Here, we present a novel computational model to study the effects of late Na+ current (INa,L) in human atrial myocytes. Simulations predict that INa,L promotes intracellular accumulation of Ca2+, with subsequent dysregulation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) signaling and ryanodine receptor 2-mediated Ca2+ release. Although INa,L plays a small role in regulating atrial myocyte excitability at baseline, CaMKII-dependent enhancement of the current promoted arrhythmogenic dynamics.
Keywords: late sodium current, atrial fibrillation, calcium/calmodulin-dependent protein kinase II, calcium handling, mathematical modeling, arrhythmia
Abstract
Atrial fibrillation (AF) affects more than three million people per year in the United States and is associated with high morbidity and mortality. Both electrical and structural remodeling contribute to AF, but the molecular pathways underlying AF pathogenesis are not well understood. Recently, a role for Ca2+/calmodulin-dependent protein kinase II (CaMKII) in the regulation of persistent “late” Na+ current (INa,L) has been identified. Although INa,L inhibition is emerging as a potential antiarrhythmic strategy in patients with AF, little is known about the mechanism linking INa,L to atrial arrhythmogenesis. A computational approach was used to test the hypothesis that increased CaMKII-activated INa,L in atrial myocytes disrupts Ca2+ homeostasis, promoting arrhythmogenic afterdepolarizations. Dynamic CaMKII activity and regulation of multiple downstream targets [INa,L, L-type Ca2+ current, phospholamban, and the ryanodine receptor sarcoplasmic reticulum Ca2+-release channel (RyR2)] were incorporated into an existing well-validated computational model of the human atrial action potential. Model simulations showed that constitutive CaMKII-dependent phosphorylation of Nav1.5 and the subsequent increase in INa,L effectively disrupt intracellular atrial myocyte ion homeostasis and CaMKII signaling. Specifically, increased INa,L promotes intracellular Ca2+ overload via forward-mode Na+/Ca2+ exchange activity, which greatly increases RyR2 open probability beyond that observed for CaMKII-dependent phosphorylation of RyR2 alone. Increased INa,L promotes atrial myocyte repolarization defects (afterdepolarizations and alternans) in the setting of acute β-adrenergic stimulation. We anticipate that our modeling efforts will help identify new mechanisms for atrial NaV1.5 regulation with direct relevance for human AF.
NEW & NOTEWORTHY Here, we present a novel computational model to study the effects of late Na+ current (INa,L) in human atrial myocytes. Simulations predict that INa,L promotes intracellular accumulation of Ca2+, with subsequent dysregulation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) signaling and ryanodine receptor 2-mediated Ca2+ release. Although INa,L plays a small role in regulating atrial myocyte excitability at baseline, CaMKII-dependent enhancement of the current promoted arrhythmogenic dynamics.
Listen to this article’s corresponding podcast at http://ajpheart.podbean.com/e/camkii-dependent-regulation-of-atrial-late-sodium-current-and-excitability/.
INTRODUCTION
The number of patients with atrial fibrillation (AF) is expected to exceed 15.9 million in the United States by the year 2050 (31). Patients with AF face high morbidity and mortality as well as increased risk of stroke and heart failure (3). The present AF therapies include a combination of anticoagulants with catheter ablation and surgical- or drug-based therapies to maintain sinus rhythm or ventricular rate (19, 37) but face important limitations related to low efficacy attributable to off-target ion channel effects or proarrhythmic impact on ventricular tissue (10, 44, 55).
Recently, there has been growing interest in antiarrhythmic drug therapies targeting “late” Na+ current (INa,L), a low-amplitude current that persists throughout the duration of the action potential (AP) attributable to failed/incomplete voltage-dependent inactivation (12, 46). Enhancement of INa,L is well documented in ventricular myocytes of animal models and human patients with congenital or acquired cardiac arrhythmia (20, 28, 32, 43, 52). More recently, there have been reports supporting a potential role for increased INa,L in human AF (27, 38) with INa,L blockers such as ranolazine or GS-458967 showing promise for suppressing atrial arrhythmias in preclinical models (4, 13, 17, 39–41, 63, 64). Previous work from our group has shown that Ca2+/calmodulin-dependent protein kinase II (CaMKII) is an upstream regulator of INa,L in cardiac myocytes through phosphorylation of Ser571 in the DI-DII linker of Nav1.5 (Fig. 1A) (16, 24). Furthermore, the effects of INa,L on arrhythmogenic activity are attenuated by CaMKII inhibition (26). Although INa,L is thought to promote triggered Ca2+ activity, the mechanistic link between increased INa,L and dysregulation of Ca2+ handling proteins [e.g., the ryanodine receptor sarcoplasmic reticulum (SR) Ca2+-release channel (RyR2)] remains to be defined. Furthermore, the specific role of INa,L in atrial arrhythmogenesis, attributable in part to lack of mathematical models of the atrial AP that incorporate CaMKII-dependent signaling, is underexplored.
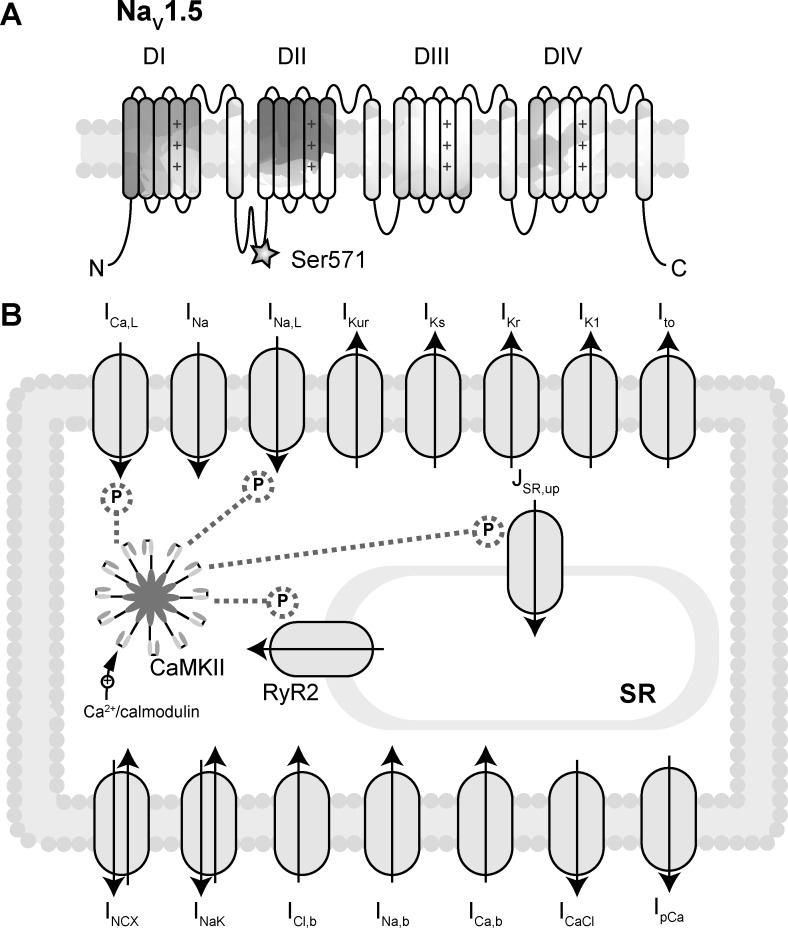
Fig. 1.
Mathematical model of Ca2+/calmodulin-dependent protein kinase (CaMKII) signaling in human atrial myocyte. A: the structure of voltage-gated Na+ channel α-subunit Nav1.5. Ser571 in the DI-DII loop is critically important for CaMKII-dependent regulation of late Na+ current (INa,L) (16). B: schematic of key plasma membrane ion currents and sarcoplasmic reticulum (SR) protein channels in the Grandi atrial cell model (18). ICa,L, L-type Ca2+ current; INa, fast Na+ current; IKur, ultrarapid delayed rectifier K+ current; IKs, slow delayed rectifier K+ current; IKr, rapid delayed rectifier K+ current; IK1, inward rectifier K+ current; Ito, 4-aminopyridine-sensitive transient outward K+ current; JSR,up, Ca2+ uptake into the SR; INCX, Na+/Ca2+ exchanger current; INaK, Na+-K+ pump ATPase; ICl,b, background Cl− current; INa,b, background Na+ current; ICa,b, background Ca2+ current; ICaCl, Ca2+-activated Cl− current; IpCa, sarcolemmal Ca2+ pump. CaMKII activation by Ca2+/calmodulin and downstream targeting of RyR2, Iup, ICa,L, and INa,L is included in this updated model.
In this study, we used a computational approach to test the hypothesis that disruption of Nav1.5 phosphorylation and a CaMKII-facilitated increase in INa,L contribute to intracellular Ca2+ accumulation and irregular atrial cell membrane excitability. We incorporated formulations for CaMKII-dependent regulation of INa,L and other targets important for intracellular Ca2+ cycling [L-type Ca2+ current (ICa,L), phospholamban (PLB), and RyR2] into an established mathematical model of the human atrial cell (Fig. 1B). We then explored the downstream effects of CaMKII-dependent phosphorylation of INa,L on intracellular ion homeostasis and other downstream CaMKII targets in atrial myocytes. Finally, we defined the contribution of CaMKII-facilitated INa,L to atrial proarrhythmia associated with acute β-adrenergic receptor (β-AR) stimulation.
METHODS
Ion channel kinetics were simulated using an existing well-validated model of the human atrial cell (18). Modifications to the original equations are provided in appendix a. Briefly, the original atrial AP model was modified to include a previously defined formulation for INa,L (9). CaMKII activation kinetics were also incorporated based on a mathematical description previously developed by our group that accounts for CaMKII activation by dynamic changes in Ca2+/calmodulin as well as modulation by autophosphorylation and/or oxidation (9). CaMKII was assumed to catalyze a transition from nonphosphorylated to phosphorylated Nav1.5 populations, based on previously described population-based modeling techniques (48), where the maximal conductance of INa,L was fit to experimental data from two different Scn5a knockin mouse models: 1) mice expressing Nav1.5 with the CaMKII phosphorylation site replaced by alanine (Nav1.5-S571A) to model 100% nonphosphorylated channels (phospho-resistant) and 2) mice expressing Nav1.5 with the CaMKII phosphorylation site replaced by glutamic acid (Nav1.5-S571E) to model 100% phosphorylated channels (phospho-mimetic) (16) (Fig. 2A). The wild-type (WT) Nav1.5 model transitions between phosphorylated and nonphosphorylated fractions based on the dynamic activity of CaMKII. A similar approach was taken to model CaMKII targeting of RyR2 using experimental data from RyR2-S2814D (phospho-mimetic) and RyR2-S2814A (phospho-resistant) mouse models (Fig. 2B) (45, 53, 57). CaMKII effects on PLB and ICa,L were also incorporated into the model based on their importance for normal Ca2+ cycling. ICa,L conductance was made dependent on CaMKII to produce a maximal change of 16% facilitation when pacing frequency increased from 1 to 2 Hz, within the range of experimentally measured values (1, 29). CaMKII effects on PLB were incorporated by introducing CaMKII dependence to the half-maximal saturation constant for Ca2+ binding to SERCA2a [altering Ca2+ uptake into the SR (JSR,up)], consistent with previous experimental and modeling studies (22). Although other CaMKII targets likely regulate atrial excitability in response to stress (47), as a first approximation we focused on Nav1.5, ICa,L, PLB, and RyR2 to address the specific role of dysregulation of Ca2+ cycling.
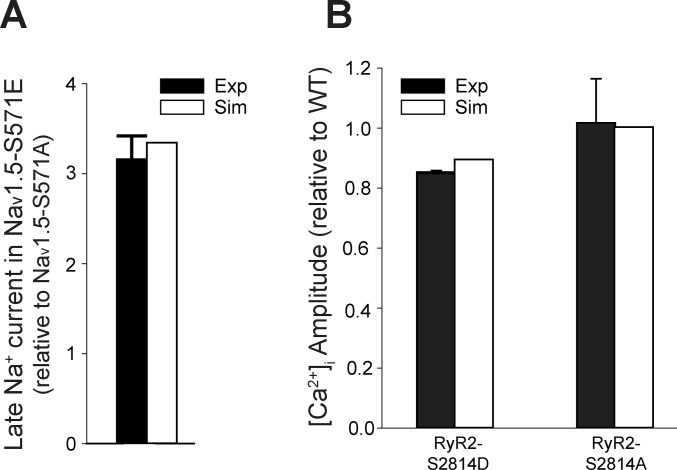
Fig. 2.
Simulated Ca2+/calmodulin-dependent protein kinase (CaMKII)-dependent regulation of late Na+ current (INa,L) and ryanodine receptor sarcoplasmic reticulum (SR) Ca2+-release channel (RyR2) compared with experimental data. A: experimentally measured (16) and simulated INa,L (expressed as ratio of levels in Nav1.5-S571E to Nav1.5-S571A). B: experimentally measured (53, 57) and simulated intracellular Ca2+ concentration ([Ca2+]i) amplitude [ratio relative to the wild-type (WT) model] in RyR2-S2814D and RyR2-S2814A models. Simulations used Grandi human atrial model (18) with modifications as described in methods.
AP duration (APD) after 490 s of pacing (to allow for the model to reach steady-state condition) was compared with experimental data recorded at body temperature from human atrial myocytes in a sinus rhythm over a range of pacing frequencies to verify that modifications did not disturb agreement of simulated and experimental APD (Fig. 3) (15, 36). The maximal conductance value for INa,L in the WT model was selected to yield APD at 90% repolarization (APD90) in the physiological range at baseline. In a subset of simulations, the effects of β-AR stimulation were simulated according to a previously published approach with changes to slow delayed rectifier K+ current (IKs), ultrarapid delayed rectifier K+ current (IKur), troponin I, JSR,up, ICa,L, and RyR2 (18, 42). Model parameter sensitivity analysis was performed using random model parameter perturbation and regression, as previously described (51). Briefly, random scale factors for ICa,L, inward rectifier K+ current (IK1), rapid delayed rectifier K+ current (IKr), IKs, IKur, fast Na+ current (INa), Na+/Ca2+ exchanger current (INCX), Na+-K+-ATPase current (INaK), INa,L, transient outward K+ current (Ito), SR Ca2+ release, and JSR,up were selected randomly for 600 independent simulations under basal conditions and in the presence of β-AR stimulation. The following steady-state model outputs were saved for each simulation and used for regression analysis: maximal diastolic intracellular Na+ concentration ([Na+]i), maximal intracellular Ca2+ concentration ([Ca2+]i), maximal Ca2+ concentration in the SR ([Ca2+]SR), and maximal CaMKII activity. To avoid artifacts induced by perturbation procedure, the final analysis included only simulations that produced an AP with a resting membrane potential more negative than −70 mV, an APD90 of <1,000 ms, and no alternans (criteria were met by 479 simulations under basal conditions and 379 simulations in the presence of β-AR stimulation).
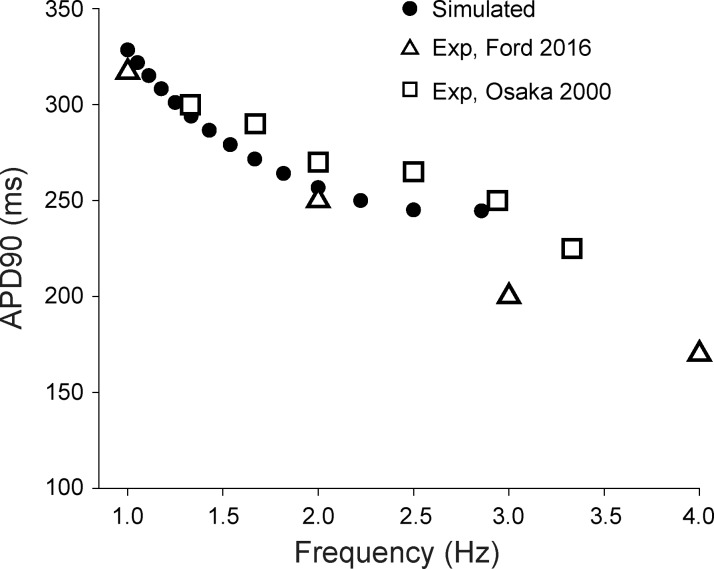
Fig. 3.
Simulated action potential duration (APD) compared with experimental data from human atrial myocytes. Simulated (black circle) and experimentally measured (15, 36) wild-type APDs at 90% repolarization (APD90) over a range of pacing frequencies are shown.
Computer code was written in C++ 11 and compiled using GNU compiler collection (gcc) for Linux. Model equations were solved numerically using the forward Euler method and a time step of 5 µs. The full model is available for download and use on a cross-platform, threaded application with graphic user interface called “LongQt” (https://hundlab.engineering.osu.edu/research/LongQt, version 0.2) (35). All other computer simulations were performed on a Dell PowerEdge R515 server (Dual 6 core, 32 GB RAM running CentOS-6.2). A single simulation to steady state required 9 min of computational time using these resources. Analysis was performed using MATLAB R2016b on a MacBook Pro with a 2.5-GHz Intel Core i7 processor.
RESULTS
Effect of CaMKII-dependent INa,L on atrial myocyte intracellular ion homeostasis.
CaMKII regulates the rate dependence of cardiac Ca2+ handling with implications for human disease (22). Previous studies have shown that CaMKII phosphorylation of Ser571 in the Nav1.5 DI-DII linker increases INa,L (21, 24). Therefore, we hypothesized that constitutive CaMKII-dependent phosphorylation of Nav1.5 would delay atrial AP repolarization and promote Ca2+ dysregulation. Consistent with our hypothesis, APD was prolonged in the simulated Nav1.5-S571E atrial myocyte compared with Nav1.5-S571A or WT atrial myocytes (Fig. 4A). At the same time, simulations predicted an increase in intracellular Ca2+ (cytoplasmic and SR) and CaMKII activity in Nav1.5-S571E compared with WT atrial myocytes (Fig. 4, B–D). On the basis of previous work showing an important role for CaMKII-dependent RyR2 phosphorylation in AF susceptibility (6, 25, 57), we also evaluated the effects of constitutive (RyR2-S2814D) or ablated (RyR2-S2814A) RyR2 phosphorylation at Ser2814 (CaMKII site) on atrial membrane excitability and Ca2+ handling. Consistent with experiments (53), our simulations predicted a decrease in steady-state [Ca2+]SR in RyR2-S2814D myocytes compared with WT or RyR2-S2814A myocytes, without any detectable difference in the AP, Ca2+ transient amplitude (slight, offsetting decrease in both peak and maximum diastolic [Ca2+]i), or CaMKII activity (slight decrease in peak) (Fig. 4, E–H).
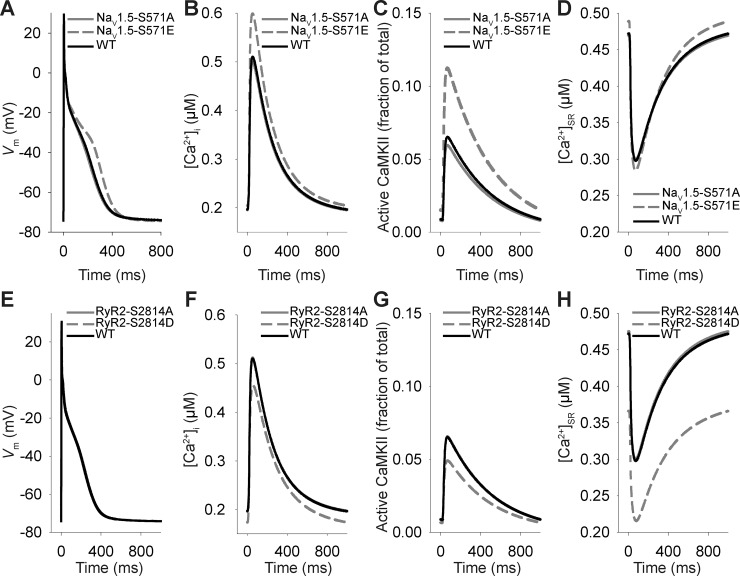
Fig. 4.
Effects of Ca2+/calmodulin-dependent protein kinase (CaMKII)-dependent phosphorylation of Nav1.5 and ryanodine receptor sarcoplasmic reticulum (SR) Ca2+-release channel (RyR2) on atrial myocyte membrane excitability and Ca2+ cycling. A−D: steady-state action potentials, intracellular Ca2+ concentration ([Ca2+]i), fraction of active CaMKII subunits, and Ca2+ concentration in the SR ([Ca2+]SR) transients at 1-Hz pacing frequency for simulated wild-type (WT), phospho-resistant Nav1.5-S571A, and phospho-mimetic Nav1.5-S571E atrial myocytes. E−H: steady-state action potentials, [Ca2+]i, fraction of active CaMKII subunits, and [Ca2+]SR transients at 1-Hz pacing frequency for simulated WT, phospho-resistant RyR2-S2814A, and phospho-mimetic RyR2-S2814D atrial myocytes. Vm, membrane potential.
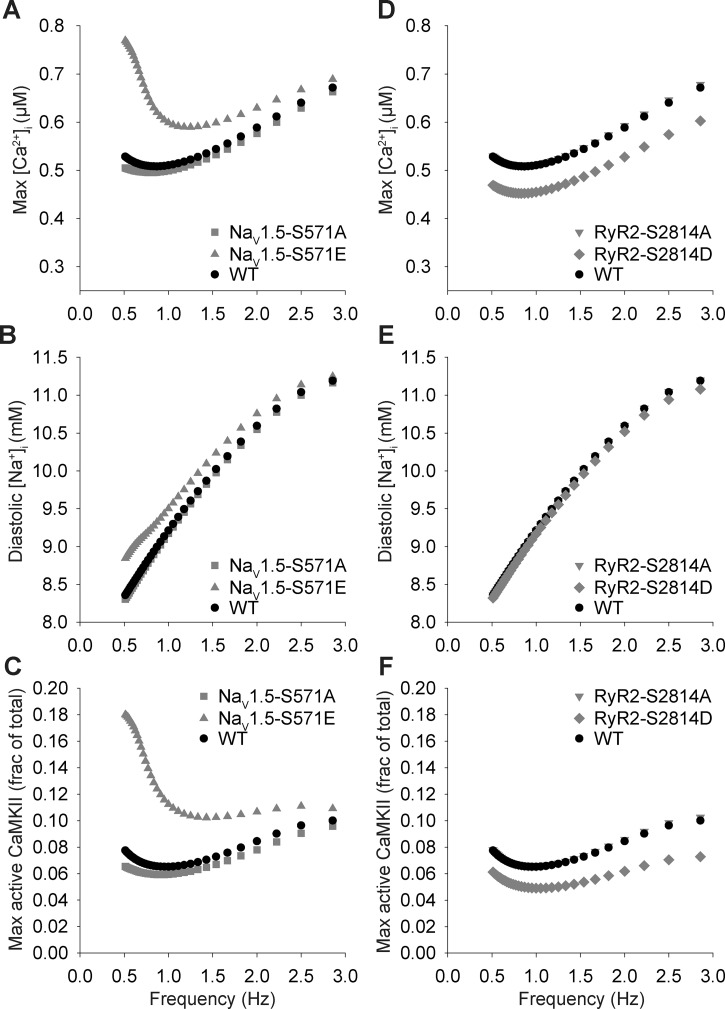
To determine the role of CaMKII-dependent INa,L in the regulation of intracellular ion homeostasis in atrial myocytes, we compared [Ca2+]i, [Na+]i, and CaMKII activity in phospho-mimetic Nav1.5-S571E, phospho-resistant Nav1.5-S571A, and WT models over a range of pacing frequencies. Interestingly, the Nav1.5-S571E model showed a small (<10%) increase in [Na+]i compared with Nav1.5-S571A and WT models, which resulted in a substantial increase in [Ca2+]i and CaMKII activity, especially at pacing frequencies of <1 Hz (Fig. 5). For comparison, the phospho-mimetic RyR2-S2814D model yielded a decrease in [Ca2+]i and CaMKII activity compared with the simulated WT model (Fig. 5). These simulation results indicate that small changes in [Na+]i induced by CaMKII-dependent hyperphosphorylation of Nav1.5 and increased INa,L have strong effects on Ca2+ handling and CaMKII activity in atrial myocytes. Furthermore, the model predicts that constitutive phosphorylation of Nav1.5 alone has a greater impact on [Ca2+]i and CaMKII than constitutive phosphorylation of RyR2 alone. Together, these data indicate that a CaMKII-dependent increase in INa,L is sufficient to produce defects in atrial AP repolarization and Ca2+ handling and likely resides upstream of RyR2 in promoting atrial arrhythmogenesis. Furthermore, our simulations predict that atrial INa,L and CaMKII reside in a feedback loop linked to Ca2+ homeostasis (33).
Fig. 5.
Effects of Ca2+/calmodulin-dependent protein kinase (CaMKII)-dependent phosphorylation of Nav1.5 and ryanodine receptor sarcoplasmic reticulum (SR) Ca2+-release channel (RyR2) on atrial myocyte ion concentration and CaMKII rate dependence. A–C: steady-state peak intracellular Ca2+ concentration ([Ca2+]i), diastolic intracellular Na+ concentration ([Na+]i), and maximal CaMKII activity values over a range of pacing frequencies in simulated wild-type (WT), Nav1.5-S571A, and Nav1.5-S571E myocytes. D−F: steady-state peak [Ca2+]i, diastolic [Na+]i, and maximal CaMKII activity values over a range of pacing frequencies in simulated WT, RyR2-S2814A, and RyR2-S2814D myocytes.
Effect of CaMKII-dependent INa,L on RyR2 function and Ca2+ cycling.
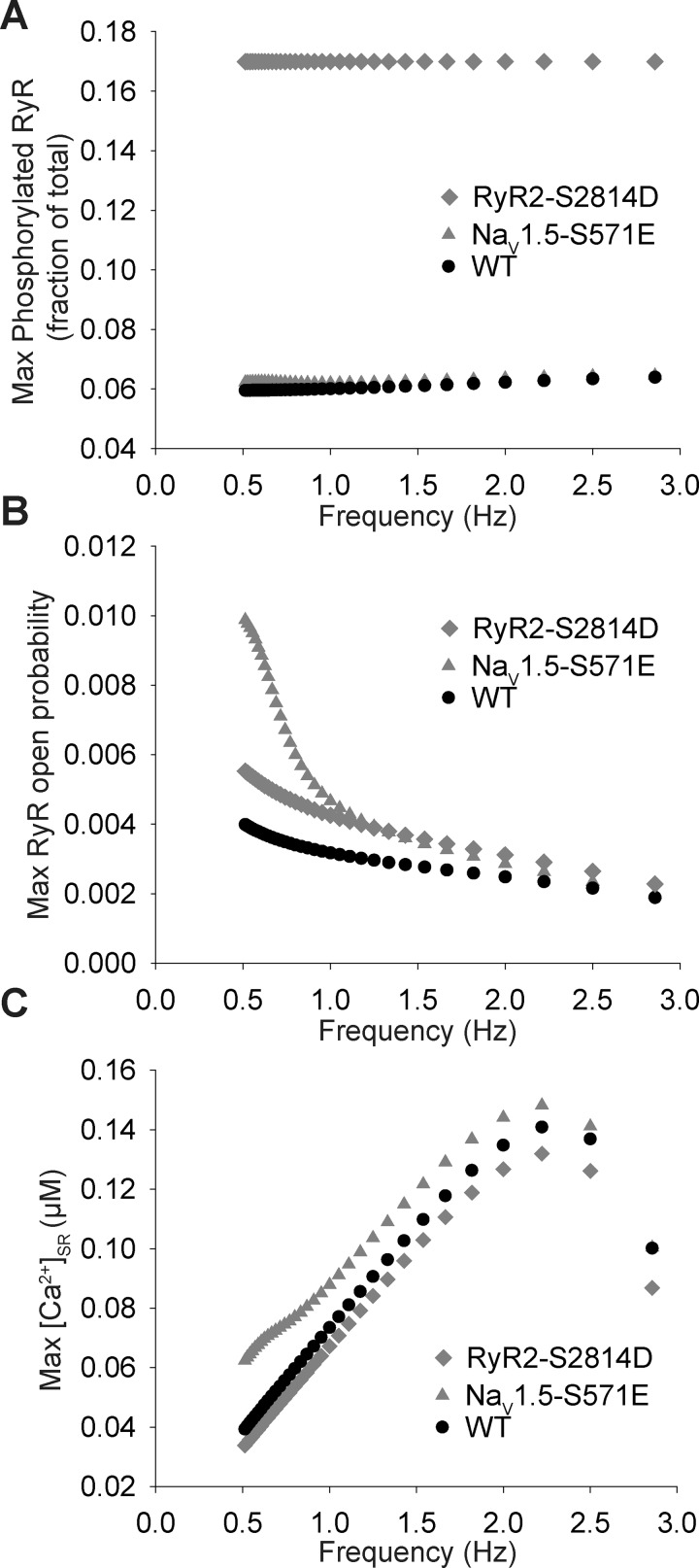
On the basis of our results showing altered Ca2+ homeostasis attributable to hyperphosphorylated Nav1.5, we sought to more fully characterize CaMKII effects on RyR2 function and SR Ca2+ release. The phospho-mimetic Nav1.5-S571E model produced only a small increase in phosphorylated RyR2 compared with the WT or RyR2-S2814D model (Fig. 6A). However, despite the small effect on phosphorylated RyR2, the Nav1.5-S571E model showed a dramatic increase in RyR2 open probability compared with the WT model, especially at pacing frequencies of <1 Hz. Surprisingly, Nav1.5 phosphorylation increased RyR2 open probability even beyond that observed in the RyR2-S2814D model, despite the negligible effect on phosphorylated RyR2, reflecting the greater SR Ca2+ load in the Nav1.5-S571E model compared with the RyR2-S2814D model (Fig. 6C). These data indicate that increasing Ca2+ load (downstream of increased [Na+]i) has a greater overall effect on RyR2 open probability than RyR2 phosphorylation alone.
Fig. 6.
Late Na+ current (INa,L) increases Ca2+/calmodulin-dependent protein kinase (CaMKII)-dependent ryanodine receptor sarcoplasmic reticulum (SR) Ca2+-release channel (RyR2) phosphorylation and open probability. A: maximum phosphorylation of RyR2. B: maximum RyR2 open probability. C: maximum Ca2+ concentration in the SR ([Ca2+]SR) over a range of frequencies for wild-type (WT), Nav1.5-S571E, and RyR2-S2814D simulated mouse models.
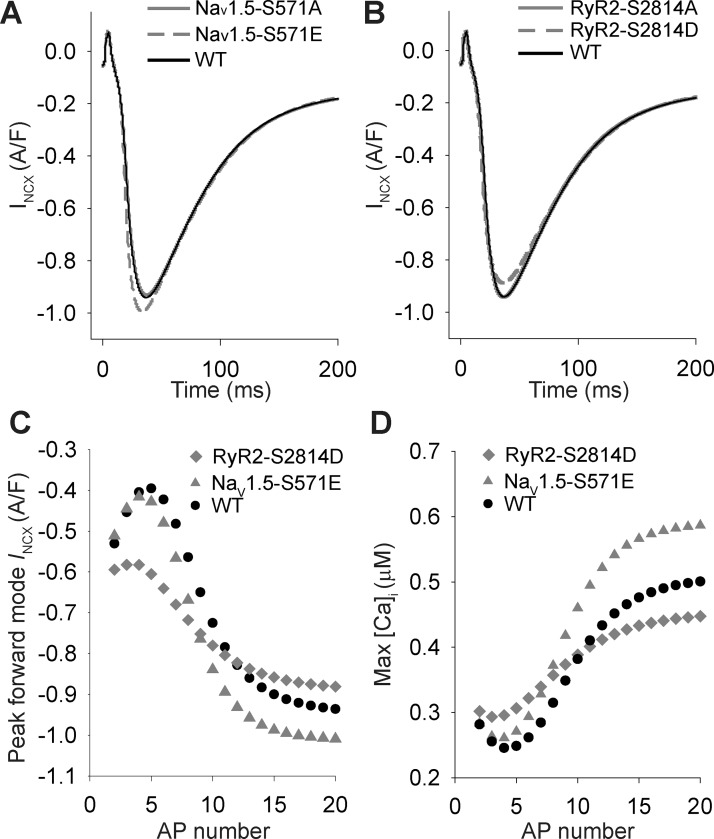
At steady state, our simulations predicted a net decrease in [Ca2+]i with constitutive RyR2 phosphorylation but an increase with Nav1.5 phosphorylation (Fig. 4). In an effort to understand the differential response of steady-state Ca2+ to constitutive RyR2 or Nav1.5 phosphorylation, we compared the behavior of INCX in the two models both at steady state and transiently during the onset of pacing (Fig. 7). The phospho-mimetic RyR2-S2814D model showed a slight decrease in forward-mode INCX at steady state compared with the Nav1.5-S571E or WT models (Fig. 7, A and B), consistent with the reduced steady-state Ca2+ load and SR Ca2+ release. However, our simulations predicted that initially peak [Ca2+]i and forward-mode INCX are potentiated in the RyR2-S2814D model compared with the other models (Fig. 7, C and D), which leads to intracellular Ca2+ depletion within 10–20 beats. In contrast, constitutive Nav1.5 phosphorylation produces a gradual increase in [Ca2+]i due to accumulation of [Na+]i and change in the driving force for INCX (reflected as small initial decrease in reverse-mode INCX during the first 3–5 pacing beats). These results demonstrate that RyR2 phosphorylation increases Ca2+ release transiently, but ultimately forward-mode INCX produces a steady-state condition where intracellular Ca2+ (cytosolic and SR) is depleted.
Fig. 7.
Role of the Na+/Ca2+ exchanger in altered Ca2+ homeostasis induced by phosphorylation of Nav1.5 and ryanodine receptor sarcoplasmic reticulum (SR) Ca2+-release channel (RyR2). A: Na+/Ca2+ exchanger current (INCX) values over time at steady-state 1-Hz pacing frequency for simulated wild-type (WT), Nav1.5-S571A (superimposed on WT), and Nav1.5-S571E models. B: INCX values over time at steady-state 1-Hz pacing frequency for simulated WT, RyR2-S2814A (superimposed on WT), and RyR2-S2814D models. C and D: peak forward-mode INCX (C) and maximum intracellular Ca2+ concentration ([Ca2+]i; D) during action potential from initiation of simulation for WT, Nav1.5-S571E, and RyR2-S2814D models.
Effect of CaMKII-dependent INa,L on atrial myocyte excitability.
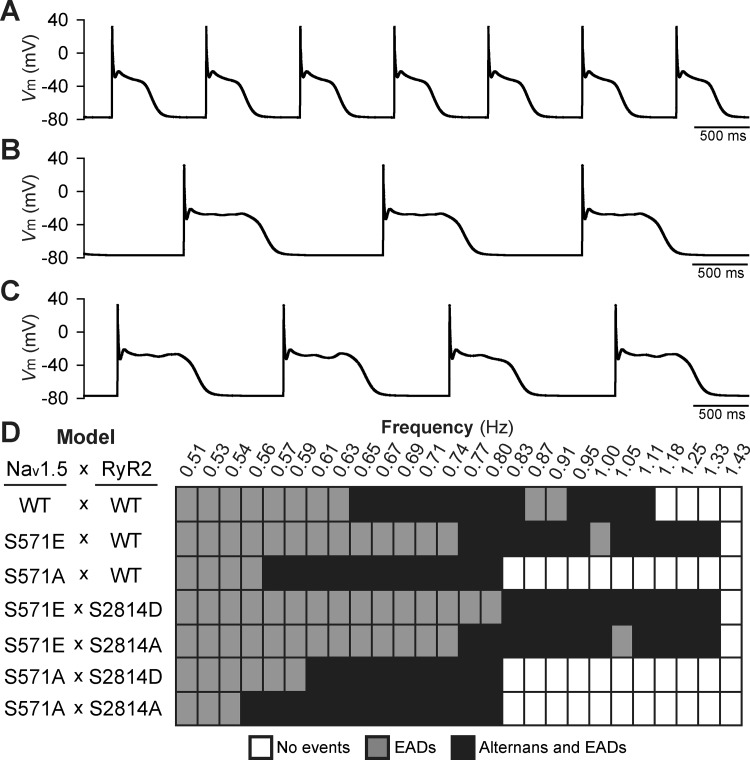
Under baseline conditions, proarrhythmic cellular afterdepolarizations were not observed in phospho-mimetic Nav1.5-S571E, phospho-resistant Nav1.5-S571A, or WT models over a range of pacing frequencies (up to 3.3 Hz; data not shown). However, proarrhythmic repolarization defects (early afterdepolarizations with or without alternans) emerged at slower pacing (below 1.3 Hz) in WT and mutant models in the presence of simulated acute β-AR stimulation (Fig. 8). Notably, the phospho-mimetic Nav1.5-S571E model produced repolarization defects over a wider range of pacing frequencies compared with the WT or phospho-resistant Nav1.5-S571A models. To determine whether proarrhythmia associated with constitutive Nav1.5 phosphorylation acted through positive feedback on CaMKII and subsequent phosphorylation of RyR2, excitability was assessed in models with phospho-mimetic or phospho-resistant changes to both Nav1.5 and RyR2 (Nav1.5-S571E × RyR2-S2814D, Nav1.5-S571E × RyR2-S2814A, Nav1.5-S571A × RyR2-S2814D, and Nav1.5-S571A × RyR2-S2814A). Constitutive phosphorylation or ablation of the RyR2-S2814 model altered the repolarization morphology but failed to normalize the increase in range over which repolarization defects were observed in the Nav1.5-S571E model (compare Nav1.5-S571E × RyR2-S2814D and Nav1.5-S571E × RyR2-S2814A with Nav1.5-S571E and WT in Fig. 8). Similarly, altering phosphorylation status of RyR2 had little impact on behavior in the Nav1.5-S571A model. These results indicate that CaMKII-dependent phosphorylation of Nav1.5 promotes atrial myocyte proarrhythmia in response to acute β-AR stimulation. Furthermore, our simulations indicate that constitutive Nav1.5 phosphorylation promotes atrial repolarization defects primarily by increasing SR Ca2+ load (and therefore RyR2 open probability) as opposed to enhancing RyR2 phosphorylation status.
Fig. 8.
Afterdepolarization activity under conditions of acute β-adrenergic stimulation. Simulated steady-state wild-type (WT) action potentials (APs) for three different pacing frequencies in the presence of β-adrenergic stimulation showed the following behavior: normal repolarization (no proarrhythmia events' A), AP with early afterdepolarizations (EADs' B), and AP alternans and EADs (C). D: summary of steady-state AP behavior observed over a range of pacing frequencies (0.51–1.43 Hz) for the following models: 1) normal Nav1.5 and ryanodine receptor sarcoplasmic reticulum (SR) Ca2+-release channel (RyR2) (WT × WT); 2) phospho-mimetic or phospho-resistant Nav1.5 on the background of normal RyR2 (S571E × WT and S571A × WT, respectively); and 3) phospho-mimetic or phospho-resistant Nav1.5 on the background of phospho-mimetic or phospho-resistant RyR2 (S571E × S2814D, S571E × S2814A, S571A × S2814D, and S571A × S2814A, respectively). Vm, membrane potential.
Relative contribution of INa,L to the regulation of atrial ion homeostasis and CaMKII activity.
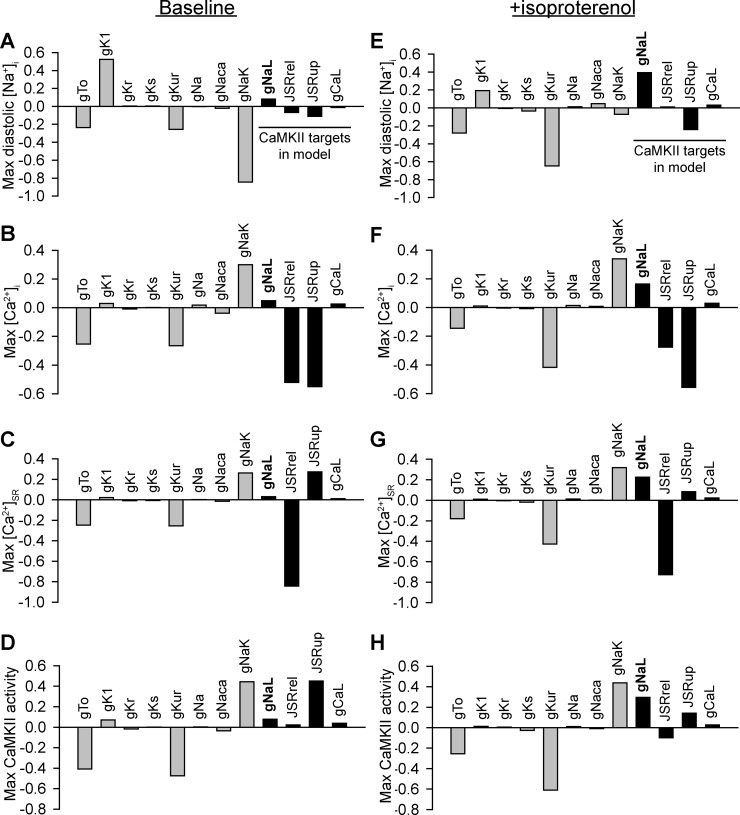
To provide insight into the importance of INa,L in regulating atrial myocyte physiology relative to other major atrial ion currents, a parameter sensitivity analysis was conducted on the model under baseline conditions and in the presence of β-AR stimulation (Fig. 9). Although perturbation of INa,L had only a limited effect on intracellular ion concentrations or CaMKII activity at baseline, its impact was greatly enhanced in the setting of β-AR stimulation. In fact, INa,L was associated with positive regression coefficients for intracellular Na+, Ca2+, and CaMKII activity, meaning an increase in INa,L (as in response to CaMKII activation) is expected to increase all of these properties. In contrast, RyR2 flux rate (JSRrel) showed negative regression coefficients for the same properties, whereas SR Ca2+ uptake (JSRup) showed a combination of positive and negative values. Finally, ICa,L showed positive but relatively small regression coefficients. In summary, of all CaMKII targets included in the model (INa,L, JSRrel, JSRup, and ICa,L), our analysis predicts that an increase in INa,L is a relatively efficient way of promoting intracellular ion accumulation and positive feedback on CaMKII.
Fig. 9.
Relative contribution of late Na+ current to changes in atrial myocyte ion homeostasis and Ca2+/calmodulin-dependent protein kinase (CaMKII) signaling. Regression coefficients showed how changes in the model parameters affected ion homeostasis and CaMKII activity in baseline wild-type (WT) simulated cells (A–D) and in the presence of the β-adrenergic agonist isoproterenol (E–H). Shown are parameter sensitivities of ion channel conductance parameters affecting maximum diastolic intracellular Na+ concentration ([Na+]i; A and E), maximum intracellular Ca2+ concentration ([Ca2+]i; B and F), maximum Ca2+ concentration in the sarcoplasmic reticulum ([Ca2+]SR; C and G), and maximum fraction of activated CaMKII subunits (D and H). Abbreviations are as follows: gTo, maximal conductance (gMax) of the transient outward K+ current; gK1, gMax of the inward rectifier K+ current; gKr, gMax of the rapid delayed rectifier K+ current; gKs, gMax of the slow delayed rectifier K+ current; gKur, gMax of the ultrarapid K+ current; gNa, gMax of the rapid Na+ current; gNaca, maximal transport rate of the Na+/Ca2+ exchanger; gNaL, gMax of the late Na+ current; JSRrel, maximal Ca2+ release flux from the junctional sarcoplasmic reticulum; JSRup, maximal Ca2+ uptake rate into the sarcoplasmic reticulum; gCaL, gMax of the L-type Ca2+ channel.
DISCUSSION
In this study, we used mathematical modeling to explore the role of CaMKII-dependent INa,L in the regulation of atrial myocyte ion homeostasis and membrane excitability. Our simulations led to a number of important findings, including 1) a CaMKII-dependent increase in INa,L promotes significant accumulation of intracellular Ca2+ (cytosolic and SR) in atrial myocytes; 2) INa,L-induced Ca2+ accumulation, in turn, enhances atrial CaMKII activity and phosphorylation of downstream targets (e.g., RyR2); 3) CaMKII phosphorylation of atrial Nav1.5 (and the subsequent increase in INa,L) alone produces greater intracellular Ca2+ accumulation, CaMKII activation, and increase in RyR2 open probability compared with RyR2 phosphorylation alone; and 4) increased INa,L promotes proarrhythmic behavior under conditions of acute β-AR stimulation in atrial myocytes primarily by increasing intracellular Ca2+ stores. Although previous studies have examined the role of CaMKII as a molecular driver for Ca2+ homeostasis and cardiac dysfunction (47), our effort represents a novel approach to dissect the complex effects of CaMKII on downstream targets in atrial cells.
Atrial myocytes from human patients with AF show increased CaMKII activity and altered INa,L compared with sinus rhythm, although the pathophysiological relevance remains unclear (6, 13, 16). In parallel, enhanced INCX activity has been measured in human AF myocytes, which has been attributed to higher [Na+]i (8). Furthermore, increased INa,L in Nav1.5-S571E or through application of the INa,L enhancer ATX-II promotes abnormal [Ca2+]i handling and SR Ca2+ leak (13, 16). These findings are consistent with predictions of our model that a CaMKII-dependent increase in INa,L promotes accumulation of intracellular Na+ and Ca2+ together with enhanced INCX activity in atrial myocytes. Furthermore, these data support the notion that INCX is a critical node linking CaMKII-dependent INa,L to dysregulation of intracellular Ca2+ homeostasis, promoting arrhythmogenesis in AF.
It is important to note that studies in preclinical models and human myocytes indicate that dysregulation of multiple CaMKII targets (not just Nav1.5) occurs in AF and likely contributes to arrhythmogenesis (54, 56, 60). For example, impaired CaMKII-dependent regulation of RyR2 has been demonstrated to alter Ca2+ cycling and increase atrial ectopy and AF susceptibility in the mouse (7, 30, 50, 53). Specifically, increased diastolic SR Ca2+ leak through phosphorylated RyR2 has been proposed as an important causal factor in AF (57). In our simulations, constitutive CaMKII-dependent RyR2 phosphorylation produced a transient elevation in SR Ca2+ release (due to higher RyR2 open probability), which ultimately produced a decrease in intracellular Ca2+ (cytosolic and SR) via activation of forward-mode INCX. In contrast, constitutive phosphorylation of Nav1.5 (and the subsequent increase in INa,L) produced a gradual but sustained increase in intracellular Na+ and Ca2+ in the model. Together, these results highlight distinct but potentially synergistic pathways for altering intracellular Ca2+ cycling. Future studies with available mouse models (e.g., Nav1.5-S571E/A or RyR2-S2814D/A) will be necessary to test these model predictions and further dissect the relative roles of Nav1.5 and other CaMKII targets in atrial arrhythmogenesis.
It is interesting to note that our simulations predicted a complex relationship between CaMKII-dependent INa,L and atrial excitability with both pro- and antiarrhythmic effects. Namely, CaMKII-induced INa,L prolongs atrial APD, which promotes formation of early afterdepolarizations at slow rates and in the presence of isoproterenol but also would be expected to decrease the likelihood for sustained reentrant arrhythmia. In fact, atrial APD shortening attributable to ion channel remodeling is commonly thought to favor sustained AF in human patients and animal models. It is possible that enhanced INa,L increases susceptibility to atrial arrhythmia triggers but that other factors are required to create a substrate for arrhythmia maintenance (e.g., APD shortening, fibrosis, or cell-to-cell uncoupling). It is also important to note that, although our simulations indicate a role for CaMKII-enhanced INa,L in proarrhythmic afterdepolarizations and alternans in response to a β-AR agonist, the relationship between β-AR stimulation and alternans has been found in separate studies to be both positive (β-AR-dependent exacerbation) (34) or negative (β-AR-dependent suppression) (14). At the same time, it is interesting to note that, although our simulations predict repolarization defects with β-AR stimulation, these occur only at slower rates (<1.33 Hz). However, β-AR blockers (expected to slow rate) remain a widely used rate control therapy in the treatment of AF (19, 23). Together, these data point to the complex web of factors underlying an arrhythmia phenotype and highlight the need for computational modeling to help tackle these high-dimensional problems.
Recent advances in the development of personalized, whole heart computer models represent an exciting direction for the field (2, 49, 61, 62). These advanced models have been used to study the effects of a wide range of disease factors on cardiac AP propagation, including heterogeneous changes in cell electrophysiology, coupling, and tissue structure. It will be interesting going forward to incorporate dynamic cell signaling pathways (such as the CaMKII pathway studied here) with these higher-dimensional models to consider how acute signaling interacts with more chronic changes in cell excitability or tissue structure. With this goal in mind, it is important to note that the updated formulations incorporated into this model for CaMKII targeting of INa,L, PLB, ICa,L, and RyR2 are compatible with other atrial cell models used in organ-level simulations (5, 11) and can help elucidate the impact of CaMKII on organ-level AF properties (e.g., rotor stabilization and conduction block). This effort is particularly exciting and poised to inform organ-level simulations as well as other research directions because of the availability of transgenic mouse models (e.g., RyR2-S2814D/A and Nav1.5-S571E/A mice) to test model predictions.
Limitations.
Although the model used in our study incorporated components important for the CaMKII-dependent behavior under investigation, it is nonetheless a simplification of the physiological system. Namely, CaMKII targets a number of membrane ion channels not included in this analysis, including Ito and IK1 (47). Furthermore, CaMKII has been demonstrated to regulate not just INa,L but also INa by reducing channel availability (58). Although inclusion of these and other CaMKII targets could potentially yield further insights into atrial arrhythmia mechanisms, our sensitivity analysis indicates that these targets are unlikely to contribute to the ion homeostasis defects observed in AF (Fig. 9). In the instance of Ito, for example, the model predicted a negative relationship between channel conductance and intracellular Na+, Ca2+, and CaMKII, meaning that these values would be expected to decrease in response to an increase in Ito (as observed with CaMKII activation). For IK1 and INa, the regression coefficients were relatively small and, in the case of INa, work against ion accumulation with CaMKII activation (CaMKII decreases INa). At the same time, regression analysis of the model highlights the fact that precise values of certain model parameters may influence absolute values for ion concentrations and CaMKII activity in the model. For example, we were surprised to find that intracellular Na+, Ca2+, and CaMKII were relatively sensitive to IKur as well as INaK (less surprising), indicating that small discrepancies in values of conductances/flux rates for these elements would be expected to have a relatively large impact on model predictions. It is also important to note that electrical and structural remodeling changes characteristic of persistent AF (59) are not incorporated into this model. Undoubtedly, it will be interesting to study the interaction between acute effects studied here and changes in tissue structure. Finally, atrial myocyte cell excitability is part of a complex network of signaling mechanisms, and incorporation of CaMKII signaling with PKA (to address cross talk and individual effects) would strengthen the prediction of CaMKII effects in this study.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-114893 (to T. Hund), HL-134824 (to T. Hund), HL-135096 (to T. Hund), and HL-129766 (to B. Onal), by the James S. McDonnell Foundation (to T. Hund), by the Saving Tiny Hearts Society (to T. Hund), and by a TriFit Challenge grant from the Ross Heart Hospital and Davis Heart and Lung Research Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.O., D.G., and T.J.H. conceived and designed research; B.O. and D.G. performed experiments; B.O., D.G., and T.J.H. analyzed data; B.O., D.G., and T.J.H. interpreted results of experiments; B.O. prepared figures; B.O. drafted manuscript; B.O., D.G., and T.J.H. edited and revised manuscript; B.O., D.G., and T.J.H. approved final version of manuscript.
APPENDIX: MODEL EQUATIONS
This appendix contains the model equations added to simulate CaMKII phosphorylation of INa,L and RyR2. See the original publications for complete model equations and variable/parameter definitions (9, 22, 45, 48).
CaMKII module.
Persistent Na+ current.
For WT:
RyR Ca2+ release and SERCA2a flux targeting by CaMKII.
L-type Ca2+ channel.
REFERENCES
- 1.Barrère-Lemaire S, Piot C, Leclercq F, Nargeot J, Richard S. Facilitation of L-type calcium currents by diastolic depolarization in cardiac cells: impairment in heart failure. Cardiovasc Res 47: 336–349, 2000. doi: 10.1016/S0008-6363(00)00107-3. [DOI] [PubMed] [Google Scholar]
- 2.Bayer JD, Roney CH, Pashaei A, Jaïs P, Vigmond EJ. Novel radiofrequency ablation strategies for terminating atrial fibrillation in the left atrium: an aimulation study. Front Physiol 7: 108, 2016. doi: 10.3389/fphys.2016.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 98: 946–952, 1998. doi: 10.1161/01.CIR.98.10.946. [DOI] [PubMed] [Google Scholar]
- 4.Carneiro JS, Bento ASA, Bacic D, Nearing BD, Rajamani S, Belardinelli L, Verrier RL. The selective cardiac late sodium current inhibitor GS-458967 suppresses autonomically triggered atrial fibrillation in an intact porcine model. J Cardiovasc Electrophysiol 26: 1364–1369, 2015. doi: 10.1111/jce.12824. [DOI] [PubMed] [Google Scholar]
- 5.Chang KC, Trayanova NA. Mechanisms of arrhythmogenesis related to calcium-driven alternans in a model of human atrial fibrillation. Sci Rep 6: 36395, 2016. doi: 10.1038/srep36395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Müller FU, Schmitz W, Schotten U, Anderson ME, Valderrábano M, Dobrev D, Wehrens XH. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest 119: 1940–1951, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang DY, Li N, Wang Q, Alsina KM, Quick AP, Reynolds JO, Wang G, Skapura D, Voigt N, Dobrev D, Wehrens XH. Impaired local regulation of ryanodine receptor type 2 by protein phosphatase 1 promotes atrial fibrillation. Cardiovasc Res 103: 178–187, 2014. doi: 10.1093/cvr/cvu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christ T, Kovács PP, Acsai K, Knaut M, Eschenhagen T, Jost N, Varró A, Wettwer E, Ravens U. Block of Na+/Ca2+ exchanger by SEA0400 in human right atrial preparations from patients in sinus rhythm and in atrial fibrillation. Eur J Pharmacol 788: 286–293, 2016. doi: 10.1016/j.ejphar.2016.06.050. [DOI] [PubMed] [Google Scholar]
- 9.Christensen MD, Dun W, Boyden PA, Anderson ME, Mohler PJ, Hund TJ. Oxidized calmodulin kinase II regulates conduction following myocardial infarction: a computational analysis. PLOS Comput Biol 5: e1000583, 2009. doi: 10.1371/journal.pcbi.1000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connolly SJ, Camm AJ, Halperin JL, Joyner C, Alings M, Amerena J, Atar D, Avezum Á, Blomström P, Borggrefe M, Budaj A, Chen SA, Ching CK, Commerford P, Dans A, Davy JM, Delacrétaz E, Di Pasquale G, Diaz R, Dorian P, Flaker G, Golitsyn S, Gonzalez-Hermosillo A, Granger CB, Heidbüchel H, Kautzner J, Kim JS, Lanas F, Lewis BS, Merino JL, Morillo C, Murin J, Narasimhan C, Paolasso E, Parkhomenko A, Peters NS, Sim KH, Stiles MK, Tanomsup S, Toivonen L, Tomcsányi J, Torp-Pedersen C, Tse HF, Vardas P, Vinereanu D, Xavier D, Zhu J, Zhu JR, Baret-Cormel L, Weinling E, Staiger C, Yusuf S, Chrolavicius S, Afzal R, Hohnloser SH; PALLAS Investigators . Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med 365: 2268–2276, 2011. doi: 10.1056/NEJMoa1109867. [DOI] [PubMed] [Google Scholar]
- 11.Courtemanche M, Ramirez RJ, Nattel S. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am J Physiol Heart Circ Physiol 275: H301–H321, 1998. [DOI] [PubMed] [Google Scholar]
- 12.De Ferrari GM, Maier LS, Mont L, Schwartz PJ, Simonis G, Leschke M, Gronda E, Boriani G, Darius H, Guillamón Torán L, Savelieva I, Dusi V, Marchionni N, Quintana Rendón M, Schumacher K, Tonini G, Melani L, Giannelli S, Alberto Maggi C, Camm AJ; RAFFAELLO Investigators (see Online Supplementary Appendix for List of Participating Centers and Investigators) . Ranolazine in the treatment of atrial fibrillation: results of the dose-ranging RAFFAELLO (Ranolazine in Atrial Fibrillation Following an Electrical Cardioversion) study. Heart Rhythm 12: 872–878, 2015. doi: 10.1016/j.hrthm.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Fischer TH, Herting J, Mason FE, Hartmann N, Watanabe S, Nikolaev VO, Sprenger JU, Fan P, Yao L, Popov AF, Danner BC, Schöndube F, Belardinelli L, Hasenfuss G, Maier LS, Sossalla S. Late INa increases diastolic SR-Ca2+-leak in atrial myocardium by activating PKA and CaMKII. Cardiovasc Res 107: 184–196, 2015. doi: 10.1093/cvr/cvv153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Florea SM, Blatter LA. Regulation of cardiac alternans by β-adrenergic signaling pathways. Am J Physiol Heart Circ Physiol 303: H1047–H1056, 2012. doi: 10.1152/ajpheart.00384.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford J, Milnes J, El Haou S, Wettwer E, Loose S, Matschke K, Tyl B, Round P, Ravens U. The positive frequency-dependent electrophysiological effects of the IKur inhibitor XEN-D0103 are desirable for the treatment of atrial fibrillation. Heart Rhythm 13: 555–564, 2016. doi: 10.1016/j.hrthm.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glynn P, Musa H, Wu X, Unudurthi SD, Little S, Qian L, Wright PJ, Radwanski PB, Gyorke S, Mohler PJ, Hund TJ. Voltage-gated sodium channel phosphorylation at Ser571 regulates late current, arrhythmia, and cardiac function in vivo. Circulation 132: 567–577, 2015. doi: 10.1161/CIRCULATIONAHA.114.015218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong M, Zhang Z, Fragakis N, Korantzopoulos P, Letsas KP, Li G, Yan GX, Liu T. Role of ranolazine in the prevention and treatment of atrial fibrillation: a meta-analysis of randomized clinical trials. Heart Rhythm 14: 3–11, 2017. doi: 10.1016/j.hrthm.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Grandi E, Pandit SV, Voigt N, Workman AJ, Dobrev D, Jalife J, Bers DM. Human atrial action potential and Ca2+ model: sinus rhythm and chronic atrial fibrillation. Circ Res 109: 1055–1066, 2011. doi: 10.1161/CIRCRESAHA.111.253955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heist EK, Mansour M, Ruskin JN. Rate control in atrial fibrillation: targets, methods, resynchronization considerations. Circulation 124: 2746–2755, 2011. doi: 10.1161/CIRCULATIONAHA.111.019919. [DOI] [PubMed] [Google Scholar]
- 20.Huang H, Zhao J, Barrane F-Z, Champagne J, Chahine M. Nav1.5/R1193Q polymorphism is associated with both long QT and Brugada syndromes. Can J Cardiol 22: 309–313, 2006. doi: 10.1016/S0828-282X(06)70915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hund TJ, Koval OM, Li J, Wright PJ, Qian L, Snyder JS, Gudmundsson H, Kline CF, Davidson NP, Cardona N, Rasband MN, Anderson ME, Mohler PJ. A β(IV)-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J Clin Invest 120: 3508–3519, 2010. doi: 10.1172/JCI43621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hund TJ, Rudy Y. Rate dependence and regulation of action potential and calcium transient in a canine cardiac ventricular cell model. Circulation 110: 3168–3174, 2004. doi: 10.1161/01.CIR.0000147231.69595.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kettlewell S, Burton FL, Smith GL, Workman AJ. Chronic myocardial infarction promotes atrial action potential alternans, afterdepolarizations, and fibrillation. Cardiovasc Res 99: 215–224, 2013. doi: 10.1093/cvr/cvt087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koval OM, Snyder JS, Wolf RM, Pavlovicz RE, Glynn P, Curran J, Leymaster ND, Dun W, Wright PJ, Cardona N, Qian L, Mitchell CC, Boyden PA, Binkley PF, Li C, Anderson ME, Mohler PJ, Hund TJ. Ca2+/calmodulin-dependent protein kinase II-based regulation of voltage-gated Na+ channel in cardiac disease. Circulation 126: 2084–2094, 2012. doi: 10.1161/CIRCULATIONAHA.112.105320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li N, Chiang DY, Wang S, Wang Q, Sun L, Voigt N, Respress JL, Ather S, Skapura DG, Jordan VK, Horrigan FT, Schmitz W, Müller FU, Valderrabano M, Nattel S, Dobrev D, Wehrens XHT. Ryanodine receptor-mediated calcium leak drives progressive development of an atrial fibrillation substrate in a transgenic mouse model. Circulation 129: 1276–1285, 2014. doi: 10.1161/CIRCULATIONAHA.113.006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang F, Fan P, Jia J, Yang S, Jiang Z, Karpinski S, Kornyeyev D, Pagratis N, Belardinelli L, Yao L. Inhibitions of late INa and CaMKII act synergistically to prevent ATX-II-induced atrial fibrillation in isolated rat right atria. J Mol Cell Cardiol 94: 122–130, 2016. doi: 10.1016/j.yjmcc.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Maier LS, Sossalla S. The late Na current as a therapeutic target: where are we? J Mol Cell Cardiol 61: 44–50, 2013. doi: 10.1016/j.yjmcc.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Maltsev VA, Silverman N, Sabbah HN, Undrovinas AI. Chronic heart failure slows late sodium current in human and canine ventricular myocytes: implications for repolarization variability. Eur J Heart Fail 9: 219–227, 2007. doi: 10.1016/j.ejheart.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mangoni ME, Fontanaud P, Noble PJ, Noble D, Benkemoun H, Nargeot J, Richard S. Facilitation of the L-type calcium current in rabbit sino-atrial cells: effect on cardiac automaticity. Cardiovasc Res 48: 375–392, 2000. doi: 10.1016/S0008-6363(00)00182-6. [DOI] [PubMed] [Google Scholar]
- 30.Mazzocchi G, Sommese L, Palomeque J, Felice JI, Di Carlo MN, Fainstein D, Gonzalez P, Contreras P, Skapura D, McCauley MD, Lascano EC, Negroni JA, Kranias EG, Wehrens XH, Valverde CA, Mattiazzi A. Phospholamban ablation rescues the enhanced propensity to arrhythmias of mice with CaMKII-constitutive phosphorylation of RyR2 at site S2814. J Physiol 594: 3005–3030, 2016. doi: 10.1113/JP271622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 114: 119–125, 2006. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 32.Moreau A, Krahn AD, Gosselin-Badaroudine P, Klein GJ, Christé G, Vincent Y, Boutjdir M, Chahine M. Sodium overload due to a persistent current that attenuates the arrhythmogenic potential of a novel LQT3 mutation. Front Pharmacol 4: 126, 2013. doi: 10.3389/fphar.2013.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morotti S, Edwards AG, McCulloch AD, Bers DM, Grandi E. A novel computational model of mouse myocyte electrophysiology to assess the synergy between Na+ loading and CaMKII. J Physiol 592: 1181–1197, 2014. doi: 10.1113/jphysiol.2013.266676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng GA, Brack KE, Patel VH, Coote JH. Autonomic modulation of electrical restitution, alternans and ventricular fibrillation initiation in the isolated heart. Cardiovasc Res 73: 750–760, 2007. doi: 10.1016/j.cardiores.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Onal B, Gratz D, Hund T. LongQt: a cardiac electrophysiology simulation platform. MethodsX 3: 589–599, 2016. doi: 10.1016/j.mex.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osaka T, Itoh A, Kodama I. Action potential remodeling in the human right atrium with chronic lone atrial fibrillation. Pacing Clin Electrophysiol 23: 960–965, 2000. doi: 10.1111/j.1540-8159.2000.tb00881.x. [DOI] [PubMed] [Google Scholar]
- 37.Piccini JP, Fauchier L. Rhythm control in atrial fibrillation. Lancet 388: 829–840, 2016. doi: 10.1016/S0140-6736(16)31277-6. [DOI] [PubMed] [Google Scholar]
- 38.Poulet C, Wettwer E, Grunnet M, Jespersen T, Fabritz L, Matschke K, Knaut M, Ravens U. Late sodium current in human atrial cardiomyocytes from patients in sinus rhythm and atrial fibrillation. PLoS One 10: e0131432, 2015. doi: 10.1371/journal.pone.0131432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reiffel JA, Camm AJ, Belardinelli L, Zeng D, Karwatowska-Prokopczuk E, Olmsted A, Zareba W, Rosero S, Kowey P; HARMONY Investigators . The HARMONY Trial. Circ Arrhythm Electrophysiol 8: 1048–1056, 2015. doi: 10.1161/CIRCEP.115.002856. [DOI] [PubMed] [Google Scholar]
- 40.Rosa GM, Dorighi U, Ferrero S, Brunacci M, Bertero G, Brunelli C. Ranolazine for the treatment of atrial fibrillation. Expert Opin Investig Drugs 24: 825–836, 2015. doi: 10.1517/13543784.2015.1036984. [DOI] [PubMed] [Google Scholar]
- 41.Scirica BM, Belardinelli L, Chaitman BR, Waks JW, Volo S, Karwatowska-Prokopczuk E, Murphy SA, Cheng ML, Braunwald E, Morrow DA. Effect of ranolazine on atrial fibrillation in patients with non-ST elevation acute coronary syndromes: observations from the MERLIN-TIMI 36 trial. Europace 17: 32–37, 2015. doi: 10.1093/europace/euu217. [DOI] [PubMed] [Google Scholar]
- 42.Shannon TR, Wang F, Bers DM. Regulation of cardiac sarcoplasmic reticulum Ca release by luminal [Ca] and altered gating assessed with a mathematical model. Biophys J 89: 4096–4110, 2005. doi: 10.1529/biophysj.105.068734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shinlapawittayatorn K, Du XX, Liu H, Ficker E, Kaufman ES, Deschênes I. A common SCN5A polymorphism modulates the biophysical defects of SCN5A mutations. Heart Rhythm 8: 455–462, 2011. doi: 10.1016/j.hrthm.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh BN, Connolly SJ, Crijns HJGM, Roy D, Kowey PR, Capucci A, Radzik D, Aliot EM, Hohnloser SH; EURIDIS and ADONIS Investigators . Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med 357: 987–999, 2007. doi: 10.1056/NEJMoa054686. [DOI] [PubMed] [Google Scholar]
- 45.Soltis AR, Saucerman JJ. Synergy between CaMKII substrates and β-adrenergic signaling in regulation of cardiac myocyte Ca2+ handling. Biophys J 99: 2038–2047, 2010. doi: 10.1016/j.bpj.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sossalla S, Kallmeyer B, Wagner S, Mazur M, Maurer U, Toischer K, Schmitto JD, Seipelt R, Schöndube FA, Hasenfuss G, Belardinelli L, Maier LS. Altered Na+ currents in atrial fibrillation effects of ranolazine on arrhythmias and contractility in human atrial myocardium. J Am Coll Cardiol 55: 2330–2342, 2010. doi: 10.1016/j.jacc.2009.12.055. [DOI] [PubMed] [Google Scholar]
- 47.Swaminathan PD, Purohit A, Hund TJ, Anderson ME. Calmodulin-dependent protein kinase II: linking heart failure and arrhythmias. Circ Res 110: 1661–1677, 2012. doi: 10.1161/CIRCRESAHA.111.243956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terrenoire C, Clancy CE, Cormier JW, Sampson KJ, Kass RS. Autonomic control of cardiac action potentials: role of potassium channel kinetics in response to sympathetic stimulation. Circ Res 96: e25–e34, 2005. doi: 10.1161/01.RES.0000160555.58046.9a. [DOI] [PubMed] [Google Scholar]
- 49.Trayanova NA. Whole-heart modeling: applications to cardiac electrophysiology and electromechanics. Circ Res 108: 113–128, 2011. doi: 10.1161/CIRCRESAHA.110.223610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uchinoumi H, Yang Y, Oda T, Li N, Alsina KM, Puglisi JL, Chen-Izu Y, Cornea RL, Wehrens XHT, Bers DM. CaMKII-dependent phosphorylation of RyR2 promotes targetable pathological RyR2 conformational shift. J Mol Cell Cardiol 98: 62–72, 2016. doi: 10.1016/j.yjmcc.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Unudurthi SD, Wu X, Qian L, Amari F, Onal B, Li N, Makara MA, Smith SA, Snyder J, Fedorov VV, Coppola V, Anderson ME, Mohler PJ, Hund TJ. Two-pore K+ channel TREK-1 regulates sinoatrial node membrane excitability. J Am Heart Assoc 5: e002865, 2016. doi: 10.1161/JAHA.115.002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, Kamp TJ, Makielski JC. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol 38: 475–483, 2005. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 53.van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, Wang Q, De Almeida AC, Skapura DG, Anderson ME, Bers DM, Wehrens XH. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation 122: 2669–2679, 2010. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res 85: 428–436, 1999. doi: 10.1161/01.RES.85.5.428. [DOI] [PubMed] [Google Scholar]
- 55.Verrier RL, Kumar K, Nieminen T, Belardinelli L. Mechanisms of ranolazine’s dual protection against atrial and ventricular fibrillation. Europace 15: 317−324, 2013. doi: 10.1093/europace/eus380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XHT, Nattel S, Dobrev D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation 129: 145–156, 2014. doi: 10.1161/CIRCULATIONAHA.113.006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XH, Dobrev D. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 125: 2059–2070, 2012. doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wagner S, Dybkova N, Rasenack ECL, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest 116: 3127–3138, 2006. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolf RM, Glynn P, Hashemi S, Zarei K, Mitchell CC, Anderson ME, Mohler PJ, Hund TJ. Atrial fibrillation and sinus node dysfunction in human ankyrin-B syndrome: a computational analysis. Am J Physiol Heart Circ Physiol 304: H1253–H1266, 2013. doi: 10.1152/ajpheart.00734.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yeh Y-H, Wakili R, Qi X-Y, Chartier D, Boknik P, Kääb S, Ravens U, Coutu P, Dobrev D, Nattel S. Calcium-handling abnormalities underlying atrial arrhythmogenesis and contractile dysfunction in dogs with congestive heart failure. Circ Arrhythm Electrophysiol 1: 93–102, 2008. doi: 10.1161/CIRCEP.107.754788. [DOI] [PubMed] [Google Scholar]
- 61.Zahid S, Cochet H, Boyle PM, Schwarz EL, Whyte KN, Vigmond EJ, Dubois R, Hocini M, Haïssaguerre M, Jaïs P, Trayanova NA. Patient-derived models link re-entrant driver localization in atrial fibrillation to fibrosis spatial pattern. Cardiovasc Res 110: 443–454, 2016. doi: 10.1093/cvr/cvw073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zahid S, Whyte KN, Schwarz EL, Blake RC III, Boyle PM, Chrispin J, Prakosa A, Ipek EG, Pashakhanloo F, Halperin HR, Calkins H, Berger RD, Nazarian S, Trayanova NA. Feasibility of using patient-specific models and the “minimum cut” algorithm to predict optimal ablation targets for left atrial flutter. Heart Rhythm 13: 1687–1698, 2016. doi: 10.1016/j.hrthm.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Wang H-M, Wang Y-Z, Zhang Y-Y, Jin X-X, Zhao Y, Wang J, Sun YL, Xue GL, Li PH, Huang QH, Yang BF, Pan ZW. Increment of late sodium currents in the left atrial myocytes and its potential contribution to the increased susceptibility of atrial fibrillation in castrated male mice. Heart Rhythm 14: 1073−1080, 2017. doi: 10.1016/j.hrthm.2017.01.046. [DOI] [PubMed] [Google Scholar]
- 64.Zou D, Geng N, Chen Y, Ren L, Liu X, Wan J, Guo S, Wang S. Ranolazine improves oxidative stress and mitochondrial function in the atrium of acetylcholine-CaCl2 induced atrial fibrillation rats. Life Sci 156: 7–14, 2016. doi: 10.1016/j.lfs.2016.05.026. [DOI] [PubMed] [Google Scholar]