Abstract
Background
The efficacy, safety and immunogenicity risk of switching between an originator biologic and a biosimilar or from one biosimilar to another are of potential concern.
Objectives
The aim was to conduct a systematic literature review of the outcomes of switching between biologics and their biosimilars and identify any evidence gaps.
Methods
A systematic literature search was conducted in PubMed, EMBASE and Cochrane Library from inception to June 2017. Relevant societal meetings were also checked. Peer-reviewed studies reporting efficacy and/or safety data on switching between originator and biosimilar products or from one biosimilar to another were selected. Studies with fewer than 20 switched patients were excluded. Data were extracted on interventions, study population, reason for treatment switching, efficacy outcomes, safety and anti-drug antibodies.
Results
The systematic literature search identified 63 primary publications covering 57 switching studies. The reason for switching was reported as non-medical in 50 studies (23 clinical, 27 observational). Seven studies (all observational) did not report whether the reasons for switching were medical or non-medical. In 38 of the 57 studies, fewer than 100 patients were switched. Follow-up after switching went beyond 1 year in eight of the 57 studies. Of the 57 studies, 33 included statistical analysis of disease activity or patient outcomes; the majority of these studies found no statistically significant differences between groups for main efficacy parameters (based on P < 0.05 or predefined acceptance ranges), although some studies observed changes for some parameters. Most studies reported similar safety profiles between groups.
Conclusions
There are important evidence gaps around the safety of switching between biologics and their biosimilars. Sufficiently powered and appropriately statistically analysed clinical trials and pharmacovigilance studies, with long-term follow-ups and multiple switches, are needed to support decision-making around biosimilar switching.
Electronic supplementary material
The online version of this article (10.1007/s40259-017-0256-z) contains supplementary material, which is available to authorized users.
Key Points
| We believe that sufficiently powered and appropriately statistically analysed trials and pharmacovigilance studies, with long-term follow-ups and multiple switching sequences, are still needed to support decision-making around biosimilar interchangeability. |
| In the interim, switching should remain a clinical decision made by the treating physician and the patient based on available evidence and individual patient circumstances. |
Introduction
Biological medicines (‘biologics’) are derived from living organisms, often by using recombinant DNA technology. Biologics differ from traditional, small molecule medicines, which are generally structurally simple, chemically synthesised and easily characterised analytically. Post-translational modifications of biologics are particularly vulnerable to manufacturing process conditions, and maintaining batch-to-batch consistency is essential [1]. Small process changes (‘evolution’) or unintentional deviations (‘drift’) can lead to changes in the biological end product and, ultimately, to product divergence, a concept that has particular relevance in the setting of biosimilars [2].
Quality assessment of biologics in terms of efficacy and safety requires physicochemical and functional assays, pharmacokinetic and pharmacodynamic evaluations, toxicity testing in animals and clinical pharmacology [3–5]. Unwanted immune responses are a concern, both in terms of adverse events (e.g. anaphylaxis, infusion reactions) and neutralisation of either the therapeutic biologic or the endogenous counterparts by neutralising antibodies, and have terminated development of some biologics [6].
Biosimilars have established similarity to the biologic reference product in terms of safety and efficacy. There are stringent regulatory requirements for demonstration of biosimilarity—including demonstration of comparable physicochemical characteristics, biological activity, efficacy and safety/immunogenicity—and these are laid down in the respective US Food and Drug Administration (FDA) and European Union (EU) guidance documents, and the approval process of biosimilars in these highly regulated markets is rigorous [4, 5, 7–10]. However, unlike generics of small molecule medicines (which can be fully characterised structurally, thus allowing for the same drug substance to be produced), biosimilars of biologic reference products can differ in terms of the overall structure of their drug substance and are similar to, but not identical to, the originator product. This is because biologics are large and structurally complex, meaning that, unlike the characterisation of generics, current analytical methodology may not be able to detect or characterise all relevant structural and functional differences between biologics, a distinction that is also noted in the FDA guidance on demonstrating biosimilarity and the Australian Therapeutic Goods Administration (TGA) biologic nomenclature consultation [3, 11]. Data on the efficacy and safety of initiating therapy with a biosimilar versus with an originator biologic, generated as part of regulatory approval and post-approval phases, have been widely reviewed [12–14] and are not the focus of the current article.
‘Interchangeability’ is the medical practice of changing one medicine for another that is expected to achieve the same clinical effect in a given clinical setting and in any patient, on the initiative, or with the agreement, of the prescriber [15, 16]. ‘Switching’ refers to the treating physician exchanging one medicine with another medicine with the same therapeutic intent in a given patient [17]. Substitution is the practice of dispensing one medicine instead of another equivalent and interchangeable medicine at the pharmacy level without consulting the prescriber [15]. Implied in this is that a biosimilar has been shown to produce the same result as the originator in any patient and that there are no signals suggesting loss of efficacy or increased toxicity if multiple switches are made in the cases in which the medicine must be administered on many occasions.
Definitions of biosimilarity and interchangeability used in the USA, the EU and Australia are summarised in Supplementary Table 1 (see the electronic supplementary material) [4, 5, 7–10]. For a reference product and a biosimilar to be considered interchangeable, the FDA requires sponsors to demonstrate that the risk in terms of side effects or diminished efficacy of switching is not greater than the risk of using the reference product without such alternating or switching [18]. Recently published FDA draft guidance on the specific data required to achieve a designation of interchangeability states that for products that are administered more than once, marketing applications are expected to include data from switching studies in appropriate conditions of use [19]. In the EU, substitution policies are within the remit of each member state [4]. However, officials from Finnish, Dutch, German and Norwegian national regulatory agencies concluded in a recent ‘current opinion’ article, published in BioDrugs, that “biosimilars licensed in the EU are interchangeable” [17]. In Australia, the TGA has responsibility for marketing authorisation (focusing on safety, efficacy and quality), with the Pharmaceutical Benefit Advisory Committee (PBAC) advising the government about whether a drug should be subsidised (focusing on cost-benefit) [20]. The PBAC has proposed that biosimilar substitution at the pharmacy level is acceptable when there is “absence of data to suggest significant differences in clinical effectiveness or safety compared with the originator product” that argue against such action [8]. The interchangeability of an originator biologic and a biosimilar or between biosimilars is considered by the PBAC on a case-by-case basis [8].
Switching between an originator biologic and a biosimilar medicine or between biosimilars can be categorised as either medical or non-medical. Medical switching is initiated by the prescriber because of medical concerns such as adverse events, or convenience of dosing or administration. For example, the incidence of injection site reactions in phase 3 clinical trials was lower with biosimilar than originator tumour necrosis factor (TNF) inhibitors, a difference that was explained by a lack of l-arginine in the formulation and of latex in the needle shield of the biosimilar products [21]. Non-medical switching occurs because of non-medical concerns, including treatment cost or availability. Recommendations from professional societies around switching are summarised in Supplementary Table 2 [22–37]. Importantly, across the USA, Canada, Europe and Australia, professional societies state that the decision to switch has to be taken by the treating physician. In the USA, for example, the American Academy of Dermatology (AAD) and the American College of Rheumatology (ACR) support switching only if it is deemed suitable by the prescribing provider [22, 26], and the Australian Rheumatology Association (ARA) position is that the decision to prescribe any medication should rest with the prescriber, in consultation with an informed patient, and substitution should not occur without the knowledge and consent of the patient [35]. The Australian Diabetes Society and Australian Diabetes Educators Association (in a collective statement with Diabetes Australia), the Gastroenterological Society of Australia and the Australian Inflammatory Bowel Disease Association strongly oppose recommendations of biosimilars as interchangeable on the grounds of patient safety [23, 33]. This is in stark contrast with the Pharmaceutical Society of Australia and the Pharmacy Guild of Australia, who support substitution by the pharmacist if the patient agrees and if the prescribing physician has not specifically indicated that no substitution should occur [38, 39].
Ongoing areas of scientific debate and potential concern around biosimilars are (1) extrapolation of approval of biosimilars across multiple clinical indications, (2) presence of impurities or chemical alterations not detected with standard comparability testing [40–42], and (3) whether the efficacy, safety and immunogenicity risk of therapy are affected when patients are switched between originator and biosimilar products or from one biosimilar to another, in particular when multiple switching occurs. This article will focus on the third point, namely the key principles around biosimilarity and interchangeability, and analyse the available evidence around switching between originator biologics and biosimilars or between biosimilars based on a systematic review of the published literature, identifying any significant evidence gaps.
Methods
Systematic Literature Searches
A systematic literature review was conducted in PubMed, EMBASE and Cochrane Library to 10 June 2017 to identify studies reporting efficacy and/or safety data on switching between originator biologics and biosimilars or between biosimilars, using the following search string: ((biosimilars OR biosimilar OR biosimilarity OR “subsequent entry biologic” OR “subsequent entry biologics” OR “similar biotherapeutic product” OR “similar biotherapeutic products” OR “similar biological medicinal product” OR “similar biological medicinal products” OR “follow-on biologic” OR “follow-on biologics”) AND (equivalent OR equivalence OR comparability OR substitute OR substitution OR substitutability OR switch OR switched OR switching OR interchange OR interchanged OR interchangeable OR interchangeability)). Records in PubMed were retrieved using the title and abstract (‘tiab’) search filter. Records in EMBASE were retrieved using the ‘ex/mj’ syntax to capture synonyms and apply major focus for all terms.
The following meetings from the main societies representing key therapeutic areas for biosimilars were checked for relevant abstracts published in 2015 or 2016: American Association of Clinical Endocrinologists (AACE); AAD; ACR; American Diabetes Association; American Gastroenterology Association; American Society of Clinical Oncology (ASCO); American Society of Nephrology; European Crohn’s and Colitis Organisation; European Renal Association/European Dialysis and Transplant Association European Society for Medical Oncology; and European League against Rheumatism. In addition, ASCO was also checked for relevant abstracts published in 2017.
The reference lists of identified primary articles and literature reviews were hand searched for any additional, potentially relevant articles. To supplement the systematic searches, an exploratory literature search was undertaken to support the overview section around the principles of biologics, biosimilarity and interchangeability.
Study Eligibility and Data Extraction
Studies were excluded if they presented data on switching only between different classes of originator biologics and biosimilars or between different classes of biosimilars. Pharmacokinetic/pharmacodynamic cross-over studies in healthy volunteers were included if more than one dose of study drug was administered and if data were reported separately for the post-washout/post-switch study period. Studies comprising fewer than 20 switch patients were excluded. No exclusion criteria were applied regarding study type (clinical or observational), retrospective or prospective analysis or publication date.
Data were extracted on treatment, indication, study design, comparators, patient numbers and demographics (age, sex), reason for treatment switching, efficacy, safety and anti-drug antibodies (ADAs).
All data were tabulated separately by study type (clinical or observational) and reason for switching (medical or non-medical). No meta-analyses were performed given the heterogeneity of study designs, interventions, populations and methods of analysis.
Role of Funding Source
The funding source had no role in the conduct of the present study.
Results
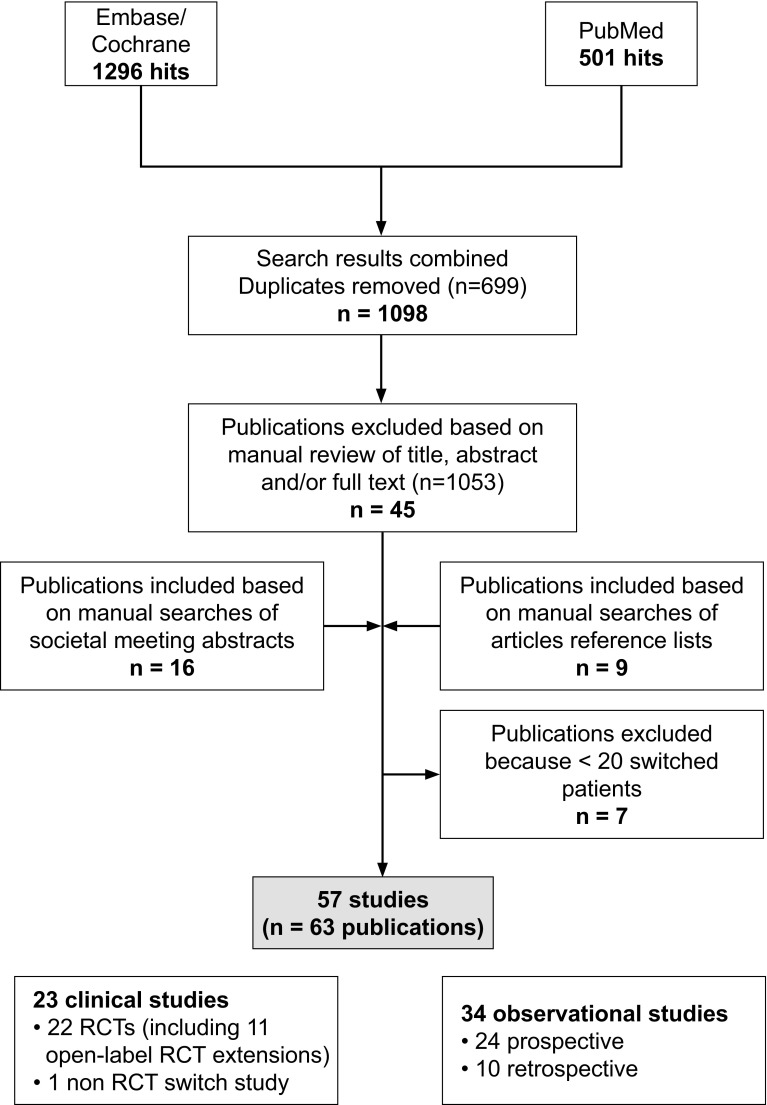
The systematic literature search identified 63 primary publications, covering 57 switching studies that reported efficacy and/or safety data [43–105]. A flow chart of the systematic searches is shown in Figure 1. Forty-one of the identified studies were reported as full articles; the remaining 16 studies were covered only in abstract form. In terms of design, 23 were clinical studies [22 randomised controlled trials (RCTs), including 11 open-label extensions of RCTs; and one non-RCT cross-over study] and 34 were observational (24 prospective, 10 retrospective).
Fig. 1.
Flow chart of systematic literature searches. RCT randomised controlled trial
Patient demographics and other study characteristics are summarised in Supplementary Table 3 [43–105]. The number of included patients per study ranged from 20 to 802, except for a retrospective chart review with N = 3018 [72] and an insurance database study with N = 6177 [76]. The average age, where reported, ranged from younger than 10 years to 71 years, and the proportion of men, where reported, ranged from 0% to 87%.
The reason for switching was reported as non-medical in 50 studies, of which 23 were clinical studies (Table 1) and 27 were observational studies (Table 2). The remaining seven studies did not report whether the reasons for switching were medical or non-medical; all were observational (Table 3). None of the studies reported having only medical reasons for switching.
Table 1.
Clinical studies (non-medical switching)
| Reference | Patients | Patients switched, n | Pre-switch treatment, duration | Post-switch treatment, duration | Efficacy | Safety | ADAs |
|---|---|---|---|---|---|---|---|
| Jorgensen et al. 2017 [53] | Mixed (CD, UC, SpA, rheumatoid arthritis, PsA, chronic plaque psoriasis) | 240 | INX RP, mean duration 6.8 years | CT-P13, follow-up 52 weeks | INX RP vs CT-P13: disease worsening, 26 vs 30%; adjusted risk difference − 4.4% (95% CI − 12.7 to − 3.9) within pre-specified non-inferiority margin of 15%; adjusted RR: 1.17 (95% CI 0.82–1.52). Remission: 61% in each group; adjusted rate difference: 0.6% (95% CI − 7.5% to 8.8%) | INX RP vs CT-P13: ≥ 1 TEAE: 70 vs 68%; infusion reactions: 4 vs 2%; serious TEAEs 10 vs 9%; discontinuation because of AE: 4 vs 3%. No deaths | At any time point, INX RP vs CT-P13: 11% vs 13% |
| Kay et al. 2015 (abstracts and poster) [55, 56] | Rheumatoid arthritis (N = 189) | 53 | INX RP (Remicade®), 16 weeks (study also included patients on BOW015 throughout, with no switch) | BOW015, 38 weeks | INX RP/BOW015 vs BOW015/BOW015: similar disease activity and disability scores (shown as graph; statistical analysis NR) | INX RP/BOW015 vs BOW015/BOW015, patients with ≥ 1 TEAE: 53 vs 48%; SAE: 6 vs 7%; infusion reaction: 2 vs 4%; discontinuation due to TEAE: 0 vs 1% | INX RP/BOW015 vs BOW015/BOW015, week 30: 47 vs 42%; at week 58: 66 vs 65% |
| Park et al. 2017 [61] | AS (N = 174) (PLANETAS open-label extension) | 86 | INX RP, 54 weeks (study also included patients on CT-P13 throughout, with no switch) | CT-P13, 48 weeks | Clinical response maintained at similar levels before and after switch, and similar in switch and non-switch groups (all P NS) | INX RP/CT-P13 vs CT-P13/CT-P13, proportion of patients with ≥ 1 TEAE during extension study: 71 vs 49%; considered related to study drug: 39 vs 22% | INX RP/CT-P13 vs CT-P13/CT-P13, proportion of patients with ADAs at week 102: 27 vs 23% (P NS) (all patients with ADAs also had nADAs) |
| Smolen et al. 2016 (abstract) [69] | Rheumatoid arthritis (N = 396) | 94 | INX RP, 54 weeks (study also included patients on INX RP or SB2 throughout, with no switch) | SB2, 24 weeks | Response rates were maintained and similar between groups (shown on graph) | Incidence of AEs and infusion-related reactions, INX RP/INX RP: 36 and 2%; SB2/SB2: 40 and 4%; INX RP/SB2: 36 and 3% | Newly developed ADAs, INX RP/INX RP: 15%; SB2/SB2: 14%; INX RP/SB2: 15% |
| Tanaka et al. 2017 [70] | With rheumatoid arthritis (N = 104) | 33 | INX RP, duration 54 weeks | CT-P13, duration 105 weeks | Mean DAS28 at end of follow-up, maintenance vs switch group: 3.2 vs 4.0; P NR); discontinuation because of lack of efficacy: 3 vs 3% | ≥1 AE, maintenance vs switch group: 90 vs 88%; discontinuation because of AE: 11 vs 24% | Maintenance vs switch group at end of follow-up: 16 vs 17%. New ADA post switch: 3 vs 3% |
| Yoo et al. 2017 [73] | Rheumatoid arthritis (N = 302) (PLANETRA open-label extension) | 144 | INX RP, 54 weeks (study also included patients on CT-P13 throughout, with no switch) | CT-P13, 48 weeks | Clinical response maintained at similar levels before and after switch, and similar in switch and non-switch groups (all P NS based on 95% CIs) | INX RP/CT-P13 vs CT-P13/CT-P13, proportion of patients with ≥ 1 TEAE during extension study: 54 vs 54%; considered related to study drug: 19 vs 22% | INX RP/CT-P13 vs CT-P13/CT-P13, proportion of patients with ADAs at week 102: 45 vs 40% (P NS) (all patients with ADAs also had nADAs) |
| Haag-Weber et al. 2009 [74] | With renal anaemia (N = 386) | 137 | ESA RP (Eprex/Erypo), 28 weeks (study also included patients on HX575 throughout, with no switch) | HX575, 28 weeks | Haemoglobin values, range, HX575/HX575 vs ESA RP/HX575: 11.6–11.9 g/dL vs 11.5–12.1 g/dL (CI for between-group difference in change from baseline within pre-specified equivalence boundaries); weekly dosage remained constant (median dosage change in both groups: 0%) | NR separately for post-switch period, except for number of deaths [9 (3.6%) in HX575/HX575 group; 5 (3.6%) in ESA RP/HX575 group] | NR separately for post-switch period |
| Harzallah et al. 2015 [75] | With renal anaemia (N = 53) | 53 | 1st epoetin NR, then epoetin alfa RP (Hemax; pre-switch), 15 days | Biosimilar epoetin alfa (Epomax), 43 days | Pre-switch vs post-switch haemoglobin levels, blood pressure and median drug doses similar (shown as graph; statistical analysis NR) | Pre-switch vs post-switch differences in AEs NR; 5 patients discontinued after switch: 2 because of abdominal pain (considered unrelated to study drug) and reason NR for other 3 (all had stable haemoglobin levels at drop-out) | NR |
| Wiecek et al. 2010 [81], Wizeman et al. 2008 [82] (two studies) | With renal anaemia (N = 481) | 481 (118 + 121 + 242, across 2 trials and open-label extension) | Epoetin alfa or epoetin zeta, ≥ 12 weeks | Epoetin zeta or epoetin alfa, ≥ 12 weeks | Pre- vs post switch: median haemoglobin levels and doses maintained within pre-specified equivalence margins | Incidence and nature of TEAEs similar between groups and appeared unaffected by switch | None detected |
| Hadjiyianni et al. 2016 [84], Ilag et al. 2016 [85] | With T1DM (N = 452 [ELEMENT 1]) | 207 | Insulin glargine RP (IGlar; Lantus®), duration NR (study also included patients on IGlar throughout, with no switch) | LY IGlar, 24 weeks | No significant differences in efficacy parameters through 52 weeks, but greater weight gain in IGlar/LY than IGlar/IGlar group (1.0 vs 0.2 kg; P = 0.035) | No significant difference in TEAEs and serious AEs | ELEMENT 1: no significant difference in ADA incidence |
| Hadjiyianni et al. 2016 [84], Ilag et al. 2016 [85], Rosenstock et al. 2015 [86] | With T2DM [N = 299 (ELEMENT 2)] | 155 | Insulin glargine RP (IGlar; Lantus®), duration NR (study also included patients on IGlar throughout, with no switch) | LY IGlar, 24 weeks | No significant differences in efficacy parameters through 52 weeks | No significant differences in TEAEs, but significantly fewer patients in IGlar/LY IGlar than IGlar/IGlar group experienced ≥ 1 SAE (3 vs 8%, P = 0.03) | Overall (any time post-baseline) ADA incidence significantly higher in IGlar/LY IGlar than IGlar/IGlar group (19 vs 8%, P = 0.01) (potentially because of baseline imbalances), but not when measured at 24-week time point |
| Romer et al. 2011 [92] (modelling Belleli et al. 2015 [91]) | With growth disturbances (N = 163) | 45 | Genotropin, 9 months (studies also included patients on Omnitrope® throughout, with no switch) | Omnitrope® (powder and/or solution), up to 75 months | Pre- vs post-switch: similar predicted (model-based analysis) vs observed growth data; height and velocity nearly identical and parallel between switch vs non-switch groups | TEAEs, 1st 9 months (before switch): in 36 patients (22%); 9–18 months: in 29 patients (18%) | 1st 9 months (before switch): in 2 patients (1%); 9–18 months: in 1 patient (0.6%) |
| Engert et al. 2009 [93] | Undergoing chemotherapy (N = 92) | 29 | Filgrastim RP, 1 chemotherapy cycle (study also included patients on XM02 throughout, with no switch) | XM02, ≤ 5 chemotherapy cycles | Febrile neutropenia incidence across all cycles, filgrastim RP/XM02 vs XM02/XM02: 41 vs 32 (P NS) | AE profile reported as being similar between groups (actual post-switch data NR) | NR |
| Gatzemeier et al. 2009 [94] | Undergoing chemotherapy (N = 240) | 79 | Filgrastim RP, 1 chemotherapy cycle (study also included patients on XM02 throughout, with no switch) | XM02, ≤ 5 chemotherapy cycles | Febrile neutropenia incidence in cycle 4, filgrastim RP/XM02 vs XM02/XM02: 3.3 vs 4.3% (P NS) | Data specifically for time period after switch NR | NR |
| Krendyukov et al. 2017 (abstract) [95] | Undergoing chemotherapy (N = 218) | 107 | Filgrastim RP or EP2006, 1 cycle (study also included patients on filgrastim RP or EP2006 throughout, with no switch) | 6 cycles of alternating treatment, switching between filgrastim RP and EP2006 | Switched vs reference, febrile neutropenia: 3.4 vs 0% (95% CI − 9.65 to 4.96; within predefined non-inferiority margin); infections: 9.3 vs 9.9%. Hospitalisation due to febrile neutropenia, n = 1 (switched patient in cycle 6) | Switched vs reference (all cycles), TEAEs related to filgrastim: 42.1 vs 39.2%; musculoskeletal or connective tissue disorders related to filgrastim: 35.5 vs 39.2% (including bone pain: 30.8 vs 33.3%) | None detected |
| Papp et al. 2017 [97]; Gooderham et al. 2016, (abstract) [96] | With plaque psoriasis (N = 350) | 77 | Adalimumab, 16 weeks (study also included patients on adalimumab or ABP501 throughout, with no switch) | ABP501, 36 weeksa | NR post-switch | TEAEs, 4 weeks post-switch: continued ABP501 vs continued adalimumab vs switched to ABP501: 23 vs 19% vs 16% | At week 20, continued ABP501 vs continued adalimumab vs switched to ABP501: 55 vs 60 vs 65%; nADAs: 7 vs 11 vs 13% |
| Weinblatt et al. 2016 (abstract) [98] | With rheumatoid arthritis (N = 506) | 125 | Adalimumab, 24 weeks (study also included patients on adalimumab or SB5 throughout, with no switch) | SB5, 28 weeks | Adalimumab/adalimumab vs adalimumab/SB5 vs SB5/SB5, response at 52 weeks: 71 vs 81 vs 77% (P NR); mean change in modified Sharp score: 0.50 vs 0.25 vs 0.17 (P NR) | Adalimumab/adalimumab vs adalimumab/SB5 vs SB5/SB5, ≥ 1 TEAE: 33 vs 38 vs 32%; serious infection: 0 vs 2 vs 0%; injection site reactions: 2 vs 0 vs 0% | Adalimumab/adalimumab vs adalimumab/SB5 vs SB5/SB5, incidence: 18 vs 17 vs 16% |
| Nasanov et al. 2016 (abstract) [99] | With rheumatoid arthritis (N = 160) | NR (approx. 80, based on study design) | RTX RP, 24 weeks, or BCD-020, 24 weeks | BCD-020 24 weeks or RTX RP 24 weeks | RTX RP/RTX RP vs RTX RP/BCD-020, ACR20 at week 48: 92.3 vs 77.8% (P = 0.25); BCD-020/RTX RP vs BCD-020/BCD020: 89.3 vs 96.0%, (P = 0.61). RTX RP/RTX RP, RTX RP/BCD-020, BCD-020/RTX RP, BCD-020/BCD-020 ACR70 at week 48: 34.6, 40.7, 39.3, 40.0% | AEs, RTX RP/RTX RP, RTX RP/BCD-020, BCD-020/RTX RP, BCD-020/BCD-020: 38, 57, 63, 44% | RTX RP/RTX RP, RTX RP/BCD-020, BCD-020/RTX RP, BCD-020/BCD-020: 0 vs 0 vs 0 vs 4% |
| Park et al. 2017 [60] | With rheumatoid arthritis (N = 58) | 20 | Rituximab, up to 72 weeks (study also included patients on CT-P10 throughout, with no switch) | CT-P10, up to 56 weeks | CT-P10/CT-P10 vs rituximab/CT-P10, mean change at week 24 vs baseline, after 1st course, DAS28-CRP: − 2.2 ± 1.15 vs − 2.2 ± 1.16 (P NS); DAS28-ESR: − 2.7 ± 1.17 vs − 2.4 ± 1.33 (P NS). Percentage achieving good or moderate EULAR-ESR and EULAR-CRP responses similar between groups for each time point (week 8, 16 and 24) | CT-P10/CT-P10 vs rituximab/CT-P10, AE: 24 vs 20%; SAE: 3 vs 5%; infusion-related reaction: 3 vs 5%; infection: 8 vs 10% | CT-P10/CT-P10 vs rituximab/CT-P10: 13 vs 15% (all since pre-switch). nADAs, n = 1 |
| Emery et al. 2016 (abstract) [101, 102] | With rheumatoid arthritis (N = 245) | 119 | ETN RP, 52 weeks (study also included patients on SB4 throughout, with no switch) | SB4, 48 weeks | Weeks 52–100, ETN RP/SB4 vs SB4/SB4, ACR20: 79 vs 78%; ACR50: 61 vs 60%; ACR70: 42 vs 43% (statistical analysis NR) | Weeks 52–100, ETN RP/SB4: ≥ 1 TEAE, n = 58 (49%); ≥ 1 SAE, n = 2 (2%); serious infections, n = 1 (1%); death, n = 0 (0%); SB4/SB4: ≥ 1 TEAE, n = 60 (48%); ≥ 1 SAE, n = 6 (5%); serious infections, n = 1 (1%); death (from hepatic cancer), n = 1 (1%) | Weeks 52–100, ETN RP/SB4: n = 1 (1%); SB4/SB4: n = 1 (1%) |
| Griffiths et al. 2017 [103] | With chronic plaque psoriasis (N = 531) | 196 | GP2015 or ETN, 12 weeks (study also included patients re-randomised to same treatment, with different dosing schedule, with no switch) | Patients with PASI improvement ≥ 50% re-randomised to series of 3 treatment switches to week 30, then continuation on last assigned treatment to week 52 | Baseline to week 52, mean scores and percentage changes in PASI score at all time points similar between continued GP2015 and ETN groups, and between pooled continued and pooled switched groups. Response rates increased over time in all treatment groups until week 30 and then remained stable to week 52 | ≥1 TEAE up to week 52, continued GP2015 vs continued ETN switched GP2015 vs switched ETN: 60 vs 57 vs 61 vs 59%. AEs of special interest, continued GP2015 vs continued ETN switched GP2015 vs switched ETN: 11 vs 5 vs 11 vs 5% | Non-nADAs: 1% in the switched ETN group at week 36 (receiving GP2015 for 12 weeks at time of finding); 2% in continued ETN group |
| Strowitzki et al. 2016 [104] | Undergoing ovarian stimulation (N = 147) | 67 | FSH RP (Gonal–f), 1 cycle in RCT (study also included patients on Ovaleap throughout, with no switch) | Ovaleap, 1 cycle in RCT plus ≤ 2 cycles in extension study | Gonal-f/Ovaleap vs Ovaleap/Ovaleap, mean number of oocytes retrieved, cycle 2: 12.1 vs 12.0; cycle 3, mean: 15.0 vs 12.3 (P NR) | Frequency of TEAEs similar in 2 groups; there were 2 drug-related TEAEs, both in Ovaleap group: 1 injection-site erythema, pruritis and haematoma, 1 lower abdominal pain | Detected in 6 patients (none with nADAs); NR separately for 2 groups |
ABP501 adalimumab biosimilar, ACR American College of Rheumatology, ADAs anti-drug antibodies, AE adverse event, AS ankylosing spondylitis, BCD-020 rituximab biosimilar, BOW015 infliximab biosimilar, CD Crohn’s disease, CI confidence interval, CRP C-reactive protein, CT-P10 biosimilar rituximab, CT-P13 biosimilar infliximab, DAS28 Disease Activity Score in 28 joints, EP2006 filgrastim biosimilar, ESA erythropoietin-stimulating agent, ESR erythrocyte sedimentation rate, ETN etanercept, EULAR European League Against Rheumatism, FSH follicle-stimulating hormone, GP2015 etanercept biosimilar, HX575 epoetin alfa biosimilar, INX infliximab, LY IGlar LY2963016 insulin glargine, nADAs neutralising anti-drug antibodies, NR not reported, NS not significant, PASI Psoriasis Area and Severity Index, PsA psoriatic arthritis, RCT randomised controlled trial, RR relative risk, RP reference product, RTX rituximab, SAE serious adverse event, SB2 infliximab biosimilar, SB4 etanercept biosimilar, SB5 adalimumab biosimilar, SpA spondyloarthritis, T1DM type 1 diabetes mellitus, T2DM type 2 diabetes mellitus, TEAE treatment-emergent adverse event, UC ulcerative colitis, XM02 filgrastim biosimilar
aOf 175 patients on adalimumab, those with PASI of ≥ 50 at 16 weeks were re-randomized 1:1 to remain on adalimumab or switch to ABP501
Table 2.
Observational studies on non-medical switching
| Reference | Patients | Patients switched, n | Pre-switch treatment, duration | Post-switch treatment, duration | Efficacy | Safety | ADAs |
|---|---|---|---|---|---|---|---|
| Abdalla et al. 2017 [43] | With inflammatory arthritis (N = 34) | 34 | INX RP, mean duration 70 months | Biosimilar INX, mean follow-up 16 months | 6 months before vs 6 months after switch: HAQ score, patient global assessment of disease activity, number of disease flares or medication dose similar (all P NS). Pain (29 mm vs 38 mm; P = 0.028) and CRP values (1.95 vs 4.0 mg/L; P = 0.001) significantly higher during longer follow-up | No significant difference in number of SAEs (2 vs 3), AEs (numbers NR) or infusion reactions (1 vs 1) during follow-up periods. 5 patients discontinued INX | NR |
| Ala et al. 2016 (abstract) [44] | With IBD (N = 20) | 20 | INX RP, duration NR | CT-P13, 6 months post-switch | Most patients remained in clinical remission or improved during follow-up (actual numbers NR) | No significant AEs reported post-switch | NR |
| Arguelles-Arias et al. 2017 [45] | With IBD (N = 120) | 98 | Originator INX, median duration 297 weeks, or INX-naive | CT-P13, follow-up 6 months | Remained in clinical remission, switched vs naïve, CD, 3 months: 88 vs 67%; 6 months: 84 vs 50%. UC, 3 months: 92 vs 44%; 6 months: 91 vs 67% | NR separately for the 2 treatment groups | NR |
| Bennett et al. 2016 (abstract) [46] | With IBD (N = 104) | 104 | INX RP, median time 162 weeks | CT-P13, follow-up 6 months | Pre-switch vs post-switch, median CRP: 2 vs 2 (P = 0.81); median DAS: 1 vs 1 (P = 0.44); remission: 85 vs 81% (P = 0.51) | After switch: 4 patients had AEs (2 minor infusion reactions, 2 skin disease); 19 patients stopped treatment (6 electively, 9 loss of response, 4 for other reasons) | Pre-switch vs post-switch: 29 vs 27 patients (P = 0.88) |
| Benucci et al. 2017 [47] | With SpA (N = 41) | 41 | Originator INX, median duration 74 months | Biosimilar INX, follow-up 6 months | 6 months before vs 6 months after switch, median BASDAI (2.6 vs 2.7), BASFI (2.2 vs 2.3), ASDAS-CRP (1.3 vs 1.4); DAS28-CRP (2.7 vs 2.7), MASES (0.2 vs 0.4), VAS pain (17 vs 18) (all P > 0.05). Median duration of morning stiffness 7.2 vs 5.8 (P = 0.02) | No significance difference in AEs (Fisher test P = 1.0; actual data NR). 1 patient stopped biosimilar INX because of severe palmoplantar psoriasis | 6 months before vs 6 months after switch: 27.3 vs 27.8 ng/mL (P = 0.98) |
| Buer et al. 2017 [48] | With IBD (N = 143) | 143 | Originator INX, CD, median 87 months; UC, median 57 months | CT-P13, follow-up 6 months | Median change in DAS (HGBI for CD, PMS for UC), 0–3 months and 3–6 months: all 0. Clinical remission baseline vs 6 months, CD: 87 vs 81%; UC: 88 vs 95%. No significant change in CRP, Hb, FC, INX dose, dose interval or plasma INX levels post-switch. All P NS | Post-switch: AEs in 17 patients (3 with UC, 14 with CD); 5 infusion reactions (leading to treatment discontinuation in 1 patient) | Baseline vs new post-switch: n = 2 vs n = 3 patients |
| Dapavo et al. 2016 [49] | With plaque psoriasis (N = 35) | 30 | INX RP, median duration 237 weeks | CT-P13, median follow-up 23 weeks with median 4 cycles | Pre- vs post-switch (end of follow-up), PASI score: P NS (actual values NR); VAS scores: P NS (actual values NR) | Post-switch: herpes zoster (1 patient) | NR |
| Fiorino et al. 2017 [50] | With IBD (N = 547) | 97 | INX RP, duration NR; mean number of infusions 18 [study also included patients with no treatment (naive) or other biologic pre-switch] | CT-P13 switch group, median follow-up 6.6 months | INX RP/CT-P13 vs naive/CT-P13 vs other/CT-P13, estimated probability of response at 24 weeks: 79 vs 74 vs 63%. Primary failure: 0 vs 10 vs 11% (P = 0.005). Estimated probability of treatment persistence at 24 weeks: 92 vs 82 vs 71% | SAEs, INX RP/CT-P13 vs naive/CT-P13 vs other/CT-P13: 12 vs 7 vs 22%; infusion reactions: 7 vs 3 vs 15%. IRR naïve vs switched for SAEs and infusion reactions leading to drug withdrawal: 1.10 (P NS) and 1.88 (P NS), respectively | NR |
| Gentileschi et al. 2016 (letter to editor) [51] | With rheumatic diseases (N = 23) | 23 | INX RP (Remicade®), mean duration: 72 months | CT-P13 (Inflectra®), mean 1.7 months | 7/23 patients (30%) relapsed after switch (5 switched back to INX RP, of whom 4 improved) | NR | NR |
| Glintborg et al. 2016 (abstract) [105] and 2017 [52] | With rheumatoid arthritis, SpA or PsA (N = 802) | 802 | INX RP, duration 6.8 years | CT-P13, median follow-up 413 days | Disease activity, 3 months pre- vs post-switch: no clinically relevant differences. unchanged in majority of patients | Post-switch treatment discontinuation: 132 patients (lack of effect, n = 71, AEs, n = 37) | Baseline vs post-switch (data from 2016 abstract, n = 96): 15 vs 16% (P = 0.7) |
| Jung et al. 2015 [54] | With IBD (N = 110) | 36 | INX RP, duration NR (study also included naive patients started straight on CT-P13 treatment, with no switch) | CT-P13, duration up to 54 weeks | INX RP/CT-P13: efficacy maintained in 31/36 patients, CT-P13 discontinued in 3 patients because of lack of efficacy and in 1 patient who wanted to switch back to INX RP (reason NR) | INX RP/CT-P13: AEs, n = 1 patient (skin rash and arthralgia) | NR |
| Nikiphorou et al. 2015 [58] | With established rheumatic diseases (N = 39) | 39 | INX RP (Remicade®), mean duration 4 years | CT-P13, median follow-up 11 months | Patient-reported outcomes (including pain, fatigue) similar pre-switch vs post-switch (all P NS) | 11 patients (28%) discontinued CT-P13: ADAs [sample taken before switch, n = 3; re-activation of tuberculosis, n = 1; new-onset neurofibromatosis, n = 1; subjective reasons (details NR), n = 6 (5 restarted INX RP)] | Not assessed after switching |
| Rahmany et al. 2016 (abstract) [62] | With IBD (N = 78) | 78 | INX RP; median treatment duration: CD 46 months, UC 25 months | CT-P13, 4–6 months | Pre- vs post-switch, remission, CD: 67 vs 72%; UC: 60 vs 85%; mean CRP: 5.4 vs 5.6 (P NS); UC: 3.1 vs 3.0% (P NS) | 1 infusion reaction. Rate of mild AEs similar pre- and post-switch (actual data NR). Post-switch treatment discontinuation: 5 patients (3 biological class switch, 1 infusion reaction, 1 deep remission) | NR |
| Razanskaite et al. 2017 [63] | With IBD (N = 143) | 143 | INX RP, median number of infusions 10 | CT-P13, follow-up: ≥ 3 infusions | Pre- vs post-switch, median IBD-Control-8 score: 11 vs 14 (P = 0.041). Mean CRP, albumin levels, platelet count, white cell count: all P NS. Treatment persistence INX RP historical cohort vs switch cohort: P NS | Pre- vs post-switch, joint pains: 26 vs 14%; headaches: 22 vs 17%; infections: 13 vs 9% | Pre- vs post-switch: 40 vs 40% of patients (P NS) |
| Schmitz et al. 2017 [67] | With rheumatoid arthritis, PsA, AS, SpA, psoriasis or arthritis with UC (N = 27) | 27 | INX RP, median duration 143 months | CT-P13, duration approximately 12 months | Median INX trough levels, CRP levels, DAS28: all P NS | Post-switch, therapy discontinuation because of AEs: 2 patients (hyperventilation, suspected vasculitis); because of higher disease activity: 2 patients | Post-switch: no new detected |
| Sheppard et al. 2016 (abstract) [65] | With rheumatic diseases (N = 25) | 25 | INX RP, duration NR | CT-P13, duration NR | 1 patient (4%) reported loss of disease control post-switch; no significant difference in CRP values before and after switch (actual data NR) | 4 patients (16%) developed AEs after switching: flu-like symptoms (n = 1), inflamed foreskin (n = 1), pruritic rash (n = 2); all were switched back to INX RP | NR |
| Sieczkowska et al. 2016 [66] | With paediatric IBD (N = 39) | 39 | INX RP, mean duration 67 weeks | CT-P13, mean follow-up 8 months | Remission rate, at baseline: 69%; after 1st CT-P13 infusion: 75%; after 2nd CT-P13 infusion: 83%; statistically significant improvement in disease activity index | After switch: 3 patients (8%) with infusion reactions (1 leading to discontinuation), 1 with varicella zoster (leading to discontinuation), 7 with infections of upper respiratory tract | NR |
| Smits et al. 2016 [68] | With IBD (N = 83) | 83 | INX RP, median duration 25 months | CT-P13, duration 16 weeks | Baseline (at switch) vs 16 weeks post-switch: no significant changes in efficacy parameters (disease activity; percentage in clinical remission; inflammatory biomarkers), but 4 patients needed additional medication to control increased disease activity | 24 patients (29%) reported AEs; 6 patients (7%) had suspected drug-related AEs: pruritis after infusion (n = 2); dizziness/tingling sensation after 1st or 2nd infusion (n = 2); warm sensation after 2nd infusion (n = 1); arthralgia (n = 1) | ADAs detected in 7 patient (8%), of whom 5 also had ADAs at baseline |
| Tweehuysen et al. 2016 (abstract) [71] | With rheumatoid arthritis, PsA, or SpA (N = 192) | 192 | Innovator INX, duration NR | Biosimilar INX, follow-up 6 months | Baseline vs 6 months for rheumatoid arthritis and PsA, mean DAS28-CRP: 2.2 (SD 0.9) vs 2.2 (SD 0.84) (P NS); for SpA, mean BASDAI: 3.8 vs 4.3 (change + 0.5, 95% CI 0.12–0.89; P = 0.01). Median CRP levels: 1.5 mg/L vs 1.0 mg/L (P NS). Discontinuation because of loss of efficacy: 18% | Post-switch treatment discontinuation: 23 because of AEs (most frequent: fatigue, n = 10; malaise, n = 5; headache, n = 3), 2 because of infusion reaction. No SAEs reported | Baseline vs 6 months: 47 vs 38% |
| Yazici et al. 2016 (abstract) [72] | With rheumatoid arthritis (N = 3018) | 148 | INX RP, duration NR | CT-P13, mean 9–12 months | Therapy discontinuation, continuation vs switched groups: 38% (average time to discontinuation 256 days) vs 82% (average time to discontinuation 124 days; 70% switched back to INX RP) | NR | NR |
| Lopez et al. 2014 (abstract) [77] | Undergoing chemotherapy (N = 28) | 28 | Epoetin alfa, darbepoetin alfa; 12 months | Epoetin zeta, 12-months | Mean Hb, pre-switch epoetin alfa vs post-switch: 10.8 vs 11.1 g/dL (P NS); pre-switch darbepoetin vs post-switch: 9.9 vs 10.9 g/dL (P = 0.01). Post-switch dose increase to maintain target Hb: 46% of patients | NR | NR |
| Morosetti et al. 2017 [79]a | With chronic renal failure (N = 87) | 87 | ESA, 6 months | Biosimilar, 6 months | Pre- vs post-switch Hb, ferritin, transferrin saturation: all P NS; mean monthly epoetin consumption/patient: 52,460 vs 49,517 Ul (P NS) | No changes in incidence of thrombosis or cardiovascular events | NR |
| Balili et al. 2015 (abstract) [83] | With T2DM (N = 24) | 24 | Humulin 70/30, duration NR | Wosulin 70/30, duration NR | Pre- to post-switch mean HbA1c decreased by 1.3%, total insulin dose/day increased by 2.4 units. Target HbA1c was achieved (specific results NR) | NR | NR |
| Segal et al. 2013 [87] | With T1DM or T2DM (N = 77) | 77 | Actraphane, Humulin 30/70 or Insuman, insulin duration 7 years | Biosulin 30/70, 6 months | Baseline (at switch) vs post-switch: no significant change in HbA1c, neither overall nor in subgroups by type of diabetes; small, significant increase in insulin dose | No severe hypoglycaemic episodes reported; other details NR | NR |
| Flodmark et al. 2013 [88] (comment: Ekelund et al. 2014 [134]) | With growth disturbances (N = 98) | 98 | Genotropin RP, duration NR (graph suggests up to 2 years) | Omnitrope®, duration NR (graph suggests up to 1.5–2 years) | Growth in height after switch: modelled vs actual data suggest no impact of switch on growth | Number of patients with TEAEs in 12 months after switch: 18 patients; injection site pain: 18 (of whom 6 subsequently switched back to originator preparation) | NR |
| Gila et al. 2014 (abstract) [89] | With growth disturbances (N = 20) | 20 | rhGH RP, mean duration 38 months | Omnitrope®, follow-up 36 months | Height velocity, 18 months before switch: 9 ± 2 cm/year; at switch: 6 ± 1 cm/year; 18 months after switch: 7 ± 3 cm/year (statistical analysis NR) | No TEAEs reported after switch; 3 patients had transitory problems with Omnitrope® device | NR |
| Rashid et al. 2014 [90] | With growth disturbances (N = 103) | 103 | Non-Omnitrope® rhGH (Humatrope®, Norditropin®, Nutropin®, Saizen®), duration ≥ 15 months | Omnitrope®, duration 15 months | Height velocity, 6 months before switch: 6.4 cm/year; at switch: 6.0 cm/year; 6 months after switch: 5.9 cm/year; 12 months after switch: 5.3 cm/year (study authors comment that this decline is expected/associated with advancing age) | NR | NR |
ADA anti-drug antibody, AE adverse event, AS ankylosing spondylitis, ASDAS Ankylosing Spondylitis Disease Activity Score, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, BASFI Bath Ankylosing Spondylitis Functional Index, CD Crohn’s disease, CRP C-reactive protein, CT-P13 biosimilar infliximab, DAS Disease Activity Score, ESA erythropoietin-stimulating agent, FC faecal calprotectin, HAQ Health Assessment Questionnaire, Hb haemoglobin, HbA1c haemoglobin A1c, HGBI Harvey–Bradshaw Index, IBD inflammatory bowel disease, INX infliximab, IRR incidence rate ratio, MASES Maastricht Ankylosing Spondylitis Enthesitis Score, NR not reported, NS not significant, PASI Psoriasis Area and Severity Index, PMS Partial Mayo Score, PsA psoriatic arthritis, rhGH recombinant human growth hormone, RP reference product, SAE serious adverse event, SD standard deviation, SpA spondyloarthritis, T1DM type 1 diabetes mellitus, T2DM type 2 diabetes mellitus, TEAE treatment-emergent adverse event, UC ulcerative colitis, VAS visual analogue scale
aData extracted from English-language abstract (full text in Italian)
Table 3.
Observational studies, reason for switching NR
| Reference | Patients | Patients switched, n | Pre-switch treatment, duration | Post-switch treatment, duration | Efficacy | Safety | ADAs |
|---|---|---|---|---|---|---|---|
| Kolar et al. 2017 [57] | With IBD (N = 74) | 74 | INX RP, mean duration 3 years | Biosimilar INX, duration 24 weeks | Baseline (at switch) vs 56 weeks post-switch, CRP: 4.3 vs 3.3 mg/L (P = 0.89); faecal calprotectin: 135 vs 199 μg/g (P = 0.17); stable disease activity: 72 vs 78% (P NR). Median difference in both HBI and SCCAI from week 0 to week 56 = 0 | Frequency and type of AE similar before and after switch; no infusion reactions reported in switch cohort. Treatment discontinuation because of AE, CD: 2 patients (arthralgia, fibrous dysplasia in maxilla); UC: 2 patients (lack of response) | ADA positive: 9.5 vs 6% (P = 0.54 |
| Park et al. 2015 [59] | With IBD (N = 173) | 60 | INX RP, duration: NR (study also included naive patients started straight on CT-P13 treatment, with no switch) | CT-P13, duration: 30 weeks | Disease worsened (remission not maintained/achieved) in 11% of patients in switch group during 30-week follow-up | Treatment-related TEAEs of special interest, n for naive vs switch, anaphylactic reaction: 0 vs 1; tuberculosis: 1 vs 0; infusion-related reaction: 0 vs 3; no cases of pneumonia or malignancy | NR |
| Rubio et al. 2016 (abstract) [64] | With rheumatic diseases (N = 78) | 53 | INX RP (Remicade®), duration NR (study also included naive patients started straight on biosimilar INX, with no switch) | Biosimilar INX (Remsima®), follow-up 9 months | 4 patients (5%) in switch group and 1 patient (4%) in naive group discontinued because of loss of efficacy | In switch group, 1 patient discontinued because of cutaneous leishmaniasis. In naive group, 1 patient each discontinued because of photosensitivity, bronchospasm, urticaria | NR |
| Hörbrand et al. 2013 [76] | With CKD (N = 6177) | 507 | Originator short-acting epoetin alfa, epoetin beta, epoetin delta, epoetin theta, long-acting darbepoetin alfa, methoxy polyethylene glycol epoetin beta, duration ≥ 3 months | Biosimilar short-acting epoetin alfa, epoetin zeta, duration ≥ 3 months | No change in median defined daily doses consumption when switched | NR | NR |
| Minutolo et al. 2016 [78] | Under-going haemodialysis (N = 149) | 149 | Reference epoetin, darbepoetin, duration NR (analysis 12 weeks) | Biosimilar epoetin (HX575, SB309), duration 24 weeks | Baseline vs post-switch, haemoglobin: 11.1 g/dL vs 11.0 g/dL (P NS); dose: 8765 IU/week vs 10,886 IU/week (P = 0.001) | NR | NR |
| Ohta et al. 2014 [80] | With renal anaemia (N = 30) | 30 | Epoetin beta, duration 3 months | Epoetin kappa, duration 3 months | Haemoglobin levels predominantly stable (shown graphically, statistical analysis NR) | NR | NR |
| Roy et al. 2013 [100] | With non-Hodgkin’s B cell lymphoma (N = 223) | 29 | Rituximab RP (Mabthera®) or biosimilar (Reditux™) (≥ 4 cycles with 1 brand) | Rituximab biosimilar (Reditux™) or RP (Mabthera®) (≥ 4 cycles with 1 brand) | All cycles with Mabthera® vs ≥ 4 cycles with Mabthera® (i.e. includes switched patients), complete remission: 75 vs 74%; partial remission: 14 vs 14%; 5-year survival: 66 vs 64%; 5-year progression-free survival: 72 vs 71%. All cycles with Reditux™ vs ≥ 4 cycles with Reditux™ (i.e. includes switched patients), complete remission: 82 vs 80%; partial remission: 13 vs 17%; 5-year survival: 76 vs 71%; 5-year progression-free survival: 81 vs 75% | NR separately for switch group | NR |
ADA anti-drug antibody, AE adverse event, CD Crohn’s disease, CKD chronic kidney disease, CRP C-reactive protein, CT-P13 biosimilar infliximab, HBI Harvey-Bradshaw index, HX575 epoetin alfa biosimilar, IBD inflammatory bowel disease, INX infliximab, NR not reported, NS not significant, RP reference product, SB309 epoetin zeta, SCCAI Simple Clinical Colitis Activity Index, TEAE treatment-emergent adverse event, UC ulcerative colitis
Non-medical Switching: Clinical Efficacy and Safety Data
Of the 23 clinical, non-medical switching studies (Table 1) [53, 55, 56, 60, 61, 69, 70, 73–75, 81, 82, 84–86, 91–99, 101–104], 22 reported data on main efficacy parameters in switched versus non-switched or pre-switch versus post-switch groups. The originator biologics/biosimilars assessed were infliximab (six studies [53, 55, 56, 61, 69, 70, 73]), erythropoietin-stimulating agents (ESAs) (four studies [74, 75, 81, 82]), filgrastim [93–95] (three studies), insulin [84–86], adalimumab [96–98], rituximab [60, 99] and etanercept [101–103] (two studies each), and genotropin [83, 92] and follicle stimulating hormone [104] (one study each).
Of these, 15 included statistical analyses of disease activity or patient outcomes in switched versus non-switched groups, pre-switch versus post-switch groups or observed versus predicted outcomes [53, 60, 61, 73, 74, 81, 82, 84–86, 91–95, 99], with five studies specifying a formal equivalence or non-inferiority margin [53, 74, 81, 82, 95]. There were no statistically significant differences between groups (based on P < 0.05 or predefined acceptance ranges), with the exception of a significant weight gain that was observed in patients with type 1 diabetes mellitus switched to biosimilar compared with patients remaining on originator insulin (+ 1.0 kg vs + 0.2 kg, respectively; P < 0.05) in the open-label extension of the ELEMENT 1 RCT, although the range of weight changes was wide in both groups [84]. The NOR-SWITCH infliximab trial was conducted across six indications and used a non-inferiority margin of 15% (adapted from rheumatoid arthritis studies), with results suggesting no loss of efficacy and no unexpected treatment-emergent adverse events (TEAEs) [53]. Eight studies reported similar outcomes between groups, but failed to provide a formal statistical analysis of between-group comparisons [55, 56, 69, 70, 75, 98, 102–104].
Most studies reported similar safety profiles between groups. An exception was the PLANETAS extension study of infliximab in patients with ankylosing spondylitis, in which TEAEs were considerably more frequent in the switched group [71% (60 patients out of 84) vs 49% (44 patients out of 90) in the group remaining on originator biologic] and included TEAEs that were considered related to the study drug [39% (33 patients out of 84) vs 22% (20 patients out of 90)] [61].
Non-medical Switching: Real-world Efficacy and Safety Data
All of the 27 observational, non-medical switching studies reported data on main efficacy parameters after switching compared with baseline/before switching (Table 2) [43–52, 54, 58, 62, 63, 65–68, 71, 72, 77, 79, 83, 87–90, 105]. The originator biologics/biosimilars assessed were infliximab (20 studies [43–52, 54, 58, 62, 63, 65–68, 71, 72]), ESAs (two studies [77, 79]), insulin (two studies [83, 87]) and growth hormone (three studies [88–90]).
Of 16 studies that reported statistical analyses, 13 found no statistically significant differences for main efficacy parameters [43, 46–49, 58, 62, 67, 68, 71, 77, 79, 87], although six of these observed changes for some parameters (in favour of the pre-switch and post-switch treatments in five studies and one study, respectively). There was a need for additional medication to control increased disease activity after switching from originator to biosimilar infliximab in four of 83 patients with inflammatory bowel disease (IBD) [68] and in 13 of 28 patients undergoing chemotherapy and switching epoetins [77]; a significant increase in median C-reactive protein values (from 1.95 to 4.0 mg/L; P < 0.05) and median pain scores (from 28.8 to 38.1 mm on a scale of 0–100; P < 0.05) in patients with IBD switching from originator to biosimilar infliximab [43]; a significant increase in disease activity (mean Bath Ankylosing Spondylitis Activity Index, from 3.8 to 4.3; P < 0.05) in patients with spondyloarthritis after switching to biosimilar infliximab [71]; a small, significant increase in total daily insulin dose (from 0.62 to 0.65 U/kg/day; P < 0.05) in patients with type 1 or type 2 diabetes mellitus switched to a biosimilar insulin [87]; but a significant decrease (i.e. improvement) in duration of morning stiffness [median duration, from 7.2 to 5.8 (no units provided); P = 0.02] in patients with arthritis switching from originator to biosimilar infliximab [47]. In the three remaining studies that included statistical analyses, IBD disease activity significantly improved in paediatric patients switched from originator to biosimilar infliximab (P < 0.05; actual values not reported) [66], but significantly worsened in adults who underwent a similar switch (median IBD-Control-8 score, from 11 to 14; P < 0.05) [63], and primary failure was significantly lower in patients with IBD switched from originator to biosimilar infliximab than in patients switched from other biologics or who were treatment naïve (0 vs 11 and 10%, respectively; P < 0.05) [50].
Eight studies reported that switching generally had no noticeable effect, but did not provide a formal statistical analysis of between-group comparisons or based this conclusion on modelling [44, 45, 52, 65, 83, 88–90]. Two studies reported on switching from infliximab originator to biosimilar. In one, relapse was reported in seven of 23 patients with rheumatic diseases within about 2 months after switching [51], and in the other, three of 36 patients with IBD discontinued within 1 year of switching because of loss of efficacy [54].
In terms of safety data after switching, eight studies reported no concerns or similar safety profiles before and after switching [43, 44, 47, 50, 62, 63, 79, 87, 89], six studies reported no general safety data [45, 51, 72, 77, 83, 90], and 12 studies reported adverse events such as injection site pain, acute hypersensitivity reactions, rash and infusion reactions after switching (although most did not provide comparative data from before switching) [46, 48, 49, 52, 54, 58, 65–68, 71, 88].
Switching, Reasons Not Reported: Efficacy and Safety Data
All seven observational studies that did not include reasons for switching assessed efficacy parameters after switching compared with baseline/before switching (Table 3) [57, 59, 64, 76, 78, 80, 100]. Three involved infliximab [57, 59, 64], three ESAs [76, 78, 80] and one rituximab [100].
Two studies included statistical analyses, both of which found no significant differences for main efficacy parameters [57, 78]. In one of these studies, on ESAs in patients on haemodialysis, switching to a biosimilar resulted in higher mean drug dose requirements (from 8765 to 10,886 IU/week; P < 0.05) to maintain haemoglobin levels [78]. Of the five studies not reporting a formal statistical analysis of between-group comparisons, four reported that switching had no noticeable effect [64, 76, 80, 100] and one reported disease worsening in five of 46 evaluable patients with IBD (11%) during 30 weeks of follow-up after switching from infliximab originator to biosimilar [59].
In terms of safety data after switching, one study reported similar patterns to before switching [57], one (in patients with IBD) reported three cases of infusion reaction and one of pneumonia in the switch group, which comprised 60 patients (vs none in the treatment-naive group, which comprised 113 patients) [59], one reported adverse events (cutaneous leishmaniasis) only when these were a reason for treatment discontinuation [64], and four studies did not report safety data [76, 78, 80, 100].
Switching: Immunogenicity Data
The relative frequency of ADAs in biologic originator and biosimilars was reported in 27 studies, of which 18 were clinical studies [53, 55, 56, 60, 61, 69, 70, 73, 81, 82, 84–86, 91, 92, 95–99, 101–104] and nine were observational [46–48, 52, 57, 63, 67, 68, 71, 105]. In 26 studies (17 clinical: follow-up 12–52 weeks [53, 55, 56, 60, 61, 69, 70, 73, 81, 82, 84, 85, 91, 92, 95–99, 101–103]; nine observational: follow-up 6–13 months [46–48, 52, 57, 63, 67, 68, 71, 105]), there were either no cases of ADAs, or the incidence of ADAs was similar in both groups.
A significantly higher proportion of patients with ADAs in the switched group than the non-switched group (19% vs 8% overall; P = 0.01) was reported in a clinical study of insulin in type 2 diabetes mellitus (ELEMENT 2) during 24 weeks of follow-up [84–86].
Comparative data regarding neutralising ADAs were reported in six studies (all clinical); none of which observed any clinically relevant [60, 96, 97, 104] or significant [61, 73, 96] differences between groups. No studies reported any treatment failure related to the development of ADAs.
Discussion
Clinical Considerations
This systematic literature review reveals important evidence gaps around the safety of switching between originator biologics and biosimilars or between biosimilars. There is a paucity of data from large studies of sufficient statistical power for efficacy, safety and immunogenicity risk considerations. There is little information on immunogenicity outside of clinical studies, with only nine of 34 included observational studies (26%) reporting such data. There is a general lack of robustness of evidence and no long-term data, with only eight of the included studies having a post-switch follow-up period beyond 1 year. This is important because patients may be exposed to treatment long-term and, although safety issues such as ADAs occur most commonly early on during treatment with biologics [106], they have been reported to arise only after the first year of treatment in some patients [107, 108]: a meta-analysis of 68 studies on incidence of ADAs to TNF inhibitors found that these first developed as early as 2 weeks, but also as late as 3 years, after treatment initiation [108]. There is large heterogeneity in the design of available studies including the number of switches and permutations of switch scenarios, use of formal equivalence or non-inferiority margins, and assessment of safety and immunogenicity risk, making it difficult to draw conclusions from the data.
Demonstration of interchangeability is a complex process that requires clinical studies in addition to those conducted to establish biosimilarity [109]. In the absence of specifically designed cross-over or multiple switch studies, demonstration of biosimilarity is not adequate to establish interchangeability [109]. The study duration needs to be clinically informed and will not be the same in every setting. Long-term studies are needed if treatment is chronic. A particular challenge when designing interchangeability studies is how to define the non-inferiority or equivalence margin, a topic that is covered by the recent FDA draft guidance [19]. In most studies in the current review, analyses around interchangeability were fairly limited, for example, taking a lack of a statistically significant difference as a lack of need for concern even when confidence intervals were wide [60]. Only five studies specified a formal equivalence or non-inferiority margin [53, 74, 81, 82, 95]. Formal, standardised definitions around margins are needed.
To demonstrate interchangeability, a study needs to show that even multiple switches between products deemed biosimilar do not affect patient safety or treatment effectiveness. The FDA draft guidance on interchangeability states that the switching arm should incorporate at least two separate exposure periods to each of the two products (i.e. at least three switches, with each switch crossing over to the alternate product) [19]. The EGALITY (etanercept) and NOR-SWITCH (infliximab) trials focused specifically on the impact of switching between these TNF-α originator biologics and their corresponding biosimilars, but NOR-SWITCH was not designed to assess the effects of multiple switches [53, 103]. In the EGALITY study, patients with chronic plaque psoriasis either continued with their original therapy or underwent three treatment switches at 6-weekly intervals [103]. TEAEs were shown to be similar between groups, ADAs were low titre and transient, and no neutralising antibodies were detected. Although the study follow-up period was short and numbers small (200 switched patients), the study is an important addition to the evidence.
In NOR-SWITCH, 240 patients were switched across six diseases, with numbers per disease indication ranging from 16 patients for psoriatic arthritis to 77 patients for Crohn’s disease. Disease worsening, the primary endpoint in NOR-SWITCH, was defined differently across the six diseases, with the data then pooled across indications for analysis. The study showed similar results for switched and non-switched groups for disease worsening and safety although, as the authors of the study commented, it was not powered to demonstrate non-inferiority within each diagnostic group, and thus the pooled analysis used does not allow conclusions about individual diseases [53, 110].
Both EGALITY and NOR-SWITCH were relatively small, short-duration studies, and larger studies with longer-term follow-up are still needed, in particular for patients with rheumatic diseases and IBD who are exposed to treatments over many years. In general, once interchangeability is deemed to be established, the relevant information will need to be passed on to healthcare providers to allow them to make individualised treatment decisions for their patients.
Differences exist regarding the regulatory approval pathways for biosimilars in different parts of the world. The regulatory framework around biosimilars is robust in the highly regulated markets of Europe, the USA, Canada, Australia and Japan, with South Korea also having positioned itself as a high-quality global biosimilars leader, while in emerging markets in south-east Asia, Latin America and Africa the regulatory pathways for biosimilars are still evolving [111]. These differences need to be taken into account when interpreting results from switching studies. Almost all of the studies identified for inclusion in the current review originated from highly regulated markets, with the exception of seven studies that included emerging markets [75, 83, 87, 93, 94, 99, 100], of which six reported similar outcomes for switched and non-switched groups and one, from South Africa, reported a requirement for increased insulin dose post-switch [87].
While not the focus of the current review, changing from one originator biologic to another is relatively common in clinical practice, most likely because of inefficacy or intolerability issues with the initial treatment [112, 113]. It is important to note, however, that this scenario is very different from switching between an originator biologic and its biosimilar or between different biosimilars of the same biologic: for example, two originator biologics targeting a given molecule may bind different epitopes, whereas a biologic and its biosimilar or two biosimilars of the same biologic should bind the same epitope. This difference probably explains why ADAs to infliximab demonstrate identical reactivity towards its biosimilar, whereas ADAs to adalimumab do not cross-react with infliximab or its biosimilar [114].
Glycosylation Patterns
Originator biologics and their biosimilars are often glycoproteins, which may be inherently immunogenic, whereas small molecules and their generics are only sometimes immunogenic, usually due to haptenating endogenous proteins [115]. There are no well-designed investigations into the clinical implications of glycosylation differences that may occur in production of biosimilars [41, 116]. The terminal sugars of glycans attached to immunoglobulin heavy chain have been shown to be critical for safety and/or efficacy of therapeutic monoclonal antibodies. For example, removal of fucose residues from these glycans can result in up to a 100-fold increase in affinity of antibodies to natural killer cells, which mediate antibody-dependent cellular cytotoxicity [117, 118]. In addition, a high degree of galactosylation has been shown to promote activation of complement in vitro [119]. Different patterns of glycosylation have been shown to influence the pharmacodynamic and pharmacokinetic behaviour of biologics, sometimes resulting in a reduction of the serum half-life of therapeutic antibodies [120]. Such changes have been used purposely as part of the product development process, such as when two extra glycosylation sites were engineered into recombinant human erythropoietin (EPO), resulting in a new, stand-alone product—darbepoetin alfa, with a threefold longer half-life—that could be administered less frequently [121]. A recent study has also demonstrated that the biological activity of EPO glycoforms varies depending on the glycosylation site occupancy pattern [122].
At present, no clinical studies have shown adverse effects of immunogenicity associated with therapeutics with high levels of non-glycosylated heavy chains; however, Fc glycan structural elements may be involved in adverse immune reactions. A high prevalence of hypersensitivity reactions to cetuximab, a monoclonal antibody approved for use in colorectal cancer and squamous-cell carcinoma of the head and neck, has been reported [123]. NeuGc, a non-human sialic acid that is found on some therapeutic proteins, can reduce efficacy due to rapid clearance of the biotherapeutic, and has been reported to cause hypersensitivity. In summary, production processes can change the glycosylation profiles of product biosimilars [41], which may potentially affect safety and efficacy. To date, the clinical implications of these changes are not well understood.
Immunogenicity Risk
If glycoproteins are sufficiently different from endogenous analogues, then patients may not exhibit immunological tolerance to them, thus rendering such glycoproteins immunogenic. In addition, variations can occur as a result of insertion of xenogeneic amino acid sequences when biologics are made in xenogeneic cells, which can enhance their immunogenicity [124, 125]. Even when fully humanised and glycosylated, therapeutic monoclonal antibodies can be immunogenic because they form immune complexes with target proteins, undergo phagocytosis and potentially activate inflammation [126]. Even within the human population, genes encoding the constant domains of immunoglobulins are highly polymorphic (known as allotypes), and each therapeutic is made from only one variant, which, therefore, may be recognised as non-self in individuals who lack that polymorphism [40, 41, 127–129]. Therefore, tolerance to an originator biologic does not necessarily predict immunological tolerance to its biosimilar and vice versa.
Significant effects of ADAs can be divided into those that neutralise the therapeutic action of the drug and/or its endogenous analogue and those that mediate hypersensitivity reactions to the drug. For example, EPO-induced ADA that neutralises endogenous EPO has been implicated in rare cases of pure red cell aplasia [130]. Unsurprisingly, there is also some evidence that ADAs arising against the reference product can also react to its biosimilars [114]. For example, in a retrospective analysis of 250 patients with arthritis who received either a reference biologic (infliximab) or a biosimilar, none of whom had undergone switching, ADAs against infliximab (detected in 126 patients) cross-reacted in in vitro antibody assays with either of two biosimilar products [114]. In the current review, no studies reported any treatment failure related to the development of ADAs. Comparative data on neutralising drug antibodies were reported in only three studies.
Regulatory Considerations
Ongoing post-marketing pharmacovigilance and accurate documentation of the specific product used by each patient are important. The current lack of standardisation of naming conventions for biosimilars poses several potential concerns. A unique name for the reference product and its biosimilar supports traceability in relation to pharmacovigilance and adverse event reporting [11, 131, 132]. Prescribers need to be able to distinguish between the reference product and its biosimilar to avoid prescribing errors. Naming conventions vary across regions, with the EU licensing biosimilars under the same international non-proprietary name (INN) as the originator and the FDA using a non-specific four-letter suffix (suggested by the sponsor) added to the non-proprietary name. The Australian TGA currently uses the locally approved name without any unique identifier for a biologic and its biosimilar, but is considering various options [11]; hence, at present, traceability may be difficult when multiple switches occur. A study on the traceability of biopharmaceuticals in spontaneous reporting systems over the period 2004–2010 found that, of six biosimilars approved in Europe at the time (sold under 12 different trade names), five contained the same INN as the innovator [133]. The product name was clearly identifiable for 90–96% of biopharmaceuticals for which biosimilars were available in the EU, although batch traceability was poorly maintained [133]. Given the evidence gaps and the difficulty of conducting randomised trials, particularly in the post-marketing setting, there is a need to establish or use pre-existing registries and administrative data, and to develop novel approaches to understanding the effectiveness data being generated by what are, in effect, natural, real-world experiments.
Conclusions
In summary, results from this systematic literature review show important evidence gaps around the safety of switching between originator biologics and biosimilars or between biosimilars. While emergent evidence from the EGALITY and NOR-SWITCH studies suggests that switching between TNF-α biologics and their corresponding biosimilars can be safe and efficacy is not compromised, we believe that sufficiently powered and appropriately statistically analysed clinical trials and pharmacovigilance studies, with long-term follow-ups and multiple switching sequences, are needed to support decision-making around biosimilar interchangeability. In the interim, switching should remain a clinical decision made by the treating physician and the patient based on available evidence and individual patient circumstances. Global harmonisation of the regulatory approach to these agents would facilitate an improved understanding of the safety and efficacy of biosimilars.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Funding
Administrative and medical writing support was provided by Ms Donna Bartlett (Allori Pty Ltd, Australia) and Dr. Anja Becher (Sydney, Australia), and was funded through Medicines Australia with specific support from the Australian affiliates of AbbVie, Amgen, Eli Lilly, Janssen, Novo Nordisk, Roche and Sanofi. The sponsors were given the opportunity to review the final version of the article for accuracy pertaining to sponsored products and compliance with individual processes; however, they did not influence the content. The authors were responsible for all content and editorial decisions and received no honoraria related to the development of this manuscript.
Compliance with Ethical Standards
Conflict of Interest
Ross McKinnon: Education and advisory service fees in relation to biosimilars for AbbVie and Sanofi Australia. Matthew Cook: Advisory board for Roche and Baxter. Winston Liauw: Advisory board for NPS MedicineWise. Mona Marabani: Consultancy, speaker fees or conference support from AbbVie, BMS, UCB, MSD, Pfizer, Janssen, Novartis and Sanofi. Ian Marschner: Research grant and consulting fees from Janssen-Cilag and consulting fees from Pfizer, AbbVie and Generic Health. Nicolle Packer reports having no conflicts of interest. John Prins holds clinical and scientific advisory board positions and/or has received speaking fees from Pfizer, Merck and Novo Nordisk.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s40259-017-0256-z) contains supplementary material, which is available to authorized users.
References
- 1.Reusch D, Tejada ML. Fc glycans of therapeutic antibodies as critical quality attributes. Glycobiology. 2015;25(12):1325–1334. doi: 10.1093/glycob/cwv065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramanan S, Grampp G. Drift, evolution, and divergence in biologics and biosimilars manufacturing. BioDrugs. 2014;28(4):363–372. doi: 10.1007/s40259-014-0088-z. [DOI] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration. Scientific considerations in demonstrating biosimilarity to a reference product: guidance for industry. 2015. http://www.fda.gov/downloads/DrugsGuidanceComplianceRegulatoryInformation/Guidances/UCM291128.pdf. Accessed 5 Sep 2016.
- 4.European Medicines Agency (EMA). Guideline on similar biological medicinal products. 2014. http://www.ema.europaeu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176768.pdf. Accessed 5 Sep 2015.
- 5.Australian Therapeutic Goods Administration (TGA). Regulation of biosimilar medicines (Version 2.0, December 2015). https://www.tga.gov.au/sites/default/files/evaluation-biosimilars-151217_0.pdf. Accessed 5 Sep 2016.
- 6.US Food and Drug Administration (FDA). Guidance for industry: immunogenicity assessment for therapeutic protein products. 2014. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm338856.pdf. Accessed 6 Sep 2016.
- 7.Australian Government Department of Health. Biosimilar medicines—factsheet for healthcare professionals. 2016. http://www.pbs.gov.au/publication/factsheets/biosimilars/biosimilars-factsheet-healthcare-professionals.pdf. Accessed 8 Sep 2016.
- 8.Australian Pharmaceutical Benefits Scheme (PBS) Pharmaceutical Benefit Advisory Committee (PBAC). PBAC special meeting minutes. 2015. http://www.pbs.gov.au/industry/listing/elements/pbac-meetings/pbac-outcomes/2015-04/2015-04-biosimilars.pdf. Accessed 8 Sep 2016.
- 9.US Food and Drug Administration (FDA). Biosimilars: questions and answers regarding implementation of the biologics price competition and innovation act 2009: guidance for industry. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm444661.pdf. Accessed 8 Sep 2016.
- 10.European Medicines Agency (EMA). Biosimilars in the EU: information guide for healthcare professionals. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/Leaflet/2017/05/WC500226648.pdf. Accessed 5 Oct 2017.
- 11.Australian Therapeutic Goods Administration (TGA). Consultation: nomenclature of biological medicines (Version 1.0, July 2017). https://www.tga.gov.au/sites/default/files/consultation-nomenclature-biological-medicines.pdf. Accessed 24 Oct 2017.
- 12.Papamichael K, Van Stappen T, Jairath V, Gecse K, Khanna R, D’Haens G, et al. Review article: pharmacological aspects of anti-TNF biosimilars in inflammatory bowel diseases. Aliment Pharmacol Ther. 2015;42(10):1158–1169. doi: 10.1111/apt.13402. [DOI] [PubMed] [Google Scholar]
- 13.Dörner T, Strand V, Cornes P, Goncalves J, Gulacsi L, Kay J, et al. The changing landscape of biosimilars in rheumatology. Ann Rheum Dis. 2016;75(6):974–982. doi: 10.1136/annrheumdis-2016-209166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinemann L, Home PD, Hompesch M. Biosimilar insulins: guidance for data interpretation by clinicians and users. Diabetes Obes Metab. 2015;17(10):911–918. doi: 10.1111/dom.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Commission. Consensus information paper: what you need to know about biosimilar medicinal products. 2013. http://ec.europa.eu/DocsRoom/documents/8242. Accessed 8 Sep 2016.
- 16.Declerck P, Endrenyi L, Chow SC. Interchangeability, switchability and substitution of biosimilar products. In: Endrenyi LDPJ, Chow SC, editors. Biosimilar drug product development. London and New York: CRC Press/Taylor & Francis; 2017. [Google Scholar]
- 17.Kurki P, van Aerts L, Wolff-Holz E, Giezen T, Skibeli V, Weise M. Interchangeability of biosimilars: a European perspective. BioDrugs. 2017;31(2):83–91. doi: 10.1007/s40259-017-0210-0. [DOI] [PubMed] [Google Scholar]
- 18.US Food and Drug Administration. Therapeutic biologic applications (BLA): information for industry (biosimilars). 2016. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/ucm241720.htm. Accessed 5 Sep 2016.
- 19.US Food and Drug Administration. Considerations in demonstrating interchangeability with a reference product: guidance for industry (draft). 2017. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM537135.pdf. Accessed 25 Jan 2017.
- 20.Allens Linklaters. Costs before caution—Australia’s unique approach to the interchangeability of biosimilars. 2016. http://www.allens.com.au/pubs/pdf/healthcare/BiosimilarsWhitePaper-CostsbeforeCaution.pdf. Accessed 8 Sep 2016.
- 21.Kay J, Isaacs JD. Clinical trials of biosimilars should become more similar. Ann Rheum Dis. 2017;76(1):4–6. doi: 10.1136/annrheumdis-2015-208113. [DOI] [PubMed] [Google Scholar]
- 22.American College of Rheumatology (ACR). Position statement: biosimilars. 2016. http://www.rheumatology.org/Portals/0/Files/Biosimilars-Position-Statement.pdf. Accessed 11 Aug 2016.
- 23.Australian Diabetes Society (ADS) and Diabetes Australia and Australian Diabetes Educators Association (ADEA). 2015. Position statement: use of ‘biosimilar’ insulins for diabetes. https://diabetessociety.com.au/documents/BiosimilarSubstitutionPositionStatementDAADSADEATBFV20150911.pdf. Accessed 5 Sep 2016.
- 24.American Society of Clinical Oncology. ASCO policy brief: biosimilars. 2015. https://www.asco.org/sites/new-wwwascoorg/files/content-files/advocacy-and-policy/documents/2013_biosilimars%20New.pdf. Accessed 5 Sep 2016.
- 25.National Rheumatoid Arthritis Society (NRAS). NRAS position paper on biosimilars—revised June 2016. http://www.nras.org.uk/data/files/About%20RA/How%20is%20RA%20managed/NRAS%20Biosimilars%20Position%20Paper%20Final.pdf. Accessed 5 Sep 2016.
- 26.American Academy of Dermatology. Position paper on generic therapeutic & biosimilar substitution. 2013. https://www.aad.org/Forms/Policies/Uploads/PS/PS-Generic%20Therapeutic%20and%20%20Biosimilar%20Substitution.pdf. Accessed 6 Oct 2016.
- 27.Tabernero J, Vyas M, Giuliani R, Arnold D, Cardoso F, Casali PG, et al. Biosimilars: a position paper of the European Society for Medical Oncology, with particular reference to oncology prescribers. ESMO Open. 2017;1(6):e000142. doi: 10.1136/esmoopen-2016-000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Annese V, Vecchi M. Use of biosimilars in inflammatory bowel disease: statements of the Italian Group for Inflammatory Bowel Disease. Dig Liver Dis. 2014;46(11):963–968. doi: 10.1016/j.dld.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 29.Fiorino G, Girolomoni G, Lapadula G, Orlando A, Danese S, Olivieri I. The use of biosimilars in immune-mediated disease: a joint Italian Society of Rheumatology (SIR), Italian Society of Dermatology (SIDeMaST), and Italian Group of Inflammatory Bowel Disease (IG-IBD) position paper. Autoimmun Rev. 2014;13(7):751–755. doi: 10.1016/j.autrev.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Abad Hernández MA, Andreu JL, Caracuel Ruiz MA, Belmonte Serrano MA, Diaz-Gonzalez F, Moreno Muelas JV. Position paper from the Spanish Society of Rheumatology on biosimilar drugs. Reumatol Clin. 2015;11(5):269–278. doi: 10.1016/j.reuma.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Klein AV, Wang J, Bedford P. Subsequent entry biologics (biosimilars) in Canada: approaches to interchangeability and the extrapolation of indications and uses. GaBi J. 2014;3(3):150–154. doi: 10.5639/gabij.2014.0303.033. [DOI] [Google Scholar]
- 32.Devlin SM, Bressler B, Bernstein CN, Fedorak RN, Bitton A, Singh H, et al. Overview of subsequent entry biologics for the management of inflammatory bowel disease and Canadian Association of Gastroenterology position statement on subsequent entry biologics. Can J Gastroenterol. 2013;27(10):567–571. doi: 10.1155/2013/327120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gastroenterological Society of Australia (GESA) and Australian Inflammatory Bowel Disease Association (AIBDA). GESA-AIBDA position on biosimilars substitution. 2015. http://www.gesa.org.au/files/editor_upload/File/NEWS/Position%20on%20Biosimilars%20GESA_AIBDA.pdf. Accessed 6 Oct 2016.
- 34.Arthritis Australia. Biosimilars and biologics: what you need to know. 2015. http://arthritisaustralia.com.au/indexphp/63-news/301-biosimilars-and-biologics-what-you-need-to-know.html. Accessed 6 Oct 2016.
- 35.Australian Rheumatology Association (ARA). ARA position on the introduction of biosimilars for the treatment of rheumatic diseases. 2016. http://rheumatology.org.au/rheumatologists/documents/20161124%20ARA%20position%20statement%20biosimilars%20Nov%2016%20final.pdf. Accessed 13 Dec 2016.
- 36.Danese S, Gomollon F. ECCO position statement: the use of biosimilar medicines in the treatment of inflammatory bowel disease (IBD) J Crohns Colitis. 2013;7(7):586–589. doi: 10.1016/j.crohns.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 37.European League Against Rheumatism (EULAR). Biosimilars: what do patients need to consider? 2015. http://www.eular.org/myUploadData/files/Biosimilars_2015.pdf. Accessed 5 Sep 2016.
- 38.Pharmaceutical Society of Australia. Biosimilar medicines: position statement. 2015. http://www.psa.org.au/downloads/ent/uploads/filebase/policies/biosimilar-medicines-position-statement.pdf. Accessed 6 Oct 2016.
- 39.The Pharmacy Guild of Australia. Policy: biosimilar medicines. 2015. https://www.guild.org.au/docs/default-source/public-documents/issues-and-resources/Policy-and-Position-Statements/20151026-guild-biosimilar-medicines.pdf?sfvrsn=0. Accessed 6 Oct 2016.
- 40.Hausberger A, Lamanna WC, Hartinger M, Seidl A, Toll H, Holzmann J. Identification of low-level product-related variants in filgrastim products presently available in highly regulated markets. BioDrugs. 2016;30(3):233–242. doi: 10.1007/s40259-016-0169-2. [DOI] [PubMed] [Google Scholar]
- 41.Mastrangeli R, Satwekar A, Cutillo F, Ciampolillo C, Palinsky W, Longobardi S. In-vivo biological activity and glycosylation analysis of a biosimilar recombinant human follicle-stimulating hormone product (Bemfola) compared with its reference medicinal product (GONAL-f) PLoS One. 2017;12(9):e0184139. doi: 10.1371/journal.pone.0184139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bracewell DG, Francis R, Smales CM. The future of host cell protein (HCP) identification during process development and manufacturing linked to a risk-based management for their control. Biotechnol Bioeng. 2015;112(9):1727–1737. doi: 10.1002/bit.25628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdalla A, Byrne N, Conway R, Walsh T, Mannion G, Hanly M, et al. Long-term safety and efficacy of biosimilar infliximab among patients with inflammatory arthritis switched from reference product. Open Access Rheumatol. 2017;9:29–35. doi: 10.2147/OARRR.S124975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ala K, Avery P, Wilson R, Prowse A, Shutt J, Jupp J, et al. Early experience with biosimilar infliximab at a district general hospital for an entire Crohns disease patient cohort switch from Remicade to Inflectra. Gut. 2016;65:A81. doi: 10.1136/gutjnl-2016-312388.145. [DOI] [Google Scholar]
- 45.Arguelles-Arias F, Guerra Veloz MF, Perea Amarillo R, Vilches-Arenas A, Castro Laria L, Maldonado Perez B, et al. Effectiveness and safety of CT-P13 (biosimilar infliximab) in patients with inflammatory bowel disease in real life at 6 months. Dig Dis Sci. 2017;62(5):1305–1312. doi: 10.1007/s10620-017-4511-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bennett KJ, Heap GA, Hawkins S, Ahmad T. Prospective evaluation of the safety and efficacy of switching stable patients with inflammatory bowel disease from Remicade™ to biosimilar infliximab (IFX) Gut. 2016;65:A146. doi: 10.1136/gutjnl-2016-312388.263. [DOI] [Google Scholar]
- 47.Benucci M, Gobbi FL, Bandinelli F, Damiani A, Infantino M, Grossi V, et al. Safety, efficacy and immunogenicity of switching from innovator to biosimilar infliximab in patients with spondyloarthritis: a 6-month real-life observational study. Immunol Res. 2017;65(1):419–422. doi: 10.1007/s12026-016-8843-5. [DOI] [PubMed] [Google Scholar]
- 48.Buer LC, Moum BA, Cvancarova M, Warren DJ, Medhus AW, Hoivik ML. Switching from Remicade® to Remsima® is well tolerated and feasible: a prospective, open-label study. J Crohns Colitis. 2017;11(3):297–304. doi: 10.1093/ecco-jcc/jjw166. [DOI] [PubMed] [Google Scholar]
- 49.Dapavo P, Vujic I, Fierro MT, Quaglino P, Sanlorenzo M. The infliximab biosimilar in the treatment of moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75(4):736–739. doi: 10.1016/j.jaad.2016.04.068. [DOI] [PubMed] [Google Scholar]
- 50.Fiorino G, Manetti N, Armuzzi A, Orlando A, Variola A, Bonovas S, et al. The PROSIT-BIO cohort: a prospective observational study of patients with inflammatory bowel disease treated with infliximab biosimilar. Inflamm Bowel Dis. 2017;23(2):233–243. doi: 10.1097/MIB.0000000000000995. [DOI] [PubMed] [Google Scholar]
- 51.Gentileschi S, Barreca C, Bellisai F, Biasi G, Brizi MG, De Stefano R, et al. Switch from infliximab to infliximab biosimilar: efficacy and safety in a cohort of patients with different rheumatic diseases. Response to: Nikiphorou E, Kautiainen H, Hannonen P, et al. Clinical effectiveness of CT-P13 (infliximab biosimilar) used as a switch from Remicade (infliximab) in patients with established rheumatic disease. Report of clinical experience based on prospective observational data. Expert Opin Biol Ther. 2015;15:1677–1683. Expert Opin Biol Ther. 2016:1–2.
- 52.Glintborg B, Sorensen IJ, Loft AG, Lindegaard H, Linauskas A, Hendricks O, et al. A nationwide non-medical switch from originator infliximab to biosimilar CT-P13 in 802 patients with inflammatory arthritis: 1-year clinical outcomes from the DANBIO registry. Ann Rheum Dis. 2017;76:1426–1431. doi: 10.1136/annrheumdis-2016-210742. [DOI] [PubMed] [Google Scholar]
- 53.Jorgensen KK, Olsen IC, Goll GL, Lorentzen M, Bolstad N, Haavardsholm EA, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet. 2017;389(10086):2304–2316. doi: 10.1016/S0140-6736(17)30068-5. [DOI] [PubMed] [Google Scholar]
- 54.Jung YS, Park DI, Kim YH, Lee JH, Seo PJ, Cheon JH, et al. Efficacy and safety of CT-P13, a biosimilar of infliximab, in patients with inflammatory bowel disease: a retrospective multicenter study. J Gastroenterol Hepatol. 2015;30(12):1705–1712. doi: 10.1111/jgh.12997. [DOI] [PubMed] [Google Scholar]
- 55.Kay J, Chopra A, Lassen C, Shneyer L, Wyand M. BOW015, a biosimilar infliximab: disease activity and disability outcomes from a phase 3 active comparator study in patients with active rheumatoid arthritis on stable methotrexate doses. Ann Rheum Dis. 2015;74(Suppl 2):462–463. doi: 10.1136/annrheumdis-2015-eular.6727. [DOI] [Google Scholar]
- 56.Kay J, Lassen C, Trokan L, Wyand M. Safety profile of BOW015, a biosimilar infliximab, in healthy subjects and patients with active rheumatoid arthritis. Ann Rheum Dis. 2015;74(Suppl 2):706. [Google Scholar]
- 57.Kolar M, Duricova D, Bortlik M, Hruba V, Machkova N, Mitrova K, et al. Infliximab biosimilar (Remsima) in therapy of inflammatory bowel diseases patients: experience from one tertiary inflammatory bowel diseases centre. Dig Dis. 2017;35(1–2):91–100. doi: 10.1159/000453343. [DOI] [PubMed] [Google Scholar]
- 58.Nikiphorou E, Kautiainen H, Hannonen P, Asikainen J, Kokko A, Rannio T, et al. Clinical effectiveness of CT-P13 (infliximab biosimilar) used as a switch from Remicade (infliximab) in patients with established rheumatic disease. Report of clinical experience based on prospective observational data. Expert Opin Biol Ther. 2015;15(12):1677–1683. doi: 10.1517/14712598.2015.1103733. [DOI] [PubMed] [Google Scholar]
- 59.Park SH, Kim YH, Lee JH, Kwon HJ, Lee SH, Park DI, et al. Post-marketing study of biosimilar infliximab (CT-P13) to evaluate its safety and efficacy in Korea. Expert Rev Gastroenterol Hepatol. 2015;9(Suppl 1):35–44. doi: 10.1586/17474124.2015.1091309. [DOI] [PubMed] [Google Scholar]
- 60.Park W, Suh CH, Shim SC, Molina FFC, Jeka S, Medina-Rodriguez FG, et al. Efficacy and safety of switching from innovator rituximab to biosimilar CT-P10 compared with continued treatment with CT-P10: results of a 56-week open-label study in patients with rheumatoid arthritis. BioDrugs. 2017;31(4):369–77. doi: 10.1007/s40259-017-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park W, Yoo DH, Miranda P, Brzosko M, Wiland P, Gutierrez-Urena S, et al. Efficacy and safety of switching from reference infliximab to CT-P13 compared with maintenance of CT-P13 in ankylosing spondylitis: 102-week data from the PLANETAS extension study. Ann Rheum Dis. 2017;76:346–354. doi: 10.1136/annrheumdis-2015-208783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rahmany S, Cotton S, Garnish S, Brown M, Saich R, Lloyd D, et al. In patients with IBD switching from originator infliximab (Remicade) to biosimilar infliximab (CT-P13) is safe and effective. Gut. 2016;65:A89. [Google Scholar]
- 63.Razanskaite V, Bettey M, Downey L, Wright J, Callaghan J, Rush M, et al. Biosimilar infliximab in inflammatory bowel disease: outcomes of a managed switching programme. J Crohns Colitis. 2017;11(6):690–696. doi: 10.1093/ecco-jcc/jjw216. [DOI] [PubMed] [Google Scholar]
- 64.Rubio E, Ruiz A, Lopez J, Lopez-Chozas JM, Bermudez L, Aguilera C, et al. Prospective study of 78 patients treated with infliximab biosimilar Remsima®. Ann Rheum Dis. 2016;75(Suppl 2):1006. doi: 10.1136/annrheumdis-2016-eular.5688. [DOI] [Google Scholar]
- 65.Sheppard M, Hadavi S, Hayes F, Kent J, Dasgupta B. Preliminary data on the introduction of the infliximab biosimilar (CT-P13) to a real world cohort of rheumatology patients. Ann Rheum Dis. 2016;75(Suppl 2):1011. doi: 10.1136/annrheumdis-2016-eular.5252. [DOI] [Google Scholar]
- 66.Sieczkowska J, Jarzebicka D, Banaszkiewicz A, Plocek A, Gawronska A, Toporowska-Kowalska E, et al. Switching between infliximab originator and biosimilar in paediatric patients with inflammatory bowel disease. Preliminary observations. J Crohns Colitis. 2016;10(2):127–132. doi: 10.1093/ecco-jcc/jjv233. [DOI] [PubMed] [Google Scholar]
- 67.Schmitz EMH, Benoy-De Keuster S, Meier AJL, Scharnhorst V, Traksel RAM, Broeren MAC, et al. Therapeutic drug monitoring (TDM) as a tool in the switch from infliximab innovator to biosimilar in rheumatic patients: results of a 12-month observational prospective cohort study. Clin Rheumatol. 2017;36(9):2129–34. doi: 10.1007/s10067-017-3686-6. [DOI] [PubMed] [Google Scholar]
- 68.Smits LJ, Derikx LA, de Jong DJ, Boshuizen RS, van Esch AA, Drenth JP, et al. Clinical outcomes following a switch from Remicade® to the biosimilar CT-P13 in inflammatory bowel disease patients: a prospective observational cohort study. J Crohns Colitis. 2016;10:1287–1293. doi: 10.1093/ecco-jcc/jjw087. [DOI] [PubMed] [Google Scholar]
- 69.Smolen JS, Choe J-Y, Prodanovic N, Niebrzydowski J, Staykov I, Dokoupilova E, et al. Comparable safety and immunogenicity and sustained efficacy after transition to SB2 (an infliximab biosimilar) vs ongoing infliximab reference product in patients with rheumatoid arthritis: results of phase III transition study. Ann Rheum Dis. 2016;75(Suppl 2):488. [Google Scholar]
- 70.Tanaka Y, Yamanaka H, Takeuchi T, Inoue M, Saito K, Saeki Y, et al. Safety and efficacy of CT-P13 in Japanese patients with rheumatoid arthritis in an extension phase or after switching from infliximab. Mod Rheumatol. 2017;27(2):237–245. doi: 10.1080/14397595.2016.1206244. [DOI] [PubMed] [Google Scholar]
- 71.Tweehuysen L, Van Den Bemt BJF, Van Ingen IL, De Jong AJL, Van Der Laan WH, Van Den Hoogen FHJ, et al. Clinical and immunogenicity outcomes after switching treatment from innovator infliximab to biosimilar infliximab in rheumatic diseases in daily clinical practice. Arthritis Rheumatol. 2016;68:821–823. [Google Scholar]
- 72.Yazici Y, Xie L, Ogbomo A, Parenti D, Goyal K, Teeple A, et al. A descriptive analysis of real-world treatment patterns in a Turkish rheumatology population that continued innovator infliximab (Remicade) therapy or switched to biosimilar infliximab. Arthritis Rheumatol. 2016;68:2903–2906. [Google Scholar]
- 73.Yoo DH, Prodanovic N, Jaworski J, Miranda P, Ramiterre E, Lanzon A, et al. Efficacy and safety of CT-P13 (biosimilar infliximab) in patients with rheumatoid arthritis: comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study. Ann Rheum Dis. 2017;76:355–363. doi: 10.1136/annrheumdis-2015-208786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haag-Weber M, Vetter A, Thyroff-Friesinger U. Therapeutic equivalence, long-term efficacy and safety of HX575 in the treatment of anemia in chronic renal failure patients receiving hemodialysis. Clin Nephrol. 2009;72(5):380–390. [PubMed] [Google Scholar]
- 75.Harzallah A, Zouaghi K, Dridi A, Boubaker K, Beji S, Ayari M, et al. Therapeutic efficacy of a biosimilar epoetin alfa in hemodialysis patients. Saudi J Kidney Dis Transpl. 2015;26(1):78–82. doi: 10.4103/1319-2442.148744. [DOI] [PubMed] [Google Scholar]
- 76.Hörbrand F, Bramlage P, Fischaleck J, Hasford J, Brunkhorst R. A population-based study comparing biosimilar versus originator erythropoiesis-stimulating agent consumption in 6,117 patients with renal anaemia. Eur J Clin Pharmacol. 2013;69(4):929–936. doi: 10.1007/s00228-012-1412-5. [DOI] [PubMed] [Google Scholar]
- 77.Lopez MJ, del García Busto N, Carrascosa O, Mejía L, Antonino G, De La Vega I, et al. Biosimilar epoetin zeta in the treatment of chemotherapy-induced anaemia. Eur J Hosp Pharm. 2014;21:A38–A39. doi: 10.1136/ejhpharm-2013-000436.94. [DOI] [Google Scholar]
- 78.Minutolo R, Borzumati M, Sposini S, Abaterusso C, Carraro G, Santoboni A, et al. Dosing penalty of erythropoiesis-stimulating agents after switching from originator to biosimilar preparations in stable hemodialysis patients. Am J Kidney Dis. 2016;68(1):170–172. doi: 10.1053/j.ajkd.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 79.Morosetti M, Dominijanni S, Calzona AB, Zappalà L, Nicolais R, Di Turi R. Switch to biosimilars in hemodialysis patients: efficacy, safety and cost analysis in a single centre. Ricerca e Pratica. 2017;33(1):5–12. [Google Scholar]
- 80.Ohta S, Yasuno N, Inomoto Y, Matsuda K, Nakagawa Y, Sasagawa I, et al. Efficacy of once or twice weekly administration of epoetin kappa in patients receiving hemodialysis: a retrospective study. Exp Ther Med. 2014;7(1):27–30. doi: 10.3892/etm.2013.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wiecek A, Ahmed I, Scigalla P, Koytchev R. Switching epoetin alfa and epoetin zeta in patients with renal anemia on dialysis: posthoc analysis. Adv Ther. 2010;27(12):941–952. doi: 10.1007/s12325-010-0080-z. [DOI] [PubMed] [Google Scholar]
- 82.Wizemann V, Rutkowski B, Baldamus C, Scigalla P, Koytchev R. Comparison of the therapeutic effects of epoetin zeta to epoetin alfa in the maintenance phase of renal anaemia treatment. Curr Med Res Opin. 2008;24(3):625–637. doi: 10.1185/030079908X273264. [DOI] [PubMed] [Google Scholar]
- 83.Balili CAV, Mendoza ESR, Mercado-Asis LB. Shifting to biosimilar insulin preparation: impact on glycemic control and cost. Endocrine Reviews. 2015;36:abstract THR-659.
- 84.Hadjiyianni I, Dahl D, Lacaya LB, Pollom RK, Chang CL, Ilag LL. Efficacy and safety of LY2963016 insulin glargine in patients with type 1 and type 2 diabetes previously treated with insulin glargine. Diabetes Obes Metab. 2016;18(4):425–429. doi: 10.1111/dom.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ilag LL, Deeg MA, Costigan T, Hollander P, Blevins TC, Edelman SV, et al. Evaluation of immunogenicity of LY2963016 insulin glargine compared with Lantus(R) insulin glargine in patients with type 1 or type 2 diabetes mellitus. Diabetes Obes Metab. 2016;18(2):159–168. doi: 10.1111/dom.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosenstock J, Hollander P, Bhargava A, Ilag LL, Pollom RK, Zielonka JS, et al. Similar efficacy and safety of LY2963016 insulin glargine and insulin glargine (Lantus(R)) in patients with type 2 diabetes who were insulin-naive or previously treated with insulin glargine: a randomized, double-blind controlled trial (the ELEMENT 2 study) Diabetes Obes Metab. 2015;17(8):734–741. doi: 10.1111/dom.12482. [DOI] [PubMed] [Google Scholar]
- 87.Segal D, Tupy D, Distiller L. The Biosulin equivalence in standard therapy (BEST) study—a multicentre, open-label, non-randomised, interventional, observational study in subjects using Biosulin 30/70 for the treatment of insulin-dependent type 1 and type 2 diabetes mellitus. S Afr Med J. 2013;103(7):458–460. doi: 10.7196/SAMJ.6345. [DOI] [PubMed] [Google Scholar]
- 88.Flodmark CE, Lilja K, Woehling H, Jarvholm K. Switching from originator to biosimilar human growth hormone using dialogue teamwork: single-center experience from Sweden. Biol Ther. 2013;3:35–43. doi: 10.1007/s13554-013-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gila AG, Garcia MP. Switching from the original to the biosimilar recombinant human GH-Omnitrope®: an experience of a single paediatric centre in Spain. Horm Res Paediatr. 2014;82:414. [Google Scholar]
- 90.Rashid N, Saenger P, Wu YL, Woehling H, Frankel M, Lifshitz F, et al. Switching to Omnitrope® from other recombinant human growth hormone therapies: a retrospective study in an integrated healthcare system. Biol Ther. 2014;4(1–2):27–39. doi: 10.1007/s13554-014-0017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Belleli R, Fisch R, Renard D, Woehling H, Gsteiger S. Assessing switchability for biosimilar products: modelling approaches applied to children’s growth. Pharm Stat. 2015;14(4):341–349. doi: 10.1002/pst.1691. [DOI] [PubMed] [Google Scholar]
- 92.Romer T, Zabransky M, Walczak M, Szalecki M, Balser S. Effect of switching recombinant human growth hormone: comparative analysis of phase 3 clinical data. Biol Ther. 2011;1:5. doi: 10.1007/s13554-011-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Engert A, Griskevicius L, Zyuzgin Y, Lubenau H, del Giglio A. XM02, the first granulocyte colony-stimulating factor biosimilar, is safe and effective in reducing the duration of severe neutropenia and incidence of febrile neutropenia in patients with non-Hodgkin lymphoma receiving chemotherapy. Leuk Lymphoma. 2009;50(3):374–379. doi: 10.1080/10428190902756081. [DOI] [PubMed] [Google Scholar]
- 94.Gatzemeier U, Ciuleanu T, Dediu M, Ganea-Motan E, Lubenau H, Del Giglio A. XM02, the first biosimilar G-CSF, is safe and effective in reducing the duration of severe neutropenia and incidence of febrile neutropenia in patients with small cell or non-small cell lung cancer receiving platinum-based chemotherapy. J Thorac Oncol. 2009;4(6):736–740. doi: 10.1097/JTO.0b013e3181a52964. [DOI] [PubMed] [Google Scholar]
- 95.Krendyukov A, Harbeck N, Gascon P, Gattu S, Li Y, Blackwell KL. Safety and efficacy of alternating treatment with EP2006, a filgrastim biosimilar, and reference filgrastim for the prevention of severe neutropenia, in patients with breast cancer receiving myelosuppressive chemotherapy. J Clin Oncol. 2017;35:abstr10116. doi: 10.1093/annonc/mdx638. [DOI] [PubMed] [Google Scholar]
- 96.Gooderham M, Spelman L, Kaliaperumal A, Costanzo A, Narbutt J, Zhang N, et al. Single transition from adalimumab to ABP 501: evaluation of immunogenicity in a phase 3 study in subjects with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;74(5):AB275. [Google Scholar]
- 97.Papp K, Bachelez H, Costanzo A, Foley P, Gooderham M, Kaur P, et al. Clinical similarity of biosimilar ABP 501 to adalimumab in the treatment of patients with moderate to severe plaque psoriasis: a randomized, double-blind, multicenter, phase III study. J Am Acad Dermatol. 2017;76(6):1093–1102. doi: 10.1016/j.jaad.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 98.Weinblatt M, Baranauskaite A, Niebrzydowski J, Dokoupilova E, Zielinska A, Sitek-Ziolkowska K, et al. Sustained efficacy and comparable safety and immunogenicity after transition to SB5 (an adalimumab biosimilar) vs continuation of the adalimumab reference product in patients with rheumatoid arthritis: results of phase III study EUCLAR 2016; 8–11 June 2016; London UK (abstract FRI0161). Arthritis Rheumatol. 2016;68(suppl 10):abstract 604.
- 99.Nasonov E, Mazurov V, Plaksina T, Nesmeyanova O, Knyazeva L, Eremeeva A, et al. Interchangeability of innovator rituximab and its biosimilar: results from international controlled comparative 1-year study in patients with active rheumatoid arthritis. Arthritis Rheumatol. 2016;68:2046–2047. [Google Scholar]
- 100.Roy PS, John S, Karankal S, Kannan S, Pawaskar P, Gawande J, et al. Comparison of the efficacy and safety of rituximab (Mabthera) and its biosimilar (Reditux) in diffuse large B-cell lymphoma patients treated with chemo-immunotherapy: a retrospective analysis. Indian J Med Paediatr Oncol. 2013;34(4):292–298. doi: 10.4103/0971-5851.125238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Emery P, Vencovský J, Sylwestrzak A, Leszczyñski P, Porawska W, Stasiuk B, et al. Additional efficacy results of SB4 (etanercept biosimilar) up to week 100: comparison between continuing SB4 and switching from reference etanercept (Enbrel®) to SB4. Arthritis Rheumatol. 2016;68(suppl 10):789–790. [Google Scholar]
- 102.Emery P, Vencovsky J, Sylwestrzak P, Leszczynski P, Porawska W, Stasiuk B, et al. Long-term safety and efficacy of SB4 (etanercept biosimilar) in patients with rheumatoid arthritis: comparison between continuing SB4 and switching from etanercept reference product to SB4. Ann Rheum Dis. 2016;75(Suppl 2):236. doi: 10.1136/annrheumdis-2017-211591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Griffiths CEM, Thaci D, Gerdes S, Arenberger P, Pulka G, Kingo K, et al. The EGALITY study: a confirmatory, randomized, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, vs. the originator product in patients with moderate-to-severe chronic plaque-type psoriasis. Br J Dermatol. 2017;176(4):928–938. doi: 10.1111/bjd.15152. [DOI] [PubMed] [Google Scholar]
- 104.Strowitzki T, Kuczynski W, Mueller A, Bias P. Safety and efficacy of Ovaleap® (recombinant human follicle-stimulating hormone) for up to 3 cycles in infertile women using assisted reproductive technology: a phase 3 open-label follow-up to main study. Reprod Biol Endocrinol. 2016;14(1):31. doi: 10.1186/s12958-016-0164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Glintborg B, Kringelbach T, Hogdall E, Sorensen IJ, Jensen DV, Loft AG, et al. Non-medical switch from originator to biosimilar infliximab among patients with inflammatory rheumatic disease—impact on S-infliximab and antidrug-antibodies. Results from the National Danish Rheumatologic Biobank and the Danbio Registry. Ann Rheum Dis. 2016;75(Suppl 2):224. [Google Scholar]
- 106.Bartelds GM, Krieckaert CL, Nurmohamed MT, van Schouwenburg PA, Lems WF, Twisk JW, et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA. 2011;305(14):1460–1468. doi: 10.1001/jama.2011.406. [DOI] [PubMed] [Google Scholar]
- 107.Pascual-Salcedo D, Plasencia C, Ramiro S, Nuno L, Bonilla G, Nagore D, et al. Influence of immunogenicity on the efficacy of long-term treatment with infliximab in rheumatoid arthritis. Rheumatology (Oxford). 2011;50(8):1445–1452. doi: 10.1093/rheumatology/ker124. [DOI] [PubMed] [Google Scholar]
- 108.Thomas SS, Borazan N, Barroso N, Duan L, Taroumian S, Kretzmann B, et al. Comparative immunogenicity of TNF inhibitors: impact on clinical efficacy and tolerability in the management of autoimmune diseases. A systematic review and meta-analysis. BioDrugs. 2015;29(4):241–258. doi: 10.1007/s40259-015-0134-5. [DOI] [PubMed] [Google Scholar]
- 109.Dowlat HA, Kuhlmann MK, Khatami H, Ampudia-Blasco FJ. Interchangeability among reference insulin analogues and their biosimilars: regulatory framework, study design and clinical implications. Diabetes Obes Metab. 2016;18(8):737–746. doi: 10.1111/dom.12676. [DOI] [PubMed] [Google Scholar]
- 110.Goll GL, Olsen IC, Jorgensen KK, Lorentzen M, Bolstad N, Haavardsholm EA, et al. Biosimilar infliximab (CT-P13) is not inferior to originator infliximab: results from a 52-week randomized switch trial in Norway Arthritis Rheumatol. 2016;68 (suppl 10) abstract 19L.
- 111.Gautam A. Market watch: strategies for biosimilars in emerging markets. Nat Rev Drug Discov. 2017;16(8):520–521. doi: 10.1038/nrd.2017.113. [DOI] [PubMed] [Google Scholar]
- 112.Marciano I, Ingrasciotta Y, Giorgianni F, Bolcato J, Chinellato A, Pirolo R, et al. How did the introduction of biosimilar filgrastim influence the prescribing pattern of granulocyte colony-stimulating factors? Results from a multicentre, population-based study, from five Italian centres in the years 2009–2014. BioDrugs. 2016;30(4):295–306. doi: 10.1007/s40259-016-0175-4. [DOI] [PubMed] [Google Scholar]
- 113.Ingrasciotta Y, Giorgianni F, Bolcato J, Chinellato A, Pirolo R, Tari DU, et al. How much are biosimilars used in clinical practice? A retrospective Italian population-based study of erythropoiesis-stimulating agents in the years 2009–2013. BioDrugs. 2015;29(4):275–284. doi: 10.1007/s40259-015-0132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ruiz-Arguello MB, Maguregui A, Ruiz Del Agua A, Pascual-Salcedo D, Martinez-Feito A, Jurado T, et al. Antibodies to infliximab in Remicade-treated rheumatic patients show identical reactivity towards biosimilars. Ann Rheum Dis. 2016;75(9):1693–1696. doi: 10.1136/annrheumdis-2015-208684. [DOI] [PubMed] [Google Scholar]
- 115.Li E, Ramanan S, Green L. Pharmacist substitution of biological products: issues and considerations. J Manag Care Spec Pharm. 2015;21(7):532–539. doi: 10.18553/jmcp.2015.21.7.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.DePalma A. Glycosylation affirmed as quality metric. GEN Select. 2014;34(13):5263. [Google Scholar]
- 117.Chung S, Quarmby V, Gao X, Ying Y, Lin L, Reed C, et al. Quantitative evaluation of fucose reducing effects in a humanized antibody on Fcgamma receptor binding and antibody-dependent cell-mediated cytotoxicity activities. MAbs. 2012;4(3):326–340. doi: 10.4161/mabs.19941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kanda Y, Yamada T, Mori K, Okazaki A, Inoue M, Kitajima-Miyama K, et al. Comparison of biological activity among nonfucosylated therapeutic IgG1 antibodies with three different N-linked Fc oligosaccharides: the high-mannose, hybrid, and complex types. Glycobiology. 2007;17(1):104–118. doi: 10.1093/glycob/cwl057. [DOI] [PubMed] [Google Scholar]
- 119.Houde D, Peng Y, Berkowitz SA, Engen JR. Post-translational modifications differentially affect IgG1 conformation and receptor binding. Mol Cell Proteomics. 2010;9(8):1716–1728. doi: 10.1074/mcp.M900540-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yu M, Brown D, Reed C, Chung S, Lutman J, Stefanich E, et al. Production, characterization, and pharmacokinetic properties of antibodies with N-linked mannose-5 glycans. MAbs. 2012;4(4):475–487. doi: 10.4161/mabs.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Egrie JC, Browne JK. Development and characterization of novel erythropoiesis stimulating protein (NESP) Nephrol Dial Transplant. 2001;16(Suppl 3):3–13. doi: 10.1093/ndt/16.suppl_3.3. [DOI] [PubMed] [Google Scholar]
- 122.Murakami M, Kiuchi T, Nishihara M, Tezuka K, Okamoto R, Izumi M, et al. Chemical synthesis of erythropoietin glycoforms for insights into the relationship between glycosylation pattern and bioactivity. Sci Adv. 2016;2(1):e1500678. doi: 10.1126/sciadv.1500678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11):1109–1117. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Burska AN, Hunt L, Boissinot M, Strollo R, Ryan BJ, Vital E, et al. Autoantibodies to posttranslational modifications in rheumatoid arthritis. Mediators Inflamm. 2014;492873(10):492873. doi: 10.1155/2014/492873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhong X, Wright JF. Biological insights into therapeutic protein modifications throughout trafficking and their biopharmaceutical applications. Int J Cell Biol. 2013;273086(10):273086. doi: 10.1155/2013/273086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vincent FB, Morand EF, Murphy K, Mackay F, Mariette X, Marcelli C. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis. 2013;72(2):165–178. doi: 10.1136/annrheumdis-2012-202545. [DOI] [PubMed] [Google Scholar]
- 127.Beck A, Reichert JM. Approval of the first biosimilar antibodies in Europe: a major landmark for the biopharmaceutical industry. MAbs. 2013;5(5):621–623. doi: 10.4161/mabs.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Magnenat L, Palmese A, Fremaux C, D’Amici F, Terlizzese M, Rossi M, et al. Demonstration of physicochemical and functional similarity between the proposed biosimilar adalimumab MSB11022 and Humira(R) MAbs. 2017;9(1):127–139. doi: 10.1080/19420862.2016.1259046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jefferis R, Lefranc MP. Human immunoglobulin allotypes: possible implications for immunogenicity. MAbs. 2009;1(4):332–338. doi: 10.4161/mabs.1.4.9122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Locatelli F, Del Vecchio L, Pozzoni P. Pure red-cell aplasia “epidemic”—mystery completely revealed? Perit Dial Int. 2007;27(2):S303–S307. [PubMed] [Google Scholar]
- 131.US Food and Drug Administration. Nonproprietary naming of biological products: guidance for industry. 2017. https://www.fda.gov/downloads/drugs/guidances/ucm459987.pdf. Accessed 17 Oct 2017.
- 132.Grampp G, Felix T. Pharmacovigilance considerations for biosimilars in the USA. BioDrugs. 2015;29(5):309–321. doi: 10.1007/s40259-015-0137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vermeer NS, Straus SM, Mantel-Teeuwisse AK, Domergue F, Egberts TC, Leufkens HG, et al. Traceability of biopharmaceuticals in spontaneous reporting systems: a cross-sectional study in the FDA Adverse Event Reporting System (FAERS) and EudraVigilance databases. Drug Saf. 2013;36(8):617–625. doi: 10.1007/s40264-013-0073-3. [DOI] [PubMed] [Google Scholar]
- 134.Ekelund M, Bidad C, Gomez R. To the editor; a commentary on “Switching from originator to biosimilar human growth hormone using a dialogue teamwork: single-center experience from Sweden”. Biol Ther. 2014;4(1–2):69–71. doi: 10.1007/s13554-014-0015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.