Abstract
Red blood cell (RBC)-derived adenosine triphosphate (ATP) has been proposed as an integral component in the regulation of oxygen supply to skeletal muscle. In ex vivo settings RBCs have been shown to release ATP in response to a number of stimuli, including stimulation of adrenergic receptors. Further evidence suggested that ATP release from RBCs was dependent on activation of adenylate cyclase (AC)/cyclic adenosine monophosphate (cAMP)-dependent pathways and involved the pannexin 1 (Panx1) channel. Here we show that RBCs express Panx1 and confirm its absence in Panx1 knockout (−/−) RBCs. However, Panx1−/− mice lack any decrease in exercise performance, challenging the assumptions that Panx1 plays an essential role in increased blood perfusion to exercising skeletal muscle and therefore in ATP release from RBCs. We therefore tested the role of Panx1 in ATP release from RBCs ex vivo in RBC suspensions. We found that stimulation with hypotonic potassium gluconate buffer resulted in a significant increase in ATP in the supernatant, but this was highly correlated with RBC lysis. Next, we treated RBCs with a stable cAMP analog, which did not induce ATP release from wild-type or Panx1−/− mice. Similarly, multiple pharmacological treatments activating AC in RBCs increased intracellular cAMP levels (as measured via mass spectrometry) but did not induce ATP release. The data presented here question the importance of Panx1 for exercise performance and dispute the general assumption that ATP release from RBCs via Panx1 is regulated via cAMP.
Keywords: red blood cells, ATP, cAMP, pannexin 1, purinergic signaling, luciferin/luciferase assay, hemolysis, hypoxic vasodilation, exercise
roles for extracellular nucleotides in vasodilatory signaling pathways have long been established (2, 40). ATP has been recognized as an important autocrine/paracrine regulator of cell signaling and function involving P2X (inotropic) and P2Y (metabotropic) receptors causing changes in membrane permeability (Na+, Ca2+) and potential (2, 40). A well-established hypothesis is that erythrocyte-derived ATP release into the lumen of small blood vessels is an integral component in balancing supply of oxygen to skeletal muscle (9–11). The discoveries in the mid-1990s that hypoxia (11) and mechanical deformation (34) each caused ATP release from red blood cells (RBCs) inspired an array of follow-up studies to understand the mechanism(s) underlying these phenomena, including studies proposing mechanisms of mechanosensing and mechanotransduction in RBCs (12, 38, 39). As summarized recently (32), the reported pathway proposes that hemoglobin desaturation signals via Gαi [and not Gαs] to activate adenylyl cyclase (AC) and produce cyclic adenosine monophosphate (cAMP), which activates protein kinase A (PKA) to stimulate ATP release through pannexin 1 (Panx1) in a cystic fibrosis transmembrane conductance regulator (CFTR)-dependent mechanism. Indeed, RBCs were shown to express Panx1 on their surface, and Panx1 was proposed to be the main channel regulating ATP release from RBCs in response to high extracellular K+ and hypotonic stress (19). Panx1 was also identified as the likely route of hypoxia-driven ATP release from RBCs, as assessed by inhibiting the latter with three pharmacological inhibitors of Panx1 (36). Because of the well-known off-target effects of Panx1 inhibitors, the experiments were confirmed using RBCs from Panx1−/− mice (25). However, in contrast to the studies implicating CFTR (33) and cAMP (35) in ATP release from RBCs, Panx1-dependent ATP release was not initially tested downstream of mechanical stimulation, so the connectivity between these elements of the reported pathway is unclear (35). More recently, the hypothesized connection between mechanical stimulation of RBCs and Panx1-dependent ATP release was explored with a single nonspecific inhibitor of Panx1 (5). Moreover, adding to the general confusion in the field, an independent group could not confirm the presence of Panx1 on mouse RBCs (21), and hemolysis was proposed recently as the main factor responsible for ATP release from RBCs (30).
In this work, the expression and membrane localization of Panx1 was analyzed on mouse RBCs, while the role of cAMP signaling on ATP release was studied in RBCs from Panx1−/− mice, wild-type (WT) mice, and humans. The data presented here confirm the presence of Panx1 on the RBC membrane; however, they do not confirm the general assumption that ATP release from RBCs via Panx1 is regulated by cAMP. A critical discussion on the pitfalls encountered in assessment of ATP release from RBCs via Panx1 is provided.
MATERIALS AND METHODS
Materials
Rabbit polyclonal antibodies against the second extracellular loop (EL2) and carboxy-terminus (CT) of Panx1 were a generous gift from Silvia Penuela and Dale Laird (University of Western Ontario, London, Canada). Rabbit monoclonal antibody against the amino-terminus (NT) of Panx1 was purchased from Thermo Fisher (no. 487900). Spectrin mouse monoclonal antibody was purchased from Sigma Aldrich (no. S3396). Connexin 43 (Cx43) rabbit polyclonal antibody was purchased from Sigma Aldrich (no. C6219).
Human Subjects
Human blood samples were acquired from healthy male and female volunteers between 20 and 30 yr old in concordance with the study protocol approved by the ethics committee of the Heinrich-Heine University. All subjects gave written informed consent in accordance with the declaration of Helsinki.
Animals
Mice were handled according to approved animal protocols at the University of Virginia. Panx1−/− (24) and wild-type control (C57Bl/6 background) mice of both sexes aged between 9 and 14 wk were used for this study.
Forced Exercise Capacity Test
In accordance with established methods (18), mice were acclimatized to the treadmill for three consecutive days before the experimental day. Acclimatization consisted of 10 min of treadmill running at 0% incline and speed of 13 m/min each day. On the day of testing, the treadmill was set to 5% incline. Running speed was set at 13 m/min for 30 min and increased by 2.7 m/min every 30 min (maximum of 27 m/min) until perceived exhaustion, as determined by refusal to run despite encouragement for 20 s. Blood lactate was measured immediately before and following the acute exercise test to verify exhaustion.
Voluntary Exercise Capacity
As published previously (18), mice were randomly selected and housed individually in cages with running wheels. Mice were allowed free access to the running wheel, food, and water. Sedentary mice were kept in communal cages. Daily running distance was recorded via computer monitoring for 14 days.
Blood Collection and Separation of RBCs
Human whole blood was collected into heparin-coated tubes and RBCs were immediately centrifuged for 10 min, at 800 g and 4°C (Hettich, Rotina 38R, 2140 rpm). Plasma and buffy coat were aspirated. The RBC pellets were washed three times with Hanks’ buffered salt solution (HBSS) containing calcium and 0.5% bovine serum albumin (BSA) by centrifugation at 300 g for 10 min at 4°C. Washed RBCs were re-suspended to 20% hematocrit (hct) in HBSS containing 0.5% BSA and used for experiments. To collect mouse blood, mice were anesthetized and exsanguinated via cardiac puncture with syringes coated with EDTA (no. E5134, Sigma-Aldrich) before use to prevent coagulation. Whole blood was centrifuged for 5 min, at 500 g and room temperature (RT) (Fisher, accuSpin Micro 17, 2300 rpm), after which plasma and buffy coat were removed by aspiration. The remaining RBC pellet was washed three times in a modified Krebs-Henseleit (K-H) buffer (1.2 mM KH2PO4, 5.0 mM KCl, 1.2 mM MgSO4, 1.6 mM CaCl2, 118 mM NaCl, 24.8 mM NaHCO3, 10 mM glucose) and centrifuged for 5 min at 500 g between each wash. Washed RBCs were resuspended to 5% hct in K-H buffer and used for experiments.
Detection and Localization of Panx1 on RBC Membrane by Immunostaining
For detection of Panx1 by immunohistochemistry, mouse afferent arteriole kidney sections were fixed in 4% paraformaldehyde, treated with Histoclear (Sigma Aldrich), progressive ethanol washes, and H2O2, then incubated with primary antibodies (Panx1 NT, Cx43, IgG control, or no primary) overnight at 4°C, and subsequently biotin-conjugated anti-rabbit IgG secondary antibody then 3,3′-diaminobenzidine (DAB) solution, and finally hematoxylin and eosin staining before mounting. DAB-stained kidney slides were imaged using a Nikon Eclipse TS100 microscope at ×20 objective. For detection of Panx1 by immunofluorescence, washed RBCs were blocked (PBS + 10% fish skin gelatin, 0.25% Triton X-100, 0.5% BSA, 10% donkey serum) and incubated with primary antibodies (Panx1 EL2 and CT) while rocking overnight at 4°C, and AlexaFluor donkey anti-rabbit secondary antibodies while rocking for 2 h at RT. RBCs were then mounted onto slides via Cytospin for 5 min at 1,000 g, mounting medium was added, and a coverslip was applied. Slides were imaged using an Olympus IX81/FV1000 confocal microscope at ×20 objective.
Western Blot Analysis of Panx1 Expression in RBCs
Washed RBCs were lysed in 10 times their volume of hypotonic RBC lysis buffer with two changes of buffer (three washes total), and RBC ghost membranes were collected by centrifuging for 5 min at 3,000 g. RBC ghosts were added to RIPA buffer (50 mM Tris·HCl, 150 mM NaCl, 5 mM EDTA, 0.5% Triton X-100, 1% sodium deoxycholate, 20 mM NaF, 1 mM NaVO4, pH 7.4, supplemented with 10 µl/ml each of protease inhibitor cocktail PI and phosphatase inhibitor cocktails P2 and P3), mixed with Laemmli buffer and subjected to SDS-PAGE on a 4–12% Bis-Tris Midi gel (no. WG1401BX10, Invitrogen) followed by transfer to nitrocellulose membrane. Blots were blocked in TBS-T [0.2% Tween in TBS (50 mM Tris, 150 mM NaCl, pH 7.53)] containing 3% BSA and incubated overnight with primary antibodies at 4°C, followed by fluorescently tagged secondary antibodies (goat anti-mouse and goat anti-rabbit; LICOR) for 1 h at RT. Both primary and secondary antibody incubations took place in 5% BSA in TBS-T. Blots were imaged via fluorescence using a LICOR Odyssey imaging system.
Extraction and Measurement of Intracellular cAMP
For extraction of intracellular nucleotides, cells were lysed and protein precipitated by addition of an ice-cold solution of 20% acetonitrile, 40% methanol, and 40% water to the RBC pellet, followed by incubation at 60°C for 10 min. Samples were then cooled on ice and centrifuged at 3,000 g for 30 min at 4°C to remove cellular debris. The supernatant was removed and kept separately while cell pellets were washed with the extraction solution and centrifuged again as described above. The supernatants of both extraction steps were combined and taken to dryness in a Speedvac system. Purines were separated by reversed-phase liquid chromatography (Waters UPLC BEH C18 column, 1.7 µm beads; 2.1 × 150 mm; Milford, MA) and assayed using a triple quadrupole mass spectrometer (TSQ Quantum-Ultra; ThermoFisher Scientific, San Jose, CA) operating in the selected reaction monitoring mode with a heated electrospray ionization source essentially as described (15). The mobile phase consisted of a linear gradient of 1% acetic acid in water (pH 3; mobile phase A) and 100% methanol (mobile phase B). The mobile phase flow rate was 300 µl/min and was delivered with a Waters Acquity ultra-performance liquid chromatographic system. The gradient (A/B) was as follows: from 0 to 2 min, 99.6%/0.4%; from 2 to 3 min, to 98.0%/2.0%; from 3 to 4 min, to 85.0%/15.0%; from 4 to 6.5 min, to 99.6%/0.4%. Instrument settings were as follows: sample tray temperature, 10°C; column temperature, 50°C; ion spray voltage, 4.0 kV; ion transfer tube temperature, 350°C; source vaporization temperature, 320°C; Q2 CID gas, argon at 1.5 mTorr; sheath gas, nitrogen at 60 psi; auxiliary gas, nitrogen at 35 psi; Q1/Q3 width, 0.7/0.7 units full-width half-maximum; scan width, 0.6 units; scan time, 0.01 s.
Protocols for Induction of ATP Release
Hypotonic potassium gluconate stimulation.
Washed mouse RBCs were resuspended in potassium gluconate (KGlu) solution (150 mM KGlu in H2O) containing 300 µM ARL 67156 trisodium (ARL) (no. 1283, Tocris) at 1% hct and incubated for 30 min at RT. Afterwards, samples were diluted with an equal volume of either 150 mM KGlu solution or H2O and incubated at RT for 5 min at RT.
Stimulation with 8Br-cAMP.
Washed RBCs were resuspended in K-H buffer at 10% hct and mixed with an equal volume of K-H buffer containing either 200 µM carbenoxolone (CBX) (no. 154930, MP Biomedicals) or vehicle to reach 5% hct and a final concentration of CBX of 100 µM. RBCs were incubated at RT for 120 min. 8Br-cAMP (100 µM) or vehicle was added and samples were incubated for 30 min at RT before analysis. A 50 µl sample was removed for ATP analysis without centrifugation.
Induction of intracellular cAMP production.
Washed human RBCs were diluted to 20% hct in 0.5% BSA HBSS. Samples were pretreated with the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX) or left untreated as a control for 10 min at 37°C with gentle shaking, and then treated for 10 min at 37°C with isoproterenol, forskolin, vehicle control (DMSO), or left untreated for control, as indicated. RBCs were pelleted by centrifugation (10 min at 300 g at RT). ATP in the supernatant was assessed as described below in Measurement of ATP in the cell supernatant. Intracellular nucleotides were extracted from RBC pellets and cAMP was assessed by liquid chromatography mass spectrometry as described above in Extraction and Measurement of Intracellular cAMP.
Measurement of intracellular ATP.
Washed RBCs at 5% hct in K-H buffer were lysed in 10× volume of H2O, then vortexed at top speed for 10 s and sonicated 10 × 1 s to ensure thorough lysis. An aliquot of this lysed suspension was diluted 10× in K-H buffer for analysis as described below in Measurement of ATP in the cell supernatant.
Measurement of ATP in the cell supernatant.
Extracellular ATP release was measured using a luciferin-luciferase assay as described previously (35, 36) by mixing equal parts crude firefly tail extract (10 mg/ml in diH2O, no. FLE250, Sigma Aldrich) and d-luciferin (0.5 mg/ml in diH2O, no. L9504, Sigma Aldrich). Alternately, a luciferase assay kit [ATP Bioluminescence Assay Kit HS II (Roche) or ATP Determination Kit (no. A22066, Invitrogen)] was also used as indicated in the figure legends. For mouse experiments, 50 µl of each sample was transferred into a well of a 96-well plate (no. 12565501, Fisher), 50 µl of luciferase assay mixture (Roche) was automatically injected into each sample and the resulting luminescence was measured in a FLUOstar Omega plate reader (BMG Labtech). For human experiments, 200 µl of luciferase assay mixture (Invitrogen) was injected into 20 µl sample. A freshly prepared dilution curve of ATP standard in the experimental buffer was run alongside each set of experimental samples. ATP levels in the supernatant were normalized to total protein concentration in the RBC suspension, which was taken as a measure of cell concentration. Total protein was assessed by BCA assay (kit no. 23227, Pierce) for mouse experiments or by Lowry assay (kit no. 5000116, Bio-Rad) for human experiments.
Evaluation of Hemolysis
Following removal of an aliquot for extracellular ATP analysis, samples were centrifuged for 5 min at 500 g at RT to pellet RBCs. A 50 µl aliquot of the supernatant of each sample was transferred into a well of a 96-well plate. A broad-spectrum absorbance reading was collected from each sample. Cell-free hemoglobin was measured by absorbance at 570 nm, which corresponds to an isosbestic point of oxy- (oxyHb) and deoxyhemoglobin (deoxyHb) spectra near a local peak absorbance of each species. Net absorbance (ΔAbs) was calculated by subtracting out a background absorbance measurement at 700 nm.
Statistics
Data are expressed as means ± SE. ATP release and intracellular cAMP data were compared by one-way ANOVA with multiple comparisons using Tukey’s test. A significance cutoff of P < 0.05 was imposed. Shapiro-Wilks tests were performed to confirm normality of data.
RESULTS
Mouse RBCs Express Panx1
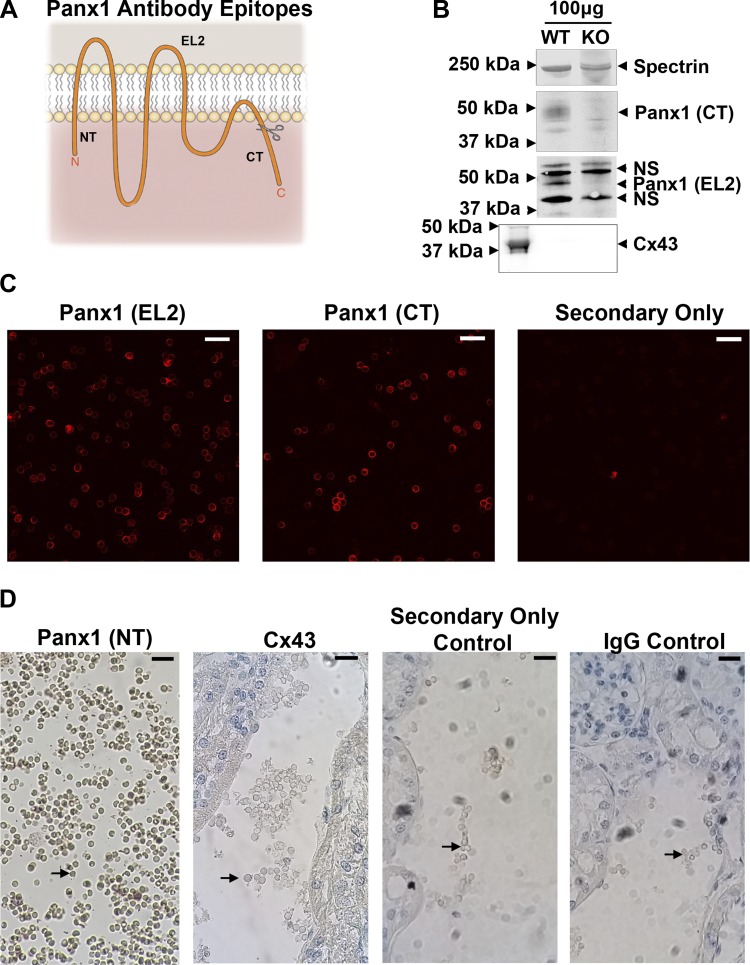
To examine the role of Panx1 in ATP release from RBCs, we first sought to confirm that RBCs express Panx1, as this protein has been separately reported to be either present (19) or absent (21) on the membrane of mouse RBCs. Using three antibodies targeting epitopes on the NH2 terminus (NT), second extracellular loop (EL2), and COOH terminus (CT) of mouse Panx1 (Fig. 1A), we examined Panx1 expression on RBCs via Western blotting, immunofluorescence, and immunohistochemistry. Comparison of RBC ghost lysates via Western blotting with anti-Panx1 CT and anti-Panx1 EL2 antibodies confirmed expression of Panx1 in RBCs of WT, but not Panx1−/− mice (Fig. 1B). Confocal immunofluorescence microscopy of RBCs using anti-Panx1 CT and anti-Panx1 EL2 antibodies (Fig. 1C) further clarified localization of Panx1 to the plasma membrane of RBCs. We additionally analyzed the presence of Panx1 in situ on RBCs trapped in the blood vessels of nonperfused mouse kidney sections by DAB staining (Fig. 1D). Using the anti-Panx1 NT antibody, we detected DAB staining on the surface of RBCs; however, we detected no DAB staining using Cx43 antibody, an IgG control, or a secondary antibody-only control. While plasma membrane localization might be expected given the absence of intracellular organelles in RBCs, its confirmation nevertheless provides for the reported participation of Panx1 in the purported release of intracellular ATP across the plasma membrane into the extracellular environment. No expression of Cx43 was detected via Western blotting in WT or Panx1−/− RBCs (Fig. 1B) or DAB staining in WT RBCs (Fig. 1D), ruling out Cx43 hemichannels as possible alternative ATP release channels in this setting.
Fig. 1.
Mouse RBCs express pannexin 1 (Panx1). A: topological schematic of Panx1 indicates the location of the epitopes targeted by the three antibodies used. Scissors indicate caspase cleavage site on COOH terminus. B: analysis of protein content in red blood cell (RBC) ghosts via Western blotting with carboxy-terminus (CT) and second extracellular loop (EL2) antibodies shows Panx1 expression in wild-type (WT) but not Panx1−/− (KO) RBCs. No connexin 43 (Cx43) expression was detected. Far left Cx43 band is 20 µg mouse lung lysate, used as a positive control. NS, nonspecific bands. C: confocal immunofluorescence microscopy of RBCs using CT and EL2 antibodies shows expression of Panx1 in RBCs. Scale bar, 20 µm. D: 3,3′-diaminobenzidine (DAB) staining of RBCs trapped in afferent arteriole kidney tissue slices using amino-terminus (NT) antibody confirms Panx1 expression on both endothelium and RBCs, stained dark brown. Arrows denote RBCs. No DAB staining was detected when sections were probed with Cx43 antibody, IgG antibody, or secondary antibody only. Scale bar, 20 µm.
Genetic Deletion of Panx1 Does Not Impair Exercise Capacity in Mice
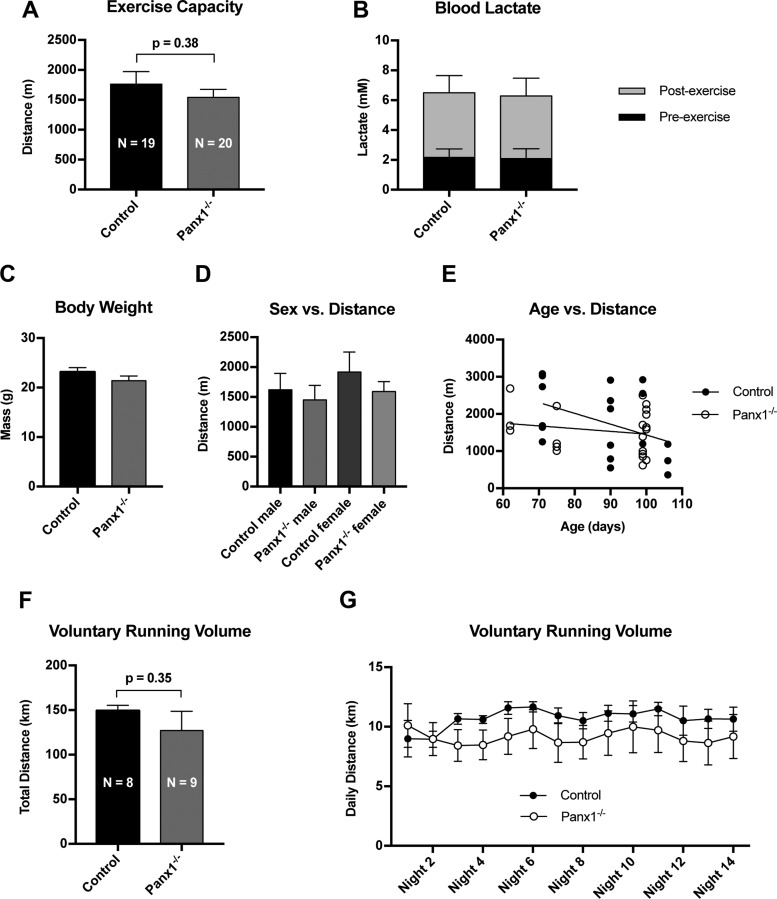
Panx1 in RBCs has been previously described as a necessary component of hemoglobin deoxygenation-induced ATP release from RBCs (36), a process believed to be particularly important for the regulation of blood perfusion in skeletal muscle (10); however, this role for Panx1 has only been tested in vitro. To examine the role of Panx1 in an exercise setting in vivo, we subjected Panx1−/− and control mice to an acute forced exercise capacity test (18). Panx1−/− exhibited no reduction in endurance capacity compared with control mice (Fig. 2A), and both groups showed the same level of exertion via blood lactate measurements (Fig. 2B). Panx1−/− and control groups exhibited similar body weight (Fig. 2C). There were no effects seen due to sex (Fig. 2D) or age (Fig. 2E) of the mice. When individually housed in cages with a wheel available, Panx1−/− also showed no significant difference from control mice in total distance run over the course of 2 wk (Fig. 2F) or at daily time points (Fig. 2G).
Fig. 2.
Genetic deletion of Panx1 does not impair exercise capacity in mice. A: Panx1−/− and Cre− Panx1fl/fl mice subjected to forced acute exercise via incremental treadmill test showed no significant difference in exercise capacity. N = 20 (Panx1−/−) and N = 19 (control). B: blood lactate measurements before and after exercise show equal exertion during treadmill test. C-E: comparisons of mass (C), sex (D), and age (E) across all mice tested. F: Panx1−/− and Cre- Panx1fl/fl mice housed individually in cages with running wheels showed no difference in voluntary running activity over a 2-wk period. N = 9 (Panx1−/−) and N = 8 (control). G: daily running data from F.
Background Concentration of ATP in the Supernatant and the Luciferin-Luciferase Assay
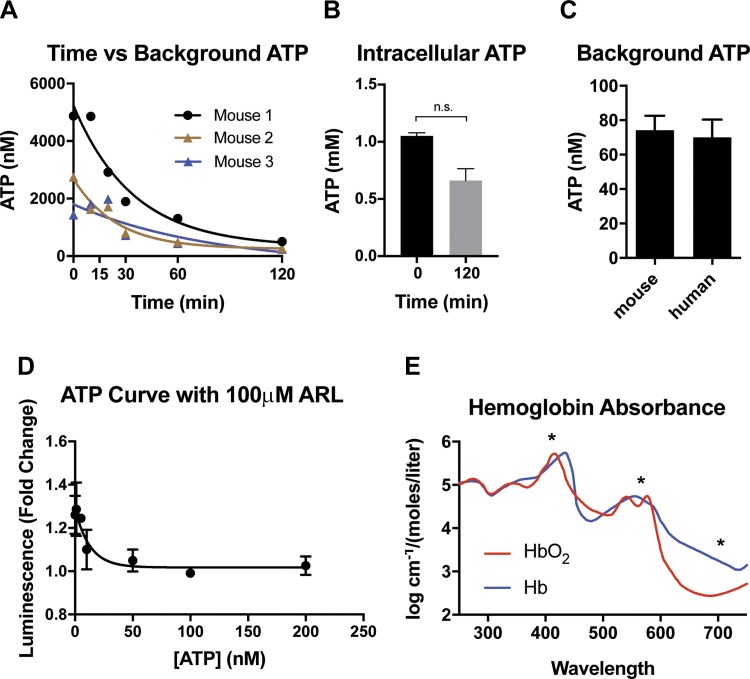
Because of the variety of experimental setups used previously in measuring ATP release from RBCs, we first carefully optimized the experimental conditions and tested a few notable variables to identify possible sources of variation in ATP measurements. To minimize extracellular ATP concentrations and increase precision of measurement, after isolating and washing RBCs we measured changes in extracellular ATP concentration with time in the experimental preparation, as determined immediately after washing and after different time intervals of incubation at RT on the bench top, up to 120 min. We observed a time-dependent decrease in extracellular ATP concentration (Fig. 3A). As the extracellular concentration of ATP decreases with time, choice of incubation time influences the level of extracellular ATP encountered in the supernatant of control cells. Minimization of extracellular ATP in control cells enables more precise measurement of experimentally induced ATP release. Notably, while we observed a slight decrease in intracellular ATP content after 120 min incubation, RBCs did not experience significant rundown of ATP gradient (Fig. 3B). Furthermore, mouse and human RBCs exhibited similar background levels of extracellular ATP following isolation and washing (Fig. 3C). To further optimize the assay, we also compared a commercially available luciferase assay kit to a crude mixture of raw luciferin and luciferase, as was utilized earlier in the literature. While crude enzymatic preparation exhibited a stronger signal than the kit assay, both assays showed linear responses across the range of ATP concentrations being tested (data not shown). Additionally, we tested the impact of the commonly used ecto-ATPase inhibitor ARL 67156 trisodium (ARL) on the luciferase assay. While previous research has shown that RBCs possess only minor ecto-ATPase activity, (23) ARL is a common component of extracellular ATP assays. As a structural analog of ATP, ARL could conceivably interfere with the luciferase assay. Across the range of ATP concentrations encountered in our experiments, ARL had no adverse effect on the ability of the luciferase assay to detect ATP vs. the assay in its absence (Fig. 3D). However, based on the slow timescale of extracellular ATP degradation in the absence of ARL in the sample preparation observed in Fig. 3A, and the previously reported low ecto-ATPase activity of ~28 fmol × (106 cells min)−1 on human RBCs (23), we deemed use of ARL unnecessary in our experiments.
Fig. 3.
Methodological considerations for measuring ATP release from RBCs in vitro. A: decay of extracellular ATP in washed mouse RBC samples at room temperature. N = 3. R2 = 0.93 (mouse 1), 0.94 (mouse 2), 0.68 (mouse 3). B: intracellular ATP concentration in mouse RBCs at start and end of timeframe in A. C: background ATP content in samples of mouse (N = 4) and human (N = 7) RBCs at 25% hct, using luciferase assay built from scratch. D: effects of ARL 67156 trisodium (ARL) on luciferase assay built from scratch, independent of RBCs. E: optical absorbance curves for oxyhemoglobin (HbO2) and hemoglobin (Hb). Asterisks indicate absorbance peak at 405 nm, isosbestic point at 570 nm, and background measurement at 700 nm.
Stimulation of ATP Release by Hypotonic Potassium Gluconate Solution Causes Hemolysis of Mouse RBCs
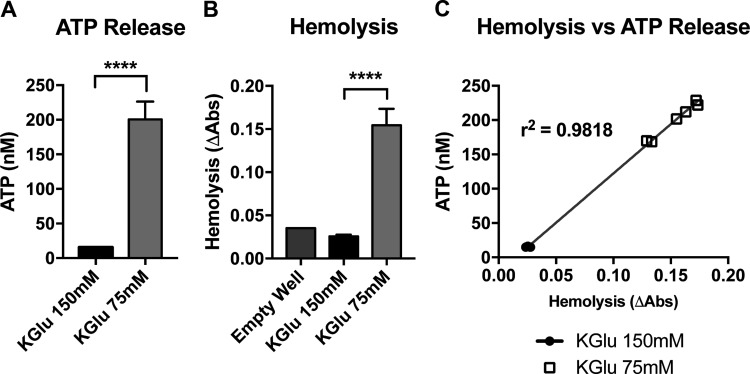
The first examination of Panx1-dependent ATP release in mouse RBCs took place under stimulation with high-potassium solution or hypo-osmotic stress (19), or by treatment with hypotonic KGlu solution (25). Substitution of KGlu in place of physiologic extracellular ions complicates the experimental study of Panx1 channels in RBCs. Since the physiologic concentration gradients of K+, Na+, Ca2+, and Cl− are reversed, and since gluconate is not a rapidly permeant anion like Cl−, a KGlu-based buffer would be expected to cause perturbations in ionic influx and efflux (8), altered pH balance (16) and cell volume (14, 37). When we exposed RBCs from WT mice to KGlu as previously done to activate Panx1-mediated ATP release (25), we indeed observed an approximately 10-fold increase in extracellular ATP (Fig. 4A); however, we also observed a large increase in RBC lysis (Fig. 4B). To determine whether increases in ATP release were due to hemolysis of RBCs as observed with other treatments/conditions (including substitution of NaCl with KCl in the supernatant) (30), we examined the presence of hemoglobin in the supernatant of the RBCs treated with KGlu by UV-visible spectrophotometry by analyzing the absorbance of oxyhemoglobin (oxyHb) at 570 nm minus the background assessed at 700 nm. In Fig. 3E the absorption spectrum of oxyHb and deoxyHb is depicted, where the highest absorbance peak at 405 nm, the isosbestic point at 570 nm and the background reading at 700 nm are marked with asterisks. Following treatment of mouse RBCs with hypotonic KGlu solution, we found that hemolysis and extracellular ATP levels were highly correlated across all samples with r2 = 0.98 (Fig. 4C), indicating that the overwhelming source of extracellular ATP in our experiment was lytic release of cellular contents, not mechanistically controlled ATP release.
Fig. 4.
Hypotonic stimulation of mouse RBCs in vitro fails to stimulate controlled ATP release. A: extracellular ATP concentration resulting from in vitro stimulation of RBCs with hypotonic, high-K+ buffer or isotonic, high-K+ buffer, measured via Roche luciferase assay kit. B: hemoglobin absorbance readings from the supernatant of the samples in A as a measurement of cell-free hemoglobin attributable to RBC lysis. C: X–Y plot of hemolysis against ATP release from A and B. R2 values indicate the extent to which changes extracellular ATP are attributable to hemolysis. ****P < 0.0001.
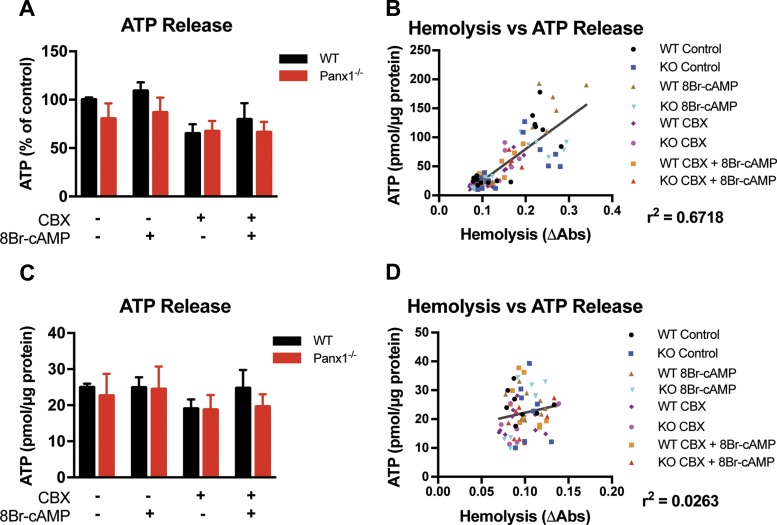
Treatment with 8Br-cAMP Fails to Stimulate Controlled ATP Release From Mouse RBCs
Given the currently understood pathway of Panx1-dependent RBC ATP release lying downstream of cAMP-dependent PKA activation (32), we next examined pharmacologic stimulation of mouse RBCs with an active cAMP analog, 8Br-cAMP, similar to the conditions used in early examination of the reported pathway (35). Our experimental setup included both wild-type and Panx1−/− RBCs, as well as the Panx1 inhibitor CBX, seeking to connect these upstream steps to the more recent identification of Panx1 as the conduit of subsequent ATP release (36). We hypothesized that stimulation of WT RBCs with 8Br-cAMP should induce ATP release that could be inhibited by genetic deletion or pharmacologic inhibition of the Panx1 channel. However, we observed no change in extracellular ATP concentration across all conditions with similar hemolysis levels (Fig. 5C). Applying the hemolysis calculation described in Fig. 3E, we report the influence of hemolysis on our ATP release measurements in Fig. 5, A and B. Including all raw data points from the original data set, we found no changes in extracellular ATP across stimulation conditions (Fig. 5A); however, we observed a range of hemolysis measurements and a clear correlation between elevated hemolysis and higher extracellular ATP readings (Fig. 5B). Based on the clustering of data, we imposed a cutoff of ΔAbs = 0.15, excluding ATP release data for all samples with hemolysis measurements exceeding the cutoff. While in this experiment our finding of no significant change in extracellular ATP concentrations across stimulation conditions remained unchanged (Fig. 5C), the r2 value showing the relationship between hemolysis and extracellular ATP was reduced from 0.67 to 0.03, indicating that our corrected ATP data are fully independent of the influence of hemolysis (Fig. 5D).
Fig. 5.
Pharmacologic stimulation of mouse RBCs in vitro fails to stimulate controlled ATP release. A: extracellular ATP concentration resulting from in vitro stimulation of WT and Panx1−/− RBCs with the active cyclic AMP analog 8Br-cAMP, with or without pretreatment with the Panx1 inhibitor carbenoxolone (CBX), using luciferase assay built from scratch. N = 5, n = 15. B: X–Y plot of hemolysis against ATP release from the experiment in A. C: replotting of data in A after removing data points in which hemolysis exceeded a cutoff of change in optical density of 0.15. N = 3, n = 9. Br-cAMP, 8-bromo cyclic adenosine monophosphate. D: X–Y plot of hemolysis against ATP release from the experiment in C. R2 values indicate the extent to which changes in extracellular ATP are attributable to hemolysis.
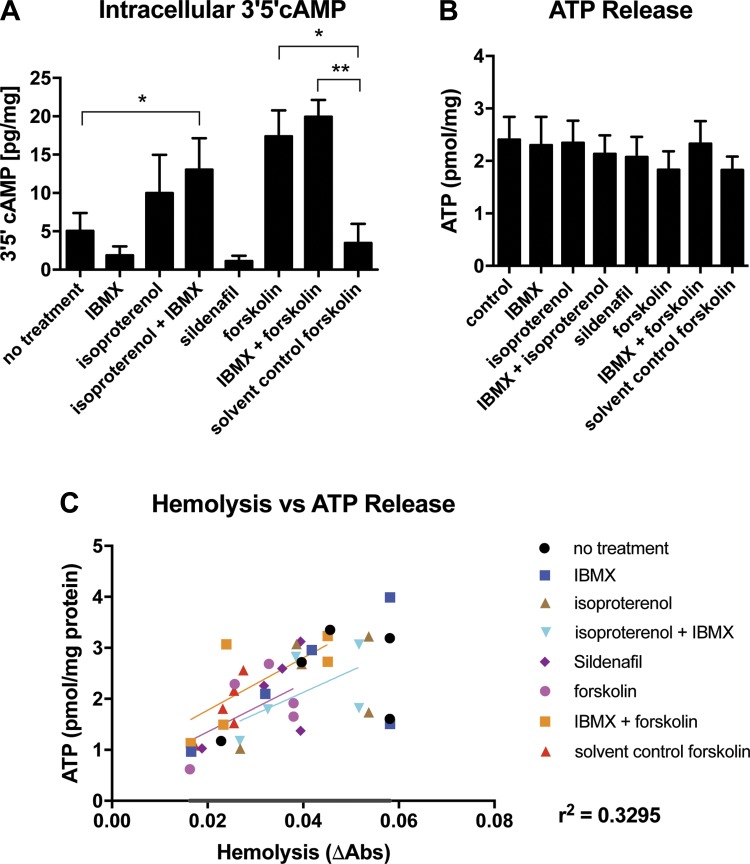
Pharmacologic Stimulation of Intracellular cAMP Production Does Not Induce ATP Release From Human RBCs
Having failed to identify ATP release from mouse RBCs in vitro following stimulation with an active cAMP analog, we next turned to human RBCs, in which the bulk of trailblazing experiments was performed (35, 36). Exposure of RBCs to a variety of pharmacologic agents including the combination of β-adrenergic agonist isoproterenol + the nonspecific phosphodiesterase inhibitor IBMX (though neither alone), the AC activator forskolin, and the combination of forskolin + IBMX, all increased intracellular cAMP levels significantly (Fig. 6A). Based on a recent report that PDE5 inhibition contributes to ATP release through a parallel cAMP-dependent pathway (4), we also tested sildenafil as a stimulation condition, but we saw no resulting increase in intracellular cAMP. While forskolin, forskolin + IBMX, and isoproterenol + IBMX elevated cAMP, none of the conditions tested resulted in an increase in extracellular ATP (Fig. 6B). In this experiment, we observed a weak relationship (r2 = 0.3295) between hemolysis and extracellular ATP (Fig. 5C); however, since the amount of hemolysis observed was minimal and no difference in extracellular ATP was observed between conditions, hemolysis-driven ATP release does not appear to have affected these results meaningfully. Taken together, these data show that increases in intracellular cAMP by modulation of its synthesis or breakdown did not increase ATP release from human RBCs.
Fig. 6.
Pharmacologic stimulation of human RBCs in vitro stimulates a rise in intracellular cAMP but fails to stimulate controlled ATP release. A: 3′,5′-cAMP concentrations measured by mass spectrometry following in vitro stimulation of RBCs with pharmacologic activators. B: extracellular ATP concentrations resulting from the experiment in A, using Invitrogen luciferase assay kit. C: X–Y plot of hemolysis against ATP release from the experiment in B. R2 value indicates the extent to which changes in extracellular ATP are attributable to hemolysis. *P < 0.05, **P < 0.01.
DISCUSSION
Are cAMP-Dependent Signaling and Panx1 in RBCs Part of the Same Pathway?
Panx1 was proposed to be the main channel responsible for ATP efflux from RBCs (19, 25, 36); however, its presence on RBCs could not be confirmed by others (21). We here show that RBCs express Panx1 and lack Cx43, as assessed by Western blot analysis, immunofluorescence, and immunohistochemistry. However, we found that the combination of high-potassium solution and hypo-osmotic stress, a treatment used previously to assess the functional role of Panx1 in RBCs (25) induced hemolysis-derived ATP release [as also shown by others (30)] instead of regulated ATP release.
According to the current paradigm (13, 32) a single signaling pathway leading from RBC deoxygenation to ATP release involves activation of AC to produce cAMP (35), culminating in Panx1 activation (6, 22, 32, 35, 36). In contrast, we found that treatment of RBCs with a cAMP analog did not induce ATP release from either WT or Panx1−/− RBCs. Moreover, untreated extracellular ATP concentrations were similar between WT and Panx1−/− RBCs, and between untreated and CBX-treated WT RBCs. Our findings suggest that Panx1 is not responsible either for controlled ATP release in response to cAMP stimulation, nor for any controlled ATP release that may contribute to the baseline ATP levels in our experimental setup. Therefore, taking into consideration that, to the best of our knowledge, no direct evidence exists to support the participation of PKA in this pathway, and the lack of response to cAMP assessed here, it appears to us unlikely that cAMP and Panx1 are in fact closely connected within the same pathway.
These findings also call into question the role of CFTR, which was proposed to participate in the ATP release pathway downstream of RBC deformation (33). Unfortunately, the study examining CFTR involvement relied upon nonspecific drugs (1, 25–29), and no rescue experiments were performed to validate specificity. Furthermore, it is difficult to speculate on the molecular mechanism underpinning the proposed interaction of CFTR and Panx1, leaving unclear how exactly CFTR might be expected to participate in the proposed pathway.
Taken together, multiple questions remain about the relationships among these various signaling elements (AC, cAMP, PKA, CFTR, Panx1) and whether they all lie in a single pathway connecting hypoxia/RBC deformation to ATP release from RBCs. Future studies must integrate these components to identify whether physiological stimuli drive ATP release from RBCs by activation of one pathway, or multiple pathways, and which signaling components do or do not interact.
Role of Panx1 in Exercise
Because of its dramatic increase in blood flow during exercise (3), skeletal muscle is recognized as an ideal context for study of luminal ATP release as a vasodilatory modulator (10, 11, 20). However, the role of Panx1 in hypoxia-induced ATP release from RBCs, a process believed to be particularly important for the regulation of blood perfusion in skeletal muscle, has not previously been studied in this physiologic context. Based on the previously reported role of Panx1 in gating ATP release from RBCs in response to increased hemoglobin deoxygenation, we hypothesized that Panx1−/− mice would exhibit reduced endurance capacity as a result of inadequate blood perfusion to active skeletal muscle. However, Panx1−/− and control mice performed similarly in both acute and chronic tests of endurance capacity. The lack of any decrease in exercise performance following genetic deletion of Panx1 challenges the assumption that Panx1 regulates increased blood perfusion to exercising skeletal muscle. This result could indicate either that Panx1 does not play an essential role in this pathway of ATP release from RBCs, or that the pathway in question does not make a significant contribution to muscle performance during exercise. Additional investigation into the effects of Panx1 deletion on blood flow in exercising skeletal muscle, measuring changes in blood flow as an end point, will help to elaborate on this finding.
Hemolysis or ATP Release From RBCs?
Among the main issues concerning the assessment of ATP release from RBCs are well-known methodological aspects underlying the ex vivo measurement of ATP release from RBCs, including: RBC concentration and integrity, incubation buffer, incubation time (to match pathways in question), as well as protocol of ATP measurement and purity of luciferase preparation.
Loss of RBC membrane integrity (hemolysis) is one of the main confounding factors concerning assessment of ATP release from RBCs and has been a recent topic of significant debate in the field (17, 30, 31) concerning reproducibility issues. Controlled versus lytic sources of ATP release can be distinguished by assessing cell-free Hb concentration in the supernatant of samples. While early papers in the field reference hemolysis readings at a variety of wavelengths (35), the 405 nm oxyhemoglobin absorbance peak (marked with asterisk in Fig. 3E) appears to be the most popular and current measurement (36). However, since the absorbance of oxyhemoglobin is ~30% higher than that of deoxyhemoglobin at the 405 nm wavelength, this measurement may be affected by the oxygenation status of a sample. Alternatively, the presence of Hb in the supernatant of cells can be assessed by measuring absorbance at 570 nm, where an isosbestic point for oxy- and deoxyhemoglobin lies close to the peak absorption of each species, as well as to the absorbance of methemoglobin (not shown). In addition, background absorbance at 700 nm can be subtracted to obtain more reproducible net absorbance readings (ΔAbs = 570 nm minus 700 nm).
Minor hemolysis during blood collection and RBC isolation process is nearly inevitable. To control for hemolysis in our experiments, we calculated a relative increase in ΔAbs compared with the average hemolysis measurements of the untreated control in an experiment, and we excluded all experiments where we found more than 0.05 increase in ΔAbs as compared with baseline hemolysis. This ΔAbs reflects a lysis of roughly 0.05% hct, a modest percentage but one that can have a significant effect on the ATP levels observed.
In summary, assessment of ATP release from RBCs in suspension is deceptively challenging due to serious and often inevitable methodological pitfalls such as hemolysis. Moreover, since RBCs lack a nucleus, classical loss-of-function and rescue-of-function experiments are only feasible by using pharmacological inhibitors and activators (which lack specificity) or in gene-targeted mouse models. Although detection of cytoplasmic proteins in RBCs is technically demanding because of the presence of high concentrations of hemoglobin (7), proof of the presence of a certain protein in RBCs needs to be presented by Western blot analysis or other proteomic approaches, not only on the basis of the effects of pharmacological inhibitors/stimulators [e.g., the participation of proteins like PKA (35) in ATP release has been proposed without being proven at the protein level]. Therefore, additional investigation is needed to understand the physiological role of regulated ATP release, and the mechanisms underpinning its regulation, especially using genetic animal models.
Novelty of Findings
Recent discussion of hemolysis-derived ATP release from RBCs (30) left lingering questions about the study of ATP release from RBCs. The data presented here resolve multiple details that until now remained contentious or unproven. Whereas Sikora et al. (30) showed ATP release to be indistinguishable from hemolysis following treatment with cAMP agonists [a source of resultant debate (17, 31)], our results show that cAMP agonists fail to induce any ATP release and offer paired mass spectrometry measurements to confirm elevation of intracellular cAMP following treatment. This finding refutes the published consensus pathway (32), providing much-needed correction to the previously untested assumption that Panx1 activation occurs downstream of cAMP activation in RBCs. Our study also specifically examines the highly cited and now-canonical RBC Panx1 stimulation condition of hypotonic, high-potassium buffer (25), showing that the resulting ATP release is driven by hemolysis. Finally, we now provide physiologic data from Panx1−/− mice, showing that genetic deletion of Panx1 has no effect on endurance exercise capacity. These findings provide key details to facilitate further refinement of existing models of ATP release from RBCs.
Conclusion: Challenges and Perspectives on Regulated ATP Release From RBCs
The data presented here exclude the role of cAMP in Panx1-dependent ATP release induced by pharmacologic activation of AC, but not necessarily of Panx1 in the effects of hypoxia and shear stress (17, 30, 31). We found that stimulation of the pathway via endogenous or exogenous cAMP failed to induce ATP release from WT or Panx1−/− RBCs. As no direct evidence has been presented of a role for PKA, nor has the previously reported role of CFTR (33) been conclusively proven to connect cAMP to Panx1 activation, it now appears unlikely that RBC deformation and hypoxia stimulate the same signaling pathway in RBCs (32). While we are confident that Panx1 is expressed in RBCs, we argue that the previously proposed cAMP-dependent pathway as the central hub for controlled ATP release cannot fully explain all the effects of the different stimuli reported to activate ATP release from RBCs. We also find no effect on endurance capacity as a result of genetic deletion of Panx1 from mice, calling into question the physiologic importance of Panx1 in this pathway’s support of muscle function. A few key questions remain: is RBC deformability in fact a prerequisite for hypoxic ATP release? Are other laboratories able to reproduce stimulation of non-hemolytic ATP release downstream of cAMP stimulation, and if so, is the mechanism PKA and Panx1-dependent? Finally, is CFTR directly involved in cAMP-dependent or Panx1-dependent ATP release from RBCs, and if so how? Further experimentation with Panx1−/− RBCs exposed to hypoxic conditions or shear stress would be helpful to confirm or deny the role of Panx1 in hypoxic or mechanotransduction-dependent pathways. Future in vitro and in vivo work will need to tease out more details to identify the true connectivity of this reported pathway, as well as confirm its physiological relevance in supply of oxygen to skeletal muscle.
GRANTS
This work was supported by the German Research Council (DFG CO 1305/2-1 to M. M. Cortese-Krott; L. Diederich is a scholar of the IRTG1902 to M. Kelm, M. M. Cortese-Krott, and B. E. Isakson, and C. Panknin is a scholar of the SFB1116 to M. M. Cortese-Krott and M. Kelm), the Susanne-Bunnenberg-Stiftung of the Düsseldorf Heart Center (to M. Kelm), as well as the NIH (Grants NS070003, DK068575, DK079307, DK091190, and HL109002 to E. K. Jackson; R01-AR050429 to Z. Yan, and HL120840 to B. E. Isakson), the American Heart Association Pre-Doctoral Award (A. S. Keller), and the American Diabetes Association 1-16-PDF-030 (J. C. Drake).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.S.K., L.D., M.M.C.-K., and B.E.I. conceived and designed research; A.S.K., L.D., C.P., L.J.D., J.C.D., R.S., E.K.J., and Z.Y. performed experiments; A.S.K., L.D., C.P., L.J.D., J.C.D., R.S., E.K.J., Z.Y., M.M.C.-K., and B.E.I. analyzed data; A.S.K., L.D., C.P., L.J.D., J.C.D., R.S., Z.Y., M.K., M.M.C.-K., and B.E.I. interpreted results of experiments; A.S.K. prepared figures; A.S.K. and M.M.C.-K. drafted manuscript; A.S.K., J.C.D., M.K., M.M.C.-K., and B.E.I. edited and revised manuscript; A.S.K., L.D., E.K.J., M.K., M.M.C.-K., and B.E.I. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Christian M. Kramer, Maximilian Ziegler, and Thirumakal Manokaran for help with experiments.
Present address of B. E. Isakson: Robert M. Berne Cardiovascular Research Center, University of Virginia School of Medicine, Charlottesville, VA 22908 (e-mail: brant@virginia.edu).
REFERENCES
- 1.Balderas E, Ateaga-Tlecuitl R, Rivera M, Gomora JC, Darszon A. Niflumic acid blocks native and recombinant T-type channels. J Cell Physiol 227: 2542–2555, 2012. doi: 10.1002/jcp.22992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berne RM. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol 204: 317–322, 1963. [DOI] [PubMed] [Google Scholar]
- 3.Boushel R, Langberg H, Green S, Skovgaard D, Bülow J, Kjaer M. Blood flow and oxygenation in peritendinous tissue and calf muscle during dynamic exercise in humans. J Physiol 524: 305–313, 2000. doi: 10.1111/j.1469-7793.2000.t01-2-00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowles EA, Moody GN, Yeragunta Y, Stephenson AH, Ellsworth ML, Sprague RS. Phosphodiesterase 5 inhibitors augment UT-15C-stimulated ATP release from erythrocytes of humans with pulmonary arterial hypertension. Exp Biol Med (Maywood) 240: 121–127, 2015. doi: 10.1177/1535370214547155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cinar E, Zhou S, DeCourcey J, Wang Y, Waugh RE, Wan J. Piezo1 regulates mechanotransductive release of ATP from human RBCs. Proc Natl Acad Sci USA 112: 11783–11788, 2015. doi: 10.1073/pnas.1507309112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connors BW. Tales of a dirty drug: carbenoxolone, gap junctions, and seizures. Epilepsy Curr 12: 66–68, 2012. doi: 10.5698/1535-7511-12.2.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortese-Krott MM, Kelm M. Endothelial nitric oxide synthase in red blood cells: key to a new erythrocrine function? Redox Biol 2: 251–258, 2014. doi: 10.1016/j.redox.2013.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotterrell D, Whittam R. The influence of the chloride gradient across red cell membranes on sodium and potassium movements. J Physiol 214: 509–536, 1971. doi: 10.1113/jphysiol.1971.sp009446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietrich HH, Ellsworth ML, Sprague RS, Dacey RG Jr. Red blood cell regulation of microvascular tone through adenosine triphosphate. Am J Physiol Heart Circ Physiol 278: H1294–H1298, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Ellsworth ML. Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med Sci Sports Exerc 36: 35–41, 2004. doi: 10.1249/01.MSS.0000106284.80300.B2. [DOI] [PubMed] [Google Scholar]
- 11.Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol Heart Circ Physiol 269: H2155–H2161, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Erkens R, Suvorava T, Kramer CM, Diederich L, Kelm M, Cortese-Krott MM. Modulation of local and systemic heterocellular communication by mechanical forces: a role of eNOS. Antioxid Redox Signal 26: 917-935, 2017. doi: 10.1089/ars.2016.6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldman D, Fraser GM, Ellis CG, Sprague RS, Ellsworth ML, Stephenson AH. Toward a multiscale description of microvascular flow regulation: O2-dependent release of ATP from human erythrocytes and the distribution of ATP in capillary networks. Front Physiol 3: 246, 2012. doi: 10.3389/fphys.2012.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunn RB, Dalmark M, Tosteson DC, Wieth JO. Characteristics of chloride transport in human red blood cells. J Gen Physiol 61: 185–206, 1973. doi: 10.1085/jgp.61.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson EK, Ren J, Mi Z. Extracellular 2′,3′-cAMP is a source of adenosine. J Biol Chem 284: 33097–33106, 2009. doi: 10.1074/jbc.M109.053876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jennings ML. Proton fluxes associated with erythrocyte membrane anion exchange. J Membr Biol 28: 187–205, 1976. doi: 10.1007/BF01869697. [DOI] [PubMed] [Google Scholar]
- 17.Kirby BS, Schwarzbaum PJ, Lazarowski ER, Dinenno FA, McMahon TJ. Liberation of ATP secondary to hemolysis is not mutually exclusive of regulated export. Blood 125: 1844–1845, 2015. doi: 10.1182/blood-2014-11-609610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laker RC, Drake JC, Wilson RJ, Lira VA, Lewellen BM, Ryall KA, Fisher CC, Zhang M, Saucerman JJ, Goodyear LJ, Kundu M, Yan Z. AMPK phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat Commun 8: 548, 2017. doi: 10.1038/s41467-017-00520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci USA 103: 7655–7659, 2006. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCullough WT, Collins DM, Ellsworth ML. Arteriolar responses to extracellular ATP in striated muscle. Am J Physiol Heart Circ Physiol 272: H1886–H1891, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Melhorn MI, Brodsky AS, Estanislau J, Khoory JA, Illigens B, Hamachi I, Kurishita Y, Fraser AD, Nicholson-Weller A, Dolmatova E, Duffy HS, Ghiran IC. CR1-mediated ATP release by human red blood cells promotes CR1 clustering and modulates the immune transfer process. J Biol Chem 288: 31139–31153, 2013. doi: 10.1074/jbc.M113.486035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michalski K, Kawate T. Carbenoxolone inhibits Pannexin1 channels through interactions in the first extracellular loop. J Gen Physiol 147: 165–174, 2016. doi: 10.1085/jgp.201511505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montalbetti N, Leal Denis MF, Pignataro OP, Kobatake E, Lazarowski ER, Schwarzbaum PJ. Homeostasis of extracellular ATP in human erythrocytes. J Biol Chem 286: 38397–38407, 2011. doi: 10.1074/jbc.M111.221713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poon IK, Chiu YH, Armstrong AJ, Kinchen JM, Juncadella IJ, Bayliss DA, Ravichandran KS. Unexpected link between an antibiotic, pannexin channels and apoptosis. Nature 507: 329–334, 2014. doi: 10.1038/nature13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu F, Wang J, Spray DC, Scemes E, Dahl G. Two non-vesicular ATP release pathways in the mouse erythrocyte membrane. FEBS Lett 585: 3430–3435, 2011. doi: 10.1016/j.febslet.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ripoll C, Lederer WJ, Nichols CG. On the mechanism of inhibition of KATP channels by glibenclamide in rat ventricular myocytes. J Cardiovasc Electrophysiol 4: 38–47, 1993. doi: 10.1111/j.1540-8167.1993.tb01210.x. [DOI] [PubMed] [Google Scholar]
- 27.Schultz BD, DeRoos AD, Venglarik CJ, Singh AK, Frizzell RA, Bridges RJ. Glibenclamide blockade of CFTR chloride channels. Am J Physiol Lung Cell Mol Physiol 271: L192–L200, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Scott-Ward TS, Li H, Schmidt A, Cai Z, Sheppard DN. Direct block of the cystic fibrosis transmembrane conductance regulator Cl(-) channel by niflumic acid. Mol Membr Biol 21: 27–38, 2004. doi: 10.1080/09687680310001597758. [DOI] [PubMed] [Google Scholar]
- 29.Sharonova IN, Dvorzhak AY. Blockade of GABAA receptor channels by niflumic acid prevents agonist dissociation. Biochemistry (Mosc), Suppl A: Membr Cell Biol 7: 37–44, 2013. doi: 10.1134/S1990747812050169. [DOI] [Google Scholar]
- 30.Sikora J, Orlov SN, Furuya K, Grygorczyk R. Hemolysis is a primary ATP-release mechanism in human erythrocytes. Blood 124: 2150–2157, 2014. doi: 10.1182/blood-2014-05-572024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sikora J, Orlov SN, Furuya K, Grygorczyk R. Response: Hemolysis is a primary and physiologically relevant ATP release mechanism in human erythrocytes. Blood 125: 1845–1846, 2015. doi: 10.1182/blood-2015-01-622159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sprague RS, Ellsworth ML. Erythrocyte-derived ATP and perfusion distribution: role of intracellular and intercellular communication. Microcirculation 19: 430–439, 2012. doi: 10.1111/j.1549-8719.2011.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol Heart Circ Physiol 275: H1726–H1732, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. ATP: the red blood cell link to NO and local control of the pulmonary circulation. Am J Physiol Heart Circ Physiol 271: H2717–H2722, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol Cell Physiol 281: C1158–C1164, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Sridharan M, Adderley SP, Bowles EA, Egan TM, Stephenson AH, Ellsworth ML, Sprague RS. Pannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytes. Am J Physiol Heart Circ Physiol 299: H1146–H1152, 2010. doi: 10.1152/ajpheart.00301.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vitvitsky VM, Frolova EV, Martinov MV, Komarova SV, Ataullakhanov FI. Anion permeability and erythrocyte swelling. Bioelectrochemistry 52: 169–177, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Wan J, Forsyth AM, Stone HA. Red blood cell dynamics: from cell deformation to ATP release. Integr Biol 3: 972–981, 2011. doi: 10.1039/c1ib00044f. [DOI] [PubMed] [Google Scholar]
- 39.Wan J, Ristenpart WD, Stone HA. Dynamics of shear-induced ATP release from red blood cells. Proc Natl Acad Sci USA 105: 16432–16437, 2008. doi: 10.1073/pnas.0805779105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolf MM, Berne RM. Coronary vasodilator properties of purine and pyrimidine derivatives. Circ Res 4: 343–348, 1956. doi: 10.1161/01.RES.4.3.343. [DOI] [PubMed] [Google Scholar]