Abstract
The metabolic stress placed on skeletal muscle by aerobic exercise promotes acute and long-term health benefits in part through changes in gene expression. However, the transducers that mediate altered gene expression signatures have not been completely elucidated. Regulated in development and DNA damage 1 (REDD1) is a stress-induced protein whose expression is transiently increased in skeletal muscle following acute aerobic exercise. However, the role of this induction remains unclear. Because REDD1 altered gene expression in other model systems, we sought to determine whether REDD1 induction following acute exercise altered the gene expression signature in muscle. To do this, wild-type and REDD1-null mice were randomized to remain sedentary or undergo a bout of acute treadmill exercise. Exercised mice recovered for 1, 3, or 6 h before euthanization. Acute exercise induced a transient increase in REDD1 protein expression within the plantaris only at 1 h postexercise, and the induction occurred in both cytosolic and nuclear fractions. At this time point, global changes in gene expression were surveyed using microarray. REDD1 induction was required for the exercise-induced change in expression of 24 genes. Validation by RT-PCR confirmed that the exercise-mediated changes in genes related to exercise capacity, muscle protein metabolism, neuromuscular junction remodeling, and Metformin action were negated in REDD1-null mice. Finally, the exercise-mediated induction of REDD1 was partially dependent upon glucocorticoid receptor activation. In all, these data show that REDD1 induction regulates the exercise-mediated change in a distinct set of genes within skeletal muscle.
Keywords: muscle fatigue, protein metabolism, neuromuscular junction
aerobic exercise places a large metabolic stress on the skeletal muscle, leading to a variety of acute and long-term health benefits mediated in part by changes in the types of genes expressed (3, 49). For example, the exercise-mediated induction of genes related to muscle oxidative capacity and mitochondrial biogenesis contribute to the ability of the skeletal muscle to generate ATP aerobically and, in turn, the ability to sustain prolonged muscular activity (3). Likewise, the exercise-mediated induction of glucose transporter 4 (GLUT4) in muscle enhances the capacity to maintain postprandial glucose levels (49). Although the regulatory pathways that control gene expression following exercise are becoming recognized, there are still many unknown molecular transducers that contribute to this effect.
In skeletal muscle, sustained expression of the stress-induced protein, regulated in development and DNA damage 1 (REDD1; a.k.a. ddit4, RTP801, dig2), is typically observed during pathological conditions in which skeletal muscle mass and metabolism are perturbed (7, 15, 18–20, 45, 54, 56). Many of these effects are thought to be reconciled predominantly by repressed signaling through the mechanistic target of rapamycin in complex 1 (mTORC1) (18, 20). Paradoxically, REDD1 expression is highly induced in skeletal muscle by an acute bout of aerobic exercise (22, 41, 44, 46), whereas changes in mTORC1 signaling are inconsistent (4, 14, 22, 41). These data suggest that REDD1 may regulate exercise-sensitive metabolic processes within the skeletal muscle that are distinct from the regulation of mTORC1 signaling.
In other models, modulation of REDD1 expression results in altered gene expression. For example, prolonged deletion of REDD1 in mouse embryonic fibroblasts increased the expression of genes involved with glycolysis, such as pyruvate dehydrogenase kinase 1 (24). Further, inhibiting REDD1 induction following acute, topical glucocorticoid administration negated the change in gene expression related to various processes, such as antigen processing/presenting and DNA replication (2). Furthermore, REDD1 is localized in the nucleus (33, 38), providing additional support for REDD1 in the regulation of gene expression. Because REDD1 is among the genes induced in skeletal muscle by acute aerobic exercise (44), we hypothesized that the induction of REDD1 facilitates the change in the gene expression signature in an mTORC1-independent manner. We utilized a genetic model and an unbiased microarray approach with subsequent RT-PCR validation to show that preventing the REDD1 induction within the skeletal muscle altered the exercise-induced change in the expression of a specific set of genes with previously designated functions in skeletal muscle biology. Importantly, this occurred in an mTORC1-independent manner. Thus, these data strongly suggest that REDD1 is a molecular transducer that regulates a specific gene signature following acute exercise.
METHODS
Animals.
Male, REDD1-null mice (REDD1−/−) aged 12 wk were generated as previously described (17, 18, 20, 56), and permission to use them was generously granted by Dr. Elena Feinstein (Quark Pharmaceuticals). REDD1−/− mice were bred in the Pennsylvania State University College of Medicine animal facility. Male wild-type, age-matched B6/129F1 control mice (REDD1+/+) were obtained from Taconic (Hudson, NY). Female REDD1+/+ and REDD1−/− mice aged 8–12 wk were generated and housed as previously described (51) and used to determine maximal treadmill exercise performance. Male C57Bl/6 mice aged 12 wk were obtained from Envigo (Indianapolis, IN) or from a breeding colony within the vivarium at the University of Central Florida for use in the glucocorticoid receptor inhibition experiments. All mice were housed in a temperature- (25°C) and light (12:12-h light-dark)-controlled environment and had access to food and water ad libitum. The Institutional Animal Care and Use Committee of the Pennsylvania State University College of Medicine, the University of Central Florida, and Massachusetts General Hospital approved the animal facilities and all experimental protocols.
Experimental design and aerobic treadmill exercise protocol.
REDD1+/+ and REDD1−/− mice were acclimated to a Columbus Instruments rodent treadmill (Columbus, OH) for the 2 days immediately before experimentation. Acclimation included 10 (10 m/min), 5 (15 m/min), and 5 min (18 m/min) all at a 5% incline. The maximal treadmill speed of this exercise paradigm corresponds to an intensity previously reported to be ∼60% of maximal O2 consumption in mice (43). On the day of experimentation, mice were deprived of food 3 h before experimentation but had access to water ad libitum. At the time of experimentation, mice were randomized to remain sedentary or undergo an acute bout of treadmill exercise consisting of a warmup [5 (10 m/min) and 5 min (15 m/min)] followed by 50 min (18 m/min) at a 5% incline. A similar protocol has been shown to increase REDD1 content in skeletal muscle (44). Mice randomized to the sedentary condition remained in their cages next to the treadmill. Immediately after completion of the exercise session, mice were returned to their cages, where they remained fasted but had unlimited access to water, until euthanization at 1, 3, or 6 h post-completion of the exercise session. The sedentary mice were euthanized immediately before the 1 h postexercise group was euthanized. At euthanization, mice were anesthetized with isoflurane (2–3%), and the plantaris and gastrocnemius muscles were excised, flash-frozen in liquid nitrogen, and stored at −80°C. Initial analyses (i.e., Western blotting and microarray) were performed on the plantaris, as this muscle undergoes a high degree of molecular adaptation following long-term aerobic exercise training (34).
Treadmill exercise performance test.
Treadmill exercise capacity was assessed as previously described (46). Briefly, mice were acclimated to an Exer 6 rodent treadmill (Columbus Instruments, Columbus, OH) for the 2 days immediately before the exercise test. Acclimation on day 1 included 5 min at 8 m/min. Acclimation on day 2 included 5 min at 8 m/min and 5 min at 10 m/min. For the exercise test, mice ran for 40 min at 10 m/min, at which point the treadmill speed increased 1 m/min every 5 min until exhaustion. Exhaustion was defined as ≥5 s on an electric shock grid without attempting to run.
Glucocorticoid receptor inhibition.
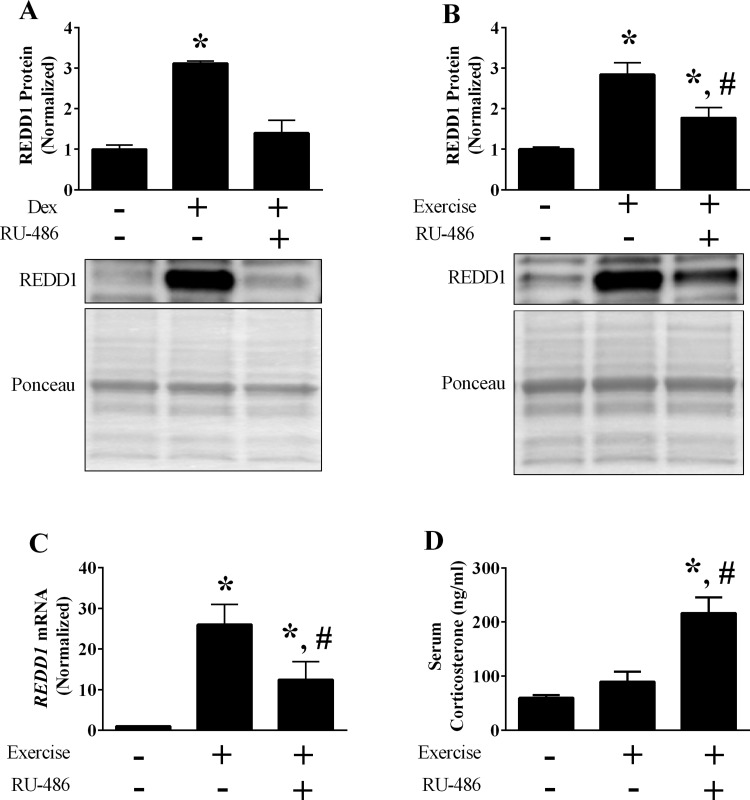
RU-486 (cat. no. M8046; Sigma-Aldrich, St. Louis, MO) diluted in polyethylene glycol 400 (PEG; cat. no. MPX1286B2; Fisher Scientific, Pittsburgh, PA) at a dose of 8 mg/kg body wt was used to inhibit the glucocorticoid receptor, as previously described (31). Preliminary experiments confirmed receptor blockade, as dexamethasone (DEX; cat. no. D1756; Sigma-Aldrich) failed to increase REDD1 expression in skeletal muscle following RU-486 treatment (Fig. 5A). Specifically, mice were given a subcutaneous injection of RU-486 or vehicle (PEG) 24 and 2 h before receiving a single intraperitoneal injection of DEX (4 mg/kg body wt) diluted in physiological saline. Plantaris muscle samples were collected 2 h post-DEX injection to mimic the time point of the subsequent aerobic exercise experiments (i.e., 1 h of exercise + 1 h of recovery). Following confirmation of receptor blockade, a new set of mice were acclimated to the treadmill for 2 days, as described above. Immediately after the second acclimation period, mice were given the first injection of RU-486 or vehicle. Twenty-four hours later, mice were given a second injection of RU-486 or vehicle, and food was withheld for the remainder of the study. Two hours following the injection, a subset of mice were subjected to a single bout of treadmill exercise as described above and euthanized 1 h following completion of the bout.
Fig. 5.
Role of glucocorticoid signaling on REDD1 induction following acute exercise. A and B: the protein content of REDD1 following glucocorticoid administration (A) or acute exercise (B) was determined by Western blot analysis. C: mRNA content of REDD1 following acute aerobic exercise was determined by RT-PCR. D: serum corticosterone was determined by ELISA; n = a total of 3–8 mice/group generated from 1 to 2 independent experiments. *Significant difference compared with non-glucocorticoid-treated or sedentary group; #significant difference compared with exercise vehicle-treated group. P ≤ 0.05 for all analysis.
Serum corticosterone.
Serum corticosterone levels were determined by ELISA (cat. no. 501320; Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s procedures, except that the sample purification procedure was omitted. Whole blood was allowed to clot on ice for ≥30 min, followed by centrifugation (2,000 g for 10 min at 4°C) and storage at −80°C. For analysis, serum was diluted 1:200 into the ELISA buffer supplied with the kit to obtain absorbance readings within the standard curve. Data are presented as nanograms per milliliter.
RNA Extraction, cDNA synthesis, and RT-PCR.
Plantaris muscle samples were homogenized in 500 µl of Zymo Tri Reagent (Irvine, CA), and RNA was isolated using a Zymo RNA Miniprep kit (cat. no. R2071) with on-column DNase treatment. RNA quantity was determined spectrophotometrically by a 260- to 280-nm ratio, and cDNA was synthesized from RNA totaling 1–1.75 ng using a High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA). RT-PCR was conducted on a QuantStudio5 thermal cycler (Thermo Fisher Scientific, Waltham, MA) using PowerUp SYBR Green Master Mix (Thermo Fisher Scientific, Waltham, MA). The conditions for RT-PCR with SYBR Green included an initial 2 min at 50°C and 2 min at 95°C, followed by 40 cycles that included a 15-s denature step at 95°C, a 15-s annealing step at 55°C, and a 1-min extension step at 72°C within each cycle. A melt curve analysis was performed for each primer pair to ensure that a single product was efficiently amplified, and the product sizes for each primer pair were verified via agarose gel electrophoresis. Junb mRNA expression was determined using TaqMan predesigned probes (Mm04243546_s1; Thermo Fisher Scientific) and TaqMan Fast Advanced Master Mix (Thermo Fisher Scientific), following the manufacturer’s recommended RT-PCR cycling conditions for the QuantStudio5 thermal cycler. Relative expression levels of each target gene were normalized by using the ΔΔCT method with GAPDH as the control gene since GAPDH expression was not altered by genotype or exercise. Primer sequences for all SYBR Green reactions are listed in Table 1.
Table 1.
Primer sequences for RT-PCR using SYBR Green
| Gene Symbol | Forward (5′–3′) | Reverse (5′–3′) | Amplicon Size, bp |
|---|---|---|---|
| Orm1 | TCGGGAGTCTCAAACAATAGGTG | GGTCAAAGGCAAGCATGAAG | 161 |
| Serpina3m | CAATGACTATGTGAGCAATCAGACC | ACCTTCACAGATCTCTTCTCATCC | 183 |
| Slc22a3 | GACTTGCTTCGTGATCGTGAC | GAAAGCTGGGCAGAGAGATG | 167 |
| PGC-1α | AAGACGGATTGCCCTCATTT | AGTGCTAAGACCGCTGCATT | 191 |
| Hsp70 | TGGTGCTGACGAAGATGAAG | ATGATCCGCAGCACGTTTAG | 154 |
| GAPDH | GTTGTCTCCTGCGACTTCA | TGCTGTAGCCGTATTCATTG | 124 |
| REDD1 | TGGTGCCCACCTTTCAGTTG | GTCAGGGACTGGCTGTAACC | 121 |
| REDD2 | CCAGCCTCAAGGACTTCTTC | TCTTCAATGACTGTCGTTCC | 133 |
Orm1, orosomucoid 1; Serpina3m, serine peptidase inhibitor clade A member 3M; Slc22a3, solute carrier family 22 member 3; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-1α; Hsp70, heat shock protein family A member 1A; REDD1 and -2, regulated in development and DNA damage 1 and 2, respectively.
Microarray and microarray data analysis.
Total RNA (n = 3/condition) from the plantaris was subjected to microarray analysis at the Analytical Genomics Core within the Sanford Burnham Prebys Medical Discovery Institute (Orlando, FL). The quality of total RNA was assessed by the Agilent Bioanalyzer Nano Chip (Agilent Technologies; Santa Clara, CA). Labeled, single-stranded cDNA (sscDNA) was generated using 300 ng of total RNA according to the Whole Transcript Sense Target Labeling Assay protocol using the Affymetrix (Santa Clara, CA) GeneChip WT PLUS Reagent kit (cat. no. 902281). Then, 5.5 µg of sscDNA was fragmented and labeled using Affymetrix WT Terminal according to the manufacturer’s protocol. The labeled sscDNA was then hybridized onto GeneChip Mouse Gene 2.0 ST Arrays (Affymetrix), which analyzes 28,000 coding transcripts and ∼7,000 noncoding transcripts using ∼760,000 probe sets (on average 27 probes/gene). The staining and washing of the arrays were conducted using an Affymetrix Fluidics 450 station and scanned using Affymetrix GeneChip Command Console Software and GeneChi Scanner 3000 7G. All procedures were conducted according to the manufacturer’s instructions.
Basic microarray data analysis was performed at the Bioinformatics Core at the Sanford Burnham Prebys Medical Discovery Institute (La Jolla, CA). Raw data files from Mouse Gene ST arrays were imported into Partek’s Genomic Software for microarray expression analysis (Partek, Chesterfield, MO). No interrogating probes or control probes were skipped. The Robust Microarray Average method was used in Partek for data processing (5, 25, 26, 57), which included background correction, quantile normalization, and median polish correction. Signals for probes were Log2 transformed to create data sets closer to the normal distribution. Affymetrix quality control charts were analyzed in line form, and box and whisker plots were analyzed to verify that no outliers were detected. Additional quality control and checks for outliers were provided by Principal Component Analysis (PCA), with no outliers detected. Following this, lists of differentially expressed genes (DEGs) were generated between groups using the following conditions: 1) exercise-REDD1+/+ vs. sedentary-REDD1+/+, 2) exercise-REDD1−/− vs. exercise-REDD1+/+, and 3) sedentary-REDD1−/− vs. sedentary-REDD1+/+. ANOVA analysis was used to define DEGs using two criteria. Initial DEG criteria was assessed using rigorous P values (fold change ≥1.5 and P value with false discovery rate <0.05), which produced only handfuls of DEGs for each comparison. Because of this, a flexible P value (fold change ≥1.5 and P value without false discovery rate <0.05) resulted in several-hundred DEGs for every comparison. The gene lists using the flexible P-value were used in the subsequent analysis. Gene expression data has been made available at GEO (https://www.ncbi.nlm.nih.gov/geo) for this study (GSE97415).
Western blot analysis.
Whole muscle protein from the plantaris was extracted by glass-on-glass homogenization in 10 volumes (10 µl/mg tissue) of RIPA buffer (cat. no. 89900; Thermo Fisher Scientific, Waltham, MA) consisting of 25 mM Tri·HCl (pH 7.6), 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% SDS and supplemented with 5 mM NaVO4, 20 mM NaF, and 10 µl/ml protease inhibitor cocktail (no. P8340; Sigma-Aldrich) or a buffer consisting of 50 mM HEPES (pH 7.4), 0.1% Triton-X 100, 4 mM EGTA, 10 mM EDTA, 15 mM Na4P2O7, 100 mM β-glycerophosphate, 25 mM NaF, 5 mM Na3VO4, and 10 µl/ml protease inhibitor cocktail (no. P8340; Sigma-Aldrich). The muscle extract was centrifuged at 10,000 g for 10 min, and the protein content of the supernatant fraction was quantified by the Bradford method (6) or use of a DC protein assay (Bio-Rad, Hercules, CA). For analysis, proteins in the supernatant were fractionated, transferred to PVDF membranes, and stained with Ponceau-S to confirm effective transfer and equal protein loading. Membranes were incubated with appropriate antibodies overnight at 4°C. Antibodies against REDD1 (cat. no. 10638-1-AP) were obtained from ProteinTech (Chicago, IL). Antibodies against GAPDH (cat. no. sc-32233) were obtained from Santa Cruz Biotechnology (Dallas, TX). Antibodies against Histone H2B (cat. no. 8135), phospho-eIF4E-binding protein 1 (4E-BP1; Ser65; cat. no. 9451), phospho-s6 ribosomal protein (Ser240/244; cat. no. 2215), total s6 ribosomal protein (cat. no. 2217), phospho-ULK1 (Ser757; cat. no. 6888), and total ULK1 (cat. no. 8054) were obtained from Cell Signaling Technology (Danvers, MA). Antibodies against total 4E-BP1 were custom made by Bethyl Laboratories (Montgomery, TX) and kindly provided by Dr. Scot R. Kimball (Pennsylvania State University College of Medicine). Following incubation with appropriate secondary antibodies (cat. no. A120-101P or A90-116P, Bethyl Laboratories, Montgomery, TX), the antigen-antibody complex was visualized by enhanced chemiluminescence (Clarity Reagent; Bio-Rad, Hercules, CA) on a Bio-Rad ChemiDoc Touch imaging system. All blots were quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
Cytosolic/nuclear fraction separation.
Whole gastrocnemius muscle samples were homogenized using glass on glass in 10 volumes (10 µl/mg tissue) of buffer (referred here after as buffer A) consisting of 10 mM NaCl, 1.5 mM MgCl2, 20 mM HEPES, 20% glycerol, 0.1% Triton-X 100, 1 mM DTT, and 10 µl/ml protease inhibitor cocktail (no. P8340; Sigma-Aldrich). Samples were centrifuged for 5 min at 2,400 g at 4°C. The supernatant was collected and saved as the cytosolic-enriched fraction. This fraction was further centrifuged three times, each for 5 min at 3,500 g and 4°C, to pellet and remove any remaining noncytosolic material. The pellet containing the nuclear enriched fraction was then gently washed three times in buffer A. Between each wash, the nuclear pellet was centrifuged for 5 min at 2,400 g at 4°C. The final nuclear pellet was then resuspended in lysis buffer A containing 5 M NaCl and rocked at 4°C for 1.5 h. The sample was then centrifuged for 15 min at 21,000 g at 4°C. The supernatant was collected and saved as the nuclear enriched fraction. The protein content of each fraction was quantified by the Bradford method (6), and equal quantities of protein from each fraction were subjected to Western blot analysis, as described above.
Statistical analysis.
Data are presented as means ± SE. Differences in REDD1 protein expression in the plantaris of REDD1+/+ mice following recovery were analyzed by one-way ANOVA with Fisher’s least significant difference (LSD) post hoc test. Differences in REDD1 protein expression in the nuclear/cytosolic-enriched fractions were analyzed by two-way ANOVA, using exercise and cellular fraction as the two factors. The mRNA expression of REDD2 and indices of mTORC1 signaling were analyzed by two-way ANOVA, using time and genotype as the two factors. Exercise capacity was evaluated by Student’s t-test. Differences in gene expression following exercise were analyzed by two-way ANOVA, using exercise and genotype as the two factors, by one-way ANOVA, or by Kruskill-Wallis if the normality assumption was violated. Fisher’s LSD was used post hoc when an interaction was detected by two-way ANOVA or if a significant F value was detected by one-way ANOVA. Dunn’s multiple comparison test was used if a significant H value was detected following Kruskill-Wallis. Because of the smaller sample size, and as previously utilized (40), detection of an interaction at P ≤ 0.1 was accepted for two-way ANOVA. Using this approach, only Junb mRNA expression required this alternative threshold, as the P value for the interaction was P = 0.056. Post hoc analysis was used to analyze Serpina3m gene expression analysis from the RU-486 experiments, although the Kruskill-Wallis H value reached only P = 0.06, as our previous experiments showed that Serpina3m gene expression was increased after exercise (i.e., Fig. 4C). With the exception of these modified thresholds, significance was set at P ≤ 0.05 for all other analyses.
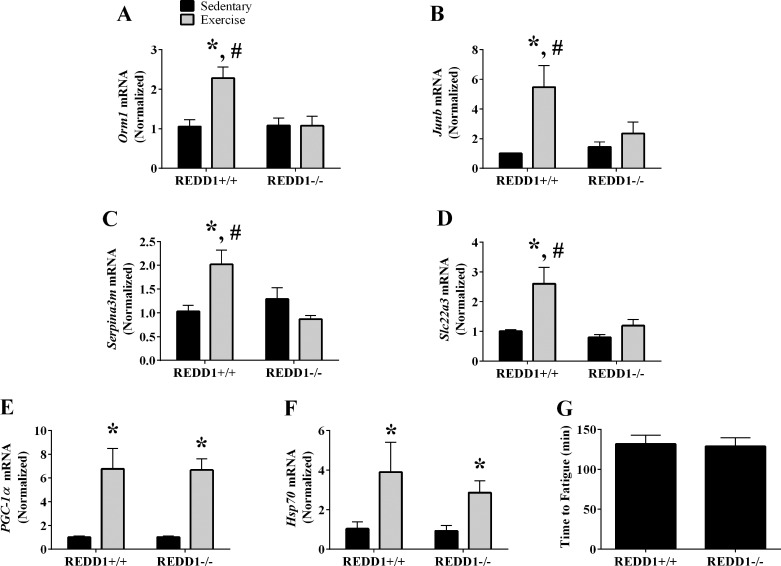
Fig. 4.
Validation of REDD1-sensitive genes following acute aerobic exercise. A–F: the relative mRNA content of orosomucoid 1 (Orm1; A), Junb (B), serine peptidase inhibitor clade A member 3M (Serpina3m; C), solute carrier family 22 member 3 (Slc22a3; D), peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α; E), and heat shock protein family A member 1A (Hsp70; F) from the plantaris was determined by RT-PCR. G: exercise capacity of REDD1+/+ and REDD1−/− mice was determined on a rodent treadmill; n = a total of 4–6/group generated from 1 to 3 independent experiments. *Significant difference compared with sedentary condition within genotype; #significant difference between genotypes within the same condition (sedentary or exercise). P ≤ 0.10 for detection of an interaction. P ≤ 0.05 for all other analysis.
RESULTS
Disruption of REDD1 does not alter mTORC1 signaling following acute aerobic exercise.
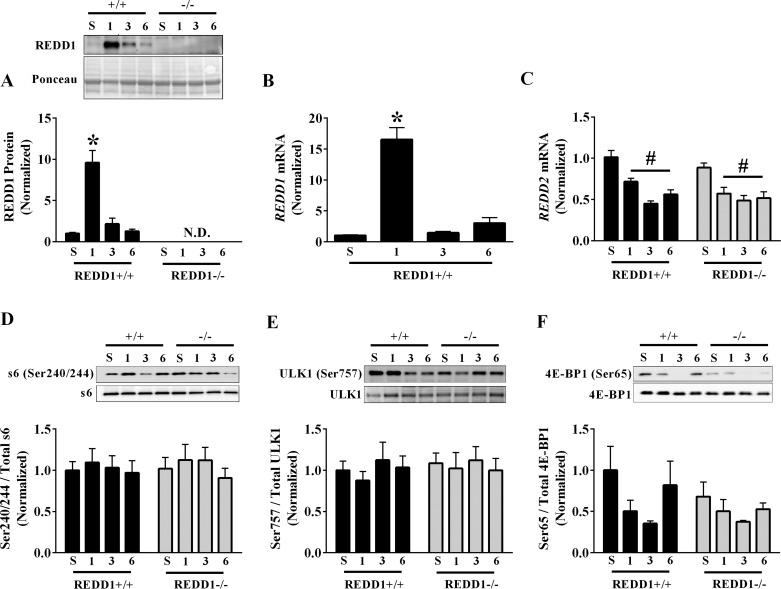
Consistent with others (22, 41, 44), acute aerobic exercise profoundly increased REDD1 protein expression in the plantaris by 860% at the 1-h postexercise time point (Fig. 1A). This induction was highly transient, as REDD1 expression returned to baseline at the 3- and 6-h postexercise time points (Fig. 1A). The induction of REDD1 protein was likely mediated by a change in the corresponding message, as REDD1 mRNA content was increased 1,550% at the 1-h postexercise time point before returning to baseline values at 3 and 6 h of recovery (Fig. 1B). Also, expression of REDD2 was decreased at each time point in the postexercise recovery period, and this was independent of genotype (Fig. 1C).
Fig. 1.
Regulated in development and DNA damage 1 (REDD1) expression and mechanistic target of rapamycin in complex 1 (mTORC1) signaling following acute aerobic exercise. A: REDD1 protein expression from the plantaris muscle in the sedentary and 1-, 3-, and 6-h postexercise conditions was determined by Western blot analysis. B and C: the relative mRNA content of REDD1 (B) and REDD2 (C) from the plantaris muscle in the sedentary and 1-, 3-, and 6-h postexercise conditions was determined by RT-PCR. D–F: the phosphorylated-to-total protein ratio for ribosomal protein S6 (Ser240/244; D), uncoordinated-like kinase 1 (ULK1) (Ser757; E), and eIF4E-binding protein 1 (4E-BP1) (Ser65; F) within the plantaris muscle in sedentary and 1-, 3-, and 6-h postexercise conditions was determined by Western blot analysis. +/+, REDD1+/+; −/−, REDD1−/−; S, sedentary; 1, 1 h; 3, 3 h; 6, 6 h (n = a total of 4–6/group generated from 3 independent experiments). *Significant difference compared with corresponding sedentary values; #main effect of time. P ≤ 0.05 for all analysis.
Because REDD1 is a known repressor of mTORC1 signaling, we assessed whether disruption of REDD1 altered the phosphorylation of various substrates downstream of mTORC1. Although we were unable to detect phosphorylation of the 70-kDa ribosomal protein S6 kinase 1 (p70S6K1) on Thr389 in any condition, which was likely due to the fasted state of the mice, we found no detectable difference in the phosphorylation of the p70S6K1 substrate S6 ribosomal protein (Ser240/244) by either exercise or genotype (Fig. 1D). Likewise, neither exercise nor genotype altered the phosphorylation of uncoordinated-like kinase 1 (ULK1) on Ser757 (Fig. 1E). Interestingly, there was a main effect to decrease phosphorylation of the eIF4E-binding protein 1 (4E-BP1) on Ser65 in both REDD1+/+ and REDD1−/− mice only at 1 and 3 h postexercise, but this main effect was abolished when the 6-h time point was included in the analysis (Fig. 1F). This exercise-mediated reduction in 4E-BP1 phosphorylation, without a corresponding change in other mTORC1 substrates, is consistent with previous reports (16, 22, 41, 55). In all, these data show that preventing induction of REDD1 in skeletal muscle following an acute bout of aerobic exercise does not alter mTORC1 signaling.
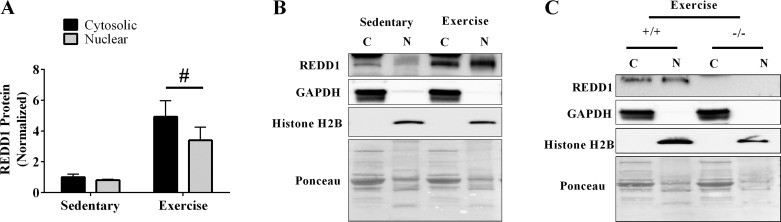
REDD1 expression is induced in both nuclear and cytosolic-enriched fractions 1 h postexercise.
To gain a better understanding of the REDD1 induction following acute aerobic exercise, we determined the fractional distribution 1 h into the postexercise recovery when expression was elevated. Gastrocnemius muscles from sedentary REDD1+/+ mice and REDD1+/+ mice were separated into cytosolic and nuclear enriched fractions. Importantly, others have shown that the exercise-mediated induction of REDD1 protein in the gastrocnemius exhibits a pattern of expression in the postexercise recovery similar to that observed in the plantaris (see Fig. 1A and Refs. 22, 41, and 44). In the sedentary condition, REDD1 protein was detected in both the cytosolic and nuclear enriched fractions, with no significant difference observed between the fractions (Fig. 2, A and B). Interestingly, exercise increased REDD1 protein in both the cytosolic (392%) and nuclear (240%) enriched fractions to a similar magnitude. Validity of our REDD1 measurement was confirmed in control experiments utilizing gastrocnemius fractional extracts from REDD1+/+ and REDD1−/− mice 1 h postexercise (Fig. 2C).
Fig. 2.
Fractional distribution of the REDD1 in the sedentary and postexercise conditions. A: REDD1 protein expression was determined in the cytosolic and nuclear enriched fractions of the gastrocnemius of REDD1+/+ mice from sedentary and 1-h postexercise conditions by Western blot analysis. B: representative Western blot. C: representative Western blots from control experiments. C, cytosolic; N, nuclear; +/+, REDD1+/+; −/−, REDD1−/− (n = a total of 4/group generated from 2 independent experiments). #Main effect of exercise. P ≤ 0.05 for all analysis
REDD1 regulates the exercise-mediated gene expression signature.
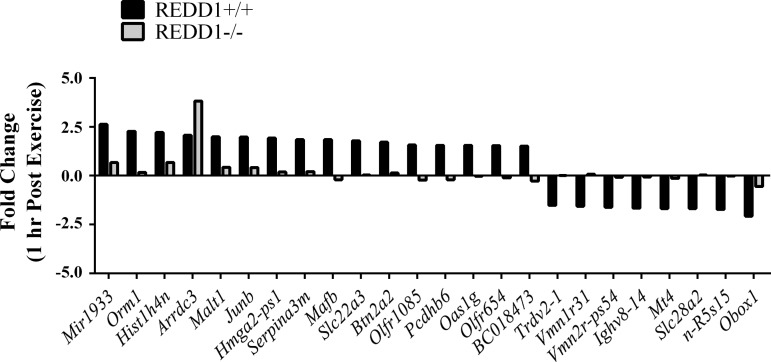
Because REDD1 expression was induced in the nuclear enriched fraction, we next sought to determine whether disruption of REDD1 altered the exercise-induced gene expression signature. Using an unbiased approach, total RNA from the plantaris of REDD1+/+ and REDD1−/− mice in the sedentary and 1-h postexercise conditions was subjected to microarray analysis. To confirm the validity of the methodology, the list of DEGs from exercise-REDD1+/+ vs. sedentary-REDD1+/+ was searched for putative genes induced by acute exercise. Indeed, an increase in peroxisome proliferator-activated receptor-γ coactivator-1α (Ppargc1a; a.k.a. PGC-1α), heat shock protein family A member 1A (Hspa1a; a.k.a. Hsp70), and REDD1 (Ddit4) was detected by the microarray (39, 44). To compare the magnitude of REDD1 induction to other altered genes, we generated a list of the top 10 genes exhibiting the greatest induction following acute exercise (Table 2) and a list of the top 10 genes exhibiting the greatest repression following acute exercise (Table 3). Interestingly, of all DEGs identified by the microarray, REDD1 exhibited the greatest fold change, supporting an important role for REDD1 following the exercise stimulus.
Table 2.
Top 10 genes exhibiting increased expression 1 h post-aerobic exercise in the plantaris of REDD1+/+ mice determined by microarray analysis
| Gene Symbol | RefSeq | Fold Change | P Value |
|---|---|---|---|
| Ddit4 | NM_029083 | 6.49065 | 0.00001 |
| Otud1 | NM_027715 | 4.99101 | 0.0007 |
| Nr4a3 | NM_001307989 | 4.32013 | 0.004 |
| Nr4a2 | NM_001139509 | 4.19124 | 0.005 |
| Mt2 | NM_008630 | 3.98769 | 0.00003 |
| Slc25a25 | NM_001164357 | 3.78215 | 0.0004 |
| Btg2 | NM_007570 | 3.57261 | 0.01 |
| Atf3 | NM_007498 | 3.35136 | 0.02 |
| Ciart | NM_001033302 | 3.34383 | 0.0005 |
| Sik1 | NM_010831 | 3.28446 | 0.00001 |
Table 3.
Top 10 genes exhibiting decreased expression 1 h post-aerobic exercise in the plantaris of REDD1+/+ mice determined by microarray analysis
| Gene Symbol | RefSeq | Fold Change | P Value |
|---|---|---|---|
| Zfp273 | NM_198322 | −2.31408 | 0.006621 |
| Rassf9 | NM_146240 | −2.30025 | 1.55E-05 |
| Trav6d-3 | OTTMUST00000035995 | −2.20007 | 8.12E-05 |
| Obox1 | NM_027802 | −2.07174 | 0.000152 |
| Aplnr | NM_011784 | −2.06461 | 0.001068 |
| Mir181a-2 | NR_029568 | −2.04873 | 0.000307 |
| BC094916 | NM_001024721 | −2.00509 | 0.032251 |
| n-R5s29 | ENSMUST00000082836 | −1.98531 | 0.036761 |
| Krtap6-5 | NM_130856 | −1.96854 | 0.028312 |
| Igkv5-45 | OTTMUST00000132854 | −1.95646 | 0.045261 |
To determine whether REDD1 regulated the skeletal muscle gene expression signature following acute exercise, we generated a list of genes that were 1) increased by exercise in REDD1+/+ mice (exercise-REDD1+/+ vs. sedentary-REDD1+/+), 2) altered by the disruption of REDD1 following exercise (exercise-REDD1−/− vs. exercise-REDD1+/+), and 3) not altered by disruption of REDD1 in the sedentary condition (sedentary-REDD1−/− vs. sedentary-REDD1+/+). This strategy identified 24 genes whose normal exercise-mediated change was altered in REDD1−/− mice (Fig. 3). Using RT-PCR, we validated the microarray output for those identified genes previously implicated in skeletal muscle biology (8, 32, 36, 47). Specifically, preventing the induction of REDD1 negated the exercise-mediated increase in orosomucoid 1 (Orm1), Junb, serine peptidase inhibitor clade A member 3M (Serpina3m), and solute carrier family 22 member 3 (Slc22a3) (Fig. 4, A–D). Although the expression of these genes was affected by disruption of REDD1, the induction of the putative exercise-responsive and intensity-dependent genes PGC-1α and Hsp70 (39, 53) was unaffected (Fig. 4, E and F), supporting specificity of the REDD1-sensitive gene signature and a similar exercise stimulus. In further support of a similar exercise stimulus, there was no difference in overall exercise capacity between REDD1+/+ and REDD1−/− mice (Fig. 4G). Together, these data indicate that REDD1 induction is required for the exercise-mediated change in the expression of a unique set of genes related to skeletal muscle biology.
Fig. 3.
Identification of REDD1-sensitive genes following acute aerobic exercise. RNA was isolated from the plantaris of REDD1+/+ and REDD1−/− mice 1 h postexercise and subjected to microarray analysis to identify genes that were altered by disruption of REDD1. The exercise-induced fold change for each gene is illustrated by genotype; n = 3/genotype.
REDD1 induction is partially dependent upon a functional glucocorticoid receptor.
Of the identified REDD1-sensitive genes, Orm1 is a presumed target of glucocorticoid signaling (35). Because glucocorticoids are increased by aerobic exercise (10, 21) and induce REDD1 expression in skeletal muscle (31, 52), this prompted us to test whether glucocorticoid signaling contributed to the REDD1 induction and change in the gene expression signature following acute aerobic exercise. Upon confirmation that RU-486 sufficiently blocked the glucocorticoid induction of REDD1 in skeletal muscle (Fig. 5A), mice treated with RU-486 or vehicle only were subjected to a single bout of aerobic exercise. Inhibition of the glucocorticoid receptor significantly blunted but did not prevent the induction of REDD1 protein following exercise (Fig. 5B). This was likely mediated by a change in the corresponding transcript, as REDD1 mRNA content showed a similar expression pattern following glucocorticoid receptor blockade (Fig. 5C). Importantly, these reductions were not due to reduced glucocorticoid production, as serum corticosterone levels were significantly elevated in the mice treated with RU-486 (Fig. 5D).
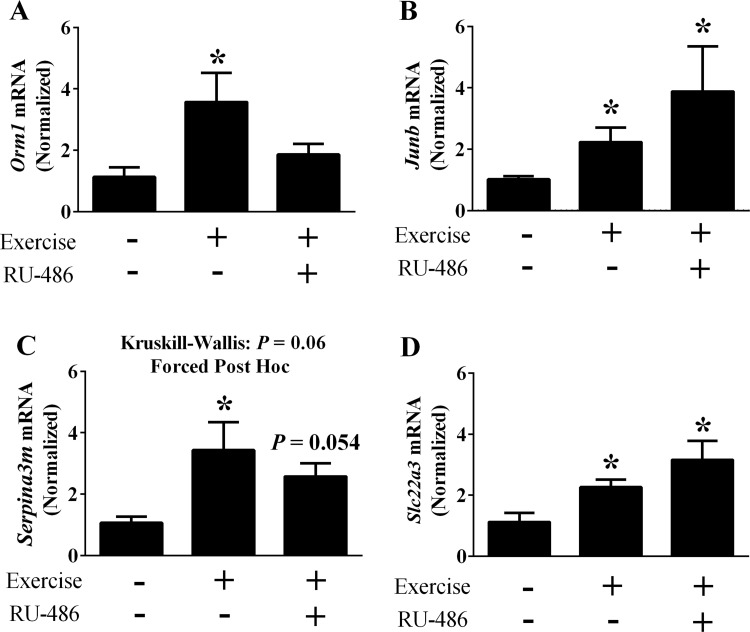
Consistent with Orm1 as a glucocorticoid target gene, RU-486 impaired the exercise-mediated increase in Orm1 mRNA expression (Fig. 6A). In contrast, the induction of Junb and Slc22a3 mRNA following exercise were maintained despite receptor blockade (Fig. 6, B and D). While the overall H value for Serpina3m did not reach significance, a strong trend was observed (P = 0.06). Because we observed induction of Serpina3m in earlier experiments (see Fig. 4C), we utilized post hoc analysis to confirm that Serpina3m mRNA was also induced by exercise in vehicle treated mice (Fig. 6C). However, following receptor inhibition only a trend (P = 0.054) for an increase was detected (Fig. 6C). These data show that glucocorticoids contribute to the exercise-mediated induction of REDD1, but signaling through this receptor does not regulate all of the previously identified REDD1 sensitive genes. Thus, it is likely that REDD1 induction coupled with the activation of upstream signals initiate changes in the specific gene expression signature.
Fig. 6.
Role of glucocorticoid signaling on REDD1-sensitive gene expression following acute exercise. The mRNA content of Orm1 (A), Junb (B), Serpina3m (C), and Slc22a3 (D) following acute aerobic exercise was determined by RT-PCR; n = a total of 4–8 mice/group generated from 2 independent experiments. *Significant difference compared with sedentary group. P ≤ 0.05 for all analysis except for Serpina3m, in which post hoc analysis was performed despite an H value of P = 0.06.
DISCUSSION
In skeletal muscle, studies have principally focused on REDD1 as a negative regulator of mTORC1 signaling during pathological and catabolic conditions (7, 15, 17–20, 29, 54). In contrast, the results herein support an alternative mTORC1-independent role for REDD1 in the regulation of exercise-induced changes in gene expression. Specifically, four of the 24 REDD1-sensitive genes identified and validated herein have previously been implicated in regulating various aspects of skeletal muscle biology. Thus, the exercise-mediated induction of REDD1 and the subsequent change in the gene expression signature may contribute to such long-term adaptations, including enhanced work capacity (28), muscle hypertrophy (30), neuromuscular junction size (12), or the action of Metformin in skeletal muscle (37). For instance, Orm1 is a secretory protein produced by skeletal muscle, and increased Orm1 levels in circulation have been shown to enhance exercise capacity (32). Alternatively, Junb expression promotes skeletal muscle hypertrophy by enhancing protein accretion (47), and increased expression may contribute to the increase in muscle size reported following aerobic exercise training (37). Although the role of Serpina3m in skeletal muscle is less understood, Serpina3m is likely involved in neuromuscular junction maintenance and/or stability, as its expression is induced and localized at the motor endplate following denervation, and this effect is augmented in a rodent model of enhanced reinnervation (36). Finally, Slc22a3 is a member of the organic cation transporters expressed on the cell membrane of various tissues, including skeletal muscle (8). Slc22a3 expression increased the Metformin-mediated activation of the 5′-AMP-activated protein kinase (AMPK) (8). Consequently, an exercise-mediated induction of Slc22a3 may potentiate the function of the drug in skeletal muscle, and it may also regulate the uptake of other cationic molecules following exercise.
Alhough we show a role for the glucocorticoid receptor in the induction of REDD1 following exercise (Fig. 5, B and D), the fact that REDD1 was still induced to some degree suggests that other factors contribute. Based upon our data and others’, (see Figs. 1B and 5B and Ref. 46), the induction is likely mediated by a change in the corresponding REDD1 mRNA transcript. Accordingly, other positive effectors of REDD1 gene transcription were shown to be activated/increased by exercise, including hypoxia inducible factor-1α (HIF-1α) (1). However, the exercise-mediated activation of HIF-1α persisted up through 6 h into the postexercise recovery (1), which is significantly longer than the REDD1 induction observed herein and as previously reported (44). In addition, expression of REDD2, which can be mildly induced by hypoxia in a manner independent of HIF-1α (48), was decreased at each time point during recovery (Fig. 1C), suggesting a limited role of hypoxia in this response. Increased production of Interleukin-6 (IL-6) is a potential contributing factor, as IL-6 alters REDD1 expression (45) and circulating IL-6 levels are increased by acute exercise (42). Moreover, the transcription factor signal transducer and activator of transcription 1 (STAT1) is activated by IL-6 signaling (9), and the Serpina3m promoter contains a predicted STAT1 binding site. It is also likely that changes in expression of REDD1-sensitive genes depend upon exercise intensity in a manner similar to the intensity-dependent changes in glucocorticoid production (23). Therefore, the magnitude of REDD1 induction, as well as that of glucocoriticoid-target genes (e.g., Orm1), could depend on exercise intensity. How signals generated by the yet to be identified factors might contribute to the induction of REDD1 and its target genes, particularly relative to changes in exercise intensity, remains to be determined.
Though the current findings support the premise that REDD1 alters gene expression, a mechanistic explanation(s) remains elusive. For instance, it is unknown whether the changes in expression of the identified genes were due to a change in the rate of transcription, the rate of mRNA turnover, or both. In support for a REDD1-mediated change in transcription, we and others showed that REDD1 is localized in the nucleus and that the induction of REDD1 following exercise occurs in the nuclear enriched fraction (see Fig. 2 and Refs. 33 and 38). However, we are unaware of any reports identifying REDD1 as a transcription factor. In addition, a DNA-binding motif within the protein sequence has not yet been described. Likewise, the possibility of REDD1 acting as a gene transcriptional coactivator has yet to be explored. Based upon other work related to the REDD1 mechanism of action, it can be hypothesized that REDD1 would alter the activation of signaling proteins/transcription factors through posttranslational modification, particularly phosphorylation (11, 13). Upon confirming the list of DEGs, we identified transcription factors predicted to bind to the promoter regions of the confirmed genes. However, we were unable to detect a change in phosphorylation of the identified transcription factors by either exercise or genotype (data not shown), necessitating further analysis.
Whereas changes in REDD1 expression are usually inversely associated with mTORC1 signaling (17, 18, 20), the REDD1 induction observed here and by others following acute exercise did not correlate well with repressed mTORC1 signaling (22, 41). Furthermore, we observed only repressed phosphorylation of the mTORC1 substrate 4E-BP1 (Ser65) at the 1- and 3-h postexercise time points, and this occurred even in the absence of REDD1 (i.e., Fig. 1F), suggesting that REDD1 and mTORC1 regulate divergent processes in the postexercise recovery period. Interestingly, previous work suggests that mTORC1 may also contribute to the regulation of gene expression in the postexercise recovery, and those genes are distinct from those regulated by REDD1. For example, treating mice with rapamycin before acute exercise augmented the mRNA induction of PGC-1α, pyruvate dehydrogenase kinase 4 (PDK4), and transcription factor A, mitochondrial (TFAM) in the postexercise recovery (44). Accordingly, in our study, PGC-1α induction was similar in both REDD1+/+ and REDD1−/− mice, suggesting that this mTORC1 mode of regulation, in combination with other known activators of PGC-1α such as calcineurin and AMPK (27, 50), was intact and did not require REDD1.
In summary, we describe an alternative, mTORC1-independent function of REDD1 in the regulation of the skeletal muscle gene expression signature following acute aerobic exercise. Specifically, we identified a change in the expression of Orm1, Junb, Serpina3m, and Slc22a3, each of which have been implicated in skeletal muscle biology. Thus, in addition to the negative connotations generally associated with long-term changes in REDD1 expression in skeletal muscle, we provide evidence that the transient induction of REDD1 following acute exercise, which is partially dependent upon signals induced by glucocorticoids, may be beneficial by facilitating changes in gene expression.
GRANTS
This work was supported by startup funds from the University of Central Florida and Florida State University (B. S. Gordon) as well as by National Institutes of Health Grants F32-AA-023422 to J. L. Steiner and RO1-CA-122589 to L. W. Ellisen.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.S.G., D.L.W., and P.M.C. conceived and designed research; B.S.G., J.L.S., M.L.R., S.Q., L.W.E., and S.S.G. performed experiments; B.S.G., J.L.S., M.L.R., S.Q., L.W.E., A.M.E., and D.L.W. analyzed data; B.S.G., J.L.S., D.L.W., and P.M.C. interpreted results of experiments; B.S.G., S.Q., and L.W.E. prepared figures; B.S.G., S.S.G., and A.M.E. drafted manuscript; B.S.G., J.L.S., M.L.R., S.S.G., A.M.E., D.L.W., and P.M.C. edited and revised manuscript; B.S.G., J.L.S., M.L.R., S.S.G., A.M.E., D.L.W., and P.M.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Michael Dennis and Gina Dieter for breeding the REDD1−/− mice as well as Dr. Kurt Steiner for valuable discussion.
REFERENCES
- 1.Ameln H, Gustafsson T, Sundberg CJ, Okamoto K, Jansson E, Poellinger L, Makino Y. Physiological activation of hypoxia inducible factor-1 in human skeletal muscle. FASEB J 19: 1009–1011, 2005. doi: 10.1096/fj.04-2304fje. [DOI] [PubMed] [Google Scholar]
- 2.Baida G, Bhalla P, Kirsanov K, Lesovaya E, Yakubovskaya M, Yuen K, Guo S, Lavker RM, Readhead B, Dudley JT, Budunova I. REDD1 functions at the crossroads between the therapeutic and adverse effects of topical glucocorticoids. EMBO Mol Med 7: 42–58, 2015. doi: 10.15252/emmm.201404601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballmann C, Tang Y, Bush Z, Rowe GC. Adult expression of PGC-1α and -1β in skeletal muscle is not required for endurance exercise-induced enhancement of exercise capacity. Am J Physiol Endocrinol Metab 311: E928–E938, 2016. doi: 10.1152/ajpendo.00209.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beelen M, Zorenc A, Pennings B, Senden JM, Kuipers H, van Loon LJC. Impact of protein coingestion on muscle protein synthesis during continuous endurance type exercise. Am J Physiol Endocrinol Metab 300: E945–E954, 2011. doi: 10.1152/ajpendo.00446.2010. [DOI] [PubMed] [Google Scholar]
- 5.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19: 185–193, 2003. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 6.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Britto FA, Begue G, Rossano B, Docquier A, Vernus B, Sar C, Ferry A, Bonnieu A, Ollendorff V, Favier FB. REDD1 deletion prevents dexamethasone-induced skeletal muscle atrophy. Am J Physiol Endocrinol Metab 307: E983–E993, 2014. doi: 10.1152/ajpendo.00234.2014. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Pawlikowski B, Schlessinger A, More SS, Stryke D, Johns SJ, Portman MA, Chen E, Ferrin TE, Sali A, Giacomini KM. Role of organic cation transporter 3 (SLC22A3) and its missense variants in the pharmacologic action of metformin. Pharmacogenet Genomics 20: 687–699, 2010. doi: 10.1097/FPC.0b013e32833fe789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi YS, Eto D, Yang JA, Lao C, Crotty S. Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. J Immunol 190: 3049–3053, 2013. doi: 10.4049/jimmunol.1203032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman MA, Garland T Jr, Marler CA, Newton SS, Swallow JG, Carter PA. Glucocorticoid response to forced exercise in laboratory house mice (Mus domesticus). Physiol Behav 63: 279–285, 1998. doi: 10.1016/S0031-9384(97)00441-1. [DOI] [PubMed] [Google Scholar]
- 11.Dennis MD, Coleman CS, Berg A, Jefferson LS, Kimball SR. REDD1 enhances protein phosphatase 2A-mediated dephosphorylation of Akt to repress mTORC1 signaling. Sci Signal 7: ra68, 2014. doi: 10.1126/scisignal.2005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deschenes MR, Maresh CM, Crivello JF, Armstrong LE, Kraemer WJ, Covault J. The effects of exercise training of different intensities on neuromuscular junction morphology. J Neurocytol 22: 603–615, 1993. doi: 10.1007/BF01181487. [DOI] [PubMed] [Google Scholar]
- 13.DeYoung MP, Horak P, Sofer A, Sgroi D, Ellisen LW. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev 22: 239–251, 2008. doi: 10.1101/gad.1617608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edgett BA, Fortner ML, Bonen A, Gurd BJ. Mammalian target of rapamycin pathway is up-regulated by both acute endurance exercise and chronic muscle contraction in rat skeletal muscle. Appl Physiol Nutr Metab 38: 862–869, 2013. doi: 10.1139/apnm-2012-0405. [DOI] [PubMed] [Google Scholar]
- 15.Favier FB, Costes F, Defour A, Bonnefoy R, Lefai E, Baugé S, Peinnequin A, Benoit H, Freyssenet D. Downregulation of Akt/mammalian target of rapamycin pathway in skeletal muscle is associated with increased REDD1 expression in response to chronic hypoxia. Am J Physiol Regul Integr Comp Physiol 298: R1659–R1666, 2010. doi: 10.1152/ajpregu.00550.2009. [DOI] [PubMed] [Google Scholar]
- 16.Gautsch TA, Anthony JC, Kimball SR, Paul GL, Layman DK, Jefferson LS. Availability of eIF4E regulates skeletal muscle protein synthesis during recovery from exercise. Am J Physiol Cell Physiol 274: C406–C414, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Gordon BS, Liu C, Steiner JL, Nader GA, Jefferson LS, Kimball SR. Loss of REDD1 augments the rate of the overload-induced increase in muscle mass. Am J Physiol Regul Integr Comp Physiol 311: R545–R557, 2016. doi: 10.1152/ajpregu.00159.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon BS, Steiner JL, Lang CH, Jefferson LS, Kimball SR. Reduced REDD1 expression contributes to activation of mTORC1 following electrically induced muscle contraction. Am J Physiol Endocrinol Metab 307: E703–E711, 2014. doi: 10.1152/ajpendo.00250.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon BS, Steiner JL, Williamson DL, Lang CH, Kimball SR. Emerging role for regulated in development and DNA damage 1 (REDD1) in the regulation of skeletal muscle metabolism. Am J Physiol Endocrinol Metab 311: E157–E174, 2016. doi: 10.1152/ajpendo.00059.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon BS, Williamson DL, Lang CH, Jefferson LS, Kimball SR. Nutrient-induced stimulation of protein synthesis in mouse skeletal muscle is limited by the mTORC1 repressor REDD1. J Nutr 145: 708–713, 2015. doi: 10.3945/jn.114.207621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hackney AC, Viru A. Twenty-four-hour cortisol response to multiple daily exercise sessions of moderate and high intensity. Clin Physiol 19: 178–182, 1999. doi: 10.1046/j.1365-2281.1999.00157.x. [DOI] [PubMed] [Google Scholar]
- 22.Hayasaka M, Tsunekawa H, Yoshinaga M, Murakami T. Endurance exercise induces REDD1 expression and transiently decreases mTORC1 signaling in rat skeletal muscle. Physiol Rep 2: e12254, 2014. doi: 10.14814/phy2.12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill EE, Zack E, Battaglini C, Viru M, Viru A, Hackney AC. Exercise and circulating cortisol levels: the intensity threshold effect. J Endocrinol Invest 31: 587–591, 2008. doi: 10.1007/BF03345606. [DOI] [PubMed] [Google Scholar]
- 24.Horak P, Crawford AR, Vadysirisack DD, Nash ZM, DeYoung MP, Sgroi D, Ellisen LW. Negative feedback control of HIF-1 through REDD1-regulated ROS suppresses tumorigenesis. Proc Natl Acad Sci USA 107: 4675–4680, 2010. doi: 10.1073/pnas.0907705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 31: e15, 2003. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4: 249–264, 2003. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 27.Jäger S, Handschin C, St.-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA 104: 12017–12022, 2007. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeppesen TD, Schwartz M, Olsen DB, Wibrand F, Krag T, Dunø M, Hauerslev S, Vissing J. Aerobic training is safe and improves exercise capacity in patients with mitochondrial myopathy. Brain 129: 3402–3412, 2006. doi: 10.1093/brain/awl149. [DOI] [PubMed] [Google Scholar]
- 29.Kelleher AR, Kimball SR, Dennis MD, Schilder RJ, Jefferson LS. The mTORC1 signaling repressors REDD1/2 are rapidly induced and activation of p70S6K1 by leucine is defective in skeletal muscle of an immobilized rat hindlimb. Am J Physiol Endocrinol Metab 304: E229–E236, 2013. doi: 10.1152/ajpendo.00409.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konopka AR, Harber MP. Skeletal muscle hypertrophy after aerobic exercise training. Exerc Sport Sci Rev 42: 53–61, 2014. doi: 10.1249/JES.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumari R, Willing LB, Jefferson LS, Simpson IA, Kimball SR. REDD1 (regulated in development and DNA damage response 1) expression in skeletal muscle as a surrogate biomarker of the efficiency of glucocorticoid receptor blockade. Biochem Biophys Res Commun 412: 644–647, 2011. doi: 10.1016/j.bbrc.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lei H, Sun Y, Luo Z, Yourek G, Gui H, Yang Y, Su DF, Liu X. Fatigue-induced orosomucoid 1 acts on C-C chemokine receptor type 5 to enhance muscle endurance. Sci Rep 6: 18839, 2016. doi: 10.1038/srep18839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin L, Stringfield TM, Shi X, Chen Y. Arsenite induces a cell stress-response gene, RTP801, through reactive oxygen species and transcription factors Elk-1 and CCAAT/enhancer-binding protein. Biochem J 392: 93–102, 2005. doi: 10.1042/BJ20050553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lira VA, Okutsu M, Zhang M, Greene NP, Laker RC, Breen DS, Hoehn KL, Yan Z. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J 27: 4184–4193, 2013. doi: 10.1096/fj.13-228486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo Z, Lei H, Sun Y, Liu X, Su DF. Orosomucoid, an acute response protein with multiple modulating activities. J Physiol Biochem 71: 329–340, 2015. doi: 10.1007/s13105-015-0389-9. [DOI] [PubMed] [Google Scholar]
- 36.Macpherson PC, Farshi P, Goldman D. Dach2-Hdac9 signaling regulates reinnervation of muscle endplates. Development 142: 4038–4048, 2015. doi: 10.1242/dev.125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malin SK, Stephens BR, Sharoff CG, Hagobian TA, Chipkin SR, Braun B. Metformin’s effect on exercise and postexercise substrate oxidation. Int J Sport Nutr Exerc Metab 20: 63–71, 2010. doi: 10.1123/ijsnem.20.1.63. [DOI] [PubMed] [Google Scholar]
- 38.Michel G, Matthes HWD, Hachet-Haas M, El Baghdadi K, de Mey J, Pepperkok R, Simpson JC, Galzi JL, Lecat S. Plasma membrane translocation of REDD1 governed by GPCRs contributes to mTORC1 activation. J Cell Sci 127: 773–787, 2014. doi: 10.1242/jcs.136432. [DOI] [PubMed] [Google Scholar]
- 39.Milne KJ, Noble EG. Exercise-induced elevation of HSP70 is intensity dependent. J Appl Physiol (1985) 93: 561–568, 2002. doi: 10.1152/japplphysiol.00528.2001. [DOI] [PubMed] [Google Scholar]
- 40.Mobley CB, Mumford PW, Kephart WC, Conover CF, Beggs LA, Balaez A, Yarrow JF, Borst SE, Beck DT, Roberts MD. Effects of testosterone treatment on markers of skeletal muscle ribosome biogenesis. Andrologia 48: 967–977, 2016. doi: 10.1111/and.12539. [DOI] [PubMed] [Google Scholar]
- 41.Murakami T, Hasegawa K, Yoshinaga M. Rapid induction of REDD1 expression by endurance exercise in rat skeletal muscle. Biochem Biophys Res Commun 405: 615–619, 2011. doi: 10.1016/j.bbrc.2011.01.078. [DOI] [PubMed] [Google Scholar]
- 42.Northoff H, Berg A. Immunologic mediators as parameters of the reaction to strenuous exercise. Int J Sports Med 12, Suppl 1: S9–S15, 1991. doi: 10.1055/s-2007-1024743. [DOI] [PubMed] [Google Scholar]
- 43.Pastva A, Estell K, Schoeb TR, Schwiebert LM. RU486 blocks the anti-inflammatory effects of exercise in a murine model of allergen-induced pulmonary inflammation. Brain Behav Immun 19: 413–422, 2005. doi: 10.1016/j.bbi.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Philp A, Schenk S, Perez-Schindler J, Hamilton DL, Breen L, Laverone E, Jeromson S, Phillips SM, Baar K. Rapamycin does not prevent increases in myofibrillar or mitochondrial protein synthesis following endurance exercise. J Physiol 593: 4275–4284, 2015. doi: 10.1113/JP271219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puppa MJ, Gao S, Narsale AA, Carson JA. Skeletal muscle glycoprotein 130’s role in Lewis lung carcinoma-induced cachexia. FASEB J 28: 998–1009, 2014. doi: 10.1096/fj.13-240580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiao S, Dennis M, Song X, Vadysirisack DD, Salunke D, Nash Z, Yang Z, Liesa M, Yoshioka J, Matsuzawa S, Shirihai OS, Lee RT, Reed JC, Ellisen LW. A REDD1/TXNIP pro-oxidant complex regulates ATG4B activity to control stress-induced autophagy and sustain exercise capacity. Nat Commun 6: 7014, 2015. doi: 10.1038/ncomms8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raffaello A, Milan G, Masiero E, Carnio S, Lee D, Lanfranchi G, Goldberg AL, Sandri M. JunB transcription factor maintains skeletal muscle mass and promotes hypertrophy. J Cell Biol 191: 101–113, 2010. doi: 10.1083/jcb.201001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reiling JH, Hafen E. The hypoxia-induced paralogs Scylla and Charybdis inhibit growth by down-regulating S6K activity upstream of TSC in Drosophila. Genes Dev 18: 2879–2892, 2004. doi: 10.1101/gad.322704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ren JM, Semenkovich CF, Gulve EA, Gao J, Holloszy JO. Exercise induces rapid increases in GLUT4 expression, glucose transport capacity, and insulin-stimulated glycogen storage in muscle. J Biol Chem 269: 14396–14401, 1994. [PubMed] [Google Scholar]
- 50.Schaeffer PJ, Wende AR, Magee CJ, Neilson JR, Leone TC, Chen F, Kelly DP. Calcineurin and calcium/calmodulin-dependent protein kinase activate distinct metabolic gene regulatory programs in cardiac muscle. J Biol Chem 279: 39593–39603, 2004. doi: 10.1074/jbc.M403649200. [DOI] [PubMed] [Google Scholar]
- 51.Sofer A, Lei K, Johannessen CM, Ellisen LW. Regulation of mTOR and cell growth in response to energy stress by REDD1. Mol Cell Biol 25: 5834–5845, 2005. doi: 10.1128/MCB.25.14.5834-5845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang H, Kubica N, Ellisen LW, Jefferson LS, Kimball SR. Dexamethasone represses signaling through the mammalian target of rapamycin in muscle cells by enhancing expression of REDD1. J Biol Chem 281: 39128–39134, 2006. doi: 10.1074/jbc.M610023200. [DOI] [PubMed] [Google Scholar]
- 53.Wen X, Wu J, Chang JS, Zhang P, Wang J, Zhang Y, Gettys TW, Zhang Y. Effect of exercise intensity on isoform-specific expressions of NT-PGC-1 α mRNA in mouse skeletal muscle. BioMed Res Int 2014: 402175, 2014. doi: 10.1155/2014/402175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williamson DL, Dungan CM, Mahmoud AM, Mey JT, Blackburn BK, Haus JM. Aberrant REDD1-mTORC1 responses to insulin in skeletal muscle from Type 2 diabetics. Am J Physiol Regul Integr Comp Physiol 309: R855–R863, 2015. doi: 10.1152/ajpregu.00285.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williamson DL, Kubica N, Kimball SR, Jefferson LS. Exercise-induced alterations in extracellular signal-regulated kinase 1/2 and mammalian target of rapamycin (mTOR) signalling to regulatory mechanisms of mRNA translation in mouse muscle. J Physiol 573: 497–510, 2006. doi: 10.1113/jphysiol.2005.103481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williamson DL, Li Z, Tuder RM, Feinstein E, Kimball SR, Dungan CM. Altered nutrient response of mTORC1 as a result of changes in REDD1 expression: effect of obesity vs. REDD1 deficiency. J Appl Physiol (1985) 117: 246–256, 2014. doi: 10.1152/japplphysiol.01350.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu ZJ, Irizarry RA, Gentleman R, Martinez-Murillo F, Spencer F. A model-based background adjustment for oligonucleotide expression arrays. J Am Stat Assoc 99: 909–917, 2004. doi: 10.1198/016214504000000683. [DOI] [Google Scholar]