Abstract
The soluble receptor for advanced glycation end products (sRAGE) may be protective against inflammation associated with obesity and type 2 diabetes (T2DM). The aim of this study was to determine the distribution of sRAGE isoforms and whether sRAGE isoforms are associated with risk of T2DM development in subjects spanning the glucose tolerance continuum. In this retrospective analysis, circulating total sRAGE and endogenous secretory RAGE (esRAGE) were quantified via ELISA, and cleaved RAGE (cRAGE) was calculated in 274 individuals stratified by glucose tolerance status (GTS) and obesity. Group differences were probed by ANOVA, and multivariate ordinal logistic regression was used to test the association between sRAGE isoform concentrations and the proportional odds of developing diabetes, vs. normal glucose tolerance (NGT) or impaired glucose tolerance (IGT). When stratified by GTS, total sRAGE, cRAGE, and esRAGE were all lower with IGT and T2DM, while the ratio of cRAGE to esRAGE (cRAGE:esRAGE) was only lower (P < 0.01) with T2DM compared with NGT. When stratified by GTS and obesity, cRAGE:esRAGE was higher with obesity and lower with IGT (P < 0.0001) compared with lean, NGT. In ordinal logistic regression models, greater total sRAGE (odds ratio, 0.91; P < 0.01) and cRAGE (odds ratio, 0.84; P < 0.01) were associated with lower proportional odds of developing T2DM. Reduced values of sRAGE isoforms observed with both obesity and IGT are independently associated with greater proportional odds of developing T2DM. The mechanisms by which each respective isoform contributes to obesity and insulin resistance may reveal novel treatment strategies for diabetes.
Keywords: receptor for advanced glycation end products, type 2 diabetes, obesity, insulin resistance, glucose tolerance
the study of advanced glycation end products (AGE) and their receptor (RAGE) has maintained scientific interest over the past several decades given evidence implicating them both as important contributors to the development and progression of complications associated with diabetes (8, 33, 45). Initiation of inflammation and generation of reactive oxygen species as a consequence of RAGE activation are well documented (39). Despite numerous attempts, targeting RAGE directly as a therapeutic strategy has largely been unsuccessful (11). However, RAGE signaling can be interrupted, in vivo, by directed proteolytic cleavage of the RAGE ectodomain [cleaved RAGE (cRAGE); 16, 32], thus creating a soluble isoform of RAGE (sRAGE) that is released from the cell and appears into the circulation (32). In addition, alternative splicing of the RAGE gene at exon 9 produces a truncated COOH-terminal protein product [endogenous secretory RAGE (esRAGE)] that is expelled from the cell via exocytosis (56). This heterogeneous pool of solubilized receptors, collectively termed total sRAGE, serves to downregulate the inflammatory response by absorbing excess RAGE ligands, thus attenuating cell membrane RAGE signaling. The production of soluble receptors, as a general concept, is regarded as a common feature of cytokine biology with significant implications for inflammatory disease progression and therapy. Thus maintaining high levels of circulating sRAGE isoforms is apparently advantageous for the organism (14, 17, 48). This is exemplified, in part, by data demonstrating that sRAGE isoforms are decreased in inflammatory conditions such as type 2 diabetes mellitus (T2DM), coronary artery disease, and neurodegenerative diseases (14, 48, 54), while treatment with recombinant sRAGE suppresses atherosclerosis and vascular dysfunction in animal models of diabetic coronary artery disease (34).
Given this evidence, efforts have been made to establish the efficacy of sRAGE isoforms as biomarkers for diabetes and associated complications. However, existing clinical data are equivocal, possibly because of low sample size, lack of metabolic control measures, and incomplete phenotyping. For example, several studies have demonstrated no difference or even elevated total sRAGE levels in T2DM compared with body mass index (BMI)-matched controls with no relationship to basic measures of insulin sensitivity such as homeostatic model assessment of insulin resistance (HOMA-IR; 4, 18). Alternatively, attenuated total sRAGE has been independently reported with obesity, prediabetes, and T2DM (5, 13, 40), and low total sRAGE was associated with greater risk of developing T2DM and cardiovascular mortality of nondiabetic individuals (40).
What these prior studies lack are normative values of sRAGE isoforms derived from a population of young, lean, and physically active adults, which is generally regarded as the ideal state of human health. Furthermore, no studies have yet examined the independent effects of body composition or obesity on all sRAGE isoforms, nor have sRAGE isoforms been comprehensively examined across the glucose tolerance continuum, which underlies the natural history of T2DM. In addition, the relationship between sRAGE isoforms and insulin sensitivity remains ambiguous, potentially because of reliance on fasting indexes of insulin sensitivity, such as HOMA-IR. Finally, cRAGE and esRAGE data are seldom reported together, and the ratio of cRAGE to esRAGE (cRAGE:esRAGE) has yet to be explored as a potential index for insulin resistance or risk of developing T2DM. The latter may be particularly insightful given the mechanistic differences by which cRAGE and esRAGE are generated in vivo. Therefore our aim was to characterize total sRAGE, cRAGE, esRAGE, and cRAGE:esRAGE in a young, lean, healthy reference group, as well as individuals stratified according to glucose tolerance status (GTS), obesity, or both. We hypothesized that sRAGE isoforms would be reduced with impaired glucose tolerance (IGT) and T2DM and further reduced in the presence of obesity compared with a lean, healthy reference group. Furthermore, we assessed whether the circulating concentrations of sRAGE isoforms were associated with greater odds of developing T2DM.
MATERIALS AND METHODS
Study design and subjects.
This data set examines 274 individuals from whom we have quantified circulating sRAGE isoform concentrations. Demographic and clinical data from some subjects participating in this work have been published (25, 31, 41, 42, 53). However, this is the first reporting of the sRAGE data in these subjects. Our intent was to examine sRAGE isoforms and insulin sensitivity in a population of overweight and obese subjects that spanned the glucose tolerance continuum [normal glucose tolerance (NGT), IGT, and T2DM] and directly contrast these observations with a group of young, lean, healthy controls (LHC) who performed at least 120 min of moderate intensity physical activity per week. We interpret the LHC group to represent an optimal state of health and thus provide a benchmark of “normal” sRAGE isoform concentrations. Potential participants underwent medical screening to determine their eligibility for the study, which included a medical history assessment, electrocardiogram, and blood chemistry screening. Evidence of prior or current chronic pulmonary, hepatic, renal, gastrointestinal, or hematological disease, weight loss (>2 kg within 6 mo), smoking, and contraindication to an exercise test were used as exclusion criteria. Blood glucose following a 2-h oral glucose tolerance test (OGTT) was used to stratify subjects by GTS according to the American Diabetes Association (ADA; 2). However, T2DM stratification relied on ADA criteria, prior clinical diagnosis, or use of prescription antidiabetic medication. BMI was used to stratify subjects by obesity status (lean, <25 kg/m2; overweight, 25–29 kg/m2; or obese, >29 kg/m2). Subjects were recruited by newspaper/radio advertisement from the local municipal areas in Chicago, IL; Cleveland, OH; and Copenhagen, Denmark. All subjects provided oral and written informed consent before participation, and the methods were approved by local ethics committees at all locations (Institutional Review Boards of the University of Illinois at Chicago and Cleveland Clinic and the Scientific Ethics Committee of the Capital Region of Denmark).
Pretest control period.
Tests took place in the Clinical Research Units of the University of Illinois at Chicago and Cleveland Clinic and at the Clinical Research Laboratory of the Centre of Inflammation and Metabolism, Rigshospitalet, Denmark. Subjects being treated with antidiabetic drugs withheld their medications for at least 24 h before metabolic testing. Diet and physical activity records were taken in an outpatient setting, and all subjects were instructed to abstain from consuming alcohol 48 h before their visit and not to consume caffeine within 24 h of their visit. Subjects also abstained from structured exercise for at least 24 h before metabolic testing.
Clinical procedures.
Height and weight were measured using standard techniques. Whole body adiposity was estimated using dual-energy X-ray absorptiometry (Lunar iDXA; GE Healthcare, Madison, WI). Subjects performed an incremental treadmill exercise test to determine their maximal oxygen consumption (V̇o2max) as described previously (43). The V̇o2max test was conducted at least 48 h before subsequent metabolic assessments. On a separate day, following an 8–10-h overnight fast, subjects came to the laboratory, and an antecubital venous cannula was placed for baseline blood collection. Subjects ingested 75 g of anhydrous glucose dissolved in 300-ml water (standard OGTT). Following glucose ingestion, regular venous blood samples were collected for 2 h. Blood was centrifuged at 2,000 g for 15 min at room temperature, and respective serum/plasma was stored at −80°C until analysis. In addition, insulin sensitivity was measured in 80 subjects via hyperinsulinemic (40 mU·m−2·min−1)-euglycemic (5 mmol/l) clamp. The methods of the hyperinsulinemic-euglycemic clamp were described previously (31, 53).
Blood analyses.
Glucose concentrations were measured using a bedside analyzer (YSI Stat; YSI, Yellow Springs, OH; and ABL; Radiometer Medical, Brønshøj, Denmark); insulin concentrations were determined by electrochemiluminescence immunoassay (E-modular; Roche, Switzerland) and radioimmunoassay (Millipore, Billerica, MA); glycated hemoglobin (HbA1c) levels were determined by high-performance liquid chromatography (HPLC; Tosoh G7 analyzer; San Francisco, CA). High-sensitivity C-reactive protein (hs-CRP) was determined via ELISA (Alpha Diagnostics International, San Antonio, TX). Total sRAGE concentrations were measured in plasma samples by commercial ELISA (R&D Systems, Minneapolis, MN) as per the manufacturer’s protocol. This measure of total human sRAGE levels includes both the cleaved (cRAGE) and spliced variants (esRAGE). A monoclonal antibody raised against the NH2 terminus of the extracellular domain of RAGE, comprising amino acids 24–344, was used to detect the sRAGE in the sample (R&D Systems). Plasma esRAGE concentrations were measured separately by commercial ELISA (As One International, Mountain View, CA) as per the manufacturer’s protocol. A monoclonal antibody raised against human esRAGE, recognizing amino acids 332–347, was used to detect esRAGE in the sample (B-Bridge International). Plasma cRAGE concentrations were then determined by subtracting esRAGE from total sRAGE as previously described (47, 55). The sRAGE ratio (cRAGE:esRAGE) was derived by the quotient of cRAGE to esRAGE and expressed in arbitrary units. All samples were analyzed in duplicate.
Statistics.
All data were tested for normality using Shapiro-Wilk’s test. Parametric or nonparametric statistical tests were applied accordingly. Subject characteristics for each group were compared using a one-way ANOVA. One-way ANOVA was also used to compare mean sRAGE isoform data between groups. The effects of obesity (lean, overweight, and obese) and glucose tolerance status (NGT, IGT, and T2DM) on sRAGE isoforms were determined via two-way ANOVA. Bonferroni/Dunn post hoc tests were used for multiple comparisons when appropriate. Multivariate ordinal regression modeling was used to determine whether sRAGE isoforms could predict risk of diabetes progression using stratification by glucose tolerance status and adjustment for age, race, and obesity (proportional odds model; 52). Caucasian was used as the reference for race, and lean was used as the reference for obesity status. Total sRAGE, esRAGE, cRAGE, and cRAGE:esRAGE were used to construct models. The values for total sRAGE, cRAGE, and esRAGE were multiplied by 100 before entering them into the models. To avoid colinearity, we did not generate a stepwise model that included all sRAGE measures in the model. Homogeneity of the odds ratios was confirmed for all variables before performing ordinal regression. Bivariate correlation analyses were performed using Pearson or Spearman correlation coefficients. SPSS v24 (IBM, Armonk, NY) and SAS (Cary, NC) were used to perform statistical analyses. P < 0.05 was considered significant, and data are presented as means ± SD.
RESULTS
Subject characteristics.
Table 1 shows subject characteristics stratified by GTS. Markers of glycemic control (HbA1c, 2-h OGTT glucose, and fasting glucose] were progressively increased across the glucose tolerance continuum. The IGT and T2DM groups were of similar age, BMI, and fitness level (V̇o2max; Table 1; P > 0.05). By design, compared with the IGT and T2DM groups, the NGT group was younger, leaner (BMI), more fit (V̇o2max), and had superior glycemic control apart from 2-h OGTT glucose incremental area under the curve (iAUC), which was not different from T2DM (Table 1). Further details of subject characteristics including sex and race frequencies in each group are provided in Table 2.
Table 1.
Metabolic characteristics
| Variable | NGT | IGT | T2DM |
|---|---|---|---|
| Sex (M/F), n | 79/71 | 10/20 | 47/47 |
| Age, yr | 39 ± 17 | 61 ± 10b | 57 ± 9b |
| Body mass index, kg/m2 | 27.0 ± 6.2 | 34.8 ± 4.8b | 32.6 ± 7.3b |
| V̇o2max, ml·kg−1·min−1 | 32.6 ± 10.4 | 23.3 ± 6.4b | 26.3 ± 6.6b |
| Body fat, % | 33.0 ± 9.4 | 43.2 ± 8.1b | 36.0 ± 9.5d |
| Fat mass, kg | 29.0 ± 12.9 | 40.7 ± 8.5b | 31.7 ± 12.4d |
| Lean body mass, kg | 55.3 ± 12.1 | 54.2 ± 12.0 | 57.9 ± 11.5 |
| 2-h OGTT glucose, mg/dl | 114 ± 22.5 | 162 ± 16.9b | 281 ± 67.4b,e |
| 2-h OGTT glucose iAUC, AU | 4,201 ± 2,083 | 7,322 ± 2,639a | 5,133 ± 5,567c |
| HbA1c, % | 5.4 ± 0.46 | 5.7 ± 0.52 | 7.1 ± 1.6b,e |
| HbA1c, mmol/mol | 35.7 ± 4.98 | 38.5 ± 5.71 | 54.5 ± 17.7b,e |
| Fasting glucose,f mg/dl | 93 ± 10.9 | 97 ± 11.7 | 151 ± 61.4b,e |
| Fasting insulin,f mU/l | 9.5 ± 6.3 | 15.9 ± 11.6a | 13.5 ± 6.6b |
| HOMA-IR,f AU | 2.2 ± 1.6 | 4.7 ± 5.0b | 5.0 ± 3.2b |
| Matsuda index,f AU | 4.7 ± 3.1 | 2.5 ± 1.5b | 3.1 ± 1.9b |
| GDR,f mg·kg−1·min−1 | 4.9 ± 2.3 | 2.9 ± 1.2a | 2.6 ± 0.96a |
| hs-CRP, mg/l | 2.2 ± 1.9 | 2.8 ± 1.6 | 2.6 ± 2.6 |
Values are means ± SD. Normally distributed data were analyzed by one-way ANOVA and Bonferroni adjustments for multiple comparisons. NGT (n = 150), normal glucose tolerance; IGT (n = 30), impaired glucose tolerance; T2DM (n = 94), type 2 diabetes mellitus; M/F, male/female; V̇o2max, maximal aerobic fitness; 2-h OGTT glucose, blood glucose at 2-h time point of oral glucose tolerance test; iAUC, incremental area under the curve; AU, arbitrary units; HbA1c, glycated hemoglobin; HOMA-IR, homeostatic model assessment of insulin resistance; GDR, hyperinsulinemic-euglycemic clamp-derived glucose disposal rate; hs-CRP, high-sensitivity C-reactive protein.
P < 0.01 and
P < 0.0001 vs. NGT;
P < 0.05,
P < 0.01, and
P < 0.0001 vs. IGT.
Nonnormally distributed variables were analyzed using Kruskal-Wallis test and Bonferroni adjustments for multiple comparisons.
Table 2.
Descriptive demographics
| NGT |
IGT |
T2DM |
||||
|---|---|---|---|---|---|---|
| Variable | n | % | n | % | n | % |
| Age, yr | 39 ± 17 | 61 ± 10† | 57 ± 9† | |||
| Young (18–35 yr) | 88 | 59 | 1 | 3 | 1 | 1 |
| Middle aged (36–64 yr) | 44 | 29 | 19 | 63 | 68 | 72 |
| Old (≥65 yr) | 18 | 12 | 10 | 33 | 25 | 27 |
| Sex | ||||||
| Male | 79 | 53 | 10 | 35 | 47 | 50 |
| Female | 71 | 47 | 20 | 67 | 47 | 50 |
| Race | ||||||
| White | 107 | 71 | 21 | 70 | 63 | 67 |
| Black | 17 | 11 | 7 | 23 | 31 | 33 |
| Hispanic | 10 | 7 | 2 | 7 | 0 | 0 |
| Asian | 16 | 11 | 0 | 0 | 0 | 0 |
| Obesity, kg/m2 | 27.0 ± 6.2 | 34.8 ± 4.8* | 32.6 ± 7.3* | |||
| Lean (18–24 kg/m2) | 74 | 49 | 1 | 3 | 15 | 16 |
| Overweight (25–29 kg/m2) | 27 | 18 | 2 | 7 | 24 | 26 |
| Obese (≥30 kg/m2) | 49 | 33 | 27 | 90 | 55 | 59 |
Age and obesity values are means ± SD. Frequencies of demographic descriptors of individuals are grouped by glucose tolerance status: NGT, normal glucose tolerance (n = 150); IGT, impaired glucose tolerance (n = 30); and T2DM, type 2 diabetes mellitus (n = 94).
P < 0.05 and
P < 0.0001 vs. NGT.
sRAGE isoforms are attenuated with impaired glucose tolerance.
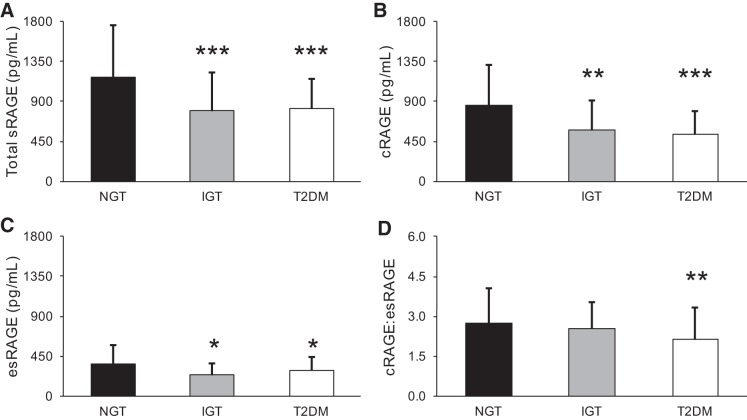
When stratified by GTS, NGT individuals had 33 ± 37% greater total sRAGE compared with IGT individuals (P < 0.05) and 31 ± 29% greater total sRAGE compared with T2DM individuals (P < 0.05; Fig. 1A). cRAGE and esRAGE, which comprise total sRAGE, were lower to a similar extent in IGT and T2DM compared with NGT individuals (P < 0.05; Fig. 1, B and C). However, cRAGE:esRAGE was only lower in T2DM compared with NGT subjects, pointing to a disproportionate lack of cRAGE in T2DM individuals (P < 0.05; Fig. 1D). This observation is significant considering that cRAGE made up 63 ± 12.5% of total sRAGE in subjects with T2DM.
Fig. 1.
Soluble receptor for advanced glycation end products (sRAGE) isoforms according to glucose tolerance status. Subjects were stratified by glucose tolerance status: normal glucose tolerance (NGT, n = 150), impaired glucose tolerance (IGT, n = 30), or type 2 diabetes mellitus (T2DM, n = 94). Comparisons between groups were made for total sRAGE (A), cleaved RAGE (cRAGE; B), endogenous secretory RAGE (esRAGE; C), and cRAGE:esRAGE ratio (D). Differences between groups were analyzed by one-way ANOVA and Bonferroni post hoc tests as necessary. Bars represent means ± SD. *P < 0.05, **P < 0.01, and ***P < 0.0001 vs. NGT.
Increased circulating sRAGE isoforms are associated with reduced proportional odds of developing diabetes.
We had hypothesized that reduced sRAGE isoforms may underlie the natural history of T2DM according to progression across the glucose tolerance continuum. Using ordinal logistic regression analysis (Table 3), total sRAGE (model 1), cRAGE (model 2), esRAGE (model 3), and cRAGE:esRAGE (model 4) were combined with other independent variables (age, race, and obesity) to form each respective model. As expected, and shown previously, both age and race were associated with greater proportional odds (Table 3) for the development of T2DM (19). For total sRAGE, cRAGE, and cRAGE:esRAGE, each was independently associated with the proportional odds for progression across the glucose tolerance continuum to T2DM, whereas esRAGE was not. A 100 pg/ml increase in total sRAGE was associated with a 9% reduction in the proportional odds of developing T2DM, whereby the same increase in cRAGE was associated with a 16% reduction (Table 3). Additionally, every 1-unit increase in cRAGE:esRAGE predicted a 26% decreased risk of diabetes progression (Table 3). The model demonstrating the greatest reduction in proportional odds was model 2, which included cRAGE isoforms (C-statistic, 0.805; Table 3).
Table 3.
Soluble RAGE isoforms and proportional odds for developing type 2 diabetes mellitus
|
Model 1 |
Model 2 |
Model 3 |
Model 4 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P |
| Age | 1.06 | 1.03–1.08 | <0.001 | 1.06 | 1.03–1.08 | <0.001 | 1.07 | 1.05–1.10 | <0.001 | 1.06 | 1.04–1.09 | <0.001 |
| Race | ||||||||||||
| Black | 3.43 | 1.69–6.96 | <0.001 | 4.11 | 1.93–8.75 | <0.001 | 3.57 | 1.74–7.31 | <0.001 | 4.04 | 1.91–8.53 | <0.001 |
| Other (Hispanic/Asian) | 0.30 | 0.06–6.96 | 0.139 | 0.31 | 0.06–1.55 | 0.155 | 0.36 | 0.07–1.72 | 0.199 | 0.40 | 0.08–1.93 | 0.255 |
| Obesity | ||||||||||||
| Overweight | 1.39 | 0.58–3.35 | 0.459 | 1.58 | 0.64–3.87 | 0.322 | 1.29 | 0.53–3.16 | 0.576 | 1.79 | 0.72–4.45 | 0.208 |
| Obese | 1.08 | 0.46–2.50 | 0.864 | 1.34 | 0.57–3.15 | 0.505 | 1.06 | 0.45–2.53 | 0.890 | 1.68 | 0.69–4.07 | 0.253 |
| Total sRAGE | 0.91 | 0.85–0.97 | 0.003 | — | — | — | — | — | — | — | — | — |
| cRAGE | — | — | — | 0.84 | 0.77–0.92 | <0.001 | — | — | — | |||
| esRAGE | — | — | — | 0.93 | 0.78–1.10 | 0.374 | — | — | — | |||
| cRAGE:esRAGE | — | — | — | — | — | — | — | — | — | 0.74 | 0.58–0.96 | 0.022 |
| C-statistic | 0.782 | 0.805 | 0.773 | 0.784 | ||||||||
Total soluble receptor for advanced glycation end products (sRAGE), cleaved RAGE (cRAGE), endogenous secretory RAGE (esRAGE), and sRAGE ratio (cRAGE:esRAGE) were used to construct models 1, 2, 3, and 4, respectively. The values for total sRAGE, cRAGE, and esRAGE were multiplied by 100 before entering them into the models. Models were corrected for age and race where Caucasian and lean were used as reference, respectively. OR, odds ratio; CI, confidence interval.
Relationships with sRAGE isoforms and metabolic variables.
Bivariate correlation analyses between sRAGE variables and metabolic variables are presented in Table 4. Total sRAGE, cRAGE, and esRAGE negatively correlated with BMI and percent body fat, with esRAGE having the strongest relationships between both variables. In addition, all sRAGE variables were positively correlated with cardiorespiratory fitness (V̇o2max). Positive correlations between cRAGE:esRAGE, V̇o2max, and BMI again demonstrate that the proportion of cRAGE and esRAGE isoforms, rather than just the independent quantity of each, is related to fitness level and body weight status.
Table 4.
Correlations between sRAGE isoforms and metabolic characteristics
| Total sRAGE, pg/ml |
cRAGE, pg/ml |
esRAGE, pg/ml |
cRAGE:esRAGE |
|||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| Age, yr | −0.368 | <0.001 | −0.387 | <0.001 | −0.206 | 0.001 | −0.254 | <0.0001 |
| V̇o2max, ml·kg−1·min−1 | 0.231 | 0.002 | 0.291 | <0.001 | 0.156 | 0.039 | 0.202 | 0.007 |
| Body mass index, kg/m2 | −0.225 | <0.001 | −0.158 | 0.010 | −0.288 | <0.001 | 0.140 | 0.023 |
| Body fat, % | −0.288 | <0.001 | −0.227 | 0.001 | −0.311 | <0.001 | −0.004 | 0.953 |
| Lean body mass, kg | 0.066 | 0.351 | 0.075 | 0.297 | −0.058 | 0.414 | 0.136 | 0.058 |
| Fat mass, kg | −0.211 | 0.003 | −0.130 | 0.071 | −0.312 | <0.001 | 0.101 | 0.158 |
| 2-h OGTT glucose, mg/dl | −0.233 | 0.002 | −0.292 | <0.001 | −0.075 | 0.332 | −0.253 | 0.001 |
| 2-h OGTT glucose iAUC, AU | −0.068 | 0.185 | 0.078 | 0.300 | −0.279 | <0.001 | 0.424 | <0.001 |
| HbA1c, % | −0.200 | 0.006 | −0.183 | 0.013 | −0.153 | 0.036 | −0.001 | 0.989 |
| Fasting glucose,* mg/dl | −0.292 | <0.001 | −0.337 | <0.001 | −0.134 | 0.046 | −0.233 | <0.001 |
| Fasting insulin,* mU/l | −0.184 | 0.006 | −0.200 | 0.003 | −0.107 | 0.116 | −0.068 | 0.322 |
| HOMA-IR,* AU | −0.255 | <0.001 | −0.291 | <0.001 | −0.121 | 0.075 | −0.154 | 0.024 |
| Matsuda index,* AU | 0.214 | 0.005 | 0.183 | 0.018 | 0.187 | 0.015 | −0.007 | 0.928 |
| GDR,* mg·kg−1·min−1 | 0.472 | <0.001 | 0.343 | 0.003 | 0.594 | <0.001 | −0.276 | 0.018 |
| C-reactive protein, mg/l | −0.220 | 0.012 | −0.138 | 0.119 | −0.274 | 0.002 | 0.140 | 0.113 |
Bivariate correlation analyses were used to examine relationships between soluble receptor for advanced glycation end products (sRAGE) isoforms and metabolic parameters. Pearson correlation coefficients were performed unless noted. cRAGE, cleaved cRAGE:esRAGE, endogenous secretory RAGE; V̇o2max, maximal aerobic fitness; 2-h OGTT glucose, blood glucose at 2-h time point of oral glucose tolerance test; iAUC, incremental area under the curve; AU, arbitrary units; HbA1c, glycated hemoglobin; HOMA-IR, homeostatic model assessment of insulin resistance; GDR, hyperinsulinemic-euglycemic clamp-derived glucose disposal rate.
Analyzed by Spearman’s rho.
Apart from 2-h OGTT glucose iAUC, total sRAGE and cRAGE negatively correlated with clinical markers of glycemic control (2-h OGTT glucose, HbA1c, fasting glucose, fasting insulin, and HOMA-IR). On the other hand, esRAGE negatively correlated with 2-h OGTT glucose iAUC, HbA1c, and fasting glucose whereas sRAGE ratio positively correlated with 2-h OGTT glucose iAUC and negatively correlated with 2-h OGTT glucose, fasting glucose, and HOMA-IR. Finally, total sRAGE, esRAGE, and cRAGE all positively correlated with Matsuda index; however, the strongest associations with insulin sensitivity were found between clamp-derived glucose disposal rate (GDR) and total sRAGE (rho = 0.472, P < 0.001), cRAGE (rho = 0.343, P = 0.003), and esRAGE (rho = 0.594, P < 0.001). GDR also negatively correlated with cRAGE:esRAGE (rho = −0.276, P = 0.018).
sRAGE isoforms are reduced with worsening obesity status.
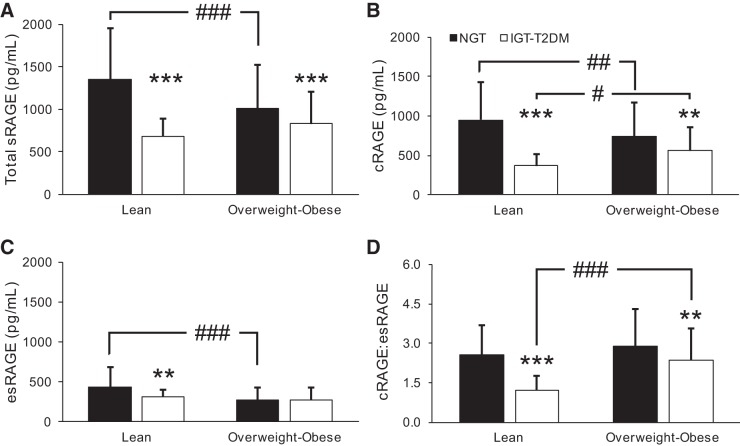
Because the glucose tolerance groups were heterogeneous with regard to obesity, we further stratified by obesity status to isolate the sRAGE phenotype of lean NGT individuals. Because of low sample size in the overweight subgrouping, the IGT group was combined with T2DM (IGT-T2DM), and overweight was combined with obese (overweight-obese; Fig. 2). Using a two-way (glucose tolerance × obesity) ANOVA, obesity status displayed a group effect for esRAGE (P = 0.001) and cRAGE:esRAGE (P < 0.0001). A group effect was also seen for GTS on total sRAGE (P < 0.0001), cRAGE (P < 0.0001), esRAGE (P = 0.026), and cRAGE:esRAGE (P < 0.0001), and an interaction effect was observed for total sRAGE (P = 0.002), cRAGE (P = 0.001), esRAGE (P = 0.048), and cRAGE:esRAGE (P = 0.032).
Fig. 2.
Effects of glucose tolerance and body mass index on soluble receptor for advanced glycation end products (sRAGE) isoforms. Subject groups were collapsed into normal glucose tolerance (NGT) vs. impaired glucose tolerance-type 2 diabetes mellitus (IGT-T2DM) designations and further stratified by body mass index (lean vs. overweight-obese). Lean, NGT, n = 74; overweight-obese, NGT, n = 76; lean, IGT-T2DM, n = 16; overweight-obese, IGT-T2DM, n = 105. Group comparisons were made for total sRAGE (A), cleaved RAGE (cRAGE; B), endogenous secretory RAGE (esRAGE; C), and cRAGE:esRAGE ratio (D) using two-way ANOVA and Bonferroni post hoc tests as necessary. Bars represent means ± SD. **P < 0.01 and ***P < 0.0001 vs. NGT; #P < 0.05, ##P < 0.01, and ###P < 0.0001 vs. lean.
Lean, NGT individuals displayed the highest concentration of total sRAGE (Fig. 2A), cRAGE (Fig. 2B), and esRAGE (Fig. 2C) compared with all other subgroups. The largest deviation from lean, NGT individuals when examining cRAGE was found in lean, IGT-T2DM (61 ± 16%). However, the largest deviation of esRAGE from lean, NGT individuals was found in overweight-obese, IGT-T2DM individuals (36 ± 36%). Comparison of cRAGE:esRAGE between groups revealed that the largest ratio exists in the overweight-obese, NGT group (Fig. 2D). This increase in cRAGE:esRAGE ratio indicates a preferential decrease in esRAGE related to worsening obesity status. Full analyses of the individual group stratifications and alternative subgroupings were performed and statistically interrogated via ANOVA. However, these analyses did not offer any insight beyond the results presented here.
We also analyzed the concentration of sRAGE isoforms across obesity status alone by stratifying individuals into lean, overweight, or obese groups. Individuals who were overweight or obese had similar concentrations of sRAGE isoforms and 24–35% lower concentrations of sRAGE isoforms compared with lean individuals (P < 0.05). The NGT group was significantly younger than the IGT and T2DM groups (Table 2). Last, we examined the effect of age on sRAGE isoforms by stratifying individuals into young (18–35 yr), middle-aged (36–64 yr), and older (≥65 yr) groups. Concentration of sRAGE isoforms were similar between middle-aged and older individuals but were 25–45% lower compared with young individuals (P < 0.05). Interestingly, older individuals had a lower cRAGE:esRAGE ratio compared with both young and middle-aged individuals (P < 0.05). Given that this analysis demonstrated a significant effect of age on sRAGE isoforms, we examined the effect of GTS on sRAGE measures while covarying for age as a continuous variable. The results of this analysis eliminated all significant effects of GTS on esRAGE and cRAGE:esRAGE concentration (P > 0.05). In addition, the differences between total sRAGE and cRAGE in NGT compared with IGT groups that exist in Fig. 1 were also resolved. However, even after controlling for age, individuals with T2DM still possess significantly lower total sRAGE and cRAGE compared with NGT individuals. All sRAGE isoforms were also negatively correlated with age (Table 4).
DISCUSSION
To our knowledge, the present study is the first to report circulating concentrations of both major sRAGE isoforms (cRAGE and esRAGE) in the context of obesity and T2DM. Our primary finding was that lean, NGT individuals possessed the greatest concentration of sRAGE isoforms compared with states of obesity, IGT, T2DM, or both. These findings are in accord with previous reports of lower sRAGE with obesity (5, 13, 18) and impaired glucose tolerance (3, 12, 22, 46). Importantly, we also demonstrate for the first time that reduced circulating concentrations of sRAGE isoforms are associated with greater proportional odds for the development of T2DM.
To this end, we developed ordinal logistic regression models using the sRAGE isoforms and cRAGE:esRAGE as independent variables to determine the proportional odds ratio of progression across the glucose tolerance continuum to T2DM. GTS is interpreted as having set thresholds along a range of possible outcomes according to ADA criteria for the diagnosis of T2DM, thus meeting the assumption needed for ordinal regression (52). The application of this type of statistical model allows for hypothesizing movement along a known continuum (using proportional odds) without longitudinal follow-up. Importantly, our analyses revealed that a 100 pg/ml increase in total sRAGE and cRAGE resulted in a marked risk reduction for progression across the glucose tolerance continuum. For calibration, 100 pg/ml represents 12% of the cRAGE concentration in lean NGT individuals. Given our regression model, the lower cRAGE observed in IGT subjects (276 pg/ml) equates to ~44% increased proportional odds of progression toward T2DM. Our sample size was relatively small, and our sampling was cross-sectional, so these data must be interpreted with caution. However, Selvin et al. reported similar findings in a sample of 1,200 individuals without T2DM, whereby those in the lowest quartile of total sRAGE concentration had an increased risk of developing T2DM 18 yr later (hazard ratio, 1.64; 95% confidence interval, 1.10–2.44; 40). Furthermore, relationships between modulation of sRAGE and health outcomes have been reported, whereby increased sRAGE, following a 12-wk aerobic exercise intervention, was associated with reduced C-reactive protein and improved aerobic fitness (9). Given the financial and time burden for longitudinal studies such as the latter, the application of ordinal regression models has merit for identification and characterization of novel targets such as sRAGE isoforms. Here, we expand on previous observations by demonstrating cRAGE as the isoform with the greatest ability to predict risk of progression across the glucose tolerance continuum whereas esRAGE did not predict risk of progression. These data suggest dichotomous roles for cRAGE and esRAGE isoforms and their relevance to T2DM.
In line with this notion, we provide novel evidence of a disproportionate loss of cRAGE and esRAGE in the case of T2DM and obesity, respectively. Although both cRAGE and esRAGE were significantly lower in IGT and T2DM compared with NGT individuals, only the T2DM group possessed a significantly lower cRAGE:esRAGE ratio. Additionally, when examining the effects of obesity and GTS on sRAGE measures, there was a significant effect of GTS on cRAGE:esRAGE ratio whereby impaired glucose tolerance tended to result in a lower ratio, implying a preferential loss of cRAGE (Fig. 2). The lean, IGT-T2DM group stratification also possessed the lowest concentration of cRAGE compared with all other perturbations. Additionally, cRAGE correlated with 2-h OGTT glucose and HOMA-IR whereas esRAGE did not. Collectively, these data suggest that loss of cRAGE is strongly influenced by IGT and T2DM.
The observed cRAGE phenotype may be mediated by a preferential attenuation of cRAGE-producing mechanisms with IGT and T2DM, specifically the proteolytic cleavage of the RAGE ectodomain via the enzyme a disintegrin and metalloproteinase 10 (ADAM10) or other matrix metalloproteinases (16, 32, 37). ADAM10 is the primary enzyme responsible for cRAGE production (37). Retinoic acid receptor-β (RARβ) positively regulates ADAM10 transcription by binding to its promoter site (28, 49). Deacetylation of RARβ is necessary for this action and is mediated by the deacetylase activity of sirtuin 1 (SIRT1; 10, 28). SIRT1 plays a role in β cell insulin secretion and insulin sensitivity in other tissues such as fat and skeletal muscle (27). Importantly, SIRT1 expression is reduced in T2DM and is also downregulated by RAGE signaling (21, 50). Activation of RAGE signaling occurs via binding of its ligands such as AGEs. These RAGE ligands are known to be elevated in the T2DM condition and have been related to insulin resistance (45). Specifically, exposure to the RAGE ligands reduces SIRT1 protein expression in the liver, skeletal muscle, and adipose tissue, resulting in the development of insulin resistance in these tissues (7).
In the present study, cRAGE correlated to GDR (r = 0.343, P = 0.003; Table 4), and its reduction was strongly associated with the proportional odds for progression through the glucose tolerance continuum (Table 3). GDR is the gold standard measure for insulin-mediated glucose disposal, which is largely dictated by the insulin sensitivity of the skeletal muscle. Therefore failure of the cRAGE-producing mechanisms such as RARβ, SIRT1, and ADAM10 in the skeletal muscle may allow for excessive RAGE signaling to promote the development of insulin resistance in skeletal muscle. This may help explain why higher cRAGE is strongly related to insulin sensitivity and lower cRAGE is related to the progression toward T2DM.
Interestingly, we show that esRAGE is preferentially lost with obesity. We found a significant group effect of obesity, whereby overweight-obese individuals possessed a higher cRAGE:esRAGE ratio compared with lean individuals suggesting a preferential loss of esRAGE. Although we did not see any difference in cRAGE:esRAGE when stratifying by obesity status alone, esRAGE was 35% lower in Obese compared with lean individuals whereas cRAGE was 24% lower in obese compared with lean individuals. In addition, the overweight-obese, IGT-T2DM group resulted in the lowest concentration of esRAGE (Fig. 2). Both BMI and body fat percentage displayed stronger correlations with esRAGE compared with cRAGE (Table 4).
Production of esRAGE is regulated by the activity of two antagonistic splicing factors, heterogeneous nuclear ribonuclear protein-A1 (hnRNPA1) and transformer-2β (TRA2β; 29). TRA2β promotes esRAGE production whereas hnRNPA1 suppresses this activity. Both TRA2β and hnRNPA1 are regulated by MAPK activity (1, 6, 51), which is well known to be activated by RAGE signaling and exacerbated in obesity and insulin resistance (24). In addition, RAGE expression plays a critical role in adipose differentiation, hypertrophy, and inflammation (15, 33, 44). Therefore adipose expansion and subsequent adipokine-mediated inflammation may be suppressing the splicing mechanisms that regulate esRAGE.
In support of this notion, TRA2β is reduced in the liver and skeletal muscle of obese, IGT-T2DM individuals (29, 35). In the present study, we found lower concentrations of esRAGE in obese individuals compared with lean. Additionally, esRAGE was correlated to GDR (r = 0.594, P < 0.001) and body fat percentage (r = −0.311, P < 0.001). These data suggest that both adipose tissue and skeletal muscle may be involved in RAGE splicing and that the mechanisms involved become dysfunctional with obesity and insulin resistance. However, future studies are needed to identify the tissue- or cell-specific sources of sRAGE isoform production and what mechanisms are responsible for promoting and attenuating their release into the circulation.
These findings demonstrate that the study of sRAGE isoforms remains an important area of research given both old and new data (30) reporting the potential role of sRAGE to impart physiological benefit and protection from cardiovascular and metabolic disease. It is evident that the mechanisms of sRAGE production are tightly regulated and that relatively small changes in circulating concentrations are linked to the natural history of T2DM. Herein, we are the first to characterize the circulating concentrations of the two most prominent sRAGE isoforms across the glucose tolerance continuum and demonstrate that total sRAGE, cRAGE, and cRAGE:esRAGE were associated with the proportional odds for progression across the glucose tolerance continuum using ordinal logistic regression. Our data are, admittedly, limited by not being age matched across all groups, as others have demonstrated that chronological age plays a significant role in sRAGE concentrations (36). However, juxtaposition of the T2DM phenotype against a young, lean, healthy phenotype demonstrates the degree to which circulating sRAGE isoforms in obesity, states of impaired glucose tolerance, and advanced age deviate from optimum health. To tease the effect of age away from these other factors, we compared circulating sRAGE isoform concentrations across GTS while covarying for age. This analysis revealed that sRAGE remained significantly reduced in T2DM despite the age difference between the T2DM and LHC groups. However, covarying for age did eliminate differences in total sRAGE and cRAGE between NGT and IGT, as well as eliminate all differences previously observed for esRAGE and cRAGE:esRAGE. This, in addition to the inverse correlations between sRAGE measures and age, implicates age to effect circulating sRAGE. However, the fact that differences were still realized between T2DM and LHC individuals indicates that the T2DM phenotype, regardless of age, is characterized in part by reduced total sRAGE and cRAGE.
We also acknowledge that the limitations of stratifying our data by BMI are such that BMI is less sensitive in detecting obesity than body fat percentage (38). However, when we stratified by body fat percentage cutoffs as previously reported by Romero-Corral et al., the findings were consistent with the data that is currently reported using BMI (38).
We were also unable to genotype our participants because of limited sample. This would have been an interesting addition to our data since multiple single-nucleotide polymorphisms (SNPs) have been identified for RAGE and have been implicated in the development of obesity and inflammation (23, 26). The SNP that involves glycine-serine switch at codon 82 (G82S) occurs in the ligand-binding domain of RAGE and enhances its ability to promote RAGE activation (20). Kim et al. demonstrated that lean and obese individuals with the S/S genotype possessed lower sRAGE compared with those with the G/S and G/G genotypes (26). Obese individuals in this cohort with the S/S genotype also possessed higher BMI and greater circulating C-reactive protein compared with those with the G/S and G/G genotypes (26). Our data demonstrate lower sRAGE isoform concentrations in obese and T2DM individuals compared with lean, healthy individuals. Unfortunately, we do not know whether the G82S SNP is partly responsible for these differences in our sample on sRAGE. However, the frequency of glycine and serine alleles has not been previously shown to be different in T2DM compared with healthy individuals (23). Nevertheless, future studies should examine the effect of RAGE SNPs on the risk of obesity and diabetes development and whether this risk is related to sRAGE concentrations. The mechanism of how sRAGE concentrations are altered by SNPs in RAGE is also unclear and warrants future study. In addition, Kim et al. only examined the relationship between the G82S SNP and total sRAGE concentrations and did not discriminate between the esRAGE and cRAGE isoforms (26). We have demonstrated here that dysregulation of these isoforms is associated with different phenotypes, and therefore these isoforms are likely under parallel regulation.
In conclusion, the disproportionate reductions of cRAGE and esRAGE in T2DM and obesity, respectively, require further mechanistic study as our data implicate adiposity and insulin sensitivity, or both, to play a role in sRAGE biology. These findings suggest the presence of a failure in the sRAGE-producing mechanisms with the onset of T2DM and obesity that requires further study. sRAGE was also strongly associated with the proportional odds ratio for progression through the glucose tolerance continuum, asserting sRAGE as a potential biomarker for T2DM. The long-term benefits for reporting these data are 1) to help direct research efforts toward elucidating failed mechanisms underpinning the discrepancy in sRAGE isoform expression in T2DM and obesity and 2) to determine the efficacy of targeting these mechanisms for treatment of T2DM and obesity.
GRANTS
This work was supported by American Diabetes Association-Junior Faculty Award 1-14-JF-32 (J. M. Haus), National Center for Advancing Translational Sciences Clinical and Translational Science Award Grants 24989 and 29879, Central Society for Clinical and Translational Research, University of Chicago Diabetes Research Training Center (National Institute of Diabetes and Digestive and Kidney Diseases Grant 20595), and National Institutes of Health Grants R01-AG-12834 (J. P. Kirwan), R01-NR-007760 (L. Quinn), and R01-DK-109948 (J. M. Haus). Additional funding from European Foundation for the Study of Diabetes/Amylin (T. P. J. Solomon) contributed to this work.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.R.M., E.W., J.P.K., L.Q., T.P.J.S., and J.M.H. conceived and designed research; E.R.M., V.S.S., J.T.M., B.K.B., S.F., K.K., C.E.F., S.K., J.P.K., L.Q., T.P.J.S., and J.M.H. performed experiments; E.R.M., V.S.S., J.T.M., B.K.B., E.W., S.F., K.K., C.E.F., S.K., J.P.K., L.Q., T.P.J.S., and J.M.H. analyzed data; E.R.M., E.W., T.P.J.S., and J.M.H. interpreted results of experiments; E.R.M., E.W., and J.M.H. prepared figures; E.R.M., J.P.K., L.Q., T.P.J.S., and J.M.H. drafted manuscript; E.R.M., V.S.S., J.T.M., B.K.B., E.W., S.F., K.K., C.E.F., S.K., J.P.K., L.Q., T.P.J.S., and J.M.H. edited and revised manuscript; E.R.M., V.S.S., J.T.M., B.K.B., E.W., S.F., K.K., C.E.F., S.K., J.P.K., L.Q., T.P.J.S., and J.M.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Karia Coleman, Victoria Meyers, Alec Chaves, Giamila Fantuzzi, and Kelly Fuller of the University of Illinois at Chicago for expert technical assistance.
REFERENCES
- 1.Akaike Y, Masuda K, Kuwano Y, Nishida K, Kajita K, Kurokawa K, Satake Y, Shoda K, Imoto I, Rokutan K. HuR regulates alternative splicing of the TRA2β gene in human colon cancer cells under oxidative stress. Mol Cell Biol 34: 2857–2873, 2014. doi: 10.1128/MCB.00333-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association 2. Classification and diagnosis of diabetes. Diabetes Care 39, Suppl 1: S13–S22, 2016. doi: 10.2337/dc16-S005. [DOI] [PubMed] [Google Scholar]
- 3.Basta G, Sironi AM, Lazzerini G, Del Turco S, Buzzigoli E, Casolaro A, Natali A, Ferrannini E, Gastaldelli A. Circulating soluble receptor for advanced glycation end products is inversely associated with glycemic control and S100A12 protein. J Clin Endocrinol Metab 91: 4628–4634, 2006. doi: 10.1210/jc.2005-2559. [DOI] [PubMed] [Google Scholar]
- 4.Biswas SK, Mohtarin S, Mudi SR, Anwar T, Banu LA, Alam SM, Fariduddin M, Arslan MI. Relationship of soluble RAGE with insulin resistance and beta cell function during development of type 2 diabetes mellitus. J Diabetes Res 2015: 150325, 2015. doi: 10.1155/2015/150325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brix JM, Höllerl F, Kopp HP, Schernthaner GH, Schernthaner G. The soluble form of the receptor of advanced glycation endproducts increases after bariatric surgery in morbid obesity. Int J Obes 36: 1412–1417, 2012. doi: 10.1038/ijo.2012.107. [DOI] [PubMed] [Google Scholar]
- 6.Buxadé M, Parra JL, Rousseau S, Shpiro N, Marquez R, Morrice N, Bain J, Espel E, Proud CG. The Mnks are novel components in the control of TNF alpha biosynthesis and phosphorylate and regulate hnRNP A1. Immunity 23: 177–189, 2005. doi: 10.1016/j.immuni.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Cai W, Ramdas M, Zhu L, Chen X, Striker GE, Vlassara H. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc Natl Acad Sci USA 109: 15888–15893, 2012. doi: 10.1073/pnas.1205847109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassese A, Esposito I, Fiory F, Barbagallo AP, Paturzo F, Mirra P, Ulianich L, Giacco F, Iadicicco C, Lombardi A, Oriente F, Van Obberghen E, Beguinot F, Formisano P, Miele C. In skeletal muscle advanced glycation end products (AGEs) inhibit insulin action and induce the formation of multimolecular complexes including the receptor for AGEs. J Biol Chem 283: 36088–36099, 2008. doi: 10.1074/jbc.M801698200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi KM, Han KA, Ahn HJ, Hwang SY, Hong HC, Choi HY, Yang SJ, Yoo HJ, Baik SH, Choi DS, Min KW. Effects of exercise on sRAGE levels and cardiometabolic risk factors in patients with type 2 diabetes: a randomized controlled trial. J Clin Endocrinol Metab 97: 3751–3758, 2012. doi: 10.1210/jc.2012-1951. [DOI] [PubMed] [Google Scholar]
- 10.Corbett GT, Gonzalez FJ, Pahan K. Activation of peroxisome proliferator-activated receptor α stimulates ADAM10-mediated proteolysis of APP. Proc Natl Acad Sci USA 112: 8445–8450, 2015. doi: 10.1073/pnas.1504890112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deane RJ. Is RAGE still a therapeutic target for Alzheimer’s disease? Future Med Chem 4: 915–925, 2012. doi: 10.4155/fmc.12.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Pino A, Urbano F, Zagami RM, Filippello A, Di Mauro S, Piro S, Purrello F, Rabuazzo AM. Low endogenous secretory receptor for advanced glycation end-products levels are associated with inflammation and carotid atherosclerosis in prediabetes. J Clin Endocrinol Metab 101: 1701–1709, 2016. doi: 10.1210/jc.2015-4069. [DOI] [PubMed] [Google Scholar]
- 13.Dozio E, Briganti S, Delnevo A, Vianello E, Ermetici F, Secchi F, Sardanelli F, Morricone L, Malavazos AE, Corsi Romanelli MM. Relationship between soluble receptor for advanced glycation end products (sRAGE), body composition and fat distribution in healthy women. Eur J Nutr (August 13, 2016). doi: 10.1007/s00394-016-1291-0. [DOI] [PubMed] [Google Scholar]
- 14.Falcone C, Emanuele E, D’Angelo A, Buzzi MP, Belvito C, Cuccia M, Geroldi D. Plasma levels of soluble receptor for advanced glycation end products and coronary artery disease in nondiabetic men. Arterioscler Thromb Vasc Biol 25: 1032–1037, 2005. doi: 10.1161/01.ATV.0000160342.20342.00. [DOI] [PubMed] [Google Scholar]
- 15.Gaens KH, Goossens GH, Niessen PM, van Greevenbroek MM, van der Kallen CJ, Niessen HW, Rensen SS, Buurman WA, Greve JW, Blaak EE, van Zandvoort MA, Bierhaus A, Stehouwer CD, Schalkwijk CG. Nε-(carboxymethyl)lysine-receptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arterioscler Thromb Vasc Biol 34: 1199–1208, 2014. doi: 10.1161/ATVBAHA.113.302281. [DOI] [PubMed] [Google Scholar]
- 16.Galichet A, Weibel M, Heizmann CW. Calcium-regulated intramembrane proteolysis of the RAGE receptor. Biochem Biophys Res Commun 370: 1–5, 2008. doi: 10.1016/j.bbrc.2008.02.163. [DOI] [PubMed] [Google Scholar]
- 17.Grossin N, Wautier MP, Meas T, Guillausseau PJ, Massin P, Wautier JL. Severity of diabetic microvascular complications is associated with a low soluble RAGE level. Diabetes Metab 34: 392–395, 2008. doi: 10.1016/j.diabet.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Guclu M, Ali A, Eroglu DU, Büyükuysal SO, Cander S, Ocak N. Serum levels of sRAGE are associated with body measurements, but not glycemic parameters in patients with prediabetes. Metab Syndr Relat Disord 14: 33–39, 2016. doi: 10.1089/met.2015.0078. [DOI] [PubMed] [Google Scholar]
- 19.Haffner SM. Epidemiology of type 2 diabetes: risk factors. Diabetes Care 21, Suppl 3: C3–C6, 1998. doi: 10.2337/diacare.21.3.C3. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann MA, Drury S, Hudson BI, Gleason MR, Qu W, Lu Y, Lalla E, Chitnis S, Monteiro J, Stickland MH, Bucciarelli LG, Moser B, Moxley G, Itescu S, Grant PJ, Gregersen PK, Stern DM, Schmidt AM. RAGE and arthritis: the G82S polymorphism amplifies the inflammatory response. Genes Immun 3: 123–135, 2002. doi: 10.1038/sj.gene.6363861. [DOI] [PubMed] [Google Scholar]
- 21.Huang KP, Chen C, Hao J, Huang JY, Liu PQ, Huang HQ. AGEs-RAGE system down-regulates Sirt1 through the ubiquitin-proteasome pathway to promote FN and TGF-β1 expression in male rat glomerular mesangial cells. Endocrinology 156: 268–279, 2015. doi: 10.1210/en.2014-1381. [DOI] [PubMed] [Google Scholar]
- 22.Huang M, Que Y, Shen X. Correlation of the plasma levels of soluble RAGE and endogenous secretory RAGE with oxidative stress in pre-diabetic patients. J Diabetes Complications 29: 422–426, 2015. doi: 10.1016/j.jdiacomp.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Hudson BI, Stickland MH, Grant PJ. Identification of polymorphisms in the receptor for advanced glycation end products (RAGE) gene: prevalence in type 2 diabetes and ethnic groups. Diabetes 47: 1155–1157, 1998. doi: 10.2337/diabetes.47.7.1155. [DOI] [PubMed] [Google Scholar]
- 24.Jialal I, Adams-Huet B, Pahwa R. Selective increase in monocyte p38 mitogen-activated protein kinase activity in metabolic syndrome. Diab Vasc Dis Res 13: 93–96, 2016. doi: 10.1177/1479164115607829. [DOI] [PubMed] [Google Scholar]
- 25.Karstoft K, Winding K, Knudsen SH, James NG, Scheel MM, Olesen J, Holst JJ, Pedersen BK, Solomon TP. Mechanisms behind the superior effects of interval vs continuous training on glycaemic control in individuals with type 2 diabetes: a randomised controlled trial. Diabetologia 57: 2081–2093, 2014. doi: 10.1007/s00125-014-3334-5. [DOI] [PubMed] [Google Scholar]
- 26.Kim OY, Jo SH, Jang Y, Chae JS, Kim JY, Hyun YJ, Lee JH. G allele at RAGE SNP82 is associated with proinflammatory markers in obese subjects. Nutr Res 29: 106–113, 2009. doi: 10.1016/j.nutres.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Kitada M, Koya D. SIRT1 in type 2 diabetes: mechanisms and therapeutic potential. Diabetes Metab J 37: 315–325, 2013. doi: 10.4093/dmj.2013.37.5.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HR, Shin HK, Park SY, Kim HY, Lee WS, Rhim BY, Hong KW, Kim CD. Cilostazol suppresses β-amyloid production by activating a disintegrin and metalloproteinase 10 via the upregulation of SIRT1-coupled retinoic acid receptor-β. J Neurosci Res 92: 1581–1590, 2014. doi: 10.1002/jnr.23421. [DOI] [PubMed] [Google Scholar]
- 29.Liu XY, Li HL, Su JB, Ding FH, Zhao JJ, Chai F, Li YX, Cui SC, Sun FY, Wu ZY, Xu P, Chen XH. Regulation of RAGE splicing by hnRNP A1 and Tra2β-1 and its potential role in AD pathogenesis. J Neurochem 133: 187–198, 2015. doi: 10.1111/jnc.13069. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Yu M, Zhang L, Cao Q, Song Y, Liu Y, Gong J. Soluble receptor for advanced glycation end products mitigates vascular dysfunction in spontaneously hypertensive rats. Mol Cell Biochem 419: 165–176, 2016. doi: 10.1007/s11010-016-2763-5. [DOI] [PubMed] [Google Scholar]
- 31.Mahmoud AM, Szczurek MR, Blackburn BK, Mey JT, Chen Z, Robinson AT, Bian JT, Unterman TG, Minshall RD, Brown MD, Kirwan JP, Phillips SA, Haus JM. Hyperinsulinemia augments endothelin-1 protein expression and impairs vasodilation of human skeletal muscle arterioles. Physiol Rep 4: e12895, 2016. doi: 10.14814/phy2.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metz VV, Kojro E, Rat D, Postina R. Induction of RAGE shedding by activation of G protein-coupled receptors. PLoS One 7: e41823, 2012. doi: 10.1371/journal.pone.0041823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monden M, Koyama H, Otsuka Y, Morioka T, Mori K, Shoji T, Mima Y, Motoyama K, Fukumoto S, Shioi A, Emoto M, Yamamoto Y, Yamamoto H, Nishizawa Y, Kurajoh M, Yamamoto T, Inaba M. Receptor for advanced glycation end products regulates adipocyte hypertrophy and insulin sensitivity in mice: involvement of Toll-like receptor 2. Diabetes 62: 478–489, 2013. doi: 10.2337/db11-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ Jr, Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med 4: 1025–1031, 1998. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- 35.Pihlajamäki J, Lerin C, Itkonen P, Boes T, Floss T, Schroeder J, Dearie F, Crunkhorn S, Burak F, Jimenez-Chillaron JC, Kuulasmaa T, Miettinen P, Park PJ, Nasser I, Zhao Z, Zhang Z, Xu Y, Wurst W, Ren H, Morris AJ, Stamm S, Goldfine AB, Laakso M, Patti ME. Expression of the splicing factor gene SFRS10 is reduced in human obesity and contributes to enhanced lipogenesis. Cell Metab 14: 208–218, 2011. doi: 10.1016/j.cmet.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prakash J, Pichchadze G, Trofimov S, Livshits G. Age and genetic determinants of variation of circulating levels of the receptor for advanced glycation end products (RAGE) in the general human population. Mech Ageing Dev 145: 18–25, 2015. doi: 10.1016/j.mad.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Raucci A, Cugusi S, Antonelli A, Barabino SM, Monti L, Bierhaus A, Reiss K, Saftig P, Bianchi ME. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). FASEB J 22: 3716–3727, 2008. doi: 10.1096/fj.08-109033. [DOI] [PubMed] [Google Scholar]
- 38.Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, Allison TG, Batsis JA, Sert-Kuniyoshi FH, Lopez-Jimenez F. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes 32: 959–966, 2008. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest 108: 949–955, 2001. doi: 10.1172/JCI200114002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selvin E, Halushka MK, Rawlings AM, Hoogeveen RC, Ballantyne CM, Coresh J, Astor BC. sRAGE and risk of diabetes, cardiovascular disease, and death. Diabetes 62: 2116–2121, 2013. doi: 10.2337/db12-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solomon TP, Haus JM, Kelly KR, Cook MD, Filion J, Rocco M, Kashyap SR, Watanabe RM, Barkoukis H, Kirwan JP. A low-glycemic index diet combined with exercise reduces insulin resistance, postprandial hyperinsulinemia, and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. Am J Clin Nutr 92: 1359–1368, 2010. doi: 10.3945/ajcn.2010.29771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solomon TP, Knudsen SH, Karstoft K, Winding K, Holst JJ, Pedersen BK. Examining the effects of hyperglycemia on pancreatic endocrine function in humans: evidence for in vivo glucotoxicity. J Clin Endocrinol Metab 97: 4682–4691, 2012. doi: 10.1210/jc.2012-2097. [DOI] [PubMed] [Google Scholar]
- 43.Solomon TP, Malin SK, Karstoft K, Knudsen SH, Haus JM, Laye MJ, Kirwan JP. Association between cardiorespiratory fitness and the determinants of glycemic control across the entire glucose tolerance continuum. Diabetes Care 38: 921–929, 2015. doi: 10.2337/dc14-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song F, Hurtado del Pozo C, Rosario R, Zou YS, Ananthakrishnan R, Xu X, Patel PR, Benoit VM, Yan SF, Li H, Friedman RA, Kim JK, Ramasamy R, Ferrante AW Jr, Schmidt AM. RAGE regulates the metabolic and inflammatory response to high-fat feeding in mice. Diabetes 63: 1948–1965, 2014. doi: 10.2337/db13-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su XD, Li SS, Tian YQ, Zhang ZY, Zhang GZ, Wang LX. Elevated serum levels of advanced glycation end products and their monocyte receptors in patients with type 2 diabetes. Arch Med Res 42: 596–601, 2011. doi: 10.1016/j.arcmed.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Tam XH, Shiu SW, Leng L, Bucala R, Betteridge DJ, Tan KC. Enhanced expression of receptor for advanced glycation end-products is associated with low circulating soluble isoforms of the receptor in type 2 diabetes. Clin Sci (Lond) 120: 81–89, 2011. doi: 10.1042/CS20100256. [DOI] [PubMed] [Google Scholar]
- 47.Tang SC, Yeh SJ, Tsai LK, Hu CJ, Lien LM, Peng GS, Yang WS, Chiou HY, Jeng JS. Cleaved but not endogenous secretory RAGE is associated with outcome in acute ischemic stroke. Neurology 86: 270–276, 2016. doi: 10.1212/WNL.0000000000002287. [DOI] [PubMed] [Google Scholar]
- 48.Thomas MC, Woodward M, Neal B, Li Q, Pickering R, Marre M, Williams B, Perkovic V, Cooper ME, Zoungas S, Chalmers J, Hillis GS; ADVANCE Collaborative Group . Relationship between levels of advanced glycation end products and their soluble receptor and adverse outcomes in adults with type 2 diabetes. Diabetes Care 38: 1891–1897, 2015. doi: 10.2337/dc15-0925. [DOI] [PubMed] [Google Scholar]
- 49.Tippmann F, Hundt J, Schneider A, Endres K, Fahrenholz F. Up-regulation of the α-secretase ADAM10 by retinoic acid receptors and acitretin. FASEB J 23: 1643–1654, 2009. doi: 10.1096/fj.08-121392. [DOI] [PubMed] [Google Scholar]
- 50.Uribarri J, Cai W, Ramdas M, Goodman S, Pyzik R, Chen X, Zhu L, Striker GE, Vlassara H. Restriction of advanced glycation end products improves insulin resistance in human type 2 diabetes: potential role of AGER1 and SIRT1. Diabetes Care 34: 1610–1616, 2011. doi: 10.2337/dc11-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Houven van Oordt W, Diaz-Meco MT, Lozano J, Krainer AR, Moscat J, Cáceres JF. The MKK(3/6)-p38-signaling cascade alters the subcellular distribution of hnRNP A1 and modulates alternative splicing regulation. J Cell Biol 149: 307–316, 2000. doi: 10.1083/jcb.149.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Warner P. Ordinal logistic regression. J Fam Plann Reprod Health Care 34: 169–170, 2008. doi: 10.1783/147118908784734945. [DOI] [PubMed] [Google Scholar]
- 53.Williamson DL, Dungan CM, Mahmoud AM, Mey JT, Blackburn BK, Haus JM. Aberrant REDD1-mTORC1 responses to insulin in skeletal muscle from type 2 diabetics. Am J Physiol Regul Integr Comp Physiol 309: R855–R863, 2015. doi: 10.1152/ajpregu.00285.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu XY, Deng CQ, Wang J, Deng XJ, Xiao Q, Li Y, He Q, Fan WH, Quan FY, Zhu YP, Cheng P, Chen GJ. Plasma levels of soluble receptor for advanced glycation end products in Alzheimer’s disease. Int J Neurosci 127: 454–458, 2017. 10.1080/00207454.2016. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto Y, Miura J, Sakurai S, Watanabe T, Yonekura H, Tamei H, Matsuki H, Obata K, Uchigata Y, Iwamoto Y, Koyama H, Yamamoto H. Assaying soluble forms of receptor for advanced glycation end products. Arterioscler Thromb Vasc Biol 27: e33–e34, 2007. doi: 10.1161/ATVBAHA.107.144337. [DOI] [PubMed] [Google Scholar]
- 56.Yonekura H, Yamamoto Y, Sakurai S, Petrova RG, Abedin MJ, Li H, Yasui K, Takeuchi M, Makita Z, Takasawa S, Okamoto H, Watanabe T, Yamamoto H. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J 370: 1097–1109, 2003. doi: 10.1042/bj20021371. [DOI] [PMC free article] [PubMed] [Google Scholar]