How exercise provides benefits to cardiometabolic health remains unclear. We performed RNA sequencing in plasma during exercise to identify the landscape of small noncoding circulating transcriptional changes. Our results suggest a link between inflammation and exercise, providing rich data on circulating noncoding RNAs for future studies by the scientific community.
Keywords: extracellular RNA, exercise, miR-181b
Abstract
Exercise improves cardiometabolic and vascular function, although the mechanisms remain unclear. Our objective was to demonstrate the diversity of circulating extracellular RNA (ex-RNA) release during acute exercise in humans and its relevance to exercise-mediated benefits on vascular inflammation. We performed plasma small RNA sequencing in 26 individuals undergoing symptom-limited maximal treadmill exercise, with replication of our top candidate miRNA in a separate cohort of 59 individuals undergoing bicycle ergometry. We found changes in miRNAs and other ex-RNAs with exercise (e.g., Y RNAs and tRNAs) implicated in cardiovascular disease. In two independent cohorts of acute maximal exercise, we identified miR-181b-5p as a key ex-RNA increased in plasma after exercise, with validation in a separate cohort. In a mouse model of acute exercise, we found significant increases in miR-181b-5p expression in skeletal muscle after acute exercise in young (but not older) mice. Previous work revealed a strong role for miR-181b-5p in vascular inflammation in obesity, insulin resistance, sepsis, and cardiovascular disease. We conclude that circulating ex-RNAs were altered in plasma after acute exercise target pathways involved in inflammation, including miR-181b-5p. Further investigation into the role of known (e.g., miRNA) and novel (e.g., Y RNAs) RNAs is warranted to uncover new mechanisms of vascular inflammation on exercise-mediated benefits on health.
NEW & NOTEWORTHY How exercise provides benefits to cardiometabolic health remains unclear. We performed RNA sequencing in plasma during exercise to identify the landscape of small noncoding circulating transcriptional changes. Our results suggest a link between inflammation and exercise, providing rich data on circulating noncoding RNAs for future studies by the scientific community.
INTRODUCTION
Exercise provides numerous pleotropic benefits to health, including improvement in cardiometabolic risk, vascular function, obesity, and survival (17). These varied benefits of exercise have recently prompted consortium efforts to integrate a variety of circulating epigenetic mediators that can “transduce” the benefits of exercise to anatomically distinct target organs (e.g., the brain, heart, fat, muscle, and vasculature) (5). In this regard, extracellular RNAs (ex-RNAs) have recently been proposed as stable circulating RNA molecules that alter target gene expression in disease (12). There is precedent for changes in microRNAs (miRNAs) during acute and chronic exercise training (1–3, 6, 13, 15, 16, 21). However, no reports to our knowledge have taken an unbiased approach to identifying shifts in the small ex-RNA transcriptome during a single bout of symptom-limited exercise. Here, we investigated two groups of individuals without cardiovascular disease referred for exercise testing to prospectively identify and validate shifts in the ex-RNA transcriptome with exercise. In addition, we examined the expression of a target in circulation dysregulated during acute exercise, miR-181b, in in vitro rodent muscle tissue during exercise. Our primary aims were to 1) identify the landscape of plasma ex-RNA transcriptional shift during exercise in humans, 2) map pathways of exercise-mediated benefits to these ex-RNAs, and 3) identify how expression of these ex-RNAs (and their targets) are modulated during physiological stress in a target tissue relevant to fitness and health (skeletal muscle).
MATERIALS AND METHODS
Discovery cohort: treadmill exercise testing (n = 26).
A standard history and physical examination were performed to document a history of cardiovascular risk factors (diabetes, hypertension, hyperlipidemia, and currently or formerly smoking). Self-reported height and weight were collected to calculate body mass index (weight/height2). Blood was drawn (either via direct venipuncture or placement of an intravenous catheter) in the supine position at rest before exercise testing. A standard Bruce treadmill protocol was performed on each individual to a maximal, symptom-limited level. Continuous monitoring of blood pressure (via manual sphygmomanometer) and ECG was recorded at rest, throughout exercise, and for 10 min into recovery. Immediately after peak exercise, subjects were returned to the supine position within 30–60 s, and a peak exercise blood sample was collected. Samples were centrifuged at 1,000 g at 4°C for 15 min within 1 h of initial venipuncture for the collection of plasma followed by centrifugation of plasma at 2,000 g at 4°C for 10 min to obtain cell-free plasma and then immediately stored at −80°C. Two individuals had positive stress tests (by electrocardiographic criteria), which were later considered false positives based on a repeat imaging-based stress test.
RNA sequencing.
In our discovery cohort (n = 26), plasma samples (1 ml) were isolated using a modified mirVana PARIS protocol (AM1556, Life Technologies) with sequential phenol-chloroform extractions (11). RNA then went through the Zymo RNA Clean & Concentrator - 5 kit (R1016, Zymo Research). After that, RNA was moved forward into sample preparation using the Illumina small RNA TruSeq kit (RS-200-0048, Illumina). The kit reagents were halved at all steps. Each sample was assigned 1 of 48 possible indexes and went through 16 rounds of PCR amplification. Indexed samples were run on a gel and purified away from the adaptor band. Samples were quantified with the Agilent High Sensitivity DNA Kit (5067-4626, Agilent). The peak for the sample was integrated from 120 to 160 bp to measure the pMolarity of the product to inform sample clustering on the sequencer. Pools of 15 samples were created, denatured, and clustered on either a single-read Illumina V3 flowcell (GD-401-3001, Illumina) or a single-read rapid Illumina V2 flowcell (GD-402-4002, Illumina). The flow cells were run on the Illumina HiSeq 2500 (Illumina) for 50 cycles, with a 7-cycle indexing read. The raw data in the form of fastq files have been deposited into the exRNA Atlas (www.exRNA-atlas.org) with the Accession No. EXR-SADAS1EXER1-AN. This will be made available on dbGaP (accession number pending).
Sequence analysis pipeline.
The raw sequence files in the fastq format were checked for quality to ensure that the quality scores did not deteriorate drastically at the read ends. The adapters from the 3′-end were clipped using cutadapt version 1.10 (http://cutadapt.readthedocs.io/en/stable/guide.html). Reads shorter than 15 nt were discarded and after adapter trimming, and the 3′-bases below a quality score of 30 were trimmed as well. The reads were first mapped to the UniVec contaminants database and then to human rRNA sequences obtained from the National Center for Biotechnology Information, and those that map were removed from analysis. Reads were then aligned to the human genome and subsequently to the human transcriptome using STAR. The transcriptome consists of all transcripts present in EMSEMBL 75 along with mature miRNAs, miRNA hairpin, tRNA, and piRNA libraries. Only one mismatch was allowed, and each read was allowed to multimap to at most 40-RNA annotations. Differential expression was conducted via DeSeq2 in R, with a false discovery rate (Benjamini-Hochberg) cutoff of 0.05. All target miRNAs meeting our false discovery rate threshold were entered into Ingenuity Pathway Analysis.
Validation cohort: cardiopulmonary exercise testing.
The methods for exercise testing have been previously described (7–10, 14). Continuous assessments of right atrial pressure, pulmonary arterial pressure, pulmonary capillary wedge pressure, mean arterial pressure, and breath-by-breath gas exchange were performed during an incremental ramp protocol on an upright bicycle ergometer. Intracardiac and systemic pressures and cardiac output (via Fick approximation) were monitored throughout exercise. Breath-to-breath respiratory gas exchange was measured at rest and during exercise with a metabolic cart interfaced to the ergometer (Medical Graphics, St. Paul, MN) to measure peak O2 consumption (V̇o2; defined as the highest median O2 consumption of a 30-s interval in the last minute of exercise). First-pass radionuclide ventriculography was performed to assess left ventricular ejection fraction via a multicrystal camera-based flux (System 77, Baird, Bedford, MA) of 99mTc-labeled red blood cells in a region of interest overlying each ventricle. For analysis of ex-RNAs, blood was collected at rest and peak exercise from the pulmonary artery (a source of mixed cardiac and total peripheral venous drainage). Samples were centrifuged at 1,228 g for 10 min and stored immediately at −80°C for storage.
RNA quantification for the validation cohort (FirePlex).
In brief, plasma (40 μl) was mixed with 36 μl of Digest Buffer and 4 μl of Protease Mix and incubated at 60°C for 45 min with shaking. For each sample run, Firefly particles (35 μl) were added to a well of a 96-well filter plate and filtered. Next, 25 μl of Hybe buffer was added to each well followed by 25 μl of sample (or water in the case of no-sample controls). The plate was incubated at 37°C for 60 min with shaking. After samples had been rinsed twice with 1× rinse B and once with 1× rinse A, 75 μl of 1× labeling buffer were added to each well. The plate was incubated at room temperature for 60 min with shaking. After two rinses with rinse B followed by one rinse with 1× rinse A, a catch plate was added to the vacuum manifold and the filter plate was put under constant vacuum. Sixty-five microliters of 95°C RNAse-free water were added twice to each well to elute the ligated sample. Thirty microliters of this meltoff were added to a clean PCR plate and mixed with 20 μl of PCR master mix. The mixture underwent 27 cycles of PCR amplification followed by 6 cycles of asymmetric amplification. Next, 60 μl of of Hybe buffer was added back to each well of the original particles followed by 20 μl of the PCR product, and the plate was incubated at 37°C for 30 min with shaking. After samples had been rinsed twice with 1× rinse A, 75 μl of 1× reporting buffer were added to each well and the plate was incubated at room temperature for 15 min with shaking. After samples had been rinsed twice with 1× rinse A, 175 μl of run buffer were added to each well. Samples were then scanned on an EMD Millipore Guava 6HT flow cytometer. Flow cytometry quantification data were analyzed with the Firefly Analysis Workbench software. To determine whether a particular miRNA signal was detected, assay background was subtracted from the measured miRNA signal, and the result was compared with a target-specific detection threshold. This detection threshold was based on comparison of fluorescence intensity of sample wells to negative (water only) wells. Samples with below-detection threshold fluorescence intensity were counted as missing for the analysis (to avoid bias). The normalization probes were chosen according to the geNorm algorithm, and the data were normalized after the background was subtracted from the raw data. For our replication, mean fluorescence intensity from the Fireplex platform from 59 patients was compared for miR-181b (our prespecified primary outcome) between baseline and peak pulmonary arterial blood. The raw and normalized data are available on GEO (GEO Tracking No. 18626509; accession number pending final QC from GEO).
Animal experiments.
We investigated miR-181b expression in skeletal muscle in young (3 mo old, n = 3) and older (24 mo old, n = 3) C57BL/6 male mice after exposure to exercise. The control group for this experiment comprised age-matched, nonexercised mice (n = 3). The experimental group (exercise, young/old) was subjected to a treadmill test run at 15 m/min speed for 60 min on a variable speed treadmill system. After exercise, mice were euthanized at 0, 2, 4, or 6 h after peak exercise. Skeletal muscle was collected from young and old mice [specifically, the extensor digitorum longus (EDL) muscle from the left hindleg], which was available from both young and old mice. [Of note is that mouse exercise experiments were performed for other purposes; here, we present results from EDL tissue, given that it was provided in both old and young mice by the investigator (M. C. Chan) for analyses].
Human and animal subject approval.
All subjects provided written informed consent under approved Institutional Review Board protocols at the Beth Israel Deaconess Medical Center and Massachusetts General Hospital. Animal experiments were approved by the Institutional Aninmal Care and Use Committee Board of Massachusetts General Hospital.
RESULTS
Clinical characteristics for the discovery cohort (n = 26, symptom-limited exercise treadmill testing) and validation cohort (n = 59, cardiopulmonary exercise testing) are shown in Table 1. The discovery cohort was predominantly young to middle-aged overweight men (mean age: 44.3 yr, 62% men/38% women, body mass index: 27.0 kg/m2). The most common cardiovascular risk factor was hyperlipidemia (35%). The average exercise time was >10 min and 10 metabolic equivalents (METs), indicating vigorous effort. As expected, our validation cohort (based on clinical referrals for invasive cardiopulmonary exercise testing, with right heart catheterization on a bike ergometer) was older, more predominantly women, and similarly overweight (mean age: 51.5 yr, 75% women/25% men). The most common cardiac risk factor was, again, hyperlipidemia (24%). Overall, the validation cohort achieved an average of 7.1 METs during cycle ergometry.
Table 1.
Characteristics of the discovery (treadmill) and validation cohorts
| Clinical Variable | Discovery Cohort (n = 26) | Validation Cohort (n = 59) |
|---|---|---|
| Number of subjects/group | 26 | 59 |
| Age, yr | 44.3 ± 11.9 | 51.5 ± 14.6 |
| Male sex | 16 (62%) | 15 (25%) |
| Body mass index, kg/m2 | 27.0 ± 5.1 | 26.5 ± 4.1 |
| Diabetes | 0 (0%) | 0 (0%) |
| Hypertension | 1 (4%) | 13 (22%) |
| Hyperlipidemia | 9 (35%) | 14 (24%) |
| Smoking (currently) | 0 (0%) | 3 (5%)* |
| Exercise time, min | 11.6 ± 2.1 | 8.7 ± 2.3 |
| METs | 12.4 ± 1.7 | 7.1 ± 2.0 |
| Resting SBP, mmHg | 120 ± 12 | 126 ± 17 (n = 49) |
| Resting DBP, mmHg | 77 ± 10 | 74 ± 9 (n = 49) |
| Resting HR, beats/min | 72 ± 11 | 76 ± 16 |
| Peak exercise SBP, mmHg | 171 ± 19 | 194 ± 27 |
| Peak exercise DBP, mmHg | 69 ± 9 | 94 ± 12 |
| Peak exercise HR, beats/min | 178 ± 14 | 155 ± 20 |
Values are expressed as means ± SD or frequency (%);
n = 20 (34%) of participants in the validation cohort were current or former smokers.
METs, metabolic equivalents; SBP, systolic blood pressure; DSP, diastolic blood pressure; HR, heart rate. For the validation cohort, we had missing data for body mass index (present in n = 20), rest and exercise blood pressure, and HR (present in n = 25).
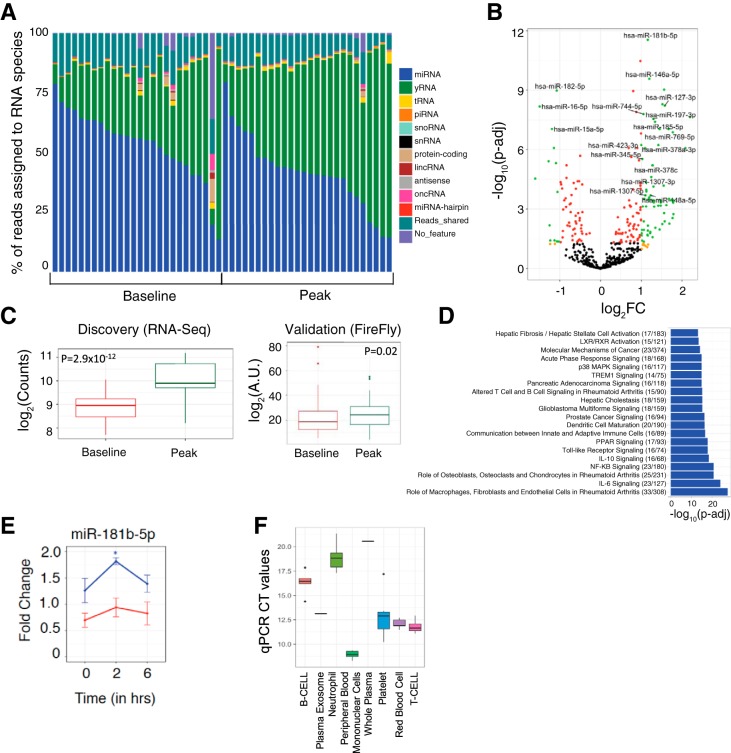
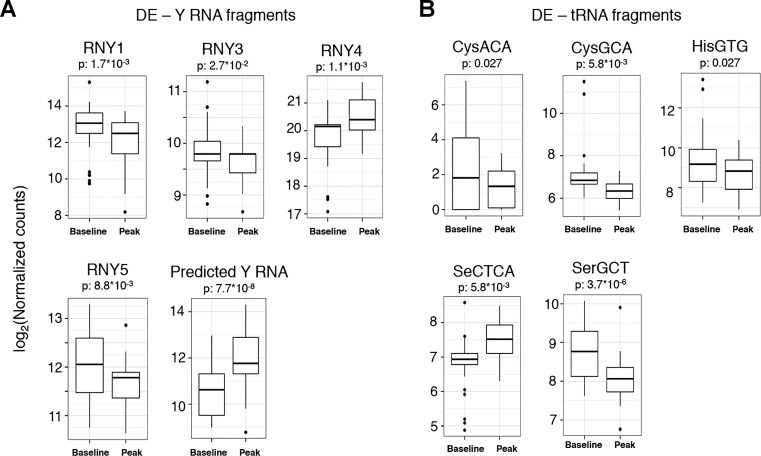
Results of RNA-seq from plasma before and after exercise in our discovery cohort are shown in Fig. 1, A and B. The average number of reads in RNA-seq across all samples was 8.5 million. We observed a diversity of ex-RNAs released during acute exercise, not only miRNAs but also non-miRNA species (Fig. 2). Given their known functional annotation and target validation, we focused on differential expression of circulating miRNAs; we found 80 miRNAs that were at least twofold different between rest and peak exercise, passing a false discovery rate threshold of P < 0.05. Of the differentially expressed miRNAs, miR-181b-5p was the most significantly altered during exercise (with a 2.2-fold increase at peak exercise, P = 2.9 × 10−12). When subjected to pathway analysis, the 80 differentially expressed miRNAs targeted genes involved in canonical pathways of inflammation, including IL-6, NF-κB, and IL-10 (Fig. 1D). For replication, we analyzed 18 miRNAs that had an average expression of >50 read counts found in RNA-seq discovery (Table 2). The results for miR-181b-5p were replicated in 59 individuals undergoing bike ergometry (P = 0.02, Fig. 1C). In addition, three other miRNAs (miR-15b-5p, miR-182-5p, and miR-185-5p) were significantly different after exercise (at a nominal P < 0.05; Table 2). To determine whether miR-181b-5p was altered in skeletal muscle tissue (a potential source of circulating miR-181b-5p with exercise), we analyzed expression in muscle in aged and young mice subjected to forced exercise (details in materials and methods). Relative to younger, nonexercised control mice, miR-181b-5p expression was increased in skeletal muscle tissue 2 h after acute exercise in young (but not old) mice (Fig. 1E). Interestingly, miR-181b was enriched in plasma exosomes and blood cells, most notably in peripheral blood monocytes (Fig. 1F), suggesting another potential source.
Fig. 1.
A: stacked-bar plot representing the percentage of reads assigned to various RNA species. B: volcano plot of the differentially expressed miRNAs. Green dots represent miRNAs that are differentially expressed at an absolute value (fold change) of ≥2 and false discovery rate adjusted <0.05 (Benjamini-Hochberg). C: plasma expression of miR-181b-5p in discovery and validation cohorts (P < 0.05 for validation using a two-sided Wilcoxon rank-sum test). D: top 20 canonical biological pathways from Ingenuity Pathway Analysis specified by miRNAs dysregulated during acute maximal exercise. The numbers in parentheses refer to the number of genes targeted by differentially expressed miRNAs over the total number of genes in a given pathway. The P values obtained from a two-tailed Fisher exact test were adjusted for multiple testing (Benjamini-Hochberg). E: miR-181b-5p fold change in murine skeletal muscle tissue after acute exercise in old (red; n = 3) and young (blue; n = 3) mice with respect to age-matched control (n = 3) mice as a function of time (0: before exercise; 2 and 6 h: after exercise). A two-tailed t-test was used to determine significance between the experimental group (exercised mouse) and age-matched control mice. *Bonferroni-adjusted P value < 0.05. F: miR-181b-5p expression in constituent cells in blood separated by flow sorting. Targeted quantitative PCR (qPCR) using the Qiagen miScript platform was performed, and the computed tomography (CT) values are presented as a box plot for 6 replicates of each cell type.
Fig. 2.
Differentially expressed Y RNA (A) and tRNA (B) fragments in human plasma during acute submaximal exercise, with the false discovery rate adjusted to <0.05 (Benjamini-Hochberg). The box plots represent log-transformed normalized read counts from small RNA sequencing.
Table 2.
Comparison of the significantly differentially expressed miRNAs in discovery (RNA-seq) and validation (FirePlex)
| RNA-Seq Discovery |
||||
|---|---|---|---|---|
| miRNA | Average expression | Log2-fold change | False discovery rate-adjusted P value | FirePlex Validation (P Value) |
| hsa-mir-15b-5p | 71.97555179 | −1.143292005 | 8.28E-07 | 5.84E-05 |
| hsa-mir-181b-5p | 843.3105733 | 1.161303094 | 2.88E-12 | 0.024750104 |
| hsa-mir-182-5p | 1829.329144 | −1.074363978 | 1.05E-09 | 0.027495955 |
| hsa-mir-185-5p | 103.7750683 | 1.339292644 | 3.91E-08 | 0.032981928 |
| hsa-mir-146a-5p | 4419.778265 | 1.198980068 | 2.69E-10 | 0.061744781 |
| hsa-mir-181d-5p | 52.09545436 | 1.096282747 | 8.28E-07 | 0.061771364 |
| hsa-mir-15a-5p | 2647.559609 | −1.192142977 | 9.06E-08 | 0.075478064 |
| hsa-mir-134-5p | 87.49682578 | 1.672958017 | 9.06E-08 | 0.189542294 |
| hsa-mir-197-3p | 130.6538653 | 1.297272994 | 2.81E-08 | 0.206127401 |
| hsa-mir-629-5p | 85.14919972 | 1.037216483 | 0.000741997 | 0.255068226 |
| hsa-mir-16-5p | 20535.39745 | −1.494068016 | 6.50E-09 | 0.262341315 |
| hsa-mir-1307-3p | 479.5764267 | 1.2469652 | 2.40E-05 | 0.266982857 |
| hsa-mir-378a-3p | 6699.24724 | 1.421244084 | 5.86E-07 | 0.306425712 |
| hsa-mir-150-3p | 99.60116946 | 1.562279446 | 9.48E-10 | 0.394843492 |
| hsa-mir-1307-5p | 118.3166763 | 1.102545357 | 0.000202236 | 0.497268916 |
| hsa-mir-769-5p | 695.5276188 | 1.77539261 | 1.32E-07 | 0.534591382 |
| hsa-mir-378c | 153.6860371 | 1.282745713 | 6.20E-06 | 0.858664858 |
| hsa-mir-127-3p | 818.452632 | 1.579631816 | 5.96E-09 | 0.946101253 |
| hsa-mir-744-5p | 869.3863559 | 1.05582934 | 1.58E-08 | NA |
| hsa-mir-146b-3p | 50.66057037 | 1.453711311 | 9.06E-08 | NA |
| hsa-mir-423-3p | 2827.735924 | 1.000334173 | 5.89E-07 | NA |
| hsa-mir-345-5p | 390.42777 | 1.027206653 | 2.95E-06 | NA |
| hsa-mir-148a-5p | 126.0809803 | 1.231604237 | 0.000203092 | NA |
| hsa-mir-6852-5p | 63.90223674 | 1.120054745 | 0.000741997 | NA |
Twenty-four miRNAs were differentially expressed in RNA-seq with an average expression of >50 read counts. Of those, 18 miRNAs were validated on an existing, custom-made FirePlex assay chip. [The 6 other miRNAs, marked not available (NA), were not initially selected in chip construction.] P values for FirePlex are based on a Wilcoxon rank-sum test and are nominal (not false discovery rate adjusted).
DISCUSSION
Here, we demonstrated a change in plasma abundance of a wide variety of ex-RNAs during acute exercise, including novel ex-RNA subtypes implicated in inflammatory signaling in cardiovascular disease (e.g., Y RNAs; see Ref. 4). Of the 80 different miRNAs that were differentially abundant by RNA sequencing, miR-181b-5p was the most significantly altered during exercise (with a 2.2-fold increase at peak exercise in 26 matched subjects, P = 2.9 × 10−12), replicated in an independent referral exercise cohort, consistent with a report in a smaller cohort (2). Skeletal muscle expression of miR-181b increased in young but not old mice upon exercise. In light of the known impact of miR-181b on inflammation in the endothelium, thrombosis, and immunity, these results demonstrate not only that exercise releases a marked diversity of ex-RNAs in humans but also that several ex-RNAs target inflammatory pathways, providing a link between ex-RNA release and anti-inflammation in fitness. Pathway analysis of differentially expressed miRNAs revealed pathways central to vascular inflammation, thrombosis, and metabolic health. Specifically, miR-181b-5p is central to vascular inflammation (18); overexpression of miR-181b-5p in the vascular endothelium has been shown to potentiate NF-κB signaling pathways, with a reduction in miR-181b-5p expression in the vascular endothelium with proinflammatory stress and rescue of this phenotype with miR-181b-5p overexpression.
The identification of “anti-inflammatory” ex-RNAs in circulation elaborated after exercise provides an additional mechanistic layer to the complex regulation of inflammation during exertion. Whereas chronic exposure to moderate exercise reduces systemic inflammation (22), demargination of acute inflammatory cells (e.g., neutrophils) has been observed during extreme exertion (e.g., long-distance running) (19, 20). Nevertheless, neutrophils do not appear to be active in response to postexercise human plasma, suggesting the presence of circulating anti-inflammatory factors (19). Although our study population certainly did not undertake extreme exercise, the elaboration of miR-181b-5p (with its well-demonstrated negative regulatory influences on central inflammatory pathways) during exercise implicates circulating ex-RNAs in potential counterregulatory responses to inflammation after acute exercise.
The limitations of our study should be viewed in the context of its design. Our primary interest was in uncovering the breadth of small noncoding RNA transcripts present in circulation during exercise. Queries on the functionality of each of the identified targets by RNA-seq require further mechanistic investigation (e.g., miR-181b) as to tissue of origin and end-organ effects. In addition, we did not directly interrogate the different mode and intensity of exercise as defining what panel of ex-RNAs may be released during exercise, although there is an emerging literature to this effect. Finally, whether these ex-RNAs are contained within exosomes or freely circulating in the plasma in other forms remains an important question: additional efforts based on tissue and plasma sequencing efforts will further clarify this matter.
The identification of a circulating set of molecules responsible for the benefits of exercise is a major goal of modern metabolic research. Given the importance of exercise across multiple organs, untargeted approaches that survey the landscape of epigenetic and genetic markers have been proposed to study how exercise alters human physiology. These results represent the first demonstration of the marked diversity of ex-RNAs released during acute exercise in humans, suggesting that inflammation may be a target of miRNAs with exercise. We await the results of consortium-wide efforts (e.g., the molecular transducers of physical activity) to provide tissue-level expression of ex-RNAs and integration with other epigenetic mediators in the molecular basis for cardiovascular benefits of exercise. Our results provide an important step in that direction, highlighting ex-RNAs as a potential mediator of inflammatory pathways across age and fitness.
GRANTS
R. Shah was supported by National Heart, Lung, and Blood Institute (NHLBI) Grants K23-HL-127099 and R01-HL-136685. G. D. Lewis was supported by NHLBI Grant R01-HL131029 and American Heart Association Grant 15GPSGC4800006. S. Das and I. Ghiran were supported by Common Fund UH3-TR000901 and NHLBI Grant U01-HL-126497 (to I. Ghiran).
DISCLOSURES
S. Das is on the Scientific Advisory Board of Dyrnamix, which had no role in this research.
R. Shah is a consultant for MyoKardia, which had no role in this research.
AUTHOR CONTRIBUTIONS
R.S., A.S.Y., I.G., G.D.L., and SD conceived and designed research; R.S., A.S.Y., A.D., A.C.-L., O.Z., E.G., J.O., P.Q.P., L.W., C.S.B., K.T., L.B., J.E.F., G.D.L., and K.V.K.-J. performed experiments; R.S., A.S.Y., A.D., O.Z., and K.V.K.-J. analyzed data; R.S., A.S.Y., J.E.F., and SD interpreted results of experiments; R.S. and A.S.Y. drafted manuscript; R.S., A.S.Y., and SD edited and revised manuscript; R.S., A.S.Y., and SD approved final version of manuscript; A.S.Y. prepared figures.
REFERENCES
- 1.Baggish AL, Hale A, Weiner RB, Lewis GD, Systrom D, Wang F, Wang TJ, Chan SY. Dynamic regulation of circulating microRNA during acute exhaustive exercise and sustained aerobic exercise training. J Physiol 589: 3983–3994, 2011. doi: 10.1113/jphysiol.2011.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banzet S, Chennaoui M, Girard O, Racinais S, Drogou C, Chalabi H, Koulmann N. Changes in circulating microRNAs levels with exercise modality. J Appl Physiol (1985) 115: 1237–1244, 2013. doi: 10.1152/japplphysiol.00075.2013. [DOI] [PubMed] [Google Scholar]
- 3.Bye A, Røsjø H, Aspenes ST, Condorelli G, Omland T, Wisløff U. Circulating microRNAs and aerobic fitness−the HUNT-Study. PLoS One 8: e57496, 2013. doi: 10.1371/journal.pone.0057496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cambier L, de Couto G, Ibrahim A, Echavez AK, Valle J, Liu W, Kreke M, Smith RR, Marbán L, Marbán E. Y RNA fragment in extracellular vesicles confers cardioprotection via modulation of IL-10 expression and secretion. EMBO Mol Med 9: 337–352, 2017. doi: 10.15252/emmm.201606924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emambokus N, Granger A, Messmer-Blust A. Exercise metabolism. Cell Metab 22: 1, 2015. doi: 10.1016/j.cmet.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 6.de Gonzalo-Calvo D, Dávalos A, Montero A, García-González Á, Tyshkovska I, González-Medina A, Soares SM, Martínez-Camblor P, Casas-Agustench P, Rabadán M, Díaz-Martínez ÁE, Úbeda N, Iglesias-Gutiérrez E. Circulating inflammatory miRNA signature in response to different doses of aerobic exercise. J Appl Physiol 119: 124–134, 2015. doi: 10.1152/japplphysiol.00077.2015. [DOI] [PubMed] [Google Scholar]
- 7.Lewis GD, Murphy RM, Shah RV, Pappagianopoulos PP, Malhotra R, Bloch KD, Systrom DM, Semigran MJ. Pulmonary vascular response patterns during exercise in left ventricular systolic dysfunction predict exercise capacity and outcomes. Circ Heart Fail 4: 276–285, 2011. doi: 10.1161/CIRCHEARTFAILURE.110.959437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis GD, Semigran MJ. Type 5 phosphodiesterase inhibition in heart failure and pulmonary hypertension. Curr Heart Fail Rep 1: 183–189, 2004. doi: 10.1007/s11897-004-0007-6. [DOI] [PubMed] [Google Scholar]
- 9.Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation 116: 1555–1562, 2007. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 10.Lewis GD, Shah RV, Pappagianopolas PP, Systrom DM, Semigran MJ. Determinants of ventilatory efficiency in heart failure: the role of right ventricular performance and pulmonary vascular tone. Circ Heart Fail 1: 227–233, 2008. doi: 10.1161/CIRCHEARTFAILURE.108.785501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgos KL, Javaherian A, Bomprezzi R, Ghaffari L, Rhodes S, Courtright A, Tembe W, Kim S, Metpally R, Van Keuren-Jensen K. Identification of extracellular miRNA in human cerebrospinal fluid by next-generation sequencing. RNA 19: 712–722, 2013. doi: 10.1261/rna.036863.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melman YF, Shah R, Das S. MicroRNAs in heart failure: is the picture becoming less miRky? Circ Heart Fail 7: 203–214, 2014. doi: 10.1161/CIRCHEARTFAILURE.113.000266. [DOI] [PubMed] [Google Scholar]
- 13.Mooren FC, Viereck J, Krüger K, Thum T. Circulating microRNAs as potential biomarkers of aerobic exercise capacity. Am J Physiol Heart Circ Physiol 306: H557–H563, 2014. doi: 10.1152/ajpheart.00711.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy RM, Shah RV, Malhotra R, Pappagianopoulos PP, Hough SS, Systrom DM, Semigran MJ, Lewis GD. Exercise oscillatory ventilation in systolic heart failure: an indicator of impaired hemodynamic response to exercise. Circulation 124: 1442–1451, 2011. doi: 10.1161/CIRCULATIONAHA.111.024141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen S, Åkerström T, Rinnov A, Yfanti C, Scheele C, Pedersen BK, Laye MJ. The miRNA plasma signature in response to acute aerobic exercise and endurance training. PLoS One 9: e87308, 2014. doi: 10.1371/journal.pone.0087308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polakovičová M, Musil P, Laczo E, Hamar D, Kyselovič J. Circulating MicroRNAs as potential biomarkers of exercise response. Int J Mol Sci 17: 1553, 2016. doi: 10.3390/ijms17101553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah RV, Murthy VL, Colangelo LA, Reis J, Venkatesh BA, Sharma R, Abbasi SA, Goff DC Jr, Carr JJ, Rana JS, Terry JG, Bouchard C, Sarzynski MA, Eisman A, Neilan T, Das S, Jerosch-Herold M, Lewis CE, Carnethon M, Lewis GD, Lima JA. Association of fitness in young adulthood with survival and cardiovascular risk: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. JAMA Intern Med 176: 87–95, 2016. doi: 10.1001/jamainternmed.2015.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, Hunninghake GM, Vera MP, MICU Registry, Blackwell TS, Baron RM, Feinberg MW, Feinberg MW. MicroRNA-181b regulates NF-κB-mediated vascular inflammation. J Clin Invest 122: 1973–1990, 2012. doi: 10.1172/JCI61495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki K, Nakaji S, Yamada M, Liu Q, Kurakake S, Okamura N, Kumae T, Umeda T, Sugawara K. Impact of a competitive marathon race on systemic cytokine and neutrophil responses. Med Sci Sports Exerc 35: 348–355, 2003. doi: 10.1249/01.MSS.0000048861.57899.04. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki K, Nakaji S, Yamada M, Totsuka M, Sato K, Sugawara K. Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc Immunol Rev 8: 6–48, 2002. [PubMed] [Google Scholar]
- 21.Uhlemann M, Möbius-Winkler S, Fikenzer S, Adam J, Redlich M, Möhlenkamp S, Hilberg T, Schuler GC, Adams V. Circulating microRNA-126 increases after different forms of endurance exercise in healthy adults. Eur J Prev Cardiol 21: 484–491, 2014. doi: 10.1177/2047487312467902. [DOI] [PubMed] [Google Scholar]
- 22.Woods JA, Wilund KR, Martin SA, Kistler BM. Exercise, inflammation and aging. Aging Dis 3: 130–140, 2012. [PMC free article] [PubMed] [Google Scholar]