Increased mucosal permeability in the gut is one of the major complications following severe burn. Here we report the novel finding that zinc finger DHHC domain-containing protein-21 (ZDHHC21) mediates gut epithelial hyperpermeability resulting from an experimental model of thermal injury. The hyperpermeability response was significantly attenuated with a pharmacological inhibitor of palmitoyl acyltransferases and in mice with genetic ablation of ZDHHC21. These findings suggest that ZDHHC21 may serve as a novel therapeutic target for treating burn-induced intestinal barrier dysfunction.
Keywords: palmitoylation, intestinal barrier function, epithelial permeability, zinc finger DHHC domain-containing protein, burn
Abstract
Clinical studies in burn patients demonstrate a close association between leaky guts and increased incidence or severity of sepsis and other complications. Severe thermal injury triggers intestinal inflammation that contributes to intestinal epithelial hyperpermeability, which exacerbates systemic response leading to multiple organ failure and sepsis. In this study, we identified a significant function of a particular palmitoyl acyltransferase, zinc finger DHHC domain-containing protein-21 (ZDHHC21), in mediating signaling events required for gut hyperpermeability induced by inflammation. Using quantitative PCR, we show that ZDHHC21 mRNA production was enhanced twofold when intestinal epithelial cells were treated with TNF-α-IFN-γ in vitro. In addition, pharmacological targeting of palmitoyl acyltransferases with 2-bromopalmitate (2-BP) showed significant improvement in TNF-α-IFN-γ-mediated epithelial barrier dysfunction by using electric cell-substrate impedance-sensing assays, as well as FITC-labeled dextran permeability assays. Using acyl-biotin exchange assay and click chemistry, we show that TNF-α-IFN-γ treatment of intestinal epithelial cells results in enhanced detection of total palmitoylated proteins and this response is inhibited by 2-BP. Using ZDHHC21-deficient mice or wild-type mice treated with 2-BP, we showed that mice with impaired ZDHHC21 expression or pharmacological inhibition resulted in attenuated intestinal barrier dysfunction caused by thermal injury. Moreover, hematoxylin and eosin staining of the small intestine, as well as transmission electron microscopy, showed that mice with genetic interruption of ZDHHC21 had attenuated villus structure disorganization associated with thermal injury-induced intestinal barrier damage. Taken together, these results suggest an important role of ZDHHC21 in mediating gut hyperpermeability resulting from thermal injury.
NEW & NOTEWORTHY Increased mucosal permeability in the gut is one of the major complications following severe burn. Here we report the novel finding that zinc finger DHHC domain-containing protein-21 (ZDHHC21) mediates gut epithelial hyperpermeability resulting from an experimental model of thermal injury. The hyperpermeability response was significantly attenuated with a pharmacological inhibitor of palmitoyl acyltransferases and in mice with genetic ablation of ZDHHC21. These findings suggest that ZDHHC21 may serve as a novel therapeutic target for treating burn-induced intestinal barrier dysfunction.
INTRODUCTION
Severe thermal injury [≥40% total body surface area (TBSA)] represents a significant clinical problem in the United States. Data from the US National Center for Injury Prevention and Control report that there are 1.2 million burn injuries per year with hospitalization required for 100,000 of those cases. Furthermore, over half of those cases result in lifelong symptoms or death. Not only does the actual burn itself impose deleterious effects, but also the systemic immune response elicited by the trauma can lead to mortality. More specifically, activation of the innate immune system increases plasma levels of a number of cytokines including TNF-α and IFN-γ (8), which are known to increase intestinal epithelial permeability. Upon clinical trauma, activation of the innate immune system leads to altered expression of signaling molecules that modify epithelial subcellular protein localization, activation, and tight junction integrity (9). As a result, burn patients that demonstrate poor intestinal barrier function are at high risk for bacterial translocation, sepsis, and mortality (2).
The intestinal epithelium represents one of the first lines of defense against bacterial translocation and sepsis. In fact, clinical studies have demonstrated a close association between intestinal barrier dysfunction and increased sepsis incidence and severity in addition to other complications (4, 27). There are ~500 different microbial species that populate the human gastrointestinal tract, some species reaching over 1 × 1010 colony-forming units per gram of luminal contents (20). Therefore increased epithelial permeability resulting from trauma can exacerbate the initial innate immune reactivity via allowing the penetration of microorganisms across the gut luminal barrier. It was reported that 60% of critically ill patients who suffer from multiple organ failure display increased intestinal barrier dysfunction (4).
Since integrity of the intestinal barrier represents a critical factor for the prognosis of burn patients, a number of key proteins that participate in mediating inflammation-driven intestinal hyperpermeability have been of interest as therapeutic targets. For example, IL-6 knockout mice had shown some improvement in the intestinal barrier following burn; however, bacterial translocation was not distinguishable from wild-type burned mice (25). Other studies showed that administering growth hormone to rats following thermal injury slightly improved villus structure but had no impact on intestinal barrier function or cellular mediators of thermal injury (14).
Interestingly, a recent study showed that inflammation-induced endothelial hyperpermeability resulted from increased palmitoyl acyltransferase (PAT) activity by the PAT zinc finger DHHC domain-containing protein-21 (ZDHHC21; 3). In that study, systemic inflammation resulted in increased total palmitoylated proteins in tissues harvested from mice experiencing systemic inflammatory response syndrome. Furthermore, inhibition of palmitoyl acyltransferase activity with 2-bromopalmitate (2-BP) prevented endothelial barrier dysfunction in response to inflammation in mice. Considering that the area of epithelial junction integrity being impacted by PATs in the context of systemic inflammation resulting from thermal injury has not been thoroughly explored, we aimed to determine whether PATs, and more specifically, ZDHHC21, play a role in thermal injury-induced epithelial barrier dysfunction in the gut.
METHODS
Animal procedures.
This study was approved by the Institutional Animal Care and Use Committee of University of South Florida, Morsani College of Medicine, and was performed in accordance with criteria outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male mice at 15–25 wk of age were anesthetized with an intramuscular injection of urethane at 1.75 g/kg. The right jugular vein and carotid artery were cannulated for infusion of drugs or solutions and continuous monitoring of blood pressure, respectively. A 40% total body surface full-thickness burn was induced over the shaved dorsal and ventral skin, as described previously (9). Briefly, an adjustable burn template that accounted for variation in body surface area was filled with boiling water and placed on the dorsal and ventral skin for 10 s each. Control groups were subjected to the same procedure with water at 37°C. After burn, animals were given a subcutaneous injection of lactated Ringer injection (Baxter Healthcare, Deerfield, IL) at 0.025 ml/g, according to the Parkland formula.
Intestinal mucosal permeability assay.
To measure actual flux of solute per square millimeter of intestinal surface area, the intestinal permeability assay was performed as previously described (9). Briefly, a midline laparotomy was performed on mice at 3 h after burn or sham treatment. A 5-cm segment of the jejunum beginning at 5 cm distal to the ligament of Treitz was dissected and tied with suture at both of the lateral ends. Two hundred microliters of FITC-labeled dextran (FD-4, mol wt 4,300, 125 mg/ml) in 0.1 M PBS (pH 7.2) were injected into the jejunum lumen and then returned to the abdominal cavity. After 30 min, blood specimens were collected by retro-orbital bleeding and then diluted in 50 mM Tris solution with 150 mM NaCl followed by centrifugation at 3,000 g for 10 min at 4°C. A standard curve of reference FITC-labeled dextran was used to determine plasma concentrations of FITC-labeled dextran using a fluorescence spectrophotometer.
Whole body systemic leakage assessment.
To evaluate overall gut barrier function in response to thermal injury in both wild-type (WT) mice with pharmacological palmitoylation inhibitor (2-BP) and ZDHHC21-ablated mice, we measured relative systemic leakage of FITC-labeled dextran via the Xenogen IVIS Spectrum scope system. Eight groups of animals (n = 3 in each group) were characterized as follows: C57BL/6, sham, burn, 2-BP, and 2-BP + burn; B6C3Fe [ZDHHC21 knockout (KO) background strain], burn and sham; and B6C3Fe with ZDHHC21 KO, burn and sham. Food was removed from cages 6 h before assay, and animals were administered 100 mg/ml FITC-labeled dextran to each mouse (44 mg/100 g body wt) by oral gavage. Indicated animals were preinjected with 2-BP followed by either 40% TBSA burning or sham burning as described above. After 3 h, isoflurane was delivered at a concentration of 4% in oxygen using a precision vaporizer for the initial induction and then maintained at 3% during the experiment.
Histology and severity scoring.
Twenty-four hours posttreatment, 10-cm sections of jejunum were cleaned with ice-cold PBS, fixed in 4% paraformaldehyde, and then coiled using the Swiss roll technique before processing for paraffin embedding. Lateral sections were cut at 5-μm thickness using a microtome and then affixed to slides for hematoxylin and eosin staining. Tissues were digitized using a Zeiss Axio Scan.Z1 slide scanner and scored with a scale from 0 to 4 (4 being most severe) on four criteria: intimal thickening, leukocyte infiltration to the lamina propria, villus length, and crypt structure, where 0 = normal, 1 = mild inflammation, 2 = moderate epithelial disruption, 3 = moderate inflammation with partial shortening of villus, and 4 = severe thickening of the lamina propria, villus disruption, and leukocyte infiltration (9). Scores were averaged among at least three animals per group, and representative images were selected for each treatment.
Cell culture.
Mouse intestinal epithelial cells (MIEpCs) from C57BL/6 mice were purchased from Cell Biologics and cultured according to the manufacturer’s instructions. Briefly, cells were plated on gelatin-coated plates and cultured with epithelial cell growth medium supplemented with insulin-transferrin-selenium, EGF, l-glutamine, penicillin/streptomycin/amphotericin B, and 5% fetal bovine serum at 37°C with 5% CO2-95% air in a humidified incubator. Cells between passages 4 and 7 were used for all experiments.
Electric cell-substrate impedance sensing.
MIEpC barrier function was determined as previously described (11, 12) by measuring cell-cell adhesive resistance to electric current using an electric cell-substrate impedance-sensing (ECIS) system (Applied Biophysics, Troy, NY). Briefly, 2 × 105 epithelial cells were seeded on ECIS electrode arrays precoated with collagen I. After cells reached 2 days postconfluence, the arrays were affixed to the ECIS system [1-V, 4,000-Hz alternating-current signal supplied through a 1-MΩ resistor to a constant-current source; in-phase voltage and out-of-phase voltage were recorded with ECMS 1.0 software (Cellular Engineering Technologies, Coralville, IA)]. ECIS tracings expressed as transepithelial electric resistance are normalized to plateaued resistance values, and comparisons were made between TNF-α-IFN-γ cocktail (100 ng/ml-500 U/ml)-treated and vehicle control (0.1% BSA in PBS)-treated MIEpC monolayers. Transepithelial electric resistance changes were recorded every 90 s. Results are representative of three independent experiments.
Transwell permeability assay.
FITC-labeled dextran (4 kDa) flux assays were conducted as previously described (10). MIEpCs (1 × 105 cells/ml) were plated on collagen I-coated polycarbonate 12-well transwell inserts (0.4-μM pore size; Corning) and allowed to reach 2 days postconfluence. The luminal (top) compartment was then treated with FITC-labeled dextran (4 kDa; 2.5 mg/ml) for 15 min, and 100-μl aliquots were obtained from each abluminal (bottom) compartment and assayed for FITC fluorescence. Samples were diluted 1:25 to yield fluorescence values within the standard curve. Permeability coefficients (Ps) were calculated using the formula Ps = [Ab]/t × 1/A × V/[Lu], where [Ab] is the abluminal concentration of FITC-labeled dextran (4 kDa), t is time in seconds for FITC-labeled dextran incubation, A is the area of the membrane in centimeters squared, V is the volume of the abluminal chamber, and [Lu] is the initial luminal concentration of FITC-labeled dextran. Results are representative of four experiments; *P < 0.05.
Quantitative polymerase chain reaction.
MIEpCs treated [cytokine cocktail, TNF-α (100 ng/ml)-IFN-γ (500 U/ml), or vehicle control, 0.1% BSA in PBS] at time points indicated in figure legends were lysed in RNAzol (Molecular Research Center) followed by RNA extraction as described by the manufacturer. The mRNA fraction was quantified, and equal masses of RNA were reverse transcribed to cDNA using random hexamers before quantitative PCR. Samples without reverse transcriptase (No RT) were run as negative control. Equal volumes of cDNA were used to quantify PTK6 message using specific primers (Bio-Rad) and SYBR master mix (Bio-Rad) according to the manufacturer’s instructions. Samples were assayed in triplicate, and No RT controls were used to ensure absence of genomic DNA (data not shown). Relative mRNA expression levels were determined using GAPDH as the housekeeping gene via CFX96 Touch Real-Time PCR detection system and software (Bio-Rad). Data are representative of four experiments; *P < 0.05.
Acyl-biotin exchange assay.
Acyl-biotin exchange assay was performed per recently developed protocols with modifications (17). MIEpCs were treated with inflammatory stimuli and then lysed with lysis buffer [50 mM Tris, 5 mM EDTA, 150 mM NaCl, 1% Nonidet P-40 (NP-40), 10% glycerol, 5 mM MgCl2, and a tablet of complete ULTRA protease inhibitor, pH 7.4] on ice. Lysates were centrifuged at 13,000 g for 10 min at 4°C. Supernatants were collected, and protein concentrations were determined using bicinchoninic acid assay. Proteins were precipitated using several volumes of ice-cold acetone for 30 min at −20°C, collected by centrifugation, and resuspended in 37°C 4% SDS buffer (4SB; 4% SDS, 5 mM EDTA, and 50 mM Tris, pH 7.4). The solution was diluted to 1% SDS using lysis buffer. Methyl methanethiosulfonate was added to a final concentration of 20 mM, and the solution was incubated for 30 min at 50°C. Proteins were washed by three sequential rounds of precipitation in ice-cold acetone followed by resuspension in 37°C 4SB. Proteins were diluted to 1% SDS and split. Both samples were treated with 1 mM biotin-p-hydroxyphenacyl diethyl phosphate (biotin-HPDP) for 60 min at room temperature. One sample was then treated with 1 M hydroxylamine (pH 7.4). The other half was treated with Tris buffer (pH 7.4) as control. Proteins were washed three times with acetone and resuspended in 4SB. The solution was diluted to 1 ml with dilution buffer (50 mM Tris, 150 mM NaCl, 5 mM EDTA, and 0.2% Triton X-100, pH 7.4). To each tube, 100 μl of prewashed streptavidin-conjugated agarose beads were added and rocked overnight at 4°C. The beads were washed three times with dilution buffer, and the captured proteins were eluted using 100-μl dilution buffer with 1% β-mercaptoethanol. Bicinchoninic acid assay was performed again, and 4× sample buffer with 2.5% β-mercaptoethanol was added to proteins. Samples were loaded into gels for subsequent Western blotting per standard protocols.
Statistical analysis.
Experiments were performed independently at least three times with experimental replicates within each assay. For experiments involving >2 groups, analysis of variance (ANOVA) was used to determine significant differences among groups, followed by post hoc Student’s t-test to determine significance between individual treatments. Significance was noted when P < 0.05. Error bars represent SE for all figures.
RESULTS
TNF-α-IFN-γ treatment of epithelial monolayers increases epithelial barrier dysfunction, and thermal injury induces intestinal epithelial structural damage in mice.
Before determining whether palmitoylation plays a role in barrier dysfunction resulting from the systemic inflammation induced by thermal injury, we first aimed to assess the physiological impact of treating mouse intestinal epithelial (MIEpC) monolayers with cytokines, TNF-α and IFN-γ known to be elevated as a result of thermal injury (8). Therefore we cultured MIEpCs to 2 days postconfluence, then treated with the cytokine cocktail (100 ng/ml TNF-α and 500 U/ml IFN-γ), and determined the change in permeability to FITC-labeled dextran (4 kDa) compared with monolayers treated with vehicle control. As shown in Fig. 1A, TNF-α-IFN-γ induced a 20% increase in permeability at the 3-h time point, and this was maintained up to 24 h. In addition to physiological assessment of cultured cells, we also compared electron micrographs of intestinal tissues from mice that were either subjected to thermal injury or sham treated. For these experiments, mice were subjected to 40% total body surface area (TBSA) thermal injury and then resuscitated and given pain management until death. Sections of small intestine (jejunum) were prepared for transmission electron microscopy and then imaged. As shown in Fig. 1B, animals that underwent sham conditions for thermal injury (left) demonstrate intact microvilli (red arrows) and joined cell-cell junctions (blue arrows). However, in intestinal tissues from mice subjected to thermal injury (right), epithelial microvilli are distorted and/or ablated (red arrows), and cell junctions are expanded (blue arrows) with passage of solute observed within the junction. These results indicate that both thermal injury in mice and TNF-α-IFN-γ treatment of epithelial monolayers induce epithelial barrier dysfunction.
Fig. 1.
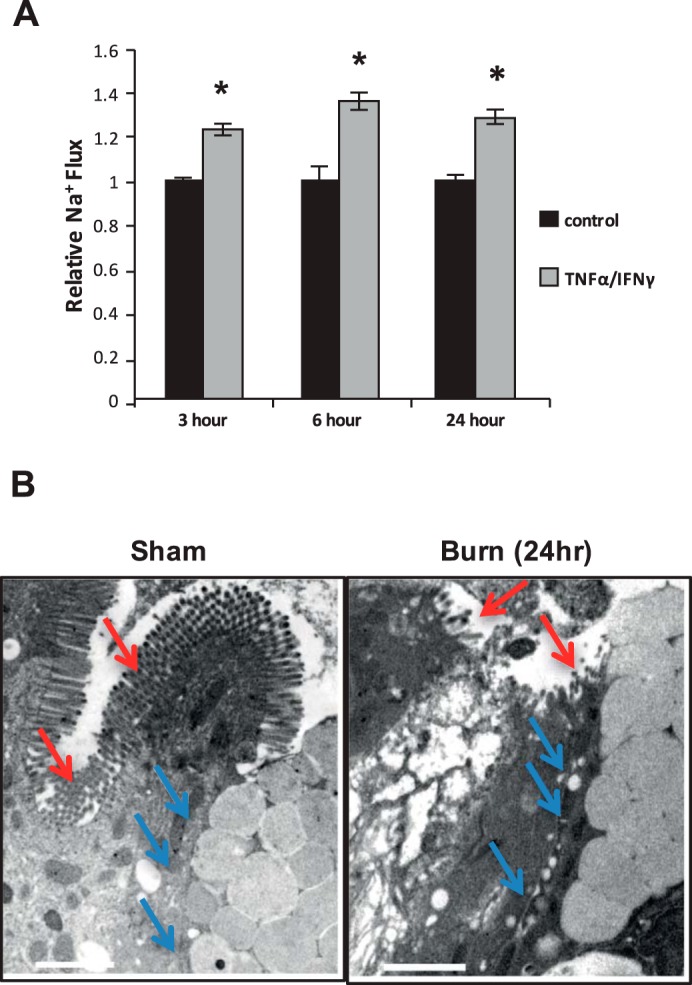
TNF-α-IFN-γ in vitro and thermal injury in vivo induce sustained barrier dysfunction for at least 24 h. A: mouse intestinal epithelial cells were grown to 2 days postconfluence on transwell inserts and then treated with TNF-α-IFN-γ for the indicated time points. FITC-labeled dextran (4 kDa; 2.5 mg/ml) was added to the luminal compartment, and abluminal samples were read on a fluorometer. B: electron micrographs of jejunum segments of animals that experienced sham burn (left) or 40% total body surface area thermal injury (right). Scale bars represent 2 µM. *P < 0.05 compared with control.
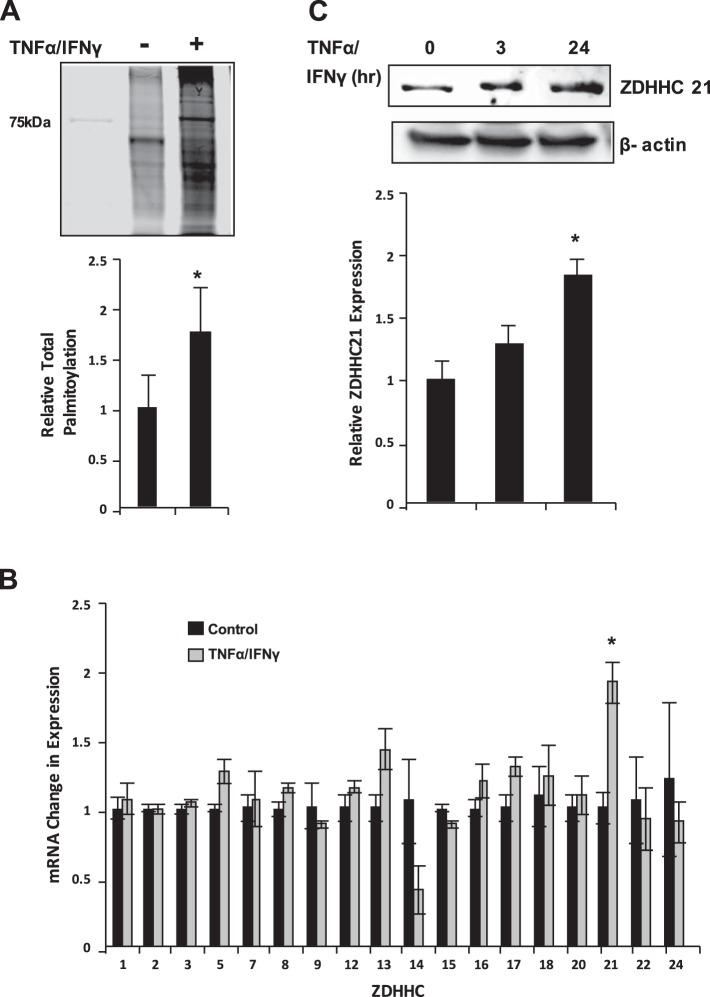
Palmitoylation and ZDHHC21 levels are elevated upon TNF-α-IFN-γ treatment.
Considering that recent studies indicate that ZDHHC play a significant role in inflammation-mediated endothelial barrier dysfunction resulting from systemic inflammatory response syndrome, we hypothesized that palmitoylation may be a critical event in epithelial barrier dysfunction in the gut resulting from severe thermal injury. Therefore we first conducted cell culture experiments to determine whether total protein palmitoylation was increased as a result of TNF-α-IFN-γ treatment. MIEpCs were treated with TNF-α-IFN-γ or vehicle control for 3 h, and palmitoylated proteins were harvested using acyl-biotin exchange assay. As shown in Fig. 2A, palmitoylated proteins were ~2-fold those of cells treated with vehicle control. Next, experiments were performed to identify which ZDHHC were expressed and/or transcriptionally regulated by TNF-α-IFN-γ. Figure 2B shows that ZDHHC21 mRNA increased twofold in response to inflammatory stimuli, followed by ZDHHC13 increase by ~40%. Similarly, as indicated in Fig. 2C, ZDHHC21 protein levels positively correlated with mRNA levels at the 3-h treatment mark and reached significance at 24-h TNF-α-IFN-γ treatment. These results suggest that ZDHHC21 is transcriptionally upregulated in response to inflammatory signals that induce epithelial barrier dysfunction.
Fig. 2.
TNF-α-IFN-γ induces barrier dysfunction and palmitoylation. A: mouse intestinal epithelial cells (MIEpCs) were treated with TNF-α-IFN-γ for 3 h and then underwent acyl-biotin exchange assay to identify total palmitoylation. B: MIEpCs were treated with TNF-α-IFN-γ for 3 h and then assayed for zinc finger DHHC domain-containing protein (ZDHHC) mRNA expression. Numbers on the x-axis represent the ZDHHC isoform detected. C: cells were treated as shown, and Western blotting was used to identify ZDHHC21 expression levels. *P < 0.05 compared with control.
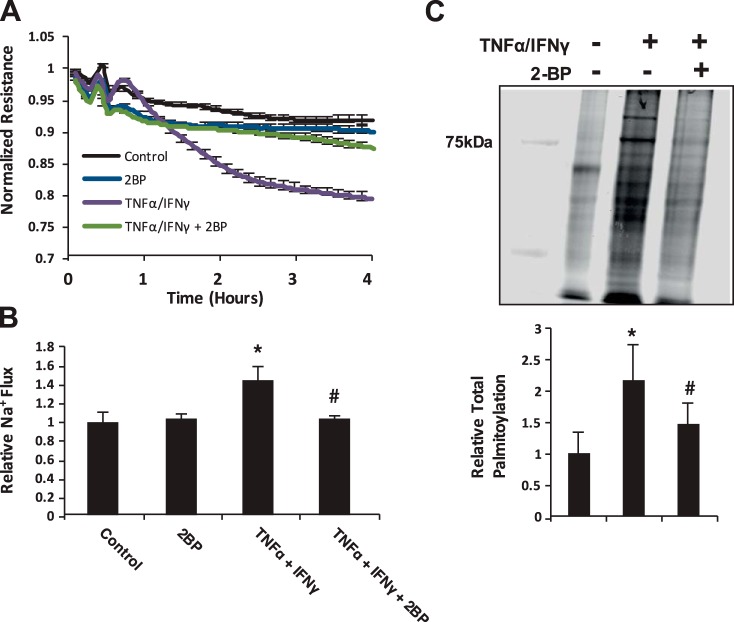
2-BP prevents epithelial barrier dysfunction and improves TNF-α-IFN-γ-mediated increase in total protein palmitoylation.
After identifying that TNF-α-IFN-γ increases total protein palmitoylation in intestinal epithelial cells, we next aimed to determine whether inhibition of total protein palmitoylation had an impact on improving barrier function. Therefore MIEpCs were grown to 2 days postconfluence on ECIS arrays, pretreated for 30 min with the general palmitoyl transferase inhibitor 2-BP (100 μM), and then treated with 100 ng/ml TNF-α-500 U/ml IFN-γ cocktail, and resistance measurements were recorded every 90 s. As shown in Fig. 3A, inhibition of palmitoylation improved barrier function similar to that of vehicle control-treated cells. Using permeability assays, results showed that 2-BP restored barrier function to control levels despite 3-h TNF-α-IFN-γ treatment (Fig. 3B). Other experiments showed that 2-BP indeed diminished levels of total protein palmitoylation by ~75% compared with monolayers treated with only TNF-α-IFN-γ for 3 h (Fig. 3C). This suggests that protein palmitoylation is an important factor in mediating epithelial permeability in response to TNF-α-IFN-γ.
Fig. 3.
2-Bromopalmitate (2-BP) prevents epithelial barrier dysfunction and improves TNF-α-IFN-γ-mediated increase in total protein palmitoylation. A: mouse intestinal epithelial cells (MIEpCs) were grown to 2 days postconfluence on electric cell-substrate impedance-sensing arrays and then treated with 100 ng/ml TNF-α and 500 U/ml IFN-γ; resistance measurements were recorded every 90 s. B: MIEpCs were grown to 2 days postconfluence on transwell inserts and then treated with TNF-α-IFN-γ for the indicated time points. FITC-labeled dextran (4 kDa; 2.5 mg/ml) was added to the luminal compartment, and abluminal samples were read on a fluorometer. C: MIEpCs were pretreated with vehicle control or 2-BP for 3 h and then TNF-α-IFN-γ for 24 h. Lysates then underwent acyl-biotin exchange assay to identify total palmitoylation. *P < 0.05 compared with control, #P < 0.05 compared with TNF-α-IFN-γ.
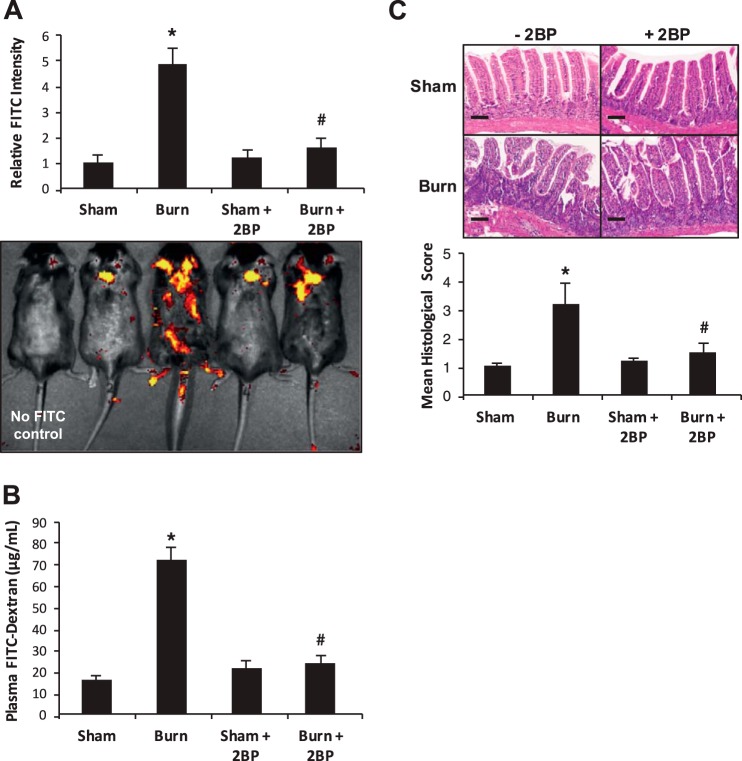
2-BP treatment attenuates burn-induced intestinal barrier dysfunction.
Considering the in vitro experiments indicating a role for ZDHHC21 in mediating inflammation-induced epithelial barrier dysfunction, we then aimed to determine whether 2-BP treatment improves intestinal barrier function following thermal injury in mice. Therefore WT mice were injected intraperitoneally with vehicle control or 2-BP (100 μM) 3 h before 40% TBSA thermal injury followed by assessment of intestinal permeability to 4-kDa FITC-labeled dextran using mucosal permeability assay and the Xenogen IVIS system for systemic leakage. As shown in Fig. 4A, thermal injury induced an ~5-fold increase in dissemination of fluorescent tracer; however, burned animals pretreated with 2-BP showed levels of dissemination similar to the sham group. Similarly, mucosal permeability assay showed that plasma from burned animals pretreated with 2-BP had a 60% reduction in detection of intestinally delivered 4-kDa FITC-labeled dextran relative to burned animals without palmitoylation inhibitor (Fig. 4B). To further characterize the impact of 2-BP in intestinal barrier dysfunction following thermal injury, we harvested segments of small intestine (jejunum) and processed tissues for hematoxylin and eosin staining. As shown in Fig. 4C, results indicated that burned animals show significant villus disorganization and infiltration of lymphocytes, yet the burned animals pretreated with 2-BP demonstrate improved villus organization and diminished lymphocyte infiltration to the lamina propria similar to that seen in sham animals. After utilizing a scoring system for quantitation as described in methods, burned 2-BP-treated animals showed diminished histological severity by ~2-fold (Fig. 4C, bottom). This suggests that 2-BP may have therapeutic potential in treating intestinal barrier dysfunction following thermal injury.
Fig. 4.
Inhibition of total palmitoylation improves intestinal hyperpermeability systemic leakage in vivo. Animals experienced 40% total body surface area thermal injury with or without intraperitoneal 2-bromopalmitate (2-BP) injection and then after 3 h underwent systemic leakage assay (A) and then mucosal permeability assay (B). C: following burn, jejunum sections from all treatment groups were processed for hematoxylin and eosin staining and then observed by microscopy and assigned histological scores as described in methods. Scale bars represent 50 μm. *P < 0.05 compared with sham, #P < 0.05 compared with burn.
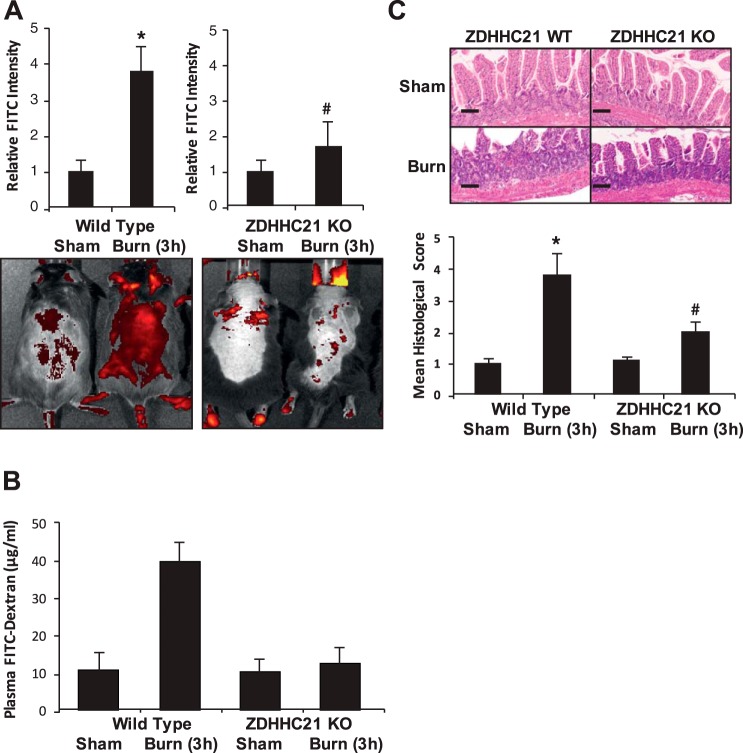
Genetic interruption of ZDHHC21-attenuated burn-induced gut hyperpermeability.
Considering that ZDHHC21 has played a role in mediating endothelial barrier dysfunction and our results indicated transcriptional regulation in response to TNF-α-IFN-γ, we pursued the hypothesis that mice with ZDHHC21 functional interruption would show improved intestinal barrier function compared with WT animals following thermal injury. Therefore B6C3Fe WT mice and B6C3Fe-ZDHHC21 functional deficiency mice were injected with vehicle control or 2-BP (100 μM) 3 h before sham or 40% TBSA thermal injury followed by assessment of intestinal permeability to 4-kDa FITC-labeled dextran using the Xenogen IVIS system for systemic leakage and mucosal permeability assay to determine intestine-to-blood flux rates. With regard to systemic leakage, WT animals subjected to thermal injury showed a fourfold increase in FITC-labeled dextran leakage, yet burned ZDHHC21 KO animals showed improved leakage comparable to that of sham animals (Fig. 5A). Similarly, mucosal permeability assays showed that burn in WT animals induced a fourfold increase in intestinal flux to 4-kDa FITC-labeled dextran, yet burned ZDHHC21 KO animals showed intestinal flux rates similar to those of sham (control) animals. To further characterize the impact of ZDHHC21 in intestinal barrier dysfunction following thermal injury, we harvested segments of small intestine (jejunum) from animals treated as shown in Fig. 5A and processed tissues for hematoxylin and eosin staining following thermal injury. As shown in Fig. 5C, the WT burned animals show significant villus disorganization and infiltration of leukocytes approximately twice as histologically severe as that seen in the burned ZDHHC21 KO animals. These results suggest that ZDHHC21 is involved in the intestinal barrier damage that occurs in response to thermal injury.
Fig. 5.
Transgenic mice null for zinc finger DHHC domain-containing protein-21 (ZDHHC21) expression show improved intestinal hyperpermeability and systemic leakage in vivo. Wild-type (WT) and ZDHHC21 knockout (KO) animals experienced 40% total body surface area thermal injury and then after 3 h underwent systemic leakage assay (A) and mucosal permeability assay (B). C: following burn, jejunum sections from all treatment groups were processed for hematoxylin and eosin staining and then observed by microscopy and assigned histological scores as described in methods. Scale bars represent 50 μm. *P < 0.05 compared with WT sham, #P < 0.05 compared with ZDHHC21 KO sham.
Electron micrographs show that KO mice have improved junction integrity following thermal injury compared with WT mice with the same treatment.
To gain a clearer understanding of the structural differences presented in mice demonstrating improved barrier function despite thermal injury, we harvested distal segments of small intestine from animals treated as shown in Fig. 5B and processed tissues for electron microscopy (Alcian Blue stain). As shown in Fig. 6, animals that underwent sham conditions for thermal injury (sham) demonstrate intact microvilli (red arrows) and joined cell-cell junctions (green arrows). However, after thermal injury (burn), epithelial microvilli are distorted and/or ablated (red arrows), and cell junctions are expanded (green arrows) with passage of solute observed within the junction. However, burned transgenic mice null for ZDHHC21 expression show improved junction connections and intact microvilli compared with the WT burned animals. These results suggest that ZDHHC21 expression plays a role in cell junction disruption that results from thermal injury.
Fig. 6.
Zinc finger DHHC domain-containing protein-21 (ZDHHC21) functional deficiency animals show minimal damage of junction architecture following burn. Animals with the indicated genotype were subjected to 40% total body surface area; following burn, sections of jejunum were processed for transmission electron microscopy. ZDHHC21 knockout (KO) animals (right) show improved villus structure, intact cell-cell junctions (green arrows), and enhanced microvillus integrity (red arrows) despite burn treatment compared with wild-type (WT) burned animals (left). Scale bar represents 1 μm.
DISCUSSION
Severe thermal injury is responsible for significant morbidity and mortality with an extremely high cost of care for patients. Despite recent advances in resuscitation and wound care, there have been no effective treatment modalities to control the edema process. Nearly 50% of extracellular fluid observed with a large burn is found in tissues remote from the wound (6), and the leak occurs rapidly within the first few hours postburn (6, 16). Since breakdown of the intestinal barrier is a critical event that contributes to the onset of systemic inflammation, systemic inflammatory response syndrome, and multiple organ failure (5, 7), a key therapeutic strategy to prevent such complications is to restore barrier function in the gut. In this study, we showed that 1) inhibition of palmitoylation improved epithelial barrier dysfunction resulting from TNF-α-IFN-γ; 2) ZDHHC21, but not other PATs, is transcriptionally responsive to inflammatory stimuli in intestinal epithelial cells; 3) mice with ZDHHC21 functional deficiency or WT mice treated with the global PAT inhibitor 2-BP show improved intestinal barrier function following thermal injury; and 4) minimal damage in epithelial junctional integrity is observed in ZDHHC21 functional deficiency mice despite subjection to thermal injury. We used cell culture as well as in vivo experiments to show that inhibition of protein palmitoylation significantly prevented intestinal epithelial barrier dysfunction due to thermal injury or inflammatory stimulation. In addition, we showed that the PAT ZDHHC21 is upregulated in response to TNF-α-IFN-γ treatment of intestinal epithelial cells, suggesting that ZDHHC21 specifically plays a role in inflammation-driven gut barrier dysfunction.
The role of palmitoylation as a posttranslational modification has largely been considered a mechanism for targeting nascent transmembrane proteins (such as claudins; 22) or proteins destined to be secreted (mucin; 23) to the plasma membrane. However, our results suggest that protein palmitoylation is a critical event that occurs in a manner consistent with signal transduction during thermal injury that results in the progression of inflammation and epithelial barrier dysfunction. Palmitoylation is a reversible posttranslational modification in which a 16-carbon saturated fatty acid is added to cysteine residues (15). This modification leads to rapid changes in protein translocation. Since many proteins that regulate permeability are commonly associated with the plasma membrane, it is not surprising that palmitoylation plays a role in epithelial hyperpermeability in response to inflammatory signals. For example, Akimzhanov and Boehning have shown that palmitoylated Lck, a tyrosine kinase, localized to lipid rafts and was required for Fas ligand-Fas receptor signaling (1). Furthermore, inhibiting Lck palmitoylation prevented tyrosine phosphorylation of its targets at the membrane (24) and attenuated the Fas ligand-mediated increase in intracellular calcium, caspase-3 activation, and subsequent apoptosis. In this context, targeting palmitoylation likely impairs the membrane microdomain targeting of kinases known to signal increased permeability via tyrosine phosphorylation of junction proteins resulting from inflammatory signals, leading to improved barrier function.
We also show that 2-BP treatment maintained the attenuation in barrier dysfunction seen with TNF-α-IFN-γ treatment of cultured cells in vitro at the 3- and 24-h time points, and these results were confirmed in vivo. This suggests that targeting palmitoylation may provide ongoing enhancement in barrier function long after the initial point of trauma. Furthermore, an improvement of barrier function was observed following 40% TBSA thermal injury in the ZDHHC21 KO animals vs. WT animals as well, which suggests that there is little, if any, redundancy in PAT substrates (at least in an inflammatory context) and supports the efficacy in targeting ZDHHC21 for purposes of enhancing the epithelial barrier during systemic inflammation.
The molecular dynamics surrounding the means by which palmitoylation as a posttranslational modification may impact signaling and/or permeability per se have not been clearly delineated thus far. It has been suggested that selectively increasing hydrophobicity of particular signaling molecules may serve as a selective process for recruiting particular signaling molecules to relevant lipid rafts required to transmit communication relative to specific extracellular cues (13, 18, 21). Specifically, it has been shown that kinases responsible for regulating tight junction integrity such as Lyn, Yes, and Fyn are palmitoylated in response to inflammatory signals, and this modification allows the required proximity for targeting tight junction proteins and increasing permeability of the epithelial barrier (19).
Considering that there are 23 PATs expressed in humans, it is likely that individual isotypes are responsible for specific functions. For example, it was shown that deletion of ZDHHC16 was lethal because of the impaired trafficking of signaling proteins during development; however, the targeted ZDHHC16 deletion in 9-wk embryos was not lethal, suggesting that ZDHHC16 plays a less critical role in development at later embryonic stages (26). Recently, a collaborative study in which we participated has reported a novel function of ZDHHC21 in mediating endothelial barrier dysfunction in inflammatory injury (3, 17); therefore we hypothesized that this PAT may also contribute to gut epithelial barrier dysfunction resulting from burn-induced systemic inflammation. Since we demonstrated that ZDHHC21, but not other PATs, was upregulated in epithelial cells stimulated with TNF-α-IFN-γ, it is logical that the total PAT inhibitor 2-BP would attenuate the inflammatory response without abrogating other mechanisms where palmitoylation plays a key role (such as cell development). Owing to the acute nature of the systemic inflammation and barrier dysfunction resulting from thermal injury, a global palmitoylation inhibitor may have utility for this type of short-term application. However, because of the broad spectrum of signaling mechanisms in which palmitoylation plays a key role, 2-BP could induce interruption of several cellular processes that may induce a cellular toxicity effect; therefore global palmitoylation inhibitor may not be a suitable long-term therapeutic option. Development of specific PAT inhibitors aimed to target ZDHHC21 may expand the utility of the therapeutic strategy not only to improve intestinal barrier function in the gut but also to minimize potential toxicity effects due to the inhibition of other PATs.
GRANTS
We acknowledge Veterans Affairs Merit Review IBX000799A and National Heart, Lung, and Blood Institute Grant 120954.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.H.W. conceived and designed research; R.J.H., C.Y.W., C.G.Y., R.A.E., and F.W. performed experiments; R.J.H., C.Y.W., C.G.Y., R.A.E., and F.W. analyzed data; R.J.H., C.Y.W., R.A.E., and M.H.W. interpreted results of experiments; R.J.H., C.Y.W., C.G.Y., and R.A.E. prepared figures; R.J.H. and C.Y.W. drafted manuscript; R.J.H. and M.H.W. edited and revised manuscript; R.J.H., C.Y.W., C.G.Y., R.A.E., F.W., and M.H.W. approved final version of manuscript.
REFERENCES
- 1.Akimzhanov AM, Boehning D. Rapid and transient palmitoylation of the tyrosine kinase Lck mediates Fas signaling. Proc Natl Acad Sci USA 112: 11876–11880, 2015. doi: 10.1073/pnas.1509929112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Ghoul WM, Khan M, Fazal N, Sayeed MM. Mechanisms of postburn intestinal barrier dysfunction in the rat: roles of epithelial cell renewal, E-cadherin, and neutrophil extravasation. Crit Care Med 32: 1730–1739, 2004. doi: 10.1097/01.CCM.0000132896.62368.01. [DOI] [PubMed] [Google Scholar]
- 3.Beard RS Jr, Yang X, Meegan JE, Overstreet JW, Yang CG, Elliott JA, Reynolds JJ, Cha BJ, Pivetti CD, Mitchell DA, Wu MH, Deschenes RJ, Yuan SY. Palmitoyl acyltransferase DHHC21 mediates endothelial dysfunction in systemic inflammatory response syndrome. Nat Commun 7: 12823, 2016. doi: 10.1038/ncomms12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De-Souza DA, Greene LJ. Intestinal permeability and systemic infections in critically ill patients: effect of glutamine. Crit Care Med 33: 1125–1135, 2005. doi: 10.1097/01.CCM.0000162680.52397.97. [DOI] [PubMed] [Google Scholar]
- 5.Deitch EA. Intestinal permeability is increased in burn patients shortly after injury. Surgery 107: 411–416, 1990. [PubMed] [Google Scholar]
- 6.Demling RH. The burn edema process: current concepts. J Burn Care Rehabil 26: 207–227, 2005. [PubMed] [Google Scholar]
- 7.Faries PL, Simon RJ, Martella AT, Lee MJ, Machiedo GW. Intestinal permeability correlates with severity of injury in trauma patients. J Trauma 44: 1031–1035, 1998. doi: 10.1097/00005373-199806000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Finnerty CC, Herndon DN, Przkora R, Pereira CT, Oliveira HM, Queiroz DM, Rocha AM, Jeschke MG. Cytokine expression profile over time in severely burned pediatric patients. Shock 26: 13–19, 2006. doi: 10.1097/01.shk.0000223120.26394.7d. [DOI] [PubMed] [Google Scholar]
- 9.Guo M, Yuan SY, Frederich BJ, Sun C, Shen Q, McLean DL, Wu MH. Role of non-muscle myosin light chain kinase in neutrophil-mediated intestinal barrier dysfunction during thermal injury. Shock 38: 436–443, 2012. doi: 10.1097/SHK.0b013e318268c731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haines RJ, Beard RS Jr, Chen L, Eitnier RA, Wu MH. Interleukin-1β mediates β-catenin-driven downregulation of claudin-3 and barrier dysfunction in CaCO2 cells. Dig Dis Sci 61: 2252–2261, 2016. doi: 10.1007/s10620-016-4145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haines RJ, Beard RS Jr, Eitner RA, Chen L, Wu MH. TNFα/IFNγ mediated intestinal epithelial barrier dysfunction is attenuated by microRNA-93 cownregulation of PTK6 in mouse colonic epithelial cells. PLoS One 11: e0154351, 2016. doi: 10.1371/journal.pone.0154351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haines RJ, Beard RS Jr, Wu MH. Protein tyrosine kinase 6 mediates TNFα-induced endothelial barrier dysfunction. Biochem Biophys Res Commun 456: 190–196, 2015. doi: 10.1016/j.bbrc.2014.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Head BP, Patel HH, Insel PA. Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim Biophys Acta 1838: 532–545, 2014. doi: 10.1016/j.bbamem.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeschke MG, Herndon DN, Finnerty CC, Bolder U, Thompson JC, Mueller U, Wolf SE, Przkora R. The effect of growth hormone on gut mucosal homeostasis and cellular mediators after severe trauma. J Surg Res 127: 183–189, 2005. doi: 10.1016/j.jss.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol 8: 74–84, 2007. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- 16.Lund T. The 1999 Everett Idris Evans memorial lecture. Edema generation following thermal injury: an update. J Burn Care Rehabil 20: 445–452, 1999. doi: 10.1097/00004630-199920060-00005. [DOI] [PubMed] [Google Scholar]
- 17.Marin EP, Derakhshan B, Lam TT, Davalos A, Sessa WC. Endothelial cell palmitoylproteomic identifies novel lipid-modified targets and potential substrates for protein acyl transferases. Circ Res 110: 1336–1344, 2012. doi: 10.1161/CIRCRESAHA.112.269514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Resh MD. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci STKE 2006: re14, 2006. doi: 10.1126/stke.3592006re14. [DOI] [PubMed] [Google Scholar]
- 19.Sato I, Obata Y, Kasahara K, Nakayama Y, Fukumoto Y, Yamasaki T, Yokoyama KK, Saito T, Yamaguchi N. Differential trafficking of Src, Lyn, Yes and Fyn is specified by the state of palmitoylation in the SH4 domain. J Cell Sci 122: 965–975, 2009. doi: 10.1242/jcs.034843. [DOI] [PubMed] [Google Scholar]
- 20.Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol 31: 107–133, 1977. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 21.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1: 31–39, 2000. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 22.Van Itallie CM, Gambling TM, Carson JL, Anderson JM. Palmitoylation of claudins is required for efficient tight-junction localization. J Cell Sci 118: 1427–1436, 2005. doi: 10.1242/jcs.01735. [DOI] [PubMed] [Google Scholar]
- 23.Wei X, Yang Z, Rey FE, Ridaura VK, Davidson NO, Gordon JI, Semenkovich CF. Fatty acid synthase modulates intestinal barrier function through palmitoylation of mucin 2. Cell Host Microbe 11: 140–152, 2012. doi: 10.1016/j.chom.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yurchak LK, Sefton BM. Palmitoylation of either Cys-3 or Cys-5 is required for the biological activity of the Lck tyrosine protein kinase. Mol Cell Biol 15: 6914–6922, 1995. doi: 10.1128/MCB.15.12.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zahs A, Bird MD, Ramirez L, Choudhry MA, Kovacs EJ. Anti-IL-6 antibody treatment but not IL-6 knockout improves intestinal barrier function and reduces inflammation after binge ethanol exposure and burn injury. Shock 39: 373–379, 2013. doi: 10.1097/SHK.0b013e318289d6c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou T, Li J, Zhao P, Liu H, Jia D, Jia H, He L, Cang Y, Boast S, Chen YH, Thibault H, Scherrer-Crosbie M, Goff SP, Li B. Palmitoyl acyltransferase Aph2 in cardiac function and the development of cardiomyopathy. Proc Natl Acad Sci U S A 112: 15666–15671, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ziegler TR, Smith RJ, O’Dwyer ST, Demling RH, Wilmore DW. Increased intestinal permeability associated with infection in burn patients. Arch Surg 123: 1313–1319, 1988. doi: 10.1001/archsurg.1988.01400350027003. [DOI] [PubMed] [Google Scholar]