Our findings show for the first time that RFVT3 is a target for posttranscriptional regulation by miR-423-5p in intestinal epithelial cells, and this regulation has functional consequences for intestinal riboflavin (RF) uptake process.
Keywords: riboflavin, SLC52A3, microRNA, transport, posttranscriptional regulation, intestinal epithelia
Abstract
Riboflavin (RF) is essential for normal cellular functions and health. Humans obtain RF from exogenous sources via intestinal absorption that involves a highly specific carrier-mediated process. We have recently established that the riboflavin transporter-3 (RFVT3) is vital for the normal intestinal RF uptake process and have characterized certain aspects of its transcriptional regulation. Little is known, however, about how this transporter is regulated at the posttranscriptional level. We address this issue by focusing on the role of microRNAs. Using bioinformatics, we identified two potential interacting miRNAs with the human (h) RFVT3-3′-UTR, and showed (using pmirGLO-hRFVT3-3′-UTR) that the hRFVT3-3′-UTR is, indeed, a target for miRNA effect. Of the two putative miRNAs identified, miR-423-5p was found to be highly expressed in intestinal epithelial cells and that its mimic affected luciferase reporter activity of the pmirGLO-hRFVT3-3′-UTR construct, and also led to inhibition in RF uptake by intestinal epithelial Caco-2 and HuTu-80 cells. Furthermore, cells transfected with mutated seed sequences for miR-423-5p showed an abrogation in inhibitory effect of the miR-423-5p mimic on luciferase activity. While miR-423-5p did not affect the level of expression of the hRFVT3 mRNA, it did lead to a significant inhibition in the level of expression of its protein. Similarly, miR-423-5p was found to affect the level of expression of the mouse RFVT3 in cultured intestinal enteroids. These findings demonstrate, for the first time, that the RFVT3 is a target for posttranscriptional regulation by miRNAs in intestinal epithelial cells and that this regulation has functional consequences on intestinal RF uptake.
NEW & NOTEWORTHY Our findings show for the first time that RFVT3 is a target for posttranscriptional regulation by miR-423-5p in intestinal epithelial cells, and this regulation has functional consequences on intestinal riboflavin (RF) uptake process.
INTRODUCTION
Riboflavin (RF; vitamin B2) is indispensable for normal human health, given that it plays a key role in cellular metabolism, proliferation, growth, and survival. In its coenzyme forms, i.e., riboflavin-5′-phosphate and flavin adenosine dinucleotide, RF plays key metabolic roles in biological oxidation-reduction reactions involving lipid, carbohydrate, and amino acid metabolism, as well as in the conversion of vitamin B6 and folate into their active forms (5). Recent studies have also found additional metabolic roles for RF by demonstrating its antioxidant properties and the ability for it to reduce cellular oxidative stress (13, 33, 36). In addition, RF has been found to have powerful anti-inflammatory properties, which allow it to assume an important role in normal immune function (20, 22, 35) and plays a role in maintaining normal intestinal homeostasis (24). Furthermore, a role for this vitamin in protein folding in the endoplasmic reticulum has been identified (47). Thus, it is not surprising that disturbances in normal RF body homeostasis leads to negative health consequences that include degenerative changes in the nervous system, anemia, and growth retardation (5, 28); it is also a risk factor for esophageal squamous cell carcinoma and gastric cardia adenocarcinoma (50). The incidence of RF deficiency or suboptimal levels of RF occurs in a variety of conditions, including chronic alcoholism (2, 21, 25, 26), inflammatory bowel diseases (7, 16), inborn errors of RF metabolism (3, 8, 11, 14, 38), and diabetes mellitus (15).
Humans (mammals) cannot synthesize RF and, thus, must obtain the vitamin from exogenous sources via intestinal absorption. The intestine, therefore, plays a central role in regulating normal body homeostasis of the vitamin. The intestinal tract is exposed to two sources of RF: a dietary and a bacterial source. Previous studies from our laboratory and others have established that the small intestinal and colonic RF absorption processes are specific and carrier-mediated (reviewed in Refs. 28 and 29). It has also been shown that all three specific RF transporters (RFVT1, RFVT2, and RFVT3; products of the SLC52A1, SLC52A2, and SLC52A3 genes, respectively) that have been identified in human/mammals (reviewed in Ref. 51), are expressed in the gut, with expression of RFVT3 being predominant (51). Live-cell confocal imaging studies from our laboratory have localized the different cellular compartments/domains at which these transporters are expressed (39). Expression of RFVT3 was found to be restricted to the apical membrane domain of the polarized absorptive epithelial cells, that of RFVT1 found to be mainly at the basolateral membrane domain, and that of RFVT2 (considered to be brain-specific; 51 and references therein) being mostly confined to intracellular vesicular structures (39–41). More recent investigations from our laboratory have utilized an intestine-specific conditional RFVT3 knockout mouse model (42) and an in vitro gene-specific silencing approach (siRNA; 39) to establish an essential role for RFVT3 in the normal intestinal RF absorption process.
Knowledge regarding how the intestinal RF absorption process is regulated has also been forthcoming. It has been shown that the intestinal RF uptake process undergoes differentiation-dependent regulation (43) and that it is adaptively regulated by the prevailing substrate level (44). In addition, aspects of the transcriptional regulation of the intestinal RF uptake process have been delineated in recent years with the cloning and characterization (both in vitro and in vivo) of the 5′-regulatory regions (promoters) of the SLC52A1 and SLC52A3 genes (9, 27). There is, however, little known about the posttranscriptional regulation of the intestinal RF absorption process, especially in regard to the potential role of microRNAs (miRNAs) in the regulatory process. Roles for these small noncoding RNAs in posttranscriptional regulation of a variety of genes in different cellular systems have been well established in recent years (1, 6, 12, 18, 34 and references therein). microRNAs bind to specific sites in the 3′-UTR of their target mRNAs leading to suppression in their translation and/or their degradation (1, 6, 12, 18, 34 and references therein). Our aim in this study was to investigate the potential role of miRNAs in posttranscriptional regulation of the predominant intestinal RF uptake system (RFVT3) and the intestinal RF uptake process. We employed human intestinal epithelial Caco-2 and HuTu-80 cells, as well as mouse intestinal enteroids in our investigations. The results showed that miR-423-5p (which is conserved in humans, rats, and mouse) interacts with the 3′-UTR region of the hRFVT3 system leading to a decrease in translational efficiency, which, in turn, resulted in reduction in intestinal RF uptake.
MATERIALS AND METHODS
Materials.
Caco-2 and HuTu-80 cells were obtained from American Type Culture Collection (Manassas, VA). 3H-RF (specific activity 12.5 Ci/mmol; radiochemical purity >97%) was purchased from Moravek Biochemicals (Brea, CA). All other cell culture reagents, solutions, specific primers for quantitative PCR amplifications, and microRNA mimics used in this study were purchased from Sigma Genosys (Woodlands, TX). TaqMan probes and primers for quantitating microRNAs were procured from Applied Biosystems (Foster City, CA).
Cloning of 3'-UTR of SLC52A3 (hRFVT3).
A 946-bp full-length fragment of the 3′-UTR-hRFVT3 was amplified from human intestinal cDNA (Clontech, Mountain View, CA) by PCR and purified using Wizard SV gel extraction kit (Promega, Madison, WI). Purified PCR product was cloned into the multiple cloning site of pmirGLO dual-luciferase miRNA target expression vector (Promega). The primer sequences flanked by SalI and XhoI sites used for PCR amplification are presented in Table 1. The resulting ligated product (pmirGLO-3′-UTR-hRFVT3) was transformed into the competent Escherichia coli (JM109; Promega), single colonies were picked from ampicillin agar plates, and plasmid DNA was prepared using a midiprep kit (Promega). Final fused pmirGLO-3′-UTR-hRFVT3 plasmid was confirmed by DNA sequencing (Laragen, Culver City, CA).
Table 1.
List of primers used in the study
| Primer | Sequence |
|---|---|
| 3′-UTR-hRFVT3 in pmirGLO vector | Forward: 5′-CCGCTCGAGCTGCACTGTCCAGCCTAG-3′ |
| Reverse: 5′-CGCGTCGACAAGCAGGGAATGCCT-3′ | |
| Real-time PCR primers hRFVT3 | |
| h-RFVT3-RT | Forward: 5′-CTGGTCTGCGTCTTCGGAATG-3′ |
| Reverse: 5′-ACCACCGTGAGGTAGGAGG-3′ | |
| m-RFVT3-RT | Forward: 5′-GGATCAGTGGAAGCCAGTG-3′ |
| Reverse: 5′-GACCTGTTAGGCAGGAAGATG-3′ | |
| hβ-actin-RT | Forward: 5′-CATCCTGCGTCTGGACCT-3′ |
| Reverse: 5′-TAATGTCACGCACGATTTCC-3′ | |
| mβ-actin-RT | Forward: 5′-ATCCTCTTCCTCCCTGGA-3′ |
| Reverse: 5′-TTCATGGATGCCACAGGA-3′ | |
| Mutant primers | Forward: 5′-GCCCCTGCCCTCTCTCCTTTACTAATCAGTCTCACAGAGGG-3′ |
| Reverse:5′-CCCTCTGTGAGACTGATTAGTAAAGGAGAGAGGGCAGGGGC-3′ |
Restriction sites are underlined, and mutated sites are in boldface italics.
Bioinformatic analyses.
The commonly used prediction algorithms TargetScan (http://www.targetscan.org/) and miRDB (http://mirdb.org/miRDB/) were used to identify the putative miRNAs targeting 3′-UTR-hRFVT3 (SLC52A3) (1, 10, 34, 40, 48). The putative potential miRNAs were chosen on the basis of their target/free energy scores and maximum “conservedness” across the human, rat, and mouse, as well as their abundant expression in the intestine.
Cell culture, transient transfection, and assay of firefly luciferase activity.
Human intestinal epithelial Caco-2 and HuTu-80 cells were grown in EMEM medium supplemented with 10% FBS, penicillin, and streptomycin at 37°C in 5% CO2-95% air environment in T-75 plastic flasks. Caco-2 and HuTu-80 cells grown in 12-well plates were transiently transfected with 1.5 µg of pmirGLO-3′-UTR-hRFVT3 alone or in combination with 50 nM of different miRNA mimics or negative control miRNA mimic (Sigma, St. Louis, MO). For transfecting miRNA mimics or negative control mimic along with 3′-UTR-hRFVT3/pmirGLO vector, we used Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA) with both of the cell lines. For transfecting mimic or negative control miRNA alone, however, we used Lipofectamine-RNAiMax reagent (Invitrogen) to achieve optimal transfection efficiency. In all transfection assays, Caco-2 and HuTu-80 cells grown up to 70–80% confluence in serum-free EMEM media were used. Forty-eight hours posttransfection, cells were lysed in passive lysis buffer (Promega), and luciferase activity was determined using the dual-luciferase assay kit (Promega) in a GLOMAX 20/20 luminometer (Promega). The 3′-UTR activity on the luciferase reporter gene was calculated as a ratio of firefly luciferase to Renilla luciferase and is expressed as a percentage of control in relative luciferase units.
Site-directed mutagenesis.
Site-directed mutations were carried out in the miR-423-5p binding site of 3′-UTR-hRFVT3 using the Quick-change XL site-directed mutagenesis kit (Agilent, La Jolla, CA) following the manufacturer’s instructions. Desired mutations were verified by direct DNA sequencing (Laragen). The primer sets used for site-directed mutagenesis are listed in Table 1.
Riboflavin uptake.
In the in vitro studies, confluent Caco-2 and HuTu-80 monolayers were used, and an initial rate (5 min) of RF uptake was examined, as described by us previously (39–41, 43). In brief, cells were incubated in Krebs-Ringer buffer (in mM: 133 NaCl, 4.93 KCl, 1.23 MgSO4, 0.85 CaCl2, 5 glucose, 5 glutamine, 10 HEPES, and 10 MES, pH 7.4) at 37°C in the presence of 3H-RF (25 nM). At the end of incubation, buffer was removed, and cells were washed twice with ice-cold Krebs-Ringer buffer, lysed using 1 N NaOH (followed by neutralization with 10 N HCl), and then counted for radioactivity in a liquid scintillation counter (Beckman Coulter). RF uptake by the carrier-mediated process was determined by subtracting uptake of 3H-RF in the presence of 1 mM unlabeled RF from total uptake (i.e., from uptake in the absence of unlabeled RF).
RNA extraction and real-time PCR.
To quantitate the RFVT3 mRNA expression level, total RNA from Caco-2 and HuTu-80 cells and mouse intestinal enteroids was extracted with Qiazol using RNeasy mini kit from Qiagen (Germantown, MD), according to the manufacturer’s instructions. Two micrograms of the total RNA was reverse transcribed to cDNA using i-Script reverse transcriptase kit (Bio-Rad, Hercules, CA), and then used for quantitative real-time PCR using specific primers (Table 1) in a CFX96 real-time i-cycler (Bio-Rad). After initial denaturation at 95°C for 5 min, the amplification program was repeated 45 times (95°C with a 30-s hold, 55°C with 15-s hold, followed by 72°C with 30-s hold for extension and fluorescence measurement). The mRNA levels of RFVT3 were normalized relative to the internal reference gene, β-actin.
Quantification of mature miRNA expression.
For quantitating the mature miRNA expression, total RNA was isolated using miRNeasy mini kit (Qiagen). Ten nanograms of total RNA was reverse transcribed per reaction to produce cDNA using the TaqMan microRNA reverse transcription kit (Applied Biosystems, Foster City, CA) following the manufacturer’s instructions. RT primers for cDNA synthesis were provided with TaqMan miRNA assay kit (Applied Biosystems). Mature miRNA levels (for miR-338-3p, miR-423-5p, and miR-760) in Caco-2, HuTu-80 cells, and mouse jejunum were quantified using TaqMan probes in Universal PCR master mix (Applied Biosystems). Constitutively expressed RNU6B miRNA was used as a reference gene.
Western blot analysis.
After transfection with miRNA mimics (48 h, 50 nM), cells were washed with ice-cold 1 × PBS and cell lysis buffer (101 Bio, Palo Alto, CA), and 1× protease cocktail inhibitor mixture (Roche, Indianapolis, IN) was added. The cells were lysed by vortexing, and the plasma membrane fraction was isolated following manufacturer’s instructions. Mouse intestinal enteroids were lysed in RIPA buffer (Sigma) after transduced with lenti-mir-423-5p or negative control. Protein concentrations were determined by using Dc protein assay kit (Bio-Rad), and the samples were frozen at −80°C until further use. To examine the level of RFVT3 protein, 40 µg of soluble membrane fraction was loaded on 4–12% premade gels (Invitrogen) and transferred to polyvinylidene difluoride (PVDF) membranes. Odyssey blocking buffer was used as a blocking buffer (LI-COR Biosciences, Lincoln, NE) for 1 h. The membranes were then probed with anti-hRFVT3 [1:200 dilution; rabbit polyclonal antipeptide antibodies were raised against peptides CEAPLSHLESRYLPAHFSP and LRLFSSADFCNLHCPA (which corresponds with 202–220 and 453–469, respectively) against the hRFVT3 peptide sequence (Alfa Diagnostic, San Antonio, TX)] and anti-Na+/K+ ATPase antibodies (1:50,000 dilution; Abcam, Cambridge, MA) in Odyssey blocking buffer (LI-COR) overnight at 4°C. For detecting mRFVT3 protein expression, the blot was probed with mRFVT3 polyclonal antibodies (1:500 dilution) (GeneTex, Irvine, CA). The specificity of the antibody was established before (42). The membranes were washed three times with the wash buffer containing 1 × PBS and 0.01% Tween-20 for 5 min each. PVDF membranes were then probed with corresponding secondary antibodies (LI-COR Biosciences) in 1:25,000 dilutions for 1 h at room temperature; then bands were visualized using Odyssey application software (version 3.0) in an Odyssey Infrared imaging system (LI-COR Biosciences). To confirm specificity of anti-RFVT3 antibody, an antibody and antigenic peptide (1:100) mixture was preincubated in PBS overnight, and then the PVDF membrane was probed with the mixture overnight. Membranes were further processed as described above.
Mouse intestinal enteroids preparation and lentivirus-mediated transfection.
For ex vivo studies, we used 10-wk-old male C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) to generate intestinal enteroids, as described previously (30–32). Mice were euthanized, and the proximal jejunum was removed and then washed with ice-cold PBS and inverted using a glass rod, with the inner lining of the intestinal tract facing out. The unwanted villus portion was scraped, and the remaining jejunal tissue was then cut into ~5-mm fragments, which go through a series of PBS washes to remove villus cells. Then, the EDTA (2.5 mM) solution was added to the tissue and placed in a high-speed rocker for 30 min. The unwanted EDTA supernatant was removed from the tissue fragments, and fresh ice-cold PBS was added. After vortexing the tissue fragments, the supernatant of fractions 2 and 3 was collected and passed through a 70-μm nylon filter (Fisher Scientific, Hampton, NH) to enrich the crypt cells. Each fraction spun down at 200 g for 5 min at 4°C to produce a pellet ideally comprising crypt cells. To culture the crypt cells, the pellet was resuspended in Matrigel (Corning, Tewksbury, MA). The crypt cell-Matrigel mixture from a single fraction was then evenly distributed in a 48-well tissue culture plate, that is 25 μl per well, and left to harden for 20 min in an incubator at 37°C. The Matrigel buttons were then submerged in 250 μl of enteroid medium, which contains a cocktail of growth factors as described before (30–32). To validate the quality of enteroids, the relative mRNA expression of mature epithelial cell markers villin, chromogranin A, and mucin 2 were measured in fresh mucosa of the mouse small intestine and enteroids. The results showed that the enteroids expressed similar levels of these markers as fresh mucosa (17, 31) (data not shown). The animal protocol was approved by the Long Beach-Veterans Affairs Medical Center Institutional Animal Care and Use Committee. The enteroids were transduced with lenti-miR-423-5p or lenti-miR-control (Biosettia, San Diego, CA) for 48 h, and total RNA and cell lysate were prepared for quantification of mouse RFVT3 mRNA and protein expression levels.
Statistical analyses.
Carrier-mediated RF uptake data presented in this study are expressed as means ± SE of multiple separate experimental determinations. Uptake was expressed as a percentage relative to simultaneously performed controls. Statistical significance was determined using the Student’s t-test with statistical significance set at less than 0.05. Firefly-luciferase assay, Western blot, and RT-PCR analyses are expressed as means ± SE of at least three to five independent experiments.
RESULTS
Prediction of putative miRNA target sites in the 3′-UTR-hRFVT3.
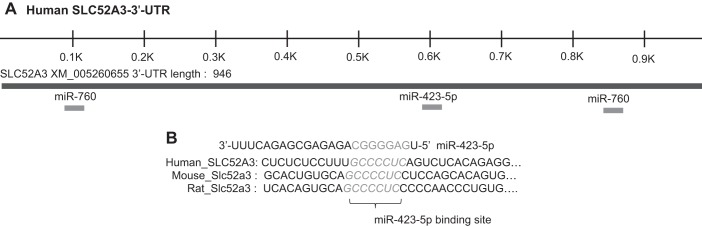
To explore possible involvement of posttranscriptional regulation of human RFVT3 via involvement of miRNAs, we subjected the 3′-UTR of hRFVT3 to commonly used bioinformatic analysis [miRDB and TargetScan programs (1, 10, 34, 48, 49)]. The analyses identify two potential interacting miRNAs based on their target and free energy scores, as well as their conservedness (among humans, rats, and mice). These two miRNAs are miR-423-5p and miR-760 (Fig. 1A). Figure 1B shows the conservation of putative miR-423-5p binding site within the human, rat, and mouse RFVT3s.
Fig. 1.
A: hRFVT3 3′-UTR and location of predicted microRNA (miRNA) seed sites. The figure is based on http://www.targetscan.org and http://mirdb.org/miRDB. B: conservation of the putative bind site of miR-423-5p in the RFVT3 3′-UTR of three mammalian species.
Demonstration that the 3′-UTR-hRFVT3 is a target for miRNAs interaction(s) in human intestinal epithelial cells.
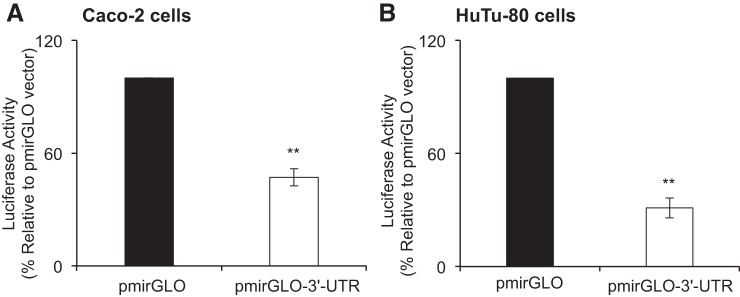
To directly demonstrate that the 3′-UTR of the hRFVT3 is a target for miRNA in intestinal epithelial cells, we cloned the 3′-UTR (946-bp) into a pmirGLO dual-luciferase miRNA target expression vector and then expressed the construct (pmirGLO-3′-UTR-hRFVT3) in two established models of human-derived intestinal epithelial cells Caco-2 and HuTu-80. The results showed a significant decrease (P < 0.01) in luciferase activity in Caco-2 (>50%) and HuTu-80 (>65%) cells that express the pmirGLO-3′-UTR-hRFVT3 compared with cells transfected with an empty vector (Fig. 2, A and B). These findings suggest that interaction may exist between certain miRNAs and the 3′-UTR region of hRFVT3.
Fig. 2.
Effect of transient transfection with pmirGLO-3′-UTR-hRFVT3 on luciferase activity. Caco-2 (A) and HuTu-80 (B) cells were transiently transfected with pmirGLO vector or the pmirGLO-3′-UTR-hRFVT3 construct, and then harvested and lysed in passive lysis buffer. Luciferase activities were measured and normalized with basal Renilla luciferase activities. The empty pmirGLO vector was set to 100% for luciferase activity. Data are expressed as means ± SE of 3–5 independent experiments (**P < 0.01).
Expression of the putative interacting miRNAs in human intestinal epithelial cells.
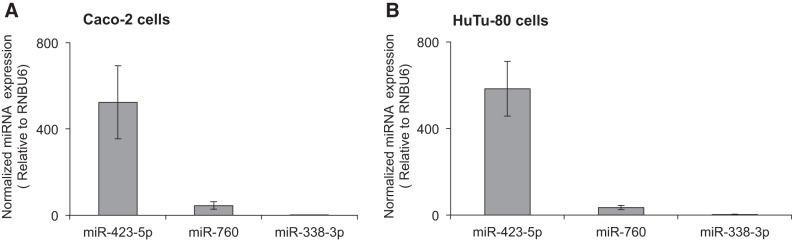
Because expression of miRNAs is tissue-specific and that their abundance in a given tissue may influence their level of involvement in posttranscriptional regulation of genes (19, 23, 37), we determined the level of expression of the two putative miRNAs that were identified as potential interactors with the 3′-UTR of hRFVT3 (miR-423-5p and miR-760) in intestinal epithelial cells using quantitative PCR [in this experiment, we also determined the level of expression of a microRNA (miR-338–3p) predicted to be outside the 3′-UTR region of hRFVT3 for comparison]. The results showed a high level of expression of miR-423-5p compared with the other two putative miRNAs (miR-760 and miR-338-3p) in both Caco-2 and HuTu-80 cells (Fig. 3, A and B). Thus, we focused our subsequent investigations on the role of miR-423-5p in posttranscriptional regulation of SLC52A3.
Fig. 3.
Expression levels of mature miRNAs in Caco-2 (A) and HuTu-80 (B) cells. MicroRNA levels were measured using TaqMan microRNA assay system, as described in materials and methods. Values of miRNA expression levels were normalized against constitutively expressed RNU6B miRNA. Data are expressed as means ± SE of 3–5 independent experiments.
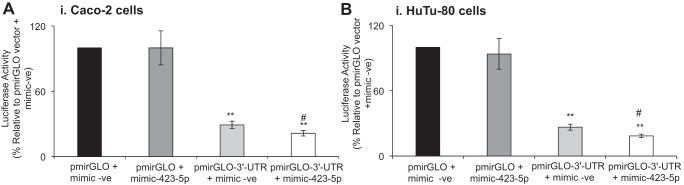
Effect of miR-423-5p mimics on luciferase reporter activity of the pmirGLO-3′-UTR-hRFVT3 construct.
In this study, we examined the effect of transfecting miR-423-5p mimic together with the pmirGLO-3′-UTR-hRFVT3 construct to determine whether the two, indeed, interact directly in intestinal epithelial cells. The results (Fig. 4) showed that when mimic-423-5p was transfected alone (no RFVT3–3′-UTR), there was no change in luciferase activity compared with negative control mimic (cells transfected with the empty vector, pmirGLO+mimic-423-5p). On the other hand, transfecting the intestinal Caco-2 and HuTu-80 cells with the mimic-423-5p (together with 3′-UTR-hRFVT3) led to a further inhibition in luciferase activity compared with cells transfected with the 3′-UTR-hRFVT3 alone (Fig. 4, A and B). These results suggest that miR-423-5p has a role in regulating hRFVT3 expression in Caco-2 and HuTu-80 cells.
Fig. 4.
Effect of transient cotransfection of mimic-423-5p with pmirGLO-3′-UTR-hRFVT3 on luciferase activity Caco-2 (A) and HuTu-80 (B) cells. Cells were transiently cotransfected with pmirGLO-vector or the pmirGLO-3'-UTR-hRFVT3 construct along with miRNA mimic (negative control mimic, mimic-423-5p, 50 nM, 48 h) and were assayed for luciferase activity, as described in materials and methods. Data are expressed as means ± SE of three independent experiments. #pmirGLO-3'-UTR-hRFVT3+mimic-423-5p is significantly decreased in the luciferase activity compared with pmirGLO-3'-UTR-hRFVT3+mimic-ve (**P < 0.01).
Effect of mutating the miR-423-5p putative site in the 3′-UTR-hRFVT3 on luciferase activity.
To further validate the interaction between miR-423-5p and its putative site in the 3′-UTR-hRFVT3 region, we mutated the interacting site, then transfected the mutated construct together with miR-423-5p mimic into Caco-2 cells and examined luciferase activity. The results (Fig. 5) showed abrogation in the inhibitory effect of the miR-423-5p mimic on luciferase activity. These findings show that the miR-423-5p putative site in the 3′-UTR-hRFVT3 has a role in the interaction between this miRNA and the 3′-UTR- hRFVT3.
Fig. 5.
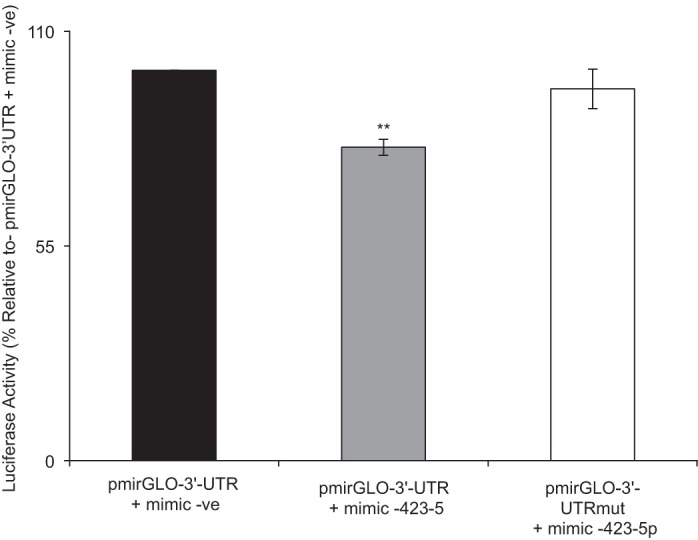
Effect of mutating the miR-423-5p putative site in the 3′-UTR-hRFVT3 on luciferase activity in Caco-2 cells. Cells were cotransfected with miR-423-5p mimic and pmirGLO-3′-UTR-hRFVT3 or mutated construct (pmirGLO-3′-UTR-hRFVT3). Luciferase activities were measured and normalized with respective Renilla luciferase activities. Data are expressed as means ± SE of at least three independent experiments (**P < 0.01).
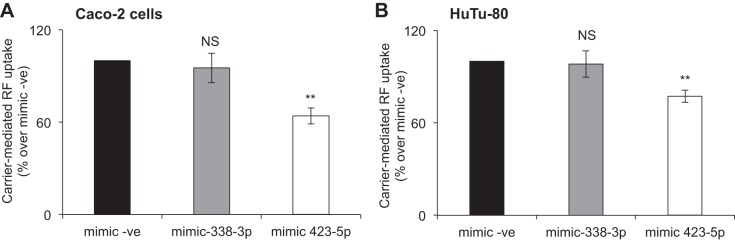
Effect of miRNA mimic on carrier-mediated RF uptake by intestinal epithelial cells.
In this investigation, we examined the effect of miR-423-5p on the functionality of hRFVT3 (the predominant RF uptake system in the gut; 39, 51) in Caco-2 and HuTu-80 cells. For this, we transfected Caco-2 and HuTu-80 cells with miR-423-5p mimic (50 nM) and then examined initial rate of 3H-RF uptake 48 h later. The results showed a significant (P < 0.01) inhibition in RF uptake in cells transfected with the miR-423-5p mimic compared with those transfected with negative control mimic (Fig. 6) [of relevance here is that transfecting cells with mimic for miR-338-3p showed no effect in RF uptake; Fig. 6, A and B]. These findings show that the interaction between miR-423-5p and the 3′-UTR-hRFVT3 has functional consequence on the overall intestinal RF uptake process.
Fig. 6.
Effect of transient transfection with miRNA mimics on carrier-mediated RF uptake in Caco-2 (A) and HuTu-80 (B) cells. Carrier-mediated RF uptake (% over mimic -ve) was measured as described in materials and methods after transfecting with miRNA mimic (negative control mimic, mimic-338-3p, or mimic-423-5p; 50 nM, 48 h). Data are expressed as means ± SE of 3–5 independent experiments (**P < 0. 01). NS, not significant.
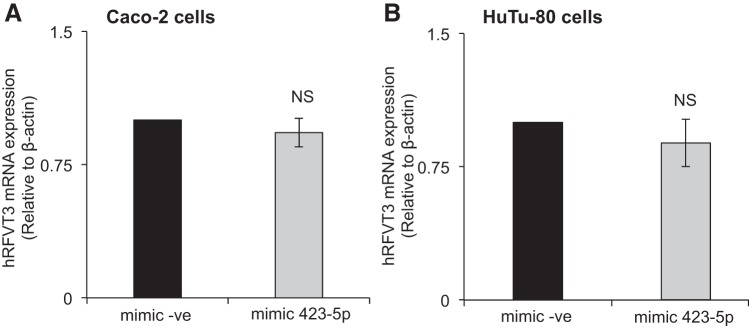
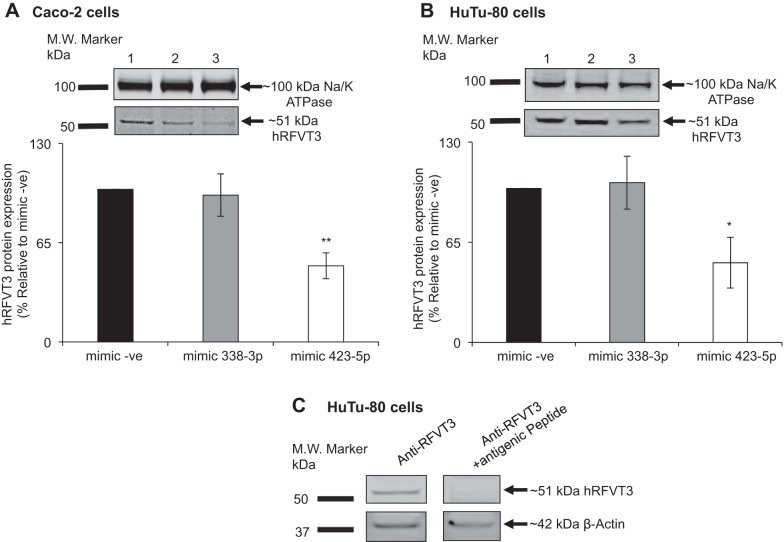
Effect of miR-423-5p on level of expression of the hRFVT3 mRNA and protein.
Because miRNAs affect gene expression via altering mRNA stability and/or its translational efficiency, we examined in this study the effect of miR-423-5p on the level of expression of hRFVT3 mRNA and protein in intestinal epithelial Caco-2 and HuTu-80 cells. Although no effect for miR-423-5p mimic was observed on the level of expression of hRFVT3 mRNA (Fig. 7, A and B), a significant (P < 0.01 for Caco-2, and P < 0.05 for HuTu-80 cells; Fig. 8, A and B, respectively) reduction in the level of expression of the hRFVT3 protein was observed in both cell types transfected with the miR-423-5p mimic [validity of the anti-hRFVT3 polyclonal antibodies was established using antigenic peptide as shown in Fig. 8C] [again transfecting cells with the miR-338-3p mimic did not affect the level of expression of the hRFVT3 protein; Fig. 8, A and B].
Fig. 7.
Effect of miR-423-5p mimic transfection on hRFVT3 mRNA expression levels. Relative hRFVT3 mRNA expression levels in Caco-2 (A) and HuTu-80 (B) cells; total RNA was extracted from cells transfected with mimic (negative control mimic or mimic-423-5p); then hRFVT3 mRNA levels were measured by quantitative real-time PCR as described in materials and methods. Values of mRNA levels for hRFVT3 were normalized against hβ-actin mRNA levels. Data are expressed as means ± SE of 3–5 independent experiments.
Fig. 8.
Effect of miR-423-5p mimic transfection on level of hRFVT3 protein expression. Relative hRFVT3 protein expression in Caco-2 (A) and HuTu-80 (B) cells. Plasma membrane fractions isolated from cells transiently transfected with mimic (negative control mimic, mimic-338-3p or mimic-423-5p) were loaded onto 4–12% Tris-Bis acrylamide premade gel, followed by transfer to PVDF membrane. Blots were processed as described in materials and methods. Lanes 1, 2, and 3 represent protein samples from negative control mimic, mimic-338-3p, and mimic-423-5p, respectively. C: Western blot results show specificity of anti-hRFVT3 antibody in HuTu-80 cells. The primary hRFVT3 antibody was probed with antigenic peptide (1:100) as described in materials and methods. Data are expressed as means ± SE of 3–5 different experiments (*P < 0.05; **P < 0.01).
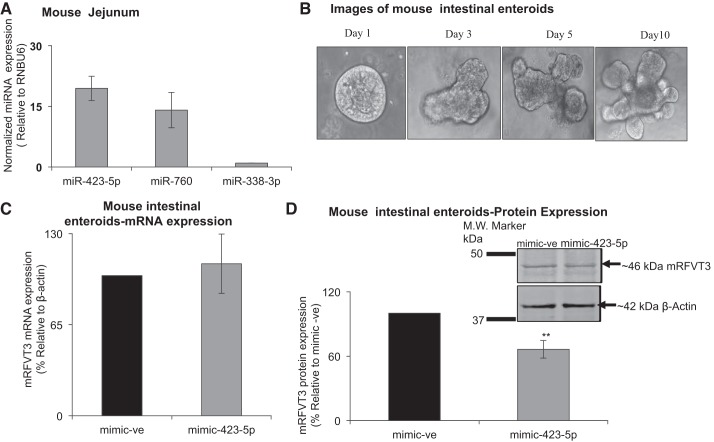
Effect of miR-423-5p on level of expression of RFVT3 in mouse intestine.
The mouse is a suitable animal model for studying different aspects of intestinal RF uptake process (42). Also, the mRFVT3 3′-UTR region contains putative binding site for miR-423-5p (Fig. 1B). Thus, we aimed in this study at determining whether miRNA-423-5p also exert posttranscriptional regulation on mRFVT3 in native intestinal epithelial cells. For this, we used intestinal enteroids derived from mouse jejunum, and we investigated the expression of miR-423-5p and miR-760 (we also determined the level of expression of miR-338-3p for comparison). The results again showed a significantly higher level of expression of miR-423-5p compared with the other miRNAs (Fig. 9A). Images of (1–10 days old) enteroids are depicted in Fig. 9B. Focusing on miRNA-423-5p, we examined the effect of transducing this miRNA into enteroids on the level of expression of mRFVT3. The results showed that while the level of mRFVT3 mRNA is not changed (Fig. 9C), a significant change occurred in the level of mRFVT3 protein in enteroids treated with mimic-423-5p compared with those transduced with negative control mimic (Fig. 9D). These results suggest that miR-423-5p has a role in posttranscriptional regulation of mRFVT3 in the native intestine, thus establishing physiological relevance to the findings with the cell culture models used.
Fig. 9.
Functional studies in mouse intestinal enteroids. A: MicroRNA expression level in mouse jejunum was determined as described in Fig. 3 legend. B: images of (1–10 days old) mouse intestinal enteroids. C: relative mRFVT3 mRNA expression levels in postmimic-423-5p treatment (48 h) using lentivirus. D: relative mRFVT3 protein expression levels in postmimic-423-5p treatment using lentivirus (48 h) (**P < 0. 01).
DISCUSSION
The physiologically and clinically important hRFVT3 plays a key role in RF absorption in the gut (3, 4, 39, 51). Studies from our laboratory and others have characterized different physiological, cellular, and molecular aspects of this important membrane transporter, including how the system is regulated at the transcriptional level, cell biology/membrane trafficking of the carrier protein, and how different conditions affect its expression (e.g., substrate availability/level, cell differentiation) (9, 27, 39, 41, 43–46). However, little is known about possible regulation of the hRFVT3 at the posttranscriptional level in the intestine or any other tissue. With the recent demonstration that microRNAs play important roles in posttranscriptional regulation of a variety of genes (including those involved in nutrient transport; 1, 6, 19, 23, 34, 37), we sought to investigate the potential role(s) of this epigenetic mechanism in posttranscriptional regulation of hRFVT3 in intestinal epithelia cells. We focus on this RF transporter because it plays a key role in the intestinal RF absorption process, as shown recently by our group using in vitro (gene-silencing approach; 39) and in vivo (use of intestine-specific RFVT3 knockout mouse model; 42) approaches. To achieve the goals of the current study, we subjected the 3′-UTR of the hRFVT3 to bioinformatics analysis and were able to identify two putative and conserved interacting miRNAs (i.e., miR-423-5p and miR-760). To directly demonstrate that the 3′-UTR of hRFVT3 is a target for miRNA interaction in intestinal epithelial cells, we then cloned and expressed the construct in Caco-2 and HuTu-80 cells. The results showed a significant decrease in luciferase activity in cells transfected with the pmirGLO-3′-UTR-hRFVT3, suggesting that the 3′-UTR region of the hRFVT3 is a potential target for microRNA effect.
Focusing on the two-predicted putative hRFVT3 interacting microRNAs, we first determined the level of their expression in intestinal epithelial cells and found that the level of miR-423-5p to be highest compared with the level of the other predicted miRNAs (i.e., miR-760 and miR-338-3p). Focusing on miR-423-5p, we then examined the effect of expressing miR-423-5p mimic along with pmirGLO-3′-UTR-hRFVT3 plasmid on luciferase activity and found a significant decrease in activity. This finding suggests that miR-423-5p may interact with the 3′-UTR-hRFVT3 in intestinal cells. In contrast to the effect of expressing miR-423-5p mimic with pmirGLO-3′-UTR-hRFVT3 on luciferase activity, transfecting a mutated 3′-UTR-hRFVT3 construct with miR-423-5p mimic abrogated the inhibitory effect of the miR-423-5p mimic on luciferase activity. The latter finding further confirms the interaction of miR-423-5p with its predicted site in the 3′-UTR-hRFVT3.
To determine whether the interaction between miR-423-5p and the 3′-UTR-hRFVT3 has a functional consequence, we examined the effect of transfecting Caco-2 and HuTu-80 cells with miR-423-5p mimic on initial rate of RF uptake. The results showed a significant inhibition in RF uptake in miR-423-5p transfected cells. This effect of miR-423-5p on RF uptake cannot be attributed to an effect of this microRNA on the other two RF transporters that are also expressed in the intestine (i.e., RFVT1, which is expressed at the BLM and RFVT2, which is mainly expressed intracellularly; 39, 40), as subjecting the 3′-UTR of these two other RF transporters to bioinformatics analysis did not identify any putative binding site for miR-423-5p in their 3′-UTR regions.
The effect of miR-423-5p mimic on RF uptake was not mediated via its effect on the level of expression of the hRFVT3 mRNA, as shown by the real-time PCR results, but rather it was found to be mediated via a significant decrease in the level of expression of the hRFVT3 protein in the intestinal epithelial cells. The latter findings suggest that miR-423-5p decrease RFVT3 expression by inhibiting the translation of the RFVT3 mRNA and not its stability. In related investigations, we extended our findings on the role of miR-423-5p in posttranscriptional regulation of RFVT3 expression with the intestinal cell lines to the native intestinal environment. For that, we used the recently established approach of mouse enteroids (30–32) and transfected these enteroids with miR-423-5p. Again, a significant decrease in mRFVT3 protein, but not mRNA, expression was observed in miR-423-5p-treated preparations.
In summary, our findings demonstrate that the RFVT3 is a target for posttranscriptional regulation by miR-423-5p in intestinal epithelial cells, and this regulation has functional consequences on intestinal RF uptake process.
GRANTS
This study was supported by grants from the Department of Veterans Affairs and the National Institutes of Health Grants DK-58057, DK-56057, and AA-018071 (to H. M. Said) and DK-107474 (to V. S. Subramanian).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.L., V.S.S., and H.M.S. conceived and designed research; R.L. and V.S.S. performed experiments; R.L. and V.S.S. analyzed data; R.L., V.S.S., and H.M.S. interpreted results of experiments; R.L. and V.S.S. prepared figures; R.L., V.S.S., and H.M.S. drafted manuscript; R.L., V.S.S., and H.M.S. edited and revised manuscript; R.L., V.S.S., and H.M.S. approved final version of manuscript.
REFERENCES
- 1.Anbazhagan AN, Priyamvada S, Kumar A, Maher DB, Borthakur A, Alrefai WA, Malakooti J, Kwon JH, Dudeja PK. Translational repression of SLC26A3 by miR-494 in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 306: G123–G131, 2014. doi: 10.1152/ajpgi.00222.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonjour JP. Vitamins and alcoholism. V. Riboflavin, VI. Niacin, VII. Pantothenic acid, and VIII. Biotin. Int J Vitam Nutr Res 50: 425–440, 1980. [PubMed] [Google Scholar]
- 3.Bosch AM, Abeling NG, Ijlst L, Knoester H, van der Pol WL, Stroomer AE, Wanders RJ, Visser G, Wijburg FA, Duran M, Waterham HR. Brown-Vialetto-Van Laere and Fazio Londe syndrome is associated with a riboflavin transporter defect mimicking mild MADD: a new inborn error of metabolism with potential treatment. J Inherit Metab Dis 34: 159–164, 2011. doi: 10.1007/s10545-010-9242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosch AM, Stroek K, Abeling NG, Waterham HR, Ijlst L, Wanders RJ. The Brown-Vialetto-Van Laere and Fazio Londe syndrome revisited: natural history, genetics, treatment and future perspectives. Orphanet J Rare Dis 7: 83, 2012. doi: 10.1186/1750-1172-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooperman JM, Lopez R. Riboflavin. In: Handbook of Vitamins: Nutritional, Biochemical and Clinical Aspects. New York: Dekker, 1984, p. 299–327. [Google Scholar]
- 6.Dalmasso G, Nguyen HT, Yan Y, Laroui H, Charania MA, Obertone TS, Sitaraman SV, Merlin D. MicroRNA-92b regulates expression of the oligopeptide transporter PepT1 in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 300: G52–G59, 2011. doi: 10.1152/ajpgi.00394.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-Banares F, Abad-Lacruz A, Xiol X, Gine JJ, Dolz C, Cabre E, Esteve M, Gonzalez-Huix F, Gassull MA. Vitamin status in patients with inflammatory bowel disease. Am J Gastroenterol 84: 744–748, 1989. [PubMed] [Google Scholar]
- 8.Green P, Wiseman M, Crow YJ, Houlden H, Riphagen S, Lin JP, Raymond FL, Childs AM, Sheridan E, Edwards S, Josifova DJ. Brown-Vialetto-Van Laere syndrome, a ponto-bulbar palsy with deafness, is caused by mutations in c20orf54. Am J Hum Genet 86: 485–489, 2010. doi: 10.1016/j.ajhg.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosal A, Sabui S, Said HM. Identification and characterization of the minimal 5′-regulatory region of the human riboflavin transporter-3 (SLC52A3) in intestinal epithelial cells. Am J Physiol Cell Physiol 308: C189–C196, 2015. doi: 10.1152/ajpcell.00342.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell 27: 91–105, 2007. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho G, Yonezawa A, Masuda S, Inui K, Sim KG, Carpenter K, Olsen RK, Mitchell JJ, Rhead WJ, Peters G, Christodoulou J. Maternal riboflavin deficiency, resulting in transient neonatal-onset glutaric aciduria Type 2, is caused by a microdeletion in the riboflavin transporter gene GPR172B. Hum Mutat 32: E1976–E1984, 2011. doi: 10.1002/humu.21399. [DOI] [PubMed] [Google Scholar]
- 12.Ikemura K, Yamamoto M, Miyazaki S, Mizutani H, Iwamoto T, Okuda M. MicroRNA-145 post-transcriptionally regulates the expression and function of P-glycoprotein in intestinal epithelial cells. Mol Pharmacol 83: 399–405, 2013. doi: 10.1124/mol.112.081844. [DOI] [PubMed] [Google Scholar]
- 13.Iwanaga K, Hasegawa T, Hultquist DE, Harada H, Yoshikawa Y, Yanamadala S, Liao H, Visovatti SH, Pinsky DJ. Riboflavin-mediated reduction of oxidant injury, rejection, and vasculopathy after cardiac allotransplantation. Transplantation 83: 747–753, 2007. doi: 10.1097/01.tp.0000256283.06469.d4. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JO, Gibbs JR, Megarbane A, Urtizberea JA, Hernandez DG, Foley AR, Arepalli S, Pandraud A, Simón-Sánchez J, Clayton P, Reilly MM, Muntoni F, Abramzon Y, Houlden H, Singleton AB. Exome sequencing reveals riboflavin transporter mutations as a cause of motor neuron disease. Brain 135: 2875–2882, 2012. doi: 10.1093/brain/aws161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kodentsova VM, Vrzhesinskaia OA, Trofimenko EV, Sokol’nikov AA, Beketova NA, Blazheevich NV, Isaeva VA, Aleĭnik SI, Trofimenko LS, Dronova VI, Spirichev VB. [Vitamin status of children with diabetes mellitus] (in Russian). Vopr Med Khim 40: 45–48, 1994. [PubMed] [Google Scholar]
- 16.Kuroki F, Iida M, Tominaga M, Matsumoto T, Hirakawa K, Sugiyama S, Fujishima M. Multiple vitamin status in Crohn’s disease. Correlation with disease activity. Dig Dis Sci 38: 1614–1618, 1993. doi: 10.1007/BF01303168. [DOI] [PubMed] [Google Scholar]
- 17.Lei NY, Jabaji Z, Wang J, Joshi VS, Brinkley GJ, Khalil H, Wang F, Jaroszewicz A, Pellegrini M, Li L, Lewis M, Stelzner M, Dunn JC, Martín MG. Intestinal subepithelial myofibroblasts support the growth of intestinal epithelial stem cells. PLoS One 9: e84651, 2014. doi: 10.1371/journal.pone.0084651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z, Rana TM. Therapeutic targeting of microRNAs: current status and future challenges. Nat Rev Drug Discov 13: 622–638, 2014. doi: 10.1038/nrd4359. [DOI] [PubMed] [Google Scholar]
- 19.Liang Y, Ridzon D, Wong L, Chen C. Characterization of microRNA expression profiles in normal human tissues. BMC Genomics 8: 166, 2007. doi: 10.1186/1471-2164-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu D, Zempleni J. Low activity of LSD1 elicits a pro-inflammatory gene expression profile in riboflavin-deficient human T Lymphoma Jurkat cells. Genes Nutr 9: 422, 2014. doi: 10.1007/s12263-014-0422-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majumdar SK, Shaw GK, Thomson AD. Vitamin utilization status in chronic alcoholics. Int J Vitam Nutr Res 51: 54–58, 1981. [PubMed] [Google Scholar]
- 22.Mazur-Bialy AI, Buchala B, Plytycz B. Riboflavin deprivation inhibits macrophage viability and activity—a study on the RAW 264.7 cell line. Br J Nutr 110: 509–514, 2013. doi: 10.1017/S0007114512005351. [DOI] [PubMed] [Google Scholar]
- 23.Nabokina SM, Ramos MB, Said HM. Mechanism(s) involved in the colon-specific expression of the thiamine pyrophosphate (TPP) transporter. PLoS One 11: e0149255, 2016. [Erratum in PLoS One 12: e01865502017, 2017]. doi: 10.1371/journal.pone.0149255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakano E, Mushtaq S, Heath PR, Lee ES, Bury JP, Riley SA, Powers HJ, Corfe BM. Riboflavin depletion impairs cell proliferation in adult human duodenum: identification of potential effectors. Dig Dis Sci 56: 1007–1019, 2011. doi: 10.1007/s10620-010-1374-3. [DOI] [PubMed] [Google Scholar]
- 25.Neville JN, Eagles JA, Samson G, Olson RE. Nutritional status of alcoholics. Am J Clin Nutr 21: 1329–1340, 1968. [DOI] [PubMed] [Google Scholar]
- 26.Rosenthal WS, Adham NF, Lopez R, Cooperman JM. Riboflavin deficiency in complicated chronic alcoholism. Am J Clin Nutr 26: 858–860, 1973. [DOI] [PubMed] [Google Scholar]
- 27.Sabui S, Ghosal A, Said HM. Identification and characterization of 5′-flanking region of the human riboflavin transporter 1 gene (SLC52A1). Gene 553: 49–56, 2014. doi: 10.1016/j.gene.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Said HM. Intestinal absorption of water-soluble vitamins in health and disease. Biochem J 437: 357–372, 2011. doi: 10.1042/BJ20110326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Said HM, Trebble T. Intestinal digestion and absorption of micronutrients. In: Slesenger and Fordtran GI and Liver Disease (10th ed.), edited by Feldman M, Friedman L, Brandt L. New York: Elsevier, 2015, p. 1765–1788. [Google Scholar]
- 30.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265, 2009. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 31.Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141: 1762–1772, 2011. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 32.Sato T, Clevers H. Primary mouse small intestinal epithelial cell cultures. Methods Mol Biol 945: 319–328, 2013. doi: 10.1007/978-1-62703-125-7_19. [DOI] [PubMed] [Google Scholar]
- 33.Sanches SC, Ramalho LN, Mendes-Braz M, Terra VA, Cecchini R, Augusto MJ, Ramalho FS. Riboflavin (vitamin B2) reduces hepatocellular injury following liver ischaemia and reperfusion in mice. Food Chem Toxicol 67: 65–71, 2014. doi: 10.1016/j.fct.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Sangani R, Periyasamy-Thandavan S, Kolhe R, Bhattacharyya MH, Chutkan N, Hunter M, Isales C, Hamrick M, Hill WD, Fulzele S. MicroRNAs-141 and 200a regulate the SVCT2 transporter in bone marrow stromal cells. Mol Cell Endocrinol 410: 19–26, 2015. doi: 10.1016/j.mce.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schramm M, Wiegmann K, Schramm S, Gluschko A, Herb M, Utermöhlen O, Krönke M. Riboflavin (vitamin B2) deficiency impairs NADPH oxidase 2 (Nox2) priming and defense against Listeria monocytogenes. Eur J Immunol 44: 728–741, 2014. doi: 10.1002/eji.201343940. [DOI] [PubMed] [Google Scholar]
- 36.Seekamp A, Hultquist DE, Till GO. Protection by vitamin B2 against oxidant-mediated acute lung injury. Inflammation 23: 449–460, 1999. doi: 10.1023/A:1021965026580. [DOI] [PubMed] [Google Scholar]
- 37.Sood P, Krek A, Zavolan M, Macino G, Rajewsky N. Cell-type-specific signatures of microRNAs on target mRNA expression. Proc Natl Acad Sci USA 103: 2746–2751, 2006. doi: 10.1073/pnas.0511045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srour M, Putorti ML, Schwartzentruber J, Bolduc V, Shevell MI, Poulin C, O’ferrall E, Buhas D, Majewski J, Brais B. Mutations in riboflavin transporter present with severe sensory loss and deafness in childhood. Muscle Nerve 50: 775–779, 2014. doi: 10.1002/mus.24224. [DOI] [PubMed] [Google Scholar]
- 39.Subramanian VS, Subramanya SB, Rapp L, Marchant JS, Ma TY, Said HM. Differential expression of human riboflavin transporters-1, -2, and -3 in polarized epithelia: a key role for hRFT-2 in intestinal riboflavin uptake. Biochim Biophys Acta 1808: 3016–3021, 2011. doi: 10.1016/j.bbamem.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subramanian VS, Kapadia R, Ghosal A, Said HM. Identification of residues/sequences in the human riboflavin transporter-2 that is important for function and cell biology Nutr Metab (Lond) 12: 13, 2015. doi: 10.1186/s12986-015-0008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subramanian VS, Rapp L, Marchant JS, Said HM. Role of cysteine residues in cell surface expression of the human riboflavin transporter-2 (hRFT2) in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 301: G100–G109, 2011. doi: 10.1152/ajpgi.00120.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subramanian VS, Lambrecht N, Lytle C, Said HM. Conditional (intestinal-specific) knockout of the riboflavin transporter-3 (RFVT-3) impairs riboflavin absorption. Am J Physiol Gastrointest Liver Physiol 310: G285–G293, 2016. doi: 10.1152/ajpgi.00340.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Subramanian VS, Ghosal A, Subramanya SB, Lytle C, Said HM. Differentiation-dependent regulation of intestinal vitamin B2 uptake: studies utilizing human-derived epithelial Caco-2 cells and native rat intestine. Am J Physiol Gastrointest Liver Physiol 304: G741–G748, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Subramanian VS, Ghosal A, Kapadia R, Nabokina SM, Said HM. Molecular mechanisms mediating the adaptive regulation of intestinal riboflavin uptake process. PLoS One 10: e0131698, 2015. doi: 10.1371/journal.pone.0131698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Subramanian VS, Sabui S, Teafatiller T, Bohl JA, Said HM. Structure/functional aspects of the human riboflavin transporter-3 (SLC52A3): role of the predicted glycosylation and substrate-interacting sites. Am J Physiol Cell Physiol 313: C228–C238, 2017. doi: 10.1152/ajpcell.00101.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomei S, Yuasa H, Inoue K, Watanabe J. Transport functions of riboflavin carriers in the rat small intestine and colon: site difference and effects of tricyclic-type drugs. Drug Deliv 8: 119–124, 2001. doi: 10.1080/107175401316906874. [DOI] [PubMed] [Google Scholar]
- 47.Tu BP, Ho-Schleyer SC, Travers KJ, Weissman JS. Biochemical basis of oxidative protein folding in the endoplasmic reticulum. Science 290: 1571–1574, 2000. doi: 10.1126/science.290.5496.1571. [DOI] [PubMed] [Google Scholar]
- 48.Wang X. miRDB: a microRNA target prediction and functional annotation database with a wiki interface. RNA 14: 1012–1017, 2008. doi: 10.1261/rna.965408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X, El Naqa IM. Prediction of both conserved and nonconserved microRNA targets in animals. Bioinformatics 24: 325–332, 2008. doi: 10.1093/bioinformatics/btm595. [DOI] [PubMed] [Google Scholar]
- 50.Wang LD, Zhou FY, Li XM, Sun LD, Song X, Jin Y, Li JM, Kong GQ, Qi H, Cui J, Zhang LQ, Yang JZ, Li JL, Li XC, Ren JL, Liu ZC, Gao WJ, Yuan L, Wei W, Zhang YR, Wang WP, Sheyhidin I, Li F, Chen BP, Ren SW, Liu B, Li D, Ku JW, Fan ZM, Zhou SL, Guo ZG, Zhao XK, Liu N, Ai YH, Shen FF, Cui WY, Song S, Guo T, Huang J, Yuan C, Huang J, Wu Y, Yue WB, Feng CW, Li HL, Wang Y, Tian JY, Lu Y, Yuan Y, Zhu WL, Liu M, Fu WJ, Yang X, Wang HJ, Han SL, Chen J, Han M, Wang HY, Zhang P, Li XM, Dong JC, Xing GL, Wang R, Guo M, Chang ZW, Liu HL, Guo L, Yuan ZQ, Liu H, Lu Q, Yang LQ, Zhu FG, Yang XF, Feng XS, Wang Z, Li Y, Gao SG, Qige Q, Bai LT, Yang WJ, Lei GY, Shen ZY, Chen LQ, Li EM, Xu LY, Wu ZY, Cao WK, Wang JP, Bao ZQ, Chen JL, Ding GC, Zhuang X, Zhou YF, Zheng HF, Zhang Z, Zuo XB, Dong ZM, Fan DM, He X, Wang J, Zhou Q, Zhang QX, Jiao XY, Lian SY, Ji AF, Lu XM, Wang JS, Chang FB, Lu CD, Chen ZG, Miao JJ, Fan ZL, Lin RB, Liu TJ, Wei JC, Kong QP, Lan Y, Fan YJ, Gao FS, Wang TY, Xie D, Chen SQ, Yang WC, Hong JY, Wang L, Qiu SL, Cai ZM, Zhang XJ. Genome-wide association study of esophageal squamous cell carcinoma in Chinese subjects identifies susceptibility loci at PLCE1 and C20orf54. Nat Genet 42: 759–763, 2010. doi: 10.1038/ng.648. [DOI] [PubMed] [Google Scholar]
- 51.Yonezawa A, Inui K. Novel riboflavin transporter family RFVT/SLC52: identification, nomenclature, functional characterization and genetic diseases of RFVT/SLC52. Mol Aspects Med 34: 693–701, 2013. doi: 10.1016/j.mam.2012.07.014. [DOI] [PubMed] [Google Scholar]