Abstract
Proteinuria is a characteristic of chronic kidney disease and also a causative factor that promotes the disease progression, in part, via activation of the intrarenal renin-angiotensin system (RAS). (Pro)renin receptor (PRR), a newly discovered component of the RAS, binds renin and (pro)renin to promote angiotensin I generation. The present study was performed to test the role of soluble PRR (sPRR) in albumin overload-induced responses in cultured human renal proximal tubular cell line human kidney 2 (HK-2) cells. Bovine serum albmuin (BSA) treatment for 24 h at 20 mg/ml induced renin activity and inflammation, both of which were attenuated by a PRR decoy inhibitor PRO20. BSA treatment induced a more than fivefold increase in medium sPRR due to enhanced cleavage of PRR. Surprisingly, this cleavage event was unaffected by inhibition of furin or a disintegrin and metalloproteinase 19. Screening for a novel cleavage enzyme led to the identification of site-1 protease (S1P). Inhibition of S1P with PF-429242 or siRNA remarkably suppressed BSA-induced sPRR production, renin activity, and inflammatory response. Administration of a recombinant sPRR, termed sPRR-His, reversed the effects of S1P inhibition. In HK-2 cells overexpressing PRR, mutagenesis of the S1P, but not furin cleavage site, reduced sPRR levels. Together, these results suggest that PRR mediates albumin-induced cellular responses through S1P-derived sPRR.
Keywords: pro(renin) receptor, albumin, renal proximal tubular cell renin activity, renin-angiotensin system
proteinuria is a common feature of chronic kidney disease (CKD), predicting an increased risk of progression to end-stage renal disease, as well as cardiovascular disease. Increasing evidence accumulated during the past two decades has demonstrated that proteinuria is not merely a marker of glomerulopathy but an important driver of renal injury, particularly the tubulointerstitial fibrosis due to glomerular damage (1, 42).
Albumin is the most abundant protein in the glomerular filtrate and is reabsorbed by the proximal tubule via megalin-cubulin-mediated endocytosis (43). The abnormal protein accumulation in the cytoplasm of the proximal tubule triggers a multitude of detrimental cellular responses, including inflammation (17, 38), oxidative stress (17, 21), fibrogenesis (7), and apoptosis (28). Additionally, proteinuria has been shown to elicit activation of the intrarenal renin-angiotensin system (RAS) via the protein kinase C-NADPH oxidase-dependent pathway (4). Indeed, inappropriate activation of the intrarenal RAS plays a key role in pathogenesis of hypertension and CKD.
In 2002, Nguyen and coworkers (25) identified the (pro)renin receptor (PRR), which binds both renin and prorenin to increase their catalytic activity. The PRR is a 39-kDa type I transmembrane receptor protein containing a proteinase cleavage site at the N-terminal domain near the transmembrane domain (5, 24). The cleavage leads to the generation of a 28-kDa soluble PRR (sPRR) and M8.9 (a subunit of vacuolar H+ ATPase, also known as ATPase 6 accessory protein 2). The circulating levels of sPRR are elevated during normal pregnancy (23, 34) and in patients with heart failure (9), gestational diabetes mellitus (34), obstructive sleep apnea syndrome (26, 27), primary epithelial ovarian cancer (13), and CKD, due to hypertension and type 2 diabetes (10). We, for the first time, report that sPRR exerts a biological function in enhancing aquaporin-2 expression and thus urine-concentrating capability (20). The cleavage mechanism for generation of sPRR appears complex, involving furin (5), a disintegrin and metalloproteinase 19 (ADAM19) (40), and site-1 protease (S1P) (22).
The present study was performed to examine a potential role of sPRR in albumin-induced cellular responses in human-cultured proximal tubular human kidney 2 (HK-2) cells. We employed a newly developed PRR inhibitor, PRO20 (the first 20 amino acid residues of the prorenin prosegment L1PTDTASFGRILLKKMPSVR20) (15), to demonstrate a role of PRR in mediating albumin overload-induced renin and proinflammatory responses. Interestingly, the albumin overload induces the production of sPRR due to the enhancement of the PRR cleavage. We further discovered S1P but not furin or ADAM19 as being responsible for generating sPRR during albumin overload.
MATERIALS AND METHODS
Reagents.
α1-Antitrypsin Portland (α1-PDX; catalog no. 126850) and furin inhibitor I (decanoyl-RVKR-chloromethylketone; catalog no. 344930) were purchased from Millipore-Sigma (St. Louis, MO). Furin inhibitor II (catalog no. SCP0148) and 4-(2-aminoethyl) benzene sulfonyl fluoride hydrochloride (AEBSF; catalog no. A8456) were purchased from Millipore-Sigma. Aprotinin [Aprot; catalog no. HY-P0017; formula: C284H432N84O79S7; Chemical Abstracts Service (CAS) #9087-70-1] and GM6001 (catalog no. HY-15768; formula: C20H28N4O4; CAS #947303-87-9) were from MedchemExpress (Sollentuna, Sweden). A protease inhibitor cocktail (PIC; catalog no. 11873580001) was purchased from Roche (Mannheim, Germany). PF-429242 (catalog no. A11230; formula: C25H35N3O2; CAS #947303-87-9) was purchased from AdooQ Bioscience (Irvine, CA). BSA (Cohn Fraction V; catalog no. 10735108001) was purchased from Roche (Indianapolis, IN). Human serum albumin (HSA; catalog no. A9731) was purchased from Millipore-Sigma. Most of the experiments were performed at Sun Yat-sen University (Guangzhou, China) except that site-directed mutagenesis and transfection experiments were conducted at the University of Utah (Salt Lake City, UT).
HK-2 cell line.
The experiments were carried out using a human renal proximal tubular epithelial cell line (HK-2). The HK-2 cells were derived from immortalized primary human proximal tubular cells isolated from the normal male adult kidney and were immortalized with the human papillomavirus (HPV 16) E6/E7 genes. The HK-2 cells were purchased from Procell Life Science (Wuhan, China). All cells were routinely authenticated by short tandem repeat profiling and tested for mycoplasma contamination. Cells were grown and maintained in Dulbecco's modified Eagle’s medium: Nutrient Mixture F-12 (DMEM/F12) containing 10% fetal bovine serum (FBS), penicillin (200 U/ml), and streptomycin (200 mg/ml; all obtained from Thermo Fisher Scientific, Waltham, MA) in a humidified atmosphere (95% air and 5% CO2). For all experiments, the cells were grown to 80–90% confluence on six-well plates and made quiescent by incubation in serum-free DMEM/F12, 24 h before stimulation with BSA (Roche, Indianapolis, IN), dissolved in serum-free DMEM/F12. The prepared BSA solution was passed through a Detoxi-Gel column (Thermo Fisher Scientific) to remove endotoxin.
Immunoblotting analysis.
Western blot analysis was carried out as previously described (32). Cells were lysed and subsequently sonicated in RIPA buffer (Shanghai Shenergy Biocolor BioScience & Technology, Shanghai, China) with PIC (Roche, Mannheim, Germany). Homogenates were centrifuged at 12,000 g for 10 min at 4°C, and protein concentrations of the supernatant were determined with the Pierce BCA Protein Assay Kit (catalog no. NCI3225CH; Thermo Fisher Scientific). Forty micrograms of protein for each sample, prepared in Laemmli sample buffer, was denatured in a boiling water bath for 10 min and then separated by SDS-PAGE, and the separated proteins were transferred to polyvinylidene fluoride membranes (GE Healthcare Life Sciences). The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline with Tween 20 (TBST) for 1 h at room temperature, followed by incubation with primary antibody (anti-PRR antibody; catalog no. HPA003156; Millipore-Sigma) overnight at 4°C. After washing with TBST, membranes were incubated with secondary antibody (goat anti-rabbit/mouse horseradish peroxidase-conjugated secondary antibody; catalog no. 31460; Thermo Fisher Scientific) for 1 h at room temperature and visualized with enhanced chemiluminescence (catalog no. 32106; Thermo Fisher Scientific). The densitometry of the bands was analyzed by using Image-Pro Plus 6.0. Exposure time was 3 min. The blot was stripped and reprobed with anti-β-actin antibody (catalog no. A1978; Millipore-Sigma). The expression of protein was calculated in relation to β-actin.
Biochemical analysis of renin, sPRR, and ANG II.
Cell culture medium was collected at the end of treatments. Renin activity in samples was determined by the delta value of the ANG I generation from the sample, incubating at 4°C and 37°C for 1 h, respectively. ANG I generation was assayed by using an ANG I Peptide Enzyme Immunoassay Kit (catalog no. S-1188; Peninsula Laboratories International, San Carlos, CA), according to the manufacturer’s instructions. The values were expressed as nanogram per milliliter per hour of newly generated ANG I. The medium concentrations of sPRR and ANG II were determined by using ELISA kits (for sPRR: catalog no. 27781, Immuno-Biological Laboratories, Gunma, Japan; for ANG II: catalog no. ADI-900-204, Enzo Life Sciences, Lausen, Switzerland).
Measurement of IL-6 and IL-8.
IL-6 (catalog no. BMS 213; eBioscience, San Diego, CA) and IL-8 (catalog no. BMS 204; eBioscience) concentrations in cell culture medium were measured by using commercial ELISA kits.
RNA interference.
The knockdown of endogenous furin (catalog no. SR303336), ADAM19 (catalog no. SR305748), proprotein convertase (PC) subtilisin/kexin type 7 (PCSK7; catalog no. SR306062), S1P (catalog no. SR305740), PCSK9 (catalog no. SR316837), or PRR (catalog no. SR306857) was performed using the predesigned small interfering RNA (siRNA) from OriGene (Rockville, MD). HK-2 cells were cultured to 80% confluence and transfected with 20 nM furin, ADAM19, PCSK7, S1P, PCSK9, PRR siRNAs, or nontargeting control siRNA (catalog no. SR30004). The siRNA was transfected 24 h before albumin treatment. Cells were harvested for RNA analyses, 48 h after siRNA transfection. Levels of sPRR in the medium were assessed by Western blotting and ELISA, 72 h after siRNA transfection. The sequences of the siRNA are shown in Table 1.
Table 1.
Sequences of custom-made siRNAs directed toward human targets
| siRNA | Sequence Information |
|---|---|
| Control siRNA | |
| Sense | CCUACGCCACCAAUUUCGU |
| Antisense | ACGAAAUUGGUGGCGUAGG |
| Furin | |
| Sense | CGAGGUGACAGAGUCAGUGGAGGCA |
| Antisense | UGCCUCCACUGCAUCUGUCACCUCG |
| ADAM19 | |
| Sense | GGAAUUAGAGGACUGAUUACGGUGA |
| Antisense | UCACCGUAAUCAGUCCUCUAAUUCC |
| PCSK7 | |
| Sense | CUCAUCUCUCUUGGGCCAA |
| Antisense | UUGGCCCAAGAGAGAUGAG |
| S1P (SKI-1) | |
| Sense | GCCACAGAACGGAGACAACAUUGAA |
| Antisense | CGGUGUCUUGCCUCUGUUGUAACUU |
| PCSK9 | |
| Sense | GCUAGCAACACCCAAAGGU |
| Antisense | ACCUUUGGGUGUUGCUAGC |
| PRR (ATP6AP2) | |
| Sense | AGAAUAUUAAGUGGAAGUGGGUGAA |
| Antisense | UUCACCCACUUCCACUUAAUAUUCU |
ADAM19, a disintegrin and metalloproteinase domain 19; PCSK7/9, proprotein convertase subtilisin/kexin type 7/9; S1P (SKI-1), subtilisin kexin isozyme 1; PRR (ATP6AP2), prorenin receptor.
Site-directed mutagenesis and transfection.
A PCR-based, site-directed mutagenesis was performed to analyze the importance of S1P and furin-mediated cleavage sites in the PRR protein. To generate the pcDNA3.1-PRR plasmid, a fragment spanning rat PRR cDNA from “ATG” to “stop codon” was amplified by PCR with the following primers: 5′-GCACCATGGCTGTGCTTGTCG-3′ (PRR forward) and 5′-TCAATCCATTCGAATCTTCTGGTTTG-3′ (PRR reverse). Amplified DNA was inserted into the pCR2.1 vector, as suggested by the manufacturer (Thermo Fisher Scientific). pCR2.1 DNA containing the insert was digested with EcoRI, and the fragment containing PRR cDNA was ligated into the pcDNA3.1 plasmid digested with EcoRI. To produce the mutation, we used site-directed mutagenesis by PCR following the established protocols (30). The forward primers used for mutating the cleavage site of S1P, furin, or S1P plus furin in PRR protein are listed as follows: S1P, 5′-CAAGGACCACCGTTGAGACGAAAC-3′; furin, 5′-CATCCCTGGTGGTGAAGTCAAG-3′; and S1P plus furin, 5′-CCCTGGTGGTGAAGTCAGTGACCATCGTTGAGAC-3′ (bold letters indicate mutation sites). The mutated DNAs were inserted into the pcDNA-3.1 plasmid the same way as the generation of pcDNA3.1-PRR. Sequences of plasmids containing the insert were verified by sequencing (University of Utah DNA Sequencing Core Facility).
HK-2 cells were cultured in DMEM/F12, supplemented with 10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin (Thermo Fisher Scientific) and 6 g/l glucose. Cells were reversely transfected during the plating (cell confluence at ∼50%) with HiPerFect Transfection Reagent (Qiagen, Beverly, MA). Plasmids were transfected in equal amounts (0.5 µg/well in a 24-well plate). Twenty-four hours after the plating, the same amount of plasmids was transfected again to the cells with Lipofectamin 3000 (Thermo Fisher Scientific). The next day, the cell culture medium was replaced with DMEM/F12 medium but without FBS, and the cells were harvested with RIPA buffer (Millipore-Sigma) containing PIC (Roche, Mannheim, Germany) after 2 days of no FBS culturing.
qRT-PCR.
Total RNA isolation and reverse transcription were performed, as previously described (29). All reactions were run in duplicate. The data are shown as a relative value, normalized by GAPDH. Oligonucleotides were designed using Primer3 software (available at http://flypush.imgen.bcm.tmc.edu/primer/primer3_www.cgi) and are shown in Table 2.
Table 2.
Primer sequences for real-time PCR
| Target Gene | Primer Sequence |
|---|---|
| Human-GAPDH, F | TCATTGACCTCAACTACATG |
| Human-GAPDH, R | CAAAGTTGTCATGGATGACC |
| Human-PRR, F | CAGACGTGGCTGCATTGTCC |
| Human-PRR, R | CTGGGGGTAGAGCCAGTTTGTT |
| Human-ACE, F | GGAGGAATATGACCGGACATCC |
| Human-ACE, R | TGGTTGGCTATTTGCATGTTCTT |
| Human-Furin, F | GCAATAATGGTCCCCATCC |
| Human-Furin, R | GCCTCCTCCTTCCAATATCC |
| Human-ADAM19, F | AAGTACCATGACAACGCCCAAT |
| Human-ADAM19, R | CATTCTCGGAGTGGTCCATGT |
| Human-PCSK1, F | CTGGATGGCATTGTGACGGAT |
| Human-PCSK1, R | GCCCCAGCTTGCACTGTAAA |
| Human-PCSK2, F | GGGAAAGGTGTTACCATTGGAA |
| Human-PCSK2, R | CCAGTCATCTGTGTACCGAGG |
| Human-PCSK4, F | CACGCAAATTCGGCTTCGTC |
| Human-PCSK4, R | ACGACAGAGCGTTTCACCC |
| Human-PCSK5, F | CCAACCACTGGGCAGTCAAA |
| Human-PCSK5, R | CTGACCTTTTAATCGTCCTGCT |
| Human-PCSK6, F | CGCAGGCCCTTTACTTCAAC |
| Human-PCSK6, R | CGGCAGCGACTGTTCTTGT |
| Human-PCSK7, F | GCAGCGTCCACTTCAACGA |
| Human-PCSK7, R | GCCCAGTCACATTGCGTTC |
| Human-PCSK9, F | AGACCCACCTCTCGCAGTC |
| Human-PCSK9, R | GGAGTCCTCCTCGATGTAGTC |
| Human-S1P, F | ACCTCGAAACAATCCATCCAGT |
| Human-S1P, R | ACTTGAGGGAACGAAAGACTTTT |
| Human-TGF-β1, F | TCCACCTGCAAGACTATCGAC |
| Human-TGF-β1, R | GAGGTATCGCCAGGAATTGTT |
| Human-IL-6, F | ACTCACCTCTTCAGAACGAATTG |
| Human-IL-6, R | CCATCTTTGGAAGGTTCAGGTTG |
| Human-IL-8, F | TTTTGCCAAGGAGTGCTAAAGA |
| Human-IL-8, R | AACCCTCTGCACCCAGTTTTC |
F, forward; R, reverse; PRR (ATP6AP2), prorenin receptor; ACE, angiotensin-converting enzyme; ADAM19, a disintegrin and metalloproteinase domain 19; PCSK1/2/4/5/6/7/9, Homo sapiens proprotein convertase subtilisin/kexin type 1/2/4/5/6/7/9; S1P, also known as SKI-1, membrane-bound transcription factor peptidase, site-1; TGF-β1: transforming growth factor-β1.
Statistical analysis.
Data are summarized as means ± SD. Prespecified hypotheses were proposed before the start of the experimental series. The sample size was determined by power analysis. One-way ANOVA, followed by Bonferroni correction, was used to analyze means between more than two groups, and unpaired Student’s t-test was used to analyze when two groups were present. GraphPad Prism 6 software was used for statistical analyses. P < 0.05 was considered statistically significant.
RESULTS
Examination of linearity of the detection system and time-course studies of BSA regulation of PRR expression.
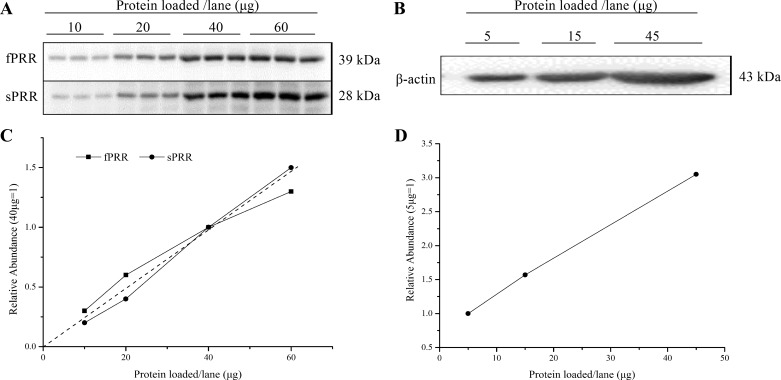
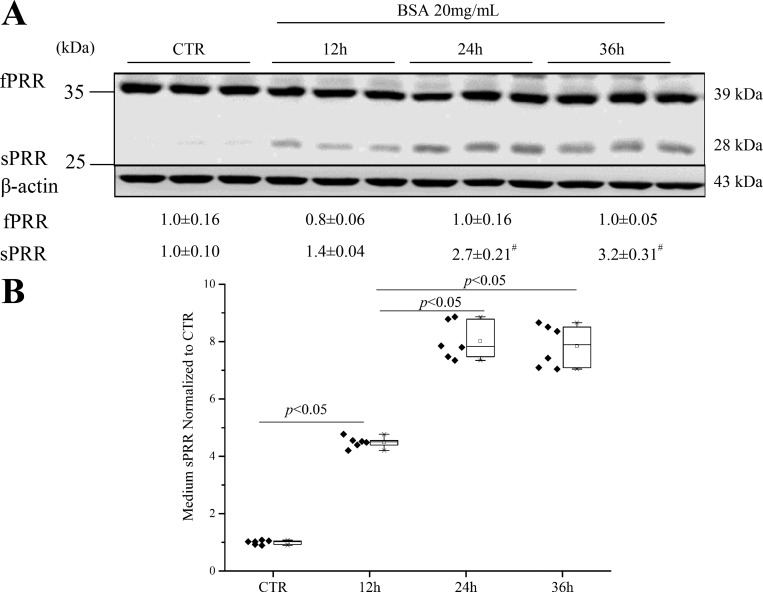
The correlation between the amount of loaded proteins and the abundance of full-length PRR (fPRR), sPRR, and β-actin was detected by immunoblotting analysis. We found that 40 μg was in the linear range for fPRR, sPRR, and β-actin in HK-2 cells (Fig. 1, A–D). The HK-2 cells were exposed to 20 mg/ml BSA for the indicated time periods, and fPRR and sPRR protein expressions were analyzed by immunoblotting. Medium sPRR was determined by ELISA and normalized by protein content. The increase in sPRR in response to BSA treatment was detected at 12 h, reaching the maximal level at 24 h (Fig. 2, A and B).
Fig. 1.
Examination of linearity of the detection system. A and C: the correlation between the amount of loaded proteins and the abundance of fPRR and sPRR detected by immunoblotting analysis. A: the blots for fPRR and sPRR. C: the line graph for A. B: the correlation between the amount of loaded proteins and the protein abundance of β-actin detected by immunoblotting analysis. D: the line graph for β-actin.
Fig. 2.
Time-course studies of BSA regulation of PRR expression. The HK-2 cells were exposed to 20 mg/ml BSA for the indicated time periods, and fPRR and sPRR protein expressions were analyzed by immunoblotting (representative blot) and densitometry analysis (A). Data are means ± SD; n = 6/group. B: ELISA detection of medium sPRR normalized by protein content. Values are presented as the median (central line), interquartile range (box), and range (whiskers); n = 6/group, #P < 0.05 vs. 12 h BSA. CTR, Control group.
BSA-induced cleavage of PRR and inhibition of PRR attenuated mRNA expression of TGF-β1, IL-6, and IL-8 and renin activity in HK-2 cells.
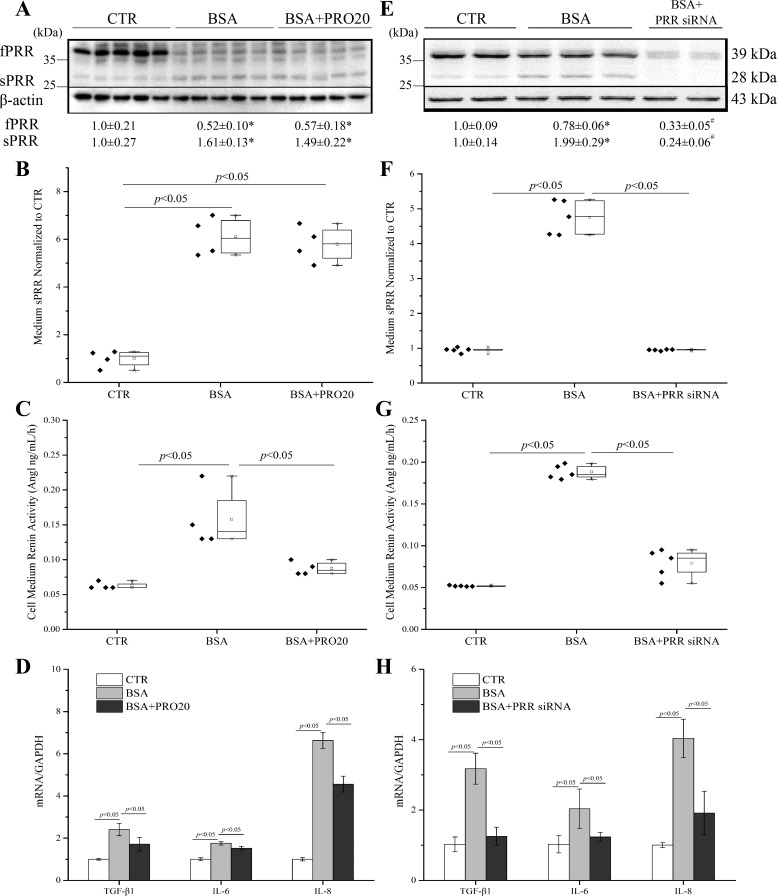
Protein droplets, seen in the cytoplasm of proximal tubular cells, are associated with acceleration of tubulointerstitial damage via the proinflammatory response (17, 35) and oxidative stress (11), which in turn, leads to excretion of chemokines and cytokines, resulting in inflammation, transformation of interstitial fibroblasts, and fibrosis (7). Exposure of HK-2 cells to high concentrations of BSA (20 mg/ml) for 24 h induced cleavage of full-length PRR (fPRR) to sPRR, as reflected by immunoblotting detection of increased sPRR protein abundance (Fig. 3A) and ELISA detection of increased medium concentration of sPRR (Fig. 3B) and reduced protein expression of fPRR (Fig. 3A). Renin activity was increased by BSA, and this increase was blunted by PRO20 (Fig. 3C). Furthermore, PRO20 partially suppressed the increases in mRNA levels of transforming growth factor-β1 (TGF-β1), IL-6, and IL-8 induced by BSA treatment (Fig. 3D). To validate these results, we employed siRNA to knock down PRR in HK-2 cells. PRR siRNA effectively reduced expression levels of fPRR and sPRR proteins (Fig. 3E) and medium sPRR (Fig. 3F) induced by BSA treatment. PRR siRNA significantly reduced BSA-induced renin activity (Fig. 3G) and the expression of TGF-β1, IL-6, and IL-8 induced by BSA treatment (Fig. 3H). To address involvement of local RAS, we examined angiotensin-converting enzyme (ACE) mRNA levels by using qRT-PCR. BSA treatment increased ACE mRNA levels (3.15 ± 0.57 in the BSA group vs. 1.00 ± 0.29 in the Control group; n = 6, P < 0.05), and this increase was suppressed by PRR siRNA (1.53 ± 0.29 in the BSA + PRR siRNA group vs. 3.15 ± 0.57 in the BSA group; n = 6, P < 0.05). Together, the consistent results demonstrated that PRR undergoes robust cleavage to produce sPRR and plays an important role in mediating albumin overload-mediated activation of renin activity and proinflammatory and profibrogenic responses.
Fig. 3.
Effects of PRO20 and siRNA-mediated PRR knockdown on renin activity and TGF-β1, IL-6, and IL-8 expression induced by BSA in HK-2 cells. The cells were treated for 24 h with vehicle, BSA (20 mg/ml) alone, or in combination with PRO20 (4 μM). PRR protein expression was analyzed by immunoblotting (A; representative image), coupled with densitometry analysis, and normalized by β-actin; n = 5/group. B: ELISA measurement of medium sPRR with normalization by total protein content; n = 4/group. C: the medium renin activity; n = 4/group. D: qRT-PCR analysis of mRNA expression of TGF-β1, IL-6, and IL-8. The values were normalized by GAPDH; n = 5/group. In a separate experiment, HK-2 cells were transfected with PRR siRNA or scrambled siRNA, followed by BSA treatment at 20 mg/ml for 24 h. The protein expression of fPRR and sPRR was analyzed by immunoblotting (E). The medium sPRR was determined by using ELISA. F: medium sPRR. G: medium renin activity. H: qRT-PCR analysis of mRNA expression of TGF-β1, IL-6, and IL-8 with normalization by GAPDH; n = 5/group. Data are means ± SD; *P < 0.05 vs. CTR; #P < 0.05 vs. BSA. B, C, F, and G: values are presented as the median (central line), interquartile range (box), and range (whiskers).
S1P, but not furin or ADAM19, contributed to PRR cleavage.
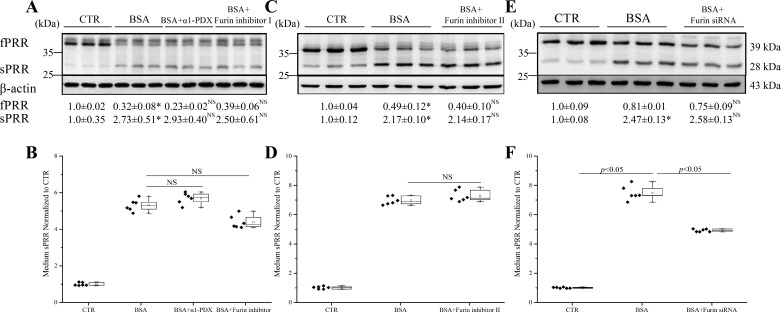
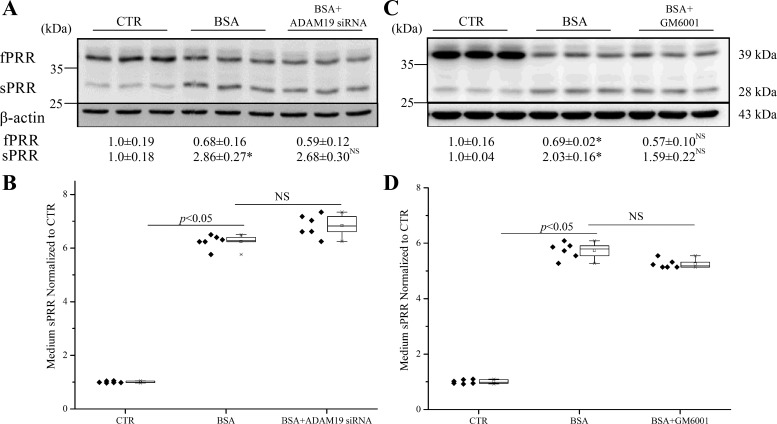
We next investigated the mechanism of PRR cleavage in response to albumin overload in HK-2 cells. It has been reported that cleavage of PRR to sPRR is mediated by furin. We therefore first examined the contribution of furin to PRR cleavage induced by albumin overload in cultured HK-2 cells with the use of furin inhibitors and siRNA. To our surprise, either furin inhibitor α1-PDX, furin inhibitor I (Fig. 4A), or furin inhibitor II (Fig. 4C) failed to suppress the cleavage of PRR, as assessed by immunoblotting analysis of reciprocal changes in fPRR and sPRR or by ELISA detection of medium sPRR (Fig. 4, B and D). Furin siRNA was highly efficient in knocking down furin mRNA expression (0.20 ± 0.09 vs. 1.0 ± 0.49; n = 12, P < 0.01). Similar to the furin inhibitors, furin siRNA was largely ineffective in suppressing the cleavage event (Fig. 4E), albeit with a modest effect in reducing medium sPRR (Fig. 4F). Overall, these data do not favor a major role of furin in mediating albumin overload-induced PRR cleavage.
Fig. 4.
Effect of furin inhibitors and siRNA on PRR cleavage induced by BSA in HK-2 cells. The cells were pretreated with α1-PDX (480 nM), furin inhibitor I (50 μM), or furin inhibitor II (25 μM) for 1 h or transfected with furin siRNA for 48 h and then treated with 20 mg/ml BSA for 24 h. The expression of fPRR and sPRR was determined by immunoblotting (A, C, and E; representative picture) coupled with corresponding densitometry analysis. B, D, and F: the concentrations of sPRR in the medium were measured by ELISA and normalized by protein content. *P < 0.05 vs. CTR; NS, nonsignificance vs. BSA. Data are means ± SD; n = 6/group. B, D, and F values are presented as the median (central line), interquartile range (box), and range (whiskers).
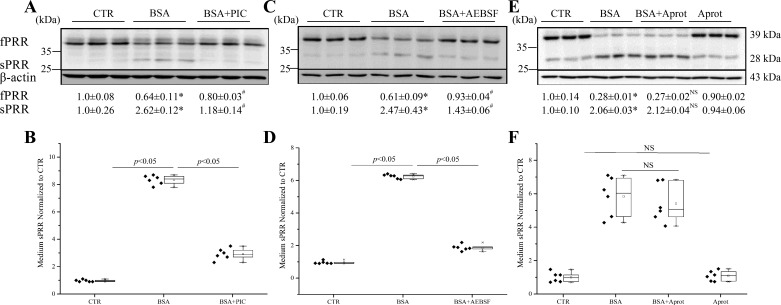
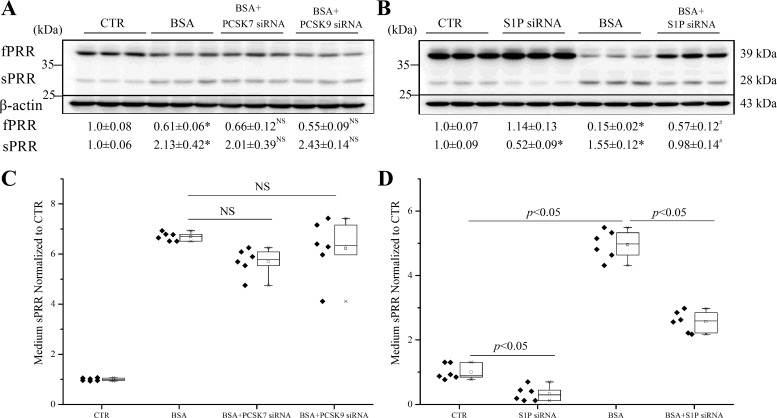
ADAM19 represents a second cleavage enzyme following furin (40). We subsequently examined the role of ADAM19 in mediating albumin overload-induced PRR cleavage in cultured HK-2 cells with the use of an siRNA or an ADAM19-specific inhibitor GM6001. The siRNA reduced ADAM19 expression by 78% in HK-2 cells (0.22 ± 0.08 vs. 1.00 ± 0.19; n = 10, P < 0.01). To our further surprise, ADAM19 siRNA was completely ineffective in suppressing albumin overload-induced cleavage of PRR, as assessed by immunoblotting analysis of the changes in fPRR and sPRR (Fig. 5A) or by ELISA detection of medium sPRR (Fig. 5B). Similarly, the parallel experiments with the use of GM6001 yield completely negative results (Fig. 5, C and D). A PIC (Roche), consisting various serine and cysteine protease inhibitors, such as PMSF, leupeptin, Aprot, pepstatin, and E-64, was used. PIC effectively suppressed PRR cleavage, as assessed by immunoblotting analysis of fPRR and sPRR expression (Fig. 6A) and ELISA detection of medium sPRR (Fig. 6B). Then, we found that a nonselective serine protease inhibitor AEBSF had a similar inhibitory effect on albumin overload-induced PRR cleavage (Fig. 6, C and D). These results suggest a protease distinct from furin and ADAM19 as a PRR cleavage enzyme. Serine proteases can be divided into two major families: those related to the trypsin or chymotrypsin fold and those that are closer to bacterial subtilisin, generally called subtilases. We found no effect of trypsin and chymotrypsin inhibitor Aprot (0.1 mM) on albumin overload-induced PRR cleavage (Fig. 6, E and F). We then turned our attention to PCs, which belong to a subtilase subfamily. PCs play an essential role in the post-translational processing of numerous inactive proteins to active proteins involved in many important biological processes. PCs comprise nine members: PC1/3, PC2, furin, PC4, PC5/6, PACE4 (paired basic amino acid cleaving enzyme 4), PCSK7, subtilisin kexin isozyme 1 (SKI-1)/S1P, and PCSK9. Among these PCs, PC1/3, PC2, PC4, PC5/6, and PACE4 exhibited no or low mRNA expression in HK-2 cells, as assessed by qRT-PCR cells, which has narrowed down the candidate PCs to furin, PCSK7, PCSK9, and S1P. Since furin was ruled out, we then focused on the involvement of PCSK7, PCSK9, and S1P by knocking down each of these three PCs using siRNA. The siRNAs against PCSK7, PCSK9, and S1P reduced the individual target mRNAs by 80~90% (PCSK7 mRNA: 0.18 ± 0.06 in the siRNA group vs. 1.0 ± 0.02 in the Control group, n = 8, P < 0.05; PCSK9 mRNA: 0.14 ± 0.04 in the siRNA group vs. 1.0 ± 0.23 in the Control group, n = 8, P < 0.05; S1P mRNA: 0.19 ± 0.03 in the siRNA group vs. 1.0 ± 0.20 in the Control group, n = 8, P < 0.05). The inhibition of PCSK7 and PCSK9 with siRNA failed to affect albumin overload-induced PRR cleavage (Fig. 7, A and C). In contrast, S1P siRNA significantly attenuated albumin overload-induced changes in fPRR and sPRR expression, even reduced the baseline level of sPRR expression, as assessed by immunoblotting analysis (Fig. 7B), and also reduced medium sPRR at basal condition and after albumin-overload treatment (Fig. 7D).
Fig. 5.
Effects of the ADAM inhibitor GM6001 and ADAM19 siRNA on PRR cleavage induced by BSA in HK-2 cells. The cells were pretreated with GM6001 (50 μM) for 1 h or transfected with ADAM19 siRNA for 48 h and then treated with 20 mg/ml BSA for 24 h. A and C: immunoblotting of fPRR and sPRR and corresponding densitometry analysis. Representative blots and quantitative densitometry analysis of fPRR and sPRR protein are shown. B and D: the concentrations of sPRR in the medium measured by ELISA and normalized by total protein content. *P < 0.05 vs. CTR; NS, no significance vs. BSA. Data are means ± SD; n = 6/group. B and D values are presented as the median (central line), interquartile range (box), and range (whiskers).
Fig. 6.
Effects of protease inhibitor PIC, a serine protease inhibitor AEBSF, and trypsin and chymotrypsin inhibitors aprotinin (Aprot; 0.1 mM) on PRR cleavage induced by BSA in HK-2 cells. The cells were pretreated with 0.4 mg/ml PIC, 80 μM AEBSF, or 0.1 mM Aprot for 1 h and then treated with 20 mg/ml BSA for 24 h. A, C, and E: immunoblotting of fPRR and sPRR and corresponding densitometry analysis. Representative blots and quantitative densitometry analysis of fPRR and sPRR protein levels are shown. B, D, and F: ELISA detection of sPRR in the medium. The values were normalized by protein content. *P < 0.05 vs. CTR; #P < 0.05 vs. BSA; NS, nonsignificance vs. BSA. Data are means ± SD; n = 6/group. B, D, and F values are presented as the median (central line), interquartile range (box), and range (whiskers).
Fig. 7.
Effects of siRNA-mediated silencing of PCSK7, PCSK9, and S1P on PRR cleavage induced by BSA in HK-2 cells. The cells were transfected with siRNA against PCSK7, PCSK9, or S1P for 48 h and then treated with 20 mg/ml BSA for 24 h. A and B: immunoblotting of fPRR and sPRR. Shown are representative blots and quantitative densitometry analysis. C and D: ELISA detection of medium sPRR. The values are normalized by protein content. *P < 0.05 vs. CTR; #P < 0.05 vs. BSA; NS, nonsignificance vs. BSA. Data are means ± SD; n = 6/group. C and D values are presented as the median (central line), interquartile range (box), and range (whiskers).
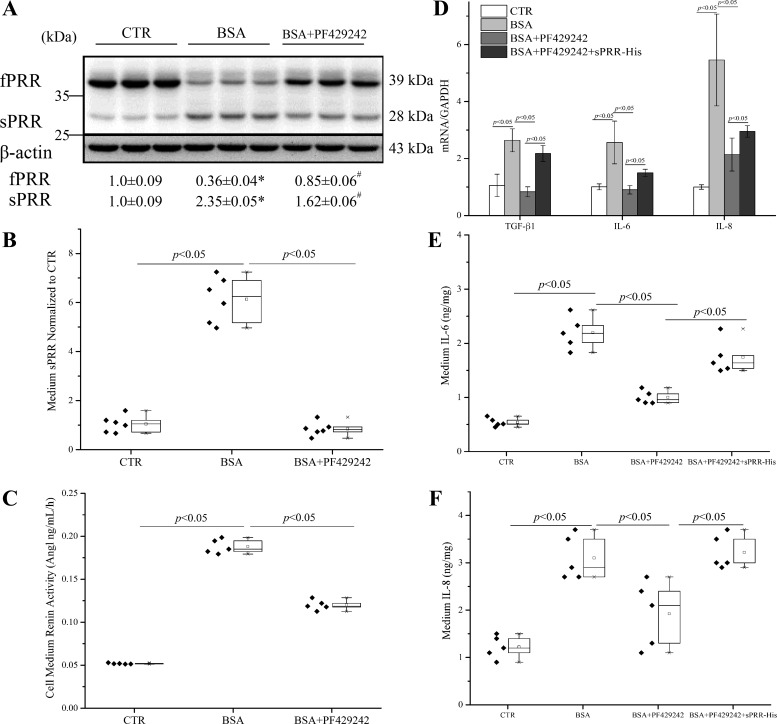
Similar results were obtained with the use of a specific S1P inhibitor, PF-429242 (Fig. 8A). Of note, PF-429242 almost completely blocked the rise of medium sPRR induced by albumin overload (Fig. 8B). These results support that S1P functions as a dominant enzyme for cleaving PRR during albumin overload in renal epithelial cells.
Fig. 8.
Effects of the S1P inhibitor PF-429242 on BSA-induced PRR cleavage, renin activity, and expression of TGF-β, IL-6, and IL-8 in HK-2 cells. The cells were pretreated with 40 μM PF-429242 for 1 h and then treated with 20 mg/ml BSA for 24 h. A: shown are representative blots and densitometry analysis. *P < 0.05 vs. CTR; #P < 0.05 vs. BSA; n = 6/group. B: ELISA detection of medium sPRR with normalization by total protein content. C: the medium renin activity; n = 5/group. D–F: for these experiments, the cells were pretreated with 40 μM PF-429242 alone or in combination with 50 nM sPRR-His for 1 h and then treated with 20 mg/ml BSA for 24 h. The expression of TGF-β1, IL-6, and IL-8 levels was determined by qRT-PCR (D); n = 5/group. Furthermore, the medium IL-6 (E) and IL-8 (F) protein concentrations were determined by using ELISA and normalized by total protein content; n = 5/group. Data are means ± SD. B, C, E, and F values are presented as the median (central line), interquartile range (box), and range (whiskers).
To probe the functional role of S1P-derived sPRR in albumin overload-induced inflammatory and fibrotic responses, we examined the effect of PF-429242 on a number of inflammatory and fibrotic markers in albumin-treated HK-2 cells. Furthermore, we examined the reversibility of the effects of PF-429242 with a recombinant sPRR, termed sPRR-His (20). PF-429242 significantly reduced BSA-induced renin activity (Fig. 8C). qRT-PCR detected parallel increases in mRNA expression of TGF-β1, IL-6, and IL-8 in BSA-loaded cells, which were all suppressed by PF-429242 and partially reversed by sPRR-His (Fig. 8D). Similar results were obtained with ELISA detection of medium IL-6 and IL-8 proteins (Fig. 8, E and F). These data suggest that S1P-derived sPRR mediates the deleterious effect of albumin overload in the renal epithelial cells.
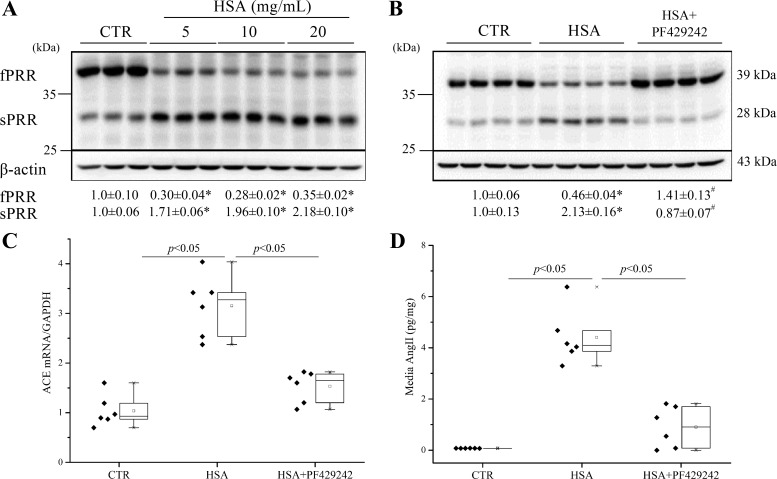
Next, we tested whether HSA and BSA have similar effects on PRR cleavage. The HK-2 cells were treated with indicated concentrations of HSA (5, 10, and 20 mg/ml) for 24 h (Fig. 9A). HSA at 5 mg/ml induced fPRR cleavage to sPRR. We also found that PF-429242 (40 µM) significantly reduced HSA-induced PRR cleavage (Fig. 9B). We subsequently determined ACE mRNA expression and ANG II levels as indices of the local RAS. HSA overload induced parallel increases in ACE mRNA and ANG II, both of which were blocked by PF-429242 (Fig. 9, C and D).
Fig. 9.
Dose course of HSA on PRR cleavage and effects of the S1P inhibitor PF-429242 on HSA-induced PRR cleavage and ANG II and expression of ACE in HK-2 cells. The cells were treated with 5, 10, and 20 mg/ml HSA for 24 h. A: shown are representative blots and densitometry analysis. *P < 0.05 vs. CTR; n = 6/group. The cells were pretreated with 40 μM PF-429242 for 1 h and then treated with 5 mg/ml HSA for 24 h. B: shown are representative blots and densitometry analysis. *P < 0.05 vs. CTR; #P < 0.05 vs. BSA; n = 4/group. C: qRT-PCR detection of ACE mRNA expression. D: ELISA detection of cell medium ANG II levels; n = 5/group. Data are means ± SD. C and D values are presented as the median (central line), interquartile range (box), and range (whiskers).
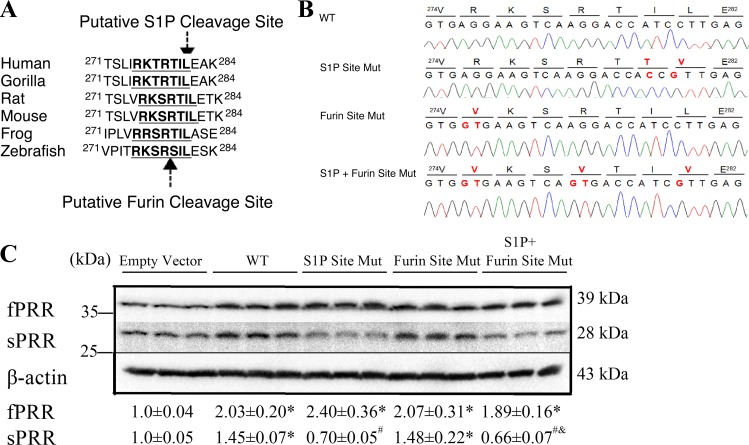
PRR contained the consensus S1P-mediated cleavage sequence “RTIL.” This site is highly conserved among different species (Fig. 10A). The predicted size of sPRR from this cleavage site is 29.46 kDa, which is quite close to its actual size of 28 kDa. This site was mutated to “VTTV” by using PCR-directed mutagenesis and validated by sequencing (Fig. 10B). This mutation led to reduced abundance of sPRR under the basal condition. In addition to the S1P cleavage site, PRR contains the furin-cleavage site. However, mutagenesis of the furin cleavage site did not affect sPRR abundance. Moreover, the combined mutagenesis of S1P and furin cleavage sites did not produce a greater effect than the single mutagenesis of the S1P cleavage site (Fig. 10C). These results have substantiated the conclusion that PRR cleavage is primarily mediated by S1P but not furin.
Fig. 10.
Blunted sPRR production by mutagenesis of S1P cleavage site in PRR protein. A: the alignment of the sequence around the S1P cleavage site in different vertebrate species. B: sequence tracks of pcDNA3.1-PRR plasmid containing wild-type (WT), mutated S1P cleavage site (S1P Site Mut), mutated furin cleavage site (Furin Site Mut), and mutated S1P and furin site (S1P + Furin Site Mut). Bold and red colored letters denote the amino acid or base substitutions. C: HK-2 cells were transfected with expression vectors containing wild-type PRR, the mutants, or an empty plasmid (Empty Vector). fPRR or sPRR abundance was detected by immunoblotting and normalized by β-actin. *P < 0.05 vs. Empty Vector; #P < 0.05 vs. WT; &P < 0.05 vs. Furin Site Mut. Data are means ± SD; n = 3/group.
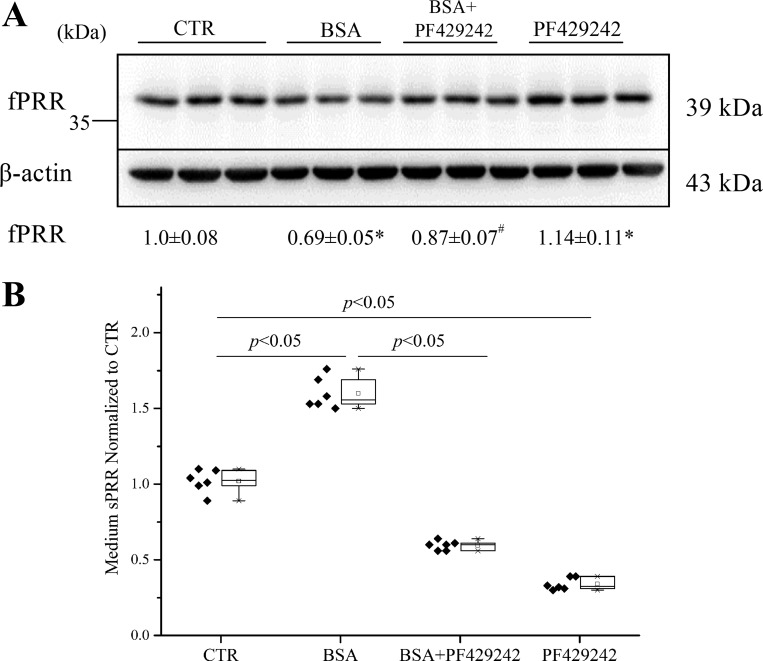
We validated some of the major findings using mouse inner medullary-collecting duct cell lines (mIMCD-K2). To confirm effects of PF-429242 (40 µM) on sPRR generation, mIMCD-K2 cells were exposed to 20 mg/ml BSA for 24 h. Like HK cells, mIMCD-K2 cells exhibited cleavage of fPRR to sPRR after exposure to albumin overload, which was effectively suppressed by PF-429242 (Fig. 11, A and B).
Fig. 11.
Effects of PF-429242 on PRR cleavage induced by BSA in mouse renal inner medullary-collecting duct cells (mIMCD-K2). The cells were treated for 24 h with control; BSA (20 mg/ml), alone or in combination with PF-429242 (40 μM); or PF-429242 (40 μM) alone. fPRR protein expression was determined by immunoblotting (A; representative blot) coupled with densitometry analysis and normalization by β-actin. *P < 0.05 vs. CTR; #P < 0.05 vs. BSA; n = 6/group. Data are means ± SD. B: ELISA detection of medium sPRR. The values are normalized by protein content; n = 6/group. B values are presented as the median (central line), interquartile range (box), and range (whiskers).
DISCUSSION
Proteinuria is well recognized as a biomarker of progressive kidney disease, such as glomerulosclerosis and diabetic nephropathy. It is also a mediator of progressive kidney damage. It has been shown that albumin overload induced activation of intrarenal RAS, but the detailed, underlying mechanism largely remains unclear (4, 14, 16). The present study attempted to investigate the role of PRR in mediating albumin overload-induced cellular responses in cultured renal epithelial cells. We found that a newly developed PRR inhibitor PRO20 effectively suppressed albumin overload-induced renin activity and a proinflammatory response. Moreover, we discovered S1P as a dominant cleavage enzyme responsible for production of sPRR that appears to contribute to albumin overload-induced cellular responses.
Activation of intrarenal RAS plays an essential role in promoting the progression of renal disease (12). It has been shown that high levels of albumin triggered the activation of the RAS in cultured renal proximal tubular cells via NADPH oxidase and protein kinase C (4). Consistent with this finding, we found that albumin overload directly stimulated renin activity in cultured renal epithelial cells. The enhancement of renin activity in response to albumin overload was effectively blocked by a PRR decoy inhibitor PRO20. This finding supports the notion that PRR functions as a regulator of the local RAS activity and thus may play a role in pathogenesis of CKD. Along this line, we recently reported that intramedullary delivery of PRO20 (32a) or collecting duct deletion of PRR attenuated the activation of intrarenal RAS during ANG II-induced hypertension (20, 31–33) and that systemic administration of PRO20 produced similar RAS-suppressing and blood pressure-lowering effects during fructose/high salt-induced hypertension (37).
PRR undergoes protease-mediated cleavage to generate sPRR. However, the cleavage mechanism remains elusive with the controversial contribution of furin (5) vs. ADAM19 (40). In fact, we found no evidence for involvement of furin or ADAM19 in mediating PRR cleavage in response to albumin overload. A major finding of the present study was the discovery of S1P as the predominant PRR cleavage enzyme. Furthermore, we confirmed that S1P-derived sPRR is functional in terms of its ability to increase renin activity and elicit a proinflammatory response. Besides PRR, S1P targets several other proteins, such as the sterol regulatory element-binding proteins (39) and low-density lipoprotein receptor (2) involved in lipid metabolism. Therefore, S1P inhibition may produce confounding effects due to modification of target proteins other than PRR. This possibility has been ruled out since the effect of S1P inhibition was reversed by administration of an exogenous sPRR-His.
The mechanism for albumin-induced activation of S1P remains elusive. Recent studies have reported that megalin/cubulin are involved in albumin-triggered tubular injury and tubulointerstitial inflammation (17), likely through lysosomal membrane permeabilization and lysosomal dysfunction (18), as well as endoplasmic reticulum stress (28). It seems reasonable to speculate that megalin/cubulin–lysosome-mediated albumin reabsorption is involved in the tubular cell activation of S1P that mediates the generation of sPRR during albumin overload. However, the relationship between S1P and the other known intracellular events, such as endoplasmic reticulum stress downstream of megalin/cubulin-mediated protein uptake, is unknown and certainly warrants further investigation.
Previous studies found that high doses of albumin activate the local RAS through the upregulation of ACE (16). ANG II is a major component of RAS, playing a key role in pathogenesis of proteinuria nephropathy through complex mechanisms, such as its direct proinflammatory actions (19). In the present study, we demonstrated that albumin overload induced an increase of ACE expression and ANG II levels, which were both blunted by S1P inhibition. These data suggest that S1P-derived sPRR may serve as an initial signal that triggers activation of the local RAS, leading to subsequent inflammation and cellular injury during albumin overload.
During our preparation for submission of this manuscript, the study of Nakagawa et al. (22) has just been published, reporting S1P as being responsible for generation of sPRR, a similar finding, as shown by the present study. It is of high significance that the two independent studies could reach a similar conclusion concerning the dominant role of S1P in the generation of sPRR. It is also important to acknowledge a number of differences between the two studies. Nakagawa et al. (22) used human PRR expressing Chinese hamster ovary cells to focus exclusively on the biochemical process for S1P-mediated PRR cleavage, whereas we probed this process by using a pathophysiologically relevant renal epithelial cell model of albumin overload-induced cellular responses. Therefore, not only did we discover S1P as the PRR cleavage enzyme, but we also defined the functional role of S1P-derived sPRR in mediating albumin-induced renin activity and proinflammatory responses. We also vigorously examined the involvement of S1P by performing mutagenesis of S1P and furin cleavage sites. Of note, we did not observe the band shift of sPRR following furin inhibition, as reported by Nakagawa et al. (22). This might be related to differences in electrophoresis conditions. Lastly, the present study is limited by the exclusive reliance on the cell culture model. A future study focusing on in vivo analysis of the role of S1P-derived sPRR in albumin overload nephropathy is certainly necessary.
In summary, the present study reports an essential role of sPRR in regulation of renin activity and proinflammatory responses in albumin-loaded renal epithelial cells. Furthermore, we discovered that albumin overload stimulated robust PRR cleavage to produce sPRR. S1P, but not furin or ADAM19, mediates the generation of sPRR during albumin overload. Together, these results suggest a potential role of S1P-derived sPRR in albumin overload-induced renal disease.
GRANTS
This work was supported by National Natural Science Foundation of China Grant No. 81630013, No. 81570377, No. 91439205 and No.31330037; Beijing Novo Nordisk Pharmaceuticals Science & Technology Co. Ltd; National Institutes of Health Grants DK094956, DK104072, and HL135851; and Merit Review Award 5I01BX002817 from the Department of Veterans Affairs. T. Yang is Research Career Scientist in the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.Y. conceived and designed research; H.F., C.X., A.L., C-J.Z., S.X., Y.C., L.Z., and M.L. performed experiments; H.F., C.X., A.L., C-J.Z., S.X., Y.C., L.Z., M.L., and L.W. analyzed data; H.F., C-J.Z., and T.Y. interpreted results of experiments; H.F. and C-J.Z. prepared figures; H.F., C-J.Z., and T.Y. drafted manuscript; W.W. and T.Y. edited and revised manuscript; T.Y. approved final version of manuscript.
REFERENCES
- 1.Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol 17: 2974–2984, 2006. doi: 10.1681/ASN.2006040377. [DOI] [PubMed] [Google Scholar]
- 2.Basu D, Huq A, Iqbal J, Hussain MM, Jiang XC, Jin W. Hepatic S1P deficiency lowers plasma cholesterol levels in apoB-containing lipoproteins when LDLR function is compromised. Nutr Metab (Lond) 12: 35, 2015. doi: 10.1186/s12986-015-0031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao W, Zhou QG, Nie J, Wang GB, Liu Y, Zhou ZM, Hou FF. Albumin overload activates intrarenal renin-angiotensin system through protein kinase C and NADPH oxidase-dependent pathway. J Hypertens 29: 1411–1421, 2011. doi: 10.1097/HJH.0b013e32834786f0. [DOI] [PubMed] [Google Scholar]
- 5.Cousin C, Bracquart D, Contrepas A, Corvol P, Muller L, Nguyen G. Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension 53: 1077–1082, 2009. doi: 10.1161/HYPERTENSIONAHA.108.127258. [DOI] [PubMed] [Google Scholar]
- 7.Eddy AA. Proteinuria and interstitial injury. Nephrol Dial Transplant 19: 277–281, 2004. doi: 10.1093/ndt/gfg533. [DOI] [PubMed] [Google Scholar]
- 9.Fukushima A, Kinugawa S, Homma T, Masaki Y, Furihata T, Abe T, Suga T, Takada S, Kadoguchi T, Okita K, Matsushima S, Tsutsui H. Increased plasma soluble (pro)renin receptor levels are correlated with renal dysfunction in patients with heart failure. Int J Cardiol 168: 4313–4314, 2013. doi: 10.1016/j.ijcard.2013.04.176. [DOI] [PubMed] [Google Scholar]
- 10.Hamada K, Taniguchi Y, Shimamura Y, Inoue K, Ogata K, Ishihara M, Horino T, Fujimoto S, Ohguro T, Yoshimoto Y, Ikebe M, Yuasa K, Hoshino E, Iiyama T, Ichihara A, Terada Y. Serum level of soluble (pro)renin receptor is modulated in chronic kidney disease. Clin Exp Nephrol 17: 848–856, 2013. doi: 10.1007/s10157-013-0803-y. [DOI] [PubMed] [Google Scholar]
- 11.Jia Y, Sun Y, Weng L, Li Y, Zhang Q, Zhou H, Yang B. Low molecular weight fucoidan protects renal tubular cells from injury induced by albumin overload. Sci Rep 6: 31759, 2016. doi: 10.1038/srep31759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 13.Kreienbring K, Franz A, Richter R, Dragun D, Heidecke H, Dechend R, Muller DN, Sehouli J, Braicu EI. Predictive and prognostic value of sPRR in patients with primary epithelial ovarian cancer. Anal Cell Pathol (Amst) 2016: 6845213, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Largo R, Gómez-Garre D, Soto K, Marrón B, Blanco J, Gazapo RM, Plaza JJ, Egido J. Angiotensin-converting enzyme is upregulated in the proximal tubules of rats with intense proteinuria. Hypertension 33: 732–739, 1999. doi: 10.1161/01.HYP.33.2.732. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Sullivan MN, Zhang S, Worker CJ, Xiong Z, Speth RC, Feng Y. Intracerebroventricular infusion of the (pro)renin receptor antagonist PRO20 attenuates deoxycorticosterone acetate-salt-induced hypertension [published correction appears in Hypertension 70: e33, 2017]. Hypertension 65: 352–361, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu BC, Gao J, Li Q, Xu LM. Albumin caused the increasing production of angiotensin II due to the dysregulation of ACE/ACE2 expression in HK2 cells. Clin Chim Acta 403: 23–30, 2009. doi: 10.1016/j.cca.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Liu D, Wen Y, Tang TT, Lv LL, Tang RN, Liu H, Ma KL, Crowley SD, Liu BC. Megalin/cubulin-lysosome-mediated albumin reabsorption is involved in the tubular cell activation of NLRP3 inflammasome and tubulointerstitial inflammation. J Biol Chem 290: 18018–18028, 2015. doi: 10.1074/jbc.M115.662064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu WJ, Xu BH, Ye L, Liang D, Wu HL, Zheng YY, Deng JK, Li B, Liu HF. Urinary proteins induce lysosomal membrane permeabilization and lysosomal dysfunction in renal tubular epithelial cells. Am J Physiol Renal Physiol 308: F639–F649, 2015. doi: 10.1152/ajprenal.00383.2014. [DOI] [PubMed] [Google Scholar]
- 19.Lombardi DM, Viswanathan M, Vio CP, Saavedra JM, Schwartz SM, Johnson RJ. Renal and vascular injury induced by exogenous angiotensin II is AT1 receptor-dependent. Nephron 87: 66–74, 2001. doi: 10.1159/000045886. [DOI] [PubMed] [Google Scholar]
- 20.Lu X, Wang F, Xu C, Soodvilai S, Peng K, Su J, Zhao L, Yang KT, Feng Y, Zhou SF, Gustafsson JA, Yang T. Soluble (pro)renin receptor via β-catenin enhances urine concentration capability as a target of liver X receptor. Proc Natl Acad Sci USA 113: E1898–E1906, 2016. doi: 10.1073/pnas.1602397113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagai J, Yamamoto A, Yumoto R, Takano M. Albumin overload induces expression of hypoxia-inducible factor 1α and its target genes in HK-2 human renal proximal tubular cell line. Biochem Biophys Res Commun 434: 670–675, 2013. doi: 10.1016/j.bbrc.2013.03.140. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa T, Suzuki-Nakagawa C, Watanabe A, Asami E, Matsumoto M, Nakano M, Ebihara A, Uddin MN, Suzuki F. Site-1 protease is required for the generation of soluble (pro)renin receptor. J Biochem 161: 369–379, 2017. doi: 10.1093/jb/mvw080. [DOI] [PubMed] [Google Scholar]
- 23.Nartita T, Ichihara A, Matsuoka K, Takai Y, Bokuda K, Morimoto S, Itoh H, Seki H. Placental (pro)renin receptor expression and plasma soluble (pro)renin receptor levels in preeclampsia [corrigendum appears in Placenta 47: 130, 2016]. Placenta 37: 72–78, 2016. doi: 10.1016/j.placenta.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen G. Renin, (pro)renin and receptor: an update. Clin Sci (Lond) 120: 169–178, 2011. doi: 10.1042/CS20100432. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002. doi: 10.1172/JCI0214276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishijima T, Tajima K, Takahashi K, Sakurai S. Elevated plasma levels of soluble (pro)renin receptor in patients with obstructive sleep apnea syndrome: association with polysomnographic parameters. Peptides 56: 14–21, 2014. doi: 10.1016/j.peptides.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Nishijima T, Tajima K, Yamashiro Y, Hosokawa K, Suwabe A, Takahashi K, Sakurai S. Elevated plasma levels of Ssoluble (pro)renin receptor in patients with obstructive sleep apnea syndrome in parallel with the disease severity. Tohoku J Exp Med 238: 325–338, 2016. doi: 10.1620/tjem.238.325. [DOI] [PubMed] [Google Scholar]
- 28.Ohse T, Inagi R, Tanaka T, Ota T, Miyata T, Kojima I, Ingelfinger JR, Ogawa S, Fujita T, Nangaku M. Albumin induces endoplasmic reticulum stress and apoptosis in renal proximal tubular cells. Kidney Int 70: 1447–1455, 2006. doi: 10.1038/sj.ki.5001704. [DOI] [PubMed] [Google Scholar]
- 29.Paliege A, Mizel D, Medina C, Pasumarthy A, Huang YG, Bachmann S, Briggs JP, Schnermann JB, Yang T. Inhibition of nNOS expression in the macula densa by COX-2-derived prostaglandin E(2). Am J Physiol Renal Physiol 287: F152–F159, 2004. doi: 10.1152/ajprenal.00287.2003. [DOI] [PubMed] [Google Scholar]
- 30.Urban A, Neukirchen S, Jaeger KE. A rapid and efficient method for site-directed mutagenesis using one-step overlap extension PCR. Nucleic Acids Res 25: 2227–2228, 1997. doi: 10.1093/nar/25.11.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang F, Lu X, Liu M, Feng Y, Zhou SF, Yang T. Renal medullary (pro)renin receptor contributes to angiotensin II-induced hypertension in rats via activation of the local renin-angiotensin system. BMC Med 13: 278, 2015. doi: 10.1186/s12916-015-0514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang F, Lu X, Peng K, Du Y, Zhou SF, Zhang A, Yang T. Prostaglandin E-prostanoid4 receptor mediates angiotensin II-induced (pro)renin receptor expression in the rat renal medulla. Hypertension 64: 369–377, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Wang F, Lu X, Peng K, Fang H, Zhou L, Su J, Nau A, Yang KT, Ichihara A, Lu A, Zhou SF, Yang T. Defining antidiuretic action of collecting duct (pro)renin receptor. I. Interaction with vasopressin/prostaglandin EP4 receptor. J Am Soc Nephrol 27: 3022–3034, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang F, Lu X, Peng K, Zhou L, Li C, Wang W, Yu X, Kohan DE, Zhu SF, Yang T. COX-2 mediates angiotensin II-induced (pro)renin receptor expression in the rat renal medulla. Am J Physiol Renal Physiol 307: F25–F32, 2014. doi: 10.1152/ajprenal.00548.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe N, Morimoto S, Fujiwara T, Suzuki T, Taniguchi K, Mori F, Ando T, Watanabe D, Kimura T, Sago H, Ichihara A. Prediction of gestational diabetes mellitus by soluble (pro)renin receptor during the first trimester. J Clin Endocrinol Metab 98: 2528–2535, 2013. doi: 10.1210/jc.2012-4139. [DOI] [PubMed] [Google Scholar]
- 35.Xiao W, Fan Y, Wang N, Chuang PY, Lee K, He JC. Knockdown of RTN1A attenuates ER stress and kidney injury in albumin overload-induced nephropathy. Am J Physiol Renal Physiol 310: F409–F415, 2016. doi: 10.1152/ajprenal.00485.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu C, Lu A, Lu X, Zhang L, Fang H, Zhou L, Yang T. Activation of renal (pro)renin receptor contributes to high fructose-induced salt sensitivity. Hypertension 69: 339–348, 2017. doi: 10.1161/HYPERTENSIONAHA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu M, Liu D, Ding LH, Ma KL, Wu M, Lv LL, Wen Y, Liu H, Tang RN, Liu BC. FTY720 inhibits tubulointerstitial inflammation in albumin overload-induced nephropathy of rats via the Sphk1 pathway. Acta Pharmacol Sin 35: 1537–1545, 2014. doi: 10.1038/aps.2014.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang J, Goldstein JL, Hammer RE, Moon YA, Brown MS, Horton JD. Decreased lipid synthesis in livers of mice with disrupted site-1 protease gene. Proc Natl Acad Sci USA 98: 13607–13612, 2001. doi: 10.1073/pnas.201524598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshikawa A, Aizaki Y, Kusano K, Kishi F, Susumu T, Iida S, Ishiura S, Nishimura S, Shichiri M, Senbonmatsu T. The (pro)renin receptor is cleaved by ADAM19 in the Golgi leading to its secretion into extracellular space. Hypertens Res 34: 599–605, 2011. doi: 10.1038/hr.2010.284. [DOI] [PubMed] [Google Scholar]
- 42.Zandi-Nejad K, Eddy AA, Glassock RJ, Brenner BM. Why is proteinuria an ominous biomarker of progressive kidney disease? Kidney Int Suppl 66, Suppl: S76–S89, 2004. doi: 10.1111/j.1523-1755.2004.09220.x. [DOI] [PubMed] [Google Scholar]
- 43.Zoja C, Benigni A, Remuzzi G. Cellular responses to protein overload: key event in renal disease progression. Curr Opin Nephrol Hypertens 13: 31–37, 2004. doi: 10.1097/00041552-200401000-00005. [DOI] [PubMed] [Google Scholar]