brain, grey matter, white matter volumes were normalized for head size and the v-scaling factor resulting from SIENAX was used to normalize subcortical structures as well. Since FIRST produces the volumes of lateralized subcortical structures, left and right volumes of each structure were combined to focus on the diffuse impact of TBI and to reduce the number of group comparisons. Last, putamen and globus pallidum volumes were collapsed to measure the volume of the striatum.
Tract-based spatial statistics (TBSS) on DTI data
Voxel-wise statistical analysis of the DTI data was performed using TBSS (Smith et al. 2006). First, the DTI maps (i.e. FA, MD, AD and RD maps) of all subjects were aligned to the most typical subject in the TC group, as determined by non-linear registrations of FA maps between all children in the TC group. Next, the DTI maps were transformed to MNI152 space using non-linear transformation and a white matter skeleton (FA > 0.2) was computed to reduce the confounding effects of misalignment and inter-subject variability in white matter tract anatomy. The DTI data of one child in the moderate/severe TBI group with major brain damage after neural resection during neurosurgery was discarded, because non-linear transformation of the damaged brain led to severe deformation of the brain in MNI space, causing unreliable alignment. The remaining subjects’ DTI maps (n = 63) were projected onto the skeleton in standard space and voxel-wise TBBS statistics were performed using randomise on the resulting maps, where threshold-free cluster enhancement and P-values as corrected for family-wise error accounted for multiple testing. Last, masks of bilateral white matter tracts were created from the FSL built-in John Hopkins University Atlas and multiplied with the FA skeleton to retrieve the following skeletonized tracts: genu, body and splenium of the corpus callosum (GCC, BCC and SCC, respectively) superior and inferior longitudinal fasciculus (SLF and ILF), inferior frontal occipital fasciculus (IFOF), anterior thalamic radiation (ATR), corticospinal tract (CST), forceps major and minor (FMa and FMi), cingulate and hippocampal parts of the cingulum bundle (CCB and HCB) and uncinate fasciculus (UF).
Functional outcome
Functional outcome was measured using Common Data Elements for neurocognitive and behavioral outcome in the chronic phase of pediatric TBI (McCauley et al. 2012). General neurocognitive functioning was operationalized as intelligence and measured by a Wechsler Intelligence Scale (WISC)-III (Kort et al. 2005) short-form (involving the Vocabulary and Block Design subtests) estimation of age-standardized full-scale IQ (FSIQ), which has shown high validity and reliability in estimating FSIQ (Sattler 2001). Attention and working memory were measured using the age-standardized score on the Digit Span subtest of the WISC-III (Wechsler 1991). Verbal learning and memory was measured using the Rey Auditory Verbal Learning Test (RAVLT; van den Burg and Kingma 1999). Age-standardized z-scores of total correct responses in the direct recall, delayed recall and recognition conditions were used to measure three aspects of verbal memory: (1) Encoding (i.e. the ability to learn new information), as measured by the direct recall condition score; (2) Retrieval (i.e. the ability to access previously learned material stored in long-term memory), as measured by the difference between the direct recall score and the delayed recall score; and (3) Consolidation (i.e. the ability to store information in long-term memory), as measured by the difference between the the delayed recall score and the recognition score.
Behavioral functioning was measured using parent and teacher ratings of internalizing problems (e.g. symptoms of anxiety and depression) and externalizing problems (e.g. aggression and symptoms of conduct disorder), obtained using the Child Behavior Checklist and the Teacher Rating Form (Verhulst and van der Ende 2013). Age- and gender- standardized T-scores of parents and teacher ratings were averaged to yield composite scores of internalizing and externalizing problems. For clarity reasons, all functional outcome scores were transformed to z-scores where lower values intuitively correspond to poorer neurocognitive performance/less behavior problems.
Procedure
The current study represents a follow-up of an earlier investigated sample of children (for more information on the recruitment of this sample, see Königs et al. 2015a). All 123 children that were eligible for the current follow-up (TBI: n = 67; TC: n = 56), were sent an information letter and were contacted by telephone to provide additional information about the study. Eleven children were not reached (TBI: n = 8; TC: n = 3) and 36 declined participation (TBI: n = 17; TC: n = 19). Main reasons not to participate were: not interested (TBI: 41%; TC: 32%), objection to MRI (TBI: 0%; TC: 21%) and no time (TBI: 24%; TC: 21%). A total of 12 children were excluded from participation due to dental braces that were incompatible with MRI (TBI: n = 2; TC: n = 6), claustrophobia (TBI: n = 2; TC: n = 1) or no show (TBI: n = 1; TC: n = 0). The remaining children in the TBI and TC groups (TBI: n = 37; TC: n = 27) did not differ from their respective recruitment cohorts in terms of age, gender and SES (TBI: ps ≥ .28; TC: Ps ≥ .07), or GCS score (TBI: p = .68).
Written informed consent was provided by parents and children aged >11 years. Trained examiners administered the neurocognitive tests in a fixed order, while parents filled out questionnaires in a waiting room. Subsequently, children were made familiar with the MRI procedure using a simulation scanner before actual MRI scanning was performed in the VU University Medical Center. Neurocognitive testing and MRI scanning were performed on the same day for all participants. Time since injury in the TBI sample ranged between 0.8 and 6.2 years. All procedures performed in this study were in accordance with the ethical standards of medical ethical committee of the VU University Medical Centre (NL37226.029.11) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statistical analysis
Statistical analyses were performed using SPSS 22.0 (IBM Corp. 2011). The dependent variables were screened for outliers (−3.29 > z-score > 3.29), which were rescaled using Windsorizing (Tabachnick and Fidell 2012). The TC, mildRF+ TBI and moderate/severe TBI groups were compared on demographic variables, injury-related variables and the prevalence of diagnosed psychiatric or learning disorders using ANOVA and chi-square tests, as appropriate.
First, we investigated the effects of TBI severity on the assessed neuroimaging parameters derived from acute CT-scans, and post-acute T1-weighted MRI and DTI. The prevalence of acute CT-scans with evident intracranial pathology was compared between groups using chi-square tests. The effects of TBI severity (TC, mildRF+ TBI and moderate/severe TBI) on the volumes of white matter, grey matter and subcortical structures in T1-weighted MRI scans were assessed using ANOVA. Next, we investigated the effect of TBI severity on whole brain white matter integrity (as assessed by DTI scans) using ANOVA on mean whole skeleton FA. In addition, regional effects of TBI on white matter integrity were assessed by voxel-wise comparisons of the skeletonized FA maps between all groups using TBSS. If one or more cluster(s) with regional group-differences in FA was/were observed, we further extracted the mean FA, MD, AD and RD values from each cluster, and used ANOVA on these DTI parameters to track down the origin of the observed effect of TBI severity on FA. A pattern of increased MD, decreased AD and/or increased RD will be interpreted as indicative of axonal degeneration and/or demyelination (Budde et al. 2011). If effects of TBI severity on MD, AD and/or RD were observed, the spatial distribution of this effect was investigated using TBSS on the relevant skeletonized maps. Overlap between each white matter tract (GCC, BCC, SCC, SLF, ILF, IFOF, ATR, CST, FMa, FMi, CCB, HCB and UF) and (the) cluster(s) of affected white matter tracts was used to assess the contribution of each white matter tract to the neuropathology of TBI (i.e. overlap as a percentage of the total cluster size of affected white matter tracts in voxels) and the extent to which each tract was affected by the neuropathology of TBI (i.e. overlap as a percentage of the total skeletonized white matter tract size).
Second, the impact of TBI severity on aspects of functional outcome was assessed. ANOVAs assessed the main effect of TBI severity on FSIQ as a measure of intelligence, Digit Span score as a measure of attention and working memory, RAVLT Encoding, Retrieval and Consolidation scores as aspects of verbal learning and memory, and ratings of internalizing and externalizing behavior problems as measures of behavioral functioning. All analyses with main effects of TBI severity were subjected to subsequent pairwise comparisons between groups (TC, mildRF+ TBI and moderate/severe TBI) using post-hoc LSD testing.
Third, the predictive value of neuroimaging parameters was investigated. Using Pearson correlations in the whole sample, relations were assessed: (1) among neuroimaging parameters with observed effects of TBI; and (2) between these neuroimaging parameters on the one hand, and neurocognitive and behavioral variables with observed effects of TBI on the other hand. Regional associations between FA within the cluster(s) of affected white matter tracts and aspects of functional outcome (with observed effects of TBI) were assessed using voxel-wise linear regression in TBSS, where threshold-free cluster enhancement and p values as corrected for family-wise error accounted for multiple testing. Scatter plots were produced for all significant associations between neuroimaging parameters and functional outcome. Finally, the role of continuous neuroimaging parameters in the observed effects of TBI on functional outcome were assessed using mediation models (Hayes 2013). All statistical analyses were two-sided, α was set at .05 and effect sizes of group differences were expressed as Cohen’s d.
Results
Patient characteristics
Demographics, injury-severity variables and prevalence of psychiatric and learning disorders are displayed for all groups in Table 1. There were no group differences on demographic variables (ps ≥ .17), except for lower SES in the mildRF+ TBI and moderate/severe TBI groups as compared to the TC group (ps ≤ .034). Regarding injury-related variables, the mechanism of injury was more likely to be a fall (as opposed to a traffic accident) in the TC group as compared to both the mildRF+ TBI and moderate/severe TBI groups (Ps < .02). As expected, the moderate/severe TBI group had lower GCS scores than the mildRF+ TBI group (p < .001), while higher prevalence of neurosurgery and longer hospital stay were observed as compared to both the mildRF+ TBI group and TC group (p ≤ .009). The TC group had higher prevalence of extracranial fractures and orthopedic surgery than both the mildRF+ TBI group (ps < .001) and the moderate/severe TBI group (ps < .001). Last, there were no group differences in the observed prevalence of psychiatric disorders (i.e. attention-deficit/hyperactivity disorder) or learning disorders (i.e. dyslexia; ps > .05).
Table 1.
Demographic and injury-related data in the TC, mildRF+ TBI and moderate/severe TBI groups
| n | Groups | Contrasts | ||
|---|---|---|---|---|
| TC | Mild RF+ TBI | Moderate/Severe TBI | ||
| 27 | 20 | 17 | ||
| Demographics | ||||
| Males, n (%) | 12 (44) | 13 (65) | 10 (59) | NS |
| Age at testing in y | 10.2 (1.5) | 10.5 (1.8) | 10.0 (1.4) | NS |
| SES | 6.3 (1.1) | 5.5 (1.3) | 5.0 (1.1) | TC > M, MS |
| Injury-related information | ||||
| Age at injury in y | 7.5 (2.2) | 7.7 (2.3) | 7.0 (1.9) | NS |
| Injury mechanism | ||||
| Fall, n (%) | 23 (85) | 11 (55) | 8 (47) | TC > M, MS |
| Traffic accident, n (%) | 4 (15) | 9 (45) | 9 (53) | TC < M, MS |
| Lowest GCS | 14.5 (0.7) | 8.4 (2.8) | M > MS | |
| Hospital Stay in d | 2.5 (2.0) | 3.5 (2.4) | 9.6 (9.3) | TC, M < MS |
| Time since injury in y | 2.7 (1.0) | 2.8 (1.1) | 3.0 (1.4) | NS |
| Range | 1.0–4.5 | 0.8–5.3 | 1.0–6.2 | |
| Extracranial fracture, n (%) | 23 (85) | 4 (20) | 2 (12) | TC > M, MS |
| > 1 Extracranial fractures, n (%) | 3 (11) | 1 (5) | 0 (0) | NS |
| Orthopedic surgery, n (%) | 22 (82) | 2 (10) | 0 (0) | TC > M, MS |
| Neurosurgery, n (%) | 0 (0) | 0 (0) | 5 (29) | TC, M < MS |
| Diagnosed conditions | ||||
| Psychiatric disorder, n (%) | 1 (4) | 2 (10) | 1 (6) | NS |
| Learning disorder, n (%) | 2 (7) | 4 (20) | 0 (0) | NS |
Data reflect mean (SD), unless otherwise indicated. TC traumatic control; TBI traumatic brain injury; RF risk factor; SES socio-economic status; NS not significant; y years; d days; GCS Glasgow Coma Scale; M mildRF+ TBI group; MS moderate/severe TBI group
Acute neuroimaging using CT
Group comparisons on the results of acute CT-scans are provided in Table 2. As expected, the mildRF+ TBI group and the moderate/severe TBI group had higher prevalence of intracranial pathology than the TC group (p < .001). Furthermore, children with moderate/severe TBI had higher prevalence of intracranial pathology than the mildRF+ TBI group (p = .035).
Table 2.
Neuroimaging data in the TC, mildRF+ TBI, moderate/severe TBI groups
| n* | Groups | ANOVA | ||||
|---|---|---|---|---|---|---|
| TC | Mild RF+ TBI | Moderate/Severe TBI | F(2,62) | p | Contrasts | |
| 27 | 20 | 17 | ||||
| Acute CT-scan | ||||||
| Intracranial Pathology, n (%) | 0 (0) | 6 (35) | 11 (65) | TC < M < MS | ||
| Normalized Brain Volume (cm3) | ||||||
| White matter | 701.0 (28.1) | 680.4 (28.1) | 675.6 (32.4) | 4.5 | .016 | TC > M, MS |
| Grey matter | 1070.0 (38.0) | 1044.5 (50.6) | 1051.8 (53.3) | 1.8 | .18 | |
| Normalized Subcortical Volume (cm3) | ||||||
| Thalamus | 21.8 (1.2) | 21.2 (1.4) | 21.0 (2.1) | 1.5 | .23 | |
| Caudate Nuclei | 10.1 (1.3) | 10.7 (0.9) | 9.8 (1.7) | 3.2 | .050 | |
| Striatum | 19.5 (1.7) | 18.5 (1.5) | 18.9 (2.0) | 1.7 | .19 | |
| Hippocampus | 10.4 (9.3) | 10.0 (1.1) | 10.6 (1.3) | 0.2 | .82 | |
| Amygdala | 3.2 (0.5) | 3.1 (0.5) | 3.3 (0.7) | 0.4 | .64 | |
| Accumbens Nuclei | 1.4 (0.3) | 1.4 (0.2) | 1.3 (0.4) | 1.1 | .33 | |
| Upper Brainstem | 26.1 (1.9) | 25.6 (2.4) | 26.2 (3.2) | 0.3 | .72 | |
| DTI Parameters | ||||||
| FA whole brain skeleton | .421 (.015) | .420 (.020) | .407 (.019) | 4.1 | .022 | TC, M > MS |
| FA whole cluster | .468 (.018) | .458 (.023) | .432 (.021) | 15.4 | <.001 | TC, M > MS |
| MD whole cluster (10−5 mm2/s) | 84.7 (2.4) | 85.3 (2.8) | 86.8 (2.7) | 3.2 | .049 | TC < MS |
| AD whole cluster (10−5 mm2/s) | 133.0 (2.2) | 132.6 (2.4) | 131.6 (2.8) | 1.8 | .18 | NS |
| RD whole cluster (105 mm2/s) | 60.6 (2.8) | 61.6 (3.5) | 64.4 (3.2) | 7.5 | .001 | TC, M < TC |
Data reflect mean (SD), unless otherwise indicated. Bold values pertain to significant results. TC traumatic control; TBI traumatic brain injury; RF risk factor; NS not significant; FA fractional anisotropy; MD mean diffusivity; AD axial diffusivity; RD radial diffusivity; M mildRF+ TBI group; MS moderate/severe TBI group
*Missing values for brain volumes (n = 4) and DTI parameters (n = 1)
Volumetric analysis of T1-weighted MRI scans
The volumetric analyses of white matter, grey matter and subcortical structures are displayed in Table 2. The results showed a significant main effect of TBI severity on white matter volume, but not on grey matter volume. Subsequent pairwise group comparisons revealed that the mildRF+ TBI group and moderate/severe TBI group both had smaller white matter volume than the TC group (p = .029, d = −0.74 and p = .009, d = −0.80, respectively). No significant main effects of TBI severity on the volumes of subcortical structures (i.e. thalamus, caudate nuclei, striatum, hippocampus, amygdala, nucleus accumbens and brainstem) were found (ps ≥ .05). Together, these findings indicate that the effects of mildRF+ TBI and moderate/severe TBI primarily manifest on the volume of white matter, rather than grey matter or subcortical structures.
White matter integrity in DTI
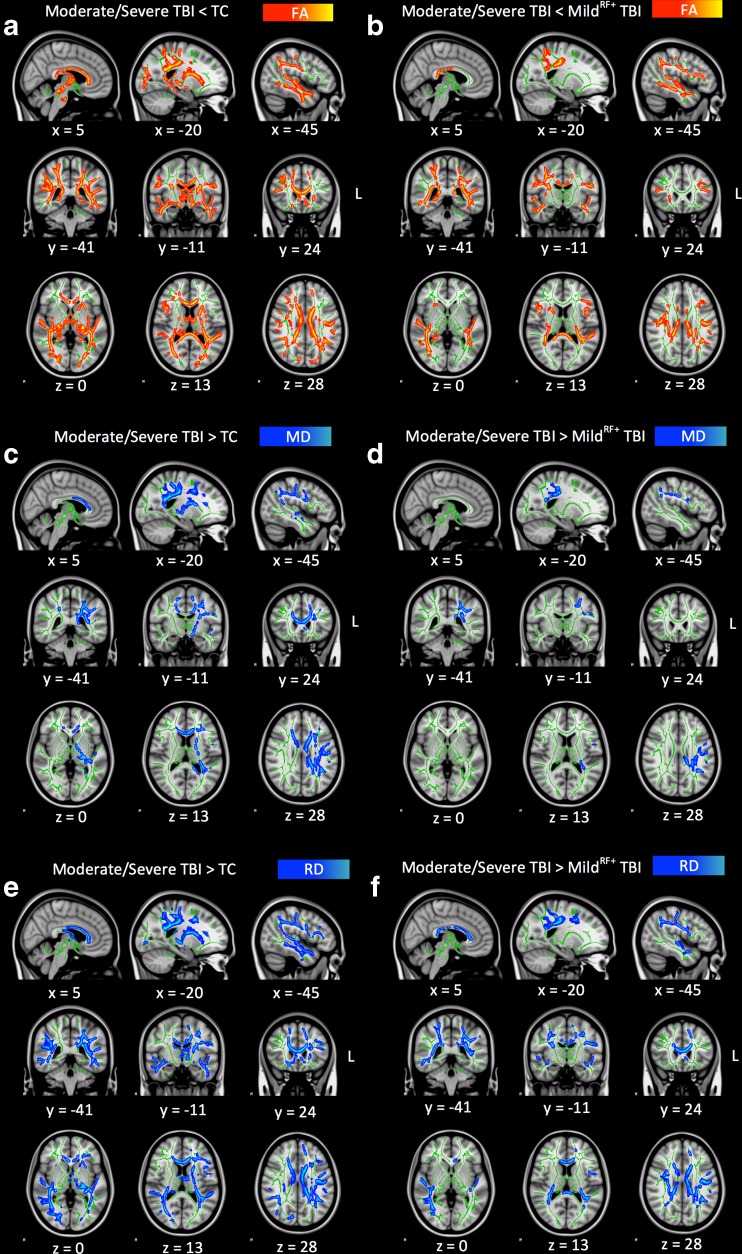
A significant effect of TBI severity on mean FA in the whole brain skeleton was found (Table 2). Subsequent pairwise group comparisons revealed that the moderate/severe TBI group had lower FA in the whole brain skeleton than both the TC group (p < .001, d = −1.88) and the mildRF+ TBI group (p < .001, d = −1.20), while no significant difference between the mildRF+ TBI group and TC group was observed (p = .10, d = −0.50). These findings indicate that children with moderate/severe TBI have diffuse white matter abnormalities. To localize regional effects of TBI on FA, voxel-wise group comparisons on the skeletonized FA maps were performed using TBSS. This analysis revealed one large cluster of lower FA in the moderate/severe TBI group as compared to the TC group as well as the mildRF+ group (Fig. 1a, b).
Fig. 1.
Voxel-wise comparison of FA, MD and RD maps using threshold-free cluster enhanced correction in TBSS. Note. Results of voxel-wise group comparisons showing the parts of the whole brain skeleton (at FA > 0.2, in green) with differences in terms of FA, MD and RD between the moderate/severe TBI group as compared to the TC group (Panels a, c and e, respectively) and mildRF+ TBI group (Panels b, d and e, respectively). Lower values in the moderate/severe TBI group as compared to other groups are displayed in red-yellow, while higher values are displayed in blue-lightblue. The results are overlaid on a MNI152 1 mm T1 brain in radiological convention (right = left), and for visualization purposes, regions in the whole brain skeleton with significant group differences were ‘thickened’ towards the full width of the white matter tract. TC trauma control group; TBI traumatic brain injury; FA fractional anisotropy; MD mean diffusivity; RD radial diffusivity
Analyses aimed at tracking down the origin of the effect of moderate/severe TBI on FA in the cluster of affected white matter tracts (Table 2), showed that the moderate/severe TBI group had higher MD and RD than the TC group (p = .015, d = 0.84 and p < .001, d = 1.32, respectively) and higher RD as compared to the mildRF+ TBI group (p < .001, d = 0.85). No differences in AD were observed between the moderate/severe TBI group and the TC group (p = .07, d = −0.60) or mildRF+ group (p = .20, d = −0.43).
Last, we investigated the regional effects of moderate/severe TBI on the skeletonized MD and RD maps as compared to both the TC group and the mildRF+ TBI group. Large clusters of higher MD and RD were observed in the moderate/severe TBI group as compared to the TC group as well as the mildRF+ TBI group (Fig. 1c-f), with high correspondence to the identified cluster of decreased FA (Fig. 1a, b).
Analyses aimed at identifying the white matter tracts involved in the neuropathology of moderate/severe TBI, revealed that all of the assessed bilateral white matter tracts were affected (see Table 3). More specifically, overlap between each white matter tract and the cluster of affected white matter tracts was used to assess the contribution of each white matter tract to the neuropathology of moderate/severe TBI (i.e. overlap as a percentage of the total cluster size of affected white matter tracts) and the extent to which each tract was affected by the neuropathology of moderate/severe TBI (i.e. overlap as a percentage of the total white matter tract size). Parts of the SLF, ILF and IFOF most prominently contributed to the neuropathology of moderate/severe TBI, while the BCC, SCC and HCB were least prominently involved. Otherwise, the GCC, BCC and SCC were most extensively affected by moderate/severe TBI, while the UF, FMi, and ATR were least extensively affected. Together, these findings indicate that children with moderate/severe TBI have widespread white matter abnormalities characterized by decreased FA, increased MD and increased RD. Furthermore, the results indicate that the SLF, ILF and IFOF are primarily involved in the white matter pathology of moderate/severe pediatric TBI, while the GCC, BCC and SCC most are the most extensively affected tracts.
Table 3.
White matter tract involvement in the neuropathology of moderate/severe TBI as measured with DTI
| White Matter Tract | % of Cluster | % of Tract Affected |
|---|---|---|
| Superior Longitudinal Fasciculus (SLF) | 24.7 | 33.7 |
| Inferior Longitudinal Fasciculus (ILF) | 23.7 | 44.7 |
| Inferior Frontal Occipital Fasciculus (IFOF) | 20.3 | 30.7 |
| Anterior Thalamic Radiation (ATR) | 15.1 | 25.1 |
| Cortical Spinal Tract (CST) | 8.0 | 30.9 |
| Forceps Major (FMa) | 7.8 | 39.6 |
| Genu of Corpus Callosum (GCC) | 5.4 | 68.8 |
| Forceps Minor (FMi) | 4.8 | 23.1 |
| Cingulate part of Cingulum Bundle (CCB) | 3.7 | 32.7 |
| Uncinate Fasciculus (UF) | 3.7 | 14.5 |
| Body of Corpus Callosum BCC | 2.9 | 66.7 |
| Splenium of Corpus Callosum (SCC) | 2.7 | 51.8 |
| Hippocampal part of Cingulum Bundle (HCB) | 2.5 | 32.1 |
Functional outcome
Analysis of neurocognitive functioning (Table 4) revealed significant main effects of TBI severity on FSIQ, Digit Span and RAVLT Encoding scores, but not on RAVLT Retrieval and Consolidation scores. These findings indicate that TBI severity affects intelligence, attention and working memory and the encoding of information into verbal memory. Subsequent pairwise group comparisons indicated that the mildRF+ TBI and moderate/severe TBI group had lower FSIQ (p = .003, d = −0.93 and p = .008, d = −0.92), Digit Span (p = .006, d = −0.92 and p = .017, d = −0.77) and RAVLT Encoding scores (p = .040 , d = −0.63 and p = .009, d = −0.89) than the TC group, while no significant differences between the mildRF+ TBI and moderate/severe TBI groups were observed (ps ≥ .53, ds ≥ −0.21).
Table 4.
Functional outcome in the TC, mildRF+ TBI and moderate/severe TBI groups
| n | Groups | ANOVA | ||||
|---|---|---|---|---|---|---|
| TC | Mild RF+ TBI | Moderate/Severe TBI | F(2,62) | p | Contrasts | |
| 27 | 20 | 17 | ||||
| Neurocognitive functioning | ||||||
| FSIQ | 0.47 (0.82) | -0.37 (1.04) | -0.32 (0.95) | 6.0 | .004 | TC > M, MS |
| Digit Span | 0.44 (0.87) | -0.36 (0.91) | -0.28 (1.08) | 5.1 | .009 | TC > M, MS |
| RAVLT Encoding | 0.40 (0.90) | -0.20 (1.05) | -0.40 (0.92) | 4.2 | .019 | TC > M, MS |
| RAVLT Retrieval | -0.03 (0.90) | 0.03 (0.90) | 0.02 (0.85) | 0.0 | .97 | |
| RAVLT Consolidation | 0.05 (0.92) | -0.04 (1.15) | -0.03 (0.98) | 0.1 | .94 | |
| Behavior Problems | ||||||
| Internalizing Problems | -0.37 (0.88) | 0.20 (0.77) | 0.46 (1.29) | 5.5 | .006 | TC < M, MS |
| Externalizing Problems | -0.37 (0.88) | 0.58 (0.84) | -0.10 (1.10) | 6.2 | .003 | TC, MS < M |
Z scores are reported. Data reflect mean (SD) unless otherwise indicated. TC traumatic control; TBI traumatic brain injury; RF risk factor; FSIQ full-scale IQ; M mildRF+ TBI group; MS moderate/severe TBI group; RAVLT Rey Auditory Verbal Learning Test
With regard to behavioral functioning, significant effects of TBI severity on ratings of internalizing problems and externalizing problems were observed (Table 4). Subsequent pairwise group comparisons revealed that, as compared to the TC group, the mildRF+ TBI and moderate/severe TBI groups had higher ratings of internalizing problems (p = .023, d = 0.85 and p = .003, d = 0.92). With regard to externalizing problems, the mildRF+ TBI group had higher ratings as compared to the TC group (p = .001, d = 1.13), while the moderate/severe TBI group had not (p = .34, d = 0.29). Comparisons between the mildRF+ TBI group and moderate/severe TBI group revealed no significant differences on ratings of internalizing problems (p = .41, d = 0.25), while the mildRF+ TBI group had higher ratings of externalizing problems as compared to the moderate/severe TBI group (p = .03, d = 0.73).
Neuroimaging parameters and functional outcome
Subsequent analyses investigated the correspondence among neuroimaging parameters with observed effects of TBI severity (i.e. intracranial pathology on acute CT-scans, white matter volume on T1-weighted MRI and FA in the cluster of affected white matter tracts). Intracranial pathology on acute CT was associated with smaller white matter volume (r = −0.29, p = .03) as well as lower FA in the cluster of white matter tracts affected by moderate/severe TBI (r = −0.26, p = .05). In turn, smaller white matter volume was associated with lower FA in the cluster of affected white matter tracts (r = .39, p = .002). These results indicate that the assessed neuroimaging parameters have a small to moderate degree of correspondence, and confirm that CT, volumetric analysis of T1-weighted MRI scans and DTI measure only partially overlapping aspects of the TBI neuropathology.
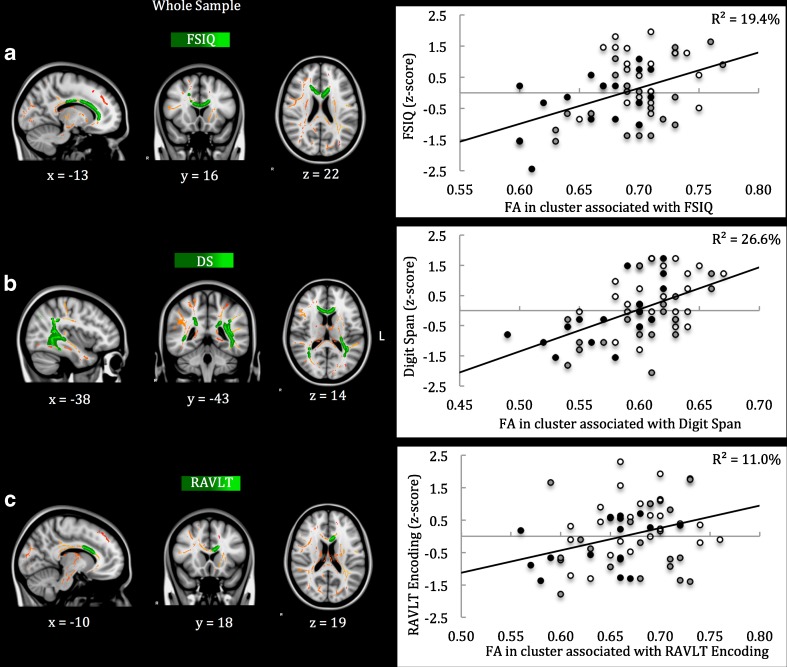
Furthermore, the relations between these neuroimaging parameters and aspects of functional outcome with observed effects of TBI severity were investigated (i.e. FSIQ, Digit Span and RAVLT Encoding scores, and ratings of internalizing and externalizing problems). This analysis revealed that intracranial pathology on acute CT scans was only related to higher ratings of internalizing problems (r = .35, p = .004). White matter volume showed no significant relations to aspects of functional outcome at all (rs ≤ .23, ps ≥ .07). In contrast, lower FA in the cluster of affected white matter tracts was associated with lower FSIQ (r = .29, p = .02), lower Digit Span score (r = .41, p < .001) and higher ratings of internalizing problems (r = −.26, p = .04; also see eFigure 1). Regional associations between FA within the cluster of affected white matter tracts and aspects of functional outcome with observed effects of TBI were investigated using TBSS (Fig. 2). The results show that lower FA in parts of: (A) the GCC, BCC and CCB was associated with lower FSIQ (r = .45, p < .001); (B) the GCC, BCC, SCC, ILF, IFOF and SLF was associated with lower Digit Span scores (r = .53, p < .001); and (C) the GCC and BCC was associated with lower RAVLT Encoding scores (r = .35, p = .005); while no regional associations between FA and ratings of internalizing or externalizing problems were identified.
Fig. 2.
Regional associations between FA and functional outcome using threshold-free cluster enhancement correction in TBSS. Note. Regional associations are displayed (in green-light green) between FA in the cluster of affected white matter tracts and FSIQ (Panel a), Digit Span score (Panel b) and RAVLT Encoding score (Panel c). The cluster of white matter tracts affected by moderate/severe TBI is displayed in red-yellow. The results are overlaid on a MNI152 1 mm T1 brain in radiological convention (right = left), and for visualization purposes, regions in the cluster of affected white matter tracts with significant associations to functional outcome were ‘thickened’ towards the full width of the white matter tract. Color of the data point in the scatter plots refers to the trauma control group (white), mildRF+ TBI group (grey) and moderate/severe TBI group (black). FSIQ full-scale IQ; DS Digit Span; RAVLT Rey Auditory Verbal Learning Test
Last, mediation models (see eFigure 2) were used to investigate the potentially mediating role of FA in the relation between moderate/severe TBI and functional outcome (FSIQ, Digit Span and RAVLT Encoding scores). As expected, these analyses showed that moderate/severe TBI was related to lower FA in the clusters with regional associations to functional outcome (Path A, see Fig. 2 for clusters) and poorer FSIQ, Digit Span and RAVLT Encoding scores (Path C). Likewise, lower FA in these clusters was related with lower FSIQ, Digit Span and RAVLT Encoding scores (Path B). Notably, lower FA in the clusters associated with FSIQ, Digit Span and RAVLT Encoding scores (Fig. 2), fully mediated the effects of moderate/severe TBI on FSIQ (B [SE] = 0.10 [0.06], 95% confidence interval [CI] = −0.01 to −0.27), Digit Span scores (B [SE] = 0.17 [0.06], 95%-CI = 0.08 to 0.31) and RAVLT Encoding scores (B [SE] = 0.12 [0.06], 95%-CI = 0.03 to 0.27), respectively (Path C′). Taken together, the results indicate that although acute and post-acute neuroimaging parameters are interrelated, DTI shows superior predictive value for functional outcome. More specifically, the results suggest that the diffuse impact of moderate/severe TBI on white matter integrity is, at a regional level, associated with intelligence, working memory and encoding of information in verbal memory, where disrupted integrity in parts of the CC was consistently associated with poorer neurocognitive functioning.
Analysis of possible confounders
Lower SES in the mildRF+ TBI group and moderate/severe TBI group as compared to the TC group could have confounded some of the reported group differences, since SES was also related to intracranial pathology on CT scans, FA in the cluster of affected white matter tracts, FSIQ, Digit Span score and RAVLT Encoding scores (rs ≥ 25, ps < .05). Therefore, the analyses were repeated after matching children with TBI to the TC group on SES. In this matching procedure, a child with (mildRF+ or moderate/severe) TBI was matched to each child in the TC group on SES (within a bandwidth of 1 unit on the 8-point SES scale), while counterbalancing for TBI severity in the matched TBI group. If multiple matches were possible using these criteria, we also matched for age and gender. The resulting matched TBI group and the TC group showed no significant differences on age, gender and SES (ps ≥ .10) and the matched TBI group had a balanced representation of mildRF+ TBI (52%) and moderate/severe TBI (48%). Repeating the analyses using the matched groups replicated all reported group differences (data available with first author), with the single exception of ratings of externalizing problems (p = .10, d = 0.46). Together, these findings indicate that SES did not account for the reported group differences, with the possible exception of higher ratings of externalizing problems in the mildRF+ TBI group as compared to the TC group.
Discussion
This study investigated the neuropathology of mildRF+ to moderate/severe TBI in children and the predictive value of neuroimaging parameters (i.e. acute CT-scans and post-acute MRI techniques) for functional outcome. The results indicate that mildRF+ to moderate/severe TBI causes white matter volume loss that is indicative of chronic white matter atrophy, while Tract-Based Spatial Statistics on DTI data revealed that moderate/severe TBI is additionally associated with widespread disruption of white matter integrity (i.e. reduced FA). Among the assessed neuroimaging parameters, DTI measures of white matter integrity (i.e. FA) most consistently displayed predictive value for functional outcome in children with TBI, both in terms of neurocognitive and behavioral functioning. The results of this study underline the potential clinical relevance of DTI parameters for the prognosis of functional outcome after pediatric TBI.
Analysis aimed at the neuropathology of TBI, indicated that 35% of children with mildRF+ TBI had intracranial pathology on acute CT-scans. Volumetric analysis of post-acute T1-weighted MRI scans showed no effects of mildRF+ TBI on cortical grey matter or subcortical structures, whereas children with mildRF+ TBI did show decreased white matter volume as compared to children with TC injury. To our best knowledge, this is the first study to show that mildRF+ TBI causes persisting white matter abnormality. Nevertheless, this finding is in line with our previous research showing that children with mildRF+ TBI are at risk of a range of neurocognitive impairments (i.e. general neurocognitive functioning, attention and visual integration) and behavior problems (i.e. internalizing and externalizing problems; Königs et al. 2015a, b). Together, these findings call for careful clinical screening for adverse neurocognitive and behavioral outcome in children with mildRF+ TBI.
It was surprising to observe an impact of mildRF+ TBI on white matter volume in the absence of corresponding effects on white matter integrity from DTI analyses. A comparable dissociation between white matter volume and white matter integrity has previously been reported in a longitudinal study investigating CC development between 3 to 12 months after complicated mild to severe pediatric TBI (Wu et al. 2010). The findings from that study suggested abnormal post-injury development of white matter at the macrostructural level (i.e. CC volume loss) thought to be caused by Wallerian degeneration as a net response to total diffuse damage to axons as well as neurons, in combination with microstructural white matter changes (i.e. FA increase) thought to reflect continued development of myelin and possible compensatory regenerative processes. In line with this reasoning, we speculate that the observed effect of mildRF+ TBI on white matter volume may have arisen as a net (Wallerian) result of subthreshold axonal as well as cortical injury. Alternatively, the absence of effects of mildRF+ TBI on white matter integrity (i.e. DTI parameters) in the post-acute phase may reflect successful regeneration of white matter at the microstructural level.
As expected, moderate/severe TBI was associated with a high prevalence of intracranial pathology on acute CT-scans (65%). Furthermore, children with moderate/severe TBI had decreased white matter volume as compared to children with TC injury, while no effects on the volumes of grey matter and subcortical structures were observed. The results of DTI analyses further indicated that children with moderate/severe TBI in addition have widespread disruptions of white matter integrity (i.e. decreased FA, and increased MD and RD), likely reflecting axonal degeneration and/or reduced myelin density (Budde et al. 2011). We further found that all assessed white matter tracts were affected by moderate/severe TBI, where parts of the SLF, ILF and IFOF were most prominently involved in the white matter pathology of moderate/severe pediatric TBI (constituting between 20 and 25% of affected white matter), while the most widespread effects of moderate/severe TBI manifested in the GCC, BCC and SCC (between 52 and 69% of tract affected). Together, these findings indicate that moderate/severe TBI in children is associated with a high risk of intracranial pathology, has a persisting detrimental impact on white matter volume and causes widespread disruption of white matter integrity. These findings are in line with the existing literature on the neuropathology of moderate to severe TBI (Sharp et al. 2014). Regarding the time interval between injury and MRI scanning in the children with TBI (0.8–6.2 years), the observed effects of TBI on white matter volume and DTI parameters may reflect not only the direct impact of TBI on white matter, but also the influence of TBI on post-injury white matter development (Ewing-Cobbs et al. 2016).
Neuroimaging parameters with observed sensitivity for TBI (i.e. acute CT, post-acute T1-weighted MRI and DTI) had small to moderate correspondence, emphasizing that these measures clearly tap into different aspects of the TBI neuropathology. With regard to the predictive value of neuroimaging parameters for functional outcome, evidence for intracranial pathology on acute CT scans was only related to higher ratings of internalizing problems, while white matter volume was not related to any aspect of functional outcome. In contrast, lower FA in the cluster of affected white matter was related to poorer neurocognitive functioning (i.e. intelligence, attention and working memory) and behavioral functioning (i.e. internalizing problems). Voxel-wise regression furthermore identified regional associations between lower FA and poorer intelligence (i.e. GCC, BCC and CCB), attention and working memory (i.e. GCC, BCC, SCC, SLF, ILF and IFOF) and encoding in verbal memory (i.e. GCC and BCC). These clusters of lower FA were subsequently found to -in statistical terms- fully mediate the effects of moderate/severe TBI on intelligence, attention and working memory, and encoding in verbal memory, respectively. Taken together, these results confirm the idea that the neuropathology of moderate/severe TBI is primarily characterized by the disruption of white matter integrity (Sharp et al. 2014). The current results further extend the literature from adult and adolescent TBI (Adamson et al. 2013; Haberg et al. 2015; Kinnunen et al. 2011), by showing that specific white matter tracts, consistently involving aspects of the corpus callosum, are likely to underlie a range of neurocognitive impairments after moderate/severe TBI. Last, the findings from this study also indicate that DTI has superior consistency in terms of the predictive value for functional outcome after pediatric TBI, as compared to more conventional neuroimaging parameters (i.e. acute CT scanning and volumetrics of T1-weighted MRI scans).
This study had some weaknesses. First, clinical research in the hospital setting is generally limited by a lack of pre-injury measurements of functioning, and therefore we relied on (suboptimal) group-wise comparisons in a cross-sectional design to assess the average impact of (mildRF+ and moderate/severe) TBI on brain structure and functional outcome. Furthermore, we assessed the predictive value of post-acute neuroimaging for functional outcome in a partly cross-sectional design, while the study of the prognostic value of neuroimaging parameters calls for a prospective longitudinal design after TBI. Given the contrasting effects of TBI on DTI parameters in the acute vs. post-acute phase (Roberts et al. 2016), the influence of time between injury and scanning on the prognostic value of DTI parameters is an important issue that remains to be investigated in future studies. Nevertheless, the results from this study highlight the sensitivity of DTI for functional outcome, relative to acute CT-scans and volumetric analysis of post-acute T1-weighted MRI scans. Second, the mildRF+ TBI group, moderate/severe TBI group and TC group consisted of relatively small samples, which may have limited statistical power for more subtle effects. For example, the difference between the mildRF+ TBI group and TC group on FA in the cluster of affected white matter tracts did not reach conventional levels of significance, despite a considerable effect size indicating lower FA in the mildRF+ TBI group (Cohen’s d = −0.50). Third, this study focused on the diffuse effects of TBI on the brain, while pediatric TBI also involves focal brain pathology with distinct heterogeneity in the severity and location of lesions (Bigler et al. 2013a). The whole brain and (group-based) ROI analyses that were used in this study are unlikely to systematically capture the relation between focal TBI pathology and functional outcome on the brain. However, the heterogeneity of focal pathology may also reduce the predictability of their consequences for functional outcome, while the current study shows that the diffuse impact of TBI on white matter integrity is consistently associated with functional outcome. Fourth and last, the tools used for the analysis of brain volume and white matter integrity have some specific limitations. With regard to volumetric analysis, the scan-rescan reliability of volume estimation has been shown to differ between neural structures (Morey et al. 2010). Some structures typically have low reliability (e.g. amygdala, accumbens) that in turn may have increased the error of measurement in the assessment of these brain volumes. With regard to TBSS, skeletonizing white matter tracts is an essential step in reducing the influence of misalignment across subjects, but also introduces anatomical inaccuracy in the FA skeleton and disregards the impact of neuropathology on the perimeter of white matter tracts (for an in-depth discussion of methodological considerations on TBSS, see Bach et al. 2014). Strong points of this study include: (1) the use of a TC group to control for pre-injury risk factors of trauma and psychological effects of hospitalization and medical procedures (Max et al. 1998); (2) the assessment of a wide range of acute and post-acute neuroimaging techniques; (3) the use of TBSS to allow a data-driven, model-free investigation of regional effects of TBI on white matter integrity, instead of a theory-driven approach (i.e. ROI analysis); (4) the investigation of TBI neuropathology in relation to crucial aspects of functional outcome in children, as assessed using Common Data Elements for outcome of pediatric TBI to maximize the clinical and research utility of the current findings; and (5) the use of a confounding analysis (which showed that group differences in SES were unlikely to account for the main findings), whilst the main findings were based on the (unmatched) samples with best representative value for the pediatric TBI population.
In conclusion, this study confirms previous literature suggesting that the core neuropathology of pediatric TBI manifests in the brain’s white matter. The results extend the literature by revealing evidence indicating that children with mildRF+ TBI are at risk of persisting white matter atrophy. Furthermore, we showed that white matter integrity as measured by DTI has superior predictive value for functional outcome, relative to acute CT-scanning and volumetric analysis on post-acute conventional (T1-weighted) MRI. More specifically, the results of this study suggest that regional disruption of white matter integrity in the corpus callosum (GCC and BCC) may importantly underlie neurocognitive impairments in children with moderate/severe TBI. Together, these findings emphasize the potential clinical relevance of DTI as an important prognostic factor of functional outcome of TBI in children, especially in terms of neurocognitive functioning.
Electronic supplementary material
Scatter plots of the significant relations between neuroimaging parameters and aspects of functional outcome in the whole study sample. Note. Color of the data point refers to the trauma control group (white), mildRF+ TBI group (grey) and moderate/severe TBI group (black). CT = computed tomography; FA = fractional anisotropy; FSIQ = full-scale intelligence quotient. (GIF 57 kb)
Mediation models testing the influence of FA on the relation between moderate/severe TBI and functional outcome. Note. TBI = traumatic brain injury; FSIQ = full-scale intelligence quotient; RAVLT = Rey Auditory Verbal Learning Test; B = raw regression coefficient; SE = standard error. *FA in the cluster of white matter tracts associated with: (1) FSIQ; (2) Digit Span score; and (3) RAVLT Encoding (Fig. 2), respectively. (GIF 35 kb)
(DOCX 23 kb)
Acknowledgements
We are grateful to Dr. J.A. van der Sluijs from the department of Pediatric Orthopedics (VU Medical Centre Amsterdam) and Dr. H.A. Heij from the Pediatric Surgical Center Amsterdam (VU University Medical Center and Academic Medical Center) for their assistance in the recruitment of participants for this study
Abbreviations
- AD
Axial diffusivity
- ATR
Anterior thalamic radiation
- BCC
Body of corpus callosum
- CCB
Cortical part of cingulum bundle
- CST
Corticospinal tract
- CT
Computed tomography
- DTI
Diffusion tensor imaging
- FA
Fractional anisotropy
- FMa
Forceps major
- FMi
Forceps minor
- FSIQ
Full-scale IQ
- FSL
Functional MRI of the brain software library
- GCC
Genu of corpus callosum
- GCS
Glasgow coma scale
- HCB
Hippocampal part of cingulum bundle
- IFOF
Inferior frontal occipital fasciculus
- ILF
Inferior longitudinal fasciculus
- LOC
Loss of consciousness
- MD
Mean diffusivity
- MRI
Magnetic resonance imaging
- PTA
Post-traumatic amnesia
- RAVLT
Rey auditory verbal learning test
- RD
Radial diffusivity
- RF
Risk factor
- SCC
Splenium of corpus callosum
- SES
Socio-economic status
- SLF
Superior longitudinal fasciculus
- TBI
Traumatic brain injury
- TBSS
Tract-based spatial statistics
- UF
Uncinate fasciculus
- WISC-III
Wechsler intelligence scale for children III
Compliance with ethical standards
Funding
This work was supported by the Netherlands’ Organization for Scientific Research (NWO, http://www.nwo.nl) grant number 022.003.010 and the Faculty of Behavioral and Movement Sciences of the Vrije Universiteit Amsterdam.
Conflicts of interest
The authors have nothing to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of medical ethical committee of the VU University Medical Centre (NL37226.029.11) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from parents of all participating children. In addition, written informed consent was obtained from children aged >11 years as required by the Dutch law on medico-scientific research (http://wetten.overheid.nl/BWBR0009408/2015-.12-17).
References
- Adamson C, Yuan W, Babcock L, Leach JL, Seal ML, Holland SK, Wade SL. Diffusion tensor imaging detects white matter abnormalities and associated cognitive deficits in chronic adolescent TBI. Brain Injury. 2013;27(4):454–463. doi: 10.3109/02699052.2012.750756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Surgeons (2004). Advanced Trauma Life Support Program for Doctors. (Committee on Trauma, Ed.) (7th ed.).
- Babikian T, Asarnow R. Neurocognitive outcomes and recovery after pediatric TBI: meta-analytic review of the literature. Neuropsychology. 2009;23(3):283–296. doi: 10.1037/a0015268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach, M., Laun, F. B., Leemans, A., Tax, C. M. W., Biessels, G. J., Stieltjes, B., & Maier-Hein, K. H. (2014). Methodological considerations on tract-based spatial statistics (TBSS). Neuroimage, 100. doi:10.1016/j.neuroimage.2014.06.021 [DOI] [PubMed]
- Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2003;50(5):1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Maxwell WL. Neuropathology of mild traumatic brain injury: relationship to neuroimaging findings. Brain Imaging and Behavior. 2012;6(2):108–136. doi: 10.1007/s11682-011-9145-0. [DOI] [PubMed] [Google Scholar]
- Bigler E, Abildskov T, Petrie J. Heterogeneity of brain lesions in pediatric traumatic brain injury. Neuropsychology. 2013;27(4):438–451. doi: 10.1037/a0032837. [DOI] [PubMed] [Google Scholar]
- Blackman JA, Rice SA, Matsumoto JA, Conaway MR, Elgin KM, Patrick PD, et al. Brain imaging as a predictor of early functional outcome following traumatic brain injury in children, adolescents, and young adults. Journal of Head Trauma Rehabilitation. 2003;18(6):493–503. doi: 10.1097/00001199-200311000-00003. [DOI] [PubMed] [Google Scholar]
- Budde M, Janes L, Gold E, Turtzo L, Frank J. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue. Brain. 2011;134(8):2248–2260. doi: 10.1093/brain/awr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Jin Y, Villalon-Reina J, Zhan L, Kernan C, Babikian T, et al. White matter disruption in moderate/severe pediatric traumatic brain injury: advanced tract-based analyses. NeuroImage: Clinical. 2015;7:493–505. doi: 10.1016/j.nicl.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Prasad MR, Swank P, Kramer L, Cox CS, Fletcher JM, et al. Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: relation to neurobehavioral outcomes. NeuroImage. 2008;42(4):1305–1315. doi: 10.1016/j.neuroimage.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing-Cobbs, L., Johnson, C., & Juranek, J. (2016). Longitudinal diffusion tensor imaging after pediatric traumatic brain injury: impact of age at injury and time since injury on pathway integrity. Human Brain Mapping, 37(11), 3929–3945. [DOI] [PMC free article] [PubMed]
- Gerlach R, Dittrich S, Schneider W, Ackermann H, Seifert V, Kieslich M. Traumatic epidural hematomas in children and adolescents: outcome analysis in 39 consecutive unselected cases. Pediatric Emergency Care. 2009;25(3):164–169. doi: 10.1097/PEC.0b013e31819a8966. [DOI] [PubMed] [Google Scholar]
- Haberg AK, Olsen A, Moen KG, Schirmer-Mikalsen K, Visser E, Finnanger TG, et al. White matter microstructure in chronic moderate-to-severe traumatic brain injury: impact of acute-phase injury-related variables and associations with outcome measures. Journal of Neuroscience Research. 2015;93(7):1109–1126. doi: 10.1002/jnr.23534. [DOI] [PubMed] [Google Scholar]
- Hayes, A. (2013). Introduction to mediation, moderation, and conditional process analysis. New York: Guilford, 3–4.
- IBM Corp . IBM SPSS statistics for windows. Armonk: IBM Corporation; 2011. [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Johnson CP, Juranek J, Kramer LA, Prasad MR, Swank PR, Ewing-Cobbs L. Predicting behavioral deficits in pediatric traumatic brain injury through uncinate fasciculus integrity. Journal of the International Neuropsychological Society : JINS. 2011;17(4):663–673. doi: 10.1017/S1355617711000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen KM, Greenwood R, Powell JH, Leech R, Hawkins PC, Bonnelle V, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain : A Journal of Neurology. 2011;134(Pt 2):449–463. doi: 10.1093/brain/awq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Königs M, Heij HA, van der Sluijs JA, Vermeulen RJ, Goslings JC, Luitse JSK, et al. Pediatric traumatic brain injury and attention deficit. Pediatrics. 2015;136(3):534–541. doi: 10.1542/peds.2015-0437. [DOI] [PubMed] [Google Scholar]
- Königs M, Weeda WD, van Heurn LWE, Vermeulen RJ, Goslings JC, Luitse JSK, et al. Impaired visual integration in children with traumatic brain injury: an observational study. PloS One. 2015;10(12):e0144395. doi: 10.1371/journal.pone.0144395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kort, W., Schittekatte, M., Dekker, P. H., Verhaeghe, P., Compaan, E. L., Bosmans, M., & Vermeir, G. (2005). WISC-IIINL Wechsler Intelligence Scale for Children. David Wechsler. Derde Editie NL. Handleiding en Verantwoording. Harcourt Test Publishers/Nederlands Instituut voor Psychologen.
- Levin HS, Wilde EA, Chu Z, Yallampalli R, Hanten GR, Li X, et al. Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. The Journal of Head Trauma Rehabilitation. 2008;23(4):197–208. doi: 10.1097/01.HTR.0000327252.54128.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Liu J. The effect of pediatric traumatic brain injury on behavioral outcomes: a systematic review. Developmental Medicine and Child Neurology. 2013;55(1):37–45. doi: 10.1111/j.1469-8749.2012.04414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, et al. Detection of blast-related traumatic brain injury in U.S. military personnel. The New England Journal of Medicine. 2011;364(22):2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max J, Koele S, Smith W., Jr Psychiatric disorders in children and adolescents after severe traumatic brain injury: a controlled study. Child & Adolescent Psychiatry. 1998;37(8):932–840. doi: 10.1097/00004583-199808000-00013. [DOI] [PubMed] [Google Scholar]
- Max J, Wilde E, Bigler E. Neuroimaging correlates of novel psychiatric disorders after pediatric traumatic brain injury. Child & Adolescent Psychiatry. 2012;51(11):1208–1217. doi: 10.1016/j.jaac.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccauley SR, Wilde EA, Bigler ED, Chu Z, Yallampalli R, Oni MB, et al. Diffusion tensor imaging of incentive effects in prospective memory after pediatric traumatic brain injury. Journal of Neurotrauma. 2011;28(4):503–516. doi: 10.1089/neu.2010.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley SR, Wilde EA, Anderson VA, Bedell G, Beers SR, Campbell TF, et al. Recommendations for the use of common outcome measures in pediatric traumatic brain injury research. Journal of Neurotrauma. 2012;29(4):678–705. doi: 10.1089/neu.2011.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn D, Levin HS, Harward H, Bruce D. Corpus Callosum lesions after closed head injury in children: MRI, clinical features and outcome. Neuroradiology. 1992;34(5):384–388. doi: 10.1007/BF00596495. [DOI] [PubMed] [Google Scholar]
- Mittl RL, Grossman RI, Hiehle JF, Hurst RW, Kauder DR, Gennarelli TA, Alburger GW. Prevalence of MR evidence of diffuse axonal injury in patients with mild head injury and normal head CT findings. AJNR. American Journal of Neuroradiology. 1994;15(8):1583–1589. [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Selgrade ES, Wagner HR, Huettel SA, Wang L, McCarthy G. Scan-rescan reliability of subcortical brain volumes derived from automated segmentation. Human Brain Mapping. 2010;31(11):NA–NA. doi: 10.1002/hbm.20973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi S, Mukherjee P. Diffusion tensor imaging of mild traumatic brain injury. The Journal of Head Trauma Rehabilitation. 2010;25(4):241–255. doi: 10.1097/HTR.0b013e3181e52c2a. [DOI] [PubMed] [Google Scholar]
- Oni MB, Wilde EA, Bigler ED, McCauley SR, Wu TC, Yallampalli R, et al. Diffusion tensor imaging analysis of frontal lobes in pediatric traumatic brain injury. Journal of Child Neurology. 2010;25(8):976–984. doi: 10.1177/0883073809356034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56(3):907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Region of interest analysis for fMRI. Social Cognitive and Affective Neuroscience. 2007;2(1):67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polinder S, Haagsma JA, van Klaveren D, Steyerberg EW, van Beeck EF. Health-related quality of life after TBI: a systematic review of study design, instruments, measurement properties, and outcome. Population Health Metrics. 2015;13(1):4. doi: 10.1186/s12963-015-0037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R, Mathias J, Rose S. Diffusion tensor imaging (DTI) findings following pediatric non-penetrating TBI: a meta-analysis. Developmental Neuropsychology. 2014;39(8):600–637. doi: 10.1080/87565641.2014.973958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, R. M., Mathias, J. L., & Rose, S. E. (2016). Relationship between diffusion tensor imaging (DTI) findings and cognition following pediatric TBI: a meta-analytic review. Developmental Neuropsychology, 1–25. doi:10.1080/87565641.2016.1186167. [DOI] [PMC free article] [PubMed]
- Sattler, J. M. (2001). Assessment of children: cognitive applications. Jerome M. Sattler, San Diego, 774.
- Sharp DJ, Scott G, Leech R. Network dysfunction after traumatic brain injury. Nature Reviews. Neurology. 2014;10(3):156–166. doi: 10.1038/nrneurol.2014.15. [DOI] [PubMed] [Google Scholar]
- Sigmund GA, Tong KA, Nickerson JP, Wall CJ, Oyoyo U, Ashwal S. Multimodality comparison of neuroimaging in pediatric traumatic brain injury. Pediatric Neurology. 2007;36(4):217–226. doi: 10.1016/j.pediatrneurol.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Statistics Netherlands (2006). Education Categorization Standard [Standaard onderwijsindeling 2006]. www.cbs.nl.
- Tabachnick, B. G., & Fidell, L. S. (2012). Using Multivariate Statistics: International Edition. Pearson.
- Teasdale G, Jennett B. Assessment and prognosis of coma after head injury. Acta Neurochirurgica. 1976;34(1–4):45–55. doi: 10.1007/BF01405862. [DOI] [PubMed] [Google Scholar]
- Treble, A., Hasan, K. M., Iftikhar, A., Stuebing, K. K., Kramer, L. A., Cox, C. S., et al. (2013). Working memory and corpus callosum microstructural integrity after pediatric traumatic brain injury: a diffusion tensor tractography study. Journal of Neurotrauma, 30(19), 1609–1619. doi:10.1089/neu.2013.2934. [DOI] [PMC free article] [PubMed]
- Van Beek L, Vanderauwera J, Ghesquiere P, Lagae L, De Smedt B. Longitudinal changes in mathematical abilities and white matter following paediatric mild traumatic brain injury. Brain Injury. 2015;9052(November):1–10. doi: 10.3109/02699052.2015.1075172. [DOI] [PubMed] [Google Scholar]
- van den Burg W, Kingma A. Performance of 225 Dutch school children on Rey’s auditory verbal learning test (AVLT): parallel test-retest reliabilities with an interval of 3 months and normative data. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists. 1999;14(6):545–559. doi: 10.1016/s0887-6177(98)00042-0. [DOI] [PubMed] [Google Scholar]
- Verhulst, F., & van der Ende, J. (2013). Handeling ASEBA Vragenlijsten voor leeftijden 6 t/m 18 jaar: CBCL/6–18, YSR & TRF. ASEBA.
- Vos P, Battistin L. EFNS guideline on mild traumatic brain injury: report of an EFNS task force. European Journal of Neurology. 2002;9(3):207–219. doi: 10.1046/j.1468-1331.2002.00407.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WISC-III: Wechsler intelligence scale for children. TX: Psychological Corporation San Antonio; 1991. [Google Scholar]
- Wilde EA, Chu Z, Bigler ED, Hunter JV, Fearing MA, Hanten G, et al. Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. Journal of Neurotrauma. 2006;23(10):1412–1426. doi: 10.1089/neu.2006.23.1412. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Newsome MR, Bigler ED, Pertab J, Merkley TL, Hanten G, et al. Brain imaging correlates of verbal working memory in children following traumatic brain injury. International Journal of Psychophysiology. 2011;82(1):86–96. doi: 10.1016/j.ijpsycho.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2006). Neurological disorders: Public health challenges. World Health Organization.
- Wozniak JR, Krach L, Ward E, Mueller BA, Muetzel R, Schnoebelen S, et al. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Archives of Clinical Neuropsychology. 2007;22(5):555–568. doi: 10.1016/j.acn.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Wilde E, Bigler E, Li X. Longitudinal changes in the corpus callosum following pediatric traumatic brain injury. Developmental Neuroscience. 2010;32(5–6):361–273. doi: 10.1159/000317058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scatter plots of the significant relations between neuroimaging parameters and aspects of functional outcome in the whole study sample. Note. Color of the data point refers to the trauma control group (white), mildRF+ TBI group (grey) and moderate/severe TBI group (black). CT = computed tomography; FA = fractional anisotropy; FSIQ = full-scale intelligence quotient. (GIF 57 kb)
Mediation models testing the influence of FA on the relation between moderate/severe TBI and functional outcome. Note. TBI = traumatic brain injury; FSIQ = full-scale intelligence quotient; RAVLT = Rey Auditory Verbal Learning Test; B = raw regression coefficient; SE = standard error. *FA in the cluster of white matter tracts associated with: (1) FSIQ; (2) Digit Span score; and (3) RAVLT Encoding (Fig. 2), respectively. (GIF 35 kb)
(DOCX 23 kb)