Abstract
The period around bariatric surgery offers a unique opportunity to characterize metabolism responses to dynamic shifts in energy, gut function, and anesthesia. We analyzed plasma acylcarnitines in obese women (n = 17) sampled in the overnight fasted/postabsorptive state approximately 1–2 wk before surgery (condition A), the morning of surgery (prior restriction to a 48-h clear liquid diet coupled in some cases a standard polyethylene glycol gut evacuation: condition B), and following induction of anesthesia (condition C). Comparisons tested if 1) plasma acylcarnitine derivatives reflective of fatty acid oxidation (FAO) and xenometabolism would be significantly increased and decreased, respectively, by preoperative gut preparation/negative energy balance (condition A vs. B), and 2) anesthesia would acutely depress markers of FAO. Acylcarnitines associated with fat mobilization and FAO were significantly increased in condition B: long-chain acylcarnitines (i.e., C18:1, ~70%), metabolites from active but incomplete FAO [i.e., C14:1 (161%) and C14:2 (102%)] and medium- to short-chain acylcarnitines [i.e., C2 (91%), R-3-hydroxybutyryl-(245%), C6 (45%), and cis-3,4-methylene-heptanoyl-(17%), etc.]. Branched-chain amino acid markers displayed disparate patterns [i.e., isobutyryl-(40% decreased) vs. isovaleryl carnitine (51% increased)]. Anesthesia reduced virtually every acylcarnitine. These results are consistent with a fasting-type metabolic phenotype coincident with the presurgical “gut preparation” phase of bariatric surgery, and a major and rapid alteration of both fat and amino acid metabolism with onset of anesthesia. Whether presurgical or anesthesia-associated metabolic shifts in carnitine and fuel metabolism impact patient outcomes or surgical risks remains to be evaluated experimentally.
Keywords: propofol, butyrobetaine, β-oxidation, xenobiotic, xenometabolomics, xenometabolite
the periods leading up to and then following bariatric surgery represent times in which there are major shifts in metabolic physiology. During the lead-in, for instance, it is common practice to place patients on a weight loss regimen, although this protocol is not universal. Furthermore, the immediate presurgical period typically includes 1 or more days of significant negative energy balance via restricting intake to clear liquids and/or gastrointestinal (GI) tract evacuation through, e.g., oral polyethylene glycol. The surgical and postsurgical episodes also involve anesthesia, healing, and then recovery. Finally, the manipulation of GI function and anatomy lead to postsurgical weight reduction and alterations in endocrinology and metabolism. The impact of these major, sometimes rapid, alterations in energy balance, GI tract status, and even anesthesia on the integrative regulation of fuel metabolism have not been studied in detail. Even less is known about the influence of the perisurgical events on microbial metabolism (xenometabolism), which would potentially be profound.
With these knowledge gaps in mind, we have leveraged targeted metabolomics tools to interrogate host metabolism longitudinally in a well-described clinical cohort of obese women, leading up to bariatric surgery. The repeated-measures experimental design enabled a comparison of plasma metabolite profiles in 1) the preoperative, overnight-fasted/postabsorptive state; 2) the phase immediately before surgery and anesthesia (following GI tract evacuation or significant negative energy balance); and 3) immediately following induction of anesthesia. The latter condition is particularly interesting from a metabolic physiology standpoint, since clinical case reports and animal model studies have led to assertions that certain anesthetics such as propofol alter in vivo fuel partitioning (i.e., in some persons, lactic acidemia, and hepatic lipid accumulation) and may perturb mitochondrial oxidative metabolism (7, 8, 12, 25). In the current report, patterns of circulating acylcarnitines and carnitine derivatives are detailed through comprehensive plasma acylcarnitine profiling. Acylcarnitines are valuable readouts of tissue metabolic status, since their concentrations in blood and urine reflect tissue pools of specific acyl-CoA metabolites, which are acted on by specific carnitine acyltransferases: the acyl-CoA pools are impacted by the net metabolic flux of fuels such as fatty acids and amino acids, making acylcarnitine profiling a widely used approach to diagnose inborn errors of metabolism (see Ref. 14). Interestingly, certain acylcarnitines, such as cis-3,4-methylene-heptanoylcarnitine, are likely derived from precursor fatty acid xenometabolites originating in the gut (27), providing an indirect snapshot of xenometabolism (28). The working model that drives our metabolomics studies in bariatric subjects predicts the following hypotheses: 1) the surgical lead-in period is characterized by an increase in fasting-associated metabolites and a decrease in xenometabolites due to typical presurgical gut evacuation protocols; and 2) Anesthesia rapidly attenuates mitochondrial fatty acid oxidation (FAO) and triggers a transition toward alternative fuels. To our knowledge, this is the first report of comprehensive metabolite patterns reflective of metabolic physiology in the perisurgical phase in a bariatric clinic setting and the first to report on the acute impact of anesthesia on acylcarnitine markers of fuel metabolism in humans.
RESEARCH DESIGN AND METHODS
Human Subjects
All studies were approved by the University of California Davis Institutional Review Board and conducted in alignment with Declaration of Helsinki guidelines. Subjects provided informed consent at the time of their preoperative evaluation (“preop”) at the University of California Davis Bariatric Surgery Clinic. Only women were enrolled for the study to minimize sex-associated experimental variance and to optimize interpretations in light of our prior acylcarnitine findings that were derived from obese female participants (i.e., Refs. 2, 28). A total of 17 nondiabetic patients participated in this study.
Experimental Design
The study design was a repeated-measures experiment testing for differences in blood analytes across three conditions: 1) the overnight-fasted/postabsorptive state at the “preop” visit (condition A); 2) the preparatory, negative energy balance and GI tract preparation phase just before surgery (condition B); and 3) in the immediate term following the onset of anesthesia (condition C). Preop visit blood samples were collected in the morning (antecubital venipuncture, EDTA vacutainers) between 10:00 and 12:00 AM from subjects asked to visit the clinic in the overnight-fasted state. Blood was kept on ice until plasma was prepared, aliquoted, and flash frozen. The blood draw occurred approximately 1–2 wk before bariatric surgery. Most subjects were compliant with the request for overnight fasting, but this was not uniform and eight less-compliant participants ate light breakfasts or had coffee several hours before the blood draw, as self reported (each item represents 1 subject with time of intake as available): coffee and creamer; sausage muffin (6:00); yogurt (7:15); sausage/egg muffin (5:40); and cereal with skim milk, cereal with 2% milk, a protein drink and decaffeinated coffee, and a protein shake (5:30). Thus these eight individuals are considered to have been in the postabsorptive condition rather than overnight fasted. To evaluate the impact of the preparatory phase for bariatric surgery, in which subjects had undergone a gut preparation protocol: subjects underwent a 2-day clear liquid diet (i.e., broths, gelatin foods, etc.) and for gastric bypass patients a bowel preparation ~24 h before surgery with GoLYTELY (polyethylene glycol-electrolyte solution; Braintree Laboratories, Braintree, MA) followed by three doses of neomycin (1 g each). No subjects reported to have been noncompliant with these protocols.
The morning of surgery, blood was collected from the intravenous catheter placed just before surgery in the nonanesthetized state. Just before surgery, enoxaparin (40 mg sc) and midazolam were administered for prophylaxis against venous thromboembolism and to help the patient relax, respectively. Induction of general anesthesia was accomplished with propofol (1.0–2.5 mg/kg; Fresenius Kabi, Lake Zurich, IL) with/without the adjunct of lidocaine (0.2–1.5 mg/kg) and paralytic agents such as rocuronium (0.6 mg/kg), vecuronium (0.05–0.1 mg/kg), or succinylcholine (0.3–1.1 mg/kg). Maintenance anesthesia was sustained with desflurane (2.5–8.5%) or sevoflurane (0.5–3%). In the operating room after induction, a second intravenous dose was started (a total of ~2 liters of Ringer lactate solution is typically given to the patient in the course of the surgery). The patients were also given dexamethasone (10 mg iv) for nausea control. Local anesthesia (0.25% bupivicaine) is typically introduced into areas where incisions are made for port placement. After surgery and skin closure, the incision sites were infiltrated with additional local anesthesia. Soon after anesthesia onset (approximately 10–30 min postonset), and before incision, a third blood sample was collected. Attempts were made at all phases of the blood draw procedures to utilize consistent timings and conditions, with the caveat that the study was conducted in a medical clinic setting, making exact timing of draws impossible for all patients.
Plasma acylcarnitine Analysis
The details of the high-performance liquid chromatography-mass spectrometry (HPLC-MS) acylcarnitine metabolite detection and analysis are presented elsewhere (16) (15). Briefly, salts and proteins were precipitated from 10–20 μl of sample (plus internal standards) through addition of organic solvents. The supernatant was applied to a mixed-mode, reversed-phase/strong cation-exchange solid-phase extraction plate (Oasis MCX; Waters, Milford, MA). Carnitine and acylcarnitines were eluted, evaporated, derivatized with pentafluorophenacyl trifluoromethanesulfonate, and injected into an ultra-HPLC-MS/MS instrument. With the use of sequential ion-exchange/reversed-phase chromatography, carnitine and γ-butyrobetaine were eluted in 4-min chromatograms; acylcarnitines were eluted in 14-min chromatograms; and optimized MRM transitions were collected for 66 acylcarnitine species and 12 acylcarnitine internal standards. Calibrants were used to construct 13-point calibration curves for absolute quantification. Low-, medium-, and high-quality control samples were used to validate that the analytic accuracy of each determination was within ±15% of the actual value for medium and high values and within ±20% for the lowest values. The limit-of-quantitation (LOQ) was 0.025 µM. In the current analysis, acylcarnitines displaying ≥0.025 µM and that were detected in at least 70% of the samples were used for data analysis.
Statistical Analysis
All statistical analyses, figures, and tables were conducted in R (version 3.1.2, http://www.r-project.org/). Group differences comparing conditions A, B, and C were examined using one-way repeated-measures ANOVA on rank-transformed acyl carnitine data with group (conditions A, B, and C) as the within-subject factor. Pairwise group comparisons were done using the Wilcoxon signed rank test, a nonparametric test to compare repeatedly measured samples. Multiple comparison adjustments were made by the Benjamini-Hochberg method with statistical significance set at an adjusted P < 0.05.
Data preprocessing for multivariate analysis.
Data were first filtered for metabolites that were present with at least 70% of values above the mass spectrometric LOQ of 0.025 µM. Any missing values of the metabolites that passed the screening were imputed with half of the LOQ values. Samples were detected as outliers if they were far outside the 95% confidence ellipse (Hotelling’s T2) in a multivariate principal component analysis plot. A total of 24 metabolites and all 17 samples passed the filtration criteria and are presented in results. To standardize across the wide range of absolute concentration data of the metabolite variables in the models, the data were centered around zero and scaled to unit variance before multivariate analysis.
Partial least squares-discriminant analysis.
Partial least squares-discriminant analysis (PLSDA), a supervised method of pattern recognition, was used to identify carnitine parameters that were significantly associated with group differences, viz. those metabolites that discriminate groups from one another. The optimum number of latent components for each PLSDA model was selected based on minimization of the cross-validated error estimates. The quality of models was validated by determining two parameters: R2 (goodness of fit) and Q2 cumulated (goodness of prediction); the latter was calculated through sevenfold internal cross validation. In all comparisons, the optimum models were further validated by repeated permutations (n = 100) of the sample identifiers, and a reference distribution of all R2 and Q2 values from the permuted PLSDA models was vetted against the actual model. Three PLSDA models with the best predictive capabilities were thus built, comparing 1) conditions A vs. B vs. C for overall group comparisons (model 1); 2) conditions A vs. B, examining the impact of fasting and presurgery gut preparations (model 2); and 3) conditions B vs. C, examining the effect of anesthesia (model 3). For each model, the major latent variables were represented in a scores scatterplot and the significant predictors were identified by their contribution to the PLS vectors in the loadings scatterplot, as well as based on their variable importance on projection (VIP) scores. Variables with a VIP value >1 are considered important for group discrimination in the predictive models (5). For model 1, an optimum two-component model captured ~57.3% of the variation in the predictor variables that correlated with ~44.4% (R2) of variation in the three groups, with a Q2 value of 38.7%. For model 2, the model had R2 and Q2 values of 57.6 and 58.8%, respectively, while for model 3, the R2 and Q2 values were 63.4 and 23.6%, respectively.
RESULTS
Participant Characteristics
Subject information and relevant clinical parameters are provided in Table 1.
Table 1.
Clinical characteristics of the obese female participants at the “preop” clinical visit approximately 1–2 wk before undergoing bariatric surgery
| Parameter | |
|---|---|
| Age, yr (range) | 41 ± 2 (21–53) |
| Body mass index, kg/m2 | 43.2 ± 1.8 |
| Plasma glucose, mg/dl | 93.4 ± 3.8 |
| Serum insulin (total), µU/ml | 42 ± 10 |
| QUICKI | 0.296 ± 0.008 |
| Serum triglycerides, mg/dl | 130 ± 17 |
| Total cholesterol, mg/dl | 194 ± 8 |
| HDL cholesterol, mg/dl | 49 ± 2 |
| LDL cholesterol, mg/dl | 117 ± 5 |
| Systolic blood pressure, mmHg | 133 ± 3 |
| Diastolic BP, mmHg | 79 ± 2 |
| Serum ALT, IU/l | 26 ± 2 |
| Serum AST, IU | 23 ± 2 |
| Steatosis score, % (range) | 18 ± 4 (5–60) |
Data are means ± SE; n = 17. Clinical values are in the overnight-fasted/postabsorptive state (see research design and methods). QUICKI, quantitative insulin sensitivity check index, calculated as 1/[log (insulin μU/ml) + log (glucose mg/dl)]. Blinded clinical pathology score was derived from histology of surgical liver biopsy (0 = <5%; 1 = mild, 5–33% steatosis; 2 = moderate, 34–66%; and 3 = severe, >66%).
Plasma Acylcarnitine Patterns
Univariate statistical analyses were conducted to determine whether there were significant differences in the plasma concentrations of specific carnitine-related factors across conditions A (overnight-fasted/postabsorptive state), B (following gut preparation, just before anesthesia on surgery day), or C (following condition B, postanesthesia onset). As shown in Table 2, there was a significant overall group effect for all detectable carnitine metabolites, meaning that the metabolites differed between at least two of the conditions. With the presurgical gut preparation phase (comparing condition B against A), many acylcarnitine species of short-chain (acetylcarnitine, R-3-hydroxybutyrylcarnitine), medium-chain (i.e., hexanoylcarnitine (C6)), and long-chain length (≥C14; i.e., cis-5-tetradecenoylcarnitine (C14:1), cis,cis-5,8-tetradecadienoylcarnitine (C14:2), palmitoylcarnitine, etc.) were significantly increased. With condition B, there were lower plasma concentrations of free carnitine, isobutyrylcarnitine (valine catabolism derivative), propionylcarnitine (potential downstream metabolite derivative of valine and isoleucine catabolism), and 2-methyl-butyrylcarnitine (isoleucine catabolism derivative), although the reduction in the latter was not statistically significant. With anesthesia, comparing condition C against B, there were further reductions in free carnitine and the branched-chain amino acid (BCAA) derivatives. Anesthesia also elicited significant reductions in the plasma concentrations of many of the short-, medium-, and long-chain acylcarnitines.
Table 2.
Univariate statistics comparing group and binary group differences in plasma carnitine metabolite concentrations in obese women during perisurgical phases of bariatric surgery
| Metabolite | Condition A | Condition B | Condition C | %Difference B vs. A | %Difference C vs. B | Group Effect* | A vs. B† | B vs. C† |
|---|---|---|---|---|---|---|---|---|
| R-3-hydroxy-butyrylcarnitine | 0.055 ± 0.016 | 0.192 ± 0.038 | 0.212 ± 0.046 | 246 | 11 | 0.055639 | 0.002014 | 0.050537 |
| cis-5-tetradecenoylcarnitine | 0.051 ± 0.005 | 0.135 ± 0.019 | 0.082 ± 0.011 | 166 | −39 | 0.001366 | 0.000183 | 0.000466 |
| cis,cis-5,8-tetradecadienoylcarnitine | 0.046 ± 0.004 | 0.095 ± 0.013 | 0.056 ± 0.006 | 105 | −41 | 0.000627 | 0.000183 | 0.000497 |
| Acetylcarnitine | 7.71 ± 0.88 | 14.79 ± 1.51 | 13.62 ± 1.50 | 92 | −8 | 0.014246 | 0.000183 | 0.017142 |
| Oleoylcarnitine | 0.102 ± 0.007 | 0.178 ± 0.014 | 0.138 ± 0.009 | 74 | −23 | 0.000298 | 0.000183 | 0.000497 |
| Lauroylcarnitine | 0.042 ± 0.004 | 0.067 ± 0.008 | 0.038 ± 0.003 | 60 | −43 | 0.000090 | 0.002014 | 0.000122 |
| Isovalerylcarnitine | 0.062 ± 0.01 | 0.095 ± 0.011 | 0.068 ± 0.009 | 53 | −28 | 0.034277 | 0.034836 | 0.021989 |
| Palmitoylcarnitine | 0.069 ± 0.003 | 0.103 ± 0.004 | 0.088 ± 0.004 | 49 | −15 | 0.000500 | 0.000183 | 0.000497 |
| Butyrylcarnitine | 0.098 ± 0.025 | 0.147 ± 0.066 | 0.092 ± 0.028 | 49 | −38 | 0.000071 | NS | 0.000105 |
| Linoleoylcarnitine | 0.058 ± 0.004 | 0.086 ± 0.008 | 0.067 ± 0.005 | 48 | −22 | 0.001312 | 0.000183 | 0.002951 |
| Hexanoylcarnitine | 0.036 ± 0.004 | 0.053 ± 0.006 | 0.035 ± 0.003 | 46 | −35 | 0.000018 | 0.001726 | 0.000092 |
| S-3-hydroxy-octanoylcarnitine | 0.049 ± 0.006 | 0.065 ± 0.006 | 0.046 ± 0.005 | 34 | −30 | 0.000209 | 0.002014 | 0.000105 |
| S-3-hydroxy-decanoylcarnitine | 0.040 ± 0.004 | 0.052 ± 0.005 | 0.034 ± 0.003 | 29 | −34 | 0.000441 | 0.034555 | 0.000366 |
| Octanoylcarnitine | 0.106 ± 0.01 | 0.126 ± 0.017 | 0.068 ± 0.005 | 19 | −46 | 0.000003 | NS | 0.000092 |
| cis-3,4-methylene-heptanoylcarnitine | 0.252 ± 0.019 | 0.294 ± 0.032 | 0.211 ± 0.023 | 17 | −28 | 0.000022 | NS | 0.000105 |
| Decanoylcarnitine | 0.194 ± 0.020 | 0.218 ± 0.031 | 0.104 ± 0.007 | 12 | −52 | 0.000005 | NS | 0.000092 |
| Total carnitine | 50.4 ± 2.6 | 55.5 ± 3.2 | 50.6 ± 2.8 | 10 | −9 | 0.000209 | 0.015808 | 0.000122 |
| Glutaroylcarnitine | 0.035 ± 0.003 | 0.039 ± 0.003 | 0.033 ± 0.003 | 9 | −14 | 0.035692 | NS | 0.036303 |
| Butyrobetaine | 0.661 ± 0.028 | 0.696 ± 0.046 | 0.597 ± 0.038 | 5 | −14 | 0.001312 | NS | 0.001602 |
| cis-4-decenoylcarnitine | 0.092 ± 0.008 | 0.093 ± 0.011 | 0.051 ± 0.004 | 1 | −46 | 0.000002 | NS | 0.000092 |
| Free carnitine | 38.9 ± 2.0 | 35.1 ± 1.9 | 33.1 ± 1.7 | −10 | −6 | 0.040898 | 0.020282 | 0.011796 |
| 2-methyl-butyrylcarnitine | 0.058 ± 0.003 | 0.051 ± 0.004 | 0.046 ± 0.004 | −12 | −10 | 0.005365 | NS | 0.000806 |
| Propionylcarnitine | 0.284 ± 0.016 | 0.215 ± 0.018 | 0.175 ± 0.016 | −24 | −18 | 0.038401 | 0.034836 | 0.003438 |
| Isobutyrylcarnitine | 0.071 ± 0.009 | 0.041 ± 0.004 | 0.037 ± 0.005 | −42 | −9 | 0.040898 | 0.006891 | 0.015436 |
Values are means ± SE. Concentrations are in µM.
Repeated-measures ANOVA on rank-transformed data, multiple comparison adjustments by Benjamini-Hochberg (BH) method.
Pairwise comparisons by Wilcoxon Sign Rank Test; P value adjusted by BH method; NS, not significant.
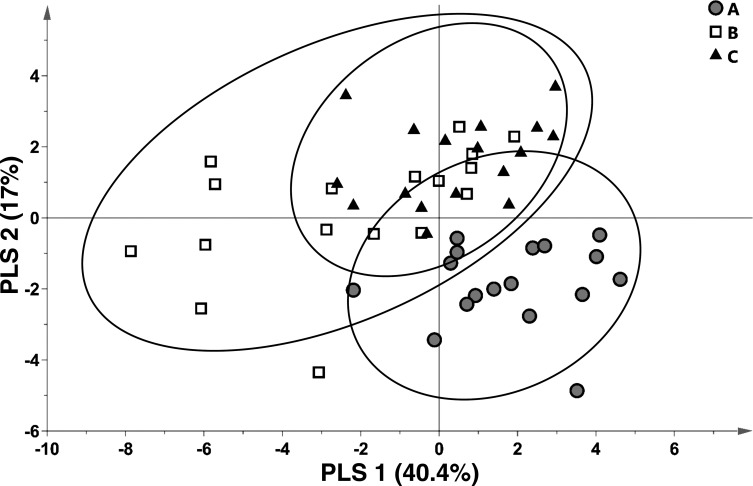
A PLSDA model (model 1) was generated that leveraged differences in within-subject carnitine metabolite concentrations across all conditions as an initial assessment of group separation or similarities. As shown in the scores plot of all the subjects (Fig. 1), variance in plasma acylcarnitine concentrations tended to discriminate condition A from conditions B and C: the latter two conditions appeared more similar to one another in that subject groupings overlapped substantially (Fig. 1). Notably, variance in the distribution of condition B subjects was greater than the other groups, as evidenced by visual inspection of the 95% confidence interval oval sizes (Fig. 1); the group variance of condition B differed from that of conditions A and C significantly (ANOVA P < 0.05) on both components 1 and 2 of the PLSDA model.
Fig. 1.
Variances in select plasma carnitine metabolites discriminate bariatric surgery patients at the preoperative overnight fasted/postabsorptive phase (condition A) from the state just before surgery in the nonanesthetized state following gut preparation (condition B), and just after surgical anesthesia induction (condition C). Depicted is a partial least squares-discriminant analysis (PLSDA; model 1) scores plot; scores from individual participants are indicated as symbols. Confidence regions of group clusters are presented as 95% confidence ellipses based on Hotelling’s T2 statistic.
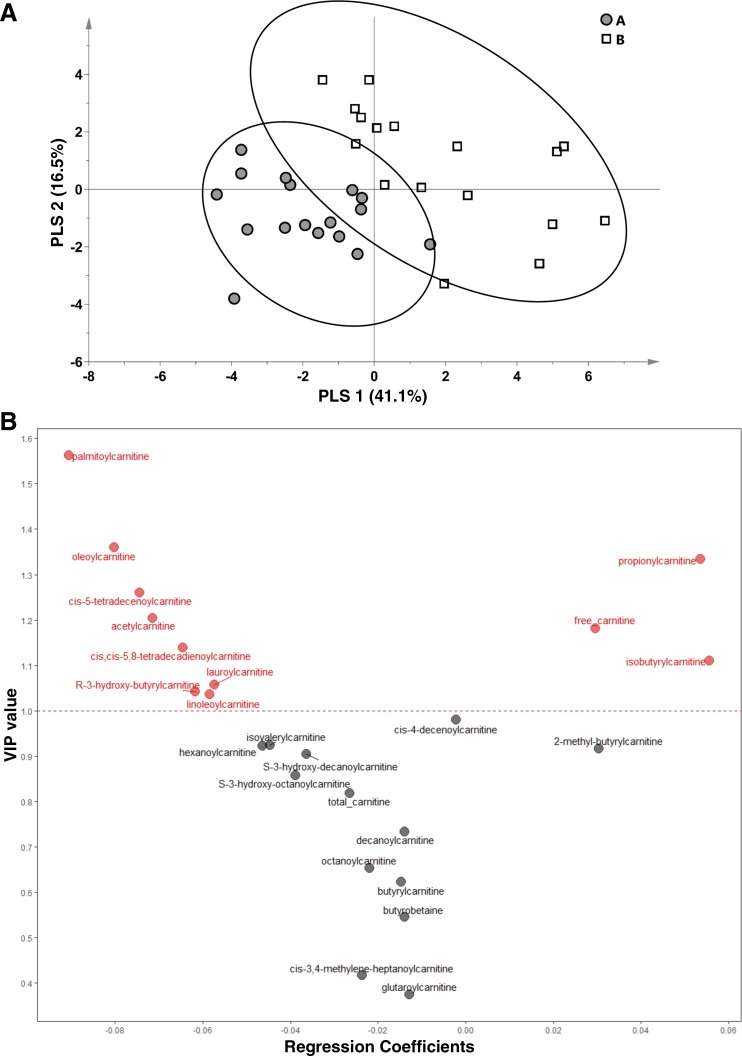
To identify the specific acylcarnitine factors that discriminated the disparate physiological states of conditions A and B, a binary group model was generated (model 2). A full summary of variables included in model 2 is provided in Table 2. Differences in distinct metabolites in this model should reflect metabolic changes due to the preoperative gut preparation phase (i.e., changes due to negative energy balance, GI tract evacuation, and/or gut microbiome shifts). Model 2 successfully discriminated subjects based on condition (Fig. 2A, scores plot). The key variables that drove separation of the groups are depicted in Fig. 2B (VIP-regression plot). This analysis indicates that the surgery gut preparation phase led to higher plasma long-chain fatty acylcarnitine and acetylcarnitine concentrations that contributed to differentiating conditions A and B (Fig. 2B, leftward variables with VIP >1). This contrasts with free carnitine, propionylcarnitine, and isobutyrylcarnitine concentrations, which were higher in condition A relative to condition B and served as discriminating variables in the model (Fig. 2B, rightward variables with VIP >1). Two additional metabolites associated with BCAA catabolism were included in the model but with lower VIPs: they displayed less robust impact on group discrimination compared with isobutyrylcarnitine. For instance, higher 2-methyl-butyrylcarnitine concentrations were associated with condition A, contrasting with higher isovalerylcarnitine in group B (Fig. 2B; see also Table 2). The similar directionality of isobutrylcarnitine and 2-methyl-butyrylcarnitine suggest shared regulation of valine and isoleucine metabolism, respectively, but the opposite pattern of isovalerylcarnitine (a leucine catabolism derivative) highlights differential effects of these conditions on BCAA metabolism.
Fig. 2.
Effect of prebariatric surgery gut preparation and coincident negative energy balance on plasma acylcarnitines in obese women. Variances in select plasma carnitine metabolites in obese women (n = 17) undergoing bariatric surgery discriminate the overnight fasted/postabsorptive state at preop examination ca. 1–2 wk before surgery (condition A) and just before surgery in the nonanesthetized state following 48 h gut preparation (condition B). A: scores plot from a PLSDA (model 2) comparing the 2 conditions: scores from individual participants are indicated as symbols. Confidence regions of group clusters are presented as 95% confidence ellipses based on Hotelling’s T2 statistic. B: PLSDA variable importance on projection (VIP) values for metabolites included in the model, plotted against regression coefficient projections of metabolite factors derived from PLS Latent Variable 1 (x-axis of A). Variables with a VIP >1 (above the dashed line) are considered to be the most robust discriminating variables in model 2. Metabolites with positive regression coefficients (rightward) were higher in condition A (thus lower in condition B), and factors with negative regression coefficients (leftward) were lower in condition A (thus higher in condition B).
With respect to markers of mitochondrial FAO, large increases in plasma R-3-hydroxy-butyrylcarnitine, cis-5-tetradecenoylcarnitine (C14:1 carnitine), and cis,cis-5,8-tetradecadienoylcarnitine (C14:2 carnitine) following the gut preparation state of condition B contributed to the discrimination of the groups (Fig. 2B, leftward variables with VIP >1). Several medium-chain acylcarnitines also typically associated with accelerated FAO (i.e., octanoylcarnitine and cis-3,4-methylene-heptanoylcarnitine) tended to be higher in condition B (see Fig. 2B; also see Table 2). However, these differences were not statistically significant and their VIP scores in PLSDA modeling were not robust in terms of serving as discriminant variables for condition B.
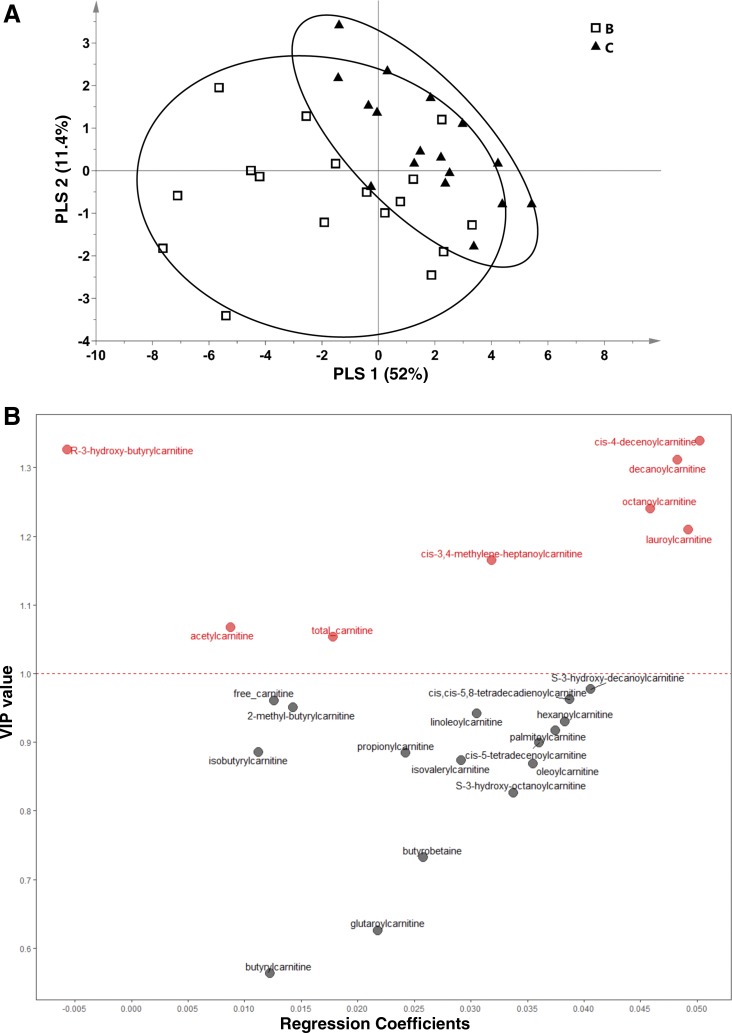
When one considers metabolomics approaches in surgical paradigms, it may be necessary in some studies to take anesthesia and other surgery-specific variables into account when interpreting results. With this in mind, we assessed plasma concentrations of acylcarnitines reflecting mitochondrial fat oxidation for effect of anesthesia. Using a binary comparison approach, PLSDA model 3 successfully discriminated conditions B and C from one another (Fig. 3A, scores plot). As illustrated by the VIP-regression plot for model 3, anesthesia was associated with a marked reduction in many carnitine metabolites: this was particularly robust for select medium-chain acylcarnitines, hydroxy-acylcarnitines, long-chain acylcarnitines, acetylcarnitine, and free carnitine (Table 2; Fig. 3B, rightward variables with VIP >1 were higher in nonanesthetized condition). One metabolite that was significantly increased in condition B relative to condition A was R-3-hydroxy-butyrylcarnitine (see Fig. 2B, top leftward variables), which was modestly increased compared with condition B (Fig. 3B and Table 2). Several BCAA-related metabolites (isobutyrylcarnitine, 2-methyl-butyrylcarnitine, and isovalerylcarnitine) were reduced in the anesthetized state (Fig. 3B, rightward variable; also see Table 2), but their overall impact as discriminating variables in the model was not robust (VIP scores <1).
Fig. 3.
Effect of acute anesthesia just before bariatric surgery on plasma acylcarnitines in obese women. Variances in select plasma carnitine metabolites in obese women (n = 17) undergoing bariatric surgery discriminate the nonanesthetized state following 48 h gut preparation and just before surgery (condition B) from the acutely anesthetized state just before commencement of surgery (condition C). A: scores plot from a PLSDA (model 3) comparing the 2 conditions: scores from individual participants are indicated as symbols. Confidence regions of group clusters are presented as 95% confidence ellipses based on Hotelling’s T2 statistic. B: PLSDA VIP values for metabolites included in the model, plotted against regression coefficients projections of metabolite factors derived from PLS latent variable 1 (x-axis of A). Variables with a VIP >1 (above the dashed line) are considered to be the most robust discriminating variables in model 2. Metabolites with a positive regression coefficient (rightward) were higher in condition B (thus lower in condition C), and R-hydroxy-butyrylcarnitine with negative regression coefficient (leftward) was higher in condition C (vs. condition B).
DISCUSSION
A targeted plasma metabolomics approach was used to characterize changes in metabolism in the preoperative and perioperative phases of bariatric surgery in obese women. The periods leading up to and following bariatric surgery offer a unique opportunity for application of metabolomics tools to interrogate metabolism, since significant shifts in host metabolic status take place during this time. Presumably, the changing conditions around and following bariatric surgery also impact gut microbe ecology and xenometabolism, as well. To our knowledge, this paper is the first report of comprehensive metabolite patterns during the perioperative time frame of bariatric surgery. Our overarching aim using metabolomics tools with this model is to explore two primary concepts. First, the gut preparation phase leading up to surgery engages a fasting-like response in terms of fuel metabolism and concurrently reduces circulating xenometabolite markers of gut microbe metabolism. The latter is premised on the fact that during this time there is a marked reduction in calorie intake and solid foods, and in some cases gut evacuation takes place, which by definition should result in loss of intestinal microbial mass. Second, anesthesia acutely attenuates FAO and increases reliance on nonlipid fuel sources. We found that comprehensive acylcarnitine profiles were consistent with major shifts in fatty acid and BCAA metabolism following presurgical bowel preparation, and with significant anesthesia-associated changes in fatty acid and/or carnitine metabolism. The impact of pre- and perisurgical conditions on xenometabolism remains to be further clarified and awaits application of other analytic platforms in this model.
It was expected that the lead-in to bariatric surgery would activate a fasting-like response in terms of lipolysis and engagement of mitochondrial long-chain fatty acid (LCFA) metabolism. To prepare the GI tract for surgery, patients undergoing Roux-en-Y (in our case, 11 subjects) have a “bowel prep” protocol that couples low energy intake from clear fluids or gelatin for 48 h, with gut evacuation (oral polyethylene glycol) commencing ~24-h before surgery. Patients who opt for a gastric sleeve protocol (in our case, 6 subjects) or gastric banding (in our case, 1 subject) must limit caloric intake to clear liquids without pulp or fiber for the 24-h period leading up to hospital check-in (but without the gut evacuation protocol). Consistent with a fasting metabolic phenotype at the immediate presurgery nonanesthetized time point (condition B), a suite of plasma long-chain fatty acylcarnitines displayed higher plasma concentrations compared with the previous preop examination; at the preop visit, subjects were in the overnight-fasted/postabsorptive state and not a bona fide fasting state. The metabolite pattern of the immediate presurgery nonanesthetized state most likely reflects active lipolysis and subsequent conversion of LCFAs to their acylcarnitine moieties by mitochondrial carnitine palmitoyltransferase-1. Heavier engagement of mitochondrial β-oxidation following the gut preparation phase was further evidenced by higher plasma concentrations of chain-shortened fatty acylcarnitines including C6–C10-acylcarnitines, C14:1 and C14:2 acylcarnitines, and cis-3,4-methylene-heptanoylcarnitine. Such patterns for long-chain and medium-chain acylcarnitines, along with acetylcarnitine, are typical of conditions in which fat catabolism is engaged: e.g., the fasted state (i.e., Refs 4, 9, 11) or during exercise (i.e., Refs. 26, 28). Enhanced β-oxidation carries with it an inherent inefficiency in terms of full combustion of LCFA-derived acetyl-CoA to CO2. Thus it follows that pools of chain-shortened CoA-metabolites naturally accumulate during active β-oxidation (13, 24). This, in turn, promotes conversion of a fraction of CoA-metabolites to their respective carnitine derivatives through the actions of carnitine acyltransferases (reviewed in Ref. 14).
Studying the presurgical gut preparation state in bariatric patients affords a unique opportunity to identify blood metabolites that emanate wholly or in part from gut microbes. This may be especially true with gut evacuation protocols, in which xenometabolism should be dramatically altered by not only dietary restriction but also loss of gut contents and bacterial load. Since the current report is limited to an acylcarnitine analytic platform, and since acylcarnitines are synthesized by the host, it is difficult to provide a full consideration of this question from the data presented herein. Notably, there were no distinct acylcarnitine pattern differences across the gut preparation protocols that differed in degree of evacuation. While speculative, this may suggest that significant negative energy balance and fasting mask some blood xenometabolite patterns that would have manifested otherwise across the gut preparation protocols. We did observe that plasma cis-3,4-methylene-heptanoylcarnitine concentrations were increased by the negative energy balance of the presurgery state condition B. This metabolite derives from cis-3,4-methylene-heptanoyl-CoA, whose origins remain to be determined; however, a reasonable hypothesis is that it derives form partial β-oxidative catabolism of a methylene-containing LCFA parent xenometabolite (27). Considering that plasma cis-3,4-methylene-heptanoylcarnitine closely tracks other medium-chain fatty acylcarnitines during the dynamic phase of acute exercise (28), we speculate that its abundance in blood is largely driven by incomplete β-oxidation of stored methylene-containing LCFAs that are released via lipolysis. With this in mind, the possibility that bariatric surgery preparation (and concurrent changes to the GI tract environment) impacts GI and blood xenometabolite profiles remains an open question, pending application of broader analytic platforms to this or other bariatric study cohorts.
Our studies also sought to characterize how anesthesia itself influences metabolic physiology. A few studies have addressed this question in humans under controlled conditions during a surgical event (i.e., Refs. 17, 19, 21); reports examining the transition from the nonanesthetized state into the anesthetized state are even more rare (21). Based on case reports of dyslipidemia in some uncommon incidents of propofol infusion syndrome, and a limited number of studies in isolated rat liver mitochondria or perfused guinea pig hearts, it has been suggested that propofol anesthesia inhibits FAO and mitochondrial oxidative phosphorylation (10, 22, and references therein). Sevoflurane (2%) reduced FAO in a perfused heart model (23). Thus we originally hypothesized that anesthesia in the bariatric patients herein would elicit reductions in acylcarnitine markers of mitochondrial LCFA catabolism pathways, including long-chain acylcarnitines, medium-chain acylcarnitines, and hydroxy-acylcarnitines. This was, in fact, the case, and could represent a shift away from FAO due to propofol and/or the flurane-based anesthetics. However, a further consideration of the literature, looking beyond observational case reports and anesthesia responses in isolated tissue, tempers the viewpoint that the plasma acylcarnitine patterns we report are truly reflecting anesthesia-associated inhibition of FAO. First, propofol infusion syndrome case reports describe pathophysiology and metabolic perturbations in many physiological systems, without dyslipidemia as a defining feature (see Refs. 10, 22). Second, in the few studies in which respiratory quotient and fat oxidation estimates were made temporally during surgical procedures that employed propofol, sevoflurane, and/or midazolam, fat combustion was steady or increased over time, and the respiratory quotient remained near 0.7 (17, 19). Based on these considerations, we favor the perspective that propofol and sevoflurane, as used clinically, do not drive a wholesale inhibition of FAO. While we cannot exclude that mitochondrial FAO was reduced due to anesthesia in the cohort we studied, it cannot be excluded that the major shifts in plasma fatty acylcarnitines and free carnitine postanesthesia also reflect changes in carnitine metabolism, including differential net partitioning into disparate tissue pools including blood, liver, and muscle; e.g., evidence for this stems from studies in animal models in which injectable or inhaled anesthetics were administered acutely, leading to significant changes in acylated carnitines in liver (generally reduced; Ref. 3), muscle (generally reduced; Refs. 11, 18), and heart (generally decreased; Ref. 18). A comparison of various anesthetics and CO2/cervical dislocation in rats revealed anesthetic-specific patterns of increased or decreased heart and liver acylcarnitines (20). While a single-sample metabolomics assessment of the blood pool is a valuable snapshot of the metabolic landscape, our interpretations are, by definition, limited, especially without concurrent assessments of calorimetry or fuel kinetics. A further caveat to interpretation of anesthesia conditions that include propofol is that this drug is infused as part of a lipid/glycerol emulsion that could itself impact fat metabolism. An additional consideration for surgery metabolism studies include other intravenous infusion components (i.e., Ringer lactate) that may contribute, over time, to changes in blood metabolite pools and fuel fluxes. Future studies are warranted that track metabolite flux, temporal changes in fuel oxidation, and tissue-level carnitine/acylcarnitine metabolism in response to anesthesia in humans.
In addition to markers of fat metabolism, we observed both presurgery fasting/gut preparation and anesthesia-associated alterations in plasma metabolites associated with mitochondrial BCAA catabolism. For instance, lower plasma isobutyrylcarnitine and propionylcarnitine were discriminating features of the immediate presurgical, nonanesthetized condition. Isobutyryl-CoA is a valine metabolite downstream of the mitochondrial branched-chain ketoacid dehydrogenase complex (BCKDC), and propionyl-CoA can be derived from further intramitochondrial catabolism of isobutyryl-CoA among other sources. Thus the isobutyryl- and propionylcarnitine results are consistent with the hypothesis that the fasting-like state of preoperative gut preparation led to attenuated tissue valine catabolism through inhibition of BCKDC. Notably, concentration of plasma 2-methyl-butyrylcarnitine (a derivative of the isoleucine metabolite 2-methyl-butyryl-CoA that is downstream of BCKDC) was also reduced, albeit not statistically significantly. The BCKDC and mitochondrial BCAA oxidation can be inhibited by engagement of FAO (i.e., in liver; Ref. 6), probably through mechanisms involving dehydrogenase inhibition by increased intramitochondrial NADH/NAD+ and perhaps acetyl-CoA (see Ref. 1). However, the proposal that the presurgical, fasting-like state dampens the BCKDC is confounded by the observation that isovalerylcarnitine (a derivative of the leucine metabolite isovaleryl-CoA downstream of BCKDC) was actually higher under these conditions. Notably, with anesthesia, the pattern of plasma isovalerylcarnitine also seemed to differ compared with isobutyrylcarnitine or 2-methyl-butyrylcarnitine. Taken together, this raises the possibility that there are differing modes of regulation of BCAA catabolism. As for the effect of anesthesia on human BCAA metabolism, only one report by Shricker et al. (21) was identified; propofol had modest effects on leucine (13C-labeled α-ketoisocaproate) turnover (significant 6% drop) and oxidation (14% reduction, not statistically significant). The current report, highlighting fasting- and anesthesia-associated changes in blood acylcarnitine BCAA derivatives, provides a framework for future experiments that more specifically address how these conditions impact BCAA fluxes and regulate tissue partitioning of BCAA metabolites into oxidative vs. anabolic pathways. The results also point to a need for better understanding of how metabolic status regulates BCAA catabolic enzymes downstream from the BCKDC.
In conclusion, targeted, quantitative metabolomics tools were used for plasma to assess the metabolic landscape of the perisurgical period in obese women undergoing bariatric surgery. The results highlight that metabolism of fats and amino acids, and possibly carnitine/acylcarnitine transport and fluxes, are significantly impacted by gut preparation/fasting and anesthesia. A strength of this study was the use of a repeated-measures design in a well-characterized cohort of human subjects and the use of a very comprehensive metabolite screen to interrogate various pathways simultaneously. There are limitations in that a larger sample size may have strengthened our ability to detect additional metabolites that differed among conditions but did not contribute to discrimination of the groups using multivariate statistical modeling. Furthermore, we sampled only the blood pool at single time points, and thus could not ascertain changes in metabolite profiles in specific tissues or the rapidity of change or fluxes of metabolic pathways. Future studies designed to explore these questions more specifically are warranted, considering the quite significant fasting- and anesthesia-associated shifts in metabolism that were observed.
GRANTS
This research was supported in part by intramural US Department of Agriculture-Agricultural Research Service Projects 5306-51530-019-00 and 6026-51000-010-05S and National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-078328 (to S. H. Adams).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.A., W.H.S., and S.H.A. conceived and designed research; M.A. and W.H.S. performed experiments; S.H.A. interpreted results of experiments; S.H.A. prepared figures; S.H.A. drafted manuscript S.B., P.E.M., M.S.S., C.L.H., and S.H.A. analyzed data; S.B., M.A., and S.H.A. edited and revised manuscript; S.B., M.A., W.H.S., P.E.M., M.S.S., C.L.H., and S.H.A. approved final version of manuscript.
REFERENCES
- 1.Adams SH. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv Nutr 2: 445–456, 2011. doi: 10.3945/an.111.000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, Hwang DH, Newman JW, Garvey WT. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr 139: 1073–1081, 2009. doi: 10.3945/jn.108.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brass EP, Hoppel CL. In vivo studies of carnitine and fatty acid metabolism: problems with use of anesthetics. Anal Biochem 110: 77–81, 1981. doi: 10.1016/0003-2697(81)90114-7. [DOI] [PubMed] [Google Scholar]
- 4.Burrage LC, Miller MJ, Wong LJ, Kennedy AD, Sutton VR, Sun Q, Elsea SH, Graham BH. Elevations of C14:1 and C14:2 plasma acylcarnitines in fasted children: a diagnostic dilemma. J Pediatr 169: 208–13.e2, 2016. doi: 10.1016/j.jpeds.2015.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chong IG, Jun CH. Performance of some variable selection methods when multicollinearity is present. Chemometr Intell Lab 78: 103–112, 2005. doi: 10.1016/j.chemolab.2004.12.011. [DOI] [Google Scholar]
- 6.Corkey BE, Martin-Requero A, Walajtys-Rode E, Williams RJ, Williamson JR. Regulation of the branched chain alpha-ketoacid pathway in liver. J Biol Chem 257: 9668–9676, 1982. [PubMed] [Google Scholar]
- 7.Cray SH, Robinson BH, Cox PN. Lactic acidemia and bradyarrhythmia in a child sedated with propofol. Crit Care Med 26: 2087–2092, 1998. doi: 10.1097/00003246-199812000-00046. [DOI] [PubMed] [Google Scholar]
- 8.De Cosmo G, Congedo E, Clemente A, Aceto P. Sedation in PACU: the role of propofol. Curr Drug Targets 6: 741–744, 2005. doi: 10.2174/138945005774574425. [DOI] [PubMed] [Google Scholar]
- 9.Hoppel CL, Genuth SM. Carnitine metabolism in normal-weight and obese human subjects during fasting. Am J Physiol Endocrinol Metab 238: E409–E415, 1980. [DOI] [PubMed] [Google Scholar]
- 10.Kam PC, Cardone D. Propofol infusion syndrome. Anaesthesia 62: 690–701, 2007. doi: 10.1111/j.1365-2044.2007.05055.x. [DOI] [PubMed] [Google Scholar]
- 11.Kerner J, Bieber LL. The effect of electrical stimulation, fasting and anesthesia on the carnitine(s) and acyl-carnitines of rat white and red skeletal muscle fibres. Comp Biochem Physiol B 75: 311–316, 1983. doi: 10.1016/0305-0491(83)90331-0. [DOI] [PubMed] [Google Scholar]
- 12.Kneiseler G, Bachmann HS, Bechmann LP, Dechene A, Heyer T, Baba H, Saner F, Jochum C, Gerken G, Canbay A. A rare case of propofol-induced acute liver failure and literature review. Case Rep Gastroenterol 4: 57–65, 2010. doi: 10.1159/000262448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopes-Cardozo M, Klazinga W, van den Bergh SG. Accumulation of carnitine esters of beta-oxidation intermediates during palmitate oxidation by rat-liver mitochondria. Eur J Biochem 83: 629–634, 1978. doi: 10.1111/j.1432-1033.1978.tb12132.x. [DOI] [PubMed] [Google Scholar]
- 14.McCoin CS, Knotts TA, Adams SH. Acylcarnitines–old actors auditioning for new roles in metabolic physiology. Nat Rev Endocrinol 11: 617–625, 2015. doi: 10.1038/nrendo.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minkler PE, Stoll MS, Ingalls ST, Kerner J, Hoppel CL. Quantitative acylcarnitine determination by UHPLC-MS/MS–going beyond tandem MS acylcarnitine “profiles”. Mol Genet Metab 116: 231–241, 2015. doi: 10.1016/j.ymgme.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minkler PE, Stoll MS, Ingalls ST, Kerner J, Hoppel CL. Validated method for the quantification of free and total carnitine, butyrobetaine, and acylcarnitines in biological samples. Anal Chem 87: 8994–9001, 2015. doi: 10.1021/acs.analchem.5b02198. [DOI] [PubMed] [Google Scholar]
- 17.Nagao Y, Tatara T, Fujita K, Sugi T, Kotani J, Hirose M. Protein sparing during general anesthesia with a propofol solution containing medium-chain triglycerides for gastrectomy: comparison with sevoflurane anesthesia. J Anesth 27: 359–365, 2013. doi: 10.1007/s00540-012-1546-8. [DOI] [PubMed] [Google Scholar]
- 18.Pessotto P, Liberati R, Petrella O, Hülsmann WC. Alteration of tissue carnitine content following anaesthesia with barbiturate and surgery in rat. Int J Clin Pharmacol Res 15: 191–199, 1995. [PubMed] [Google Scholar]
- 19.Pestaña D, García-de-Lorenzo A, Madero R. Metabolic pattern and lipid oxidation during abdominal surgery: midazolam versus propofol. Anesth Analg 83: 837–843, 1996. doi: 10.1213/00000539-199610000-00032. [DOI] [PubMed] [Google Scholar]
- 20.Petucci C, Rojas-Betancourt S, Gardell SJ. Comparison of tissue harvest protocols for the quantitation of acylcarnitines in mouse heart and liver by mass spectrometry. Metabolomics 8: 784–792, 2012. doi: 10.1007/s11306-011-0370-8. [DOI] [Google Scholar]
- 21.Schricker T, Klubien K, Carli F. The independent effect of propofol anesthesia on whole body protein metabolism in humans. Anesthesiology 90: 1636–1642, 1999. doi: 10.1097/00000542-199906000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Vasile B, Rasulo F, Candiani A, Latronico N. The pathophysiology of propofol infusion syndrome: a simple name for a complex syndrome. Intensive Care Med 29: 1417–1425, 2003. doi: 10.1007/s00134-003-1905-x. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Ko KW, Lucchinetti E, Zhang L, Troxler H, Hersberger M, Omar MA, Posse de Chaves EI, Lopaschuk GD, Clanachan AS, Zaugg M. Metabolic profiling of hearts exposed to sevoflurane and propofol reveals distinct regulation of fatty acid and glucose oxidation: CD36 and pyruvate dehydrogenase as key regulators in anesthetic-induced fuel shift. Anesthesiology 113: 541–551, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Watmough NJ, Turnbull DM, Sherratt HS, Bartlett K. Measurement of the acyl-CoA intermediates of beta-oxidation by h.p.l.c. with on-line radiochemical and photodiode-array detection. Application to the study of [U-14C]hexadecanoate oxidation by intact rat liver mitochondria. Biochem J 262: 261–269, 1989. doi: 10.1042/bj2620261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf A, Weir P, Segar P, Stone J, Shield J. Impaired fatty acid oxidation in propofol infusion syndrome. Lancet 357: 606–607, 2001. doi: 10.1016/S0140-6736(00)04064-2. [DOI] [PubMed] [Google Scholar]
- 26.Xu G, Hansen JS, Zhao XJ, Chen S, Hoene M, Wang XL, Clemmesen JO, Secher NH, Häring HU, Pedersen BK, Lehmann R, Weigert C, Plomgaard P. Liver and muscle contribute differently to the plasma acylcarnitine pool during fasting and exercise in humans. J Clin Endocrinol Metab 101: 5044–5052, 2016. doi: 10.1210/jc.2016-1859. [DOI] [PubMed] [Google Scholar]
- 27.Yang S, Minkler P, Hoppel C. cis-3,4-Methylene-heptanoylcarnitine: characterization and verification of the C8:1 acylcarnitine in human urine. J Chromatogr B Analyt Technol Biomed Life Sci 857: 251–258, 2007. doi: 10.1016/j.jchromb.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Light AR, Hoppel CL, Campbell C, Chandler CJ, Burnett DJ, Souza EC, Casazza GA, Hughen RW, Keim NL, Newman JW, Hunter GR, Fernandez JR, Garvey WT, Harper ME, Fiehn O, Adams SH. Acylcarnitines as markers of exercise-associated fuel partitioning, xenometabolism, and potential signals to muscle afferent neurons. Exp Physiol 102: 48–69, 2017. doi: 10.1113/EP086019. [DOI] [PMC free article] [PubMed] [Google Scholar]