Abstract
Augmented intratubular angiotensin (ANG) II is a key determinant of enhanced distal Na+ reabsorption via activation of epithelial Na+ channels (ENaC) and other transporters, which leads to the development of high blood pressure (BP). In ANG II-induced hypertension, there is increased expression of the prorenin receptor (PRR) in the collecting duct (CD), which has been implicated in the stimulation of the sodium transporters and resultant hypertension. The impact of PRR deletion along the nephron on BP regulation and Na+ handling remains controversial. In the present study, we investigate the role of PRR in the regulation of renal function and BP by using a mouse model with specific deletion of PRR in the CD (CDPRR-KO). At basal conditions, CDPRR-KO mice had decreased renal function and lower systolic BP associated with higher fractional Na+ excretion and lower ANG II levels in urine. After 14 days of ANG II infusion (400 ng·kg−1·min−1), the increases in systolic BP and diastolic BP were mitigated in CDPRR-KO mice. CDPRR-KO mice had lower abundance of cleaved αENaC and γENaC, as well as lower ANG II and renin content in urine compared with wild-type mice. In isolated CD from CDPRR-KO mice, patch-clamp studies demonstrated that ANG II-dependent stimulation of ENaC activity was reduced because of fewer active channels and lower open probability. These data indicate that CD PRR contributes to renal function and BP responses during chronic ANG II infusion by enhancing renin activity, increasing ANG II, and activating ENaC in the distal nephron segments.
Keywords: blood pressure, renin-angiotensin system, distal tubular Na+ transport, prorenin, renin
increased activity of the intrarenal renin-angiotensin system (RAS) in a setting of elevated arterial blood pressure (BP) increases renal vasoconstriction, fractional sodium (Na+) reabsorption, and elicits tissue fibrosis (19). The Na+ retention is due, in part, to stimulation of Na+ reabsorption in the collecting ducts (CDs), via angiotensin II (ANG II) type 1 receptor (AT1R)-dependent activation of epithelial Na+ channels (ENaC) and other distal transporters (8, 12, 16, 25). Even small adjustments in the amount of Na+ reabsorbed by the CD exert substantial impact on Na+ balance and BP. In ANG II-induced hypertensive mice, ENaC activity is increased above the physiological range and is not effectively suppressed by mineralocorticoid receptor (MR) blockade (15). This evidence highlights the role of the CD as a critical regulator of Na+ excretion and BP, and emphasizes the necessity for complete delineation of the mechanisms increasing ANG II–dependent actions on distal Na+ transport.
The prorenin receptor (PRR), a component of the RAS, activates prorenin and enhances the catalytic activity of renin, which increases formation of ANG I and conversion to ANG II (21). In the kidney, the PRR is expressed in mesangial cells, podocytes, renal vasculature, macula densa, proximal tubule (PT) cells (38), and intercalated cells of the CD (1, 4, 7). The PRR is upregulated in the CD in models of ANG II–dependent hypertension, including chronic ANG II-infused hypertension, two-kidney one-clip Goldblatt hypertension, and Ren2TGR malignant hypertension (4, 26, 27). In these experimental models of hypertension, the higher ANG II content in the renal medulla compared with cortex is associated with augmentation of prorenin in the principal cells of the CD (9, 26, 27, 29). The production and release of mainly prorenin by the principal cells occurs in response to ANG II via activation of AT1R (5, 6, 30). Activation of prorenin by PRR may be particularly important in the CD compared with renin from juxtaglomerular cells, which is predominantly in the active form.
The impact of the deletion of PRR along the nephron on BP regulation and Na+ handling remains controversial. Trepiccione et al. (44), using a mouse model with an inducible PRR deletion along the entire nephron, reported no effects on intrarenal ANG II production, ANG II–dependent BP regulation, or sodium handling in the kidney. In contrast, using the same nephron-wide PRR-deficient mouse model, Ramkumar et al. (33) demonstrated attenuated hypertensive and Na+ retention responses to chronic ANG II infusions. Therefore it is imperative to unravel the specific role of PRR in the CD in the pathogenesis of hypertension. To define the precise contribution(s) of PRR in the distal nephron segments, we generated mice with cell-specific deletion of PRR in the CD (CDPRR-KO mice) by using Cre-LoxP technology and Cre transgene driven by the Hoxb7 promoter. We hypothesize that CD PRR regulates BP by increasing intratubular renin, ANG II, and ENaC activity during ANG II-induced hypertension.
MATERIALS AND METHODS
All animal protocols were approved by the Animal Care and Use Committee of Tulane University School of Medicine and University of Texas Health Science Center at Houston (UT Health) and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. A total number of 96 mice were used for this study. By the completion of the experimental protocols (20–24 wk), no signs of disease or mortality were observed in the knockout (KO) mice.
Development of the CDPRR-KO mouse model.
Gene-targeted C57BL/6 mice with the exon 2 of the Atp6ap2 gene (encoding PRR) flanked by two loxP sites (11) were provided in collaboration with Dr. Atzuhiro Ichihara (Tokyo Women’s Medical University, Tokyo, Japan). These mice were used to establish the mouse colony with CD-specific ablation of the PRR obtained by breeding Atp6ap2-floxed mice with mice that expressed the Cre-recombinase under the control of a Hox-b7 promoter. The resultant Atp6ap2lox/Y, Hox-b7-Cre+/0 mice were named CDPRR-KO mice. Littermates of floxed PRR mice without the Hox-b7-Cre+/0 transgene were used as wild-type (WT) phenotype since no morphological or physiological differences between WT and floxed mice were found. For genotyping, tail DNA was isolated, and PCR was performed using the following primers: Atp6ap2, forward 5-GGG GGG TAA ATT GTT GAT GAG TCT TGG AGC ATA GC-3 and reverse 5-GAA GCC CAT GGA CAG TGC AGC TAC GTC TGG GAT TCG A-3′, which yields a 600-bp product from the floxed Atp6ap2 gene and a 400-bp product from the WT allele; Hox-b7, forward 5-GGA CAT GTT CAG GGA TCG CCA GGC-3; CreTag, reverse 5-GCG AAC ATC TTC AGG TTC TGC GG-3; and R2D2, reverse 5-CGA CGA TGA AGC ATG TTT AGC TG −3′, which yield a 200-bp product for the Hox-b7-Cre-positive allele. Mice were bred for at least six generations, and homozygous male KO (PRR gene is on the X chromosome) and floxed mice, 10–12 wk old, and 25- to 30-g body weight were used for all studies.
Morphological macro- and microscopic characterizations of the kidney of CDPRR-KO mice.
Mice were conscious and euthanized by rapid decapitation to avoid sympathetic related-RAS activation. After euthanasia, kidneys from WT (n = 8) and CDPRR-KO (n = 8) male mice (10–12 wk old) were immediately removed to assess macro- and microscopic characteristics. Kidneys were measured, weighted, and then fixed in 10% formaldehyde in PBS (pH = 7.4). Sections were stained with hematoxylin and eosin for microscopic examination of cortical and medullary regions and glomeruli number count. The glomeruli number was estimated using three kidney cross sections per animal separated by 100 µm (Table 1). In addition, 3-µm sections from paraffin-embedded kidneys were processed for immunohistochemistry by using a peroxidase technique, as previously described (4). Specific PRR immunostaining was assessed using a polyclonal rabbit anti-PRR antibody (cat. no. HPA 003156, Sigma, St. Louis, MO) at 1:500 dilution. Proper controls included preabsorption for 72 h with specific immune peptides by using the following sequences: GKANSVFEDLSVTLR (139–153) and KDHSPDLYSLELAGL (206–220).
Table 1.
Body weight, kidney weight, glomeruli number, as well as renal functional data in anesthetized WT and CDPRR-KO mice
| WT | CDPRR-KO | |
|---|---|---|
| Body weight, g | 25.96 ± 1.09 | 26.44 ± 1.44 |
| Kidney weight, g | 0.39 ± 0.02 | 0.32 ± 0.03* |
| Kidney weight/body weight, g | 0.015 ± 0.001 | 0.012 ± 0.001* |
| Gross estimated glomeruli number | 47.17 ± 2.34 | 35.80 ± 2.65** |
| MAP, mmHg | 84.4 ± 1.3 | 71.5 ± 3.7** |
| Plasma osmolality, mosmol/kgH2O | 324 ± 7 | 317 ± 8 |
| Urine osmolality, mosmol/kgH2O | 939 ± 49 | 577 ± 56*** |
| Urine flow, µl/min | 1.13 ± 0.07 | 2.99 ± 0.44* |
| Urinary pH | 6.23 ± 0.12 | 6.34 ± 0.16 |
| FENa+, % | 0.88 ± 0.15 | 1.54 ± 0.35* |
| FEK+, % | 1.5 ± 0.2 | 1.3 ± 1.2 |
| GFR, ml·min−1·g kidney wt−1 | 0.89 ± 0.12 | 0.54 ± 0.06* |
| RBF, ml·min−1·g kidney wt−1 | 6.49 ± 0.94 | 5.23 ± 0.75 |
| RVR, mmHg·min−1·g−1 kidney wt | 15.75 ± 1.82 | 14.04 ± 1.26 |
Data presented as means ± SE. CDPRR-KO, deletion of the prorenin receptor in the collecting duct; WT, wild-type; MAP, mean arterial pressure; FENa+, fractional excretion of sodium; FEK+, fractional excretion of potassium; GFR, glomerular filtration rate; RBF, renal blood flow; RVR, renal vascular resistance.
P < 0.05;
P < 0.01;
P < 0.001 vs. WT mice.
Renal functional studies.
For this protocol, WT (n = 8) and CDPRR-KO male (n = 6) mice were anesthetized with Inactin (100 mg/kg ip; cat. no. T133, Sigma) and then placed on a temperature-regulated surgical table to maintain a stable body temperature. Briefly, after a tracheotomy (PE-90; cat. No. 427421, Becton Dickinson, Sparks, MD), catheters were inserted into the bladder (PE-50; cat. no. 427411, Becton Dickinson) for collection of urine samples and into the carotid artery (PE-50 + PE 10; cat. no. 427401, Becton Dickinson) to record mean arterial pressure (MAP; MP 100A, Biopac System) throughout the experiment and for blood withdrawal. An additional catheter was implanted in the jugular vein (PE-50 + PE-10) for intravenous infusions. A 60-min stabilization period was allowed before baseline periods were started. Three 30-min basal clearance collections were obtained. The infusion rate did not cause volume expansion because body weight increased <4% in the mice (41). MAP was continuously recorded in anesthetized mice, and data were averaged for each clearance period. Urine flow was calculated taking together the urine volume and the length of each period and UNaV and UKV were calculated by multiplying UV by the urine sodium and potassium concentration, respectively, measured with a flame photometer (Instrumentation Laboratory 943). Blood samples were used to measure inulin, PAH, and electrolyte concentrations. Standard clearance formulas were used to calculate glomerular filtration rate (GFR) and estimated renal plasma flow (eRPF) by using the renal clearances of inulin and PAH, respectively. Standardized GFR values were similar to those obtained in anesthetized rats by using [3H]-inulin clearance (36). Renal blood flow (RBF) changes were calculated from eRPF and hematocrit values. The clearances were normalized by kidney weight. Plasma and urine osmolality were measured by vapor pressure osmometry (Vapro Osmometer, model 5600, Wescor). After completion of the study, animals were euthanized by administration of an overdose of anesthesia.
Physiological responses to changes in dietary salt intake.
To examine physiological responses to changes in salt intake, WT (n = 8) and CDPRR-KO (n = 6) male mice (10–12 wk old) were sequentially fed for 3 days on each type of diet: a normal-salt diet (NS; 0.3% NaCl; cat. no. 113781, Diets, Inc., Bethlehem, PA), a high-salt diet (HS; 4% NaCl; cat. no. 113756, Diets, Inc.), and a low-salt diet (LS; 0.001% NaCl; cat. no. 103602, Diets, Inc.). During the last 24 h on each specific diet, the mice were placed in cages for urine metabolic assessment. A similar set of mice was used for systolic blood pressure (SBP) assessment by using tail-cuff plethysmography with a Visitech BP2000 system (Visitech Systems, Apex, NC) in previously trained mice.
Physiological responses to water deprivation.
We further examined whether conscious mice have altered ability to concentrate urine. Male WT (n = 6) and CDPRR-KO (n = 6) mice were subjected to 24-h water deprivations or ad libitum tap water supplementation. During the water deprivation, animals were maintained in metabolic cages with a training period of 5 days prior water deprivation. Plasma and urine osmolality were measured by vapor pressure osmometry (Vapro Osmometer, model 5600, Wescor).
Responses to chronic ANG II infusions.
Metabolic studies, SBP and diastolic blood pressure (DBP), renin activity, and ANG II measurements were made as follows. WT (n = 8) and CDPRR-KO (n = 8) male mice were infused with ANG II (cat. no. A9525, Sigma) via subcutaneous microsmotic pumps (Alzet model 1002) at a concentration of 400 ng·kg−1·min−1 for 14 days, as previously described (8, 9). During this protocol, mice were placed in metabolic cages for metabolic assessment and 24-h urine collections. To avoid excessive stress with a potential effect on BP in the mice, a similar set of WT (n = 8) and CDPRR-KO (n = 8) mice was handled without metabolic caging. These mice were implanted with a carotid catheter with radio transmitters (model PA-C10; Dataquest A.R.T, Software 4.0, DSI, St. Paul, MN) for daily BP recordings during 2-h periods. A chronic ANG II infusion protocol started after 2 wk of recovery from surgical telemeter implantation for BP measurements. Mice were euthanized by conscious decapitation on day 14, and the kidneys were immediately removed and quickly weighed as previously described (9, 14).
Renin content and ANG II levels during chronic ANG II infusions.
Mice were placed in metabolic cages for urine collection on day 2 (baseline) and on days 3, 6, 10, and 14 of ANG II infusion. Urine samples were centrifuged, and the volume was measured and stored at −80°C. Blood samples were obtained in vials with EDTA by a submandibular bleeding method on the same days. Renin content and ANG II were measured by an ELISA (cat. no. RE53321, City State, IBL International) and EIA kit (cat. no. 589301, Cayman Chemical, Ann Arbor, MI), respectively (14, 37).
Determination of urinary AGT, renin, and ANG II.
One hundred microliters of standard samples of mouse angiotensinogen (AGT; 0.16–10 ng/ml) diluted in ELISA buffer and urine (1:25 diluted in ELISA buffer) were added into each well of the plates and sequentially incubated at 37°C for 1 h, washed, and then incubated with 100 μl/well of a horseradish peroxidase-labeled COOH-terminal antibody (1:30 diluted in antibody solution) at 37°C for 30 min. The plates were then incubated with 100 μl/well of 3,3′,5,5′-tetramethylbenzidine solution under light-protected conditions at room temperature for 30 min, followed by the administration of 100 μl/well of sulfuric acid (0.5 mol/l) to stop the reaction. Absorbance was measured at 450 nm.
Determination of urinary albumin.
Mice were placed in metabolic cages overnight (16 h) for urine collection, both the day before minipump implantation (baseline) and the day before euthanasia (ANG II). Urine volumes were determined volumetrically, and a constant fraction (0.5%) of the overnight urine was denatured in Laemmli sample buffer, resolved by SDS-PAGE, and stained with Bio-Safe Coomassie G-250 stain (Bio-Rad Laboratories) according to the manufacturer’s directions: gel was fixed (40% methanol, 10% acetic acid, 50% water) for 10 min three times then stained with Bio-Safe Coomassie G-250 stain for 1 h, and then rinsed with water for 30 min four times. Albumin was detected as the major band on the gel (between the 50- and 75-kDa molecular weight markers). The destained gel was imaged and quantified with the Odyssey Infrared Imaging System (LI-COR) and quantified by accompanying software.
Characterization of distal nephron transporters.
As described previously (22), homogenates were denatured in SDS-PAGE sample buffer for 20 min at 60°C. To assess consistency of protein loading, 15 µg of protein from each sample was resolved by SDS-PAGE, the gel was stained with Bio-Safe Coomassie G-250 stain, and density of bands in the lane quantitated with the LI-COR Odyssey System (17). The density of protein stain/lane varied <20%. For immunoblotting, each sample was run at both 20 and 40 μg/lane to verify linearity of the detection system; only one amount is shown (see Fig. 4). Specific protein abundance and phosphorylation was assessed using antibodies as described in detail (22). Primary antibodies used included the following: anti-sodium-chloride cotransporter (NCC) [1:5,000; McDonough Laboratory (22); anti-NCC phosphorylated at Ser71 (NCCpS71, Loffing, Univ. of Zurich) (40); anti-ENaC α (1:5,000), anti-ENaC β (1:15,000), and anti-ENaC γ (1:15,000; Loffing, Univ. of Zurich) (40)]. Secondary antibodies were tagged with either Alexa Fluor 680 (Invitrogen) or IRDye 800 (LI-COR, Lincoln, NE). Signals were detected with the Odyssey Infrared Imaging System (LI-COR) and quantified by accompanying software. Arbitrary density units were normalized to mean intensity of control group, defined as 1.0. Since the samples were run twice (at 1 and 1/2), the normalized values were averaged and mean values compiled for statistical analysis.
Assessment of ENaC in split-open CDs.
Six mice from each experimental group were used for this protocol. After euthanasia by CO2 plus cervical dislocation, kidneys were removed for patch-clamp experiments (n = 40–50). Patch-clamp experiments were performed in freshly isolated microdissected cortical CDs from kidney slices (<1 mm) obtained from WT and CDPRR-KO mice chronically infused with ANG II (400 ng·kg−1·min−1 for 14 days) or ANG II plus spironolactone (30 mg/kg body wt, added to drinking water for 14 days) and subsequently split open to measure ENaC activity, ENaC open probability (Po), and ENaC functional expression in situ, as previously described (16, 45). ENaC activity in principal cells was determined in cell-attached patches on the apical membrane made under voltage-clamp conditions (−Vp = −60 mV) by using standard procedures (16).
Statistical analysis.
Results are expressed as means ± SE. Grubb’s test was used to detect outliers in univariate data assumed to come from a normally distributed population. Comparisons between groups were performed using one-way ANOVA when appropriate with Tukey’s posttest. Interactions among genotypes are reported according to a two-way ANOVA by using pound (♯) symbols, and differences between treatments are reported using star (*) symbols. The Student unpaired t-test was used to compare data between CDPRR-KO and WT mice for Western blot and ENaC activity assays. P ≤ 0.05 values were considered statistically significant.
RESULTS
Renal morphological and physiological characterization of mice with specific PRR deletion in the CD.
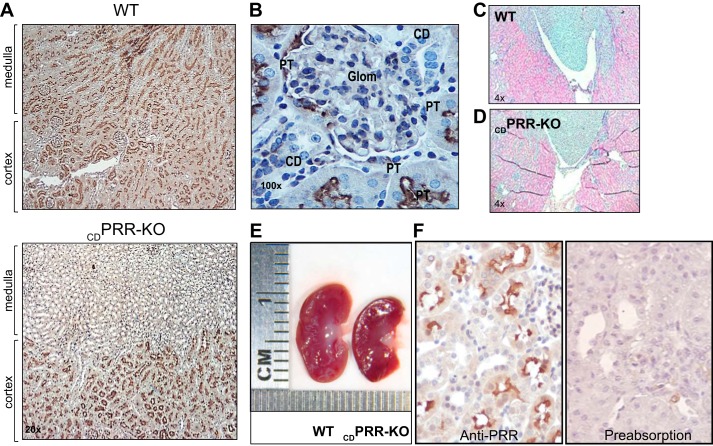
No differences were found between the kidneys from floxed and WT mice. Compared with WT mice, the kidneys from CDPRR-KO mice exhibited PRR positive immunoreactivity preferentially in cortical regions containing mainly glomeruli and PTs as previously described (38), but lesser PRR staining in the inner medullary regions containing mostly CD (Fig. 1A). These findings were further confirmed in microphotographs taken at higher magnification (Fig. 1B). At this antibody concentration, we were unable to see strong labeling in podocytes or mesangial cells as described (10). Kidneys from CDPRR-KO mice showed reduced glomeruli number (35.8 ± 2.7 vs. 47.2 ± 2.3; P < 0.01), mild hypoplasia of the renal medulla (Fig. 1, C and D), lower kidney weight (0.32 ± 0.03 vs. 0.39 ± 0.02 g; P < 0.05), and reduced kidney size compared with WT mice (Fig. 1 E). However, similar body weights were found between mice from both genotypes (26.4 ± 1.4 vs. 25.9 ± 1.1 g, P = nonsignificant). Figure 1F shows WT mouse kidney sections stained after incubations with either anti-PRR antibody (left) or preimmune peptide used at 30-fold in excess (right). The complete absence of PRR immunostaining in the section treated with preadsorbed antibody denotes the specificity of the anti-PRR antibody used in the experiments.
Fig. 1.
A: kidney sections (3 µm) from a wild-type (WT) mouse (top) showed positive prorenin receptor (PRR) immunoreactivity in glomeruli, proximal tubules, and collecting ducts (CDs) in renal cortex and medulla; however, in the kidney from a deletion of PRR in the CD (CDPRR-KO) mouse (bottom), PRR immunoreactivity was mostly detected in cortical tubules but absent in renal medulla, primarily containing collecting ducts (×20 magnification). B: high magnification of PRR immunostaining (3,3prime diaminobenzidine tetrahydrochloride; DAB; brown chromogen, ×100) in a kidney section (3 µm) from a CDPRR-KO mouse. PRR was detected in glomeruli and proximal tubules (PT) but not in the CDs of CDPRR-KO mouse kidney. Kidney sections (3 µm, ×4 magnification) hematoxylin-stained from a wild-type (WT) (C) and knockout (KO) mice (D). E: gross morphology analysis in kidneys from a WT mouse and CDPRR-KO showing kidney size and internal structure. No evidence of significant medullary hypoplasia was observed. F: preabsorption of primary PRR antibody for 72 h using preimmune peptide 30-fold excess denotes the specificity of the antibody. Glom, glomeruli.
Responses to changes in dietary sodium chloride.
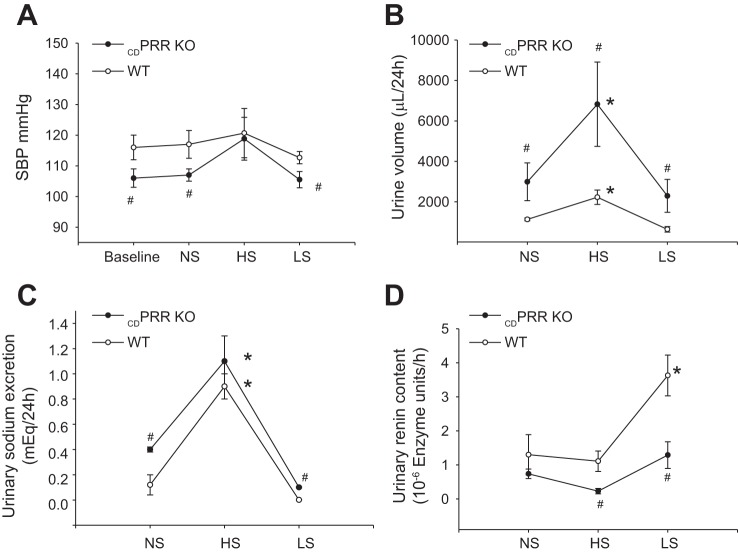
In conscious mice, changes in dietary salt (Fig. 2A) did not alter SBP in PRR-KO mice; however, SBP was lower than WT during NS (107 ± 2 vs. 117 ± 5 mmHg, P < 0.05) and LS diet (112 ± 2 vs. 105 ± 3 mmHg, P < 0.05). KO mice exhibited higher urine flow (Fig. 2B) and augmented urinary Na+ excretion during NS and LS diet, and a similar pattern during HS diet as compared with WT mice (Fig. 2C). Importantly, during HS and LS intakes, the content of renin in urine was significantly lower in KO mice compared with WT (Fig. 2D). Renin content was augmented in LS diet in WT animals but not in the KO mice. Despite the differences observed in Na+ excretion, no differences were found in food intake between WT (NS: 2.1 ± 0.3 g; HS: 2.3 ± 0.2 g; LS: 1.9 ± 0.4 g) and KO mice (NS: 2.0 ± 0.4 g; HS: 2.2 ± 0.3 g; LS: 2.3 ± 0.4 g). Table 1 summarizes renal morphology and functional data in anesthetized mice. MAP and GFR were significantly lower in PRR-KO mice than those in WT, but the lower RBF was not statistically significant. FENa+ and urine flow were greater in CDPRR-KO mice than in WT mice. Because urine osmolality was lower in CDPRR-KO mice than in WT, we further examined whether conscious mice have an impaired ability to concentrate the urine after 24-h water deprivation or ad libitum tap water supplementation. The CDPRR-KO mice subjected to water restriction showed a reduced increase in urine osmolality (1,153 ± 90 vs. 577 ± 56 mosmol/kgH2O, P = 0.032) compared with WT (939 ± 49 vs. 1,996 ± 34 mosmol/kgH2O, P = 0.0012). Taken together, these data indicate that CDPRR-KO mice have impairment in the ability to concentrate the urine and reabsorb Na+.
Fig. 2.
Physiological responses to changes in dietary salt in wild-type (WT, n = 8) and CDPRR-KO mice (n = 6). A: systolic blood pressure (SBP) measured by tail-cuff method at baseline during three consecutive days on normal-salt (NS), high-salt (HS), and low-salt (LS) diets. B: urine volume was higher in KO mice than WT during NS, HS, and LS diets. C: urine sodium excretion was higher in CDPRR-KO mice than WT at baseline and LS conditions. D: importantly, during LS diet, the content of active renin in the urine was augmented in WT mice but not in CDPRR-KO mice. Measurements were done by day 3 on each diet. Interactions among genotypes are reported according to the two-way ANOVA (#P < 0.05 vs. WT mice), and differences between treatments are reported (*P < 0.05 vs. baseline).
Responses to chronic ANG II infusion in mice with CD PRR deletion.
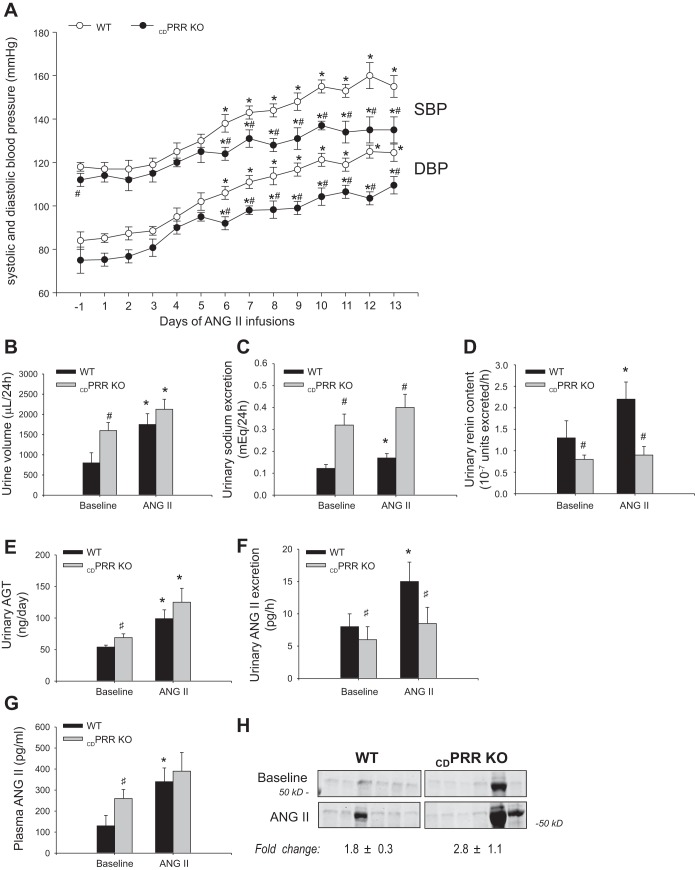
Telemetry studies in WT and CDPRR-KO mice (Fig. 3A) showed slight but significant differences in SBP at day 1 before ANG II infusion. SBP and DBP increased in WT mice starting at day 6 of ANG II infusion and progressively continued to increase until day 13, before euthanasia. In contrast, CDPRR-KO mice exhibited attenuated SBP and DBP responses compared with WT values, although both significantly started to increase by day 6. The higher urine volumes observed at baseline in CDPRR-KO mice were sustained at day 14 (Fig. 3B). The CDPRR-KO mice had higher Na+ excretion during both basal conditions and at the end of ANG II infusions (Fig. 3C), suggesting an impaired capability to reabsorb Na+.
Fig. 3.
Physiological responses to chronic ANG II infusions in wild-type (WT, n = 8) and CDPRR-KO (n = 8) mice. A: daily blood pressures (DBP and SBP) in WT and CDPRR-KO mice in response to chronic ANG II infusions (400 ng·kg−1·min−1 × 14 days). Blood pressure was measured by telemetry. Two lines show systolic (SBP) (top), and two lines show diastolic (DBP) (bottom). Analysis by ANOVA shows that both groups of mice exhibited increases in DBP and SBP after 5–6 days of ANG II infusions; however, CDPRR-KO mice showed attenuated responses compared with WT mice. *P < 0.05 vs. baseline; #P < 0.05 vs. WT mice. Urine volume (B) and urine sodium excretion (C) were higher in CDPRR-KO mice at baseline but significantly increased after ANG II infusions only in WT mice. D: urinary renin content was not different between both groups of mice at baseline. However, after ANG II infusions urinary renin content was significantly increased by ANG II infusions in WT mice but not in CDPRR-KO mice. E: urinary angiotensinogen (AGT) data is presented. F: in CDPRR-KO mice, ANG II excretion in the urine (pg/h) was reduced at baseline and after ANG II infusions. After ANG II infusions, the urinary excretion of ANG II only increased further in WT mice. G: at baseline, plasma ANG II levels (pg/ml) were higher in CDPRR-KO mice compared with WT mice, and the values increased further in both groups after ANG II infusions. *P < 0.05 vs. baseline; #P < 0.05 vs. WT mice. H: urinary albuminuria, assessed by SDS-PAGE in 0.5% of overnight urine, was similar at baseline and increased similarly in WT and CDPRR-KO mice; values shown are fold changes in ANG II/baseline assessed in the same gel which were not different between genotypes (n = 6 per group; P = 0.4, unpaired t-test).
Urinary AGT, renin, ANG II, and proteinuria during ANG II infusion.
After completion of 2 wk of ANG II infusion, AGT urinary excretion was augmented in both CDPRR-KO and WT mice (Fig. 3E); however, the ANG II content in urine was augmented in WT mice but not in CDPRR-KO mice (Fig. 3F), despite higher plasma ANG II concentrations in CDPRR-KO mice (Fig. 3G). These findings were in concert with the amount of renin content in urine, which was significantly augmented by the ANG II infusion in WT but not in the CDPRR-KO mice (Fig. 3D). At baseline, urinary albumin values overlapped in WT and CDPRR-KO mice and were increased similarly by ANG II treatment (fold-changes ANG II/baseline: WT: 1.8 ± 0.3-fold, CDPRR-KO: 2.8 ± 1.1-fold, (means ± SE, P = 0.4; Fig. 3H).
Effects of CD PRR deletion on distal sodium transporters at baseline and during ANG II infusion.
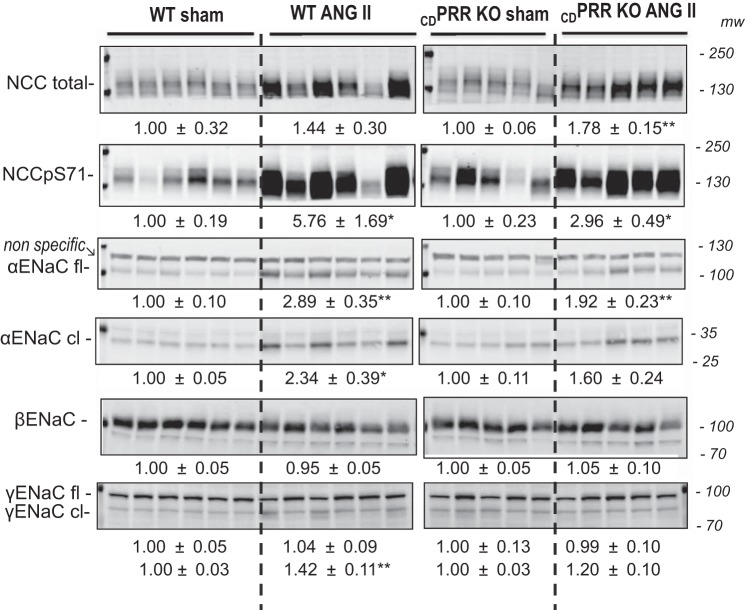
The higher Na+ excretion rates in the CDPRR-KO mice prompted examination of the effects of CD PRR deletion on the main distal sodium transporters in normal conditions and during ANG II infusion. We investigated the abundance of total NCC, NCC phosphorylated at serine 71, a marker of activation in the plasma membrane (NCCpS71), full-length ENaC α, β, and γ subunits, and cleaved (activated) forms of ENaC α and γ by using specific antibodies and a quantitative protocol (materials and methods). Figure 4 shows the immunoblots (n = 5–6 mice/group) by using specific antibodies against these transporters. No differences in the protein abundance of these transporters were found between WT and CDPRR-KO mice at baseline (noninfused-sham operated). ANG II similarly augmented NCC-pS71 and full-length αENaC in Sham and CDPRR-KO mice. Importantly, the cleaved (activated) forms of α- and γENaC were significantly augmented in WT mice infused with ANG II, but not in CDPRR-KO mice.
Fig. 4.
Representative immunoblots showing NCC and epithelial Na+ channel (ENaC) protein abundance in wild-type (WT, n = 12) and CDPRR-KO mice (n = 10) subjected to either sham surgery or chronic ANG II infusion (400 ng·kg−1·min−1 × 14 days). Immunoblots of renal homogenates indicate abundance of total NCC, NCC phosphorylated at S71, and α, β, and γENaC subunits in WT and CDPRR-KO mice. Both 20 and 40 μg of protein were run on each gel to establish linearity of the system. Only the 40-μg sample loading/lane is shown. *P < 0.05, **P < 0.01 vs. WT mice, unpaired t-test. cl, cleaved; fl, full length.
ENaC functional studies in split-opened CDs in WT and CDPRR-KO mice.
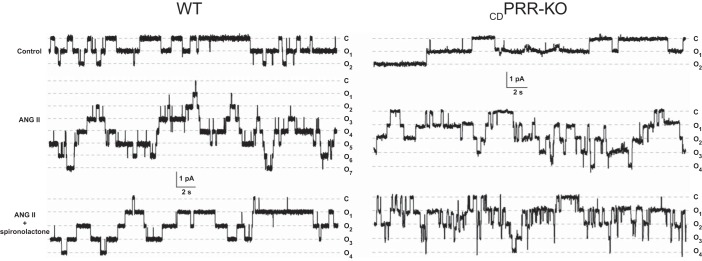
We further tested whether CD-specific PRR deletion affects functional ENaC expression (fN) and ENaC Po in WT and CDPRR-KO mice after 2 wk of infusions with vehicle (Sham n = 6), ANG II (n = 6), and ANG II plus spironolactone 30 mg/kg BW in drinking water (n = 6). Figure 5 shows representative traces of ENaC activity, which are summarized in Table 2 showing total ENaC activity (fNPo), fN, and Po in WT and CDPRR-KO mice. There were no differences in basal ENaC activity in WT and CDPRR-KO mice. ANG II markedly augmented ENaC activity by increasing both Po and the number of active channels; this response occurred predominantly in an aldosterone-independent manner. Although a similar stimulatory action of ANG II infusion on ENaC activity was observed in CDPRR-KO mice, the magnitude of this response was significantly lower. Specifically, the CDPRR-KO mice exhibited fewer fN and lower Po compared with the same conditions in WT mice. Inhibition of MR receptors with spironolactone had only a mild additional inhibitory effect and failed to reduce ENaC activity to basal levels.
Fig. 5.
A: representative continuous current traces from cell-attached patches (n = 40–50) monitoring ENaC activity in WT and CDPRR-KO mice after 2-wk infusion of vehicle (n = 6), 400 ng·kg body wt−1·min−1 ANG II (n = 6) and ANG II plus spironolactone (30 mg/kg BW; bottom row, n = 6). Patches were held at a test potential of Vh = −Vp = −60 mV. Inward Li+ currents are downward. Dashed lines indicate the respective current states marked as o1, and a c denotes the closed state. Summary of total ENaC activity, functional ENaC expression, and ENaC open probability are described in Table 2.
Table 2.
ENaC functional studies in split-open collecting ducts in WT and CDPRR-KO mice
| Group | fNPo | Po | fN |
|---|---|---|---|
| WT sham | 0.31 ± 0.06 | 0.32 ± 0.03 | 0.87 ± 0.12 |
| WT sham + ANG II | 1.63 ± 0.25* | 0.57 ± 0.04* | 3.73 ± 0.39* |
| WT sham + ANG II + spironolactone | 0.91 ± 0.09* | 0.52 ± 0.03* | 1.82 ± 0.18* |
| CDPRR-KO sham | 0.28 ± 0.05 | 0.30 ± 0.04 | 0.92 ± 0.11 |
| CDPRR-KO + ANG II | 1.11 ± 0.15*# | 0.41 ± 0.03*# | 2.47 ± 0.26*# |
| CDPRR-KO + ANG II + spironolactone | 0.87 ± 0.13* | 0.41 ± 0.03* | 2.01 ± 0.24 |
Data presented as means ± SE. Summary of total ENaC activity (fNPo), functional ENaC expression (fN), and ENaC open probability (Po) in wild-type (WT) and CDPRR-KO mice after 2 wk of infusions with vehicle (sham; n = 6), ANG II (n = 6), and ANG II plus spironolactone 30 mg/kg body wt in drinking water (n = 6).
P < 0.05 vs. sham WT split-open collecting ducts (n = 40–50);
P < 0.05 vs. WT corresponding treatment.
DISCUSSION
The specific role of the CD-derived PRR in the pathogenesis of hypertension is still controversial. The discrepancies could be due to specific strategies used for the generation of PRR KO mouse models, in which the Atp6ap2 gene has been deleted nephron-wide vs. those with specific cell-type deletion in the CD. Different interpretations might be also due to the suggested role of the PRR in kidney development (39). The absence of the Atp6ap2 gene causes increased endophagosome accumulation (18). Trepiccione et al. (44) reported the results from a mouse model with an inducible PRR deletion along the nephron by using a Pax8/rtTA/tetO/Cre strategy. In this study, no effects on intrarenal ANG II production, ANG II–dependent BP regulation, or sodium handling in the kidney were observed. In contrast, Ramkumar et al. (33) using the same nephron-wide mouse model with the Pax8/rtTA/tetO/Cre strategy demonstrated that PRR-deficient mice exhibited attenuated hypertensive and Na+ retention responses to chronic ANG II infusion. PAX8 is expressed in all kidney epithelial cells, among other tissues (43). The conditional PRR deletion by Cre-recombinase driven by the aquaporin-2 (AQP2) promoter showed that both urinary renin activity and α-ENaC abundance in the renal medulla were blunted in the null mice during chronic ANG II infusion (24). As indicated by the authors, this transgenic model with deletion of PRR from the whole CD, including principal and intercalated cells, did not show renal developmental abnormalities (24). Differences in the strategies to target PRR in the distinct nephron segments and cell types in the kidney may explain existent discrepancies. How the PRR, a receptor that is exclusively expressed in the intercalated cells, regulates ENaC, which is expressed primarily in the principal cells, remains uncertain. Therefore the specific role of PRR in the CD in the regulation of BP and in the pathogenesis of hypertension remains unclear.
To define the precise contributions of PRR in the distal nephron segments, we used mice with specific deletion of PRR in the CD (CDPRR-KO mice) using Cre-LoxP technology and a Cre transgene driven by the Hoxb7 promoter. Hoxb7 is a homeobox widely expressed in principal cells, intercalated cell types A and B, and non-A non-B and during the development of the ureteric bud (23). In this CDPRR-KO mouse model, despite grossly normal renal morphology, the mice exhibited smaller kidneys due to mild renal inner medulla hypoplasia (Fig. 1E). Our CDPRR-KO mice also had fewer glomeruli. Using a similar mouse model, Song et al. (39) reported that at developmental stage P1, kidneys from mutant mice presented with decreased nephron number, kidney hypoplasia, occasional cysts in the CD, and dilation of tubules from PT origin compared with control, suggesting that PRR was essential for ureteric bud branching and CD development. Regardless of the kidney development alterations, our transgenic mouse model has a normal life span, and it is suitable for metabolic, physiological, and renal functional studies. Despite the fact that fewer glomeruli is linked to a predisposition to the development of hypertension (9, 33), these CDPRR-KO mice showed reduced DBP and SBP responses to chronic ANG II infusion, supporting the role of CD-derived PRR in BP regulation.
In this study we provide the first evidence of renal function in CDPRR-KO mice. These mice had lower GFR and RPF along with lower resting MAP in anesthetized mice. CDPRR-KO mice exhibited higher fractional Na+ excretion and urine flow (Table 1). These differences suggest that CDPRR-KO have a greater pressure-natriuresis response than the WT mice. Therefore, the CDPRR-KO mice excrete more Na+ for a given BP, which provides a beneficial effect that mitigates the increases in SBP and DBP caused by chronic ANG II infusion (Fig. 3A). Indeed, in physiological conditions of augmented intrarenal ANG II during LS diet, CDPRR-KO mice have a blunted response. Furthermore, at baseline conditions, the urinary renin content was lower in the CDPRR-KO mice compared with WT. Plasma ANG II levels are primarily determined by the activity of renin in the plasma (PRA). It is possible that the CDPRR-KO mice have compensatory mechanisms involving increases in PRA. However, we did not measure PRA in these animals to rule out this possibility. Ramkumar et al. and Trepiccione et al. (32, 44) both reported higher plasma renin content values in CDPRR-KO mice. The alterations of the responses to ANG II in the CDPRR-KO mice are substantiated by the fact that urine renin content was augmented only in WT mice during physiological stimulation of intrarenal ANG II evoked by LS diet. We suggest that CD-derived PRR is of relevance for the activation of prorenin secreted by the distal nephron segment in response to local ANG II, particularly during ANG II dependent where PRA and juxtaglomerular renin are suppressed (20, 28). It has been shown that CD-derived PRR contributes to the regulation of water reabsorption (32, 39, 44), which might be due to alterations in the abundance of AQP2 (39). It is possible that the diminished abundance of AQP2 in the KO comply with the relative smaller renal inner medullary mass in the CDPRR-KO mice compared with WT mice.
All the components of the RAS needed for intratubular ANG II formation are augmented in the distal nephron segments during ANG II-induced hypertension (9, 19, 32). In the CD, the PRR can activate prorenin, thus contributing to the formation of ANG I and II from the AGT derived from PTs and ACE present on the luminal membranes along the CD (2, 12, 35). In the present study, the KO mice showed much higher UNaV than WT mice even before chronic ANG II infusion. This finding has not been previously reported. It is likely that this Na+ excretion response is a consequence of the decrease in intratubular ANG II content in KO mice. PRR could mediate the regulation of ENaC via a mechanism that is dependent and/or independent of intrarenal RAS (24). Here, we showed that the increase in DBP and SBP caused by ANG II infusion (400 ng·kg−1·min−1) was mitigated in CDPRR-KO mice. Importantly, CDPRR-KO mice exhibited blunted α- and γENaC cleavage, along with lower ANG II and renin content in urine compared with WT (Fig. 3, D and G). When Trepiccione et al. (44) subjected the mouse model with nephron-wide PRR deletion to chronic ANG II infusion (1,000 ng·kg−1·min−1 for 2 wk), they did not measure changes in intrarenal ANG II levels. Ramkumar et al. (32), however, in the same transgenic model (ANG II infused at 600 ng·kg−1·min−1 for 2 wk) found reduced renin content in urine but no changes in intrarenal ANG II levels. Discrepancies in the findings might be due to differences in the ANG II dosage used.
In concordance with the concept that de novo intratubular ANG II formation regulates ENaC in the CD, Ramkumar et al. (34) showed that specific deletion of the Renin 1c gene from the CD exhibit reduced ENaC protein expression even after chronic ANG II infusion, but did not provide information on the activated form. In the present study, we investigated the impact of the specific deletion of PRR in the CD on ENaC activity in isolated cortical CDs. ANG II increased ENaC activity in WT; however; the response was blunted in CDPRR-KO mice chronically infused with ANG II, which showed reduced ENaC Po and fewer active channels (fN). ANG II–dependent regulation of ENaC activity was further supported because MR inhibition with spironolactone failed to reduce ENaC activity to basal levels (Fig. 5 and Table 2). Chronic ANG II infusions cause elevation of plasma aldosterone levels (13). Despite the role of aldosterone, recent works support the independence of ANG II-driven regulation of ENaC in CD cells (3). It is likely that the attenuation of hypertension in CDPRR-KO mice is due, at least in part, to an impaired ANG II-dependent activation of ENaC (associated with reduced α and γ subunit cleavage) and reduced Na+ reabsorption, as indicated by the ENaC patch-clamp studies in the CD. Because chronic ANG II infusion has been reported to induce proteinuria with subsequent luminal proteolytic activation of γENaC by soluble proteases (42), we compared urinary albumin directly by SDS-PAGE before and after chronic ANG II infusion. Overall, the albuminuria was similar at baseline and increased similarly after ANG II in WT and CDPRR-KO mice (Fig. 3H). One mouse from each genotype exhibited high baseline proteinuria and exaggerated worsening in response to ANG II (Fig. 3H).
Despite the apparent inability to concentrate the urine, and lower mean arterial BP, CDPRR-KO mice did not manifest SBP changes during variations in dietary salt intake (Fig. 2A), similar to mice lacking the Renin 1c gene (34). Therefore, the functional effects of the interaction between CD-derived PRR and renin in the regulation of BP are not apparent during physiological alterations in salt intake.
The PRR in the CD directly targets ENaC to mediate ANG II-induced hypertension through the augmentation of αENaC, which seems to depend exclusively on a PRR-dependent intracellular pathway, likely involving vasopressin-dependent stimulation of cAMP (24). In isolated inner medullary CD, αENaC expression is markedly diminished in tubules from nephron-wide PRR-KO mice (33). The demonstration that the prorenin-dependent effect on ENaC activity is prevented by PKA (31–33) further advocates for an interaction between PRR and prorenin in the CD linked to cAMP cell signaling, which may contribute to stimulate ENaC in the principal cells. Hence the mechanisms underlying the regulation of ENaC by the PRR appear to be direct as well as indirect, likely involving paracrine mechanisms denoting the critical relevance of the functional consequences of the interactions between prorenin and the PRR in the CD.
In conclusion, it is now more apparent that the CD harbors a mechanism for the activation of locally produced prorenin, which increases intratubular ANG II content and stimulates ENaC-mediated Na+ reabsorption. In rodent models with high intrarenal ANG II content, tubular prorenin synthesis is increased significantly and secreted by the principal cells; PRR plays a critical role in the intratubular prorenin activation and subsequent ANG II formation. Potential mechanisms underlying discrepancies among phenotypes need to be more fully investigated with additional tools including in vitro approaches. Our data also suggest that the PRR in the CD is necessary for normal renal function and appropriate regulation of the mechanisms responsible for urine concentration during normal physiological conditions. During pathological settings, PRR is necessary for the regulation of Na+ handling. Thus the PRR in the CD facilitates intratubular ANG II formation and ANG II actions to synergistically potentiate the effects of aldosterone on ENaC activity, distal Na+ reabsorption, and overall BP regulation.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant R01 DK-104375, LACaTS Program Grant 1U54GM104940, and Tulane University School of Medicine, Faculty Research Pilot Funds (to M. Prieto); FONDECYT-Chile Grant 11121217 (to A. Gonzalez); NIDDK Grant R01 DK-083785 and American Heart Association (AHA)-Western States Affiliate Grant 15GRNT23160003 (to A. McDonough); AHA Grant 15SDG25550150 (to M. Mamenko); and NIDDK Grant R01 DK-095029 (to O. Pochynyuk). M. McLellan (medical student) was a scholar recipient of the Bourgeois and Ramadhyani Student Research Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.C.P. conceived and designed research; M.C.P., V.R., M. Mamenko, M.K., L.C.V., C.B.R., M. McLellan, O.G., V.B.J., A.I., A.A.M., O.M.P., and A.A.G. performed experiments; M.C.P., V.R., M.K., L.C.V., C.B.R., M. McLellan, O.G., V.B.J., A.A.M., O.M.P., and A.A.G. analyzed data; M.C.P., V.R., L.C.V., A.A.M., O.M.P., and A.A.G. interpreted results of experiments; M.C.P., V.R., L.C.V., A.A.M., O.M.P., and A.A.G. prepared figures; M.C.P. and A.A.G. drafted manuscript; M.C.P. and A.A.G. edited and revised manuscript; M.C.P. and A.A.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Molecular, Imagine, and Analytical Core, as well as the Mouse Phenotyping Core of the Tulane Renal Hypertension Center of Excellence for providing the physical infrastructure to perform some of the experiments of this study. M. Kuczeriszka was a recipient of a Polish Academy of Sciences Postdoctoral Fellowship Award. The authors also acknowledge Akemi Katsurada, Dale M. Seth, and Alexander Castillo for excellent technical assistance.
REFERENCES
- 1.Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K, Connelly KA, Yuen D, Trogadis J, Herzenberg AM, Kuliszewski MA, Leong-Poi H, Gilbert RE. The (Pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension 54: 261–269, 2009. doi: 10.1161/HYPERTENSIONAHA.109.128645. [DOI] [PubMed] [Google Scholar]
- 2.Casarini DE, Boim MA, Stella RCR, Krieger-Azzolini MH, Krieger JE, and Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol 272: F405–F409, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Chen D, Stegbauer J, Sparks MA, Kohan D, Griffiths R, Herrera M, Gurley SB, Coffman TM. Impact of angiotensin Type 1A receptors in principal cells of the collecting duct on blood pressure and hypertension. Hypertension 67: 1291–1297, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC. Soluble form of the (pro)renin receptor is augmented in the collecting duct and urine of chronic angiotensin II-dependent hypertensive rats. Hypertension 57: 859–864, 2011. doi: 10.1161/HYPERTENSIONAHA.110.167957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez AA, Liu L, Lara LS, Bourgeois CRT, Ibaceta-Gonzalez C, Salinas-Parra N, Gogulamudi VR, Seth DM, Prieto MC. PKC-α-dependent augmentation of cAMP and CREB phosphorylation mediates the angiotensin II stimulation of renin in the collecting duct. Am J Physiol Renal Physiol 309: F880–F888, 2015. doi: 10.1152/ajprenal.00155.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez AA, Liu L, Lara LS, Seth DM, Navar LG, Prieto MC. Angiotensin II stimulates renin in inner medullary collecting duct cells via protein kinase C and independent of epithelial sodium channel and mineralocorticoid receptor activity. Hypertension 57: 594–599, 2011. doi: 10.1161/HYPERTENSIONAHA.110.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez AA, Luffman C, Bourgeois CRT, Vio CP, Prieto MC. Angiotensin II-independent upregulation of cyclooxygenase-2 by activation of the (Pro)renin receptor in rat renal inner medullary cells. Hypertension 61: 443–449, 2013. doi: 10.1161/HYPERTENSIONAHA.112.196303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, Giani JF, Nguyen MTX, Riquier-Brison AD, Seth DM, Fuchs S, Eladari D, Picard N, Bachmann S, Delpire E, Peti-Peterdi J, Navar LG, Bernstein KE, McDonough AA. The absence of intrarenal ACE protects against hypertension. J Clin Invest 123: 2011–2023, 2013. doi: 10.1172/JCI65460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Villalobos RA, Satou R, Ohashi N, Semprun-Prieto LC, Katsurada A, Kim C, Upchurch GM, Prieto MC, Kobori H, Navar LG. Intrarenal mouse renin-angiotensin system during ANG II-induced hypertension and ACE inhibition. Am J Physiol Renal Physiol 298: F150–F157, 2010. doi: 10.1152/ajprenal.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ichihara A, Kaneshiro Y, Takemitsu T, Sakoda M, Itoh H. The (pro)renin receptor and the kidney. Semin Nephrol 27: 524–528, 2007. doi: 10.1016/j.semnephrol.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Kinouchi K, Ichihara A, Sano M, Sun-Wada GH, Wada Y, Kurauchi-Mito A, Bokuda K, Narita T, Oshima Y, Sakoda M, Tamai Y, Sato H, Fukuda K, Itoh H. The (pro)renin receptor/ATP6AP2 is essential for vacuolar H+-ATPase assembly in murine cardiomyocytes. Circ Res 107: 30–34, 2010. doi: 10.1161/CIRCRESAHA.110.224667. [DOI] [PubMed] [Google Scholar]
- 12.Komlosi P, Fuson AL, Fintha A, Peti-Peterdi J, Rosivall L, Warnock DG, Bell PD. Angiotensin I conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension 42: 195–199, 2003. doi: 10.1161/01.HYP.0000081221.36703.01. [DOI] [PubMed] [Google Scholar]
- 13.Li XC, Shull GE, Miguel-Qin E, Zhuo JL. Role of the Na+/H+ exchanger 3 in angiotensin II-induced hypertension. Physiol Genomics 47: 479–487, 2015. doi: 10.1152/physiolgenomics.00056.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L, Gonzalez AA, McCormack M, Seth DM, Kobori H, Navar LG, Prieto MC. Increased renin excretion is associated with augmented urinary angiotensin II levels in chronic angiotensin II-infused hypertensive rats. Am J Physiol Renal Physiol 301: F1195–F1201, 2011. doi: 10.1152/ajprenal.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mamenko M, Zaika O, Doris PA, Pochynyuk O. Salt-dependent inhibition of epithelial Na+ channel-mediated sodium reabsorption in the aldosterone-sensitive distal nephron by bradykinin. Hypertension 60: 1234–1241, 2012. doi: 10.1161/HYPERTENSIONAHA.112.200469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mamenko M, Zaika O, Prieto MC, Jensen VB, Doris PA, Navar LG, Pochynyuk O. Chronic angiotensin II infusion drives extensive aldosterone-independent epithelial Na+ channel activation. Hypertension 62: 1111–1122, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonough AA, Veiras LC, Minas JN, Ralph DL. Considerations when quantitating protein abundance by immunoblot. Am J Physiol Cell Physiol 308: C426–C433, 2015. doi: 10.1152/ajpcell.00400.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müller DN, Binger KJ, Riediger F. Prorenin receptor regulates more than the renin-angiotensin system. Ann Med 44, Suppl 1: S43–S48, 2012. doi: 10.3109/07853890.2012.660496. [DOI] [PubMed] [Google Scholar]
- 19.Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular renin-angiotensin system in hypertension. Hypertension 57: 355–362, 2011. doi: 10.1161/HYPERTENSIONAHA.110.163519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navar LG, Prieto MC, Satou R, Kobori H. Intrarenal angiotensin II and its contribution to the genesis of chronic hypertension. Curr Opin Pharmacol 11: 180–186, 2011. doi: 10.1016/j.coph.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002. doi: 10.1172/JCI0214276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen MT, Lee DH, Delpire E, McDonough AA. Differential regulation of Na+ transporters along nephron during ANG II-dependent hypertension: distal stimulation counteracted by proximal inhibition. Am J Physiol Renal Physiol 305: F510–F519, 2013. doi: 10.1152/ajprenal.00183.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patterson LT, Potter SS. Hox genes and kidney patterning. Curr Opin Nephrol Hypertens 12: 19–23, 2003. doi: 10.1097/00041552-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Peng K, Lu X, Wang F, Nau A, Chen R, Zhou SF, Yang T. Collecting duct (pro)renin receptor targets ENaC to mediate angiotensin II-induced hypertension. Am J Physiol Renal Physiol 312: F245–F253, 2017. doi: 10.1152/ajprenal.00178.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol 13: 1131–1135, 2002. doi: 10.1097/01.ASN.0000013292.78621.FD. [DOI] [PubMed] [Google Scholar]
- 26.Prieto MC, Williams DE, Liu L, Kavanagh KL, Mullins JJ, Mitchell KD. Enhancement of renin and prorenin receptor in collecting duct of Cyp1a1-Ren2 rats may contribute to development and progression of malignant hypertension. Am J Physiol Renal Physiol 300: F581–F588, 2011. doi: 10.1152/ajprenal.00433.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prieto-Carrasquero MC, Botros FT, Pagan J, Kobori H, Seth DM, Casarini DE, Navar LG. Collecting duct renin is upregulated in both kidneys of 2-kidney, 1-clip Goldblatt hypertensive rats. Hypertension 51: 1590–1596, 2008. doi: 10.1161/HYPERTENSIONAHA.108.110916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension 44: 223–239, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension 44: 223–229, 2004. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutiérrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol 289: F632–F637, 2005. doi: 10.1152/ajprenal.00462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramkumar N, Kohan DE. Role of the collecting duct renin angiotensin system in regulation of blood pressure and renal function. Curr Hypertens Rep 18: 29, 2016. doi: 10.1007/s11906-016-0638-5. [DOI] [PubMed] [Google Scholar]
- 32.Ramkumar N, Stuart D, Calquin M, Quadri S, Wang S, Van Hoek AN, Siragy HM, Ichihara A, Kohan DE. Nephron-specific deletion of the prorenin receptor causes a urine concentration defect. Am J Physiol Renal Physiol 309: F48–F56, 2015. doi: 10.1152/ajprenal.00126.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramkumar N, Stuart D, Mironova E, Bugay V, Wang S, Abraham N, Ichihara A, Stockand JD, Kohan DE. Renal tubular epithelial cell prorenin receptor regulates blood pressure and sodium transport. Am J Physiol Renal Physiol 311: F186–F194, 2016. doi: 10.1152/ajprenal.00088.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramkumar N, Stuart D, Rees S, Hoek AV, Sigmund CD, Kohan DE. Collecting duct-specific knockout of renin attenuates angiotensin II-induced hypertension. Am J Physiol Renal Physiol 307: F931–F938, 2014. doi: 10.1152/ajprenal.00367.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Redublo Quinto BM, Camargo de Andrade MC, Ronchi FA, Santos EL, Alves Correa SA, Shimuta SI, Pesquero JB, Mortara RA, Casarini DE. Expression of angiotensin I-converting enzymes and bradykinin B2 receptors in mouse inner medullary-collecting duct cells. Int Immunopharmacol 8: 254–260, 2008. doi: 10.1016/j.intimp.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 36.Reverte V, Tapia A, Baile G, Gambini J, Gíménez I, Llinas MT, Salazar FJ. Role of angiotensin II in arterial pressure and renal hemodynamics in rats with altered renal development: age- and sex-dependent differences. Am J Physiol Renal Physiol 304: F33–F40, 2013. doi: 10.1152/ajprenal.00424.2012. [DOI] [PubMed] [Google Scholar]
- 37.Shao W, Seth DM, Navar LG. Angiotensin II type 1 receptor-mediated augmentation of urinary excretion of endogenous angiotensin II in Val5-angiotensin II-infused rats. Hypertension 56: 378–383, 2010. doi: 10.1161/HYPERTENSIONAHA.110.153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siragy HM, Huang J. Renal (pro)renin receptor upregulation in diabetic rats through enhanced angiotensin AT1 receptor and NADPH oxidase activity. Exp Physiol 93: 709–714, 2008. doi: 10.1113/expphysiol.2007.040550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song R, Preston G, Ichihara A, Yosypiv IV. Deletion of the prorenin receptor from the ureteric bud causes renal hypodysplasia. PLoS One 8: e63835, 2013. doi: 10.1371/journal.pone.0063835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013. doi: 10.1038/ki.2013.14. [DOI] [PubMed] [Google Scholar]
- 41.Staudacher T, Pech B, Tappe M, Gross G, Mühlbauer B, Luippold G. Arterial blood pressure and renal sodium excretion in dopamine D3 receptor knockout mice. Hypertens Res 30: 93–101, 2007. doi: 10.1291/hypres.30.93. [DOI] [PubMed] [Google Scholar]
- 42.Svenningsen P, Andersen H, Nielsen LH, Jensen BL. Urinary serine proteases and activation of ENaC in kidney–implications for physiological renal salt handling and hypertensive disorders with albuminuria. Pflugers Arch 467: 531–542, 2015. doi: 10.1007/s00424-014-1661-5. [DOI] [PubMed] [Google Scholar]
- 43.Tong GX, Yu WM, Beaubier NT, Weeden EM, Hamele-Bena D, Mansukhani MM, O’Toole KM. Expression of PAX8 in normal and neoplastic renal tissues: an immunohistochemical study. Mod Pathol 22: 1218–1227, 2009. doi: 10.1038/modpathol.2009.88. [DOI] [PubMed] [Google Scholar]
- 44.Trepiccione F, Gerber SD, Grahammer F, López-Cayuqueo KI, Baudrie V, Păunescu TG, Capen DE, Picard N, Alexander RT, Huber TB, Chambrey R, Brown D, Houillier P, Eladari D, Simons M. Renal Atp6ap2/(Pro)renin receptor is required for normal vacuolar H+-ATPase function but not for the renin-angiotensin system. J Am Soc Nephrol 27: 3320–3330, 2016. doi: 10.1681/ASN.2015080915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaika O, Mamenko M, O’Neil RG, Pochynyuk O. Bradykinin acutely inhibits activity of the epithelial Na+ channel in mammalian aldosterone-sensitive distal nephron. Am J Physiol Renal Physiol 300: F1105–F1115, 2011. doi: 10.1152/ajprenal.00606.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]