Abstract
Background
ALZ-801 is an orally available, valine-conjugated prodrug of tramiprosate. Tramiprosate, the active agent, is a small-molecule β-amyloid (Aβ) anti-oligomer and aggregation inhibitor that was evaluated extensively in preclinical and clinical investigations for the treatment of Alzheimer’s disease (AD). Tramiprosate has been found to inhibit β-amyloid oligomer formation by a multi-ligand enveloping mechanism of action that stabilizes Aβ42 monomers, resulting in the inhibition of formation of oligomers and subsequent aggregation. Although promising as an AD treatment, tramiprosate exhibited two limiting deficiencies: high intersubject pharmacokinetic (PK) variability likely due to extensive gastrointestinal metabolism, and mild-to-moderate incidence of nausea and vomiting. To address these, we developed an optimized prodrug, ALZ-801, which retains the favorable efficacy attributes of tramiprosate while improving oral PK variability and gastrointestinal tolerability. In this study, we summarize the phase I bridging program to evaluate the safety, tolerability and PK for ALZ-801 after single and multiple rising dose administration in healthy volunteers.
Methods
Randomized, placebo-controlled, phase I studies in 127 healthy male and female adult and elderly volunteers included [1] a single ascending dose (SAD) study; [2] a 14-day multiple ascending dose (MAD) study; and [3] a single-dose tablet food-effect study. This program was conducted with both a loose-filled capsule and an immediate-release tablet formulation, under both fasted and fed conditions. Safety and tolerability were assessed, and plasma and urine were collected for liquid chromatography-mass spectrometry (LC-MS) determination and non-compartmental PK analysis. In addition, we defined the target dose of ALZ-801 that delivers a steady-state plasma area under the curve (AUC) exposure of tramiprosate equivalent to that studied in the tramiprosate phase III study.
Results
ALZ-801 was well tolerated and there were no severe or serious adverse events (AEs) or laboratory findings. The most common AEs were transient mild nausea and some instances of vomiting, which were not dose-related and showed development of tolerance after continued use. ALZ-801 produced dose-dependent maximum plasma concentration (C max) and AUC exposures of tramiprosate, which were equivalent to that after oral tramiprosate, but with a substantially reduced intersubject variability and a longer elimination half-life. Administration of ALZ-801 with food markedly reduced the incidence of gastrointestinal symptoms compared with the fasted state, without affecting plasma tramiprosate exposure. An immediate-release tablet formulation of ALZ-801 displayed plasma exposure and low variability similar to the loose-filled capsule. ALZ-801 also showed excellent dose-proportionality without accumulation or decrease in plasma exposure of tramiprosate over 14 days. Based on these data, 265 mg of ALZ-801 twice daily was found to achieve a steady-state AUC exposure of tramiprosate equivalent to 150 mg twice daily of oral tramiprosate in the previous phase III trials.
Conclusions
ALZ-801, when administered in capsule and tablet forms, showed excellent oral safety and tolerability in healthy adults and elderly volunteers, with significantly improved PK characteristics over oral tramiprosate. A clinical dose of ALZ-801 (265 mg twice daily) was established that achieves the AUC exposure of 150 mg of tramiprosate twice daily, which showed positive cognitive and functional improvements in apolipoprotein E4/4 homozygous AD patients. These bridging data support the phase III development of ALZ-801in patients with AD.
Electronic supplementary material
The online version of this article (doi:10.1007/s40262-017-0608-3) contains supplementary material, which is available to authorized users.
Key Points
| We summarize the phase I bridging program to evaluate the safety, tolerability and pharmacokinetics (PK) of ALZ-801 in healthy volunteers. |
| ALZ-801 is an orally available, valine-conjugated prodrug of tramiprosate with substantially improved PK properties and gastrointestinal tolerability compared with the parent compound. |
| Oral ALZ-801 represents an advanced and markedly improved clinical candidate for the treatment of Alzheimer’s disease. |
Introduction
Alzheimer’s disease (AD) remains an enormous healthcare challenge, with limited therapeutic options other than symptomatic management with acetylcholinesterase inhibitors (AChEIs; e.g. donepezil) and the N-methyl-d-aspartate (NMDA) receptor antagonist memantine for moderate-to-severe stages of the disease [1]. Major limitations of the current symptomatic therapies include their temporary efficacy window, modest clinical effect on cognition and function, and lack of modification of the course of disease progression. Therefore, newer therapeutics with the potential to improve cognitive or functional endpoints over and above the existing treatments, or slow disease progression, will be of significant value to patients and their caretakers.
A number of biomarker and interventional studies have improved our understanding of the pathophysiology of AD. Two major risk factors for late-onset AD are brain β-amyloid (Aβ) accumulation and apolipoprotein E4 (APOE4) genotype, both believed to mediate pathogenesis of the disease [2]. Familial AD caused by a direct result of mutations leading to overproduction of Aβ provides a strong case for the pathogenic role of Aβ in AD. Despite the controversies over the ‘amyloid hypothesis’ [3, 4], the recent clinical data with anti-Aβ antibodies, in particular aducanumab (Biogen clinical candidate in phase III development), have provided encouraging proof-of-concept evidence that targeting soluble Aβ aggregates (i.e. Aβ oligomers) may produce cognitive and functional benefits in AD patients [5, 6]. According to the amyloid hypothesis, AD pathogenesis is caused by the generation of Aβ peptides after cleavage of the amyloid precursor peptide, with subsequent formation of Aβ oligomers and fibrils, and ultimately deposition in amyloid plaques. This amyloidogenic cascade leads to neuronal dysfunction and death, which underlie the progressive cognitive and behavioral decline in AD patients. Thus, strategies aimed at preventing the formation of Aβ oligomers and fibrils represent potential therapeutic value.
Tramiprosate, a 3-amino-1-propanesulfonic acid, was initially identified as an inhibitor of Aβ aggregation from screening a series of ionic sulfate and sulfonate molecules [7]. As a glycosaminoglycan mimetic, tramiprosate binds preferentially to soluble Aβ species, preventing the formation of fibrillary structures, and their deposition and neurotoxicity [7–9]. This mechanism of action of tramiprosate, previously studied extensively [7–10], has been further elaborated in our recent work using molecular approaches, including ion mobility spectrometry-mass spectrometry (IMS-MS), nuclear magnetic resonance (NMR), and molecular dynamics [11]. Overall, the accumulating evidence indicates that amyloid causes neurotoxicity following a dynamic, conformational transition from soluble monomers to toxic oligomers, and then transitioning to insoluble fibrils (i.e. elongation phase) followed by the deposition of plaques. Tramiprosate interacts with the soluble Aβ monomers, maintains them in a stable conformational state, and prevents the progression of the amyloid cascade, thereby preventing the formation of toxic oligomer species. In vivo, chronic oral tramiprosate treatment of TgCRND8 mice has been shown to achieve a significant reduction (~ 30%) in brain amyloid plaque load, as well as both soluble and insoluble Aβ40 and Aβ42 species [7].
The tramiprosate phase III program comprised 2025 patients with mild-to-moderate AD, and evaluated two active doses, 100 mg twice daily and 150 mg twice daily. In the phase III North American study, the clinical efficacy of tramiprosate was evaluated in a randomized, double-blind, placebo-controlled study in 1052 patients with AD, where it was administered as an add-on to standard symptomatic therapy over 18 months [12–15]. While the study did not meet the primary endpoints in the intent-to-treat population, the statistical analysis showed an unanticipated high variance between clinical sites and poor model fit. The data were subsequently re-analyzed based on a prespecified plan using a repeated measures covariate model with the APOE4 genotype as a covariate. This analysis unmasked a novel and clinically meaningful drug-mediated effect in 599 patients (~ 60% of the study population) who were APOE4 carriers (APOE4-positive; APOE4 homozygous or APOE4 heterozygous), a statistically significant and sustained cognitive improvement in response to tramiprosate (150 mg twice daily) compared with placebo, as measured by the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-cog). While no consistent efficacy was seen in APOE4-negative patients (APOE4 non-carriers), a 2-point ADAS-cog difference in APOE4 carriers and robust 4-point difference in APOE4 homozygous (APOE4/4) patients were observed compared with placebo controls, in response to oral treatment with tramiprosate [12].
Recent clinical and nonclinical data have extended our understanding of the role of APOE4 in AD pathogenesis and helped define the impact of APOE4 genotype on efficacy. Amyloid positron emission tomography (PET) imaging from the recent large AD trials confirms high rates of negative amyloid scans in APOE4-negative patients, while APOE4 homozygotes are predominantly (98%) amyloid-positive [16]. APOE4 homozygotes have also been found to have a significantly higher burden of soluble amyloid oligomers compared with non-carriers [17, 18].
Based on the understanding that amyloid toxicity plays an important role early in the disease, further sensitivity analyses of the subgroup of APOE4/4 patients with mild AD [Mini-Mental State Examination (MMSE) score of 22 and above] showed a robust and sustained efficacy signal on both cognition (as measured by ADAS-cog) and function [as measured by the Clinical Dementia Rating Scale—Sum of Boxes (CDR-SB)] [19].
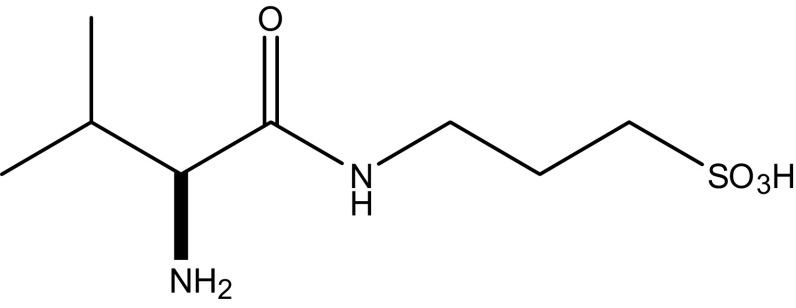
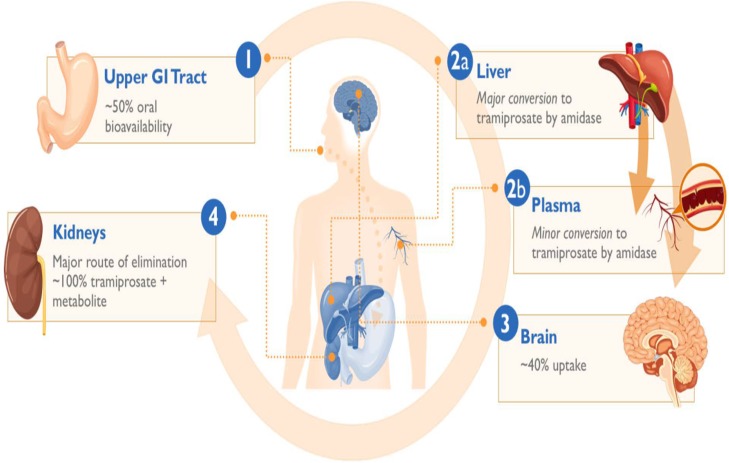
While oral tramiprosate exhibited promising activity in this susceptible AD population, it displayed two limiting pharmaceutical properties: high interindividual pharmacokinetic (PK) variability, and mild-to-moderate incidence of nausea and vomiting most likely due to direct local gastrointestinal irritation [20]. To exploit its positive efficacy attributes, we have developed a novel valine-conjugated prodrug of tramiprosate, ALZ-801 (3-(l-valyl) amino-1-propanesulfonic acid) (Fig. 1), with the goal of improving oral PK variability and tolerability for optimal delivery of the active agent—tramiprosate—to achieve an area under the curve (AUC) target exposure necessary for clinical efficacy. Oral ALZ-801 is rapidly absorbed from the gastrointestinal tract and undergoes rapid and completed cleavage of valine moiety via an hepatic or plasma amidase, leading to the release of tramiprosate in plasma, which is subsequently metabolized to its major primary metabolite NRM5074 and eliminated by renal excretion (Fig. 2). ALZ-801 has been evaluated in both single ascending dose (SAD) and 14-day multiple ascending dose (MAD) trials, which confirmed these favorable attributes. Thus, oral ALZ-801 represents an advanced and substantially improved clinical candidate for AD treatment.
Fig. 1.
Chemical structure of ALZ-801
Fig. 2.
Schematic illustration of the oral absorption, conversion to tramiprosate, distribution and elimination characteristics of ALZ-801 prodrug. GI gastrointestinal
In this paper, we summarize the phase I bridging program to evaluate the safety, tolerability, and PK for ALZ-801 in healthy elderly volunteers. This program included [1] a SAD study; [2] a 14-day MAD study; and [3] a single-dose tablet food-effect study. In addition, we also determined the clinical dose of ALZ-801, with the goal of delivering a steady-state plasma AUC exposure of tramiprosate equivalent to the tramiprosate 150 mg twice-daily exposure used in the phase III program.
Methods
Study Subjects and Conduct
A total of 127 male and female healthy adult and elderly volunteers were enrolled in the ALZ-801 phase I trials, including Study 1 (Table 1), which was conducted by Bellus Health (formerly Neurochem, Inc.) at Quintiles (London UK), and Studies 2 and 3, which were sponsored by Alzheon, Inc. and conducted at Quotient Clinical (Nottingham, UK). Patient written consent and appropriate approvals from the Ethics Committee and the Medicines and Healthcare Products Regulatory Agency (MHRA) were obtained prior to study initiation. The study was conducted in accordance with the International Conference on Harmonization (ICH) Good Clinical Practice, the Medicines for Human Use (Clinical Trials) Regulations, and the ethical principles of the Declaration of Helsinki.
Table 1.
Overview of the completed ALZ-801 phase I clinical program
| Study | Design | Treatments | Subjects | Objectives |
|---|---|---|---|---|
| Study 1: ALZ-801 SAD |
Double-blind, placebo-controlled, single ascending dose (14-day washout) | Substudy 1: Two-way crossover study to compare ALZ-801 (100 mg in a loose-filled capsule) and tramiprosate (equimolar dose of 58 mg in a loose-filled capsule) | 8 healthy adult males and 8 healthy elderly males/females | Safety, tolerability, and PK of single doses |
| Substudy 2: Designed to evaluate up to four doses of ALZ-801 loose-filled capsule in four sequential groups (only 300 mg was conducted) | 15 healthy elderly males/females | |||
| Substudy 3: Double-dummy, parallel study to compare ALZ-801 (172 mg in a loose-filled capsule) and tramiprosate (equimolar dose of 100 mg in a capsule or modified-release tablet) | 36 healthy elderly males/females randomized to each treatment in a 1:1:1 fashion | |||
| Study 2: ALZ-801 MAD |
Double-blind, placebo-controlled, multiple ascending dose |
Cohort A: Days 1–7: 171 mg ALZ-801 loose capsule or placebo once daily on day 1 and twice daily on days 2–7 Days 8–14: 256.5 mg ALZ-801 or placebo once daily; fasted |
9 healthy males/females received the drug; 3 healthy males/females received placebo |
Safety, tolerability, and PK of multiple doses |
|
Cohort B: Days 1–7: 256.5 mg ALZ-801 loose capsule or placebo once daily on day 1 and twice daily on days 2–7 Days 8–14: 340 mg ALZ-801 or placebo once daily; fasted |
9 healthy males/females received the drug; 3 healthy males/females received placebo |
|||
|
Cohort C: Days 1–7: 256.5 mg ALZ-801 loose capsule or placebo once daily on day 1 and twice daily on days 2–7 Days 8–14: 340 mg ALZ-801 or placebo once daily; fed |
9 healthy males/females received the drug; 3 healthy males/females received placebo |
|||
|
Cohort D: Days 1–6: 265 mg ALZ-801 tablet or placebo once daily on day 1 and twice daily on days 2–6 Day 7: 265 mg ALZ-801 tablet or placebo once daily; fed |
9 healthy males/females received the drug; 3 healthy males/females received placebo |
|||
| Study 3: ALZ-801 tablet PK and food effect | Open label, four-period, sequential, single ascending dose (14-day washout) | ALZ-801 tablet, single dose: (a) 171 mg, fasted (b) 205 mg, fasted (c) 205 mg, fed (d) 342 mg, fed |
12 healthy adult and elderly, male and female subjects | Safety, tolerability, and PK of immediate-release tablet; food effect |
SAD single ascending dose, MAD multiple ascending dose, PK pharmacokinetics
Drug Substance and Drug Product
ALZ-801 is a synthetic (S)-3-(2-amino-3-methylbutanamide) propane-1-sulfonic acid (Fig. 1) with one chiral moiety, with a molecular mass of 238.3 g/mole. ALZ-801 is a white powder, pKa 7.69, with a solubility in water > 200 mg/ml, and was synthesized and release-tested by Shanghai SynTheAll Pharmaceutical Co. Ltd, China. ALZ-801 capsules and tablets were manufactured and release-tested by Quotient Clinical (Nottingham, UK). The capsules contained ALZ-801 white powder only, while the tablets were comprised of ALZ-801 white powder and inert common excipients: microcrystalline cellulose (Avicel® PH102), polyvinylpyrrolidone, aerosol, and magnesium stearate. ALZ-801 tablets exhibited dissolution and disintegration characteristics typical for immediate-release dosing.
Study Design
The phase I bridging program for ALZ-801 comprised three components, as outlined in Table 1. Study 1 was a double-blind, randomized, placebo-controlled SAD study (doses of 100, 200 and 300 mg), and included three substudies: [1] a two-way crossover study to compare the safety, tolerability, and PK following a single dose of ALZ-801 (100 mg in a loose-filled capsule) and tramiprosate (equimolar dose of 58 mg in a loose-filled capsule) in eight healthy adult male subjects (20–75 years of age, inclusive) and eight healthy elderly male and female subjects (> 50 years of age; with a 14-day washout period); [2] a SAD study for up to four doses of ALZ-801 loose-filled capsules in four sequential groups of 15 healthy elderly male and female subjects (> 50 years of age; the starting dose of 300 mg was determined following review of the data from Substudy 1, and no further dose-escalation was conducted); and [3] a double-dummy, parallel study to compare the safety, tolerability and PK following a single oral administration of either ALZ-801 (172 mg in a loose-filled capsule) or tramiprosate (equimolar dose of 100 mg in a capsule or modified-release coated tablet). Thirty-six healthy elderly male and female subjects (> 50 years of age) were randomized to receive one of the three treatments in a ratio of 1:1:1.
Study 2 was a double-blind, randomized, placebo-controlled, 14-day MAD study in elderly adults, including two substudies: [1] Cohorts A, B, and C comprised a parallel-group study of the safety, tolerability, and PK in plasma and urine of MAD ALZ-801 in healthy male or female subjects aged 50–75 years, inclusive; subjects were enrolled into three successive cohorts (12 per cohort) and randomized in a 3:1 ratio to receive treatment with ALZ-801 capsules (n = 9) or placebo capsules (n = 3) for 2 weeks; and [2] Cohort D was a parallel-group study of the safety, tolerability, and PK in plasma and urine of MAD ALZ-801 from an immediate-release tablet formulation in healthy male or female subjects aged 60–75 years, inclusive; 12 subjects were enrolled as a single cohort and randomized in a 3:1 ratio to receive treatment with ALZ-801 (n = 9) or placebo (n = 3) in the fed state (i.e. subjects were fed 30 min prior to dosing) for 1 week. The study sequence is shown in Figs. S1 and S2 (electronic supplementary material).
Study 3 was an open-label, four-period, sequential, SAD PK study to evaluate the safety, PK and food effect of ALZ-801 delivered as an immediate-release tablet. Twelve healthy volunteers were administered single increasing doses of the tablet in the fasted or fed state (50–64 years of age, with average age of 56 years). This study was also aimed at identifying a dose of the ALZ-801 tablet that would result in exposure similar to the historic exposure data from tramiprosate. For the single-dose tablet food-effect study, a high-fat restricted menu was provided to subjects. The start and stop time of the meal was recorded.
Safety Assessments
Safety evaluations comprised laboratory variables, vital signs, electrocardiograms (ECGs) and physical examination, and adverse events (AEs), as defined by the study protocol. Laboratory measures included hematology, clinical chemistry, and urinalysis. Blood pressure, heart rate and 12-lead ECGs were recorded by an automated recorder after the subject had been in a supine position for a minimum of 5 min. An AE could be any unfavorable or unintended sign, symptom or disease temporally associated with the administration of ALZ-801, whether or not considered related to the treatment. AEs were recorded from the first dose until discharge from the study and at the follow-up call. Any adverse experiences recorded between the time of informed consent and first dose were to be recorded with the medical history. AEs and medications were coded using the Medical Dictionary for Regulatory Activities (MedDRA; v18.1; 29).
Plasma and Urine Sample Collection, Bioanalysis, and Pharmacokinetic (PK) Evaluation
Blood samples were collected on ice into 3 mL K3 EDTA tubes, and centrifuged at 2000g for 10 min at 4 °C within 30 min of collection. The resultant plasma was transferred into polypropylene tubes, frozen within 1 h of collection, and stored at − 20 °C or below until bioanalysis. Urine samples were collected into polypropylene containers and kept at 4 °C. Each individual sample was weighed and the weight recorded along with the sample collection time. After thorough mixing, 3 × 10 mL (primary and duplicate) aliquots were removed and placed in 25 mL polypropylene containers; the remaining bulk sample was then discarded. The aliquots were immediately stored at − 20 °C until bioanalysis. Plasma and urine concentrations for ALZ-801, tramiprosate, and the metabolite NRM5074 were quantified at LGC Ltd (Fordham, Cambridgeshire, UK), using validated liquid chromatography-mass spectrometry (LC-MS) methods. The lower limit of quantification (LLOQ) in plasma was 10 ng/mL for ALZ-801 and tramiprosate, and 40 ng/mL for NRM5074. The LLOQ in urine was 30 ng/mL for ALZ-801, 100 ng/mL for tramiprosate, and 10,000 ng/mL for NRM5074. Non-compartmental PK analysis (Phoenix WinNonlin v6.3; Certara Inc., St. Louis, MO, USA), data analysis, and data reporting were performed by Quotient Clinical (Nottingham, UK). Renal clearance (CLr) of each analyte was calculated as the amount excreted (Ae)/plasma AUC.
Brain Penetration for Oral Tramiprosate and ALZ-801 in Animals
Animals were housed in a standard facility, with water and food ad libitum at all times. Brain penetration of tramiprosate after a single-dose oral administration of 100 and 500 mg/kg 14C-tramiprosate was conducted in male C57BL/6 mice (8 weeks of age, Charles River, QC, Canada; n = 3 per sampling time point). The AUC of radioactivity in the brain and plasma were assessed over 24 h, with the brain collected after intracardiac saline perfusion. In a separate study to assess the relative efficiency in delivering tramiprosate into the brain by the prodrug ALZ-801 versus tramiprosate itself, a single oral dose of ALZ-801 and tramiprosate at the molar equivalent doses of 172 and 100 mg/kg, respectively, was administered to male CD-1 mice (5–6 weeks, Charles River, QC, Canada; n = 6 per sampling time point). The 24 h brain (perfused) and plasma AUC exposures of tramiprosate were quantified by LC-MS. The brain/plasma AUC ratio was derived as an indicator of brain penetration. This work was conducted by Neurochem/Bellus Health (Laval, QC, Canada) after approval from the Institutional Animal Care and Use Committee (CISAU) of the INRS-Institut Armand-Frappier.
Bridging Clinical Dose Analysis for ALZ-801 and Brain Drug Exposure
A clinical dose regimen for ALZ-801 to be evaluated in efficacy studies was projected based on the previous tramiprosate phase III trial data showing a robust efficacy signal in APOE4/4 AD patients over 78 weeks of oral tramiprosate 150 mg twice-daily treatment [12]. The steady-state drug exposure in the brain was subsequently estimated based on the human plasma drug AUC exposure and the brain/plasma relationship derived from the above rodent models.
Data Analysis
Descriptive statistics for safety data were presented as cases or cases (%), while descriptive statistics for PK parameters [maximum plasma concentration (C max), AUC, half-life (t ½)] and graphs were expressed as mean ± standard deviation, based on arithmetic means, except for time to reach C max (T max) in humans, for which the median (range) was used.
Results
Summary of Clinical Safety and Tolerability in Human Single Ascending Dose (SAD) and Multiple Ascending Dose (MAD) Studies (Study 1)
Overall, oral administration of ALZ-801 in either capsule or tablet form was well tolerated (Tables 2, 3). There were no treatment-emergent AEs (TEAEs), serious adverse events (SAEs), or AEs leading to discontinuation, and AEs were generally mild. The most common AEs were instances of transient mild nausea and sporadic instances of vomiting, and there was no evidence of dose response in the reported AEs. The top dose of 342 mg ALZ-801 evaluated (i.e. 200 mg tramiprosate equivalent), administered once or twice daily, was well tolerated following initial titration using a reduced dose for the first week. The incidence of nausea and vomiting were markedly lower during the second week of treatment in the MAD study (Table 3B). Administration of ALZ-801 with food further reduced nausea or vomiting in some subjects. The proposed dose regimen of 265 mg twice daily for the ALZ-801 tablet (Table 3) was very well tolerated at concentrations that matched or exceeded the AUC plasma exposure equivalent to the historical tramiprosate 150 mg twice-daily data. No trends were observed in safety laboratory, vitals, or ECGs, and no dose-limiting toxicity or maximum tolerated dose (MTD) was observed over 2 weeks of dosing at doses up to 342 mg twice daily.
Table 2.
Safety summary from the ALZ-801 (tablet) phase I single dose ascending study (i.e. Study 3 in Table 1): incidence of adverse events
| System organ class | Regimen A (n = 10) | Regimen B (n = 11) | Regimen C (n = 12) | Regimen D (n = 12) |
|---|---|---|---|---|
| Subjects reporting AEs | 4 | 4 | 0 | 3 |
| Gastrointestinal disorders | 4 | 4 | 0 | 3 |
| Flatulence | 0 | 0 | 0 | 0 |
| Nausea | 4 | 4 | 0 | 3 |
| Vomiting | 1 | 1 | 0 | 0 |
| Nervous system disorders | 0 | 0 | 0 | 0 |
| Headache | 0 | 0 | 0 | 0 |
| Somnolence | 0 | 0 | 0 | 0 |
| General disorders and administration site conditions | 0 | 0 | 0 | 0 |
| Chills | 0 | 0 | 0 | 0 |
| Vascular disorders | 0 | 0 | 0 | 0 |
| Flushing | 0 | 0 | 0 | 0 |
Regimen A: 171 mg ALZ-801 tablet (equivalent to 100 mg tramiprosate), administered in the fasted state
Regimen B: 205 mg ALZ-801 tablet (equivalent to 120 mg tramiprosate), administered in the fasted state
Regimen C: 205 mg ALZ-801 tablet (equivalent to 120 mg tramiprosate), administered in the fed state (30 min after starting a high-fat breakfast)
Regimen D: 342 mg ALZ-801 as 2 × 171 mg ALZ-801 tablets (equivalent to a total dose of 200 mg tramiprosate) administered in the fed state (30 min after starting a high-fat breakfast)
A total of 12 subjects were enrolled in the study. Ten subjects were enrolled and dosed in period 1 of the study, and were successfully dosed with Regimen A. An additional two subjects were enrolled for study periods 2–4. One subject was not dosed in study period 2 due to an unrelated AE, but returned to the study for regimens c and d
AEs adverse events
Table 3.
Safety summary from the ALZ-801 (capsule, tablet) phase I multiple dose ascending study (i.e. Study 2 in Table 1)
| A. SAEs | |||
|---|---|---|---|
| Group | Subjects affected by SAE/exposed | No. of deaths (all causes) | No. of deaths resulting from AEs |
| Part 1: Cohort A, 171 and 256.5 mg ALZ-801 capsule, fasted | 0/9 | 0 | 0 |
| Part 1: Cohort B, 256.5 and 340 mg ALZ-801 capsule, fasted | 0/9 | 0 | 0 |
| Part 1: Cohort C, 256.5 and 340 mg ALZ-801 capsule, fed | 0/9 | 0 | 0 |
| Part 1: Combined placebo groups of three cohorts, fed/fasted | 0/9 | 0 | 0 |
| Part 2: Cohort D, 265 mg ALZ-801 tablet, fed | 0/9 | 0 | 0 |
| Part 2: Placebo group, fed | 0/3 | 0 | 0 |
| B. TEAEs | |||||
|---|---|---|---|---|---|
| TEAEs | Placebo | Cohort A 171 mg bid 256.5 mg qd Capsule, fasted |
Cohort B 256.5 mg bid 340 mg qd Capsule, fasted |
Cohort C 256.5 mg bid 340 mg bid Capsule, fed |
Cohort D 265 mg bid Tablet, fed |
| Nausea | |||||
| Week 1 | 1 (11.1) | 3 (33.3) | 4 (44.4) | 5 (55.6) | 3 (33.3) |
| Week 2 | 0 | 3 (33.3) | 0 | 0 | NA |
| Vomiting | |||||
| Week 1 | 0 | 0 | 2 (22.2) | 2 (22.2) | 1 (11.1) |
| Week 2 | 0 | 0 | 2 (22.2) | 0 | NA |
Data are expressed as cases (%)
The timeframe for reporting adverse events: from screening visit (day − 28 to day − 2) to follow-up visit 7–10 days after the last dose of study medication (Part 1: days 21–24; Part 2: days 14–17). Assessment type: systematic
NA not applicable, SAEs serious adverse events, TEAEs treatment-emergent adverse events, bid twice daily, qd once daily
Tablet SAD Study (Study 3)
ALZ-801 was well tolerated, with no treatment-related safety concerns identified. No deaths, serious TEAEs, severe TEAEs, or SAEs were reported, and no TEAEs led to the withdrawal of ALZ-801 treatment. Most TEAEs were mild in severity; only two participants reported moderate and transient TEAEs (nausea), both following fasted dosing with a single 171 mg dose of ALZ-801. No moderate TEAEs were reported following dosing under fed conditions. For most participants, the onset of nausea was approximately 0.5–1 h and 1.5–2.5 h following fasted and fed dosing, respectively. ALZ-801 was better tolerated when administered in the fed state; no participant reported an investigational drug-related TEAE following fed dosing with 205 mg ALZ-801, compared with four subjects following fasted dosing. ALZ-801 was also better tolerated at a higher fed dose of 342 mg (2 × 171 mg ALZ-801 tablets) compared with the lowest fasted dose. There were no clinically significant findings in any clinical laboratory, vital signs, ECG, or physical examination assessment.
Capsule and Tablet MAD Study (Study 2)
ALZ-801 was well tolerated when administered at doses of up to 340 mg twice daily. No deaths, SAEs, or severe AEs were reported, and no TEAE led to withdrawal. Following ALZ-801 dosing in the fasting state, 17 (63.0%) subjects experienced a total of 65 TEAEs, compared with 6 (66.7%) subjects following dosing with placebo reporting a total of 12 TEAEs. Most TEAEs were mild in severity, with gastrointestinal disorders being the most frequently reported TEAEs, most commonly nausea and vomiting. The incidence of nausea was comparable across cohorts with no discernable dose response; however, a few instances of vomiting were reported only at the two highest dose levels [Cohort B (fasted) and Cohort C (fed)]. Following dosing with ALZ-801 265 mg twice daily in the fed state, the incidence of TEAEs was equivalent to placebo. There were no clinically significant laboratory findings, vital signs, ECG, physical examination findings, or C-SSRS (The Columbia-Suicide Severity Rating Scale), VAS-DE (Visual Analog Scale- Drug Effect assessment) and BWSQ2 (Benzodiazepine Withdrawl Symptom Questionnaire) questionnaire scores. No subject reached the subject withdrawal criteria for QTc prolongation or safety laboratory tests.
Plasma Pharmacokinetics
SAD Study of ALZ-801 Loose-Filled Capsule (Study 1)
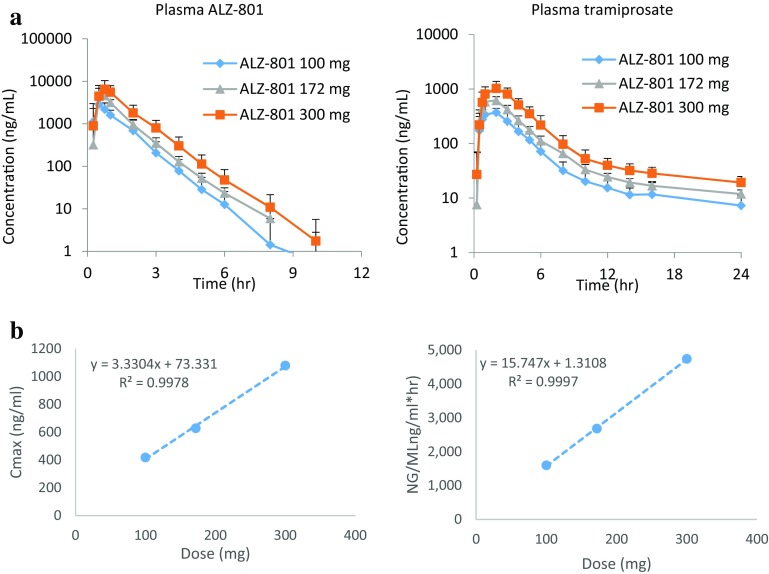
In this initial study, ALZ-801 was administered orally as single-dose capsules to fasted healthy male and female volunteers at 100, 172, and 300 mg doses (equivalent to tramiprosate 58, 100, and 175 mg) with PK evaluated over 48 h (Table 4). No gender effect was observed. ALZ-801 was absorbed rapidly: the median T max for plasma ALZ-801 and tramiprosate was 0.51–0.75 h and 1.05–2.0 h, respectively. The major primary metabolite NRM5074 was also detected at 172 and 300 mg doses, with a median T max of approximately 5 h; no additional metabolite was identified. This is consistent with the release of tramiprosate from the prodrug, followed by metabolism to NRM5074. The plasma exposure [C max, AUC from time zero to time t (AUCt)] for ALZ-801 was higher than that for tramiprosate; however, ALZ-801 showed a relatively transient presence in plasma (the apparent t ½ was 0.7–0.81 h), whereas the apparent t ½ for tramiprosate was much longer (up to ~ 18 h). Importantly, the plasma C max and AUC exposures of both ALZ-801 and tramiprosate were highly dose-proportional (Fig. 3), reflecting a linear kinetic profile.
Table 4.
Summary of plasma PK profiles for ALZ-801 (prodrug) and tramiprosate (active drug) after single dose oral administration of 100, 172 and 300 mg of ALZ-801 (capsule) in healthy human subjects in the phase I single ascending dose study (i.e. Study 1 in Table 1)
| PK parameters | ALZ-801 100 mg (equivalent to tramiprosate 58 mg) | ALZ-801 172 mg (equivalent to tramiprosate 100 mg) | ALZ-801 300 mg (equivalent to tramiprosate 175 mg) |
|---|---|---|---|
| ALZ-801 | |||
| T max (h) | 0.51 [0.25–2.00] [16] | 0.63 [0.50–1.02] [12] | 0.75 [0.48– 1.03] [11] |
| C max (ng/mL) | 3646 ± 1445 [16] | 5445 ± 1669 [12] | 8149 ± 3607 [11] |
| AUCt (ng/mL h) | 3611 ± 684 [16] | 5891 ± 1185 [12] | 9606 ± 2683 [11] |
| t ½ (h) | 0.70 ± 0.11 [13] | 0.87 ± 0.20 [12] | 0.81 ± 0.13 [9] |
| Tramiprosate | |||
| T max(h) | 1.05 [0.75–3.02] [16] | 2.00 [1.00–2.00] [12] | 2.00 [1.00–2.08] [11] |
| C max (ng/mL) | 418 ± 94.2 [16] | 628 ± 100 [12] | 1079 ± 339 [11] |
| AUCt (ng/mL h) | 1595 ± 391 [16] | 2680 ± 448 [12] | 4736 ± 1291 [11] |
| t ½ (h) | 13.4 ± 7.64 [8] | 14.9 ± 3.97 [10] | 17.8 ± 2.11 [10] |
Data are expressed as mean ± SD (N), except for T max, which is reported as median [range] (N)
t ½: the elimination kinetics in some subjects could not be determined, thus the n is different for some of the PK parameters
PK pharmacokinetic, C max maximum concentration, T max time to reach Cmax, AUC t area under the concentration–time curve from time zero to time t, t ½ half-life, SD standard deviation
Fig. 3.
a Plasma concentrations of ALZ-801 (prodrug) and tramiprosate (active drug) over time after single ascending oral administration of ALZ-801 loose-filled capsules at 100, 172, and 300 mg/kg in healthy volunteers (mean + SD, n = 11–16; Study 1 in Table 1). b Tramiprosate exposure (C max and AUCt) versus dose relationship indicates dose linearity. C max maximum concentration, AUC t area under the concentration–time curve from time zero to time t, SD standard deviation
The PK for plasma tramiprosate was compared after oral administration of ALZ-801 versus tramiprosate at equimolar doses, i.e. 100 mg of ALZ-801 versus 58 mg of tramiprosate as a capsule, and 172 mg ALZ-801 capsule versus 100 mg tramiprosate capsule or modified-release tablet (the latter shown in Table 5). Overall, the data indicate reasonably comparable plasma exposure of tramiprosate in plasma after oral administration of a molar equivalent dose of the prodrug ALZ-801 versus tramiprosate itself. However, single-dose oral administration of ALZ-801 (capsule) displayed significant improvements on the resulting plasma tramiprosate PK parameters. When compared with oral tramiprosate delivered as a capsule or modified-release tablet, oral ALZ-801 resulted in an extension of the apparent t ½ of plasma tramiprosate from 5 to 6 h to ~ 15 h, and a reduction in intersubject variability (coefficient of variation; CV%) of AUC from 41 to 17%.
Table 5.
Comparison of plasma tramiprosate PK parameters following oral administration of tramiprosate as a modified-release tablet or loose-filled capsule, and ALZ-801 as a loose-filled capsule (i.e. Study 1, Substudy 3 in Table 1)
| PK parameter | Plasma tramiprosate after tramiprosate MR tablet 100 mg |
Plasma tramiprosate after tramiprosate LF capsule 100 mg |
Plasma tramiprosate after ALZ-801 LF capsule 172 mg |
|---|---|---|---|
| C max (ng/mL) | 506 ± 187 [12] | 769 ± 228 [12] | 628 ± 100 [12] |
| T max (h) | 4.50 [2.00–5.00] [12] | 1.00 [0.75–5.00] [12] | 2.00 [1.00–2.00] [12] |
| AUCt (ng/mL h) | 2355 ± 747 [12] | 3268 ± 1128 [12] | 2680 ± 448 [12] |
| AUCinf (ng/mL h) | 2037 ± 712 [6] | 3332 ± 1369 [8] | 2875 ± 492 [10] |
| λz (h−1) | 0.1826 ± 0.1025 [6] | 0.1846 ± 0.1020 [8] | 0.0494 ± 0.0128 [10] |
| t ½ (h) | 4.99 ± 2.66 [6] | 5.90 ± 5.22 [8] | 14.9 ± 3.97 [10] |
Data are expressed as mean ± SD [N], except for T max, which is reported as median [range] (N)
t ½: the elimination kinetics in some subjects could not be determined, thus the n is different for some of the PK parameters
PK pharmacokinetic, C max maximum concentration, T max time to reach Cmax, AUC t area under the concentration–time curve from time zero to time t, AUC inf area under the concentration–time curve from time zero to infinity, t ½ half-life, SD standard deviation, MR modified-release, LF loose-filled
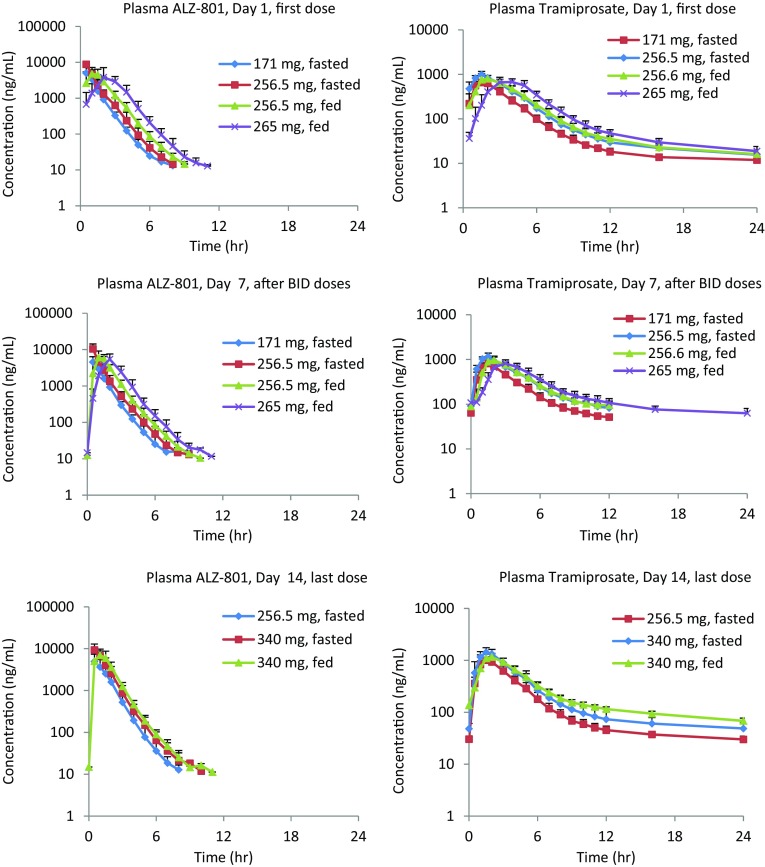
MAD Study of ALZ-801 Loose-Filled Capsule and Immediate-Release Tablet (Study 2) (Figs. 4, 5, Tables 6, 7 and electronic supplementary Table S1)
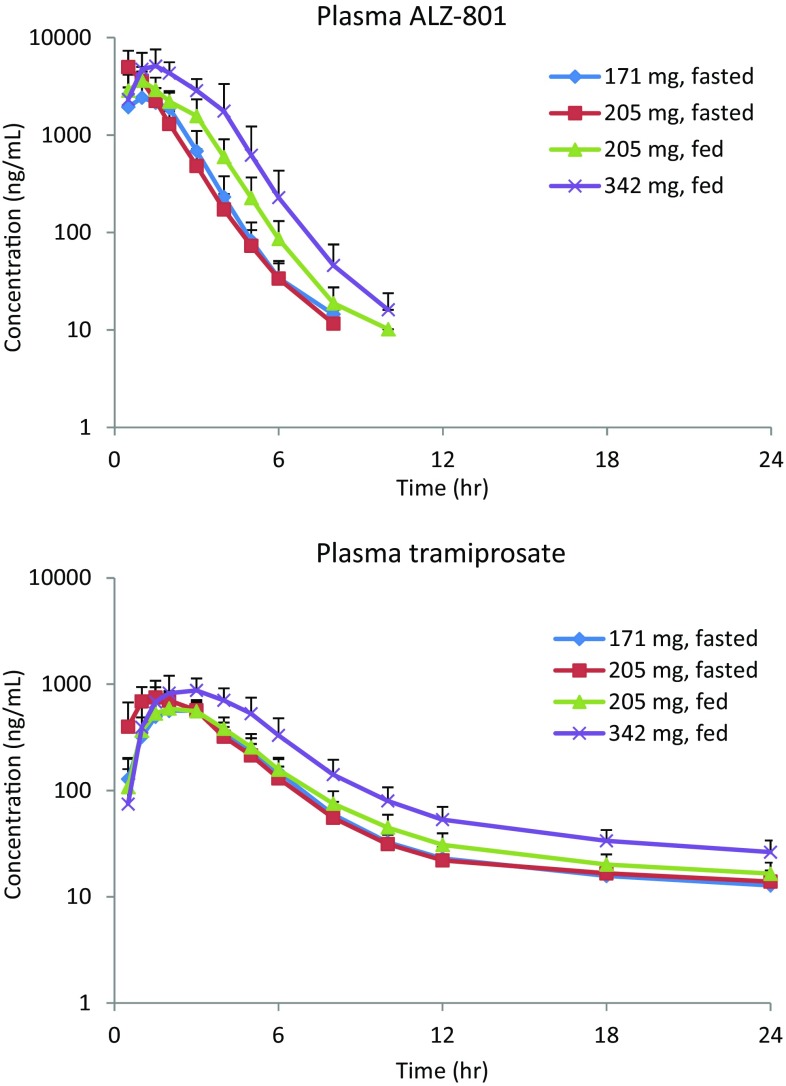
Fig. 4.
Mean pharmacokinetic time course for ALZ-801 phase I multiple ascending dose studies in healthy human volunteers under fasted and fed conditions (Study 2 in Table 1)
Fig. 5.
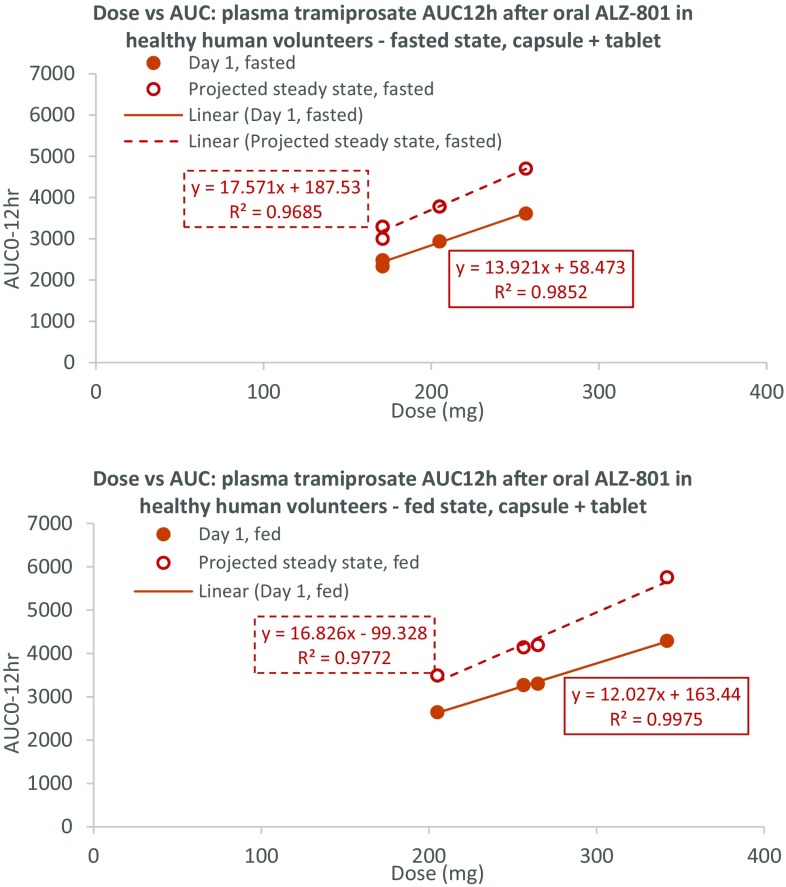
Plasma tramiprosate exposure versus dose relationships after an oral ALZ-801 tablet and capsule in healthy humans show strong dose-exposure proportionality under all conditions (Studies 2 and 3 in Table 1). AUC area under the concentration–time curve, AUC 12 AUC from time zero to 12 h
Table 6.
Steady-state T max, C max and AUC profiles for ALZ-801 (prodrug) and tramiprosate (active drug) after oral administration of ALZ-801 (capsule) over 14 days in fasted and fed healthy human subjects in the phase I multiple ascending dose study (i.e. Cohorts B and C of Study 2 in Table 1; n = 9 per group)
| Food status | Plasma analyte | Day | T max (h) | C max (ng/mL) | AUC12 (ng/mL h) | AUC24 (ng/mL h) |
|---|---|---|---|---|---|---|
| Fasted | ALZ-801 | 14 | 0.629 ± 0.229 | 10,400 ± 2880 | 13,800 ± 3350 | 13,800 ± 3350 |
| Tramiprosate | 14 | 1.634 ± 0.351 | 1480 ± 305 | 5470 ± 1220 | 6170 ± 1420 | |
| Fed | ALZ-801 | 14 | 0.944 ± 0.388 | 8830 ± 2660 | 13,800 ± 2220 | 13,800 ± 2220 |
| Tramiprosate | 14 | 1.778 ± 0.264 | 1180 ± 190 | 5230 ± 691 | 6300 ± 781 |
Dosing schedule: Days 1–7: 256.5 mg ALZ-801 capsule once daily on day 1 and twice daily on days 2–7; days 8–14: 340 mg ALZ-801 once daily
Data are expressed as mean ± SD
C max maximum concentration, T max time to reach Cmax, AUC area under the concentration–time curve, AUC 12 AUC from time zero to 12 h, AUC 24 AUC from time zero to 24 h, SD standard deviation
Table 7.
Steady-state plasma PK profiles for ALZ-801 (prodrug) and tramiprosate (active drug) after oral administration of ALZ-801 (tablet) 265 mg twice daily over 7 days in fed healthy human subjects in the phase I multiple ascending dose study (i.e. Cohort D of Study 2 in Table 1; n = 9)
| Food status | Plasma analyte | Day | T max (h) | C max (ng/mL) | AUC12 (ng/mL h) | AUC24 (ng/mL h) | t ½ (h) |
|---|---|---|---|---|---|---|---|
| Fed | ALZ-801 | 1 | 2.448 ± 0.848 | 4960 ± 2650 | 10,300 ± 2830 | 10,300 ± 2830 | 1.05 ± 0.160 |
| Tramiprosate | 1 | 3.559 ± 0.880 | 759 ± 163 | 3300 ± 608 | 3650 ± 670 | 8.94 ± 1.35 | |
| Fed | ALZ-801 | 7 | 2.114 ± 0.553 | 6460 ± 1800 | 11,200 ± 1970 | 11,200 ± 1970 | 1.11 ± 0.263 |
| Tramiprosate | 7 | 2.898 ± 0.603 | 865 ± 172 | 4100 ± 687 | 5020 ± 859 | 36.1 ± 4.15 |
Dosing schedule: Days 1–6: 265 mg ALZ-801 tablet once daily on day 1 and twice daily on days 2–6; day 7: 265 mg ALZ-801 tablet once daily
Data are expressed as mean ± SD
PK pharmacokinetic, C max maximum concentration, T max time to reach C max, AUC 12 area under the concentration–time curve from time zero to 12 h, AUC 24 area under the concentration–time curve from time zero to 24 h, t ½ half-life, SD standard deviation
ALZ-801 in plasma: Consistent with the SAD study, the ALZ-801 capsule (171 and 256.5 mg) was absorbed rapidly on day 1 under fasted conditions, with the ALZ-801 T max occurring at 0.5 h in plasma. The plasma ALZ-801 exposures (C max and AUC) were dose-proportional, and the t ½ for ALZ-801 in plasma was short, approximating 1 h. Under the fed state, absorption was slightly delayed (median T max approximated 1 h) following 256.5 mg ALZ-801 capsules, resulting in a 25% lower C max than the fasted state; however, the AUC and t ½ remained similar. In addition, oral administration of 265 mg ALZ-801 tablets in the fed state resulted in an expected delay in T max (approximately 2 h) compared with the 256.5 mg of ALZ-801 capsule, likely due to a slower dissolution. Although the dose difference between the two formulations administered in the fed state was only 3% (256.5 vs. 265 mg), the C max was 22% lower for the tablet; however, the AUC was comparable for both the tablet and capsule formulations, as was the t ½.
After repeated twice-daily administration of 171 and 256.5 mg ALZ-801 capsules in the fasted state for 6 days, the plasma ALZ-801 T max did not change. The plasma exposures of ALZ-801 increased with the dose only slightly more than dose-proportional, with 2.1- and 1.9-fold increases in C max and AUC from time zero to 12 h (AUC12), respectively, for a 1.5-fold increase in dose. There was no significant accumulation over time: mean accumulation ratios were 0.94 for both the C max and AUC12 for the 171 mg dose, and 1.19 and 1.14 for the C max and AUC12, respectively, for the 256.5 mg dose. Similar to day 1, repeated administration of 256.5 mg ALZ-801 capsules with food resulted in a slightly delayed T max and a 30% lower C max than the fasted state, but the AUC exposure remained similar. There was no evidence of accumulation over time. Compared with the 256.5 mg ALZ-801 capsule, the T max occurred later for 265 mg ALZ-801 tablets when administered with food; however, the C max and AUC12 were similar.
Due to the changes in dosing regimen between days 1 and 14, and between the cohorts, it is difficult to make direct PK comparisons. However, it was noted on day 14 that the T max for plasma ALZ-801 following dosing in the fasted state generally occurred at approximately 0.5 h, as observed on days 1 and 7, and dosing in the fed state caused a slight delay in T max. The t ½ of ALZ-801 was approximately 1 h irrespective of dosing regimen and day.
Tramiprosate in plasma: After administration of 171 and 256.5 mg ALZ-801 capsules in the fasted state on day 1, ALZ-801 was rapidly converted to tramiprosate, with quantifiable levels present at the first sampling time point of 0.5 h. The T max (1.5 h) was similar at both dose levels. As with ALZ-801, the C max and AUC exposures for plasma tramiprosate increased dose-proportionally. The t ½ for tramiprosate was similar for both doses, but considerably longer than that for ALZ-801. Following a single oral dose of 256.5 mg ALZ-801 capsules with food, there was a slight delay in the presence of quantifiable plasma tramiprosate concentrations in some subjects; however, the median T max was similar to that observed in the fasted state. There was a slight decrease (approximately 13%) in mean C max values between the fed and fasted states; however, as previously observed, the AUC values were similar, as was the half-life. After a single oral dose of 265 mg ALZ-801 tablets with food, the T max for tramiprosate occurred later than with 256.5 mg ALZ-801 capsules, and there was an approximately 12% decrease in C max. The AUC values were similar for both the tablet and capsule formulations in the fed state, as was the t ½. In addition, following single oral doses of ALZ-801 on day 1 (i.e. 171 mg capsules without food, 256.5 mg capsules both with and without food, and 265 mg tablets with food), the interindividual variability for plasma tramiprosate exposure was low, with CV% for C max and AUC ranging from 14 to 22%.
Based on the kinetics, steady state should be reached by day 7 for tramiprosate, which was confirmed by visual inspection of the predose values. On day 7, the occurrence of T max for tramiprosate was similar for both 171 and 256.5 mg capsule doses; the C max and AUC exposures increased dose-proportionally. Compared with day 1, the tramiprosate T max was similar at both doses, but the C max and AUC12 were higher, with accumulation ratios of 1.2 and 1.3, respectively. When administered with food, 256.5 mg ALZ-801 capsules on day 7 resulted in a slight delay in T max in plasma tramiprosate, and an approximate 18% lower C max than the fasted state, but AUC12 was comparable. Repeated twice-daily dosing of 256.5 mg capsules in the fed state also resulted in higher C max and AUC12 than day 1, with accumulation ratios of 1.14 and 1.27, respectively, similar to those in the fasted state. As seen on day 1, the 265 mg ALZ-801 tablets also delayed the tramiprosate T max compared with the 256.5 mg ALZ-801 capsules on day 7; the C max was 11% lower but AUC12 was similar. Consistent with the capsule, C max and AUC12 on day 7 were higher than those on day 1 for the tablet formulation, with accumulation ratios of 1.15 and 1.25, respectively. As with the day 1 data, the interindividual variability was low on day 7, with the CV% for C max and AUC ranging from approximately 15 to 26% following twice-daily administration of 171 mg ALZ-801 capsules in the fasted state, 256.5 mg capsules in the fed and fasted states, and 265 mg tablets in the fed state.
On day 14, the occurrence of plasma tramiprosate T max in the fasted state was similar to that observed on days 1 and 7, and the range over which the T max occurred in the fed state was also similar to that previously observed. The t ½ for tramiprosate was longer on day 14 compared with day 1, and was calculated out to 96 h versus 24 h on day 1.
NRM5074 (metabolite of tramiprosate) in plasma: there was a lag between the presence of quantifiable concentrations of tramiprosate and those of the metabolite (NRM5074) following a single oral dose of ALZ-801 on day 1. Numerous peaks in NRM5074 concentrations were observed, resulting in T max ranging from 2 to 16 h, although the CV% for the C max values were generally low irrespective of the dosing regimen. On days 7 and 14, although there were multiple peaks, the median T max for all dosing regimens was 5 h. As with tramiprosate, the t ½ for NRM5074 was longer on day 14 compared with day 1, and was similar for the three dose regimens.
Single-Dose ALZ-801 Immediate-Release Tablet and Food Effect (Study 3) (Figs. 6, 7)
Fig. 6.
Mean pharmacokinetic curves for the ALZ-801 immediate-release tablet in phase I, single-dose studies in healthy human volunteers (Study 3 in Table 1)
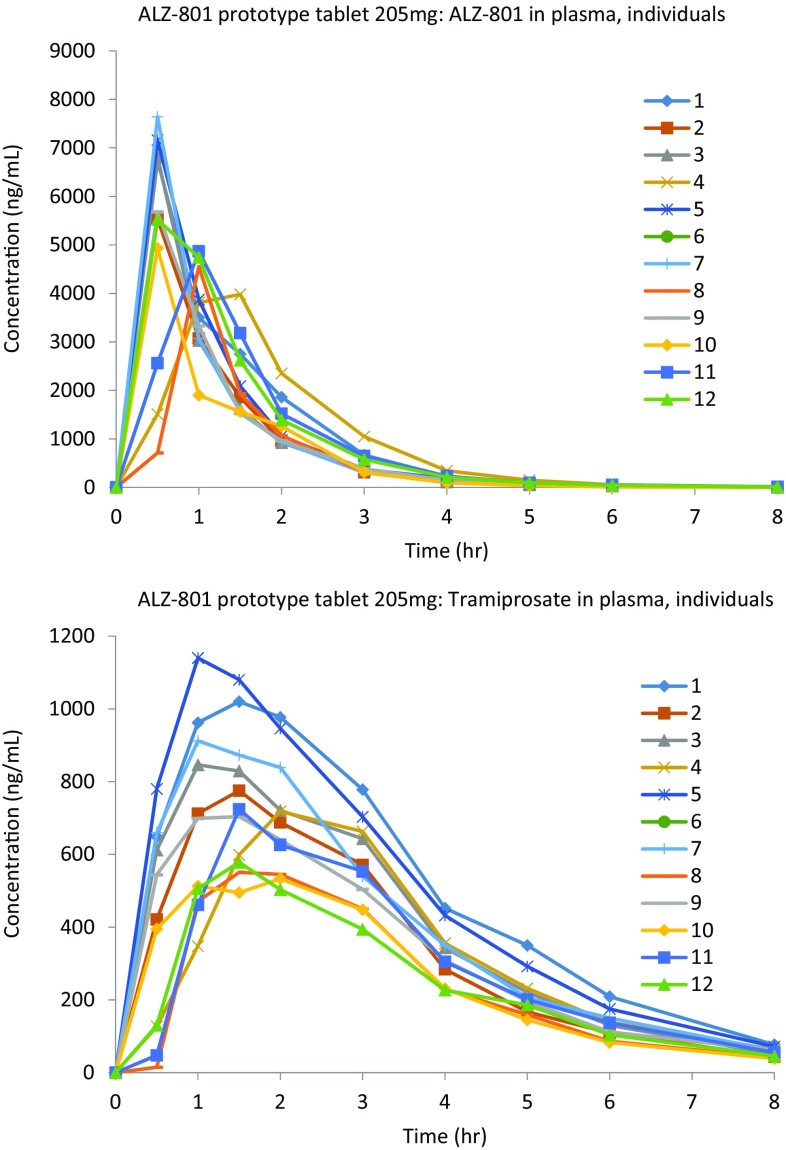
Fig. 7.
Individual ALZ-801 and tramiprosate plasma time course graphs illustrating low intersubject variability after oral administration of the ALZ-801 tablet to healthy human subjects (Study 3 in Table 1)
Following administration of the prototype tablet at doses of 171 and 205 mg in the fasted state (Regimens A and B), ALZ-801was rapidly absorbed, with no lag phase for plasma ALZ-801 and a median T max of 1.0 and 0.5 h, respectively. Elimination was efficient, with a t ½ of < 1 h, consistent across all dose levels administered. The formation of tramiprosate was rapid, with a median T max of 2.5 and 1.5 h for the 171 and 205 mg doses, respectively; the t ½ was 12.9 and 16.5 h, respectively, much longer than that for ALZ-801. Overall, the C max and AUC exposures of tramiprosate were dose-proportional, with mean C max values of 611 and 773 ng/mL, and AUCt of 2680 and 3150 ng/mL h, respectively.
Compared with the fasted condition, administration of 205 mg ALZ-801 tablets with food (Regimen C) delayed the T max of tramiprosate by approximately 30 min, resulting in a 17% reduced C max (638 ng/mL), but a relatively comparable (5% lower) AUCt of 2990 ng/mL h. A further 1.7-fold increase in the dose of ALZ-801 tablets to 342 mg (Regimen D) administered in the fed state resulted in a 1.5- and 1.7-fold increase in tramiprosate C max (978 ng/mL) and AUCt (4990 ng/mL h), demonstrating dose linearity. Interindividual variability (Fig. 7) for exposure to tramiprosate following ALZ-801 tablet administration in the fed and fasted states was low, i.e. in the region of approximately 20–35%.
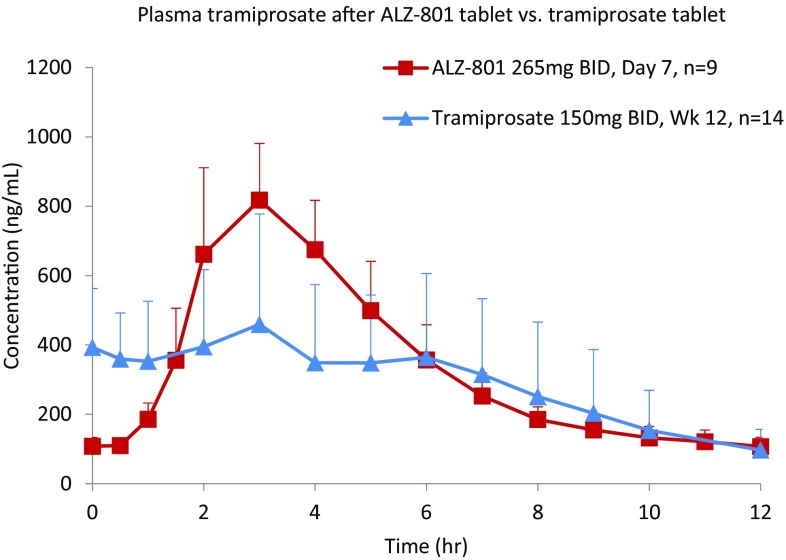
ALZ-801 Tablet versus Tramiprosate Tablet Comparison
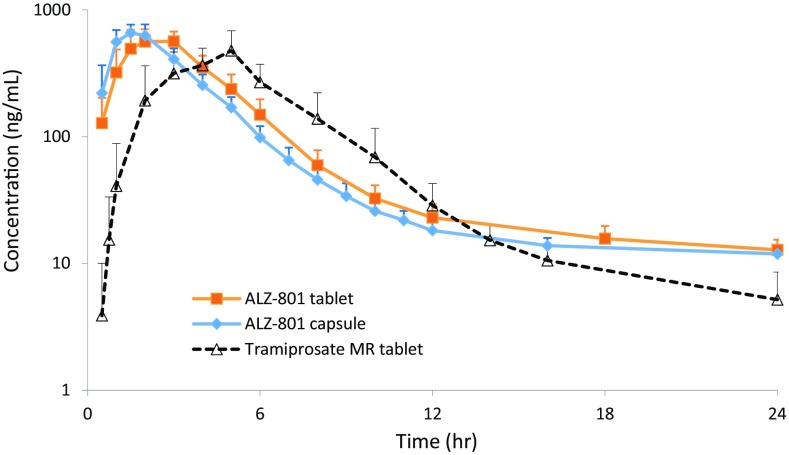
A composite comparison of the PK curves and variability for plasma tramiprosate in fasted healthy volunteers following a single dose of 171 mg ALZ-801 tablet versus loose capsule, and versus an equivalent dose of 100 mg tramiprosate modified-release tablet is shown in Fig. 8. Overall, relative to the tramiprosate tablet, oral ALZ-801 administration in either capsule or tablet formulation delivered a comparable level of plasma tramiprosate exposure, but prolonged the elimination half-life and reduced interindividual variability. When compared with the earlier phase II tramiprosate trial of 3 months’ duration [20], the steady-state plasma tramiprosate PK after 7-day twice-daily administration of the ALZ-801 tablet showed substantially lower variability (Fig. 9). A further comparison with the phase III tramiprosate study PK is shown in Fig. S3 in the electronic supplementary material.
Fig. 8.
Comparison of the pharmacokinetic curves and variability for plasma tramiprosate in fasted healthy volunteers following a single oral dose of 171 mg ALZ-801 tablet versus loose capsule, and versus a single equivalent oral dose of 100 mg tramiprosate modified-release tablet. MR modified-release
Fig. 9.
Comparison of pharmacokinetic variability of plasma tramiprosate following ALZ-801 tablet in the present phase I study versus tramiprosate tablet in an earlier phase II trial. bid twice daily
Urine Drug Recovery, Renal Clearance, and Estimate of Oral Bioavailability
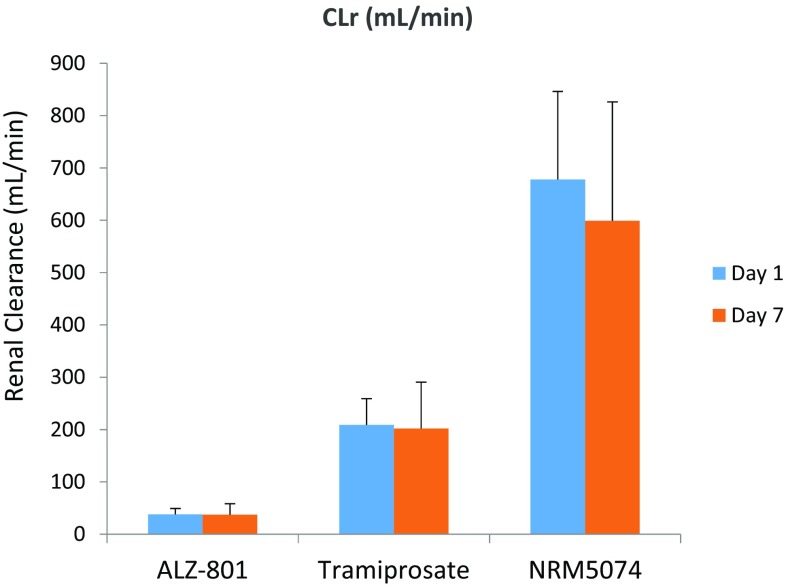
CLr of all three analytes was assessed in Study 2, which was similar for all four cohorts on both days 1 and 7, and day 14 for Cohorts A–C (representative Cohort D data shown in Fig. 10). For ALZ-801, the mean CLr (range 27.2–5.7 mL/min) across dosing cohorts and days was substantially lower than the glomerular filtration rate (GFR) of 125 mL/min in healthy humans, suggesting that renal elimination of ALZ-801 is likely to be via filtration with some reabsorption. For tramiprosate, the mean CLr (range 128–221 mL/min) was slightly higher than the GFR in most subjects, suggesting that, to some extent, active secretion contributes towards renal elimination. The mean CLr for NRM5074 (range 500–1570 mL/min) was much higher than GFR, indicating that this metabolite is renally cleared via active processes. Table 8 summarizes urinary recovery of all three analytes in Cohort D, which generated an estimated oral bioavailability of approximately 52% for the ALZ-801 tablet.
Fig. 10.
Urinary clearance of ALZ-801, tramiprosate, and NRM5074 following oral administration of ALZ-801 immediate-release tablet (Cohort D of Study 2: day 1, 265 mg once daily; days 2–6, 265 mg twice daily; and day 7, 265 mg once daily) in healthy human subjects. Data are summarized in Table 8. CLr renal clearance
Table 8.
Urinary drug clearance (CLr) and recovery of ALZ-801, tramiprosate, and NRM5074 following oral administration of ALZ-801 tablet (day 1, 265 mg once daily; days 2–6, 265 mg twice daily; and day 7, 265 mg once daily) in healthy human subjects (i.e. Cohort D of Study 2 in Table 1; n = 9)
| Time | Analytes | CLr (mL/min) | Plasma AUC24 (ng/mL h) | Mean Ae (mg) |
|---|---|---|---|---|
| Day 1 | ALZ-801 | 36.4 ± 10.7 | 10,300 ± 2830 | 22.5 |
| Tramiprosate | 201 ± 48.2 | 3650 ± 670 | 44.0 | |
| NRM5074 | 652 ± 161 | 1810 ± 348 | 70.8 | |
| Total recovery in urine | 137.3 | |||
| Day 7 | ALZ-801 | 35.9 ± 19.9 | 11,200 ± 1970 | 24.1 |
| Tramiprosate | 194 ± 85.1 | 5020 ± 859 | 58.4 | |
| NRM5074 | 575 ± 218 | 5470 ± 1030 | 188.7 | |
| Total recovery in urine | 271.3 | |||
Calculations were based on 24-h urine collections on days 1 and 7
CLr renal clearance, AUC 24 area under the concentration–time curve from time zero to 24 h, Ae amount of unchanged drug excreted into urine
Bridging Dose Analysis Based on the Phase III Tramiprosate Plasma Area Under the Curve Bioequivalence and Projection of Brain Drug Exposure
We aimed to define the clinical dose of ALZ-801 that achieves the plasma exposure of tramiprosate equivalent to the 150 mg twice-daily tramiprosate dose used in the previous phase III study [12]. In that study, the mean plasma exposure of tramiprosate at 78 weeks provided an AUC12 of 4429 ng/mL h. The accumulation factor for plasma AUC of tramiprosate after oral twice-daily dosing of ALZ-801 was approximately 1.3, which was consistent between the day 7 and day 1 data (Study 2, loose-filled capsules, MAD) and the theoretically modeled data (Study 3, immediate-release tablets, SAD). The dose versus tramiprosate exposure relationships following an oral ALZ-801 tablet after both single dose and at steady state are shown in Fig. 5. A linear regression of the steady-state dose versus AUC relationship for the two tablet doses (205 and 342 mg) under fed conditions yielded a projected dose of 262 mg twice daily for the ALZ-801 tablet. Thus, we proposed a preliminary dose of 265 mg twice daily for the ALZ-801 tablet for future phase III trials, to be administered orally under fed conditions considering the improved gastrointestinal tolerability. When compared with the totality of PK data obtained from the phase I studies (both capsule and tablet, both fasted and fed conditions), the 265 mg twice-daily dose fell within a linear dose–exposure relationship; therefore, the projected plasma tramiprosate AUC12 was approximately 4844, 4360 and 4502 ng/mL h for oral ALZ-801 under the fasted, fed, and fasted and fed combined conditions, respectively, at steady state.
Based on the brain:plasma ratio obtained from the rodent using the C14 method (i.e. ~ 40% projected after steady-state dosing) (Table 9), we further estimated the steady-state brain tramiprosate exposure following ALZ-801 tablet administration, relative to central nervous system soluble Aβ concentrations (Table 10). Overall, the average steady-state tramiprosate level after long-term administration of 265 mg ALZ-801 twice daily is expected to be over 5000- to 15,000-fold in excess of human cerebrospinal fluid (CSF) Aβ42 levels.
Table 9.
Brain penetration of tramiprosate after single-dose oral administration of tramiprosate and ALZ-801 in rodents
| A. Brain and plasma levels of radioactivity after a single oral dose of 14C-tramiprosate (100 or 500 mg/kg) in male C57BL/6 mice. N = 3 per dose/sampling time points; mean PK curves were used for calculation of PK parameters. Brains were perfused prior to bioanalysis | ||||||
|---|---|---|---|---|---|---|
| Tissue | Dose (mg/kg) | C max (µg eq/mL) | T max (h) | AUCt (µg eq/mL h) | AUCinf (µg eq/mL h) | t ½ (h) |
| Plasma | 100 | 8.01 | 2 | 68.8 | 69.3 | 3.28 |
| 500 | 28.9 | 4 | 388 | 397 | 4.43 | |
| Brain | 100 | 0.741 | 8 | 13.2 | 26.1 | 20.6 |
| 500 | 3.64 | 12 | 63.0 | NC | NC | |
| B. Comparison of brain and plasma tramiprosate exposure (mean AUCt; ng/ml h) after a single oral dose of ALZ-801 vs. tramiprosate (equivalent dose) in male CD-1 mice. N = 6 per group/sampling time points; mean PK curves were used for calculation of PK parameters. Drug concentrations were measured using an LC-MS method | |||
|---|---|---|---|
| Tissue | ALZ-801 dose (172 mg/kg) | Tramiprosate dose (100 mg/kg) | ALZ-801:tramiprosate ratio |
| Plasma | 58,758 | 32,274 | 1.8 |
| Brain | 5841 | 1861 | 3.1 |
PK pharmacokinetic, C max maximum concentration, T max time to reach Cmax, AUC t area under the concentration–time curve from time zero to time t, AUC inf area under the concentration–time curve from time zero to infinity, t ½ half-life, LC-MS liquid chromatography-mass spectrometry, NC not calculated; terminal mono-exponential phase contained only two points
Table 10.
Projected steady-state plasma and brain tramiprosate exposures following oral administration of ALZ-801 tablet 265 mg twice-daily in humans
| Parameters | Tramiprosate exposure |
|---|---|
| Plasma AUC12 | 34.8 μΜ h |
| Projected brain AUC12 | 13.9 μM h (14C method)a |
| Projected brain average concentration (C ss,av) | 550 nM (14C method)a |
| Projected multiple excess of brain drug concentration versus soluble Aβ | > 5000- to 15,000-fold |
aBased on 19% single dose and approximately 40% steady-state brain penetration based on 14C method. Multiple excess of drug exposure analyses based on CSF soluble Aβ concentrations of 0.035–0.1 nM [23, 24, 28], and comparable levels in brain parenchyma as per published human microdialysis data [21, 22]
AUC 12 area under the concentration–time curve from time zero to 12 h, Css,av average concentration at steady state
Discussion
ALZ-801 is in development as an oral anti-amyloid agent with potential disease-modifying effects for the treatment of AD. ALZ-801 is a prodrug of tramiprosate, designed to improve the PK properties and gastrointestinal tolerability through conjugation of tramiprosate with the amino acid valine. The earlier tramiprosate phase III program included 2025 patients with mild-to-moderate AD, and evaluated two active doses, 100 mg twice daily and 150 mg twice daily. In the completed phase III North American Study in 1052 AD patients, the co-primary outcomes in the overall study population did not achieve statistical significance; however, the APOE4 genotype was found to have a significant effect on efficacy [12, 19].
Recent clinical and nonclinical data have advanced our understanding of the role of APOE4 in AD pathogenesis. Amyloid PET imaging from recent large AD trials has shown high rates of negative amyloid scans in APOE4 non-carriers, while APOE4 homozygotes were predominantly (98%) amyloid-positive [16]. APOE4 homozygotes were also found to have a significantly higher burden of soluble amyloid oligomers compared with non-carriers [17, 18]. AD patients with the APOE4/4 genotype therefore represent a phenotype that is enriched with amyloid pathology and is considered most homogeneous.
With this improved understanding, prespecified post hoc analyses of the tramiprosate phase III data were performed based on the number of APOE4 alleles in three subgroups: non-carriers (predominantly APOE3/3), heterozygotes (predominantly APOE3/4), and homozygotes (APOE4/4). In the APOE4/4 subgroup, tramiprosate 150 mg twice daily showed a significant and potentially meaningful cognitive benefit over 78 weeks, supported by positive trends on CDR-SB [12]. Further sensitivity analyses in mild APOE4/4 patients (MMSE of ≥ 22) indicated that tramiprosate 150 mg twice daily displayed efficacy on ADAS-cog and CDR-SB that was large in magnitude and durable over 78 weeks (p values < 0.001 and < 0.02, respectively) [19].
Tramiprosate inhibits Aβ oligomer formation and aggregation [7–10], and interacts directly with the soluble Aβ monomers and stabilizes them in a concentration-dependent, multi-ligand enveloping fashion, thereby preventing the formation of toxic oligomer species and the progression of amyloid cascade [11]. Furthermore, the molecular stoichiometry of tramiprosate was in alignment with the clinical drug dose exposure, suggesting that this mechanism of action may mediate its observed clinical benefits in AD patients [11]. To exploit this efficacy attribute of tramiprosate while circumventing its limitations (gastrointestinal side effects and high intersubject PK variability), we developed the prodrug ALZ-801.
In the phase I studies, oral administration of ALZ-801 either as a loose-filled capsule or tablet, showed good safety and tolerability in healthy adults and elderly volunteers. There were no SAEs or TEAEs, or AEs leading to discontinuation, and no specific findings in safety laboratories, vitals, or ECGs. The gastrointestinal side effects of nausea and vomiting were significantly improved over oral tramiprosate in previous clinical trials [28], and did not exhibit dose or exposure dependency, suggesting a likely mild local upper gastrointestinal tract irritation. It is of interest to note that in the 14-day MAD study, following an initial titration with a reduced dose in the first week, the incidence of nausea and vomiting was markedly reduced during the second week, suggesting the development of tolerance on continued use. Administration of ALZ-801 with food further reduced gastrointestinal symptoms in some subjects; thus, no dose-limiting toxicity was observed over 2 weeks of dosing at doses up to 342 mg twice daily, which exceeds the proposed 265 mg dose regimen of ALZ-801 (265 mg yields tramiprosate AUC plasma exposure equivalent to oral tramiprosate 150 mg) for future pivotal efficacy trials.
The SAD and MAD PK analyses indicated that ALZ-801, dosed as either a capsule or a tablet, was absorbed rapidly on oral administration, following the release of tramiprosate in plasma likely via a hepatic or plasma amidase, which was then subsequently metabolized to its major primary metabolite NRM5074. The presence of the prodrug ALZ-801 in plasma was short (elimination t ½ ≤ 30 min) at all doses studied, whereas the elimination half-life for tramiprosate was much longer (~ 18 h). This long apparent t ½ for tramiprosate following an oral dose of the prodrug most likely reflects the consistent release of tramiprosate from the prodrug. Importantly, the plasma C max and AUC exposures for both ALZ-801 and tramiprosate were highly dose-proportional, reflecting linear kinetics.
In the MAD study, we observed no significant accumulation or decrease of plasma ALZ-801 and tramiprosate over 7 days of twice-daily administration, when plasma tramiprosate reached its steady state. The plasma AUC exposures for ALZ-801 and tramiprosate were dose-proportional at steady state. In addition, the elimination half-life for tramiprosate was prolonged on day 14 compared with day 1.
We compared the PK profiles for oral ALZ-801 versus oral tramiprosate (either in a capsule or tablet formulation). Overall, ALZ-801 delivered an equivalent exposure of tramiprosate in plasma compared with an equimolar dose of oral tramiprosate. However, ALZ-801 exhibited two substantially improved characteristics over tramiprosate: [1] a longer elimination half-life of plasma tramiprosate, which reflects the release of tramiprosate from the prodrug; and [2] a substantially reduced interindividual PK variability, which occurred in all cohorts and is presumably due to the reduced tramiprosate metabolism in the gut. Both improvements may lead to better pharmacodynamic effects.
In the presence of food, a modest, non-significant reduction in the oral absorption of ALZ-801 was observed, such that the presence of food slowed the absorption and reduced the C max of tramiprosate; however, the reduction in AUC exposure of tramiprosate was minimal and was not considered to be clinically meaningful. In addition, the presence of food did not affect the dose-proportionality of ALZ-801 or tramiprosate, which suggests that while the rate of absorption is reduced, the extent of absorption is unaffected by the administration of ALZ-801 in the fed state.
Importantly, administration of ALZ-801 in the fed state reduced the incidence of gastrointestinal symptoms while maintaining the total exposure of tramiprosate. Thus, it is likely that administration with food provides a gastroprotective local effect in the gut rather than a central effect linked to drug exposure. Additionally, the ALZ-801 tablet formulation further delayed the absorption compared with the capsule, but did not affect the AUC exposure of tramiprosate in plasma. Finally, there was no apparent age-related effect on PK parameters within the subjects studied.
We also analyzed the CLr of all three analytes in the MAD study, which was similar for all four cohorts at all time points. The data indicate that renal elimination of ALZ-801 is likely to be via filtration with some re-absorption, and that, for tramiprosate, active secretion contributes, to some extent, towards renal elimination. While the mechanism for apparent secretion of tramiprosate is unknown, no effect on the family of renal transporters, including organic anion transporter (OAT) 1 and OAT3, was observed at concentrations up to 100 mM (data on file). Since ALZ-801 is a prodrug, and no absolute oral bioavailability had been previously obtained, we estimated its bioavailability based on the urinary recovery data for the three analytes, which generated an approximate oral bioavailability of tramiprosate of 52% for the 265 mg ALZ-801 immediate-release tablet.
Based on the PK data for ALZ-801, and the clinically effective tramiprosate exposure, we projected the clinical dose for ALZ-801 to be used in future efficacy trials. While our animal model data indicate that oral ALZ-801 delivers tramiprosate into the brain more efficiently than oral tramiprosate (Table 9B), we adopted a conservative analytical approach based on plasma AUC for clinical dose projection. Our goal was to provide a plasma exposure of tramiprosate equivalent to the 150 mg twice-daily tramiprosate dose in the previous phase III study, which produced a robust cognitive and functional efficacy in APOE4/4 AD patients [12, 19]. Based on the dose-exposure linearity and a modest accumulation factor of 1.3 at steady state, we projected a dose of 265 mg twice daily for the ALZ-801 tablet, to be administered orally under fed conditions.
We further estimated brain drug exposure following long-term administration of the ALZ-801 tablet, based on the brain:plasma ratio derived from rodents. Overall, the average steady-state tramiprosate level at the ALZ-801 dose of 265 mg twice daily is projected to reach approximately 550 nM, which is over 5000- to 15,000-fold in excess of human CSF Aβ42 levels. The published brain Aβ data are variable, but the recent microanalysis studies in humans suggest that the interstitial soluble Aβ42 levels [21, 22] are comparable with those in CSF [23, 24]. Since CSF Aβ reflects diffusible peptide in the brain [25–28], its decrease as seen in the tramiprosate phase II study is considered a good indicator of changes in brain diffusible Aβ levels [20]. The predicted brain drug exposure following ALZ-801 265 mg twice daily also aligns with the previously observed CSF drug levels (i.e. 60 nM) after oral tramiprosate 150 mg twice daily for 78 weeks [11].
Together, this PK and brain penetration analysis of ALZ-801 tablets is projected to yield a steady-state CNS exposure of tramiprosate that maintains over three orders of magnitude in excess of soluble Aβ42 in the brain, which, according to molecular mechanism of action studies [11], is sufficient to block the formation of toxic oligomers and amyloid aggregation. This is consistent with our experimental IMS-MS results where a 1000-fold excess of tramiprosate completely abrogated the formation of Aβ oligomers [11]. Considering the much-improved individual PK variability of oral ALZ-801 than oral tramiprosate, a better and more consistent clinical efficacy outcome is expected in the pivotal phase III efficacy studies.
Conclusions
The phase I bridging PK data show that the oral dose of 265 mg ALZ-801 tablet, administered twice daily, yields AUC plasma exposure equivalent to oral tramiprosate 150 mg twice daily from the previous completed tramiprosate phase III trial. Moreover, the overall improvements in safety, tolerability, and PK properties of ALZ-801 support further confirmatory efficacy studies in AD patients. ALZ-801 has the promise to be a new, orally available, small molecule amyloid-targeting treatment with a favorable safety profile, and the ability to slow the progression of AD and extend the efficacy of the current standard-of-care therapies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
ALZ-801 studies were conducted at Quintiles (London, UK) and Quotient Clinical (Nottingham, UK). Tablet manufacturing and CMC work for phase I studies were conducted at Quotient Clinical (Nottingham, UK). The authors would like to thank Dr. Diane Jorkasky for her expert advice and review of the paper, and Christine Rathbun for assistance with graphs.
Author Contributions
JAH wrote the article in collaboration with JYY. All co-authors reviewed and contributed to the article.
Compliance with Ethical Standards
Funding
The studies summarized in this report were supported by Alzheon, Inc.
Conflict of interest
John A. Hey, Susan Abushakra, Petr Kocis, Aidan Power, Paul L. Kaplan, and Martin Tolar are employees of Alzheon, Inc. Jeremy Y. Yu, Mark Versavel, and John Amedio have served as consultants or advisors to Alzheon, Inc., and may own Alzheon stock options.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s40262-017-0608-3) contains supplementary material, which is available to authorized users.
References
- 1.Qaseem A, Snow V, Cross JT, Jr, Forciea MA, Hopkins R, Jr, Shekelle P, Adelman A, Mehr D, Schellhase K, Campos-Outcalt D, Santaguida P, Owens DK. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2008;148:370–378. doi: 10.7326/0003-4819-148-5-200803040-00008. [DOI] [PubMed] [Google Scholar]
- 2.Kanekiyo T, Xu H, Bu G. ApoE and Abeta in Alzheimer’s disease: accidental encounters or partners? Neuron. 2014;81:740–754. doi: 10.1016/j.neuron.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8(6):595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris GP, Clark IA, Vissel B. Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer’s disease. Acta Neuropathol Commun. 2014;2:135. doi: 10.1186/s40478-014-0135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reardon S. Antibody drugs for Alzheimer’s show glimmers of promise. Nature. 2015;523:509–510. doi: 10.1038/nature.2015.18031. [DOI] [PubMed] [Google Scholar]
- 6.Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, O’Gorman J, Qian F, Arastu M, Li M, Chollate S, Brennan MS, Quintero-Monzon O, Scannevin RH, Arnold HM, Engber T, Rhodes K, Ferrero J, Hang Y, Mikulskis A, Grimm J, Hock C, Nitsch RM, Sandrock A. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 7.Gervais F, Paquette J, Morissette C, Krzywkowski P, Yu M, Azzi M, Lacombe D, Kong X, Aman A, Laurin J, Szarek WA, Tremblay P. Targeting soluble Abeta peptide with tramiprosate for the treatment of brain amyloidosis. Neurobiol Aging. 2007;28:537–547. doi: 10.1016/j.neurobiolaging.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Martineau E, de Guzman JM, Rodionova L, Kong X, Mayer PM, Aman AM. Investigation of the noncovalent interactions between anti-amyloid agents and amyloid beta peptides by ESI-MS. J Am Soc Mass Spectrom. 2010;21:1506–1514. doi: 10.1016/j.jasms.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Caltagirone C, Ferrannini L, Marchionni N, Nappi G, Scapagnini G, Trabucchi M. The potential protective effect of tramiprosate (homotaurine) against Alzheimer’s disease: a review. Aging Clin Exp Res. 2012;24:580–587. doi: 10.3275/8585. [DOI] [PubMed] [Google Scholar]
- 10.Young LM, Saunders JC, Mahood RA, Revill CH, Foster RJ, Tu LH, Raleigh DP, Radford SE, Ashcroft AE. Screening and classifying small-molecule inhibitors of amyloid formation using ion mobility spectrometry-mass spectrometry. Nat Chem. 2015;7:73–81. doi: 10.1038/nchem.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kocis P, Tolar M, Yu J, Sinko W, Ray S, Blennow K, Fillit H, Hey JA. Elucidating the Abeta42 Anti-Aggregation Mechanism of Action of Tramiprosate in Alzheimer’s Disease: Integrating Molecular Analytical Methods. Pharmacokinetic and Clinical Data. CNS Drugs. 2017;31:495–509. doi: 10.1007/s40263-017-0434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abushakra S, Porsteinsson A, Vellas B, Cummings J, Gauthier S, Hey JA, Power A, Hendrix S, Wang P, Shen L, Sampalis J, Tolar M. Clinical benefits of tramiprosate in alzheimer’s disease are associated with higher number of APOE4 alleles: the “APOE4 gene-dose effect”. J Prev Alz Dis. 2016;3:219–228. doi: 10.14283/jpad.2016.115. [DOI] [PubMed] [Google Scholar]
- 13.Gauthier S, Aisen PS, Ferris SH, Saumier D, Duong A, Haine D, Garceau D, Suhy J, Oh J, Lau W, Sampalis J. Effect of tramiprosate in patients with mild-to-moderate Alzheimer’s disease: exploratory analyses of the MRI sub-group of the Alphase study. J Nutr Health Aging. 2009;13:550–557. doi: 10.1007/s12603-009-0106-x. [DOI] [PubMed] [Google Scholar]
- 14.Saumier D, Duong A, Haine D, Garceau D, Sampalis J. Domain-specific cognitive effects of tramiprosate in patients with mild to moderate Alzheimer’s disease: ADAS-cog subscale results from the Alphase Study. J Nutr Health Aging. 2009;13:808–812. doi: 10.1007/s12603-009-0217-4. [DOI] [PubMed] [Google Scholar]
- 15.Aisen PS, Gauthier S, Ferris SH, Saumier D, Haine D, Garceau D, Duong A, Suhy J, Oh J, Lau WC, Sampalis J. Tramiprosate in mild-to-moderate Alzheimer’s disease - a randomized, double-blind, placebo-controlled, multi-centre study (the Alphase Study) Arch Med Sci. 2011;7:102–111. doi: 10.5114/aoms.2011.20612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Degenhardt EK, Witte MM, Case MG, Yu P, Henley DB, Hochstetler HM, D’Souza DN, Trzepacz PT. Florbetapir F18 PET amyloid neuroimaging and characteristics in patients with mild and moderate Alzheimer dementia. Psychosomatics. 2016;57:208–216. doi: 10.1016/j.psym.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto T, Serrano-Pozo A, Hori Y, Adams KW, Takeda S, Banerji AO, Mitani A, Joyner D, Thyssen DH, Bacskai BJ, Frosch MP, Spires-Jones TL, Finn MB, Holtzman DM, Hyman BT. Apolipoprotein E, especially apolipoprotein E4, increases the oligomerization of amyloid beta peptide. J Neurosci. 2012;32:15181–15192. doi: 10.1523/JNEUROSCI.1542-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu-Seifert H, Siemers E, Sundell K, Price K, Han B, Selzler K, Aisen P, Cummings J, Raskin J, Mohs R. Cognitive and functional decline and their relationship in patients with mild Alzheimer’s dementia. J Alzheimers Dis. 2015;43:949–955. doi: 10.3233/JAD-140792. [DOI] [PubMed] [Google Scholar]
- 19.Abushakra S, Porsteinsson A, Scheltens P, Sadowsky C, Vellas B, Cummings J, Gauthier S, Hey JA, Power A, Wang P, Shen L, Tolar M. Clinical effects of tramiprosate in APOE4/4 homozygous patients with mild Alzheimer’s disease suggest disease modification potential. J Prev Alzheimers Dis. 2017;4:149–156. doi: 10.14283/jpad.2017.26. [DOI] [PubMed] [Google Scholar]
- 20.Aisen PS, Saumier D, Briand R, Laurin J, Gervais F, Tremblay P, Garceau D. A Phase II study targeting amyloid-beta with 3APS in mild-to-moderate Alzheimer disease. Neurology. 2006;67:1757–1763. doi: 10.1212/01.wnl.0000244346.08950.64. [DOI] [PubMed] [Google Scholar]
- 21.Herukka SK, Rummukainen J, Ihalainen J, von Und Zu, Fraunberg M, Koivisto AM, Nerg O, Puli LK, Seppala TT, Zetterberg H, Pyykko OT, Helisalmi S, Tanila H, Alafuzoff I, Hiltunen M, Rinne J, Soininen H, Jaaskelainen JE, Leinonen V. Amyloid-beta and tau dynamics in human brain interstitial fluid in patients with suspected normal pressure hydrocephalus. J Alzheimers Dis. 2015;46:261–269. doi: 10.3233/JAD-142862. [DOI] [PubMed] [Google Scholar]
- 22.Brody DL, Magnoni S, Schwetye KE, Spinner ML, Esparza TJ, Stocchetti N, Zipfel GJ, Holtzman DM. Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science. 2008;321:1221–1224. doi: 10.1126/science.1161591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ, Alzheimer’s Disease Neuroimaging, I. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol. 1999;155:853–862. doi: 10.1016/S0002-9440(10)65184-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aisen PS, Gauthier S, Vellas B, Briand R, Saumier D, Laurin J, Garceau D. Alzhemed: a potential treatment for Alzheimer’s disease. Curr Alzheimer Res. 2007;4:473–478. doi: 10.2174/156720507781788882. [DOI] [PubMed] [Google Scholar]
- 26.Barten DM, Guss VL, Corsa JA, Loo A, Hansel SB, Zheng M, Munoz B, Srinivasan K, Wang B, Robertson BJ, Polson CT, Wang J, Roberts SB, Hendrick JP, Anderson JJ, Loy JK, Denton R, Verdoorn TA, Smith DW, Felsenstein KM. Dynamics of {beta}-amyloid reductions in brain, cerebrospinal fluid, and plasma of {beta}-amyloid precursor protein transgenic mice treated with a {gamma}-secretase inhibitor. J Pharmacol Exp Ther. 2005;312:635–643. doi: 10.1124/jpet.104.075408. [DOI] [PubMed] [Google Scholar]
- 27.Best JD, Jay MT, Otu F, Churcher I, Reilly M, Morentin-Gutierrez P, Pattison C, Harrison T, Shearman MS, Atack JR. In vivo characterization of Abeta(40) changes in brain and cerebrospinal fluid using the novel gamma-secretase inhibitor N-[cis-4-[(4-chlorophenyl)sulfonyl]-4-(2,5-difluorophenyl)cyclohexyl]-1,1,1-trifl uoromethanesulfonamide (MRK-560) in the rat. J Pharmacol Exp Ther. 2006;317:786–790. doi: 10.1124/jpet.105.100271. [DOI] [PubMed] [Google Scholar]
- 28.Pannee J, Portelius E, Minthon L, Gobom J, Andreasson U, Zetterberg H, Hansson O, Blennow K. Reference measurement procedure for CSF amyloid beta (Abeta)1-42 and the CSF Abeta1-42 /Abeta1-40 ratio: a cross-validation study against amyloid PET. J Neurochem. 2016;139:651–658. doi: 10.1111/jnc.13838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.