A dobutamine-induced increase in cardiac contractility did not increase internal carotid artery blood flow despite an increase in cardiac output and arterial blood pressure. In contrast, external carotid artery blood flow and conductance increased. This external cerebral blood flow response may assist with protecting from overperfusion of intracranial blood flow.
Keywords: dobutamine, cardiac output, blood pressure, internal carotid artery, external carotid artery
Abstract
The effect of acute increases in cardiac contractility on cerebral blood flow (CBF) remains unknown. We hypothesized that the external carotid artery (ECA) downstream vasculature modifies the direct influence of acute increases in heart rate and cardiac function on CBF regulation. Twelve healthy subjects received two infusions of dobutamine [first a low dose (5 μg·kg−1·min−1) and then a high dose (15 μg·kg−1·min−1)] for 12 min each. Cardiac output, blood flow through the internal carotid artery (ICA) and ECA, and echocardiographic measurements were performed during dobutamine infusions. Despite increases in cardiac contractility, cardiac output, and arterial pressure with dobutamine, ICA blood flow and conductance slightly decreased from resting baseline during both low- and high-dose infusions. In contrast, ECA blood flow and conductance increased appreciably during both low- and high-dose infusions. Greater ECA vascular conductance and corresponding increases in blood flow may protect overperfusion of intracranial cerebral arteries during enhanced cardiac contractility and associated increases in cardiac output and perfusion pressure. Importantly, these findings suggest that the acute increase of blood perfusion attributable to dobutamine administration does not cause cerebral overperfusion or an associated risk of cerebral vascular damage.
NEW & NOTEWORTHY A dobutamine-induced increase in cardiac contractility did not increase internal carotid artery blood flow despite an increase in cardiac output and arterial blood pressure. In contrast, external carotid artery blood flow and conductance increased. This external cerebral blood flow response may assist with protecting from overperfusion of intracranial blood flow.
INTRODUCTION
The systemic circulation is an important factor in the regulation of cerebral blood flow (CBF) (15, 18, 19). For example, regional and global CBF abnormalities occur in patients with severe heart failure (1, 7, 12, 25, 33–35), suggesting that chronic cardiac dysfunction may induce alterations in cerebral perfusion and its vascular regulation. On the other hand, in healthy young subjects, changes in central blood volume or cardiac output (CO) influence CBF at rest and during exercise, and its influence is independent of cerebral autoregulation (17). In addition, acute hypotension-induced increases in CO, via rapid arterial baroreceptor unloading, affect the recovery of CBF from acute hypoperfusion (23). These findings (17, 23) suggest that changes in CO, with accompanying changes in central blood volume, influence CBF regulation. Moreover, our recent findings (31) indicate that the dynamic change in aortic pulsatile pressure is associated with that of the pulsatile component of CBF. Given these combined observations, cardiac contractility may directly contribute to changes in CBF. In the previous study (17), however, the manipulation of central blood volume perturbed the cardiopulmonary baroreflex and consequently modified the peripheral vasculature via the autonomic nervous system. In addition, orthostatic stress-induced peripheral vasoconstriction modifies dynamic pulsatile hemodynamic transition from aorta to the brain (31). Because these changes in autonomic nervous system activity may modify CBF, the direct effect of acute change in cardiac function (e.g., cardiac contractility) on CBF remains unknown.
Anatomically, the common carotid artery divides into the external carotid artery (ECA), which supplies blood to the face, scalp, skull, and cranium wall, and the internal carotid artery (ICA), which supplies blood to the majority of the cerebral cortex. ECA blood flow toward the extracranial region (i.e., cutaneous circulation) is small relative to that toward intracranial space via the ICA at rest (27). Previously, our studies (9, 13, 20, 27) have reported that changes in blood flow through the ECA in response to a variety of perturbations are different from that of the ICA or vertebral artery, perhaps because of differences in the control of blood flow through these unique beds (20, 22, 28). In addition, the vascular bed perfused by the ECA may be important for maintaining an adequate intracranial CBF. Recently, we (9) demonstrated that resistance exercise increases ECA blood flow and vascular conductance with no change in ICA blood flow attributable to reductions in ICA vascular conductance. This finding indicates that the ECA downstream vasculature responds differently from that of the ICA to acute hypertension. Importantly, an increase in ECA blood flow, as well as cerebrovascular autoregulation, may protect cerebral overperfusion, and the associated vasculature, against structural damage from resistance exercise-induced acute hypertension. On the basis of these observations, the ECA downstream vasculature may buffer the influence of acute increases in cardiac function and corresponding increases in arterial blood pressure. This response, along with cerebrovascular autoregulation, would serve to maintain intracranial CBF despite increases in perfusion pressure.
Clinically, dobutamine infusion is used as a “chemical stress test” to evaluate the effects of increased work of the heart on markers of impaired coronary perfusion (24). Dobutamine enhances cardiac contractility and heart rate (HR) and consequently increases CO and blood pressure. Because changes in CO affect cerebral perfusion (17), dobutamine-induced increases in CO may cause cerebral overperfusion. However, the effect of dobutamine infusion on CBF remains unknown. In the present study, we administered varying doses of dobutamine to examine the effect of increases in HR and cardiac contractility and the corresponding increases in CO and blood pressure on CBF regulation in humans. We hypothesized that the downstream vasculature of the ECA will dilate to a greater extent than the vasculature of the ICA, in response to dobutamine-induced increases in CO and mean arterial pressure (MAP), attributable to differing dynamic autoregulation between these beds.
METHODS
Twelve healthy subjects (7 men and 5 women, age: 26 ± 4 yr, height: 176 ± 14 cm, weight: 74 ± 21 kg; means ± SD) participated in the study. The subjects underwent medical examination, including a detailed history, and were determined free from cardiovascular, pulmonary, or kidney disease. Each subject was instructed to abstain from caffeinated beverages, strenuous physical activity, and alcohol for 24 h before the experiment. The protocol was approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas, and each subject provided written informed consent to participate according to the principles of the Declaration of Helsinki.
Experimental protocol.
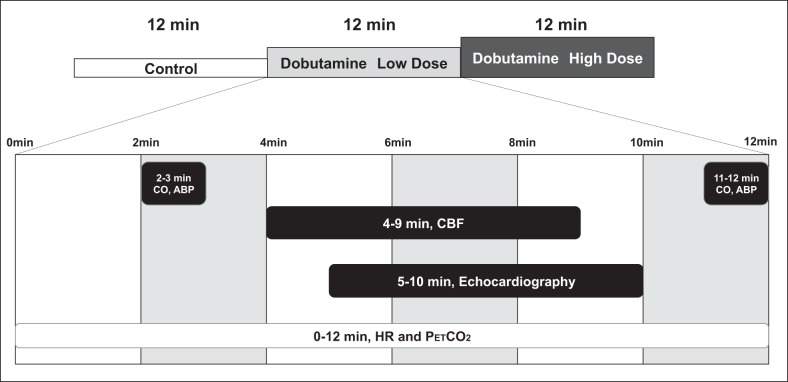
All subjects were familiarized with the equipment and procedures before any experimental sessions. On the experimental day, subjects arrived to the laboratory at least 2 h postprandial. After instrumentation, subjects rested supine, and a catheter was inserted in an antecubital vein for administration of dobutamine. Resting CO and CBF were measured for 6 min after 1 h of supine rest. Next, subjects received incremental doses of dobutamine (low dose: 5 μg·kg−1·min−1 and high dose: 15 μg·kg−1·min−1), and the same measurements were repeated for each dose. Each dose of dobutamine was administered for 12 min, with CBF, cardiac echography, CO, and arterial pressure measured at the indicated periods during the final 10 min of dobutamine administration (see Fig. 1). The effect of dobutamine on the measured variables reached a steady state by 2 min, which was maintained during the 10 min of physiological assessments through a constant infusion of the drug (8, 30).
Fig. 1.
Experimental protocol. Cardiac output (CO) and arterial blood pressure (ABP) were measured at 2–3 and 11–12 min after the onset of dobutamine infusion, with the obtained responses averaged. Cerebral blood flow (CBF), i.e., internal carotid artery, external carotid artery, and common carotid artery, were measured by Doppler ultrasound at 4–9 min after the onset of dobutamine infusion. Echocardiographic indexes of systolic function were performed 5–10 min after the onset of dobutamine infusion. Heart rate (HR) and end-tidal carbon dioxide pressure () were recorded continuously throughout the experiment. All measures were obtained at the indicated times for both the low-dose and high-dose dobutamine trials.
CBF measurements.
Blood flows from the right side of the neck were measured by trained investigators using color-coded Doppler ultrasound systems (Vivid-I, GE Healthcare, Tokyo, Japan) equipped with 12-MHz linear array transducers. ICA blood flow was measured 1.0–1.5 cm cranial to the carotid bifurcation. ECA and common carotid artery (CCA) blood flows were obtained ∼1.0–1.5 cm above the carotid bifurcation on the right ECA and ∼1.5 cm below the carotid bifurcation on the right CCA, respectively. Because neck rotation mechanically influences venous vessels (6), subjects kept their head in a neutral position as much as possible during ultrasound scanning. Each arterial flow was measured for ~45 s. The time-averaged mean flow velocity (Vmean) was calculated by the integration of velocity waveform across ~15–20 cardiac cycles to minimize any effects of respiration. Probe position remained stable so that the insonation angle did not vary (<60°) and that the sample volume was positioned in the center of the vessel and adjusted to cover its width. Vmean was calculated on the basis of velocity waveforms traced automatically by offline analysis. We used the brightness mode to measure the vessel diameter in a longitudinal aspect. Systolic and diastolic diameters were measured, and mean diameter (Dmean) was calculated as follows: [(systolic diameter × 1/3)] + [(diastolic diameter × 2/3)] (21, 26, 27, 28). Blood flow was calculated by multiplying the vessel cross-sectional area [π × (Dmean /2)2] with Vmean; blood flow = Vmean × area × 60 (ml/min) (27). Four cardiac cycles were randomly selected within 15 s (~15 cardiac cycles) of each recoding period. We calculated mean arterial diameters three times from each recording period and averaged these values for blood flow analysis. Importantly, diameter was measured at the same site as velocity, which is required to calculate an appropriate blood flow. In addition, ICA, ECA, and CCA vascular conductances were estimated from the ratio of ICA, ECA, and CCA blood flow to MAP and reported in ml·min−1·mmHg−1. ICA, ECA, and CCA flows were obtained 4–9 min after the onset of dobutamine administration. A single operator measured ICA, ECA, and CCA blood flows throughout each trial by randomly moving the Doppler probe from one artery to another on the right side neck within this timeframe (Fig. 1). However, because of time constraints during drug administration (10 min), priority was given to the measurement of ICA and ECA blood flows, with a lower priority to CCA blood flow. Thus, to provide an estimate of CCA blood flow in response to dobutamine administration, CCA blood flow from all subjects was calculated by summing the measured ICA and ECA blood flows. The data of some previous studies (27, 32) indicate that calculated CCA blood flow is almost matched with direct measured CCA.
Echocardiography.
Echocardiographic indexes of systolic function {i.e., peak septal and lateral mitral annular tissue systolic velocities [S′(s) and S′(l), respectively]} were performed using ultrasound equipment (Philips iE33, Phillips Medical Systems, Andover, MA). Tissue Doppler measurements were obtained from the apical four-chamber view with a 5.0-mm sample volume positioned at the junction of the septal mitral annulus and left ventricular (LV) wall. The best echocardiography image for each subject was selected at each stage for LV volumetric analysis. In addition, LV end-diastolic volume (EDV) and end-systolic volume (ESV) were determined using a modified Simpson’s method. Also, ejection fraction (EF) was calculated as follows: EF (%) = (EDV − ESV)/EDV × 100. Echocardiographic measurements were performed 5–10 min after the onset of dobutamine administration.
Cardiorespiratory measurements.
End-tidal carbon dioxide pressures () were sampled breath by breath (9004 Capnocheck Plus, Smiths Medical, Watford, UK), and HR was monitored via electrocardiography (Solar 8000i, GE Healthcare, Milwaukee, WI) interfaced with a cardiotachometer (CWE, Ardmore, PA). Arterial blood pressure was measured using an automated sphygmomanometer (Tango, SunTech Medical Instruments, Raleigh, NC) to identify systolic blood pressure (SBP), diastolic blood pressure (DBP), and MAP. CO was measured by inert gas rebreathing (Innocor, Innovision, Glamsbjerg, Denmark). HR during the rebreathing procedure was used to calculate stroke volume (SV) from the ratio of CO to HR (CO/HR). Total vascular conductance (TVC) was calculated from the ratio of CO to MAP (TVC = CO/MAP). COs and arterial blood pressures were obtained at 2–3 and 11–12 min after the onset of dobutamine infusion, with the obtained responses averaged.
Statistics.
Values are expressed as means ± SD. Differences in hemodynamic variables were compared by one-way repeated-measures ANOVA across baseline and the two doses of dobutamine. Two-way ANOVA with repeated measures was used to determine significant differences in the relative changes in ICA and ECA blood flows and conductances, CO, and TVC between baseline and dobutamine administration. Student-Newman-Keuls post hoc tests were used when main effects were significant. Statistical significance was set at P < 0.05. Analyses were conducted using SigmaStat (Jandel Scientific Software, SPSS, Chicago, IL).
RESULTS
HR and DBP were unchanged, but SBP, MAP, and CO increased with the low dose of dobutamine (Table 1). The high dose of dobutamine also did not change DBP, but HR, SBP, MAP, and CO increased from low-dose values. decreased with each dobutamine dose. EDV was unchanged (P = 0.634), whereas ESV gradually decreased with an increased dose of dobutamine (P < 0.001). In addition, EF as an index of cardiac contractility increased from 63% to 76% during the low dose of dobutamine and to 82% during the high dose of dobutamine (P < 0.001). Similarly, both S′(s) or S′(l) as an index of cardiac systolic function increased from baseline with low (P < 0.001) and high (P < 0.001) dobutamine doses.
Table 1.
Cardiorespiratory and cerebrovascular variables at baseline and during both low (5 μg·kg−1·min−1) and high doses (15 μg·kg−1·min−1) of dobutamine
| Baseline |
Low Dose |
High Dose |
|||||
|---|---|---|---|---|---|---|---|
| Value | n | Value | n | Value | n | PValue | |
| Hemodynamics | |||||||
| HR, beats/min | 62.000 ± 11.000 | 64.000 ± 12.000 | 95.000 ± 20.000*† | <0.001 | |||
| SBP, mmHg | 116.000 ± 9.000 | 141.000 ± 14.000* | 182.000 ± 22.000*† | <0.001 | |||
| DBP, mmHg | 65.000 ± 7.000 | 65.000 ± 6.000 | 60.000 ± 5.000 | 0.105 | |||
| MAP, mmHg | 82.000 ± 6.000 | 91.000 ± 6.000* | 101.000 ± 8.000*† | <0.001 | |||
| CO, l/min | 6.800 ± 1.900 | 8.500 ± 1.800* | 10.000 ± 1.400*† | <0.001 | |||
| , mmHg | 48.300 ± 2.700 | 44.400 ± 3.800* | 43.600 ± 2.400*† | <0.001 | |||
| TVC, l·min−1·mmHg−1 | 0.082 ± 0.017 | 0.095 ± 0.021* | 0.099 ± 0.011* | ||||
| Cerebral blood flow | |||||||
| ICA | |||||||
| Blood flow, ml/min | 317.000 ± 61.000 | 290.000 ± 50.000* | 300.000 ± 51.000* | 0.002 | |||
| Diameter, cm | 0.490 ± 0.050 | 0.480 ± 0.000 | 0.480 ± 0.050 | 0.299 | |||
| Velocity, cm/s | 28.000 ± 5.000 | 27.000 ± 6.000 | 28.000 ± 6.000 | 0.804 | |||
| Conductance, ml·min−1·mmHg−1 | 3.850 ± 0.630 | 3.200 ± 0.480* | 2.970 ± 0.340*† | <0.001 | |||
| ECA | |||||||
| Blood flow, ml/min | 115.000 ± 39.000 | 137.000 ± 45.000* | 179.000 ± 61.000*† | <0.001 | |||
| Diameter, cm | 0.380 ± 0.070 | 0.380 ± 0.070 | 0.400 ± 0.070*† | 0.008 | |||
| Velocity, cm/s | 16.000 ± 3.000 | 19.000 ± 3.000* | 23.000 ± 6.000*† | <0.001 | |||
| Conductance, ml·min−1·mmHg−1 | 1.390 ± 0.550 | 1.480 ± 0.540 | 1.720 ± 0.620*† | 0.009 | |||
| CCA | |||||||
| Blood flow, ml/min | 471.000 ± 113.000 | 460.000 ± 104.000 | 470.000 ± 102.000 | 0.694 | |||
| Diameter, cm | 0.590 ± 0.070 | 0.600 ± 0.080 | 0.570 ± 0.070 | 0.123 | |||
| Velocity, cm/s | 29.000 ± 5.000 | 27.000 ± 5.000 | 31.000 ± 7.000† | 0.039 | |||
| Conductance, ml·min−1·mmHg−1 | 5.770 ± 1.030 | 12 | 5.010 ± 0.890* | 10 | 4.730 ± 0.940* | 8 | 0.007 |
| Calculated CCA | |||||||
| Blood flow, ml/min | 429.000 ± 74.000 | 423.000 ± 69.000 | 475.000 ± 194.000*† | <0.001 | |||
| Conductance, ml·min−1·mmHg−1 | 5.250 ± 0.880 | 4.680 ± 0.730* | 4.690 ± 0.750* | 0.003 | |||
| Cardiac function | |||||||
| EDV, ml | 104.000 ± 34.000 | 111.000 ± 34.000 | 100.000 ± 43.000 | 0.634 | |||
| ESV, ml | 39.000 ± 15.000 | 26.000 ± 8.000* | 20.000 ± 12.000* | <0.001 | |||
| EF, % | 63.000 ± 4.000 | 10 | 76.000 ± 6.000* | 10 | 82.000 ± 6.000*† | 9 | <0.001 |
| S′(s), cm/s | 8.100 ± 0.700 | 11 | 11.700 ± 1.700* | 11 | 16.600 ± 3.700*† | 11 | <0.001 |
| S′(l), cm/s | 11.000 ± 1.400 | 11 | 15.700 ± 2.400* | 11 | 21.400 ± 4.600*† | 11 | <0.001 |
Data are means ± SD; n = 12 (if the number of subjects lacked, the actual number of subjects is shown at each measurement value). HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; CO, cardiac output; , end-tidal carbon dioxide pressure; TVC, total vascular conductance; ICA, internal carotid artery; ECA, external carotid artery; CCA, common carotid artery; calculated CCA, value obtained by summing measured ICA and ECA blood flows; EDV, left ventricular end-diastolic volume ; ESV, left ventricular end-systolic volume; EF, ejection fraction; S′(s), peak septal mitral annular tissue systolic velocity; S′(l), peak lateral mitral annular tissue systolic velocity.
Different from baseline (P < 0.05);
different from the low dose of dobutamine (P < 0.05).
Calculated CCA blood flow increased during the high dobutamine dose (P < 0.001; Table 1), whereas ICA blood flow decreased by ~5–8% from resting baseline during low- and high-dose infusions of dobutamine, respectively (P = 0.002). On the other hand, two-way ANOVA with repeated measures for comparison of the relative (i.e., percent) changes between different cerebral arteries showed no relative (percent) change (from the respective baselines) in ICA blood flow during dobutamine infusion (Fig. 2). In contrast, ECA blood flow profoundly increased during low-dose dobutamine and further increased during high-dose dobutamine, with these flows increasing by ~21% and 58%, respectively (P < 0.001; Table 1). When analyzed via two-way repeated-measures ANOVA, the relative change in ECA blood flow was larger than that of ICA blood flow during both low and high dobutamine doses (Fig. 2).
Fig. 2.
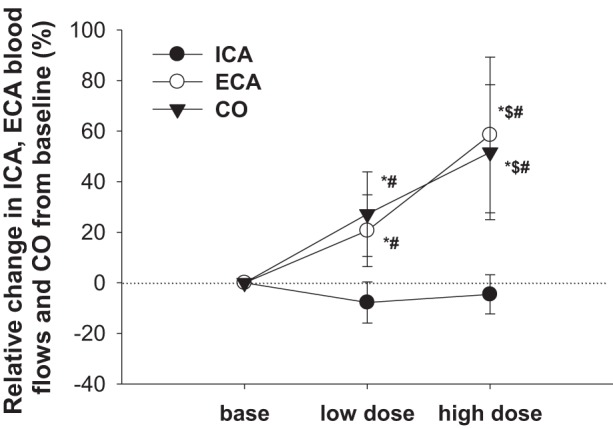
Relative change in internal carotid artery (ICA) blood flow, external carotid artery (ECA) blood flow, and cardiac output (CO) from predrug baseline (base) during administration of two doses (low dose: 5 μg·kg−1·min−1 and high dose: 15 μg·kg−1·min−1) of dobutamine. *Different from baseline (P < 0.05); $different from low-dose dobutamine (P < 0.05); #different from ICA blood flow (P < 0.05).
ECA vascular conductance did not change with the low dose of dobutamine but increased with the high dose of dobutamine (P = 0.009; Table 1). Conversely, ICA vascular conductance appreciably decreased with dobutamine (P < 0.001), indicative of a vasoconstrictor response. Calculated CCA vascular conductance also decreased during both doses of dobutamine from baseline (P = 0.003). Two-way repeated-measures ANOVA revealed that the relative increase in ECA vascular conductance was similar to that of TVC. In contrast, the relative change in ICA vascular conductance was in the opposite direction (vasoconstriction) of that observed for ECA and TVC vascular conductances during both low and high dobutamine doses.
DISCUSSION
Dobutamine increased CO via enhanced cardiac contractility and HR, which resulted in an increase in arterial blood pressure. Infusion of dobutamine decreased ICA blood flow, whereas ECA blood flow was appreciably increased. An increase in ECA vascular conductance, reflective of vasodilation in the vascular bed perfused by the ECA, may protect against overperfusion of intracranial cerebral arteries during elevated CO and arterial blood pressure. That said, we cannot rule out the possibility that the absence of an increase in ICA blood flow with dobutamine-induced increases in arterial blood pressure is due to vascular autoregulation or cerebral carbon dioxide reactivity of the vascular bed downstream of the ICA measures.
Control of the cerebral vasculature is regulated by nitric oxide, sympathetic activity, carbon dioxide, cerebrovascular autoregulation, etc., whereas systemic blood flow or pressure regulation, especially CO regulation, may also indirectly affect the cerebral vasculature (15–17, 23). These findings indicate the possibility that cardiac function contributes to CBF regulation directly or indirectly. Indeed, cardiac dysfunction in patients with heart failure occurs in parallel with impaired CBF regulation and subsequently increases the risk of cerebrovascular disease outcomes (1, 7, 12, 25, 33–35). However, a direct effect of changes in cardiac function, and the associated consequences on CO and arterial blood pressure, on CBF regulation remains unknown.
To resolve this question, in this study we administered dobutamine, a selective β1-adrenoceptor agonist, to enhance heart contractility (evidenced by increases in S′ and EF) and HR while evaluating blood flow responses through intra- and extracranial vascular beds (Table 1). Because the effect onset of dobutamine reaches steady state within 2 min and its effect remains stable across a subsequent 10-min period of assessment attributable to the steady-state infusion (8, 30), the step-change protocol (Fig. 1) was used to identify the CBF response to each dobutamine infusion in the present study. Consequently, dobutamine increased CO and arterial blood pressure, resulting in alterations in the control of CBF. Our previous study (17), which manipulated CO via modulating central blood volume, showed that loading cardiopulmonary baroreceptors, with accompanying peripheral vasodilation, may modulate the effect of cardiac function on CBF regulation indirectly. In contrast, the dobutamine infusion used in the present study increased CO via a mechanism that is not reliant on increasing central blood volume, which permitted the assessment of the effects of increasing cardiac function on CBF without a parallel vasodilation of the peripheral vasculature secondary to loading of cardiopulmonary baroreceptors. However, arterial blood pressure was elevated with dobutamine infusions, which would clearly load the arterial baroreceptors and evoke changes in the peripheral vasculature. Nevertheless, the mechanisms responsible for the changes in CBF to dobutamine-induced elevations in CO differ from CO-induced increases caused by elevations in central blood volume (17).
It is noteworthy that the present findings are in contrast to our previous work (17), showing that increasing CO augments CBF. One possible reason for this discrepancy may be the mechanism by which CO was elevated between the two studies. The prior study used volume loading to increase CO, which increases cardiopulmonary baroreceptor loading, with an absence of an increase in arterial blood pressure. Conversely, in the present study, CO was increased secondary to increases in cardiac contractility and HR, resulting in increases in arterial blood pressure and thus arterial baroreceptor loading. Elevated arterial blood pressure would engage cerebrovascular autoregulation, resulting in reductions in vascular conductance of these beds, consistent with reductions in ICA vascular conductance observed in the present study. Moreover, there are differences in autonomic regulation between these studies, given that, in the present study the arterial baroreceptors were primarily loaded, whereas, in the prior study the cardiopulmonary baroreceptors were primarily loaded. Another methodological difference between these studies is the quantification of cerebral perfusion. In the previous study (17), cerebral perfusion was assessed via transcranial Doppler of the middle cerebral artery, whereas the present study assessed volumetric flow from the ICA. Finally, the magnitude of the increase in CO with dobutamine infusion was appreciably greater in the present study relative to the prior study, where volume was administered to increase CO. Aside from these differences, we are unaware of other potential mechanisms responsible for the discrepancy between previous and present studies regarding increases in CO on CBF.
Others have likewise assessed the effects of dobutamine administration on cerebral perfusion, resulting in mixed observations. Hussain et al. (10) reported that transcranial Doppler-determined middle cerebral artery mean blood velocity increased during dobutamine (4 μg·kg−1·min−1) infusion in healthy subjects (n = 5), but not to a level that achieved significance. In nonhuman primates (2), both high and low doses of dobutamine did not change CBF or cerebral metabolic rates of oxygen and glucose utilization. Although dobutamine is less effective in monkeys than in humans (2), these previous findings are consistent with the present findings that dobutamine infusion does not increase CBF. In contrast, dobutamine infusion increases CBF in patients with vasospasm after subarachnoid hemorrhage (11) and in septic patients with altered mental status (3, 4), perhaps to protect delayed cerebral ischemia and improve the resultant outcomes. Similarly, CBF is increased by dobutamine administration in an animal model of a subarachnoid hemorrhage (14). From our findings, however, the response of CBF to dobutamine in healthy humans is different from that in these patients (3, 4, 11, 14).
The effects of dobutamine administration on ICA and ECA blood flows differed in the present study, with ICA blood flow decreased and ECA blood flow appreciably increased (Table 1). These differences are likely due to unique aspects of the regulation of the vascular beds perfused by these two vessels. For example, cerebral autoregulation is much higher within the ICA vascular bed compared with the ECA bed (20, 22), suggesting that ECA blood flow is more susceptible to changes in perfusion pressure. This is, perhaps, due to the profound autoregulatory capabilities of the vascular bed downstream to the ICA but not the ECA, resulting in an attenuation in ICA blood flow to the elevations in blood pressure observed with dobutamine. It is also noteworthy that carbon dioxide reactivity is much higher in the vascular beds downstream of the ICA relative to the ECA (28). This point is particularly relevant given the small reductions in , and thus presumably arterial Pco2, with dobutamine administration (Table 1), which likely contributes to the observed reductions in vascular conductance of the ICA. It is interesting to note that the increases in ECA blood flow were due to a combination of increases in perfusion pressure and vascular conductance. The mechanism responsible for increased ECA vascular conductance remains unknown but may be related to a neurally mediated response secondary to increases in arterial blood pressure and associated baroreceptor loading and/or flow-mediated vasodilation. Nevertheless, perhaps this vasodilatory response (as well as vasodilation of other beds throughout the vasculature) protects against overperfusion of intracranial cerebral vessels during increases in CO and accompanying increases in arterial blood pressure, as previously proposed (9, 20). Therefore, the heterogeneous regulation of the vascular beds perfused by the ICA and ECA likely contributes to the maintenance of CBF during conditions such as dobutamine-induced increases in CO and arterial blood pressure. Also, the high dose of dobutamine increased calculated CCA blood flows (P < 0.001; Table 1). This finding suggests that dobutamine-induced increases in CO caused an increase in CCA blood flow.
In patients with heart failure, cardiac β1-adrenergic sensitivity may be evaluated by a dobutamine stress echocardiogram test (24). Given the effects of dobutamine administration on CO and arterial blood pressure, administration of this drug in patients with heart failure may increase the risk of a cerebrovascular injury, given our prior findings that enhanced CO increases cerebral perfusion (17). In addition, severe heart failure causes abnormalities in CBF and its regulation (1, 5, 7, 12, 25, 33–35). For example, patients with ischemic heart failure are more likely to have impaired dynamic cerebral autoregulation compared with age-matched controls (5). Thus, particularly in these patients, a dobutamine-induced increase in arterial blood pressure may also increase cerebral perfusion. Interestingly, in contrast to our expectation, neither dose of dobutamine increased ICA blood flow, despite a substantial increase in CO and arterial blood pressure. That said, a similar study would need to be performed on individuals with heart failure to identify whether their CBF responses to dobutamine are similar to that observed herein from healthy young adults.
A limitation of the present study was that we did not assess the pulsatile nature of cerebral perfusion, which is pertinent given our recent study (31) showing that the pulsatile component of cardiac contraction affects cerebral perfusion. Indeed, dobutamine infusion increased pulsatile CBF and thus may cause pulsatile stress in the cerebral circulation despite no effect in volumetric flow. We should note that measured CCA blood flows remained unchanged from baseline (471 ± 113 ml/min; Table 1) for both doses of dobutamine (460 ± 104 and 470 ± 102 ml/min for the low and high dose, respectively, P = 0.694). However, we could not measure CCA blood flow in two subjects for the low-dose condition and in four subjects for the high-dose condition (Table 1) because of time limitations and a priority toward ICA and ECA measures, which could lead to a misinterpretation of the CCA data. Thus, we calculated CCA blood flows from the sum of the measured ICA and ECA blood.
In summary, dobutamine infusion does not increase ICA blood flow despite an increase in CO and arterial blood pressure. This response is likely due to a combination of cerebrovascular autoregulation and responsiveness to reductions in arterial Pco2 in the vascular bed downstream of the ICA. Conversely, dobutamine administration increased ECA blood flow to a level greater than that caused by the increase in blood pressure alone. Calculations suggest that ~40% of the increase in ECA blood flow at the highest dobutamine dose was due to increases in perfusion pressure, whereas the residual ~60% was due to increases in vascular conductance of the bed downstream of the ECA. A combination of cerebrovascular autoregulation and increases in ECA blood flow/vascular conductance during dobutamine infusion may assist in protecting from overperfusion of intracranial blood flow.
GRANTS
This study was supported, in part, by Japanese Ministry of Education, Culture, Sports, Science and Technology Grant-in-Aid for Scientific Research 15H003098 (to S. Ogoh), National Institute of General Medical Sciences Grant GM-068865 (to C. G. Crandall), and United States Department of Defense Grant W81XWH-12-1-0152 (to C. G. Crandall).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.O., G.M., M.S., and C.G.C. conceived and designed research; S.O., G.M., T.W., S.S., M.H., S.A.R., M.N.C., M.S., and C.G.C. performed experiments; S.O., G.M., and T.W. analyzed data; S.O., G.M., and T.W. interpreted results of experiments; S.O. prepared figures; S.O. and C.G.C. drafted manuscript; S.O., G.M., T.W., S.S., M.H., S.A.R., M.N.C., M.S., and C.G.C. edited and revised manuscript; S.O., G.M., T.W., S.S., M.H., S.A.R., M.N.C., M.S., and C.G.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We appreciate the commitment of the study participants and the staff at the Institute for Exercise and Environmental Medicine.
REFERENCES
- 1.Alves TC, Rays J, Fráguas R Jr, Wajngarten M, Meneghetti JC, Prando S, Busatto GF. Localized cerebral blood flow reductions in patients with heart failure: a study using 99mTc-HMPAO SPECT. J Neuroimaging 15: 150–156, 2005. doi: 10.1177/1051228404272880. [DOI] [PubMed] [Google Scholar]
- 2.Bandres J, Yao L, Nemoto EM, Yonas H, Darby J. Effects of dobutamine and dopamine on whole brain blood flow and metabolism in unanesthetized monkeys. J Neurosurg Anesthesiol 4: 250–256, 1992. doi: 10.1097/00008506-199210000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Berré J, De Backer D, Moraine JJ, Mélot C, Kahn RJ, Vincent JL. Dobutamine increases cerebral blood flow velocity and jugular bulb hemoglobin saturation in septic patients. Crit Care Med 25: 392–398, 1997. doi: 10.1097/00003246-199703000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Berré J, De Backer D, Moraine JJ, Vincent JL, Kahn RJ. Effects of dobutamine and prostacyclin on cerebral blood flow velocity in septic patients. J Crit Care 9: 1–6, 1994. doi: 10.1016/0883-9441(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 5.Caldas JR, Panerai RB, Haunton VJ, Almeida JP, Ferreira GS, Camara L, Nogueira RC, Bor-Seng-Shu E, Oliveira ML, Groehs RR, Ferreira-Santos L, Teixeira MJ, Galas FR, Robinson TG, Jatene FB, Hajjar LA. Cerebral blood flow autoregulation in ischemic heart failure. Am J Physiol Regul Integr Comp Physiol 312: R108–R113, 2017. doi: 10.1152/ajpregu.00361.2016. [DOI] [PubMed] [Google Scholar]
- 6.Dawson EA, Secher NH, Dalsgaard MK, Ogoh S, Yoshiga CC, González-Alonso J, Steensberg A, Raven PB. Standing up to the challenge of standing: a siphon does not support cerebral blood flow in humans. Am J Physiol Regul Integr Comp Physiol 287: R911–R914, 2004. doi: 10.1152/ajpregu.00196.2004. [DOI] [PubMed] [Google Scholar]
- 7.Gruhn N, Larsen FS, Boesgaard S, Knudsen GM, Mortensen SA, Thomsen G, Aldershvile J. Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke 32: 2530–2533, 2001. doi: 10.1161/hs1101.098360. [DOI] [PubMed] [Google Scholar]
- 8.Guglin M, Kaufman M. Inotropes do not increase mortality in advanced heart failure. Int J Gen Med 7: 237–251, 2014. doi: 10.2147/IJGM.S62549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirasawa AI, Sato K, Yoneya M, Sadamoto T, Bailey DM, Ogoh S. Heterogeneous regulation of brain blood flow during low-intensity resistance exercise. Med Sci Sports Exerc 48: 1829–1834, 2016. doi: 10.1249/MSS.0000000000000948. [DOI] [PubMed] [Google Scholar]
- 10.Hussain A, Turley A, Hawley SK, Sherriff SB, Edbrooke DL. The effect of dobutamine on middle cerebral artery blood velocity in volunteers: a preliminary study. Postgrad Med J 67, Suppl 1: S51–S55, 1991. [PubMed] [Google Scholar]
- 11.Joseph M, Ziadi S, Nates J, Dannenbaum M, Malkoff M. Increases in cardiac output can reverse flow deficits from vasospasm independent of blood pressure: a study using xenon computed tomographic measurement of cerebral blood flow. Neurosurgery 53: 1042–1051, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Kim MS, Kim JS, Yun SC, Lee CW, Song JK, Park SW, Park SJ, Kim JJ. Association of cerebral blood flow with the development of cardiac death or urgent heart transplantation in patients with systolic heart failure. Eur Heart J 33: 354–362, 2012. doi: 10.1093/eurheartj/ehr345. [DOI] [PubMed] [Google Scholar]
- 13.Miyazawa T, Horiuchi M, Ichikawa D, Subudhi AW, Sugawara J, Ogoh S. Face cooling with mist water increases cerebral blood flow during exercise: effect of changes in facial skin blood flow. Front Physiol 3: 308, 2012. doi: 10.3389/fphys.2012.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mutoh T, Mutoh T, Nakamura K, Sasaki K, Tatewaki Y, Ishikawa T, Taki Y. Inotropic support against early brain injury improves cerebral hypoperfusion and outcomes in a murine model of subarachnoid hemorrhage. Brain Res Bull 130: 18–26, 2017. doi: 10.1016/j.brainresbull.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Ogoh S, Ainslie PN. Cerebral blood flow during exercise: mechanisms of regulation. J Appl Physiol 107: 1370–1380, 2009. doi: 10.1152/japplphysiol.00573.2009. [DOI] [PubMed] [Google Scholar]
- 16.Ogoh S, Ainslie PN. Regulatory mechanisms of cerebral blood flow during exercise: new concepts. Exerc Sport Sci Rev 37: 123–129, 2009. doi: 10.1097/JES.0b013e3181aa64d7. [DOI] [PubMed] [Google Scholar]
- 17.Ogoh S, Brothers RM, Barnes Q, Eubank WL, Hawkins MN, Purkayastha S, O-Yurvati A, Raven PB. The effect of changes in cardiac output on middle cerebral artery mean blood velocity at rest and during exercise. J Physiol 569: 697–704, 2005. doi: 10.1113/jphysiol.2005.095836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogoh S, Hirasawa A, Raven PB, Rebuffat T, Denise P, Lericollais R, Sugawara J, Normand H. Effect of an acute increase in central blood volume on cerebral hemodynamics. Am J Physiol Regul Integr Comp Physiol 309: R902–R911, 2015. doi: 10.1152/ajpregu.00137.2015. [DOI] [PubMed] [Google Scholar]
- 19.Ogoh S, Hirasawa A, Sugawara J, Nakahara H, Ueda S, Shoemaker JK, Miyamoto T. The effect of an acute increase in central blood volume on the response of cerebral blood flow to acute hypotension. J Appl Physiol 119: 527–533, 2015. doi: 10.1152/japplphysiol.00277.2015. [DOI] [PubMed] [Google Scholar]
- 20.Ogoh S, Lericollais R, Hirasawa A, Sakai S, Normand H, Bailey DM. Regional redistribution of blood flow in the external and internal carotid arteries during acute hypotension. Am J Physiol Regul Integr Comp Physiol 306: R747–R751, 2014. doi: 10.1152/ajpregu.00535.2013. [DOI] [PubMed] [Google Scholar]
- 21.Ogoh S, Sato K, Okazaki K, Miyamoto T, Hirasawa A, Sadamoto T, Shibasaki M. Blood flow in internal carotid and vertebral arteries during graded lower body negative pressure in humans. Exp Physiol 100: 259–266, 2015. doi: 10.1113/expphysiol.2014.083964. [DOI] [PubMed] [Google Scholar]
- 22.Ogoh S, Sato K, Okazaki K, Miyamoto T, Hirasawa A, Shibasaki M. Hyperthermia modulates regional differences in cerebral blood flow to changes in CO2. J Appl Physiol 117: 46–52, 2014. doi: 10.1152/japplphysiol.01078.2013. [DOI] [PubMed] [Google Scholar]
- 23.Ogoh S, Tzeng YC, Lucas SJ, Galvin SD, Ainslie PN. Influence of baroreflex-mediated tachycardia on the regulation of dynamic cerebral perfusion during acute hypotension in humans. J Physiol 588: 365–371, 2010. doi: 10.1113/jphysiol.2009.180844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pousset F, Chalon S, Thomaré P, Diquet B, Lechat P. Evaluation of cardiac beta 1-adrenergic sensitivity with dobutamine in healthy volunteers. Br J Clin Pharmacol 39: 633–639, 1995. doi: 10.1111/j.1365-2125.1995.tb05723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pullicino P, Mifsud V, Wong E, Graham S, Ali I, Smajlovic D. Hypoperfusion-related cerebral ischemia and cardiac left ventricular systolic dysfunction. J Stroke Cerebrovasc Dis 10: 178–182, 2001. doi: 10.1053/jscd.2001.26870. [DOI] [PubMed] [Google Scholar]
- 26.Sato K, Fisher JP, Seifert T, Overgaard M, Secher NH, Ogoh S. Blood flow in internal carotid and vertebral arteries during orthostatic stress. Exp Physiol 97: 1272–1280, 2012. doi: 10.1113/expphysiol.2012.064774. [DOI] [PubMed] [Google Scholar]
- 27.Sato K, Ogoh S, Hirasawa A, Oue A, Sadamoto T. The distribution of blood flow in the carotid and vertebral arteries during dynamic exercise in humans. J Physiol 589: 2847–2856, 2011. doi: 10.1113/jphysiol.2010.204461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato K, Sadamoto T, Hirasawa A, Oue A, Subudhi AW, Miyazawa T, Ogoh S. Differential blood flow responses to CO2 in human internal and external carotid and vertebral arteries. J Physiol 590: 3277–3290, 2012. doi: 10.1113/jphysiol.2012.230425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sicari R, Nihoyannopoulos P, Evangelista A, Kasprzak J, Lancellotti P, Poldermans D, Voigt JU, Zamorano JL; European Association of Echocardiography . Stress echocardiography expert consensus statement: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur J Echocardiogr 9: 415–437, 2008. doi: 10.1093/ejechocard/jen175. [DOI] [PubMed] [Google Scholar]
- 31.Sugawara J, Tomoto T, Imai T, Maeda S, Ogoh S. Impact of mild orthostatic stress on aortic-cerebral hemodynamic transmission: insight from the frequency domain. Am J Physiol Heart Circ Physiol 312: H1076−H1084, 2017. doi: 10.1152/ajpheart.00802.2016. [DOI] [PubMed] [Google Scholar]
- 32.Trangmar SJ, Chiesa ST, Llodio I, Garcia B, Kalsi KK, Secher NH, González-Alonso J. Dehydration accelerates reductions in cerebral blood flow during prolonged exercise in the heat without compromising brain metabolism. Am J Physiol Heart Circ Physiol 309: H1598–H1607, 2015. doi: 10.1152/ajpheart.00525.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vogels RL, Oosterman JM, Laman DM, Gouw AA, Schroeder-Tanka JM, Scheltens P, van der Flier WM, Weinstein HC. Transcranial Doppler blood flow assessment in patients with mild heart failure: correlates with neuroimaging and cognitive performance. Congest Heart Fail 14: 61–65, 2008. doi: 10.1111/j.1751-7133.2008.07365.x. [DOI] [PubMed] [Google Scholar]
- 34.Vogels RL, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: a systematic review of the literature. Eur J Heart Fail 9: 440–449, 2007. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Vogels RL, van der Flier WM, van Harten B, Gouw AA, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Brain magnetic resonance imaging abnormalities in patients with heart failure. Eur J Heart Fail 9: 1003–1009, 2007. doi: 10.1016/j.ejheart.2007.07.006. [DOI] [PubMed] [Google Scholar]