To our best knowledge, this study is the first to investigate the involvement of the orexin system in salt-sensitive hypertension. Our results suggest that the orexin system may contribute to the Dahl model of salt-sensitive hypertension by enhancing vasopressin signaling in the hypothalamic paraventricular nucleus.
Keywords: paraventricular nucleus, orexin, salt-sensitive hypertension, sympathetic nerve activity, vasopressin
Abstract
The orexin system is involved in arginine vasopressin (AVP) regulation, and its overactivation has been implicated in hypertension. However, its role in salt-sensitive hypertension (SSHTN) is unknown. Here, we tested the hypothesis that hyperactivity of the orexin system in the paraventricular nucleus (PVN) contributes to SSHTN via enhancing AVP signaling. Eight-week-old male Dahl salt-sensitive (Dahl S) and age- and sex-matched Sprague-Dawley (SD) rats were placed on a high-salt (HS; 8% NaCl) or normal-salt (NS; 0.4% NaCl) diet for 4 wk. HS intake did not alter mean arterial pressure (MAP), PVN mRNA levels of orexin receptor 1 (OX1R), or OX2R but slightly increased PVN AVP mRNA expression in SD rats. HS diet induced significant increases in MAP and PVN mRNA levels of OX1R, OX2R, and AVP in Dahl S rats. Intracerebroventricular infusion of orexin A (0.2 nmol) dramatically increased AVP mRNA levels and immunoreactivity in the PVN of SD rats. Incubation of cultured hypothalamus neurons from newborn SD rats with orexin A increased AVP mRNA expression, which was attenuated by OX1R blockade. In addition, increased cerebrospinal fluid Na+ concentration through intracerebroventricular infusion of NaCl solution (4 µmol) increased PVN OX1R and AVP mRNA levels and immunoreactivity in SD rats. Furthermore, bilateral PVN microinjection of the OX1R antagonist SB-408124 resulted in a greater reduction in MAP in HS intake (−16 ± 5 mmHg) compared with NS-fed (−4 ± 4 mmHg) anesthetized Dahl S rats. These results suggest that elevated PVN OX1R activation may contribute to SSHTN by enhancing AVP signaling.
NEW & NOTEWORTHY To our best knowledge, this study is the first to investigate the involvement of the orexin system in salt-sensitive hypertension. Our results suggest that the orexin system may contribute to the Dahl model of salt-sensitive hypertension by enhancing vasopressin signaling in the hypothalamic paraventricular nucleus.
INTRODUCTION
Enhanced plasma arginine vasopressin (AVP) levels have been found in human subjects with hypertension (8, 40) and several animal models of experimental hypertension (9, 10, 25, 37). Dahl salt-sensitive (Dahl S) rats fed a high-salt (HS) diet have greater plasma AVP levels than Dahl salt-resistant rats fed a HS diet (4, 34, 45, 54). Elevated AVP levels may contribute to salt-induced hypertension in this model, as it has been shown that oral administration of a dual AVP receptor antagonist RWJ-676070 produced a transient decrease in mean arterial pressure (MAP) in hypertensive Dahl S rats (17). However, the central mechanisms that mediate enhanced AVP levels associated with HS-fed Dahl S rats are not well known.
Orexin A and orexin B (also known as hypocretin 1 and hypocretin 2) are neuropeptides produced in the central nervous system from the same precursor polypeptide (12, 43) whose actions are mediated by two G protein-coupled receptors termed orexin receptor-1 (OX1R) and orexin receptor-2 (OX2R) (43). Orexin A and orexin B bind OX2R with equal affinity, whereas orexin A binds to OX1R with a higher affinity than orexin B (43). The orexins are produced by neurons located exclusively in the hypothalamus, particularly in the lateral hypothalamic area, perifornical nucleus, dorsomedial hypothalamic nucleus, dorsal hypothalamic area, and posterior hypothalamic area in the rat brain (11, 39, 41). However, these orexin-containing neurons have widespread projections throughout the brain and are involved in maintaining wakefulness, feeding, emotional behavior, and reward seeking as well as accompanying autonomic and endocrine responses (42).
In terms of cardiovascular regulation, the orexin system has been implicated in AVP regulation. Intracerebroventricular administration of orexin A increases AVP mRNA in parvocellular neurons of the hypothalamic paraventricular nucleus (PVN) (a prominent region in AVP production) in conscious rats (1) and also plasma AVP levels in conscious rabbits (35). These findings are consistent with orexin-containing nerve terminals (3, 5, 6, 39, 41, 46, 55) and receptors from in situ hybridization studies (31, 33, 53) and immunohistochemistry studies (2, 7, 19) residing in many cardiovascular control regions of the brain including both magnocellular and parvocellular neurons of the PVN. Studies focusing on the action of orexin in the PVN have shown that orexin A and orexin B have excitatory actions by depolarizing both magnocellular and parvocellular PVN neurons (13, 44, 48).
Accumulating evidence indicates that hyperactivity of the orexin system contributes to several animal models of hypertension, which have recently been reviewed (21). Oral administration of the dual orexin receptor antagonist almorexant significantly decreased systolic blood pressure, diastolic blood pressure, MAP, and heart rate (HR) compared with pretreatment in conscious spontaneously hypertensive rats (SHRs) in both wakefulness and sleep during dark and light periods of the diurnal cycle (28). However, the same dose of the dual orexin receptor antagonist did not affect blood pressure or HR in Wistar-Kyoto rats (28). This study suggests that overactivation of the orexin system contributes to the maintenance of hypertension in the SHR model. Furthermore, intracerebroventricular administration or rostral ventrolateral medulla (RVLM) injection of the OX2R antagonist TCS-OX2-29 resulted in a significant reduction in MAP and HR in SHRs but not in Wistar-Kyoto rats (26). This study suggests that elevated OX2R activity in the RVLM contributes to hypertension in the SHR model. In addition, upregulation of OX1R contributes to increased firing frequency of spinal cord-projecting PVN neurons and contributes to augmented sympathetic nerve activity and hypertension in the obese Zucker rat model of obesity-related hypertension (56). However, the role orexin plays in salt-sensitive hypertension (SSHTN) is unknown.
On the basis of recent evidence that increased activity of the brain orexin system is involved in hypertension, we sought to investigate its involvement in SSHTN. Taking into account the importance of the PVN in AVP and blood pressure control and that orexin is involved in the regulation of PVN neuronal activity and AVP regulation, we hypothesized that increased activity of the orexin system in the PVN contributes to enhanced AVP signaling and the maintenance of hypertension in HS-fed Dahl S rats.
METHODS
Animals.
All rats used in this study were purchased from Charles River Laboratories (Wilmington, MA). Eight-week-old male Dahl S and age- and sex-matched Sprague-Dawley (SD) rats were placed on either a normal-salt (NS; 0.4% NaCl, 4 g/kg) diet or HS (8% NaCl, 80.0 g/kg) diet (Envigo RMS) for 4 wk and used for blood pressure measurement, brain mRNA measurement, immunohistochemistry, and sympathetic nerve activity recording. Adult male SD rats (350–500 g) were used for brain intracerebroventricular infusion and immunohistochemistry. Cultured hypothalamic neurons were obtained from newborn 1-day-old SD rats. All animals were housed with 2 rats/cage and kept on a 12:12-h light-dark cycle in a climate-controlled room. Rat chow and water were provided ad libitum. All animal experiments followed protocols that were approved by the Michigan Technological University Institutional Animal Care and Use Committee.
Blood pressure measurement.
Before diet treatment, 7-wk-old male SD and Dahl S rats were anesthetized, and telemetry transmitters (HD-S11, Data Science, St. Paul, MN) were implanted to directly measure arterial blood pressure in individual animals. Briefly, rats were anesthetized with isoflurane (2–3%), a catheter connected to a telemetry transmitter was inserted into the femoral artery, and the transmitter was placed subcutaneously (51). After 1 wk of recovery from the surgery, animals were randomly divided into two groups and were fed either the NS or HS diet. Blood pressure of conscious rats was recorded as previously described (47). Blood pressure was monitored from 9 AM to 12 PM by telemetry transducer 1 day before the different diet treatments as a baseline and twice a week at the same time window after diet treatment for 4 wk.
Real-time PCR analysis of PVN AVP, OX1R, and OX2R mRNA expression.
Eight-week-old adult male Dahl S rats and age- and sex-matched SD rats were placed on either a NS diet or a HS diet for 4 wk. After diet treatment, animals were euthanized, brains were removed, and the hypothalamic PVN was punched out with a 12-gauge needle (inner diameter: 1.5 mm). To identify hypothalamic PVN tissue, the optic tract was identified, and a 1-mm-thick brain section was taken from the rostral end point of the optic tract. Samples were frozen in liquid nitrogen and then stored at −80°C until used (24). RNA isolation from punched PVN tissues was performed using a RNeasy plus Mini kit (Qiagen) following the manufacturer’s instructions. Briefly, tissues were first lysed and homogenized using lysis buffer provided by the kit. The lysate was passed through a genomic DNA eliminator spin column, the same volume of 70% ethanol was then added to the flow through, and the sample was applied to an RNeasy MinElute spin column to bind RNA. After a wash with wash buffer, DNase I was applied to the column to digest genomic DNA. RNA was then eluted with RNase-free water. About 200−500 ng of RNA were reverse transcribed into cDNA, the cDNA was then used as template, and real-time PCR was performed to measure mRNA levels of genes of interest using the Step One Plus Real Time PCR System (Applied Biosystems, Foster City, CA). Taqman primers and probes for AVP (Rn00566449_m1), OX1R (Rn00565155_m1), and OX2R (Rn00565032_m1) were purchased (Applied Biosystems) and used for the gene assay. Data were normalized to GAPDH (Rn01775763-g1) mRNA.
AVP and OX1R immunoreactivity in the brain.
Immunofluorescence staining was performed using 25-μm coronal brain sections containing the PVN with the following protocols. Rats were deeply anesthetized and transcardially perfused with 4% paraformaldehyde (PFA) (in 1× PBS). After perfusion, the brains were removed and fixed in 4% PFA overnight at 4°C. The brains then were transferred to a tube containing 30% sucrose-PBS and kept at 4°C until the brains sank to the bottom of the tube. Brain coronal sections were then cut from the hypothalamus area using a cryostat. For immunostaining, brain sections were first washed in PBS three times for 10 min each. Brain sections were then incubated with either rabbit anti-OX1R antibody (Alomon Laboratories, Jerusalem, Israel, 1:500 dilution) or rabbit anti-AVP antibody (1:500 dilution) in PBS containing 0.5% Triton X-100 and 5% horse serum for 72 h at 4°C. Afterward, brain sections were washed with PBS three times for 10 min each. Brain sections were then incubated with secondary antibody Alexa fluor 488 donkey anti-rabbit IgG overnight at 4°C. The immunoreactivity of OX1R or AVP was observed under a Leica DMIL microscope, and micrographs were taken.
Preparation of neuronal cultures.
Primary neuronal cultures were made from the hypothalamus containing the PVN of 1-day-old SD rats essentially as previously described (30). Briefly, hypothalamic areas that contained the PVN, supraoptic nucleus, anterior nucleus, lateral nucleus, posterior nucleus, dorsomedial nucleus, and ventromedial nucleus were dissected, and brain cells were dissociated by trypsin. Cells were plated on poly-l-lysine-precoated 24-well culture plates in DMEM supplemented with 10% horse serum and 1% antibiotics including penicillin and streptomycin (DMEM-HSPS) and incubated at 37°C in a humidified incubator containing 5% CO2. Two days later, cultures were treated with 5 µM cytosine arabinoside in DMEM-HSPS for 48 h to kill astroglia and other dividing cells and then changed back to regular DMEM-HSPS. Cultures were maintained for an additional 8–10 days in the CO2 incubator before their use in experiments. These cultures have been shown to contain 90−95% neuronal cells and the remaining <5−10% were astroglial cells (30).
Measurement of orexin A-induced AVP mRNA expression in cultured brain neurons.
Primary neuronal cultures from the hypothalamus were incubated with vehicle control or differing concentrations of orexin A (10 nM, 100 nM, 1 μM, or 10 μM) in DMEM-HSPS for 6 h. The culture medium was removed, and cells were washed with cold PBS, collected, and subjected to RNA isolation. Real-time PCR was performed to measure mRNA levels of AVP. To test which orexin receptor mediates the AVP increase induced by orexin, we coincubated neuronal cultures with 1 μM orexin A with or without the OX1R antagonist SB-408124 (100 µM) or OX2Ra TCS-OX2-29 (100 µM, Tocris Bioscience) for 6 h. The mRNA level of AVP was determined by real-time quantitative PCR. Each set of experiments was performed using 3 culture wells in a 24-well plate, and all cDNA samples were assayed in duplicate. The whole experiment was repeated two to three times. Data were normalized to GAPDH mRNA.
Intracerebroventricular injections.
Adult male SD rats (350–500 g) were anesthetized with isoflurane (3%). Body temperature was held at 37°C with a water circulating pad. Animals were placed in a stereotaxic head frame, and the skull was leveled between the bregma and lambda. A 2-mm-diameter circle was drilled in the skull. The stereotaxic coordinates for intracerebroventricular injection into the left lateral ventricle were as follows: 0.8–0.9 mm caudal to the bregma, 1.4–1.8 mm lateral to the midline, and 3.2–3.8 mm ventral to the dura. Each rat received only one injection. For the intracerebroventricular injection, vehicle control (0.9% saline), orexin A (0.2 nmol dissolved in 0.9% saline, Sigma-Aldrich), and hypertonic saline (4 µmol NaCl) were injected in the same volume at a rate of 1 µl/min into the left lateral ventricle with am UltraMicroPump3 (World Precision Instruments, Sarasota, FL) with a 10-µl NanoFil syringe and 33-gauge beveled needle (World Precision Instruments). Three hours after intracerebroventricular injection, several rats were euthanized, and brain PVN tissues were punched out and received real-time PCR analysis of genes of interest. Additional rats were perfused with 4% PFA and subjected to immunofluorescence staining of AVP or OX1R, respectively.
Experiment preparation for recording of sympathetic nerve activity.
Animal surgery was performed following a previously described protocol (16). On the day of the experiment, rats were anesthetized with an intraperitoneal injection containing a mixture of α-chloralose (80 mg/kg) and urethane (800 mg/kg). Adequate depth of anesthesia was assessed before surgery by the absence of the pedal withdrawal reflex and corneal reflexes. Animals were instrumented with an arterial catheter inserted into the aorta through a femoral artery. The catheter was connected to a pressure transducer to measure arterial blood pressure. HR was obtained from the R wave of the ECG (lead I). A catheter was also placed in the left femoral vein to administer drugs. After tracheal cannulation, rats were paralyzed with gallamine triethiodide (25 mg·kg−1·h−1 iv) and artificially ventilated with oxygen-enriched room air. After paralysis, adequate depth of anesthesia was determined by lack of pressor responses to noxious foot pinch. Supplemental doses of anesthesia equal to 10% of the initial dose were given when needed. End-tidal Pco2 was continuously monitored and maintained within normal limits (35–40 mmHg) by adjusting ventilation rate (80–100 breaths/min) and/or tidal volume (2.0–3.0 ml). Body temperature was held at 37°C with a water circulating pad. All animals were allowed to stabilize at least 2 h after surgery.
Recording of sympathetic nerve activity.
Sympathetic nerve activity recording was performed according to previously described protocols (16, 50). With the use of a left flank incision, a left renal and splanchnic sympathetic nerve bundle was isolated from surrounding tissue and mounted on a stainless steel wire electrode (outer diameter: 0.127 mm, A-M Systems) and covered with a silicon-based impression material (Coltene, Light Body) to insulate the recording from body fluids. The recorded signal was directed to an alternating current amplifier (P511, Grass Technologies) equipped with half-amplitude filters (band pass: 100–1,000 Hz) and a 60-Hz notch filter. The processed signal was rectified, integrated (10-ms time constant), and digitized at a frequency of 5,000 Hz using a 1401 Micro3 analog-to-digital converter and Spike2 software (version 7.04, Cambridge Electronic Design, Cambridge, UK). Background noise was determined by a bolus injection of hexamethonium (30 mg/kg iv), a ganglionic blocker, at the end of the experiment and was subtracted from all integrated values of sympathetic nerve activity.
PVN microinjection.
PVN injections were performed as previously described (16). Animals were placed in a stereotaxic head frame, and the skull was leveled between the bregma and lambda for PVN injections. A small piece of the skull was removed so that a single-barreled glass microinjector pipette could be lowered vertically into the PVN. The stereotaxic coordinates for PVN injections were as follows: 1.2–1.6 mm caudal to the bregma, 0.5–0.7 mm lateral to the midline, and 7.0–7.4 mm ventral to the dura. After a 20-min baseline period, SB-408124 (30 pmol) was microinjected into the PVN bilaterally in a volume of 50 nl/side with a pneumatic pump (World Precision Instruments). The OX1R antagonist SB-408124 (Santa Cruz Biotechnology) was dissolved in DMSO. The interval between two bilateral injections was ∼5 min.
The volume of each injection was determined by measuring the movement of the fluid meniscus within the microinjector pipette using a dissecting microscope equipped with an eyepiece reticule. At the end of each experiment, Chicago blue dye solution (2% in saline, 50 nl) was injected into the PVN to mark the site of each injection. Brains were removed and postfixed for 5 days at room temperature in 4% PFA. Brain coronal sections containing the PVN were cut into 50-µm sections, and microinjection sites were identified under bright-field microscopy by observing the ink staining. Rats with injection site(s) not inside the PVN were excluded from data analysis.
Data analysis.
Summary data are expressed as means ± SE. For sympathetic nerve activity recording, splanchnic sympathetic nerve activity (SSNA) and renal sympathetic nerve activity (RSNA) were determined as an average of the rectified integrated signal. Baseline values of all recorded variables were obtained by averaging a 10-min segment of data recorded immediately before PVN microinjection in anesthetized rats. SSNA, RSNA, and MAP responses to OX1R antagonist SB-408124 were obtained by averaging a 2-min period centered on the maximal response. Data are presented as percent changes from baseline after subtraction of background noise determined with bolus injection of the ganglionic blocker hexamethonium (30 mg/kg). Both in vivo and in vitro data were analyzed using either one-way ANOVA or an unpaired Student's t-test. Post hoc analysis was performed with Newman-Keuls multiple-comparison test. Differences were considered statistically significant at a critical value of P < 0.05.
RESULTS
HS diet increases blood pressure in Dahl S rats.
To investigate the effect of a HS diet on blood pressure in Dahl S and SD rats, 8-wk-old male Dahl S rats and age- and sex-matched SD rats were placed on a NS or HS diet for 4 wk. Blood pressure was monitored via radiotelemetry transducer system from 9 AM to 12 PM starting 1 day before different diet treatment as a baseline and twice a week in the same time window after different diet treatment. Average MAP was calculated and is shown in Fig. 1. SD rats on the HS diet showed no change in MAP compared with NS diet-fed SD rats (HS: 106 ± 1, n = 5, vs. NS: 103 ± 2 mmHg, n = 5). Dahl S rats on a HS diet had significantly (P < 0.05) increased MAP; the blood pressure started to increase 3 days after HS diet intake and reached ~160 mmHg 4 wk after HS diet treatment (HS: 159 ± 6, n = 5, vs. NS: 111 ± 1 mmHg, n = 5).
Fig. 1.
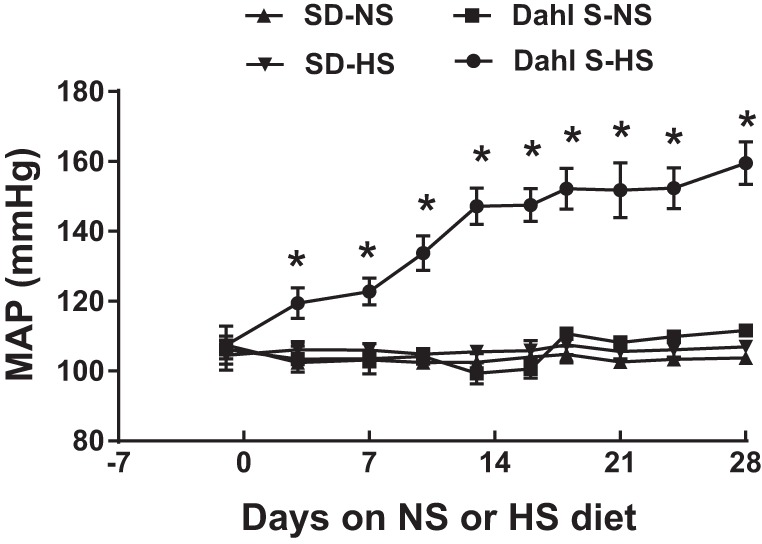
Mean arterial pressure (MAP) of Sprague-Dawley (SD) rats and Dahl salt-sensitive (Dahl S) rats on 4 wk of a normal-salt (NS; 0.4% Nacl) or high-salt (HS; 8% NaCl) diet detected by radiotelemetry. HS diet increases MAP of Dahl S rats but not SD rats. n = 5 for each group. *P ≤ 0.05 vs. NS diet.
HS diet increases PVN mRNA levels of OX1R, OX2R, and AVP in Dahl S rats.
Next, we sought to determine whether a HS diet alters PVN AVP and orexin system component expression in SD and Dahl S rats. Eight-week-old male Dahl S rats and age- and sex-matched SD rats were divided into two groups and fed either a NS or HS diet for 4 wk. Rats were then euthanized, PVN tissue was punched out, and real-time PCR was performed to assay OX1R, OX2R, and AVP mRNA levels. SD rats on a HS diet showed no significant change in PVN mRNA levels of OX1R (HS: 1.21 ± 0.06 vs. NS: 1.0 ± 0.13, n = 4–6, P > 0.05) or OX2R (HS: 0.82 ± 0.05 vs. NS: 1.0 ± 0.17, n = 4–6, P > 0.05) compared with NS diet-fed SD rats. However, SD rats with HS intake showed significantly increased PVN mRNA levels of AVP (HS: 2.14 ± 0.38 vs. NS: 1.0 ± 0.07, n = 5–8, P < 0.05) compared with SD rats with NS intake (Fig. 2). In contrast to SD rats, HS intake induced significant increases in PVN mRNA levels of OX1R (HS: 1.5 ± 0.09 vs. NS: 1.0 ± 0.09, n = 7, P < 0.05), OX2R (HS: 1.8 ± 0.3 vs. NS: 1.0 ± 0.12, n = 5, P < 0.05), and AVP (HS: 5.8 ± 0.8 vs. NS: 1.0 ± 0.2, n = 5, P < 0.05) in Dahl S rats (Fig. 2).
Fig. 2.
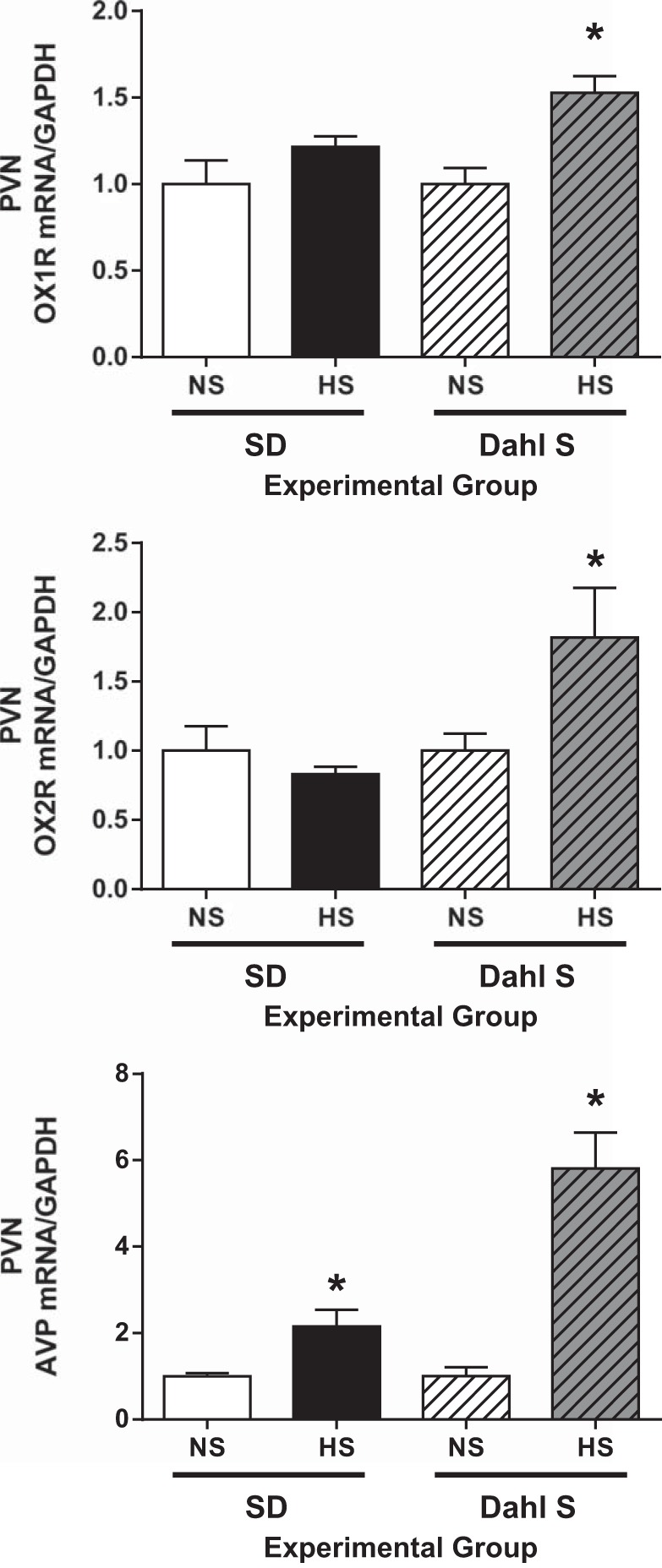
Real-time PCR analysis of orexin receptor 1 (OX1R), orexin receptor 2 (OX2R), and arginine vasopressin (AVP) mRNA expression levels in the paraventricular nucleus (PVN) between normal salt (NS; 0.4% NaCl) and high salt (HS; 8% NaCl) diet-fed Sprague-Dawley (SD) rats and Dahl salt-sensitive (Dahl S) rats. HS diet did not change OX1R or OX2R but significantly increased AVP PVN mRNA expression in SD rats. HS diet increased mRNA levels of OX1R, OX2R, and AVP in the PVN in Dahl S rats. The mRNA level in the control sample was assigned to equal 1 arbitrary unit. n = 4–8 for each group. *P ≤ 0.05 vs. NS diet.
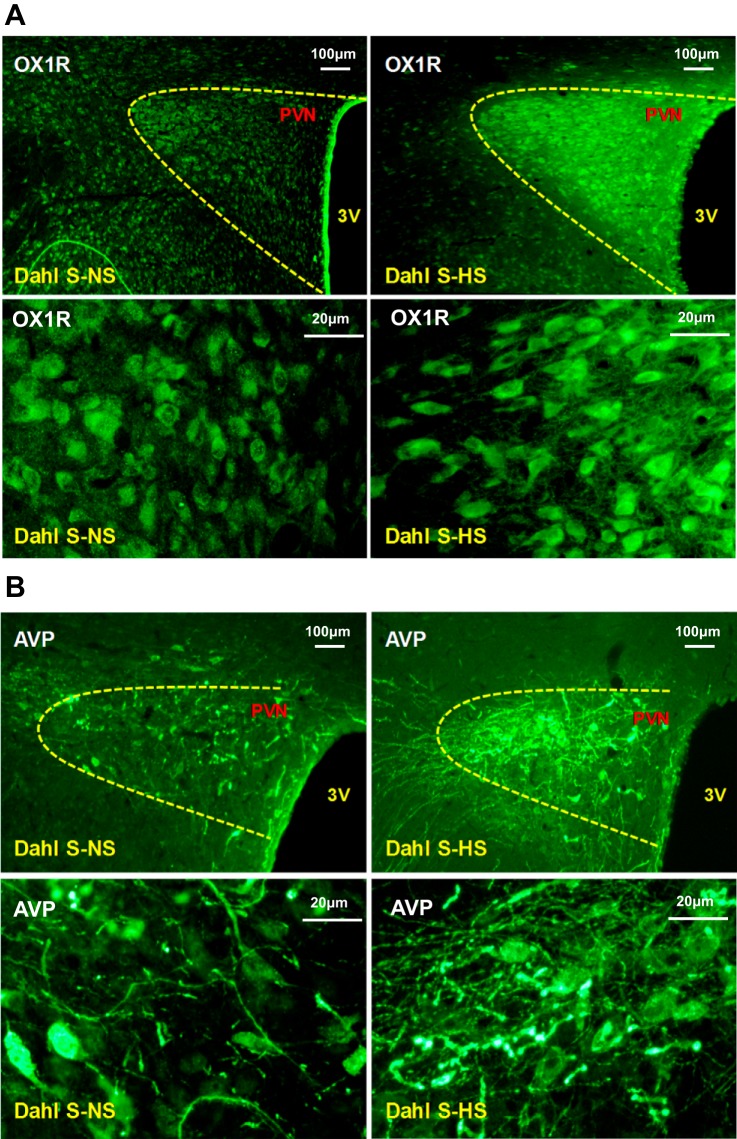
HS diet increases PVN immunoreactivity of OX1R and AVP in Dahl S rats.
The above-mentioned experiments showed that HS intake significantly increased mRNA levels of AVP and OX1R in the PVN of Dahl S rats. In this experiment, we further investigated whether or not HS diet upregulated PVN protein expression of AVP and OX1R in Dahl S rats. Immunostaining was performed using the brain coronal sections containing the PVN of Dahl S rats on either a NS or HS diet. The results showed that HS intake dramatically increased the immunoreactivity of both OX1R and AVP (Fig. 3).
Fig. 3.
A: representative immunostaining images of orexin receptor 1 (OX1R) in the paraventricular nucleus (PVN) between normal-salt (NS; 0.4% NaCl; left) and high-salt (HS; 8% NaCl; right) diet-fed Dahl salt-sensitive (Dahl S) rats. HS diet increased PVN OX1R immunoreactivity in Dahl S rats. The top row shows low-magnification images; the bottom row shows high-magnification images. B: representative immunostaining images of arginine vasopressin (AVP) in the PVN between NS and HS diet-fed Dahl S rats. HS diet increased PVN AVP immunoreactivity in Dahl S rats. The top row shows low-magnification images; the bottom row shows high-magnification images (contrasts were adjusted to enhance immunostained clusters).
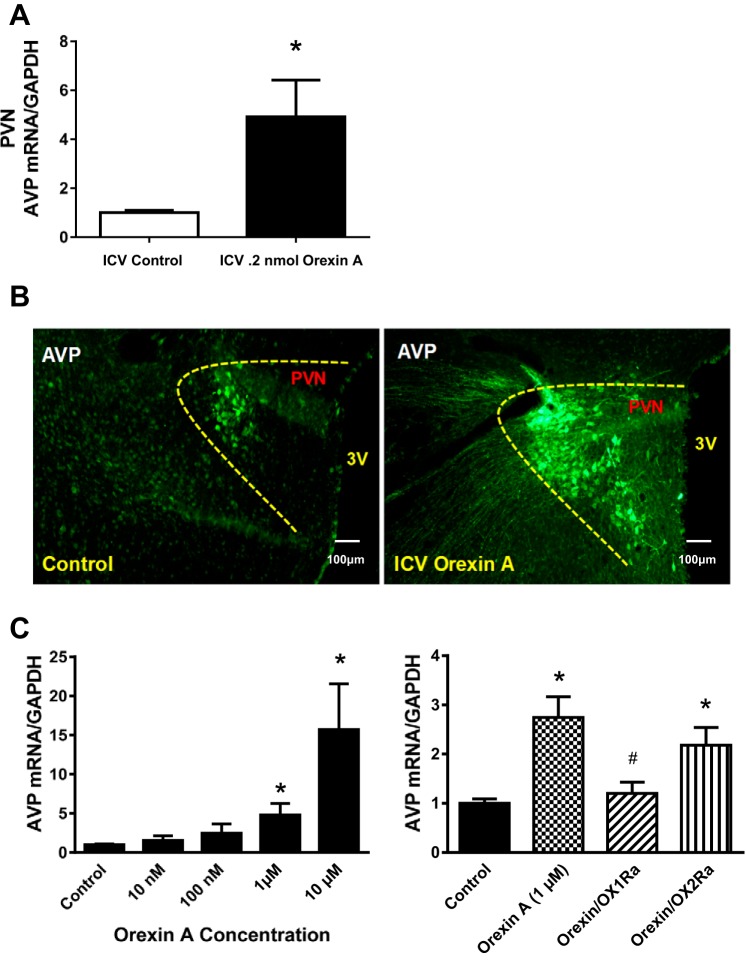
Orexin A controls PVN AVP expression, and orexin A control of AVP is mediated by OX1R in cultured hypothalamic neurons.
We expected from the experiment above that increased orexin receptor activation (OX1R or OX2R) in the PVN might be responsible for the enhanced PVN AVP levels in HS diet-fed Dahl S rats. To investigate whether or not orexin A controls PVN AVP expression in vivo, male adult SD rats received an intracerebroventricular injection of orexin A (0.2 nmol). Three hours after injection, rats were euthanized, and brain PVN tissues were punched out and received real-time PCR analysis to determine AVP mRNA expression. The results showed that AVP mRNA levels were increased 4.9-fold in orexin A-injected rats (n = 7, P < 0.05) compared with vehicle control saline-injected rats (Fig. 4A). In addition, intracerebroventricular injection of orexin A dramatically increased AVP immunoreactivity in both cell bodies and neuronal fibers (Fig. 4B). We then determined whether the orexin A-induced AVP increase occurred in PVN neurons and which orexin receptor was responsible for the regulation of AVP signaling. Primary neuronal cultures from the hypothalamus of newborn SD rats containing the PVN were incubated with differing concentrations of orexin A for 6 h. Real-time PCR was performed to assay mRNA levels of AVP. The results indicated that orexin treatment resulted in a dose-dependent increase in AVP mRNA levels (100 nM: 2.5-fold, 1 μM: 4.8-fold, and 10 μM: 15.7-fold, n = 4, Fig. 4C, left). To determine the orexin receptor type responsible for inducing increases in AVP expression, primary neuronal cultures from the hypothalamus of newborn SD rats were incubated with orexin A (1 µM), orexin A (1 µM) together with the OX1R antagonist SB-408124 (100 µM), or orexin A (1 µM) together with the OX2R antagonist TCS-OX2-29 (100 µM) for 6 h. Real-time PCR was performed to measure AVP mRNA levels. The results showed that orexin A-induced increases in AVP mRNA levels (2.7-fold) were attenuated by OX1R antagonist (1.2-fold, P < 0.05) but not OX2R antagonist (2.1 fold, n = 4; Fig. 4C, right).
Fig. 4.
A: real-time PCR analysis of paraventricular nucleus (PVN) arginine vasopressin (AVP) mRNA expression levels at 3 h after acute intracerebroventricular (ICV) infusion of vehicle control (0.9% saline) and orexin A (0.2 nmol) using male adult Sprague-Dawley (SD) rats. Intracerebroventricular injection of orexin A increased PVN AVP mRNA expression in SD rats. The mRNA level in the control sample was assigned to equal 1 arbitrary unit. n = 7–9 for each group. *P ≤ 0.05 vs. vehicle control. B: representative immunostaining images showing PVN AVP expression at 3 h after acute intracerebroventricular infusion of vehicle control (0.9% saline; left) and orexin A (0.2 nmol; right) using male adult SD rats. Intracerebroventricular infusion of orexin A increased AVP immunoreactivity in SD rats (contrast was adjusted to enhance immunostained clusters). C, left: incubation of primary neuronal cultures from the hypothalamus of newborn SD rats containing the PVN with increasing concentrations of orexin A for 6 h. Neurons were then collected, RNA was purified, and real-ime PCR was performed to assay mRNA levels of AVP. n = 4 for each group. *P ≤ 0.05 vs. control. Right, incubation of primary neuronal cultures from the hypothalamus of newborn SD rats containing the PVN with orexin A alone or coincubated with the orexin receptor 1 (OX1Ra) antagonist SB-408124 or the orexin receptor 2 antagonist (OX2Ra) TCS-OX2-29 for 6 h. Real-time PCR was performed to measure AVP mRNA levels. Orexin A-induced increases in mRNA levels of AVP were attenuated by orexin receptor 1 but not orexin receptor 2 blockade in hypothalamic neurons. The mRNA level in the control sample was assigned to equal 1 arbitrary unit. n = 4 for each group. *P ≤ 0.05 vs. control; #P ≤ 0.05 vs. orexin.
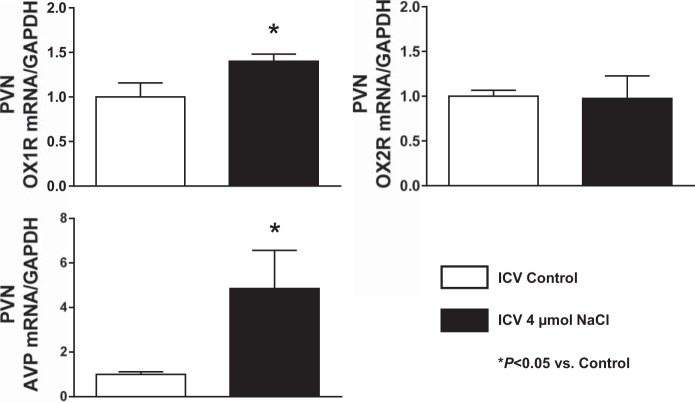
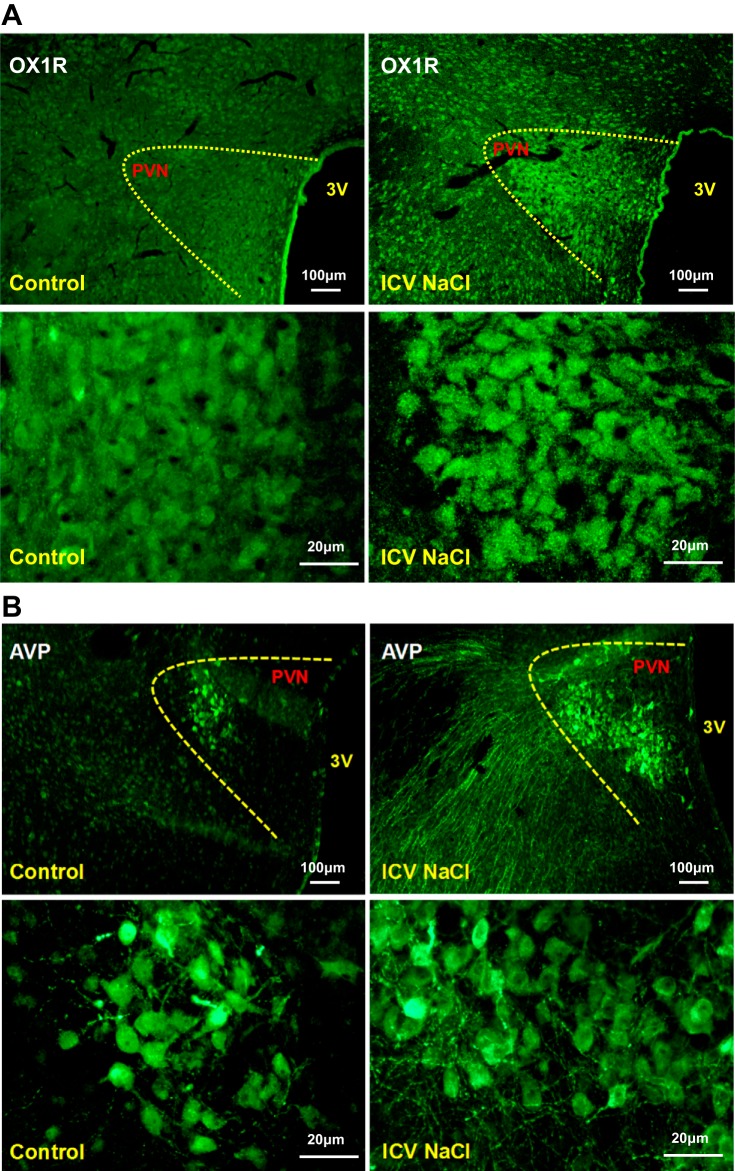
Acute intracerebroventricular injection of hypertonic saline increases PVN OX1R and AVP mRNA expression and immunoreactivity in SD rats.
The results from the experiment above suggest that orexin controls PVN AVP expression through OX1R activation. This suggests that increased PVN OX1R activation, potentially via upregulation, may cause enhanced PVN AVP signaling in HS diet-fed Dahl S rats. It has been previously shown that the cerebrospinal fluid (CSF) Na+ concentration increases in Dahl S rats on a HS diet (20, 38). Therefore, we hypothesized that an increased CSF Na+ concentration in Dahl S rats with HS intake might cause PVN OX1R upregulation leading to enhanced PVN AVP signaling. It has previously been demonstrated that the CSF Na+ concentration of Dahl S rats increased by 6 mM after 3 days on an 8% HS diet (20). Therefore, we expected that after 4 wk of 8% HS diet, CSF Na+ concentration should increase by no less than 10 mM. Assuming the entire CSF volume is 400 μl (52), intracerebroventricular injection of 4 μmol NaCl should increase the CSF Na+ concentration by 10 mM. Male adult SD rats received intracerebroventricular injection of hypertonic saline (4 μmol) to mimic the increased CSF Na+ concentration of Dahl S rats on a HS diet for 4 wk, as used in our study. Three hours after injection, PVN tissue was punched, RNA was extracted and reverse transcribed to cDNA, and real-time PCR was performed to determine PVN mRNA levels of OX1R, OX2R, and AVP. The results showed that OX1R (1.4-fold) but not OX2R (0.97-fold) PVN mRNA levels were significantly increased (P < 0.05) in hypertonic saline-injected rats compared with vehicle control-injected rats. AVP mRNA levels were significantly increased (4.8-fold) in hypertonic saline-injected rats (n = 5) compared with vehicle control-injected rats (P < 0.05; Fig. 5). Consistent with these results, immunostaining showed that 3 h after intracerebroventricular infusion of hypertonic saline (4 µmol) immunoreactivity of OX1R and AVP was also obviously increased (Fig. 6).
Fig. 5.
Real-time PCR analysis of paraventricular nucleus (PVN) orexin receptor 1 (OX1R), orexin receptor 2 (OX2R), and arginine vasopressin (AVP) mRNA expression levels at 3 h after acute intracerebroventricular (ICV) infusion of vehicle control (0.9% saline) and hypertonic saline (4 μmol) using male adult Sprague-Dawley (SD) rats. Acute intracerebroventricular injection of hypertonic saline increased PVN OX1R and AVP mRNA levels in SD rats. The mRNA level in the control sample was assigned to equal 1 arbitrary unit. n = 4–7 for each group. *P ≤ 0.05 vs. vehicle control.
Fig. 6.
A: representative images showing immunoreactivity of paraventricular nucleus (PVN) orexin receptor 1 (OX1R) 3 h after acute intracerebroventricular (ICV) infusion of vehicle control (0.9% saline; left) and hypertonic saline (4 μmol; right) using male adult Sprague-Dawley SD rats. Intracerebroventricular hypertonic saline infusion increased PVN OX1R immunoreactivity. The top row shows low-magnification images; the bottom row shows high-magnification images. B: representative images showing PVN arginine vasopressin (AVP) expression 3 h after acute intracerebroventricular infusion of vehicle control (0.9% saline; left) and hypertonic saline (4 μmol; right) using male adult SD rats. Intracerebroventricular NaCl solution increased PVN AVP immunoreactivity. The top row shows low-magnification images; the bottom row showss high-magnification images (contrast was adjusted to enhance immunostained clusters).
PVN OX1R blockade reduces blood pressure in HS diet-fed Dahl S rats.
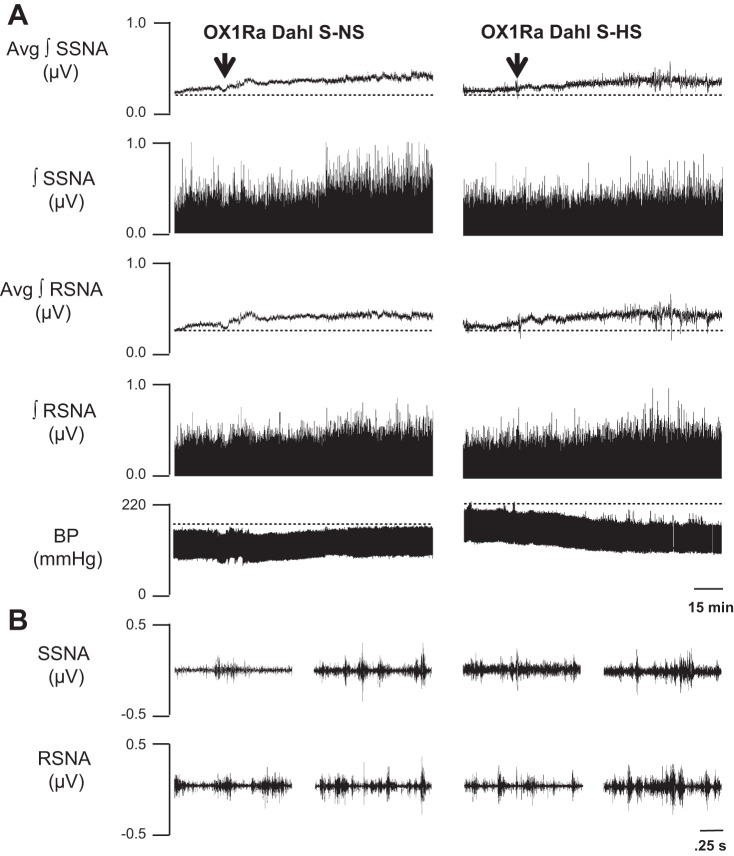
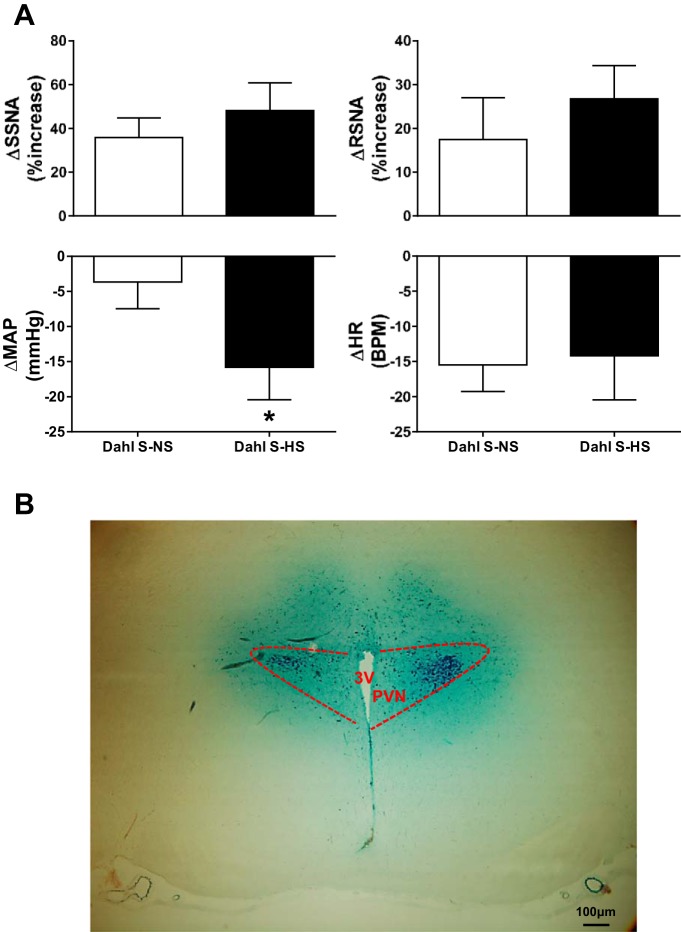
The results from the above experiments indicate that HS diet increases PVN OX1R expression in Dahl S rats, OX1R mediates AVP signaling in the PVN, and the increased CSF Na+ concentration in HS diet-fed Dahl S rats may cause PVN OX1R upregulation leading to enhanced AVP signaling. Therefore, we hypothesized that increased OX1R activation in the PVN contributes to the maintenance of hypertension in HS diet-fed Dahl S rats. To test this hypothesis, we recorded MAP, sympathetic nerve activity, and the HR response to bilateral PVN microinjection of the OX1R antagonist SB-408124 in anesthetized Dahl S rats fed a NS and HS diet for 4–5 wk. MAP before microinjection of the OX1R antagonist was 112.2 ± 3.3 mmHg in the NS group and 144.4 ± 3.4 mmHg in the HS group. The OX1R antagonist SB-408124 bilaterally injected into the PVN significantly decreased MAP in Dahl S rats fed a HS diet compared with Dahl S rats fed a NS diet (HS: −16 ± 5 mmHg, n = 5, vs. NS: −4 ± 4 mmHg, n = 6, P < 0.05). Interestingly, OX1R blockade also increased SSNA and RSNA in both NS and HS diet-fed Dahl S rats. Figure 7, A and B, left, shows an example of SSNA, RSNA, and MAP responses to the OX1R antagonist SB-408124 (30 pmol/50 nl per side) in Dahl S rats fed a NS diet (0.4% NaCl). Figure 7, A and B, right, shows an example of SSNA, RSNA, and MAP responses to SB-408124 (30 pmol/50 nl per side) in Dahl S rats fed a HS diet (8% NaCl). Maximum increases in SSNA and RSNA were greater in HS diet-fed rats compared with NS diet-fed rats but failed to reach statistical significance [SSNA: HS 48.2 ± 12.6%, n = 5, vs. NS 35.8 ± 9%, n = 5, P = 0.2; and RSNA: HS 26.7 ± 7.5%, n = 5. vs. NS 17.4 ± 10%, n = 6, P = 0.2]. Figure 8A shows PVN microinjection summary data. Figure 8B shows a single representative PVN injection.
Fig. 7.
A: representative traces showing splanchnic sympathetic nerve activity (SSNA), renal sympathetic nerve activity (RSNA), and mean arterial pressure [blood pressure (BP)] responses to bilateral paraventricular nucleus (PVN) microinjections of the orexin receptor 1 antagonist (OX1Ra) SB-408124 (30 pmol/50 nl per side) into Dahl salt-sensitive (Dahl S) rats fed a normal-salt (NS; 0.4% NaCl; left) or high-salt (HS; 8% NaCl; right) diet. PVN orexin receptor 1 blockade reduced blood pressure in Dahl S rats with HS intake. B, left: 2.5-s specimen traces of SSNA (top) and RSNA (bottom) before injection of OX1Ra into the PVN and after microinjection of OX1Ra into the PVN of a Dahl S rat on NS diet. Right, 2.5-s specimen traces of SSNA (top) and RSNA (bottom) before injection of OX1Ra into the PVN and after microinjection of OX1Ra into the PVN of a Dahl S rat on the HS diet. Avg, average; ∫, integrated.
Fig. 8.
A: summary data showing changes in splanchnic sympathetic nerve activity (ΔSSNA), renal sympathetic nerve activity (ΔRSNA), mean arterial pressure (ΔMAP), and heart rate [ΔHR; in beats/min (BPM)] in response to bilateral paraventricular nucleus (PVN) microinjections of the orexin receptor 1 antagonist SB-408124 (30 pmol/50 nl per side) in normal-salt (NS; 0.4% NaCl, n = 5–6) and high-salt (HS; 8% NaCl) diet-fed Dahl salt-sensitive (Dahl S) rats (n = 5). *P < 0.05 vs. NS diet. B: representation of a single injection (50 nl) of ink showing the injection site within the PVN.
DISCUSSION
Accumulating evidence suggests that the orexin system is involved in the central control of cardiovascular function and that its hyperactivity in the brain contributes to hypertension (26–28, 56). However, the role of the orexin system in SSHTN is not known. This study is the first to investigate the contribution of the orexin system to SSHTN. Here, we report five findings. First, OX1R, OX2R, and AVP expressions are upregulated in the PVN in Dahl S rats with HS intake. Second, intracerebroventricular administration of orexin A increases PVN AVP expression in normotensive SD rats. Third, incubation of cultured hypothalamic neurons from SD rats containing the PVN with orexin A increases AVP mRNA levels, which is attenuated by OX1R blockade. Fourth, intracerebroventricular administration of hypertonic saline increases PVN OX1R and AVP expression in SD rats. Finally, PVN OX1R antagonist injection significantly reduces MAP in Dahl S rats fed a HS diet compared with Dahl S rats fed a NS diet. Taken together, these findings indicate that increased OX1R activity in the PVN contributes to enhanced AVP signaling in Dahl S rats on a HS diet, which may contribute to the maintenance of SSHTN in Dahl S animals.
The PVN is a key site for the integration of autonomic and neuroendocrine function, and its function is essential to maintain elevated blood pressure in the Dahl model of SSHTN (14). Expressions of orexin A and B (5, 6, 39, 41, 46, 55) and their receptors (2, 7, 19, 31, 33, 53) have been found in regions of the brain involved in blood pressure regulation, including the PVN, in normotensive rats. Our study is novel in that it shows that mRNA expression levels of OX1R and OX2R and immunoreactivity of OX1R are higher in the PVN of Dahl S rats on a HS diet than in Dahl S rats on a NS diet (Figs. 2 and 3). We did not observe PVN mRNA expression of components of the orexin system in the PVN to be changed in HS diet-fed SD rats (Fig. 2). Our results also showed that Dahl S rats on a HS diet had increased PVN AVP mRNA levels and immunoreactivity consistent with previous findings that plasma AVP is elevated in Dahl S rats on a HS diet (34, 45, 54). However, we found that PVN AVP mRNA expression was also increased in HS compared with NS diet-fed SD rats, but the increase in AVP mRNA expression was much greater in Dahl S rats fed a HS diet (5.5-fold) compared with SD rats fed the same diet (1.5-fold). We propose that this enhanced AVP signaling in HS diet-fed Dahl S rats compared with HS diet-fed SD rats is at least partially mediated by activation of orexin receptors in the PVN. Overall, these results suggest that a HS diet may be linked to increased PVN orexin system activity in Dahl S rats. Consistent with previous studies and as expected, we found that a 4-wk HS (8% NaCl) diet significantly increased MAP in Dahl S rats but not in SD rats (Fig. 1) (15, 29).
On the basis of these results, we proposed that increased PVN orexin system activity might be responsible for increased AVP signaling in the PVN in HS Dahl S rats. First, we sought to confirm that orexin receptor activation controls PVN AVP expression in vivo. Intracerebroventricular infusion of orexin A significantly augmented PVN AVP mRNA expression compared with vehicle control and increased PVN AVP immunoreactivity (Fig. 4). This is consistent with previous studies showing that intracerebroventricular administration of orexin A increased PVN AVP mRNA expression in conscious rats (1) and plasma AVP levels in conscious rabbits after 90 min (35). Incubation of cultured neurons from the hypothalamus containing the PVN from newborn SD rats with orexin A resulted in a dose-dependent increase in AVP mRNA expression (Fig. 4). Coincubation with OX1R antagonist but not with OX2R antagonist attenuated this orexin-induced AVP mRNA expression response (Fig. 4). It should be noted that, although the cultured neurons used in this study included the PVN, they were a mixture of neurons from different hypothalamic nuclei. Since magnocellular neurons of the supraoptic nucleus (SON), located in the hypothalamus, also produce AVP, and we cannot exclude the possibility that the orexin system also regulates AVP production in the SON. Taken together, these results indicate that activation of OX1R rather than OX2R expressed on PVN neurons is likely responsible for regulating orexin A-induced AVP mRNA expression. Interestingly, OX1R has a one order of magnitude greater affinity for orexin A over orexin B (43), which suggests that orexin A may play a larger role in regulating PVN AVP mRNA expression than orexin B. It has previously been shown that the magnocellular division of the PVN shows OX1R immunoreactivity, and double labeling indicated that OX1R-immunoreactive neurons in the magnocellular PVN contained vasopressin (2). These results suggest that increased PVN OX1R activation, potentially via upregulation, contributes to enhanced PVN AVP signaling of Dahl S rats on a HS diet. Dahl S rats on a HS diet show an increased CSF Na+ concentration (20, 38), which precedes hypertension (20). It was found that the CSF Na+ concentration of Dahl S rats, but not Dahl salt-resistant rats and normal rats such as Wistar-Kyoto rats, increased by 6 mM after 3 days on an 8% HS diet preceding hypertension (20). Therefore, we hypothesized that the increased CSF Na+ concentration in Dahl S rats with HS intake might cause PVN OX1R upregulation leading to enhanced PVN AVP signaling. Intracerebroventricular infusion of hypertonic saline in SD rats was performed to mimic an increase in CSF Na+ concentration to levels observed in HS diet-fed Dahl S rats. The results showed that intracerebroventricular infusion of 4 µmol NaCl significantly increased PVN OX1R and AVP expression in both mRNA levels (Fig. 5) and protein expressions (Fig. 6), which suggests that increased CSF Na+ concentration, a main phenotype component of Dahl S rats on a HS diet, may cause PVN OX1R upregulation leading to enhanced AVP signaling. The link between how increased salt intake could activate the orexin system is unclear at the present time. There have been no studies that have directly determined whether orexin-producing neurons are sensitive to changes in osmolality. However, it has previously been shown that prepro-orexin mRNA is upregulated in the hypothalamus of 48-h water-deprived rats (23), indicating that orexin-producing neurons may be sensitive to changes in osmolality. Also, the lamina terminalis, which is the location of the primary central osmoreceptors, has been reported to have efferent projections to the lateral hypothalamus, where orexin-producing cell bodies are located (18, 22), which may explain why orexin neurons would be sensitive to changes in body fluid osmolality. Interestingly, it has been hypothesized that orexin neurons may be a link between the lamina terminalis sensing of perturbations in body fluid homeostasis and activating motivation and reward systems in the mesolimbic system (dopaminergic projection from the ventral tegmental area to the nucleus accumbens) to affect salt- and water appetite-motivated behavior to restore body fluid homeostasis (22).
Finally, since we found that Dahl S rats on a HS diet displayed increased PVN OX1R expression, OX1R regulates AVP expression, and increased CSF Na+ concentration may cause PVN OX1R upregulation leading to enhanced AVP signaling in HS intake Dahl S rats, we determined whether increased OX1R activation in the PVN was implicated in HS-induced hypertension in Dahl S rats. Microinjection of the OX1R antagonist SB-408124 into the PVN elicited a significantly greater reduction in MAP in HS compared with NS diet-fed Dahl S rats (Fig. 7). This result suggests that increased PVN OX1R activation contributes to the maintenance of HS-induced hypertension in Dahl S rats. This is likely through increased PVN OX1R expression, since OX1R mRNA expression was upregulated in the PVN of HS diet-fed Dahl S rats. PVN OX1R blockade also increased both SSNA and RSNA in NS and HS diet-fed rats. This sympathetic nerve activity response is likely driven by the arterial baroreflex mechanism, since experiments were performed in arterial baroreceptor-intact animals. Interestingly, there was no significant difference in RSNA and SSNA responses to PVN OX1R blockade in NS compared with HS diet-fed Dahl S rats. This could be explained by a study (36) that showed that HS intake was involved in the impairment of arterial baroreflex function in Dahl S rats. It should be noted that the present study failed to observe a parallel reduction in sympathetic nerve activity along with the reduction in MAP; therefore, it is plausible that OX1R expressed on magnocellular PVN neurons is responsible for AVP expression. Therefore, increased activation of OX1R expressed in the PVN could be expected to upregulate AVP expression, contributing to the maintenance of hypertension in Dahl S rats on a HS diet. Evidence indicates that orexin A depolarizes magnocellular PVN neurons by increasing glutamate release from presynaptic or interneuron glutamatergic neurons in the PVN (13). Also, orexin neurons may depolarize magnocellular PVN neurons directly by positively modulating glutamate receptor activity (13).
Although the contribution of AVP to the Dahl model of SSHTN is still controversial, it was recently demonstrated that oral administration of the dual AVP receptor antagonist RWJ-676070 produced a transient decrease in MAP in hypertensive Dahl S rats on a HS diet (17). This evidence indicates that upregulation of AVP could be involved in SSHTN in Dahl S rats. However, we still cannot ignore the possibility that increased OX1R activation on parvocellular PVN neurons may be responsible for increased sympathetic outflow and elevated blood pressure in Dahl S rats on a HS diet. This possibility is supported by a previous study (13) showing that orexin A is able to depolarize parvocellular PVN neurons through activation of nonselective cation channels.
Taking all results into account, we speculate that in Dahl S rats a combination of genetic abnormalities may lead to chronic activation of the orexin system in the PVN in response to elevated salt intake. It has been demonstrated that Dahl S rats have genetic functional abnormalities of the kidneys that reduce natriuresis, or the ability to excrete Na+ (32). It has been shown that in response Na+ is abnormally transported into the CSF, causing the Na+ concentration to increase (20, 38, 49). The circumventricular organs in the lamina terminalis may sense increased osmolality due to elevated CSF Na+ concentration, which may subsequently activate orexin-producing neurons located in the hypothalamus. Alternatively, increased CSF Na+ concentration may directly activate orexin-producing neurons. This may result in increased orexin release to the PVN, causing upregulation of OX1R, resulting in enhanced AVP secretion and eventually contributing to hypertension. In addition, the reduced ability of natriuresis by the kidneys of Dahl S rats may support chronic activation of the orexin system and may explain why orexin-producing neurons would not be chronically activated in SD or Dahl S rats in response to a HS diet.
Perspectives
Individuals with hypertension are at an increased risk for cardiovascular disease, the leading global cause of death. Increased dietary salt intake is a primary contributor to essential hypertension; therefore, it is of great interest to elucidate how HS intake underlies the mechanisms of SSHTN to identify new molecular targets for treatment. The present study identifies a novel mechanism whereby elevated PVN OX1R activation may be involved in the maintenance of hypertension in HS diet-fed Dahl S rats by enhancing AVP signaling.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grants R15-HL-129213 (Z. Shan) and R15-HL-122952 (Q.-H. Chen), American Heart Association Grant 11S DG-7420029 (Z. Shan), Michigan Technological University Research Excellence Fund (Z. Shan), and Portage Health Foundation Research Excellence Fund (Z. Shan). Y. Fan was supported by an Exchange Scholarship from China Scholarship Council of the Ministry of Education (File no. 201606280270).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.J.H., Y.F. and Z.S. conceived and designed research; M.J.H., Y.F., E.J., F.Z., R.A.L., N.L., and Z.S. performed experiments; M.J.H., N.L., and Z.S. analyzed data; M.J.H. and Z.S. interpreted results of experiments; M.J.H. and Z.S. prepared figures; M.J.H. drafted manuscript; M.J.H., Q.-H.C., and Z.S. edited and revised manuscript; M.J.H., Y.F., E.J., F.Z., R.A.L., J.Y., N.L., Q.-H.C., and Z.S. approved final version of manuscript.
REFERENCES
- 1.Al-Barazanji KA, Wilson S, Baker J, Jessop DS, Harbuz MS. Central orexin-A activates hypothalamic-pituitary-adrenal axis and stimulates hypothalamic corticotropin releasing factor and arginine vasopressin neurones in conscious rats. J Neuroendocrinol 13: 421–424, 2001. doi: 10.1046/j.1365-2826.2001.00655.x. [DOI] [PubMed] [Google Scholar]
- 2.Bäckberg M, Hervieu G, Wilson S, Meister B. Orexin receptor-1 (OX-R1) immunoreactivity in chemically identified neurons of the hypothalamus: focus on orexin targets involved in control of food and water intake. Eur J Neurosci 15: 315–328, 2002. doi: 10.1046/j.0953-816x.2001.01859.x. [DOI] [PubMed] [Google Scholar]
- 3.Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol 464: 220–237, 2003. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- 4.Bayorh MA, Ogbolu EC, Williams E, Thierry-Palmer M, Sanford G, Emmett N, Harris-Hooker S, Socci RR, Chu TC, Chenault VM. Possible mechanisms of salt-induced hypertension in Dahl salt-sensitive rats. Physiol Behav 65: 563–568, 1998. doi: 10.1016/S0031-9384(98)00194-2. [DOI] [PubMed] [Google Scholar]
- 5.Ciriello J, de Oliveira CV. Cardiac effects of hypocretin-1 in nucleus ambiguus. Am J Physiol Regul Integr Comp Physiol 284: R1611–R1620, 2003. doi: 10.1152/ajpregu.00719.2002. [DOI] [PubMed] [Google Scholar]
- 6.Ciriello J, Li Z, de Oliveira CV. Cardioacceleratory responses to hypocretin-1 injections into rostral ventromedial medulla. Brain Res 991: 84–95, 2003. doi: 10.1016/j.brainres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Cluderay JE, Harrison DC, Hervieu GJ. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul Pept 104: 131–144, 2002. doi: 10.1016/S0167-0115(01)00357-3. [DOI] [PubMed] [Google Scholar]
- 8.Cowley AW Jr, Skelton MM, Velasquez MT. Sex differences in the endocrine predictors of essential hypertension. Vasopressin versus renin. Hypertension 7: I151–I160, 1985. doi: 10.1161/01.HYP.7.3_Pt_2.I151. [DOI] [PubMed] [Google Scholar]
- 9.Crofton JT, Share L, Shade RE, Allen C, Tarnowski D. Vasopressin in the rat with spontaneous hypertension. Am J Physiol Heart Circ Physiol 235: H361–H366, 1978. [DOI] [PubMed] [Google Scholar]
- 10.Crofton JT, Share L, Wang BC, Shade RE. Pressor responsiveness to vasopressin in the rat with DOC-salt hypertension. Hypertension 2: 424–431, 1980. doi: 10.1161/01.HYP.2.4.424. [DOI] [PubMed] [Google Scholar]
- 11.Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci USA 96: 748–753, 1999. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS II, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 95: 322–327, 1998. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Follwell MJ, Ferguson AV. Cellular mechanisms of orexin actions on paraventricular nucleus neurones in rat hypothalamus. J Physiol 545: 855–867, 2002. doi: 10.1113/jphysiol.2002.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goto A, Ikeda T, Tobian L, Iwai J, Johnson MA. Brain lesions in the paraventricular nuclei and catecholaminergic neurons minimize salt hypertension in Dahl salt-sensitive rats. Clin Sci (Lond) 61, Suppl 7: 53s–55s, 1981. doi: 10.1042/cs061053s. [DOI] [PubMed] [Google Scholar]
- 15.Gu JW, Young E, Pan ZJ, Tucker KB, Shparago M, Huang M, Bailey AP. Long-term high salt diet causes hypertension and alters renal cytokine gene expression profiles in Sprague-Dawley rats. Beijing Da Xue Xue Bao 41: 505–515, 2009. [PubMed] [Google Scholar]
- 16.Gui L, LaGrange LP, Larson RA, Gu M, Zhu J, Chen QH. Role of small conductance calcium-activated potassium channels expressed in PVN in regulating sympathetic nerve activity and arterial blood pressure in rats. Am J Physiol Regul Integr Comp Physiol 303: R301–R310, 2012. doi: 10.1152/ajpregu.00114.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gunnet JW, Wines P, Xiang M, Rybczynski P, Andrade-Gordon P, de Garavilla L, Parry TJ, Cheung WM, Minor L, Demarest KT, Maryanoff BE, Damiano BP. Pharmacological characterization of RWJ-676070, a dual vasopressin V1A/V2 receptor antagonist. Eur J Pharmacol 590: 333–342, 2008. doi: 10.1016/j.ejphar.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Hahn JD, Swanson LW. Distinct patterns of neuronal inputs and outputs of the juxtaparaventricular and suprafornical regions of the lateral hypothalamic area in the male rat. Brain Res Brain Res Rev 64: 14–103, 2010. doi: 10.1016/j.brainresrev.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience 103: 777–797, 2001. doi: 10.1016/S0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- 20.Huang BS, Van Vliet BN, Leenen FH. Increases in CSF [Na+] precede the increases in blood pressure in Dahl S rats and SHR on a high-salt diet. Am J Physiol Heart Circ Physiol 287: H1160–H1166, 2004. doi: 10.1152/ajpheart.00126.2004. [DOI] [PubMed] [Google Scholar]
- 21.Huber MJ, Chen QH, Shan Z. The orexin system and hypertension. Cell Mol Neurobiol In press. doi: 10.1007/s10571-017-0487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurley SW, Johnson AK. The role of the lateral hypothalamus and orexin in ingestive behavior: a model for the translation of past experience and sensed deficits into motivated behaviors. Front Syst Neurosci 8: 216, 2014. doi: 10.3389/fnsys.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunii K, Yamanaka A, Nambu T, Matsuzaki I, Goto K, Sakurai T. Orexins/hypocretins regulate drinking behaviour. Brain Res 842: 256–261, 1999. doi: 10.1016/S0006-8993(99)01884-3. [DOI] [PubMed] [Google Scholar]
- 24.Larson RA, Gui L, Huber MJ, Chapp AD, Zhu J, LaGrange LP, Shan Z, Chen QH. Sympathoexcitation in ANG II-salt hypertension involves reduced SK channel function in the hypothalamic paraventricular nucleus. Am J Physiol Heart Circ Physiol 308: H1547–H1555, 2015. doi: 10.1152/ajpheart.00832.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee-Kwon WJ, Share L, Crofton JT, Shade RE, Brooks B, Muirhead EE, Manning M, Sawyer WH. Vasopressin in the rat with partial nephrectomy-salt hypertension. Clin Exp Hypertens 3: 281–297, 1981. doi: 10.3109/10641968109033665. [DOI] [PubMed] [Google Scholar]
- 26.Lee YH, Dai YW, Huang SC, Li TL, Hwang LL. Blockade of central orexin 2 receptors reduces arterial pressure in spontaneously hypertensive rats. Exp Physiol 98: 1145–1155, 2013. doi: 10.1113/expphysiol.2013.072298. [DOI] [PubMed] [Google Scholar]
- 27.Lee YH, Tsai MC, Li TL, Dai YW, Huang SC, Hwang LL. Spontaneously hypertensive rats have more orexin neurons in the hypothalamus and enhanced orexinergic input and orexin 2 receptor-associated nitric oxide signalling in the rostral ventrolateral medulla. Exp Physiol 100: 993–1007, 2015. doi: 10.1113/EP085016. [DOI] [PubMed] [Google Scholar]
- 28.Li A, Hindmarch CC, Nattie EE, Paton JF. Antagonism of orexin receptors significantly lowers blood pressure in spontaneously hypertensive rats. J Physiol 591: 4237–4248, 2013. doi: 10.1113/jphysiol.2013.256271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Limas C, Goldman P, Limas CJ, Iwai J. Effect of salt on prostaglandin metabolism in hypertension-prone and -resistant Dahl rats. Hypertension 3: 219–224, 1981. doi: 10.1161/01.HYP.3.2.219. [DOI] [PubMed] [Google Scholar]
- 30.Lu D, Raizada MK. Delivery of angiotensin II type 1 receptor antisense inhibits angiotensin action in neurons from hypertensive rat brain. Proc Natl Acad Sci USA 92: 2914–2918, 1995. doi: 10.1073/pnas.92.7.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu XY, Bagnol D, Burke S, Akil H, Watson SJ. Differential distribution and regulation of OX1 and OX2 orexin/hypocretin receptor messenger RNA in the brain upon fasting. Horm Behav 37: 335–344, 2000. doi: 10.1006/hbeh.2000.1584. [DOI] [PubMed] [Google Scholar]
- 32.Manger WM, Simchon S, Stokes MB, Reidy JJ, Kumar AR, Baer L, Gallo G, Haddy FJ. Renal functional, not morphological, abnormalities account for salt sensitivity in Dahl rats. J Hypertens 27: 587–598, 2009. doi: 10.1097/HJH.0b013e32831ffec7. [DOI] [PubMed] [Google Scholar]
- 33.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435: 6–25, 2001. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 34.Matsuguchi H, Schmid PG, Van Orden D, Mark AL. Does vasopressin contribute to salt-induced hypertension in the Dahl strain? Hypertension 3: 174–181, 1981. doi: 10.1161/01.HYP.3.2.174. [DOI] [PubMed] [Google Scholar]
- 35.Matsumura K, Tsuchihashi T, Abe I. Central orexin-A augments sympathoadrenal outflow in conscious rabbits. Hypertension 37: 1382–1387, 2001. doi: 10.1161/01.HYP.37.6.1382. [DOI] [PubMed] [Google Scholar]
- 36.Miyajima E, Buñag RD. Exacerbation of central baroreflex impairment in Dahl rats by high-salt diets. Am J Physiol Heart Circ Physiol 252: H402–H409, 1987. [DOI] [PubMed] [Google Scholar]
- 37.Möhring J, Möhring B, Petri M, Haack D. Plasma vasopressin concentrations and effects of vasopressin antiserum on blood pressure in rats with malignant two-kidney Goldblatt hypertension. Circ Res 42: 17–22, 1978. doi: 10.1161/01.RES.42.1.17. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura K, Cowley AW Jr. Sequential changes of cerebrospinal fluid sodium during the development of hypertension in Dahl rats. Hypertension 13: 243–249, 1989. doi: 10.1161/01.HYP.13.3.243. [DOI] [PubMed] [Google Scholar]
- 39.Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res 827: 243–260, 1999. doi: 10.1016/S0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- 40.Os I, Kjeldsen SE, Skjøtø J, Westheim A, Lande K, Aakesson I, Frederichsen P, Leren P, Hjermann I, Eide IK. Increased plasma vasopressin in low renin essential hypertension. Hypertension 8: 506–513, 1986. doi: 10.1161/01.HYP.8.6.506. [DOI] [PubMed] [Google Scholar]
- 41.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18: 9996–10015, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakurai T. The role of orexin in motivated behaviours. Nat Rev Neurosci 15: 719–731, 2014. doi: 10.1038/nrn3837. [DOI] [PubMed] [Google Scholar]
- 43.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92: 573–585, 1998. doi: 10.1016/S0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 44.Samson WK, Taylor MM, Follwell M, Ferguson AV. Orexin actions in hypothalamic paraventricular nucleus: physiological consequences and cellular correlates. Regul Pept 104: 97–103, 2002. doi: 10.1016/S0167-0115(01)00353-6. [DOI] [PubMed] [Google Scholar]
- 45.Serino R, Ueta Y, Hanamiya M, Nomura M, Yamamoto Y, Yamaguchi KI, Nakashima Y, Yamashita H. Increased levels of hypothalamic neuronal nitric oxide synthase and vasopressin in salt-loaded Dahl rat. Auton Neurosci 87: 225–235, 2001. doi: 10.1016/S1566-0702(00)00279-4. [DOI] [PubMed] [Google Scholar]
- 46.Shahid IZ, Rahman AA, Pilowsky PM. Orexin A in rat rostral ventrolateral medulla is pressor, sympatho-excitatory, increases barosensitivity and attenuates the somato-sympathetic reflex. Br J Pharmacol 165: 2292–2303, 2012. doi: 10.1111/j.1476-5381.2011.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shan Z, Shi P, Cuadra AE, Dong Y, Lamont GJ, Li Q, Seth DM, Navar LG, Katovich MJ, Sumners C, Raizada MK. Involvement of the brain (pro)renin receptor in cardiovascular homeostasis. Circ Res 107: 934–938, 2010. doi: 10.1161/CIRCRESAHA.110.226977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shirasaka T, Miyahara S, Kunitake T, Jin QH, Kato K, Takasaki M, Kannan H. Orexin depolarizes rat hypothalamic paraventricular nucleus neurons. Am J Physiol Regul Integr Comp Physiol 281: R1114–R1118, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Simchon S, Manger W, Golanov E, Kamen J, Sommer G, Marshall CH. Handling 22NaCl by the blood-brain barrier and kidney: its relevance to salt-induced hypertension in dahl rats. Hypertension 33: 517–523, 1999. doi: 10.1161/01.HYP.33.1.517. [DOI] [PubMed] [Google Scholar]
- 50.Stocker SD, Muntzel MS. Recording sympathetic nerve activity chronically in rats: surgery techniques, assessment of nerve activity, and quantification. Am J Physiol Heart Circ Physiol 305: H1407–H1416, 2013. doi: 10.1152/ajpheart.00173.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thunhorst RL, Xue B, Beltz TG, Johnson AK. Age-related changes in thirst, salt appetite, and arterial blood pressure in response to aldosterone-dexamethasone combination in rats. Am J Physiol Regul Integr Comp Physiol 308: R807–R815, 2015. doi: 10.1152/ajpregu.00490.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tomiyama Y, Brian JE Jr, Todd MM, Pearce W. Cerebral blood flow during hemodilution and hypoxia in rats: role of ATP-sensitive potassium channels. Stroke 30: 1942–1948, 1999. doi: 10.1161/01.STR.30.9.1942. [DOI] [PubMed] [Google Scholar]
- 53.Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett 438: 71–75, 1998. doi: 10.1016/S0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- 54.Wainford RD, Kapusta DR. Hypothalamic paraventricular nucleus G alpha q subunit protein pathways mediate vasopressin dysregulation and fluid retention in salt-sensitive rats. Endocrinology 151: 5403–5414, 2010. doi: 10.1210/en.2010-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng H, Patterson LM, Berthoud HR. Orexin-A projections to the caudal medulla and orexin-induced c-Fos expression, food intake, and autonomic function. J Comp Neurol 485: 127–142, 2005. doi: 10.1002/cne.20515. [DOI] [PubMed] [Google Scholar]
- 56.Zhou JJ, Yuan F, Zhang Y, Li DP. Upregulation of orexin receptor in paraventricular nucleus promotes sympathetic outflow in obese Zucker rats. Neuropharmacology 99: 481–490, 2015. doi: 10.1016/j.neuropharm.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]