Summary
3‐Hydroxy‐3‐methylglutaryl‐coenzyme A synthase (HMGS) in the mevalonate (MVA) pathway generates isoprenoids including phytosterols. Dietary phytosterols are important because they can lower blood cholesterol levels. Previously, the overexpression of Brassica juncea wild‐type (wt) and mutant (S359A) BjHMGS1 in Arabidopsis up‐regulated several genes in sterol biosynthesis and increased sterol content. Recombinant S359A had earlier displayed a 10‐fold higher in vitro enzyme activity. Furthermore, tobacco HMGS overexpressors (OEs) exhibited improved sterol content, plant growth and seed yield. Increased growth and seed yield in tobacco OE‐S359A over OE‐wtBjHMGS1 coincided with elevations in NtSQS expression and sterol content. Herein, the overexpression of wt and mutant (S359A) BjHMGS1 in a crop plant, tomato (Solanum lycopersicum), caused an accumulation of MVA‐derived squalene and phytosterols, as well as methylerythritol phosphate (MEP)‐derived α‐tocopherol (vitamin E) and carotenoids, which are important to human health as antioxidants. In tomato HMGS‐OE seedlings, genes associated with the biosyntheses of C10, C15 and C20 universal precursors of isoprenoids, phytosterols, brassinosteroids, dolichols, methylerythritol phosphate, carotenoid and vitamin E were up‐regulated. In OE‐S359A tomato fruits, increased squalene and phytosterol contents over OE‐wtBjHMGS1 were attributed to heightened SlHMGR2, SlFPS1, SlSQS and SlCYP710A11 expression. In both tomato OE‐wtBjHMGS1 and OE‐S359A fruits, the up‐regulation of SlGPS and SlGGPPS1 in the MEP pathway that led to α‐tocopherol and carotenoid accumulation indicated cross‐talk between the MVA and MEP pathways. Taken together, the manipulation of BjHMGS1 represents a promising strategy to simultaneously elevate health‐promoting squalene, phytosterols, α‐tocopherol and carotenoids in tomato, an edible fruit.
Keywords: isoprenoids, 3‐hydroxy‐3‐methylglutaryl‐coenzyme A synthase, mevalonate, phytosterols, squalene, tomato
Introduction
In plant cells, isoprenoids including phytosterols, sesquiterpenes, monoterpenes, cytokinins (CKs), carotenoids, vitamin E, dolichol and brassinosteroids (BRs) are generated via the mevalonate (MVA) pathway and/or the methylerythritol phosphate (MEP) pathway (Akhtar et al., 2013; Besser et al., 2009; Enfissi et al., 2010; Rodríguez‐Concepción and Gruissem, 1999; Sallaud et al., 2009). Isopentenyl diphosphate isomerase (IPI) catalyses the interconversion of isopentenyl diphosphate (IPP) and its allyl isomer dimethylallyl diphosphate (DMAPP) and provides the first key intermediate for all kinds of isoprenoids including sterols (Bach, 1995; Hemmerlin et al., 2012; Rohmer, 1999; Sacchettini and Poulter, 1997). IPP is involved in cross‐talk between the cytosolic MVA pathway and the plastidial MEP pathway (Hemmerlin et al., 2003; Laule et al., 2003). Some cross‐regulations between them are also known to occur (Hemmerlin et al., 2012 and references therein; Huchelmann et al., 2014; Liao et al., 2016 and references therein).
The MEP pathway generates monoterpenes, diterpenes, carotenoids, tocopherols and class II sesquiterpenes. Geranyl diphosphate synthase (GPS) is responsible for the biosynthesis of monoterpene precursors, GPP and some FPPs (van Schie et al., 2007). Subsequently, monoterpene synthases (MTS) act to produce monoterpenes (Besser et al., 2009). Geranylgeranyl diphosphate synthase (GGPPS) catalyses the formation of 20‐carbon geranylgeranyl diphosphate (GGPP), which is the universal precursor of carotenoids, diterpenes, gibberellins (GAs) and vitamin E (Lichtenthaler, 1999; Rohmer, 1999). GGPP reductase (GGPPR) and γ‐methyl tocopherol transferase (GMTT) are two enzymes responsible for tocopherol biosynthesis in plants (Camara and d'Harlingue, 1985; Enfissi et al., 2010). Of the four naturally occurring species of tocopherols (vitamin E), α‐tocopherol is the most important one to human health as it has higher antioxidant activity than β‐, γ‐ or δ‐tocopherols (Azzi, 2007; DellaPenna, 2005; Shintani and DellaPenna, 1998).
3‐Hydroxy‐3‐methylglutaryl‐coenzyme A synthase (HMGS), which represents the second enzyme in the MVA pathway, can be genetically engineered to overaccumulate phytosterol content (Lange et al., 2015; Liao et al., 2014b; Wang et al., 2012). The importance of Arabidopsis thaliana HMGS in sterol biosynthesis, pollen grain fertility and seed yield has been demonstrated (Bhangu‐Uhlmann, 2011; Ishiguro et al., 2010; Lange et al., 2015; Liao et al., 2014a). In Brassica juncea, four isogenes encode HMGS (Alex et al., 2000). A mutant recombinant BjHMGS1 (S359A) was reported to show a 10‐fold increase in enzyme activity in vitro (Nagegowda et al., 2004). Interestingly, for the Enterococcus faecalis HMGS mutant, A110G, the reaction rate was elevated 140‐fold, because the amino acid substitution in A110G caused repositioning of the hydroxyl group in Ser308, the equivalent to B. juncea HMGS Ser359 (Steussy et al., 2006). It was proposed that B. juncea HMGS S359A has a shorter side chain, bringing the backbone of S359A closer to the catalytic loop, expediting the reaction (Steussy et al., 2006). Transgenic Arabidopsis HMGS‐OEs driven by the CaMV 35S promoter displayed induced expression of A. thaliana 3‐HYDROXY‐3‐METHYLGLUTARYL‐COENZYME A REDUCTASE (AtHMGR), STEROL METHYLTRANSFERASE2 (AtSMT2), Δ24 STEROL REDUCTASE (AtDWF1), STEROL C‐22 DESATURASE (AtCYP710A1) and BRASSINOSTEROID‐6‐OXIDASE2 (AtBR6OX2) (Wang et al., 2012). Phytosterol content was increased in Arabidopsis OE‐wtBjHMGS1 (11.3% and 13.6% enhancements in total seedling and leaf sterol content, respectively) and OE‐S359A (26.8% and 22.3% elevations in total seedling and leaf sterol content, respectively) (Wang et al., 2012). Tobacco (Nicotiana tabacum) HMGS‐OEs driven by the CaMV 35S promoter showed an up‐regulation of NtHMGR1, NtIPI2, SQUALENE SYNTHASE (NtSQS), NtSMT1‐2, NtSMT2‐1, NtSMT2‐2, CYTOCHROME P450 MONOOXYGENASE (NtCYP85A1) and NtGGPPS2, but down‐regulation of NtIPI1, NtGGPPS1, NtGGPPS3 and NtGGPPS4 (Liao et al., 2014b). In addition, the expression of NtSQS, NtHMGR1, NtSMT2‐1 and NtCYP85A1 in tobacco S359A overexpressors (OE‐S359A) was even higher than in the wild‐type (wt) BjHMGS1 overexpressors (OE‐wtBjHMGS1) (Liao et al., 2014b). Phytosterol content was also promoted in tobacco OE‐wtBjHMGS1 (4.6% and 12.1% in total seedling and leaf sterol content, respectively) and OE‐S359A (22.9% and 18.7% in total seedling and leaf sterol content, respectively), with OE‐S359A seedlings showing higher phytosterol content than OE‐wtBjHMGS1 (Liao et al., 2014b). Furthermore, tobacco HMGS‐OEs displayed enhanced plant growth, pod size and seed yield, with OE‐S359A exhibiting a greater effect than OE‐wtBjHMGS1 (Liao et al., 2014b).
Given that dietary phytosterols have been reported to lower blood cholesterol levels and might thereby reduce the risk of heart disease (Bradford and Awad, 2007; Moreau et al., 2002; Woyengo et al., 2009), it would be strategic to genetically manipulate HMGS in an edible fruit such as tomato. The metabolic engineering of the tomato MVA pathway using HMGR had caused a 2.4‐fold increase in phytosterol content in mature transgenic T0 tomato fruits overexpressing Arabidopsis HMGR1 (HMGR1‐OE) (Enfissi et al., 2005). However, in the mature fruits of the homozygous T2 tomato HMGR1‐OE, total HMGR activity was not enhanced and only few specific phytosterols were elevated (Enfissi et al., 2005), indicating that phytosterol increase in tomato HMGR1‐OEs was not stably inherited (Enfissi et al., 2005). Furthermore, HMGR is known to be subject to regulation at transcriptional, post‐transcriptional, translational and post‐translational levels (Bach, 1986; Hemmerlin, 2013; Wong et al., 1982).
Given the positive effects of BjHMGS1 in elevating phytosterols in HMGS‐OEs of model plants such as Arabidopsis and tobacco, and the improved effect of OE‐S359A in plant growth besides sterol content, the application of S359A should be extended to benefit food crops. Tomato (Solanum lycopersicum) was selected in this study because it is a popular fruit, eaten raw and from which juice is easily extracted for human consumption. Furthermore, the technology for tomato transformation is available (Mathews et al., 2003). To comprehensively investigate the effects of HMGS in regulating isoprenoid biosynthesis in tomato, the expression of genes in tomato HMGS‐OEs involved in the biosyntheses of MVA, C10, C15 and C20 universal precursors of isoprenoids, phytosterols, BR, CKs, dolichols, monoterpenes, sesquiterpenes, MEP, carotenoids and vitamin E was analysed by quantitative reverse transcription PCR (qRT‐PCR). The effect of HMGS overexpression on the metabolic flux was subsequently investigated by gas chromatography–mass spectrometry (GC‐MS) and high‐performance liquid chromatography (HPLC). It is interesting to note that the overexpression of BjHMGS1 in tomato up‐regulated SlGPS and SlGGPPS, causing a significant elevation in fruit α‐tocopherol and carotenoids, products of the MEP pathway, besides those of the MVA pathway such as squalene and phytosterols. Overall, this study demonstrates the potential of BjHMGS1 in simultaneously promoting vitamin E, carotenoid, squalene and phytosterol production in edible plants that would benefit human health.
Results
Tomato HMGS‐OE plants showed increased growth
To check whether BjHMGS1 overexpression in verified tomato lines (Figures 1, S1 and S2) caused enhanced growth as previously observed in tobacco HMGS‐OEs (Liao et al., 2014b), the length and fresh weight of 3‐week‐old (Figure 2a–c) and 5‐week‐old (Figure 2d and e) seedlings and the height of 9‐week‐old tomato plants (Figure 2f and g) were compared amongst OE‐wtBjHMGS1, OE‐S359A and the vector‐transformed lines. Three‐week‐old OE‐wtBjHMGS1 and OE‐S359A tomato seedlings had a higher fresh weight (83% and 57%, respectively, in Figure 2a and b) and longer seedling length (16% and 19%, respectively, in Figure 2a and c) than the control, but there was no significant difference in growth between 3‐week‐old OE‐wtBjHMGS1 and OE‐S359A seedlings (Figure 2b and c). Correspondingly, 5‐week‐old transgenic OE‐wtBjHMGS1 and OE‐S359A tomato plants displayed significant increases (17% and 26%, respectively) in height over the control (Figure 2d and e). Consistently, 9‐week‐old HMGS‐OEs (OE‐wtBjHMGS1 and OE‐S359A) grew better than the control (Figure 2f); OE‐wtBjHMGS1 displayed a 22% increase in height over the control, while OE‐S359A displayed an even higher (39%) increase as shown in Figure 2g.
Figure 1.
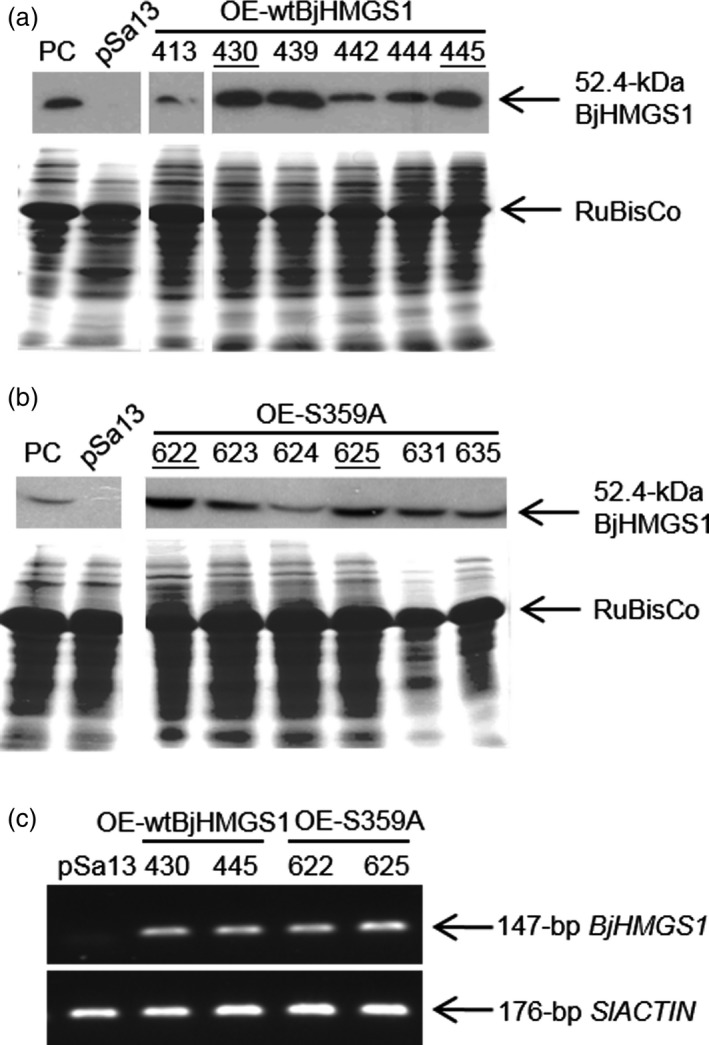
Characterization of representative transgenic tomato HMGS‐OEs. (a, b) Western blot analysis using antibodies against BjHMGS1 to verify the expression of BjHMGS1 in tomato leaves of 5‐week‐old representative wild‐type HMGS‐OEs (OE‐wtBjHMGS1) in (a) and mutant HMGS‐OEs (OE‐S359A) in (b). The cross‐reacting HMGS band is indicated by an arrowhead. Putative tomato HMGS‐OEs were designated as OE‐wtBjHMGS1 lines (413, 430, 439, 442, 444 and 445) in (a) and OE‐S359A lines (622, 623, 624, 625, 631 and 635) in (b). PC, positive control (tobacco BjHMGS1 OE line 402 as reported in Liao et al., 2014b); pSa13, vector (pSa13)‐transformed tomato. Bottom, Coomassie Blue‐stained gel of 20 μg total protein in each well. Two independent lines from each construct selected for further tests are underlined. White lines have been inserted between lanes that have been spliced together from the same original gel/blot. (c) Semiquantitative RT‐PCR analysis on representative transgenic tomato plants. BjHMGS1‐specific primers (ML1666 and ML1667) and tomato ACTIN (SlACTIN)‐specific primers (ML1688 and ML1689) were used. The PCR bands of 147‐bp BjHMGS1 and 176‐bp SlACTIN are indicated. pSa13, vector (pSa13)‐transformed control.
Figure 2.
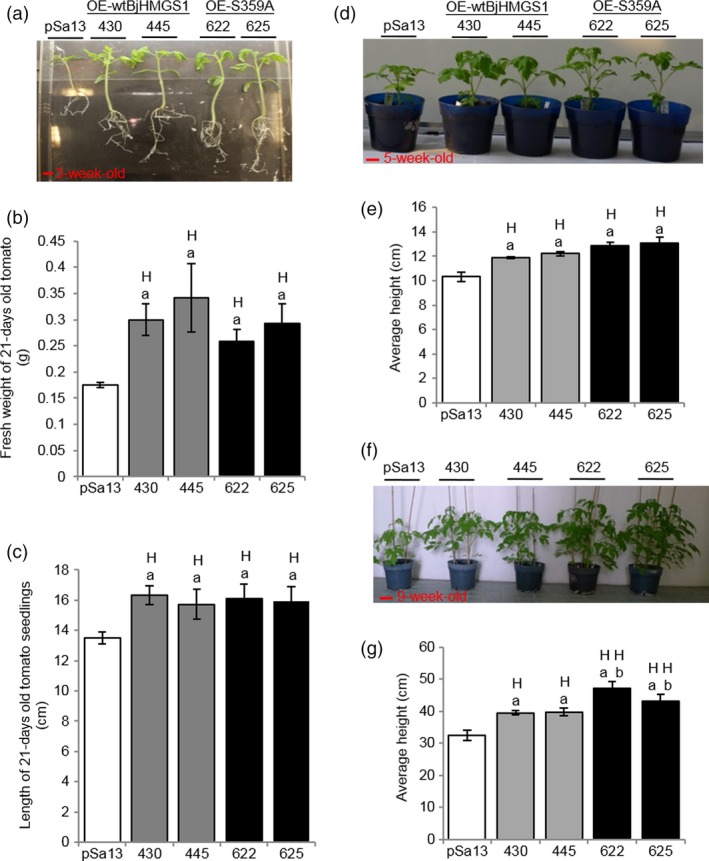
Comparison in growth between 3‐, 5‐, 9‐week‐old tomato HMGS‐OE seedlings/plants and vector‐transformed control. Two independent lines each of OE‐wtBjHMGS1 (430 and 445) and OE‐S359A (622 and 625) lines were compared to the vector‐transformed control (pSa13). (a) Representative MS plate‐grown seedlings photographed 3 weeks after germination. Bar = 1 cm. (b) Statistical analysis on fresh weight of 3‐week‐old tomato seedlings. (c) Statistical analysis on length of 3‐week‐old tomato seedlings. (d) Representative greenhouse‐grown plants photographed 5 weeks after germination. Bar = 3 cm. (e) Statistical analysis on height of 5‐week‐old tomato plants. (f) Representative greenhouse‐grown plants photographed 9 weeks after germination. Bar = 6 cm. (g) Statistical analysis on height of 9‐week‐old tomato plants. Values are mean ± SD (n = 30); bars are SD; H, value higher than the control; L, value lower than the control. ‘a’ indicates significant difference between HMGS‐OE and the vector (pSa13)‐transformed control; ‘b’ indicates significant difference between OE‐wtBjHMGS1 and OE‐S359A (P < 0.01, Student's t‐test).
Effect of HMGS overexpression on MVA‐related gene expression in tomato HMGS‐OE seedlings
Quantitative reverse transcription PCR was performed to test the effect of BjHMGS1 overexpression on the expression of genes downstream of HMGS, as well as of genes responsible for the biosyntheses of C10, C15 and C20 universal precursors of isoprenoids, and of sesquiterpenes in tomato HMGS‐OE seedlings. In OE‐wtBjHMGS1, SlSQS, SQUALENE EPOXIDASE (SlSQE) and CYCLOARTENOL SYNTHASE1 (SlCAS1) mRNAs were induced (Figure 3). In OE‐S359A, additional genes including SlHMGR1, FARNESYL DIPHOSPHATE SYNTHASE1 (SlFPS1), SlSQS, SlSQE, SlCAS1 and sesquiterpene‐related genes (SlSSTLE1 and SlSSTLH3) were up‐regulated (Figures 3 and S3). Greater expression of SlHMGR1, SlFPS1, SlSQS, SlSQE, SlCAS1, SlSSTLE1 and SlSSTLH3 in OE‐S359A than that of OE‐wtBjHMGS1 seedlings (Figures 3 and S3) corresponded to significant growth enhancement in 9‐week‐old OE‐S359A plants (Figure 2f and g). Furthermore, the expression of BR‐related genes (SlCYP85A1 and SlCYP85A3), CK‐related genes that encode type‐A response regulator proteins (SlTRR3/4, SlTRR8/9a, SlTRR8/9b and SlTRR16/17) (Shani et al., 2010) and dolichol‐related cis‐PRENYLTRANSFERASE (SlCPT3) was significantly elevated in both OE‐wtBjHMGS1 and OE‐S359A over the vector control (Figure S4a–c), coinciding with increased growth in tomato HMGS‐OEs (Figure 2). SlCYP85A3 and SlCPT3 expression in OE‐S359A was much higher than in OE‐wtBjHMGS1 (Figure S4a and c), corresponding well to better growth enhancement in 9‐week‐old OE‐S359A plants (Figure 2f and g). However, SlHMGR2 and SlIPI expression significantly decreased in all OE‐wtBjHMGS1 and OE‐S359A lines (Figure 3).
Figure 3.
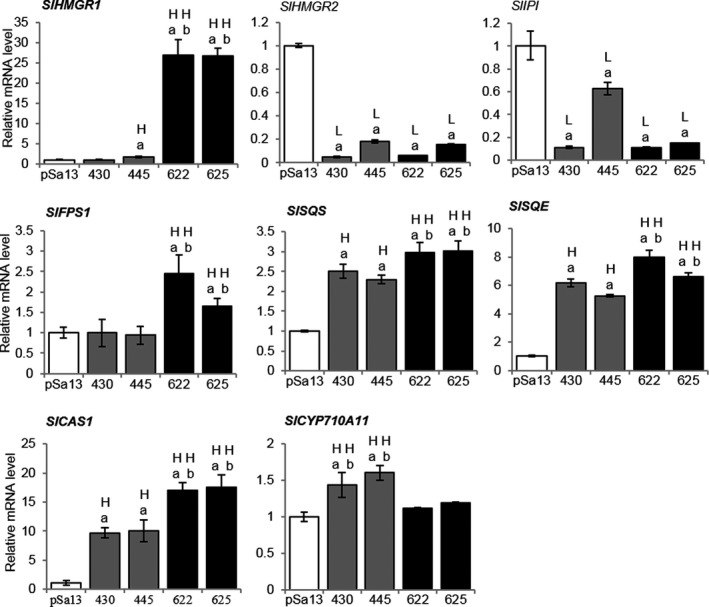
QRT‐PCR analysis on the expression of MVA‐ and phytosterol‐related genes in tomato HMGS‐OE seedlings. Total RNA was extracted from 3‐week‐old tomato seedlings of the vector (pSa13)‐transformed control, OE‐wtBjHMGS1 lines (430 and 445) and OE‐S359A lines (622 and 625). H, value higher than the control; L, value lower than the control. Values are means ± SD (n = 3). ‘a’ indicates significant difference between HMGS‐OE and the vector (pSa13)‐transformed control; ‘b’ indicates significant difference between OE‐wtBjHMGS1 and OE‐S359A (P < 0.05, Student's t‐test).
HMGS overexpression affected MEP‐related genes in tomato seedlings
MEP‐related genes [1‐DEOXY‐D‐XYLULOSE 5‐PHOSPHATE SYNTHASE1 (SlDXS1), SlDXS2 and 1‐DEOXY‐D‐XYLULOSE 5‐PHOSPHATE REDUCTOISOMERASE (SlDXR)] were slightly induced in OE‐wtBjHMGS1 and OE‐S359A in comparison with the vector control (Figure 4). The expression of genes (SlCPT1, SlCPT2, SlCPT6, SlGPS, SlGPPS‐SSU‐II, SlGGPPS1 and SlGGPPS2) leading to the biosynthesis of plastidial C10 and C20 universal precursors of isoprenoids was higher in OE‐wtBjHMGS1 and OE‐S359A than in the control (Figure S5a). SlCPT1 and SlCPT2 expression in OE‐S359A was much higher than in OE‐wtBjHMGS1 (Figure S5a), corresponding to significant growth enhancement in 9‐week‐old OE‐S359A plants (Figure 2f and g).
Figure 4.
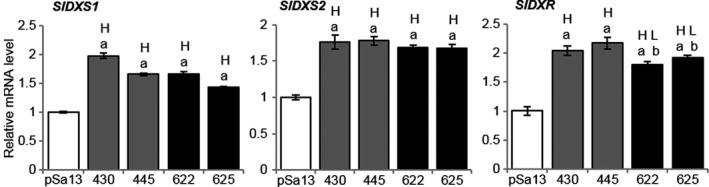
QRT‐PCR analysis on the expression of MEP pathway‐related genes in tomato HMGS‐OE seedlings. Total RNA was extracted from 3‐week‐old tomato seedlings of the vector (pSa13)‐transformed control, OE‐wtBjHMGS1 lines (430 and 445) and OE‐S359A lines (622 and 625). H, value higher than the control; L, value lower than the control. Values are means ± SD (n = 3). ‘a’ indicates significant difference between HMGS‐OE and the vector (pSa13)‐transformed control; ‘b’ indicates significant difference between OE‐wtBjHMGS1 and OE‐S359A (P < 0.05, Student's t‐test).
It is interesting to note that carotenoid‐related genes [(PHYTOENE SYNTHASE (SlPSY‐1), SlPSY‐2, ζ‐CAROTENE DESATURASE (SlZDS), CAROTENE ISOMERASE (SlCRTISO), β‐LYCOPENE CYCLASE (SlLCY‐B), β‐LYCOPENE CYCLASE (SlCYC‐B) and ε‐LYCOPENE CYCLASE (SlLCY‐E) except PHYTOENE DESATURASE (SlPDS)] and vitamin E‐related genes (SlGGPPR and SlGMTT) were up‐regulated in tomato HMGS‐OE seedlings (Figure S6a and b). Furthermore, SlPDS, SlCRTISO and SlCYC‐B expression in OE‐S359A was much higher than in OE‐wtBjHMGS1 (Figure S6a). However, there was no difference in monoterpene‐related SlMTS1 expression amongst the vector control, OE‐wtBjHMGS1 and OE‐S359A (Figure S5b).
Tomato HMGS‐OE fruits accumulated MVA‐derived squalene, sterol‐related intermediates, sterols and MEP‐derived vitamin E and carotenoids
Given the induced expression of isoprenoid‐, vitamin E‐ and carotenoid‐related genes in tomato HMGS‐OE seedlings and tomato fruit is edible, analysis on tomato HMGS‐OE fruits would inform on feasibility in the accumulation of health‐promoting end products from the MVA and MEP pathways such as phytosterols and related intermediates, vitamin E and carotenoids.
When the contents of major phytosterols (campesterol, β‐sitosterol and stigmasterol) and related intermediates (squalene, cycloartenol, 24‐methylene‐cycloartanol, cycloeucalenol, 24‐methylene‐lophenol and Δ7,22‐ergostadienol) in tomato HMGS‐OE fruits were analysed by GC‐MS, the results showed an increase in OE‐BjHMGS1 and OE‐S359A over the vector control, with the exception of squalene (in OE‐BjHMGS1 line 430) and 24‐methylene‐cycloartanol (in OE‐BjHMGS1 line 430 and OE‐S359A line 625) (Figure 5). Furthermore, squalene, cycloeucalenol, campesterol and β‐sitosterol contents in OE‐S359A were significantly higher than in OE‐BjHMGS1 (Figure 5).
Figure 5.
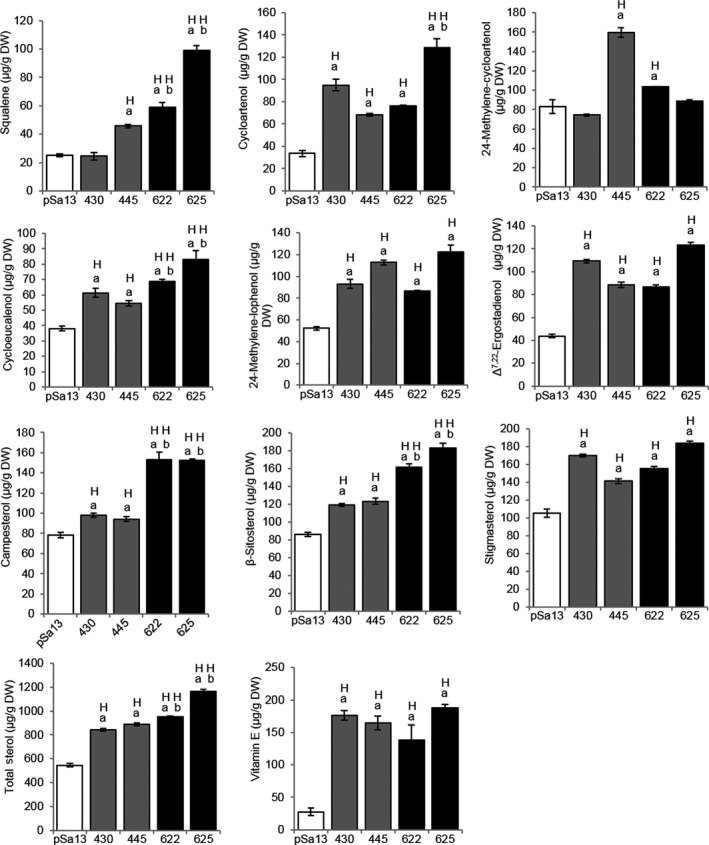
GC‐MS analysis of squalene, sterol‐related intermediate, sterol and vitamin E contents [μg/g dry weight (DW)] in 57 DAP mature tomato HMGS‐OE fruits. Lipids were extracted from the vector‐transformed control (pSa13), two independent lines of OE‐wtBjHMGS1 (430 and 445) and two independent lines of OE‐S359A (622 and 625). H, value higher than the control (P < 0.01, Student's t‐test); L, value lower than the control (P < 0.01, Student's t‐test). Values are mean ± SD (n = 8); bars are SD; ‘a’ indicates significant difference between HMGS‐OE and the vector (pSa13)‐transformed control; ‘b’ indicates significant difference between OE‐wtBjHMGS1 and OE‐S359A (P < 0.01, Student's t‐test).
In particular, such elevation was evident in squalene (average elevation of 39.2%), cycloartenol (140%), 24‐methylene‐cycloartanol (40.8%), cycloeucalenol (52.0%), 24‐methylene‐lophenol (96.8%), campesterol (22.9%), β‐sitosterol (40.1%), stigmasterol (47.7%), Δ7,22‐ergostadienol (130%) and total sterol (58.7%) in OE‐BjHMGS1 fruits over the vector control (Tables 1 and 2). Also, squalene (210%), cycloartenol (200%), 24‐methylene‐cycloartanol (15.6%), cycloeucalenol (99.5%), 24‐methylene‐lophenol (99.9%), campesterol (95.0%), β‐sitosterol (99.8%), stigmasterol (61.0%), Δ7,22‐ergostadienol (140%) and total sterol content (93.7%) increased in OE‐S359A fruits over the vector control (Tables 1 and 2). Accordingly, the average amounts of squalene (130%), cycloartenol (25.5%), cycloeucalenol (31.2%), campesterol (58.7%), β‐sitosterol (42.1%) and total sterol (22.1%) in OE‐S359A fruits were significantly higher than in OE‐BjHMGS1 (Tables 1 and 2).
Table 1.
Sterol‐related intermediate, sterol, α‐tocopherol and carotenoid profiles of tomato HMGS‐OE fruits [μg/g dry weight (DW) for sterols and α‐tocopherol, mg/g DW for carotenoids]
| Sterols | pSa13 | 430 | 445 | 622 | 625 |
|---|---|---|---|---|---|
| Squalene | 25.2 ± 0.55 | 24.5 ± 1.49 | 45.7 ± 0.71 a | 58.9 ± 1.98 a , b | 99.1 ± 2.04 a , b |
| Cycloartenol | 33.8 ± 2.79 | 94.9 ± 5.22 a | 68.2 ± 1.40 a | 76.3 ± 0.60 a | 128.4 ± 8.39 a , b |
| 24‐Methylene‐cycloartanol | 83.2 ± 6.84 | 74.5 ± 1.16 | 159.8 ± 4.84 a | 103.4 ± 0.05 a | 89.0 ± 1.49 |
| Cycloeucalenol | 38.1 ± 1.42 | 61.3 ± 3.08 a | 54.5 ± 1.65 a | 68.9 ± 1.00 a | 83.1 ± 5.68 a , b |
| 24‐Methylene‐lophenol | 52.2 ± 1.69 | 93.0 ± 4.09 a | 112.7 ± 2.18 a | 86.7 ± 0.75 a | 122.1 ± 6.37 |
| Campesterol | 78.2 ± 2.8 | 98.0 ± 2.3 a | 94.2 ± 2.3 a | 152.8 ± 7.7 a , b | 152.2 ± 1.5 a , b |
| β‐Sitosterol | 86.2 ± 2.5 | 119.2 ± 1.7 a | 123.2 ± 3.5 a | 161.3 ± 4.3 a , b | 183.3 ± 4.8 a , b |
| Stigmasterol | 105.2 ± 4.5 | 169.9 ± 1.5 a | 140.9 ± 2.5 a | 155.4 ± 1.9 a | 183.5 ± 2.6 a , b |
| Δ7,22‐Ergostadienol | 43.8 ± 1.6 | 109.2 ± 1.2 a | 88.5 ± 2.3 a | 86.7 ± 1.6 a | 123.5 ± 2.1 a , b |
| Total sterol | 546.0 ± 12.3 | 844.5 ± 10.9 a | 887.6 ± 10.7 a | 950.4 ± 10.0 a , b | 1164.2 ± 17.5 a , b |
| α‐Tocopherol | 27.5 ± 3.2 | 176.4 ± 4.4 a | 164.7 ± 6.2 a | 138.6 ± 13.4 a | 187.5 ± 3.6 a |
| Lycopene | 3.0 ± 0.1 | 10.7 ± 1.2 a | 9.3 ± 0.5 a | 6.7 ± 0.7 a , b | 6.0 ± 0.2 a , b |
| β‐Carotene | 1.5 ± 0.06 | 6.7 ± 0.09 a | 3.8 ± 0.1 a | 3.5 ± 0.3 a | 4.5 ± 0.09 a |
| Total carotenoids | 4.5 ± 0.16 | 17.4 ± 1.29 a | 13.1 ± 0.6 a | 10.2 ± 1.0 a , b | 10.5 ± 0.29 a , b |
Two independent lines for each OE genotype were analysed. For OE‐wtBjHMGS1, lines 430 and 445 were tested. For OE‐S359A, lines 622 and 625 were tested. aIndicates significant difference (P < 0.01 by the Student's t‐test) between HMGS‐OE and the vector (pSa13)‐transformed control; bIndicates significant difference (P < 0.01 by the Student's t‐test) between OE‐wtBjHMGS1 and OE‐S359A. Total sterol content was calculated from the contents of all the components in the above table. Values are mean ± SD, n = 8 for sterol and 6 for carotenoid determination.
Table 2.
Comparison in sterol‐related intermediate, sterol, α‐tocopherol and carotenoid composition in tomato fruits
| Elevation (%) in tomato fruits | |||||
|---|---|---|---|---|---|
| Sterol‐related intermediates and sterols | 430 vs pSa13 | 445 vs pSa13 | 622 vs pSa13 | 625 vs pSa13 | OE‐S359A vs OE‐wtBjHMGS1 |
| Squalene | −2.9 | 81.2 | 133.6 | 293.0 | 125.1 |
| Cycloartenol | 181.1 | 101.9 | 125.9 | 280.2 | 6.5 |
| 24‐Methylene‐cycloartanol | −10.5 | 92.1 | 24.3 | 6.9 | −17.9 |
| Cycloeucalenol | 60.9 | 43.0 | 80.8 | 118.1 | 31.2 |
| 24‐Methylene‐lophenol | 77.9 | 115.7 | 65.9 | 133.9 | 1.6 |
| Campesterol | 24.0 | 19.2 | 94.1 | 93.3 | 58.7 |
| β‐Sitosterol | 38.3 | 42.9 | 87.1 | 112.5 | 42.1 |
| Stigmasterol | 61.5 | 33.9 | 47.7 | 74.4 | 9.0 |
| Δ7,22‐Ergostadienol | 149.1 | 101.9 | 97.9 | 181.8 | 6.4 |
| Total sterol | 54.7 | 62.6 | 74.1 | 113.2 | 22.1 |
| α‐Tocopherol | 542.6 | 500.1 | 405.0 | 583.1 | −4.4 |
| Lycopene | 256.6 | 211.0 | 123.7 | 98.4 | −52.5 |
| β‐Carotene | 343.3 | 150.4 | 135.9 | 202.6 | −31.4 |
| Total carotenoids | 277.1 | 184.5 | 122.8 | 128.1 | −45.6 |
Two independent lines for each OE genotype were analysed. For tomato OE‐wtBjHMGS1, lines 430 and 445 were tested. For tomato OE‐S359A, lines 622 and 625 were tested. The data presented for OE‐S359A in comparison with OE‐wtBjHMGS1 were calculated from an average of two lines (average of 622 and 625 for OE‐S359A in comparison with average of 430 and 445 for OE‐wtBjHMGS1). Bold font indicates significant (P < 0.05) % increases, in OE‐S359A (over OE‐wtBjHMGS1) and in OE‐wtBjHMGS1 and OE‐S359A (over the vector‐transformed control pSa13).
When the molecular mechanism for the accumulation of MVA‐derived compounds in HMGS‐OE fruits was investigated, the expression of SlFPS1, SlGPS, SlSQS, SlSQE, SlCAS1, SlCYP85A1 and SlCYP85A3 was found to be significantly higher in OE‐wtBjHMGS1 and OE‐S359A than in the control, with the exception of SlSQS in OE‐wtBjHMGS1 line 430 and SlCYP85A1 in OE‐S359A line 625 (Figure S7a and b). SlFPS1, SlGPS, SlSQS and SlCYP710A11 expression in OE‐S359A was much higher than in OE‐BjHMGS1 (Figure S7a and b). Conversely, the expression of SlHMGR1, SlHMGR2 and SlIPI was down‐regulated in HMGS‐OE fruits (Figure S7a).
More interestingly, a significant increase in vitamin E (α‐tocopherol) in tomato HMGS‐OE fruit lipid extracts was noted (Figure 5) from GC‐MS analysis. Vitamin E content in OE‐BjHMGS1 and OE‐S359A was significantly higher than in the vector control (Figure 5). Its content in HMGS‐OEs increased to 138.6–187.5 μg/g dry weight, in comparison with 27.5 μg/g dry weight in the control (Figure 5), representing average increases of 521% in OE‐BjHMGS1 and 494% in OE‐S359A (Figure 5). In HPLC analysis, a significant enhancement of carotenoids (lycopene and β‐carotene) in tomato HMGS‐OE fruit was observed (Figure 6). Lycopene and β‐carotene contents in OE‐BjHMGS1 and OE‐S359A were higher than in the control (Figure 6, Tables 1 and 2). Lycopene content in HMGS‐OEs increased to 6.0–10.7 mg/g dry weight, in comparison with 3.0 mg/g dry weight in the control (Table 1), representing average elevations of 234% in OE‐BjHMGS1 and 111% in OE‐S359A (Table 2). β‐Carotene content in HMGS‐OEs went up to 3.5–6.6 mg/g dry weight, in comparison with 1.5 mg/g dry weight in the control (Table 1), representing average increases of 247% in OE‐BjHMGS1 and 169% in OE‐S359A (Table 2). However, lycopene and total carotenoid contents in OE‐S359A were significantly lower than in OE‐BjHMGS1 (Figure 6, Tables 1 and 2). Lycopene and total carotenoid contents decreased by 53% and 46%, respectively, in OE‐S359A than in OE‐wtBjHMGS1 (Table 2). The elevation in vitamin E and carotenoids in OE‐BjHMGS1 and OE‐S359A fruits (Figure 5) coincided with an up‐regulation of plastidial SlGPS and SlGGPPS1, but not of SlGGPPS2 in OE‐wtBjHMGS1 and OE‐S359A fruits (Figure S7c).
Figure 6.
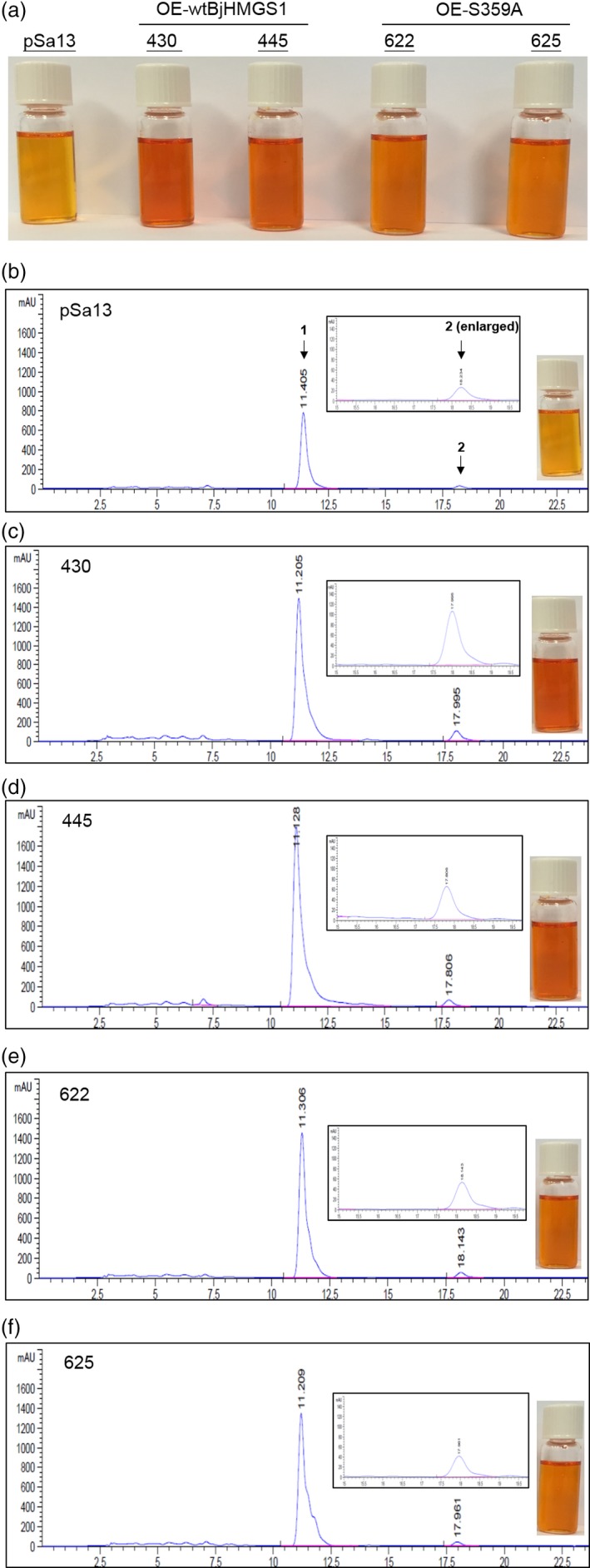
HPLC analysis of carotenoid content [μg/g dry weight (DW)] in 57 DAP mature tomato HMGS‐OE fruits. Carotenoids were extracted from the vector‐transformed control (pSa13), two independent lines of OE‐wtBjHMGS1 (430 and 445) and two independent lines of OE‐S359A (622 and 625). (a) Photographs of carotenoid extracts dissolved in chloroform; (b–f) HPLC chromatograms of carotenoids from pSa13, OE‐wtBjHMGS1 (430 and 450) and OE‐S359A (622 and 625), respectively. Peak 1, lycopene; peak 2, β‐carotene. The enlarged HPLC chromatogram of peak 2 and photograph of carotenoid extract dissolved in chloroform from each sample is presented in b–f.
Enhanced antioxidant activity of total carotenoids in tomato HMGS‐OE fruits
As α‐tocopherol and carotenoids (lycopene and β‐carotene) (Azzi, 2007; DellaPenna, 2005; Fiedor and Burda, 2014; Shintani and DellaPenna, 1998) accumulated in tomato HMGS‐OE fruits (Tables 1 and 2), they were tested for antioxidant activity in comparison with the vector control. Tomato HMGS‐OE fruits displayed significantly (P < 0.05) higher DPPH (1,1‐diphenyl‐2‐picrylhydrazyl) radical scavenging activities than the control (Figure 7). OE‐wtBjHMGS1 showed 257.9%–299.9% higher antioxidant activity than the control, while OE‐S359A was 89.5%–96.5% higher. OE‐wtBjHMGS1 possessed 82.1%–111.2% higher antioxidant activity than OE‐S359A (Figure 7), and this corresponded to a higher carotenoid content in OE‐wtBjHMGS1 than in OE‐S359A (Tables 1 and 2).
Figure 7.
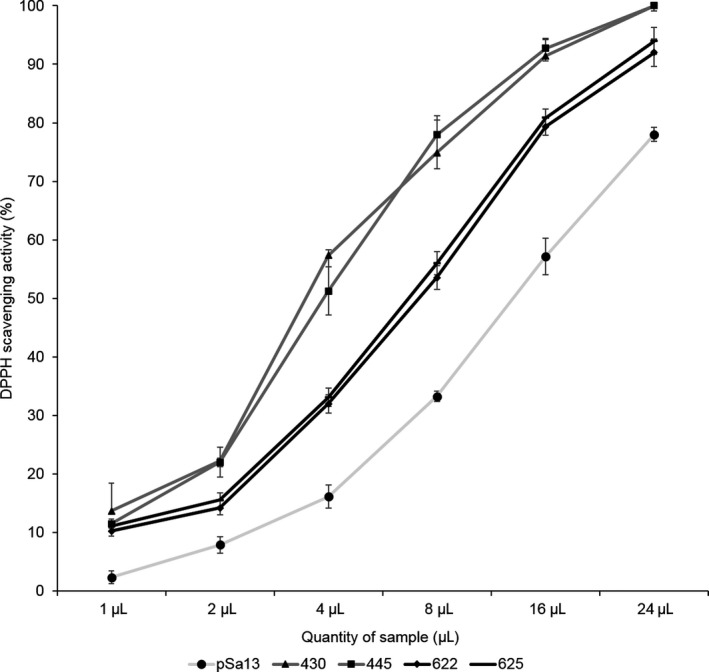
DPPH scavenging activities of carotenoids in 57 DAP mature tomato HMGS‐OE fruits. The vector‐transformed control (pSa13), two independent lines of OE‐wtBjHMGS1 (430 and 445) and two independent lines of OE‐S359A (622 and 625) were analysed. Values are means ± SD (n = 3).
Discussion
Elevation of health‐promoting components achieved via metabolic engineering of BjHMGS1 in tomato fruit
Resembling tobacco HMGS‐OEs, tomato HMGS‐OEs showed an enhanced expression of SlHMGR1, SlSQS, SlGGPPS1 and SlCYP85A1 and an increase in growth accompanied by a decline in SlHMGS2 and SlIPI expression. Furthermore, sterol content was elevated in HMGS‐OE tomato fruits, confirming that elevation of health‐promoting components was achieved via metabolic engineering of BjHMGS1 in tomato fruit and that extended phytosterol enhancement in fruits was attained using mutant S359A. New observations on tomato HMGS‐OE seedlings and fruits not previously reported for tobacco HMGS‐OE seedlings and leaves included:
Up‐regulation of genes associated with the biosyntheses of C10, C15 and C20 universal precursors of isoprenoids, phytosterols and dolichols in seedlings;
Up‐regulation of MEP‐, carotenoid‐ and vitamin E‐related genes, but down‐regulation of carotenoid‐related gene SlPDS and no apparent effect on monoterpene‐related gene (SlMTS1) expression in seedlings;
Greater expression of MVA‐related genes, genes leading to C10, C15, C20 universal precursors of isoprenoids and sesquiterpene‐related genes in OE‐S359A than that of OE‐wtBjHMGS1 seedlings, corresponding to significant growth enhancement in 9‐week‐old OE‐S359A plants, suggesting that S359A overexpression affected the expression of these isoprenoid precursor‐ and sesquiterpene‐related genes;
Up‐regulation of SlFPS1, SlSQE, SlCAS1, SlCYP85A1, SlCYP85A3, SlGPS and SlGGPPS1 in HMGS‐OE fruits, resulting in higher amounts of health‐promoting components including squalene, vitamin E (α‐tocopherol) and carotenoids; and
Elevation of fruit squalene and phytosterols in OE‐S359A in comparison with OE‐wtBjHMGS1, which was attributed to higher expression of SlHMGR2, SlFPS1, SlGPS, SlSQS and SlCYP710A11 in OE‐S359A fruits.
HMGS regulates isoprenoid biosynthesis genes in tomato HMGS‐OE seedlings
It has been reported that NtHMGR1 is a housekeeping gene, while NtHMGR2 is stress‐inducible (Hemmerlin et al., 2004; Merret et al., 2007). This corresponds well to the up‐regulation of NtHMGR1, but not NtHMGR2, in tobacco HMGS‐OE seedlings (Liao et al., 2014b). In tomato, SlHMGR1 is known to be highly expressed at the early stage of fruit development, while SlHMGR2 is confined to fruit maturation and ripening (Narita and Gruissem, 1989). Not surprisingly, SlHMGR1, but not SlHMGR2, expression closely coincided with growth‐dependent phytosterol biosynthesis (Rodríguez‐Concepción and Gruissem, 1999). In this study, the differential expression of SlHMGR1 and SlHMGR2 in tomato seedlings (Figure 3) resembles that of tomato fruits (Narita and Gruissem, 1989; Rodríguez‐Concepción and Gruissem, 1999).
Besides SlHMGR2, SlIPI was significantly down‐regulated in tomato HMGS‐OE seedlings (Figure 3), like NtIPI1 in transgenic tobacco HMGS‐OEs (Liao et al., 2014b). Besides IPI, plastid‐localized SlCPT1 and SlCPT2 prefer DMAPP as a substrate (Akhtar et al., 2013), and their mRNA expression in tomato OE‐S359A seedlings was significantly higher than in OE‐wtBjHMGS1 (Figure 3), corresponding to better growth (Figure 2). Interestingly, the increase in SlCPT1 expression was greater than that of SlCPT2 in these tomato HMGS‐OE seedlings (Figure S5a), implying that HMGS overexpression exerted a stronger effect on SlCPT1.
SlFPS1 has been proposed to play an important role in early fruit development as well as cell division and elongation (Gaffe et al., 2000). Simultaneous silencing of both FPS in Arabidopsis reduced sterol content and retarded seedling growth (Manzano et al., 2016). While NtFPPS expression was slightly elevated in tobacco seedlings (Liao et al., 2014b), SlFPS1 expression in tomato OE‐wtBjHMGS1 seedlings remained unchanged and was up‐regulated in OE‐S359A (Figure 3). Higher SlFPS1 expression observed in OE‐S359A in comparison with OE‐wtBjHMGS1 tomato seedlings (Figure 3) coincided well with a positive growth effect in 9‐week‐old OE‐S359A, suggesting that S359A overexpression was more effective in enhancing SlFPS1 expression.
Squalene, product of SQS (Abe et al., 1993; Devarenne et al., 1998, 2002; Seo et al., 2005), has been reported to confer health benefits because it possesses antitumour properties (Mathews, 1992; Newmark, 1997; Smith, 2000), and abilities in quenching singlet oxygen (Kohno et al., 1995) and reducing phenobarbital, theophylline and strychnine in animals (Kamimura et al., 1992). The overexpression of Panax ginseng SQS1 enhanced sterol and ginsenoside contents in transgenic P. ginseng roots (Lee et al., 2004). Transgenic Arabidopsis overexpressing Glycine max SQS1 demonstrated significant elevation in seed sterols (Nguyen et al., 2013). The silencing of Withania somnifera SQS down‐regulated downstream sterol pathway genes, reduced squalene, and sterol content and caused a dwarf phenotype (Singh et al., 2015). In this study, an enhanced effect of S359A on squalene production (Figure 5, Tables 1 and 2) mirrored the benefits seen in P. ginseng and Arabidopsis (Lee et al., 2004; Nguyen et al., 2013). The increase in SlSQS expression correlated with enhanced squalene and sterol accumulation in tomato HMGS‐OE fruits and higher SlSQS expression in OE‐S359A tomato seedlings and fruits corresponded well with elevated squalene and sterol contents, consistent with greater expression of NtSQS and AtSQS (Liao et al., 2014b; Wang et al., 2012). However, reports of a dwarf phenotype from the overexpression of a truncated yeast SQS or codon‐optimized Flag‐tagged yeast SQS in tobacco (Pasoreck et al., 2016; Wu et al., 2012) suggest differences do occur between applications of plant and yeast SQS.
SlGGPPS1 was highly expressed in tomato leaves in contrast to SlGGPPS2, which was induced in fruits and flowers (Ament et al., 2006). Both SlGGPPS1 and SlGGPPS2 expression was up‐regulated in tomato HMGS‐OE seedlings (Figure S5), but only NtGGPPS2 was elevated in several lines of tobacco HMGS‐OE seedlings, while NtGGPPS1, NtGGPPS3 and NtGGPPS4 remained down‐regulated in all previous lines tested (Liao et al., 2014b). These results suggest that HMGS overexpression produced inconsistent effects on GGPPS expression in tomato and tobacco seedlings. It appears that HMGS overexpression caused a stronger positive effect on GGPPS expression in tomato seedlings in comparison with tobacco. Ruiz‐Sola et al. (2016) identified one GGPPS gene encoding two differentially targeted (plastidial and cytosol) GGPPSs in Arabidopsis, implying that such could also occur in tomato and tobacco.
Co‐up‐regulation of BR‐, CK‐ and dolichol‐related genes in tomato HMGS‐OEs
In higher plants, besides sterols, BRs and CKs are essential in growth and development (He et al., 2003; Howell et al., 2003; Li et al., 1996; Shani et al., 2010; Vriet et al., 2012; Wang et al., 2012), while dolichol is important in protein glycosylation (Zhang et al., 2008). BR‐related genes were up‐regulated in Arabidopsis and tobacco HMGS‐OEs (Liao et al., 2014b; Wang et al., 2012). A BR (SlCYP85A3)‐ and a dolichol (SlCPT3)‐related gene were dramatically up‐regulated in tomato HMGS‐OE seedlings and more highly expressed in OE‐S359A than in OE‐wtBjHMGS1 (Figure S4a and c), indicating an effect from HMGS overexpression. In Caenorhabditis elegans, HMGS is significant in the miRNA pathway by regulating the function of many miRNAs during development (Shi and Ruvkun, 2012). MVA‐derived dolichols, which are involved in N‐glycosylation, are essential for the activity of miRNAs in silencing their target mRNAs (Shi and Ruvkun, 2012). More experiments are needed to address the relationship between HMGS and dolichol accumulation in plants.
The overexpression of cytosolic HMGS promotes carotenoid and vitamin E formation in plastids
The overexpression of Arabidopsis HMGR in Lavandula latifolia and that of cytosolic isopentenyl phosphate kinase in Arabidopsis and tobacco increased both MVA‐derived sterols and MEP‐derived monoterpenes and sesquiterpenes (Henry et al., 2015; Muñoz‐Bertomeu et al., 2007). Salvia miltiorrhiza HMGR overexpression in hairy roots enhanced MEP‐associated diterpene tanshinone accumulation (Kai et al., 2011). In this study, MEP‐related genes (SlDXS1, SlDXS2 and SlDXR), most carotenoid‐related genes (SlPSY‐1, SlPSY‐2, SlZDS, SlCRTISO, SlLCY‐B, SlCYC‐B and SlLCY‐E with the exception of SlPDS) and vitamin E‐related genes (SlGGPPR and SlGMTT) were up‐regulated in tomato HMGS‐OE seedlings (Figures 4 and S6), suggesting that HMGS overexpression in the cytosol can affect the biosynthesis of plastidial MEP‐related isoprenoids including carotenoids and vitamin E. Indeed, total carotenoids increased in tomato HMGS‐OE fruits (Figure 6, Tables 1 and 2) and lycopene content and total carotenoids were much higher in OE‐wtBjHMGS1 than in OE‐S359A (Figure 6, Tables 1 and 2). In contrast, the expression of SlCRTISO and SlCYC‐B in tomato OE‐S359A seedlings was significantly higher than in OE‐wtBjHMGS1 (Figure S6). These results implied that differential carotenoid‐related gene expression had probably occurred between tomato HMGS‐OE seedlings and fruits, or post‐transcriptional/post‐translational regulation may have taken effect. Furthermore, increased HMGS enzyme activity in OE‐S359A resulted in enhanced MVA‐derived squalene and phytosterols but not MEP‐derived carotenoids and vitamin E (Tables 1 and 2).
Vitamin E consists of two forms, tocopherols and tocotrienols (Brigelius‐Flohé and Traber, 1999). Previous studies manipulated enzymes in the vitamin E biosynthetic pathway to increase leaf (37%: 10‐fold) and seed (18%–1500%) vitamin E content in model plants Arabidopsis and tobacco, as well as crop plants including canola, soybean, corn, lettuce, potato and sunflower (Chen et al., 2006 and references cited therein; Del Moral et al., 2013; DellaPenna, 2005 and references cited therein; Vom Dorp et al., 2015). Also, the overexpression of zeaxanthin epoxidase in potato resulted in a two‐ to threefold elevation in α‐tocopherol (Römer et al., 2002). However, the metabolic engineering of the MVA pathway or HMGS for α‐tocopherol accumulation in a fruit crop had not been reported. We successfully demonstrated herein that manipulation of HMGS from the MVA pathway in tomato led to dramatic increase (~5‐fold) in fruit α‐tocopherol (Figure 5). This confirms that the overexpression of a cytosolic HMGS could cause an increase in plastidial GGPP‐derived vitamin E. There is evidence of cross‐talk between the MVA and MEP pathways in up‐regulated SlGGPPS1 expression in HMGS‐OE tomato fruits, promoted α‐tocopherol production. It is worth noting that there was no significant difference in the level of α‐tocopherol between OE‐wtBjHMGS1 and OE‐S359A, indicating that S359A was not superior to wtBjHMGS1 in enhancing α‐tocopherol production. Hence, metabolic engineering of HMGS from the MVA pathway can provide an alternative strategy in elevating α‐tocopherol production in a fruit crop. Also, the tomato HMGS‐OE fruits generated in this study present potential as an emerging form of beneficial food enriched in health‐promoting components including squalene, phytosterols, carotenoids and α‐tocopherol.
Experimental procedures
Plant materials and growth conditions
Wild‐type tomato (Lycopersicon esculentum Mill. cv. UC82B) seeds were obtained from Dr. WK Yip, The University of Hong Kong. Tomato seeds were surface‐sterilized in 75% ethanol for 1 min, rinsed thrice in sterilized water, soaked in 25% Clorox for 10 min and rinsed four times with sterilized water. Seeds were transferred to MS medium for 2 days at 4 °C before being moved to a tissue culture room for germination and seedling development. Tomato plants were grown at 25 °C (16‐h light)/22 °C (8‐h dark).
Generation and characterization of transgenic tomato overexpressing HMGS
Plasmids pBj134 (wt‐BjHMGS1) and pBj136 (S359A) from Wang et al. (2012) were used for Agrobacterium‐mediated tomato transformation (Mathews et al., 2003) with vector control pSa13 (Xiao et al., 2008). T1 transgenic tomato seeds were screened on MS with 50 μg/ml kanamycin and analysed by PCR followed by DNA sequencing (Liao et al., 2014b; Wang et al., 2012). T2 homozygous lines with single copy of BjHMGS1/S359A were analysed in mRNA and protein expression, plant growth and metabolite composition. Tomato total protein was extracted (Chye et al., 1999) from 3‐week‐old fresh tomato leaves and protein concentration measured (Bradford, 1976). Western blot analysis was conducted as described previously (Liao et al., 2014b; Wang et al., 2012; Xiao et al., 2010). Antibodies against BjHMGS1 were used in Western blot analysis (Wang et al., 2012). EcoRI‐digested tomato genomic DNA (40 μg) from 4‐week‐old leaves was separated on agarose gel (0.7%) by electrophoresis. Southern blot analysis (Southern, 2006) was performed using a digoxigenin‐labelled full length of BjHMGS1 cDNA probe generated by primer pair ML264 and ML276 (Wang et al., 2012). Primers are listed in Table S1.
Semiquantitative reverse transcription PCR (RT‐PCR)
Total RNA from 3‐week‐old tomato seedlings was extracted using RNeasy Plant Mini Kit (Qiagen, Hilden, Germany) followed by DNase I treatment (Qiagen). First‐strand cDNA was synthesized from 5 μg total RNA from 3‐week‐old tomato seedlings using the SuperScript First‐Strand Synthesis System (Invitrogen, Carlsbad, CA, USA). Semiquantitative reverse transcription PCR (RT‐PCR) was conducted using the PCR System (Bio‐Rad, Hercules, USA) with BjHMGS1‐specific primer pair (ML1666 and ML1667) and tomato ACTIN primer pair (ML1688 and ML1689). Tomato ACTIN (SlACTIN), which has been previously tested (EI‐Sharkawy et al., 2016; Melilli et al., 2014), was used as an internal control to estimate the amount of RNA in each sample. The conditions for PCR were as follows: denaturation at 95 °C for 5 min, followed by 35 cycles of amplification (95 °C for 15 s, 60 °C for 20 s and 72 °C for 20 s) and extension at 72 °C for 10 min. The experiment was repeated two times. Primers for RT‐PCR are listed in Table S1.
Quantitative reverse transcription PCR
Total RNA from 3‐week‐old tomato seedlings or mature red tomato fruits aged 57 days after pollination (DAP; breaker+15) was extracted using RNeasy Plant Mini Kit (Qiagen). The RNA (5 μg) was treated using DNase I (Qiagen) before reverse‐transcribed into first‐strand cDNA using the SuperScript First‐Strand Synthesis System (Invitrogen). Quantitative reverse transcription PCR (qRT‐PCR) was carried out with a StepOne Plus Real‐time PCR System (Applied Biosystems, Foster City, CA, USA) and FastStart Universal SYBR Green Master (Roche, Mannheim, Germany). The conditions for qRT‐PCR were as follows: denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Three experimental replicates for each reaction were carried out using gene‐specific primers, and tomato ACTIN was used as the internal control. The comparative CT method was used to analyse the qRT‐PCR data (Schmittgen and Livak, 2008). The relative expression was normalized to SlACTIN, and the relative mRNA level in each HMGS‐OE line in comparison with the empty vector control from three independent experiments was presented on the y‐axis. Significant differences in the relative mRNA levels between different samples were analysed by the Student's t‐test. Primers for qRT‐PCR are listed in Table S1.
Measurements of growth rate
Phenotypic changes in tomato plants were analysed (Johnston and Dore, 1929). Single‐copy T2 homozygous plants were compared in plant growth. Four‐day‐old tomato seedlings were moved to fresh MS plates and grown for 8 days. Twelve‐day‐old tomato seedlings of similar size were transferred to soil for further analysis; height measurements of 5‐week‐old and 9‐week‐old tomato plants were taken. For each OE construct, two independent lines were tested. Thirty plants per individual line were used for measurements in height.
Extraction and GC‐MS/HPLC/antioxidant activity analysis of sterols, intermediates, vitamin E and carotenoids
Extraction and analysis of sterols, intermediates, vitamin E (Babiychuk et al., 2008; Liao et al., 2014b; Schaller et al., 1995; Wang et al., 2012) and carotenoids (Fraser et al., 2000; Zanfini et al., 2010) in transgenic tomato fruits were performed. Antioxidant activity analysis was conducted by DPPH (1,1‐diphenyl‐2‐picrylhydrazyl) (Blois, 1958). See details for these analyses in Supplementary Methods.
Statistical analysis
Significant differences in data between different samples were analysed by the Student's t‐test.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
Figure S1 PCR analysis on representative transgenic tomato HMGS‐OEs.
Figure S2 Southern blot analysis of representative transgenic tomato HMGS‐OEs.
Figure S3 QRT‐PCR analysis on the expression of sesquiterpene‐related genes in tomato HMGS‐OE seedlings.
Figure S4 QRT‐PCR analysis on the expression of BR‐, cytokinin‐ and dolichol‐related genes in tomato HMGS‐OE seedlings.
Figure S5 QRT‐PCR analysis on the expression of C10 and C20 universal precursors of isoprenoid‐, and monoterpene‐related genes in tomato HMGS‐OE seedlings.
Figure S6 QRT‐PCR analysis on the expression of MEP‐derived carotenoid‐ and vitamin E‐related genes in tomato HMGS‐OE seedlings.
Figure S7 QRT‐PCR analysis on the expression of genes downstream of HMGS and plastidial GGPPSs in tomato HMGS‐OE fruits.
Table S1 Oligonucleotide primers used in this study.
Data S1 Supplementary Methods.
Data S2 Supplementary Result.
Acknowledgements
This work was supported by the Wilson and Amelia Wong Endowment Fund, Research Grants Council of Hong Kong (AoE/M‐05/12), Innovation Technology Fund of the Innovation Technology Commission (Support to Partner State Key Laboratories in Hong Kong) and HKU CRCG awards (0910159039, 1007160002, 1511159010). PL was supported by a University Postgraduate Fellowship and a Postdoctoral Fellowship from the University of Hong Kong.
References
- Abe, I. , Rohmer, M. and Prestwich, G.D. (1993) Enzymatic cyclization of squalene and oxidosqualene to sterols and triterpenes. Chem. Rev. 93, 2189–2206. [Google Scholar]
- Akhtar, T.A. , Matsuba, Y. , Schauvinhold, I. , Yu, G. , Lees, H.A. , Klein, S.E. and Pichersky, E. (2013) The tomato cis–prenyltransferase gene family. Plant J. 73, 640–652. [DOI] [PubMed] [Google Scholar]
- Alex, D. , Bach, T.J. and Chye, M.L. (2000) Expression of Brassica juncea 3‐hydroxy‐3‐methylglutaryl‐CoA synthase is developmentally regulated and stress‐responsive. Plant J. 22, 415–426. [DOI] [PubMed] [Google Scholar]
- Ament, K. , Van Schie, C.C. , Bouwmeester, H.J. , Haring, M.A. and Schuurink, R.C. (2006) Induction of a leaf specific geranylgeranyl pyrophosphate synthase and emission of (E, E)‐4,8,12‐trimethyltrideca‐1,3,7,11‐tetraene in tomato are dependent on both jasmonic acid and salicylic acid signaling pathways. Planta, 224, 1197–1208. [DOI] [PubMed] [Google Scholar]
- Azzi, A. (2007) Molecular mechanism of α‐tocopherol action. Free Radic. Biol. Med. 43, 16–21. [DOI] [PubMed] [Google Scholar]
- Babiychuk, E. , Bouvier‐Navé, P. , Compagnon, V. , Suzuki, M. , Muranaka, T. , Van Montagu, M. , Kushnir, S. et al (2008) Allelic mutant series reveal distinct functions for Arabidopsis cycloartenol synthase 1 in cell viability and plastid biogenesis. Proc. Natl Acad. Sci. USA, 105, 3163–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach, T.J. (1986) Hydroxymethylglutaryl‐CoA reductase, a key enzyme in phytosterol synthesis? Lipids, 21, 82–88. [DOI] [PubMed] [Google Scholar]
- Bach, T.J. (1995) Some new aspects of isoprenoid biosynthesis in plants – a review. Lipids, 30, 191–202. [DOI] [PubMed] [Google Scholar]
- Besser, K. , Harper, A. , Welsby, N. , Schauvinhold, I. , Slocombe, S. , Li, Y. , Dixon, R.A. et al (2009) Divergent regulation of terpenoid metabolism in the trichomes of wild and cultivated tomato species. Plant Physiol. 149, 499–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhangu‐Uhlmann, A. (2011) The mevalonate pathway: a monitoring approach in plants by systems biology tools. Ph.D. thesis, ETH Zürich.
- Blois, M.S. (1958) Antioxidant determinations by the use of a stable free radical. Nature, 181, 1199–1200. [Google Scholar]
- Bradford, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Bradford, P.G. and Awad, A.B. (2007) Phytosterols as anticancer compounds. Mol. Nutr. Food Res. 51, 161–170. [DOI] [PubMed] [Google Scholar]
- Brigelius‐Flohé, R. and Traber, M.G. (1999) Vitamin E: function and metabolism. FASEB J. 13, 1145–1155. [PubMed] [Google Scholar]
- Camara, B. and d'Harlingue, A. (1985) Demonstration and solubilization of S‐adenosylmethionine: γ‐tocopherol methyltransferase from Capsicum chromoplasts. Plant Cell Rep. 4, 31–32. [DOI] [PubMed] [Google Scholar]
- Chen, S. , Li, H. and Liu, G. (2006) Progress of vitamin E metabolic engineering in plants. Transgenic Res. 15, 655–665. [DOI] [PubMed] [Google Scholar]
- Chye, M.L. , Huang, B.Q. and Zee, S.Y. (1999) Isolation of a gene encoding Arabidopsis membrane‐associated acyl‐CoA binding protein and immunolocalization of its gene product. Plant J. 18, 205–214. [DOI] [PubMed] [Google Scholar]
- Del Moral, L. , Fernández‐Martínez, J.M. , Pérez‐Vich, B. and Velasco, L. (2013) Accumulation dynamics of seed tocopherols in sunflowers lines with modified tocopherol levels. Acta Physiol. Plant. 35, 3157–3165. [Google Scholar]
- DellaPenna, D. (2005) Progress in the dissection and manipulation of vitamin E synthesis. Trends Plant Sci. 10, 574–579. [DOI] [PubMed] [Google Scholar]
- Devarenne, T.P. , Shin, D.H. , Back, K. , Yin, S. and Chappell, J. (1998) Molecular characterization of tobacco squalene synthase and regulation in response to fungal elicitor. Arch. Biochem. Biophys. 349, 205–215. [DOI] [PubMed] [Google Scholar]
- Devarenne, T.P. , Ghosh, A. and Chappell, J. (2002) Regulation of squalene synthase, a key enzyme of sterol biosynthesis, in tobacco. Plant Physiol. 129, 1095–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Sharkawy, I. , Sherif, S. , El Kayal, W. , Jones, B. , Li, Z. , Sullivan, A.J. and Jayasankar, S. (2016) Overexpression of plum auxin receptor PslTIR1 in tomato alters plant growth, fruit development and fruit shelf‐life characteristics. BMC Plant Biol. 16, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enfissi, E.M. , Fraser, P.D. , Lois, L.M. , Boronat, A. , Schuch, W. and Bramley, P.M. (2005) Metabolic engineering of the mevalonate and non‐mevalonate isopentenyl diphosphate‐forming pathways for the production of health‐promoting isoprenoids in tomato. Plant Biotechnol. J. 3, 17–27. [DOI] [PubMed] [Google Scholar]
- Enfissi, E.M. , Barneche, F. , Ahmed, I. , Lichtlé, C. , Gerrish, C. , McQuinn, R.P. , Giovannoni, J.J. et al (2010) Integrative transcript and metabolite analysis of nutritionally enhanced DE‐ETIOLATED1 downregulated tomato fruit. Plant Cell, 22, 1190–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedor, J. and Burda, K. (2014) Potential role of carotenoids as antioxidants in human health and disease. Nutrients, 6, 466–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser, P.D. , Pinto, M.E. , Holloway, D.E. and Bramley, P.M. (2000) Application of high‐performance liquid chromatography with photodiode array detection to the metabolic profiling of plant isoprenoids. Plant J. 24, 551–558. [DOI] [PubMed] [Google Scholar]
- Gaffe, J. , Bru, J.P. , Causse, M. , Vidal, A. , Stamitti‐Bert, L. , Carde, J.P. and Gallusci, P. (2000) LEFPS1, a tomato farnesyl pyrophosphate gene highly expressed during early fruit development. Plant Physiol. 123, 1351–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, J.X. , Fujioka, S. , Li, T.C. , Kang, S.G. , Seto, H. , Takatsuto, S. , Yoshida, S. et al (2003) Sterols regulate development and gene expression in Arabidopsis. Plant Physiol. 131, 1258–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmerlin, A. (2013) Post‐translational events and modifications regulating plant enzymes involved in isoprenoid precursor biosynthesis. Plant Sci. 203–204, 41–54. [DOI] [PubMed] [Google Scholar]
- Hemmerlin, A. , Hoeffler, J.F. , Meyer, O. , Tritsch, D. , Kagan, I.A. , Grosdemange‐Billiard, C. , Rohmer, M. et al (2003) Cross‐talk between the cytosolic mevalonate and the plastidial methylerythritol phosphate pathways in tobacco bright yellow‐2 cells. J. Biol. Chem. 278, 26666–26676. [DOI] [PubMed] [Google Scholar]
- Hemmerlin, A. , Gerber, E. , Feldtrauer, J.F. , Wentzinger, L. , Hartmann, M.A. , Tritsch, D. , Hoeffler, J.F. et al (2004) A review of tobacco BY‐2 cells as an excellent system to study the synthesis and function of sterols and other isoprenoids. Lipids, 39, 723–735. [DOI] [PubMed] [Google Scholar]
- Hemmerlin, A. , Harwood, J.L. and Bach, T.J. (2012) A raison d'etre for two distinct pathways in the early steps of plant isoprenoid biosynthesis? Prog. Lipid Res. 51, 95–148. [DOI] [PubMed] [Google Scholar]
- Henry, L.K. , Gutensohn, M. , Thomas, S.T. , Noel, J.P. and Dudareva, N. (2015) Orthologs of the archaeal isopentenyl phosphate kinase regulate terpenoid production in plants. Proc. Natl Acad. Sci. USA, 112, 10050–10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell, S.H. , Lall, S. and Che, P. (2003) Cytokinins and shoot development. Trends Plant Sci. 8, 453–459. [DOI] [PubMed] [Google Scholar]
- Huchelmann, A. , Gastaldo, C. , Veinante, M. , Zeng, Y. , Heintz, D. , Tritsch, D. , Schaller, H. et al (2014) S‐carvone suppresses cellulase‐induced capsidiol production in Nicotiana tabacum by interfering with protein isoprenylation. Plant Physiol. 164, 935–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro, S. , Nishimori, Y. , Yamada, M. , Saito, H. , Suzuki, T. , Nakagawa, T. , Miyake, H. et al (2010) The Arabidopsis FLAKY POLLEN1 gene encodes a 3‐hydroxy‐3‐methylglutaryl‐coenzyme A synthase required for development of tapetum‐specific organelles and fertility of pollen grains. Plant Cell Physiol. 51, 896–911. [DOI] [PubMed] [Google Scholar]
- Johnston, E.S. and Dore, W.H. (1929) The influence of boron on the chemical composition and growth of the tomato plant. Plant Physiol. 4, 31–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai, G. , Xu, H. , Zhou, C. , Liao, P. , Xiao, J. , Luo, X. , You, L. et al (2011) Metabolic engineering tanshinone biosynthetic pathway in Salvia miltiorrhiza hairy root cultures. Metab. Eng. 13, 319–327. [DOI] [PubMed] [Google Scholar]
- Kamimura, H. , Koga, N. , Oguri, K. and Yoshimura, H. (1992) Enhanced elimination of theophylline, phenobarbital and strychnine from the bodies of rats and mice by squalane treatment. J. Pharmacobiodyn. 15, 215–221. [DOI] [PubMed] [Google Scholar]
- Kohno, Y. , Egawa, Y. , Itoh, S. , Nagaoka, S. , Takahashi, M. and Mukai, K. (1995) Kinetic study of quenching reaction of singlet oxygen and scavenging reaction of free radical by squalene in n‐butanol. Biochim. Biophys. Acta, 1256, 52–56. [DOI] [PubMed] [Google Scholar]
- Lange, I. , Poirier, B.C. , Herron, B.K. and Lange, B.M. (2015) Comprehensive assessment of transcriptional regulation facilitates metabolic engineering of isoprenoid accumulation in Arabidopsis. Plant Physiol. 169, 1595–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laule, O. , Fürholz, A. , Chang, H.S. , Zhu, T. , Wang, X. , Heifetz, P.B. , Gruissem, W. et al (2003) Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana . Proc. Natl Acad. Sci. USA, 100, 6866–6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M.H. , Jeong, J.H. , Seo, J.W. , Shin, C.G. , Kim, Y.S. , In, J.G. , Yang, D.C. et al (2004) Enhanced triterpene and phytosterol biosynthesis in Panax ginseng overexpressing squalene synthase gene. Plant Cell Physiol. 45, 976–984. [DOI] [PubMed] [Google Scholar]
- Li, J. , Nagpal, P. , Vitart, V. , McMorris, T.C. and Chory, J. (1996) A role for brassinosteroids in light‐dependent development of Arabidopsis. Science, 272, 398–401. [DOI] [PubMed] [Google Scholar]
- Liao, P. , Wang, H. , Hemmerlin, A. , Nagegowda, D.A. , Bach, T.J. , Wang, M. and Chye, M.L. (2014a) Past achievements, current status and future perspectives of studies on 3‐hydroxy‐3‐methylglutaryl‐CoA synthase (HMGS) in the mevalonate (MVA) pathway. Plant Cell Rep. 33, 1005–1022. [DOI] [PubMed] [Google Scholar]
- Liao, P. , Wang, H. , Wang, M. , Hsiao, A.S. , Bach, T.J. and Chye, M.L. (2014b) Transgenic tobacco overexpressing Brassica juncea HMG‐CoA synthase 1 shows increased plant growth, pod size and seed yield. PLoS ONE, 9, e98264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, P. , Hemmerlin, A. , Bach, T.J. and Chye, M.L. (2016) The potential of the mevalonate pathway for enhanced isoprenoid production. Biotechnol. Adv. 34, 697–713. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler, H.K. (1999) The 1‐deoxy‐D‐xylulose‐5‐phosphate pathway of isoprenoid biosynthesis in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 47–65. [DOI] [PubMed] [Google Scholar]
- Manzano, D. , Andrade, P. , Caudepón, D. , Altabella, T. , Arró, M. and Ferrer, A. (2016) Suppressing farnesyl diphosphate synthase alters chloroplast development and triggers sterol‐dependent induction of jasmonate‐ and Fe‐related responses. Plant Physiol. 172, 93–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews, J. (1992) Sharks still intrigue cancer researchers. J. Natl Cancer Inst. 84, 1000–1002. [DOI] [PubMed] [Google Scholar]
- Mathews, H. , Clendennen, S.K. , Caldwell, C.G. , Liu, X.L. , Connors, K. , Matheis, N. , Schuster, D.K. et al (2003) Activation tagging in tomato identifies a transcriptional regulator of anthocyanin biosynthesis, modification, and transport. Plant Cell, 15, 1689–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melillo, M.T. , Leonetti, P. and Veronico, P. (2014) Benzothiadiazole effect in the compatible tomato‐Meloidogyne incognita interaction: changes in giant cell development and priming of two root anionic peroxidases. Planta 240, 841–854. [DOI] [PubMed] [Google Scholar]
- Merret, R. , Cirioni, J. , Bach, T.J. and Hemmerlin, A. (2007) A serine involved in actin‐dependent subcellular localization of a stress‐induced tobacco BY‐2 hydroxymethylglutaryl‐CoA reductase isoform. FEBS Lett. 581, 5295–5299. [DOI] [PubMed] [Google Scholar]
- Moreau, R.A. , Whitaker, B.D. and Hicks, K.B. (2002) Phytosterols, phytostanols, and their conjugates in foods: structural diversity, quantitative analysis, and health‐promoting uses. Prog. Lipid Res. 41, 457–500. [DOI] [PubMed] [Google Scholar]
- Muñoz‐Bertomeu, J. , Sales, E. , Ros, R. , Arrillaga, I. and Segura, J. (2007) Up‐regulation of an N‐terminal truncated 3‐hydroxy‐3‐methylglutaryl CoA reductase enhances production of essential oils and sterols in transgenic Lavandula latifolia . Plant Biotechnol. J. 5, 746–758. [DOI] [PubMed] [Google Scholar]
- Nagegowda, D.A. , Bach, T.J. and Chye, M.L. (2004) Brassica juncea 3‐hydroxy‐3‐methylglutaryl (HMG)‐CoA synthase 1: expression and characterization of recombinant wild‐type and mutant enzymes. Biochem. J. 383, 517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita, J.O. and Gruissem, W. (1989) Tomato hydroxymethylglutaryl‐CoA reductase is required early in fruit development but not during ripening. Plant Cell, 1, 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmark, H.L. (1997) Squalene, olive oil, and cancer risk: a review and hypothesis. Cancer Epidemiol. Biomarkers Prev. 6, 1101–1103. [PubMed] [Google Scholar]
- Nguyen, H.T.M. , Neelakadan, A.K. , Quach, T.N. , Valliyodan, B. , Kumar, R. , Zhang, Z. and Nguyen, H.T. (2013) Molecular characterization of Glycine max squalene synthase genes in seed phytosterol biosynthesis. Plant Physiol. Biochem. 73, 23–32. [DOI] [PubMed] [Google Scholar]
- Pasoreck, E.K. , Su, J. , Silverman, I.M. , Gosai, S.J. , Gregory, B.D. , Yuan, J.S. and Daniell, H. (2016) Terpene metabolic engineering via nuclear or chloroplast genomes profoundly and globally impacts off‐target pathways through metabolite signalling. Plant Biotechnol. J. 14, 1862–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez‐Concepción, M. and Gruissem, W. (1999) Arachidonic acid alters tomato HMG expression and fruit growth and induces 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase‐independent lycopene accumulation. Plant Physiol. 119, 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohmer, M. (1999) The discovery of a mevalonate‐independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat. Prod. Rep. 16, 565–574. [DOI] [PubMed] [Google Scholar]
- Römer, S. , Lübeck, J. , Kauder, F. , Steiger, S. , Adomat, C. and Sandmann, G. (2002) Genetic engineering of a zeaxanthin‐rich potato by antisense inactivation and co‐suppression of carotenoid epoxidation. Metab. Eng. 4, 263–272. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Sola, M.Á. , Barja, M.V. , Manzano, D. , Llorente, B. , Schipper, B. , Beekwilder, J. and Rodríguez‐Concepción, M. (2016) A single Arabidopsis gene encodes two differentially targeted geranylgeranyl diphosphate synthase isoforms. Plant Physiol. 172, 1393–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchettini, J.C. and Poulter, C.D. (1997) Creating isoprenoid diversity. Science, 277, 1788–1789. [DOI] [PubMed] [Google Scholar]
- Sallaud, C. , Rontein, D. , Onillon, S. , Jabès, F. , Duffé, P. , Giacalone, C. , Thoraval, S. et al (2009) A novel pathway for sesquiterpene biosynthesis from Z, Z‐farnesyl pyrophosphate in the wild tomato Solanum habrochaites . Plant Cell, 21, 301–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller, H. , Grausem, B. , Benveniste, P. , Chye, M.L. , Tan, C.T. , Song, Y.H. and Chua, N.H. (1995) Expression of the Hevea brasiliensis (H.B.K) Mull. Arg. 3‐hydroxy‐3‐methylglutaryl‐coenzyme A reductase 1 in tobacco results in sterol overproduction. Plant Physiol. 109, 761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schie, C.C. , Ament, K. , Schmidt, A. , Lange, T. , Haring, M.A. and Schuurink, R.C. (2007) Geranyl diphosphate synthase is required for biosynthesis of gibberellins. Plant J. 52, 752–762. [DOI] [PubMed] [Google Scholar]
- Schmittgen, T.D. and Livak, K.J. (2008) Analyzing real‐time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108. [DOI] [PubMed] [Google Scholar]
- Seo, J.W. , Jeong, J.H. , Shin, C.G. , Lo, S.C. , Han, S.S. , Yu, K.W. , Harada, E. et al (2005) Overexpression of squalene synthase in Eleutherococcus senticosus increases phytosterol and triterpene accumulation. Phytochemistry, 66, 869–877. [DOI] [PubMed] [Google Scholar]
- Shani, E. , Ben‐Gera, H. , Shleizer‐Burko, S. , Burko, Y. , Weiss, D. and Ori, N. (2010) Cytokinin regulates compound leaf development in tomato. Plant Cell, 22, 3206–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Z. and Ruvkun, G. (2012) The mevalonate pathway regulates microRNA activity in Caenorhabditis elegans . Proc. Natl Acad. Sci. USA, 109, 4568–4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani, D. and DellaPenna, D. (1998) Elevating the vitamin E content of plants through metabolic engineering. Science, 282, 2098–2100. [DOI] [PubMed] [Google Scholar]
- Singh, A.K. , Dwivedi, V. , Rai, A. , Pal, S. , Reddy, S.G. , Rao, D.K. , Shasany, A.K. et al (2015) Virus‐induced gene silencing of Withania somnifera squalene synthase negatively regulates sterol and defence‐related genes resulting in reduced withanolides and biotic stress tolerance. Plant Biotechnol. J. 13, 1287–1299. [DOI] [PubMed] [Google Scholar]
- Smith, T.J. (2000) Squalene: potential chemopreventive agent. Expert Opin. Investig. Drugs, 9, 1841–1848. [DOI] [PubMed] [Google Scholar]
- Southern, E. (2006) Southern blotting. Nat. Protoc. 1, 518–525. [DOI] [PubMed] [Google Scholar]
- Steussy, C.N. , Robison, A.D. , Tetrick, A.M. , Knight, J.T. , Rodwell, V.W. , Stauffacher, C.V. and Sutherlin, A.L. (2006) A structural limitation on enzyme activity: the case of HMG‐CoA synthase. Biochemistry, 45, 14407–14414. [DOI] [PubMed] [Google Scholar]
- Vom Dorp, K. , Hölzl, G. , Plohmann, C. , Eisenhut, M. , Abraham, M. , Weber, A.P. , Hanson, A.D. et al (2015) Remobilization of phytol from chlorophyll degradation is essential for tocopherol synthesis and growth of Arabidopsis. Plant Cell, 27, 2846–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriet, C. , Russinova, E. and Reuzeau, C. (2012) Boosting crop yields with plant steroids. Plant Cell, 24, 842–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Nagegowda, D.A. , Rawat, R. , Bouvier‐Navé, P. , Guo, D. , Bach, T.J. and Chye, M.L. (2012) Overexpression of Brassica juncea wild‐type and mutant HMG‐CoA synthase 1 in Arabidopsis up‐regulates genes in sterol biosynthesis and enhances sterol production and stress tolerance. Plant Biotechnol. J. 10, 31–42. [DOI] [PubMed] [Google Scholar]
- Wong, R.J. , McCormack, D.K. and Russell, D.W. (1982) Plastid 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase has distinctive kinetic and regulatory features: properties of the enzyme and positive phytochrome control of activity in pea seedlings. Arch. Biochem. Biophys. 216, 631–638. [DOI] [PubMed] [Google Scholar]
- Woyengo, T.A. , Ramprasath, V.R. and Jones, P.J.H. (2009) Anticancer effects of phytosterols. Eur. J. Clin. Nutr. 63, 813–820. [DOI] [PubMed] [Google Scholar]
- Wu, S. , Jiang, Z. , Kempinski, C. , Eric Nybo, S. , Husodo, S. , Williams, R. and Chappell, J. (2012) Engineering triterpene metabolism in tobacco. Planta, 236, 867–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, S. , Li, H.Y. , Zhang, J.P. , Chan, S.W. and Chye, M.L. (2008) Arabidopsis acyl‐CoA‐binding proteins ACBP4 and ACBP5 are subcellularly localized to the cytosol and ACBP4 depletion affects membrane lipid composition. Plant Mol. Biol. 68, 571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, S. , Gao, W. , Chen, Q.F. , Chan, S.W. , Zheng, S.X. , Ma, J. , Wang, M. et al (2010) Overexpression of Arabidopsis acyl‐CoA binding protein ACBP3 promotes starvation‐induced and age‐dependent leaf senescence. Plant Cell, 22, 1463–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanfini, A. , Corbini, G. , La Rosa, C. and Dreassi, E. (2010) Antioxidant activity of tomato lipophilic extracts and interactions between carotenoids and α‐tocopherol in synthetic mixtures. LWT‐Food Sci. Technol. 43, 67–72. [Google Scholar]
- Zhang, H. , Ohyama, K. , Boudet, J. , Chen, Z. , Yang, J. , Zhang, M. , Muranaka, T. et al (2008) Dolichol biosynthesis and its effects on the unfolded protein response and abiotic stress resistance in Arabidopsis . Plant Cell, 20, 1879–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 PCR analysis on representative transgenic tomato HMGS‐OEs.
Figure S2 Southern blot analysis of representative transgenic tomato HMGS‐OEs.
Figure S3 QRT‐PCR analysis on the expression of sesquiterpene‐related genes in tomato HMGS‐OE seedlings.
Figure S4 QRT‐PCR analysis on the expression of BR‐, cytokinin‐ and dolichol‐related genes in tomato HMGS‐OE seedlings.
Figure S5 QRT‐PCR analysis on the expression of C10 and C20 universal precursors of isoprenoid‐, and monoterpene‐related genes in tomato HMGS‐OE seedlings.
Figure S6 QRT‐PCR analysis on the expression of MEP‐derived carotenoid‐ and vitamin E‐related genes in tomato HMGS‐OE seedlings.
Figure S7 QRT‐PCR analysis on the expression of genes downstream of HMGS and plastidial GGPPSs in tomato HMGS‐OE fruits.
Table S1 Oligonucleotide primers used in this study.
Data S1 Supplementary Methods.
Data S2 Supplementary Result.


