Abstract
Although all-trans-retinoic acid (ATRA) provides protection against a variety of conditions in vivo, particularly ischemia, the molecular mechanisms underpinning these effects remain unclear. The present studies were designed to assess potential mechanisms by which ATRA affords cytoprotection against renal toxicants in LLC-PK1 cells. Pretreatment of LLC-PK1 cells with ATRA (25 μM) for 24 h afforded cytoprotection against oncotic cell death induced by p-aminophenol (PAP), 2-(glutathion-S-yl)hydroquinone (MGHQ), and iodoacetamide but not against apoptotic cell death induced by cisplatin. Inhibition of protein synthesis with cycloheximide blunted ATRA protection, indicating essential cell survival pathways must be engaged before toxicant exposure to provide cytoprotection. Interestingly, ATRA did not prevent the PAP-induced generation of reactive oxygen species (ROS) nor did it alter glutathione levels. Moreover, ATRA had no significant effect on Nrf2 protein expression, and the Nrf2 inducers sulforaphane and MG132 did not influence ATRA cytoprotection, suggesting cytoprotective pathways beyond those that influence ROS levels contribute to ATRA protection. In contrast, ATRA rapidly (15 min) induced levels of the cellular stress kinases p-ERK and p-AKT at concentrations of ATRA (10 and 25 μM) required for cytoprotection. Consistent with a role for p-ERK in ATRA-mediated cytoprotection, inhibition of p-ERK with PD98059 reduced the ability of ATRA to afford protection against PAP toxicity. Collectively, these data suggest that p-ERK and its downstream targets, independent of ROS and antioxidant signaling, are important contributors to the cytoprotective effects of ATRA against oncotic cell death.
Keywords: nephrotoxicants, ATRA, Nrf2, p-ERK
retinoic acid (RA) is a fat-soluble vitamin obtained from the diet and essential for human survival. RA stimulates multiple physiological processes, including those involved in cellular growth and differentiation, immune system regulation, vision, and apoptosis. RA has also found clinical application in the treatment of cancer and acne (2). These pleiotropic effects are not mediated by retinoic acid itself but rather through its active metabolites, all-trans-retinoic acid (ATRA) and 9-cis retinoic acid (9-cis RA). ATRA is the predominant endogenous active metabolite of RA and exerts its effects through both genomic and nongenomic signaling mechanisms (1). ATRA localizes to the nucleus, where it binds to the retinoic acid receptor/retinoic acid x receptor (RAR/RXR) heterodimer to operate in a ligand-dependent manner and induce the transcription of RAR/RXR responsive genes. ATRA can also signal independently of the nuclear receptors, in multiple cell types including neuronal, lung, and embryonic stem cells, via activation of the mitogen-activated protein kinase (MAPK) and phosphotidyl inositol 3-phosphate kinase (PI3K) signaling pathways (4, 14, 34).
There is current interest in therapeutic strategies that utilize the preadministration of small molecules to afford cytoprotection. A variety of pharmacological agents and natural compounds have also shown protective effects in multiple organs (24, 40). In particular, the RA metabolites have demonstrable cytoprotective properties, with ATRA being shown to be protective against glomerular injury by increasing the expression of podocyte differentiation markers, which improve renal function (23). In the brain, ATRA pretreatment reduces cerebral ischemic injury, presumably by reducing the inflammatory markers cyclooxygenase-2 and CCAAT/enhancer binding proteins (7). In the liver, ATRA decreases ischemia reperfusion injury (IRI) through both anti-inflammatory and antioxidant mechanisms (36, 37), whereas in the heart protection occurs via a reduction in cardiomyocyte apoptosis (50). 9-cis RA is also protective against renal IRI (3). Thus in vivo studies demonstrate RA-mediated protection under a variety of conditions in multiple organs. Nonetheless, the molecular mediators underlying these protective effects are not well established.
Mitogen-activated protein kinases (MAPKs) participate in the regulation of a variety of cellular functions and include the extracellular signal-regulated kinases (ERK1/2), Jun NH2-terminal kinase (JNK), and the p38 MAPK signaling cascades. ERK1/2 is activated by growth factors and mediates cell proliferation, differentiation, and survival. JNK and p38 MAPK kinase signaling cascades are activated by cellular stress and are typically mediators of cellular toxicity (5, 41). In parallel, a number of cell survival signals are also transmitted through the PI3K-AKT cascade (30). Preconditioning strategies that activate ERK and AKT have protective effects in multiple organs. Pretreatment of LLC-PK1 renal epithelial cells with endoplasmic reticulum stress inducers enhances oxidative stress-induced ERK activation, also leading to cytoprotection (16). AKT is a critical mediator in the renal protection following brief cycles of ischemic preconditioning (20). The epidermal growth factor receptor and insulin-like growth factor-1 demonstrate protective effects through the stimulation of ERK and AKT in multiple cell types (31, 47).
Pretreatment of renal proximal tubule cells (LLC-PK1) with 11-deoxy-16,16-dimethyl PGE2 (DDM-PGE2), a stable synthetic analog of PGE2, is protective against 2,3,5-tris(glutathion-S-yl)hydroquinone (TGHQ)-induced renal toxicity (45). TGHQ is a nephrotoxic and nephrocarcinogenic metabolite of hydroquinone (22). A 24-h pretreatment time is necessary for maximal DDM-PGE2-mediated protection (45) suggestive of the need to engage specific signaling pathways to afford protection. Interestingly, the cytoprotective effect of DDM-PGE2 is mediated via the thromboxane receptor coupled to protein kinase C and nuclear factor-κ β-signaling pathways (44). During the 24-h exposure time, DDM-PGE2 induces retinol binding protein (RBP) (19). The increase in RBP synthesis suggests the engagement of ATRA and/or 9-cis RA during DDM-PGE2 cytoprotection. Furthermore, ATRA induces RBP expression (unpublished data). In the present study, we therefore sought to determine potential molecular mechanisms by which ATRA mediates cytoprotection by evaluating the expression levels of stress proteins during a 24-h ATRA pretreatment period. Specifically, we examined the contributions of reactive oxygen species (ROS) and the stress kinases p-ERK and p-AKT in mediating ATRA protection against renal epithelial cell injury. Our findings reveal that p-ERK, but not p-AKT nor reductions in ROS, contribute to ATRA cytoprotection.
MATERIALS AND METHODS
Materials.
MGHQ was synthesized and purified according to established protocols (22). PAP, iodoacetamide (IDAM), cisplatin, and ATRA were purchased from Sigma-Aldrich (St. Louis, MO). HPLC-grade solvents were obtained from Sigma-Aldrich. Glutathione (GSH) was purchased from Fisher Scientific (Pittsburgh, PA). 5,5-Dithio-bis-(2-nitrobenzoic acid) (DTNB) was a product of Sigma-Aldrich. Sulforaphane, MG132, BSO, and NAC were obtained from Sigma-Aldrich. PD98059 was supplied by InvivoGen (San Diego, CA) and LY24002 supplied by EMD Millipore (San Diego, CA). Neutral red was purchased from Amresco (Solon, OH). Caspase 3, p-ERK, ERK, p-AKT, and AKT antibodies were acquired from Cell Signaling Technologies (Danvers, MA). GAPDH was a product of Abcam (Cambridge, MA). Nrf2 and all secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell culture and treatment conditions.
The LLC-PK1 renal proximal tubule epithelial cell line was obtained from the American Type Culture Collection (Manassas, VA) and is derived from the New Hampshire minipig. LLC-PK1 cells develop characteristics of an in vivo proximal tubule. Thus high levels of the brush border membrane enzyme γ-glutamyltransferase (γ-GT) are present; the enzyme cleaves the γ-glutamyl bond in GSH (and GS-conjugates) and in concert with the subsequent activity of aminopeptidase(s) yields the corresponding cysteine conjugate for transport into the proximal tubule epithelial cells (38). LLC-PK1 cells were maintained in DMEM with 4.5 g/l glucose and supplemented with 10% FBS in a 37°C/5%CO2 humidified incubator. Cells were subcultured every 3–4 days at 90% confluence. All assays were conducted with cells plated in multiwell or 100-mm dishes, as needed, and grown to postconfluence before treatments in triplicate and repeated at least three times. Cells were subsequently washed and treated with the various agents in DMEM without FBS. γ-GT, a marker of nephrotoxicity, cleaves glutathione conjugates to their toxic metabolites in postconfluent LLC-PK1 cells (13).
Neutral red assay.
Cell viability following treatment with toxicants was measured using the neutral red lysosomal uptake assay, as previously described (27). Briefly, cells were grown in 96-well plates and allowed to grow to postconfluence before treatment. At the end of each experiment, cells were washed and then incubated with 0.25 mg/ml neutral red solution for 1 h at 37°C/5%CO2. The neutral red was then removed, and cells were washed and fixed in 1% formaldehyde/1% CaCl2, followed by extraction of neutral red using 1% acetic acid/50% ethanol solution for 15 min at room temperature in the dark to remove excess dye. The extent of lysosomal neutral red accumulation was assessed by determining absorbance at 540 nm.
CM-H2DCFDA assay.
ROS generation was determined using the CM-H2DCFDA probe (Molecular Probes, Eugene, OR). Cells were grown in 96-well black-walled clear-bottom plates and allowed to grow to postconfluence before treatments. The CM-H2DCFDA dye was prepared according to the manufacturer’s protocol, preloaded in cells for 30 min, and following toxicant addition, fluorescence was assessed at excitation and emission wavelengths of 485/515 nm, respectively.
GSH assay.
GSH levels were measured as described previously (11, 28), with modifications. After treatment, cells were lysed in 8% sulfosalicylic acid and centrifuged at 8,000 g for 10 min. The supernatant was collected and stored on ice for analysis. A standard curve for GSH was prepared at the following concentrations: 0, 3.9, 7.8, 15.6, 31.25, 62.5, 125, 250, and 500 μg. Standard or undiluted samples (20 μl) were pipetted into 96-well plates. DTNB (120 μl at 1 mg/ml) was subsequently added to each well and absorbance measured at 412 nm. GSH levels in each sample were determined using the GSH standard curve.
Western blot analysis.
Following the various treatments, cells were washed in ice cold PBS and lysed with 1× RIPA buffer containing 20 mM Tris·HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 10% NP-40, and 10% sodium deoxycholate. Complete protease and phosphatase inhibitor cocktail tablets (Roche, South San Francisco, CA) were added fresh to the buffer. Cell lysates were pelleted by centrifugation at 16,000 g for 15 min, and supernatants, containing total protein, were collected and stored at −80°C. Protein concentrations were measured using the Bio-Rad DC protein assay kit (Bio-Rad Laboratories, Hercules, CA). Aliquots (50–75 μg) of protein lysates were separated on 7.5 or 10% denaturing polyacrylamide gels (SDS-PAGE) and transferred to a nitrocellulose membrane for immunoblotting. The membranes were blocked in 5% nonfat dry milk in TBS with 0.1% Tween 20 (TBST) for 1 h and then incubated with primary antibodies overnight at 4°C in blocking solution. Primary antibody dilutions were either 1:500 or 1:1,000. Secondary antibodies were diluted to 1:3,000 in blocking solution and incubated with the membranes for 1 h at room temperature. Blots were finally developed with enhanced chemiluminescence and imaged.
Statistical analysis.
For individual comparisons, one-way ANOVA followed by Tukey’s post hoc analysis or unpaired Student’s t-test was used for most analyses. A two-way ANOVA followed by Bonferroni post hoc analysis was conducted to evaluate ATRA cytoprotection against multiple renal toxicants. All data are expressed as means ± SE, and P < 0.05 was considered to be significant.
RESULTS
ATRA-initiated signaling is required for cytoprotection.
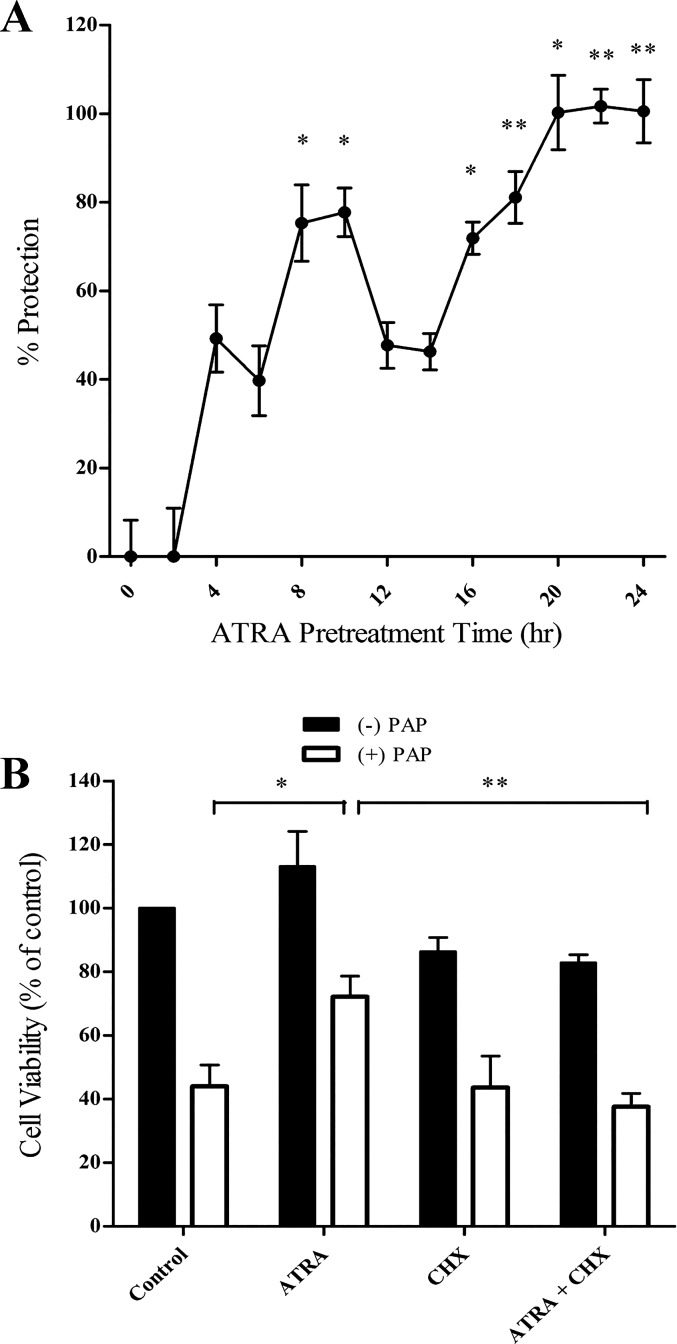
Cells were exposed to 25 μM ATRA for between 0 and 24 h followed by exposure to PAP. ATRA cytoprotection required between 8 and 12 h of pretreatment, with maximal protection occurring at 24 h, consistent with the kinetics of DDM-PGE2 cytoprotection (45). Cotreatment of cells with ATRA at the time of toxicant exposure failed to provide cytoprotection, implying that ATRA-initiated signaling is required. (Fig. 1A). Consistent with this view, addition of cycloheximide 4 h before ATRA exposure completely inhibited ATRA-mediated protection against PAP cytotoxicity (Fig. 1B).
Fig. 1.
All-trans-retinoic acid (ATRA)-mediated cytoprotection requires protein synthesis. A: LLC-PK1 cells were exposed to ATRA for increasing periods of time (0–24 h) followed by p-aminophenol (PAP; 150 μM, 3 h). Cytoprotection was determined by a neutral red lysosomal uptake assay. B: LLC-PK1 cells were pretreated with the protein synthesis inhibitor cycloheximide (CHX; 0.5 μM) for 4 h followed by ATRA (25 μM, 24 h) and subsequently exposed to PAP (150 μM, 3 h). Cell viability was assessed by lysosomal uptake of neutral red. Black bars: cells treated without PAP; open bars: cells treated with PAP (*P < 0.05; **P < 0.01; n = 4).
ATRA protects against necrotic but not apoptotic death of LLC-PK1 cells.
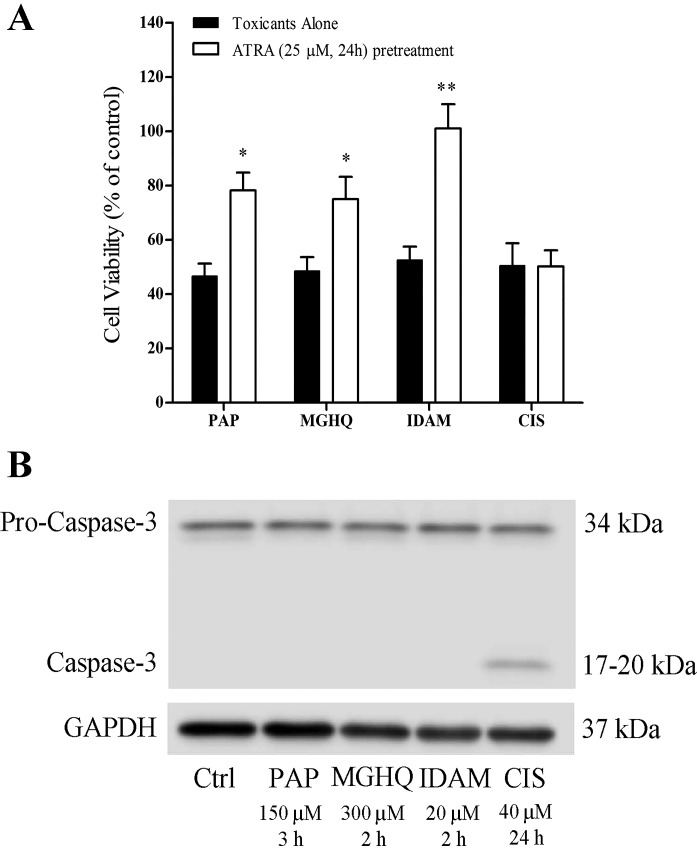
PAP was the initial model toxicant used to characterize dose and time assessments with ATRA. To determine whether ATRA-mediated cytoprotection was specific to this toxicant, LLC-PK1 cells were pretreated with ATRA for 24 h followed by exposure to multiple renal toxicants; PAP (150 μM, 3 h), 2-(glutathion-S-yl)hydroquinone (MGHQ; 300 μM, 2 h), iodoacetamide (IDAM; 20 μM, 2 h), or cisplatin (CIS; 40 μM, 24 h). ATRA protected cells against PAP-, MGHQ-, and IDAM-induced cytotoxicity but not against cisplatin (Fig. 2A). PAP, MGHQ, and IDAM all induce oncotic/necrotic cell death, whereas cisplatin induces apoptotic cell death of LLC-PK1 cells. Consistent with these data, Western blot analysis revealed caspase 3 cleavage to active fragments only in cells treated with the apoptotic toxicant cisplatin. (Fig. 2B). Collectively, the results indicate that ATRA selectively protects against oncotic/necrotic cell death but not apoptotic cell death.
Fig. 2.
ATRA protects against oncotic/necrotic but not apoptotic cell death. A: LLC-PK1 cells were exposed to PAP (150 μM, 2 h), 2-(glutathion-S-yl)hydroquinone (MGHQ; 300 μM, 2 h), iodoacetamide (IDAM; 20 μM, 2 h), or cisplatin (CIS; 40 μM, 24 h). Cell viability was assessed by the neutral red assay. Black bars: toxicants treatment alone; open bars: ATRA (25 μM, 24 h) pretreatment. *Significantly different from toxicant alone group (P < 0.05, **P < 0.01; n ≥ 3). B: caspase-3 cleavage in LLC-PK1 cells undergoing oncotic/necrotic or apoptotic cell death. LLC-PK1 cells were lysed in RIPA buffer. The anti-caspase-3 antibody detects both full-length and a cleaved fragment of caspase-3. Activation of caspase-3 requires proteolytic processing of pro-caspase-3 to into cleaved fragments. GAPDH was used as a loading control (Ctrl).
ATRA-mediated cytoprotection occurs independent of effects on ROS levels and without an apparent improvement in the antioxidant response.
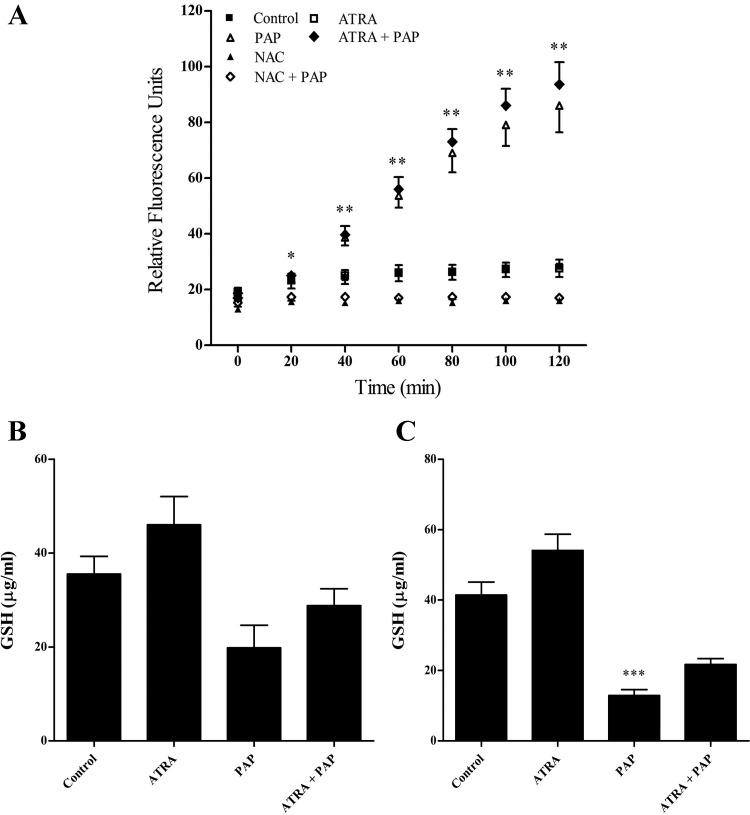
Under certain stress conditions ATRA has the ability to engage cellular antioxidant defenses (8). We therefore investigated whether ATRA-mediated cytoprotection against renal epithelial cell injury involves modulation of ROS. Cells were pretreated with ATRA, loaded with the CM-H2DCFDA dye for 30 min, and exposed to PAP treatment for 0–2 h. Exposure of cells to PAP increased ROS levels approximately sixfold, with ROS levels initially increasing at 20 min and remaining elevated until the conclusion of the experiment at 2 h. However, ATRA had no effect on PAP-induced ROS (Fig. 3A). Concomitant with the ability of PAP-induced elevations in ROS, PAP decreased GSH levels by ~80% at 2 h, and ATRA had only modest but statistically insignificant effects on preserving cellular GSH subsequent to PAP treatment (Fig. 3, B and C). Thus ATRA affords cytoprotection without apparently improving the antioxidant response.
Fig. 3.
ATRA has no significant effect on PAP-induced reactive oxygen species (ROS) generation or reduced GSH levels. A: LLC-PK1 cells were pretreated with ATRA (25 μM 24 h), loaded with CM-H2DCFDA dye, and exposed to PAP (150 μM, 0–2 h). NAC (10 mM) was cotreated with PAP and used as a positive control. LLC-PK1 cells were pretreated with ATRA (25 μM, 24 h) followed by treatment with PAP (150 μM) for 1 h (B) or 2 h (C). ■: Control cells; □: cells treated with ATRA; △: cells treated with PAP; ⧫: cells pretreated with ATRA followed by exposure to PAP; ▲: cells treated with NAC; ◇, cells pretreated with NAC followed by exposure to PAP. Statistical significance was determined from comparisons to time zero for ROS generation and vehicle-treated cells for GSH levels (*P < 0.05; **P < 0.01; ***P < 0.001; n ≥ 3).
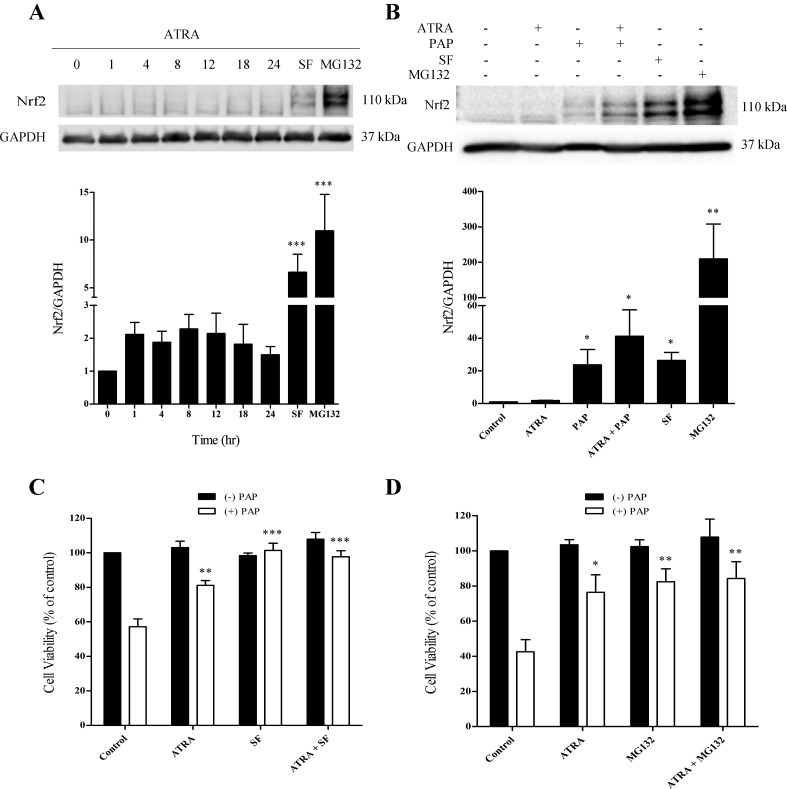
ATRA-mediated cytoprotection occurs independent of the engagement of Nrf2.
Since Nrf2 is a master regulator of the antioxidant stress response, we examined Nrf2 protein levels. ATRA had modest but statistically insignificant effects on Nrf2 protein levels during the 24-h ATRA pretreatment period (Fig. 4A). Under conditions where cells are not pretreated with ATRA, exposure to PAP generates ROS with the subsequent engagement of the Nrf2 antioxidant response, as evidenced by increases in Nrf2 protein levels (Fig. 4B). The effects of ATRA pretreatment on the PAP-induced Nrf2 response was therefore assessed. Under these conditions, the PAP-induced elevations in Nrf2 are similar, whether cells are pretreated or not with ATRA (see Fig. 6B). Interestingly, even though ATRA pretreatment protects against PAP-induced cytotoxicity in the absence of a significant increase in Nrf2, pretreatment of cells with the classic Nrf2 inducer sulforaphane also provided protection against PAP-mediated cytotoxicity (Fig. 4C). Indeed, sulforaphane offered essentially complete cytoprotection. The data reveal that LLC-PK1 cells possess multiple pathways that can be engaged to provide protection against cytotoxicants. MG132, which also elevates Nrf2 levels, but by a different mechanism to sulforaphane, acted in a similar manner to sulforaphane (Fig. 4D).
Fig. 4.
ATRA has little effect on Nrf2 expression in LLC-PK1 cells. A: LLC-PK1 cells were exposed to ATRA (25 μM) for a maximum of 24 h. B: LLC-PK1 cells were pretreated with ATRA (25 μM, 24 h) and then treated with PAP (150 μM, 1 h). Sulforaphane (5 μM, 4 h) and MG132 (10 μM, 4 h) were used as positive controls. GAPDH was used as a loading control. LLC-PK1 cells were pretreated with ATRA, sulforaphane (C), or MG132 (D), or a combination of ATRA and sulforaphane or MG132 for 24 h. ATRA (25 μM), sulforaphane (SF; 5 μM, 4 h), and MG132 (0.5 μM, 24 h). Cell viability was determined by the neutral red assay. Black bars: cells treated without PAP; open bars: cells treated with PAP. Statistical significance was determined from comparisons to vehicle-treated cells for Nrf2 induction and from cells treated with PAP alone for the cytoprotection experiments (*P < 0.05; **P < 0.01; ***P < 0.001; n ≥ 3).
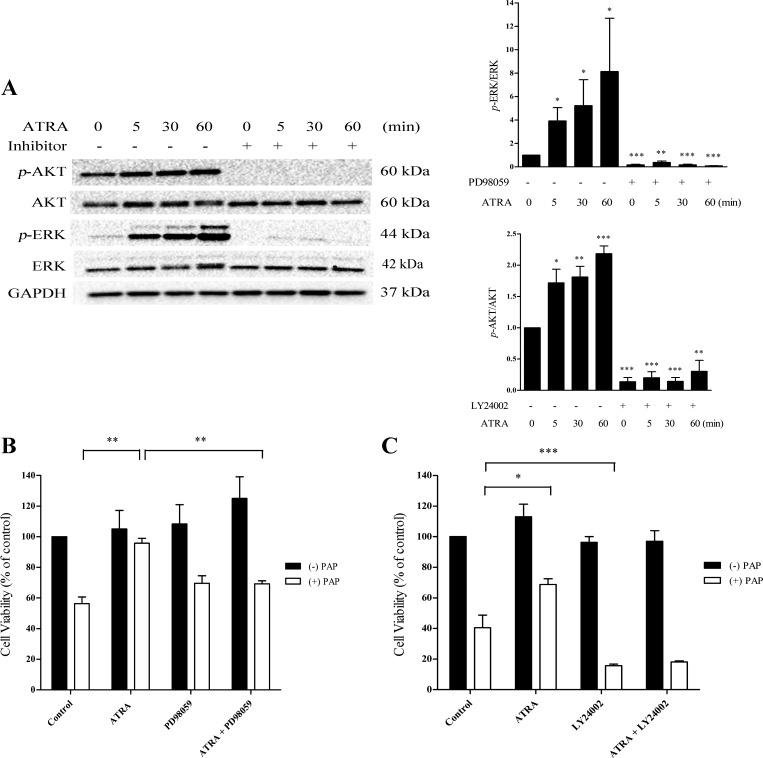
Fig. 6.
Inhibition of p-ERK antagonizes the ability of ATRA to afford cytoprotection. A: cells were pretreated with the MEK inhibitor PD98059 (50 μM) or the PI3 kinase inhibitor LY24002 (25 μM) for 30 min. ATRA (25 μM) was spiked into each well for a maximum of 60 min. Cells were pretreated with PD98059 (B; 50 μM) or LY24002 (C; 25 μM) for 30 min followed by ATRA (25 μM, 24 h). Cells were exposed to PAP (150 μM, 3 h). Cell viability was assessed by lysosomal uptake of neutral red. Black bars: cells treated without PAP; open bars: cells treated with PAP. Statistical significance was determined from comparisons between vehicle-treated control cells or inhibitor alone with ATRA plus inhibitor groups for p-ERK or p-AKT inhibition. For protection experiments, comparisons were made between PAP alone and PAP plus LY24002 or ATRA plus PAP groups (*P < 0.05; **P < 0.01; ***P < 0.001; n = 3).
ATRA increases cell survival via the MAPK-ERK pathway.
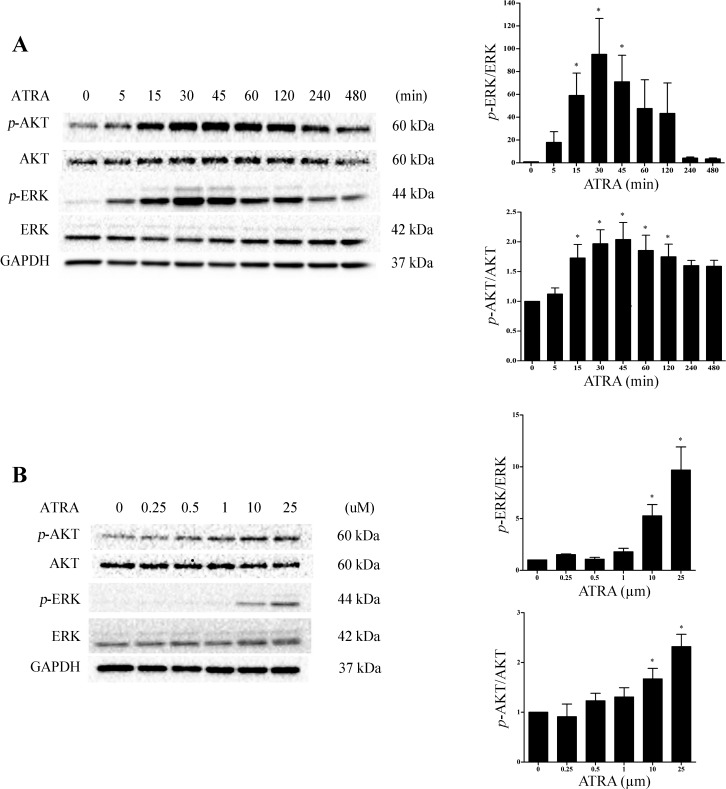
We have shown that N-acetylcysteine provides protection against the cytotoxicity of TGHQ in human proximal tubular (HK-2) cells in part via ERK1/2 activation (48). Moreover, both the MAPK-ERK and PI3K-AKT pathways can induce cellular growth and survival pathways and have been implicated in the physiological functions of ATRA. It is important to note, however, that the contribution of these pathways in the response to ATRA is very much context dependent. To determine whether either of these pathways participates in ATRA mediated cytoprotection in LLC-PK1 cells, we examined p-ERK and p-AKT expression following ATRA treatment. p-ERK and p-AKT expression was rapidly (15 min) induced after ATRA exposure. Elevations in p-ERK was transient, with levels returning to baseline between 60 and 120 min (Fig. 5A). Importantly, the concentration of ATRA required to induce p-ERK and p-AKT expression (10 and 25 μM) is consistent with the dose required for cytoprotection (Fig. 5B). Given that ATRA was capable of inducing p-ERK and p-AKT expression in LLC-PK1 cells, we next assessed their potential contribution to ATRA-mediated cytoprotection. Cells were treated for 30 min with either 50 μM PD98059, an inhibitor of the MAPK pathway, or 25 μM LY24002, a PI3K-AKT inhibitor, followed by the addition of ATRA. Both PD98059 and LY24002 essentially completely inhibited their respective targets (Fig. 6A). Cells were subsequently pretreated with either the MAPK or PI3K-AKT inhibitor for 30 min followed by exposure to ATRA for 24 h, and then PAP was added for 3 h. In the absence of the ATRA pretreatment, PD98059 had no effect on PAP-induced cytotoxicity (Fig. 6B). However, PD98059 significantly attenuated the ability of ATRA to provide protection against PAP cytotoxicity (Fig. 6B), suggesting that the ERK1/2 pathway participates in the cytoprotective response. In contrast, the PI3K-AKT inhibitor LY24002 exacerbated PAP toxicity in LLC-PK1 cells, compromising our ability to assess the contribution of AKT to ATRA cytoprotection (Fig. 6C).
Fig. 5.
ATRA induces the ERK and AKT cell survival pathways in LLC-PK1 cells. A: cells were exposed to ATRA (25 μM) for a maximum of 8 h. B: cells were exposed to ATRA (0–25 μM) for 30 min. Protein extracts were analyzed for p-ERK and p-AKT induction. p-ERK was normalized to ERK and p-AKT was normalized to AKT. GAPDH was used as a loading control. Significance was determined from comparisons to vehicle-treated control cells for p-ERK or p-AKT induction (*P < 0.05; n = 3).
DISCUSSION
In LLC-PK1 renal epithelial cells, ATRA-mediated cytoprotection is specific to chemical toxicants that induce oncotic/necrotic cell death. Thus ATRA provided protection against PAP (an oxidant)-, IDAM (an alkylating agent; Ref. 22)-, and MGHQ (an oxidant and electrophile; Ref. 22)-induced oncotic/necrotic cell death but not against cisplatin (DNA damaging agent and mitochondrial toxicant; Ref. 33)-mediated apoptotic cell death. Indeed, ATRA potentiates cisplatin-induced renal injury in a rodent model (12). Coincidentally, DDM-PGE2 is only protective against toxicants that induce oncotic/necrotic cell death and not apoptotic cell death (19).
A reduction in ROS levels via engagement of the antioxidant stress response mediates several cytoprotective processes, and ATRA protects against ischemia reperfusion injury during which ROS play a major pathological role (37). We therefore examined the role of ROS and antioxidant signaling in ATRA-mediated cytoprotection. ATRA-mediated cytoprotection does not appear to involve modulation of Nrf2 nor the antioxidant response but rather involves engagement of the ERK1/2 signaling pathway. Thus ATRA pretreatment did not decrease PAP-induced ROS generation (Fig. 3A). Furthermore, ATRA only had modest effects on PAP-induced reductions in GSH levels (Fig. 3, B and C) and Nrf2 induction (Fig. 4A). In contrast, ATRA appears to prevent angiotensin-induced apoptosis by inhibiting ROS generation and by increasing the antioxidant response (8). Why ATRA does not protect against cisplatin-induced apoptotic cell death in the current studies is unclear but may be related to the temporal nature of the stress kinase response (see below). ATRA is also protective in an in vivo model of diabetic renal injury by attenuating oxidative stress (29) further emphasizing the importance of cell context on the response to ATRA, although the reported reductions in Nrf2 in this model will require further evaluation based on the reported molecular weight (21). Moreover, an essential role for Nrf2 has been reported in a model of diabetic wound healing (25).
The apparent Nrf2-independence of the cytoprotective ATRA response is intriguing given the fact that both PAP and MGHQ generate ROS and especially since sulforaphane and MG-132, classic inducers of Nrf2, are both capable of providing protection against PAP toxicity (Fig. 4, C and D). However, the interaction among ATRA, RAR, and RXR appears complex and context dependent. Thus ATRA inhibits Nrf2 targeted gene expression in vivo and in vitro, apparently via activation of RARα (43), and ATRA inhibits Nrf2 in A549 cells (18). Consistent with these findings, RXRα reduces Nrf2 cytoprotection by binding to the Neh2 domain in the Nrf2 gene in both a ligand-dependent and -independent manner (42, 46). In contrast, “toxic” concentrations of ATRA activate Nrf2 and induce Nrf2 target genes (39). Of interest in this latter study is the finding that inhibition of MEK1/ERK MAPKs suppressed ATRA-induced Nrf2 activation.
ERK has long been considered a key modulator of organ injury regulating survival and death. On the one hand, ERK activation is associated with protection against various insults including oxidative and heat stress and tissue repair after renal ischemia reperfusion injury (16, 17, 31, 32). However, ERK has also been found to contribute to ROS-induced cell death (10, 35, 51). The mechanisms by which ERK activation modulates cell survival or death are not well characterized and are likely to be dependent on stimulus, duration of activation, cell type, and subcellular location. The temporal nature of ERK activation after exposure to stress may be particularly important. Thus, in the absence of any “preconditioning,” TGHQ-mediated ERK activation in LLC-PK1 cells is rapid and somewhat prolonged, and appears to contribute to cell death, since pharmacological inhibition of ERK prolongs cell survival via a mechanism that involves NF-κB (35). ATRA-mediated cytoprotection is also associated with the rapid phosphorylation of ERK (Fig. 5), but in this scenario inhibition of p-ERK disengages the protective effects of ATRA (Fig. 6). ATRA-induced phosphorylation of ERK is rapid and transient, implying that the recruitment of downstream targets of p-ERK is required to unveil the cytoprotective effects of ATRA pretreatment. Specific p-ERK targets are under investigation. Finally, the nuclear translocation of ERK is important for access to transcription factors necessary for cell proliferation. Cytosolic retention inhibits the survival and proliferative signals and can enhance proapoptotic proteins such as death associated protein kinase (26).
Although ATRA stimulates AKT activation, this does not appear to be involved in the protective effects. However, the contribution of p-AKT to ATRA-mediated cytoprotection could not be defined by these studies since pharmacological inhibition of p-AKT enhanced PAP cytotoxicity. However, AKT is an important component of cell survival pathways in many cell types (9, 15). Indeed, ATRA affords protection against cell death in SH-SY5Y cells via the AKT pathway (6) and activation of the PI3K-AKT pathway in SH-SY5Y cells promotes cell survival signal against epoxomicin-induced apoptosis via an upregulation of antiapoptotic factors (49). In the studies reported herein, ATRA selectively protects against necrotic but not apoptotic cell death, and this difference likely distinguishes between the consequences of ATRA-mediated AKT activation in the different cell types.
In summary, ATRA selectively protects against chemicals that induce oncotic/necrotic cell death of renal epithelial cells. Moreover, the protective process occurs in the absence of effects on ROS levels, and in a mechanism that involves activation of ERK, but not the Nrf2 pathway. Understanding the mechanism of ATRA-mediated cytoprotection, and the factors that determine whether ERK activation is manifest as a protective or harmful response, may provide insights to assist in the development of novel therapeutic interventions for conditions such as chemical-induced kidney injury, renal ischemia reperfusion injury, and hypoxia.
GRANTS
This work was supported by National Institute of Environmental Health Sciences (NIEHS) Grants R01-ES-016578 (to S. S. Lau) and P30-ES-006694 (Southwest Environmental Health Sciences Center; to S. S. Lau) and NIEHS Training Grant T32-ES-007091 (to J. M. Sapiro).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M.S., T.J.M., and S.S.L. conceived and designed research; J.M.S. performed experiments; J.M.S., T.J.M., and S.S.L. analyzed data; J.M.S., T.J.M., and S.S.L. interpreted results of experiments; J.M.S. prepared figures; J.M.S. drafted manuscript; J.M.S., T.J.M., and S.S.L. edited and revised manuscript; J.M.S., T.J.M., and S.S.L. approved final version of manuscript.
REFERENCES
- 1.Al Tanoury Z, Piskunov A, Rochette-Egly C. Vitamin A and retinoid signaling: genomic and nongenomic effects. J Lipid Res 54: 1761–1775, 2013. doi: 10.1194/jlr.R030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Álvarez R, Vaz B, Gronemeyer H, de Lera AR. Functions, therapeutic applications, and synthesis of retinoids and carotenoids. Chem Rev 114: 1–125, 2014. doi: 10.1021/cr400126u. [DOI] [PubMed] [Google Scholar]
- 3.Balasubramanian S, Jansen M, Valerius MT, Humphreys BD, Strom TB. Orphan nuclear receptor Nur77 promotes acute kidney injury and renal epithelial apoptosis. J Am Soc Nephrol 23: 674–686, 2012. doi: 10.1681/ASN.2011070646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cañón E, Cosgaya JM, Scsucova S, Aranda A. Rapid effects of retinoic acid on CREB and ERK phosphorylation in neuronal cells. Mol Biol Cell 15: 5583–5592, 2004. doi: 10.1091/mbc.E04-05-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature 410: 37–40, 2001. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 6.Cheng B, Martinez AA, Morado J, Scofield V, Roberts JL, Maffi SK. Retinoic acid protects against proteasome inhibition associated cell death in SH-SY5Y cells via the AKT pathway. Neurochem Int 62: 31–42, 2013. doi: 10.1016/j.neuint.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 7.Choi BK, Kim JH, Jung JS, Lee YS, Han ME, Baek SY, Kim BS, Kim JB, Oh SO. Reduction of ischemia-induced cerebral injury by all-trans-retinoic acid. Exp Brain Res 193: 581–589, 2009. doi: 10.1007/s00221-008-1660-x. [DOI] [PubMed] [Google Scholar]
- 8.Choudhary R, Baker KM, Pan J. All-trans retinoic acid prevents angiotensin II- and mechanical stretch-induced reactive oxygen species generation and cardiomyocyte apoptosis. J Cell Physiol 215: 172–181, 2008. doi: 10.1002/jcp.21297. [DOI] [PubMed] [Google Scholar]
- 9.Cui T, Jimenez JJ, Block NL, Badiavas EV, Rodriguez-Menocal L, Vila Granda A, Cai R, Sha W, Zarandi M, Perez R, Schally AV. Agonistic analogs of growth hormone releasing hormone (GHRH) promote wound healing by stimulating the proliferation and survival of human dermal fibroblasts through ERK and AKT pathways. Oncotarget 7: 52661–52672, 2016. doi: 10.18632/oncotarget.11024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong J, Ramachandiran S, Tikoo K, Jia Z, Lau SS, Monks TJ. EGFR-independent activation of p38 MAPK and EGFR-dependent activation of ERK1/2 are required for ROS-induced renal cell death. Am J Physiol Renal Physiol 287: F1049–F1058, 2004. doi: 10.1152/ajprenal.00132.2004. [DOI] [PubMed] [Google Scholar]
- 11.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 82: 70–77, 1959. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 12.Elsayed AM, Abdelghany TM, Akool S, Abdel-Aziz AA, Abdel-Bakky MS. All-trans retinoic acid potentiates cisplatin-induced kidney injury in rats: impact of retinoic acid signaling pathway. Naunyn Schmiedebergs Arch Pharmacol 389: 327–337, 2016. doi: 10.1007/s00210-015-1193-3. [DOI] [PubMed] [Google Scholar]
- 13.Fowler LM, Foster JR, Lock EA. Nephrotoxicity of 4-amino-3-S-glutathionylphenol and its modulation by metabolism or transport inhibitors. Arch Toxicol 68: 15–23, 1994. doi: 10.1007/BF03035706. [DOI] [PubMed] [Google Scholar]
- 14.García-Regalado A, Vargas M, García-Carrancá A, Aréchaga-Ocampo E, González-De la Rosa CH. Activation of Akt pathway by transcription-independent mechanisms of retinoic acid promotes survival and invasion in lung cancer cells. Mol Cancer 12: 44, 2013. doi: 10.1186/1476-4598-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gusscott S, Jenkins CE, Lam SH, Giambra V, Pollak M, Weng AP. IGF1R derived PI3K/AKT signaling maintains growth in a subset of human T-cell acute lymphoblastic leukemias. PLoS One 11: e0161158, 2016. doi: 10.1371/journal.pone.0161158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hung CC, Ichimura T, Stevens JL, Bonventre JV. Protection of renal epithelial cells against oxidative injury by endoplasmic reticulum stress preconditioning is mediated by ERK1/2 activation. J Biol Chem 278: 29317–29326, 2003. doi: 10.1074/jbc.M302368200. [DOI] [PubMed] [Google Scholar]
- 17.Jang HS, Han SJ, Kim JI, Lee S, Lipschutz JH, Park KM. Activation of ERK accelerates repair of renal tubular epithelial cells, whereas it inhibits progression of fibrosis following ischemia/reperfusion injury. Biochim Biophys Acta 1832: 1998–2008, 2013. doi: 10.1016/j.bbadis.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Jayakumar S, Pal D, Sandur SK. Nrf2 facilitates repair of radiation induced DNA damage through homologous recombination repair pathway in a ROS independent manner in cancer cells. Mutat Res 779: 33–45, 2015. doi: 10.1016/j.mrfmmm.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Jia Z, Person MD, Dong J, Shen J, Hensley SC, Stevens JL, Monks TJ, Lau SS. Grp78 is essential for 11-deoxy-16,16-dimethyl PGE2-mediated cytoprotection in renal epithelial cells. Am J Physiol Renal Physiol 287: F1113–F1122, 2004. doi: 10.1152/ajprenal.00138.2004. [DOI] [PubMed] [Google Scholar]
- 20.Joo JD, Kim M, D’Agati VD, Lee HT. Ischemic preconditioning provides both acute and delayed protection against renal ischemia and reperfusion injury in mice. J Am Soc Nephrol 17: 3115–3123, 2006. doi: 10.1681/ASN.2006050424. [DOI] [PubMed] [Google Scholar]
- 21.Lau A, Tian W, Whitman SA, Zhang DD. The predicted molecular weight of Nrf2: it is what it is not. Antioxid Redox Signal 18: 91–93, 2013. doi: 10.1089/ars.2012.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau SS, Hill BA, Highet RJ, Monks TJ. Sequential oxidation and glutathione addition to 1,4-benzoquinone: correlation of toxicity with increased glutathione substitution. Mol Pharmacol 34: 829–836, 1988. [PubMed] [Google Scholar]
- 23.Li X, Dai Y, Chuang PY, He JC. Induction of retinol dehydrogenase 9 expression in podocytes attenuates kidney injury. J Am Soc Nephrol 25: 1933–1941, 2014. doi: 10.1681/ASN.2013111150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lieberthal W, Tang M, Lusco M, Abate M, Levine JS. Preconditioning mice with activators of AMPK ameliorates ischemic acute kidney injury in vivo. Am J Physiol Renal Physiol 311: F731–F739, 2016. doi: 10.1152/ajprenal.00541.2015. [DOI] [PubMed] [Google Scholar]
- 25.Long M, Rojo de la Vega M, Wen Q, Bharara M, Jiang T, Zhang R, Zhou S, Wong PK, Wondrak GT, Zheng H, Zhang DD. An essential role of NRF2 in diabetic wound healing. Diabetes 65: 780–793, 2016. doi: 10.2337/db15-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle 8: 1168–1175, 2009. doi: 10.4161/cc.8.8.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mertens JJ, Gibson NW, Lau SS, Monks TJ. Reactive oxygen species and DNA damage in 2-bromo-(glutathion-S-yl) hydroquinone-mediated cytotoxicity. Arch Biochem Biophys 320: 51–58, 1995. doi: 10.1006/abbi.1995.1341. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther 187: 211–217, 1973. [PubMed] [Google Scholar]
- 29.Molina-Jijón E, Rodríguez-Muñoz R, Namorado MC, Bautista-García P, Medina-Campos ON, Pedraza-Chaverri J, Reyes JL. All-trans retinoic acid prevents oxidative stress-induced loss of renal tight junction proteins in type-1 diabetic model. J Nutr Biochem 26: 441–454, 2015. doi: 10.1016/j.jnutbio.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 30.Mundi PS, Sachdev J, McCourt C, Kalinsky K. AKT in cancer: new molecular insights and advances in drug development. Br J Clin Pharmacol 82: 943–956, 2016. doi: 10.1111/bcp.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niederlechner S, Baird C, Petrie B, Wischmeyer E, Wischmeyer PE. Epidermal growth factor receptor expression and signaling are essential in glutamine’s cytoprotective mechanism in heat-stressed intestinal epithelial-6 cells. Am J Physiol Gastrointest Liver Physiol 304: G543–G552, 2013. doi: 10.1152/ajpgi.00418.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ong Q, Guo S, Duan L, Zhang K, Collier EA, Cui B. The timing of Raf/ERK and AKT activation in protecting PC12 cells against oxidative stress. PLoS One 11: e0153487, 2016. doi: 10.1371/journal.pone.0153487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan H, Chen J, Shen K, Wang X, Wang P, Fu G, Meng H, Wang Y, Jin B. Mitochondrial modulation by epigallocatechin 3-gallate ameliorates cisplatin induced renal injury through decreasing oxidative/nitrative stress, inflammation and NF-κB in mice. PLoS One 10: e0124775, 2015. doi: 10.1371/journal.pone.0124775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Persaud SD, Lin YW, Wu CY, Kagechika H, Wei LN. Cellular retinoic acid binding protein I mediates rapid non-canonical activation of ERK1/2 by all-trans retinoic acid. Cell Signal 25: 19–25, 2013. doi: 10.1016/j.cellsig.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramachandiran S, Huang Q, Dong J, Lau SS, Monks TJ. Mitogen-activated protein kinases contribute to reactive oxygen species-induced cell death in renal proximal tubule epithelial cells. Chem Res Toxicol 15: 1635–1642, 2002. doi: 10.1021/tx0200663. [DOI] [PubMed] [Google Scholar]
- 36.Rao J, Qian X, Wang P, Pu L, Zhai Y, Wang X, Zhang F, Lu L. All-trans retinoic acid preconditioning protects against liver ischemia/reperfusion injury by inhibiting the nuclear factor kappa B signaling pathway. J Surg Res 180: e99–e106, 2013. doi: 10.1016/j.jss.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Rao J, Zhang C, Wang P, Lu L, Zhang F. All-trans retinoic acid alleviates hepatic ischemia/reperfusion injury by enhancing manganese superoxide dismutase in rats. Biol Pharm Bull 33: 869–875, 2010. doi: 10.1248/bpb.33.869. [DOI] [PubMed] [Google Scholar]
- 38.Sepúlveda FV, Burton KA, Pearson JD. The development of gamma-glutamyltransferase in a pig renal-epithelial-cell line in vitro. Relationship to amino acid transport. Biochem J 208: 509–512, 1982. doi: 10.1042/bj2080509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan KP, Kosuge K, Yang M, Ito S. NRF2 as a determinant of cellular resistance in retinoic acid cytotoxicity. Free Radic Biol Med 45: 1663–1673, 2008. doi: 10.1016/j.freeradbiomed.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Tao S, Rojo de la Vega M, Quijada H, Wondrak GT, Wang T, Garcia JG, Zhang DD. Bixin protects mice against ventilation-induced lung injury in an NRF2-dependent manner. Sci Rep 6: 18760, 2016. doi: 10.1038/srep18760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian W, Zhang Z, Cohen DM. MAPK signaling and the kidney. Am J Physiol Renal Physiol 279: F593–F604, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Liu K, Geng M, Gao P, Wu X, Hai Y, Li Y, Li Y, Luo L, Hayes JD, Wang XJ, Tang X. RXRα inhibits the NRF2-ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2. Cancer Res 73: 3097–3108, 2013. doi: 10.1158/0008-5472.CAN-12-3386. [DOI] [PubMed] [Google Scholar]
- 43.Wang XJ, Hayes JD, Henderson CJ, Wolf CR. Identification of retinoic acid as an inhibitor of transcription factor Nrf2 through activation of retinoic acid receptor alpha. Proc Natl Acad Sci USA 104: 19589–19594, 2007. doi: 10.1073/pnas.0709483104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weber TJ, Monks TJ, Lau SS. DDM-PGE2-mediated cytoprotection in renal epithelial cells by a thromboxane A 2 receptor coupled to NF-kB. Am J Physiol Renal Physiol 278: F270–F278, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Weber TJ, Monks TJ, Lau SS. PGE2-mediated cytoprotection in renal epithelial cells: evidence for a pharmacologically distinct receptor. Am J Physiol Renal Fluid 273: F507–F515, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Wu J, Wang H, Tang X. Rexinoid inhibits Nrf2-mediated transcription through retinoid X receptor alpha. Biochem Biophys Res Commun 452: 554–559, 2014. doi: 10.1016/j.bbrc.2014.08.111. [DOI] [PubMed] [Google Scholar]
- 47.Yang X, Wei A, Liu Y, He G, Zhou Z, Yu Z. IGF-1 protects retinal ganglion cells from hypoxia-induced apoptosis by activating the Erk-1/2 and Akt pathways. Mol Vis 19: 1901–1912, 2013. [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang F, Lau SS, Monks TJ. The cytoprotective effect of N-acetyl-L-cysteine against ROS-induced cytotoxicity is independent of its ability to enhance glutathione synthesis. Toxicol Sci 120: 87–97, 2011. doi: 10.1093/toxsci/kfq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H, Xiong Z, Wang J, Zhang S, Lei L, Yang L, Zhang Z. Glucagon-like peptide-1 protects cardiomyocytes from advanced oxidation protein product-induced apoptosis via the PI3K/Akt/Bad signaling pathway. Mol Med Rep 13: 1593–1601, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu Z, Zhu J, Zhao X, Yang K, Lu L, Zhang F, Shen W, Zhang R. All-Trans Retinoic Acid Ameliorates Myocardial Ischemia/Reperfusion Injury by Reducing Cardiomyocyte Apoptosis. PLoS One 10: e0133414, 2015. doi: 10.1371/journal.pone.0133414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhuang S, Kinsey GR, Yan Y, Han J, Schnellmann RG. Extracellular signal-regulated kinase activation mediates mitochondrial dysfunction and necrosis induced by hydrogen peroxide in renal proximal tubular cells. J Pharmacol Exp Ther 325: 732–740, 2008. doi: 10.1124/jpet.108.136358. [DOI] [PubMed] [Google Scholar]