We propose that local circuits in the enteric nervous system sense and regulate intestinal ammonia. We show that ammonia modifies enteric neuromuscular transmission to increase motility in human, pig, and mouse intestine model systems. The mechanisms underlying the effects of ammonia on enteric neurotransmission include GABAergic pathways that are regulated by enteric glial cells. Our new data suggest that myenteric glial cells sense local ammonia and directly modify neurotransmission by releasing GABA.
Keywords: enteric glia, gut, enteric nervous system, ammonia, GABA
Abstract
Impaired gut motility may contribute, at least in part, to the development of systemic hyperammonemia and systemic neurological disorders in inherited metabolic disorders, or in severe liver and renal disease. It is not known whether enteric neurotransmission regulates intestinal luminal and hence systemic ammonia levels by induced changes in motility. Here, we propose and test the hypothesis that ammonia acts through specific enteric circuits to influence gut motility. We tested our hypothesis by recording the effects of ammonia on neuromuscular transmission in tissue samples from mice, pigs, and humans and investigated specific mechanisms using novel mutant mice, selective drugs, cellular imaging, and enzyme-linked immunosorbent assays. Exogenous ammonia increased neurogenic contractions and decreased neurogenic relaxations in segments of mouse, pig, and human intestine. Enteric glial cells responded to ammonia with intracellular Ca2+ responses. Inhibition of glutamine synthetase and the deletion of glial connexin-43 channels in hGFAP::CreERT2+/−/connexin43f/f mice potentiated the effects of ammonia on neuromuscular transmission. The effects of ammonia on neuromuscular transmission were blocked by GABAA receptor antagonists, and ammonia drove substantive GABA release as did the selective pharmacological activation of enteric glia in GFAP::hM3Dq transgenic mice. We propose a novel mechanism whereby local ammonia is operational through GABAergic glial signaling to influence enteric neuromuscular circuits that regulate intestinal motility. Therapeutic manipulation of these mechanisms may benefit a number of neurological, hepatic, and renal disorders manifesting hyperammonemia.
NEW & NOTEWORTHY We propose that local circuits in the enteric nervous system sense and regulate intestinal ammonia. We show that ammonia modifies enteric neuromuscular transmission to increase motility in human, pig, and mouse intestine model systems. The mechanisms underlying the effects of ammonia on enteric neurotransmission include GABAergic pathways that are regulated by enteric glial cells. Our new data suggest that myenteric glial cells sense local ammonia and directly modify neurotransmission by releasing GABA.
INTRODUCTION
Ammonia/ammonium (NH3/) are cytotoxic waste products of protein metabolism that are primarily produced by urease positive bacteria in the intestine (53). Luminal concentrations of ammonia within the colon are estimated to range between 4 and 70 mM (55), and intestinal ammonia released with bicarbonate plays important physiological roles that include contributing to the alkaline pH of the colonic luminal environment and the buffering of short-chain fatty acids produced by the microbiota. The negative effects of chronically high levels of ammonia are usually avoided because it is restricted to the portal circulation and detoxified in the liver to generate urea (48).
However, severe, systemic consequences arise if the balance of ammonia detoxification in the liver is disturbed and renal excretion of nitrogenous waste is perturbed. Under these circumstances, ammonia clearance in the intestine can be limited and ammonia is permitted to escape into the general circulation (11, 49). The resulting systemic hyperammonemia plays a major role in the pathogenesis of a number of acquired and inherited metabolic neurological disorders including hepatic encephalopathy, Reye’s syndrome, valproate encephalopathy, organic acidurias, and idiopathic hyperammonemia (7, 10, 46, 48, 52).
Hepatic encephalopathy, in particular, is extremely common and affects up to 75% of patients with end stage liver disease (50). Despite the high prevalence of these disorders, current therapies are limited and poorly tolerated by patients. More effective therapies are clearly needed, but their development is hindered by the poor understanding of peripheral mechanisms that regulate ammonia in key organs like the intestine.
It is feasible that intestinal motility could play an important role in the regulation of systemic levels of ammonia. Dysmotility often precedes the development of hepatic encephalopathy, and constipation from whatever cause can be considered an important and common precipitant of the syndrome (38). Constipation impairs the intestinal clearance of ammonia and rapidly increases the concentration of ammonia in the portal venous blood (49). The synthetic nonabsorbable disaccharide lactulose (β-galactosidofructose) is a mainstay therapy for overt hepatic encephalopathy that functions to decrease the time available for ammonia absorption by promoting peristalsis and altering osmotic concentrations (12, 14). Lactulose also promotes ammonia clearance by lowering the colonic pH to form ammonium within the colon, displacing urease-producing bacteria with nonurease-producing Lactobacillus (47), increasing the incorporation of ammonia by gut bacteria and decreasing the formation of potentially toxic short-chain fatty acids (12). These observations suggest that the impairment of intestinal motility contributes, at least in part, to the development of systemic hyperammonemia. How the intestine senses and regulates local levels of luminal and local tissue content of ammonia is unknown.
The neural circuits that control gut motility are located in the enteric nervous system (ENS). These neural circuits are ideally positioned to transduce changes in local ammonia levels to modify intestinal motility. Given the profound effects of ammonia in the brain (17, 45, 46, 54), we hypothesized that direct effects of ammonia on the ENS might substantively contribute to changes in gut motility. We tested our hypothesis by analyzing the acute effects of ammonia on the neuronal control of gut contractility in segments of mouse, pig, and human intestine. Our data indicate novel mechanisms whereby local ammonia acts through glial GABAergic signaling to regulate neuromuscular transmission in the ENS. We propose that alterations to this novel mechanism of glial ammonia sensing and neural modification play a significant role in the development of a number of neurological disorders associated with hyperammonemia.
MATERIALS & METHODS
Animals.
Animal protocols received approval from the Michigan State University (MSU) Institutional Animal Care and Use Committee. Segments of ileum and colon were collected from 9- to 15-wk-old male and female mice. Wild-type (WT) C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). GFAP::hM3Dq transgenic mice were bred in house (35). hGFAP::CreERT2+/−/Cx43f/f transgenic mice were generated by mating hGFAP::CreERT2+/− mice [Jackson Laboratories, (GFAP-cre/ERT2)505Fmv/J, RRID:IMSR_JAX:012849] with CX43f/f mice (Jackson Laboratories, B6.129S7-Gja1tm1Dlg/J, RRID:IMSR_JAX:008039) as described previously (6). Cre recombinase was induced with tamoxifen citrate chow (40 mg/kg; Charles River) for 2 wk. Animals were returned to normal chow for 1 wk to clear tamoxifen before experiments. Mice were maintained on a 12:12-h light:dark cycle with ad libitum access to food and water. Segments of pig jejunum were collected from female pigs aged 28 wk by the Food Science and Human Nutrition Department at MSU.
Human tissue.
Experimental protocols involving human tissue were approved by the MSU Institutional Review Board. Samples of human jejunum were collected from individuals undergoing Roux-en-Y gastric bypass surgery for weight loss. Segments of bowel were placed in chilled DMEM/F-12 during transfer to the laboratory. Tissue samples were collected from six individuals (4 females and 2 males), with a median age of 36 (27–45) yr and body mass index of 44 (30–54) kg/m2.
Calcium imaging.
Intracellular Ca2+ fluxes were measured as described previously (35). Briefly, whole mount preparations of myenteric plexus were prepared by dissecting the mucosa and circular muscle from segments of mouse ileum. Live preparations were placed in laminar-flow recording chambers and loaded with Fluo-4-AM (4 μM; Life Technologies) for 30 min at 37°C in a dark incubator (95% air-5% CO2). Tissues were imaged through the ×40 water-immersion objective (LUMPlan N, 0.8 n.a.) of an upright Olympus BX51WI fixed-stage microscope (Olympus, Center Valley, PA). Images were acquired at 1 Hz using a Neo sCMOS digital camera (Andor, South Windsor, CT) and Andor IQ3 software. Drugs were dissolved in buffer maintained at 34°C and were bath applied using a gravity flow perfusion system at a rate of 3 ml/min.
Contractility studies.
Longitudinally oriented muscle strips were mounted in organ baths, and one end was attached to force transducer (Grass Instruments, Quincy, MA). Electrical field stimulation (EFS) was supplied by two platinum electrodes and a GRASS stimulator (S88; GRASS telefactor, West Warwick, RI). Data were charted with LabChart 8 software (ADInstruments, Colorado Springs, CO) as described previously (35). Tissue segments were equilibrated for 20 min under 0.5-g initial tension (mice) or 1-g tension (pig and human). Neurogenic relaxations were studied in tissues precontracted with 5 μM prostaglandin F2-α (PGF2α). Relaxations were induced when the contractile response to PGF2α was stable for at least 5 min. Tetrodotoxin (TTX; 0.3 μM) was applied to block neurogenic responses.
Colonic migrating motor complexes.
Mouse colons were removed and placed in a bath containing DMEM/F-12 maintained at 37°C. Luminal contents were flushed, and a stainless steel rod (1.5-mm diameter) was inserted into the lumen. The tissue was secured at both ends using silk suture, and force transducers (Grass Instruments) were placed 2 cm apart and attached by metal hooks. The initial tension was adjusted to 0.5 g, and the development of spontaneous colonic migrating motor complexes (CMMCs) was monitored over an acclimatization period of 20 min using LabChart 8. The last 6-min interval of the 20-min acclimatization period was used as baseline. Drugs were added cumulatively after the acclimatization period, and CMMCs were recorded for an additional 10-min interval.
GABA release measurements.
GABA was measured in supernatants collected from myenteric plexus whole mount preparations from the colons of WT and GFAP::hM3Dq mice. Enteric glia were specifically excited by exposing tissue isolated from GFAP::hM3Dq mice to clozapine-N-oxide (CNO; 10 μM) in the presence of TTX (0.3 μM). Control tissue was isolated from WT littermates and exposed to CNO in the presence of TTX. Supernatants were collected (200-μl total volume) after 10 min, and GABA release was quantified using a Gamma Aminobutyric Acid ELISA Kit (MyBioSource, San Diego, CA). The ELISA reaction was measured using a microtiter plate reader read at 450 nm and sample values were quantified by plotting on a standard curve.
Solutions.
Muscle contractility studies were performed in normal Krebs buffer consisting of the following (in mmol/l): 117 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, and 11 glucose. CMMC studies were conducted in DMEM/F12 (Life Technologies) supplemented with l-glutamine and HEPES. Ca2+ imaging experiments were performed in modified Krebs buffer consisting of the following (in mmol/l): 121 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 10 HEPES, 21.2 NaHCO3, 1 pyruvic acid, and 8 glucose (pH adjusted to 7.4 with NaOH) with 3 μM nicardipine and 1 μM scopolamine. No significant alterations to the pH of Krebs buffer were observed with the addition of 5 mM NH4Cl.
Chemicals and reagents.
NH4Cl, PGF2α, PAPA NONOate, methionine sulfoximine (MSO), bicuculline, strychnine, nipecotic acid, (S)-SNAP 5114, TTX, and adenosine diphosphate were purchased from Sigma-Aldrich (St. Louis, MO). CNO was obtained from the National Institute on Drug Abuse Drug Supply Program at the National Institutes of Health. Drugs were bath applied via a gravity-fed perfusion system in Ca2+ imaging experiments, directly added to organ baths for isometric muscle tension recordings and CMMC recordings and directly added to plate wells for GABA release studies.
Statistical analysis.
Data were analyzed using Prism 6 (GraphPad Software, La Jolla, CA) and are shown as means ± SE. Contractility studies were analyzed by two-way ANOVA with a Bonferroni posttest. Remaining data were analyzed by Student’s t-test and one-way ANOVA with a Bonferroni posttest. P < 0.05 was considered statistically significant.
RESULTS
We studied the effects of ammonia on enteric neuromuscular transmission in segments of mouse, human and pig intestine ex vivo. We conducted our all of our studies using the ionized, protonated, form of ammonia (NH4Cl) because over 98% of ammonia exists in this form at physiological pH (40).
Effects of ammonia on enteric neuromuscular transmission.
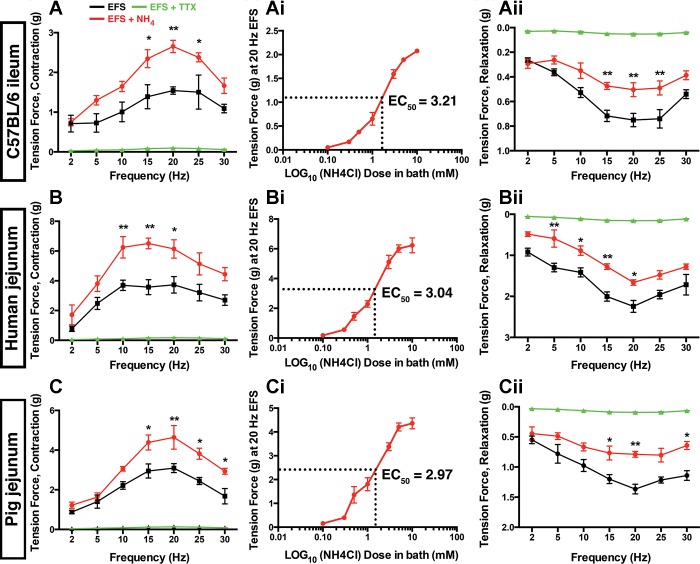
We initially studied how ammonia affects the neuronal control of gut contractility in isometric muscle tension recordings using EFS to drive neurogenic contractions and relaxations. EFS induced a frequency-dependent contractile response that peaked between 10 and 20 Hz in intestinal segments from mice, humans, and pigs (Fig. 1, A–C). EFS-induced contractions were neurogenic in all cases because they were abolished by pretreatment with TTX (0.3 μM). NH4Cl (5 mM) did not stimulate relaxations or contractions in the absence of EFS (Fig. 2). However, preincubation with NH4Cl significantly enhanced contractions at frequencies above 10 Hz in all species (Fig. 1, A–C). For example, NH4Cl increased the maximal force generated by 20-Hz EFS in segments of mouse intestine by 73% and by 65 and 50% in the human and pig intestine, respectively (P < 0.01). Dose-response experiments conducted at 20 Hz showed that the maximal facilitatory effect of NH4Cl is reached when concentrations rise to near 10 mM and EC50 values were near 3 mM in intestinal segments from all species tested (Fig. 1, Ai–Ci). Our original 5-mM concentration is a well-established concentration to study hyperammonemia in the brain (45) and it fit well within this range, so we reasoned that it was appropriate to continue using this concentration for the remainder of our experiments.
Fig. 1.
NH4Cl alters neuromuscular transmission in the mouse, human and pig intestine. Electrical field stimulation (EFS; 20 V, 0.3 ms, 2-30 Hz) frequency-response curves for neurogenic contractions and relaxations in segments of ileum and jejunum from mice (A), humans (B), and pigs (C) in the presence (red) or absence (black) of NH4Cl (5 mM). Contractions (A, B, and C) and relaxations (Aii, Bii, and Cii) driven by EFS are abolished in the presence of tetrodotoxin (TTX; 0.3 μM; green). Ai, Bi, and Ci: dose-response curves showing the effect of ascending concentrations of NH4Cl on contractions driven by 20-Hz EFS in mice, humans and pigs. EC50 values for NH4Cl are given for each at right. Data are means ± SE and were analyzed by two-way ANOVA and Bonferonni’s post hoc test. *P < 0.05, **P < 0.01 vs. control (n = 5 in each group).
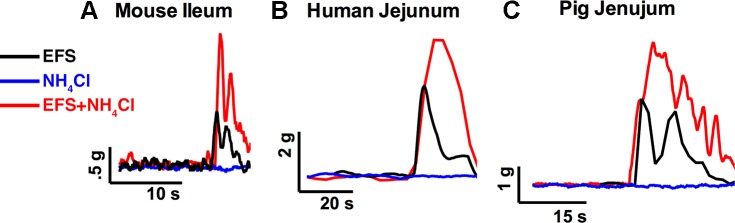
Fig. 2.
Effects of NH4Cl on neurogenic contractions in mice, humans, and pigs. Representative traces from isometric muscle tension recordings in organ baths showing the effect of NH4Cl on contractions driven by 20 Hz EFS in segments of mouse ileum (A), human jejunum (B), and pig jejunum (C).
We tested the effect of NH4Cl on inhibitory neuromuscular transmission by inducing neurogenic relaxations with EFS in segments of ileum and jejunum precontracted with PGF2α (Fig. 1, Aii–Cii). Contrary to the effects on contractions, NH4Cl significantly attenuated neurogenic relaxations elicited at 20 Hz EFS by 39% in mice, 43% in pigs, and 29% in humans. Artificially increasing the overall inhibitory tone by incubating samples in the nitric oxide donor PAPA NONOate (100 μM) was sufficient to overcome the effects of NH4 on both contractions and relaxations (Fig. 3). Taken together, these results show that elevated levels of ammonia significantly modify enteric neuromuscular transmission with the main outcome being enhanced contractility.
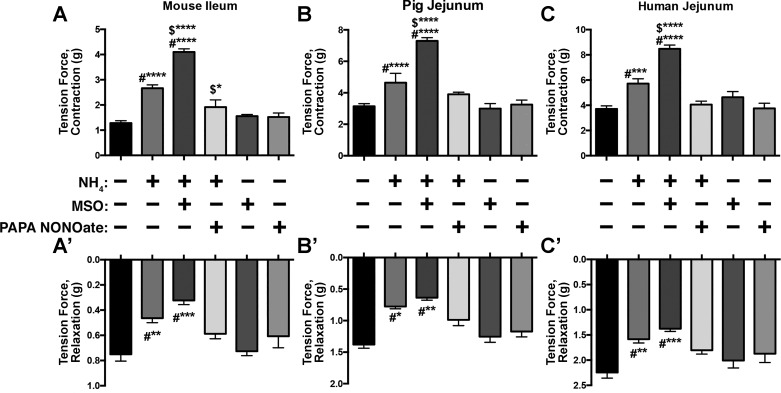
Fig. 3.
The effects of NH4Cl on neuromuscular transmission are potentiated by the inhibition of glial glutamine synthetase and abolished in the presence of an nitric oxide (NO) donor. Summary data showing the effects of methionine sulfoximine (MSO; 1 mM), a glutamine synthetase inhibitor, and PAPA NONOate (100 μM), a NO donor, on the potentiation of neurogenic contractions (A, B, and C) and impairment of neurogenic relaxations (A’, B’, and C’) caused by NH4Cl in segments of mouse ileum (A), pig (B), and human (C) jejunum. Experimental: n = 5 per group; drug control: n = 4 per group. #*P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001, compared with control; $*P < 0.05, ****P < 0.001, compared with NH4 or MSO, two-way ANOVA. No significant effect of drug control alone.
Enteric glia regulate the effects of ammonia on neuromuscular transmission.
In the brain, ammonia is primarily detoxified by the glial enzyme glutamine synthetase (34, 54), and in the gut, glutamine synthetase is expressed by glial cells (26). The inhibition of glutamine synthetase with MSO (1 mM) potentiated the effect of NH4Cl on contractions in the mouse ileum, the human jejunum, and the pig jejunum (P < 0.0001) (Fig. 3, A–C). The inhibition of glutamine synthetase had a more modest effect on the reduction in neurogenic relaxations driven by NH4Cl but still significantly decreased relaxations in mouse ileum, the human jejunum, and the pig ileum (P < 0.01) beyond the effect of NH4Cl alone (Fig. 3, A’–C’). Therefore, the detoxification of ammonia by glial glutamine synthetase is an important mechanism that regulates the effects of ammonia on enteric circuits.
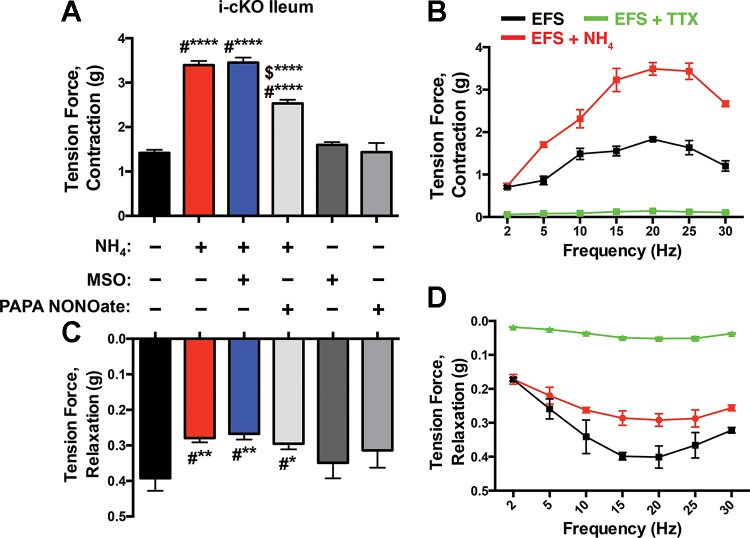
The main mechanism by which ammonia causes neuronal dysfunction in the cortex is by interfering with astrocytic buffering through gap junction-coupled networks (45). Enteric glia, like astrocytes, buffer neuroactive compounds by redistribution through glial networks coupled by gap junctions composed of connexin-43 (Cx43) (36). Therefore, we reasoned that interfering with glial buffering by ablating Cx43 should enhance the effects of ammonia on enteric neuromuscular transmission. We tested this possibility by assessing the effect of NH4Cl in intestinal segments isolated from transgenic mice with a conditional ablation of Cx43 in enteric glia (hGFAP::CreERT2+/−/Cx43f/f; i-cKO) (36). The effects of NH4Cl on both neurogenic contractions and relaxations were exaggerated in tissue from mice lacking glial Cx43 (Fig. 4). Interestingly, these responses were similar in magnitude to those obtained in the presence of MSO in WT tissue and MSO had no further effect on NH4Cl-altered contractions and relaxations in tissue from mice lacking glial Cx43 (Fig. 4). Increasing nitrergic tone with PAPA NONOate prevented a portion of the changes induced by NH4Cl in mice lacking glial Cx43, but the effect was, however, less robust than in tissue from WT mice (compare Fig. 3A with Fig. 4, A and C). In summary, enteric glia regulate the availability of ammonia in the ENS and the effects of ammonia on neuromuscular transmission are accentuated when glial detoxification and buffering mechanisms are impaired.
Fig. 4.
The conditional ablation of glial connexin-43 exacerbates the effects of NH4Cl on neuromuscular transmission. Neurogenic contractions (A and B) and relaxations (C-D) of the mouse ileum driven by EFS (20 V, 0.3 ms, 20 Hz) in hGFAP::CreERT2+/−/Cx43f/f mice. NH4Cl was more efficacious at potentiating neurogenic contractions (A and B) and diminishing relaxations (C and D) in hGFAP::CreERT2+/−/Cx43f/f mice than wild-type (WT) mice (see Fig. 1). MSO (1 mM) did not further potentiate the effects of NH4Cl and PAPA NONOate (100 μM) partially antagonized the effects of NH4Cl. Data are means ± SE and were analyzed by two-way ANOVA and Bonferonni’s post hoc test. *P < 0.05, **P < 0.01, #****P < 0.001, compared with control; $****P < 0.001, compared with NH4 or MSO (n = 5 in each group).
Integrative effects of ammonia on CMMCs.
Our muscle tension data above suggest that ammonia enhances gut contractility through effects on the ENS in the small intestine of humans, pigs, and mice.
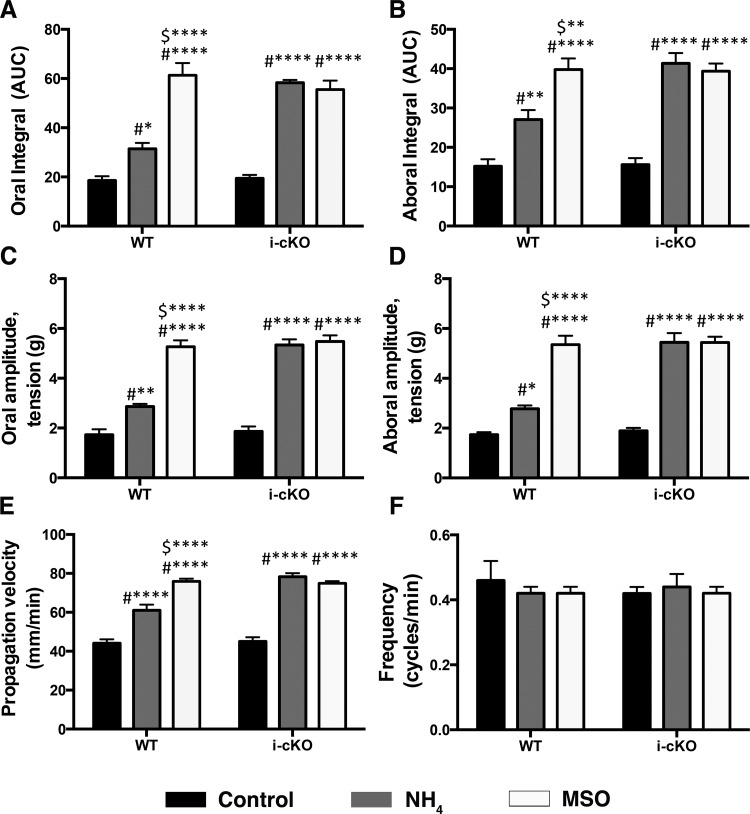
To test how ammonia affects motility reflexes in the colon, where luminal concentrations of ammonia would presumably be higher than the small bowel, we measured the effects of ammonia on CMMCs in mice. In agreement with our muscle tension studies in the small intestine, our results show that the amplitude and integral of oral and aboral contractions of the colon were increased in the presence of NH4Cl (oral integral by 70%, aboral integral by 78%, oral amplitude by 66%, and aboral amplitude by 60%) (Fig. 5, A–F). CMMCs propagated more rapidly in the presence of NH4Cl (control = 44.1 ± 0.1 mm/s, NH4Cl = 61.1 ± 2.9 mm/s, P < 0.05) but occurred at the same frequency (control = 0.46 ± 0.06 cycles/min, NH4Cl = 0.42 ± 0.02 cycles/min, P > 0.05), indicating that NH4Cl primarily affected neural transmission rather than pacemaker functions.
Fig. 5.
The inhibition, or ablation, of glial detoxification and buffering mechanisms enhances the effect of NH4Cl on mouse colonic migrating motor complexes (CMMCs). A–F: summary data showing the effects of NH4Cl of on CMMC characteristics in WT and hGFAP::CreERT2+/−/Cx43f/f mice (i-cKO) in the presence (white bars) or absence (gray bars) of the glial glutamine synthetase inhibitor MSO. AUC, area under the curve. Parameters shown include the integral of oral (A) and aboral (B) contractions, amplitude of oral (C) and aboral (D) contractions, CMMC frequency (E), and CMMC propagation velocity (F). Data are means ± SE and were analyzed by two-way ANOVA and Bonferonni’s post hoc test. #*P < 0.05, **P < 0.01, ****P < 0.001, compared with control; $**P < 0.01 and ****P < 0.001, compared with NH4 (n = 5 in each group).
The effects of NH4Cl on CMMCs were enhanced when glial detoxification by glutamine synthetase was inhibited with MSO and when glial buffering through Cx43 gap junctions was ablated in Cx43 i-cKO mice (Fig. 5). The ablation of glial Cx43 increased the effect of NH4Cl on propagation velocity by a further 36%, oral amplitude by 121%, oral integral by 130%, aboral amplitude by 188%, and aboral integral by 87%. The inhibition of glial glutamine synthetase with MSO also significantly enhanced the effects of NH4Cl in WT colons but had no further effect in colons from mice lacking glial Cx43. Together, these results show that ammonia acts through enteric circuits to increase CMMC speed without a change in CMMC frequency and that enteric glia contribute to the effect of ammonia. Interestingly, we did not observe significant differences in CMMCs between control mice and those lacking glial Cx43. This was surprising given our previous work where we showed impairments in neuromuscular transmission and colonic motility in these animals (36). This outcome may suggest that glial signaling involving connexin-43 has a more prominent role in evoked contractile responses such as propulsive motility than housekeeper forms of motility such as CMMCs.
Mechanisms underlying the effect of ammonia on enteric circuits.
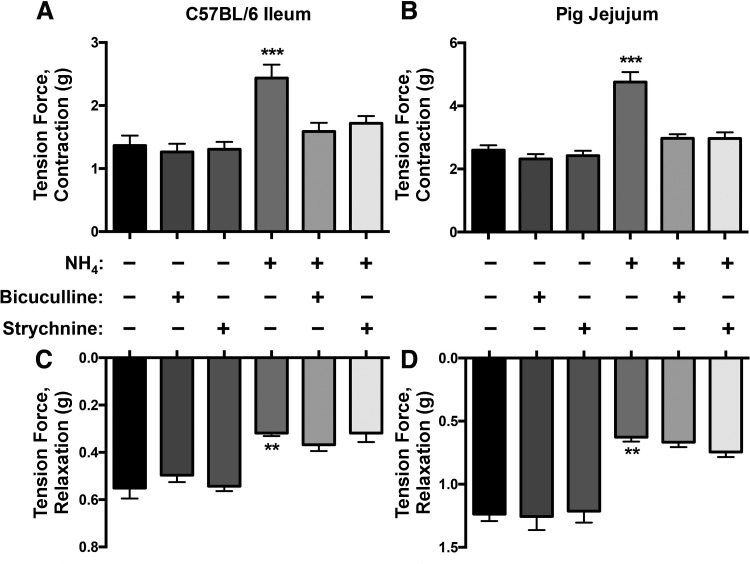
Ammonia may increase neuronal excitability through multiple mechanisms such as disrupting the ionic balance of the extracellular environment and direct actions on ion channels. However, many of the most prominent effects of ammonia on neurotransmission in the brain are mediated through actions on GABAergic signaling (8, 20, 45, 51). Interestingly, the effects of GABA on myenteric neurons match the effects we observed with ammonia because enteric GABA is primarily excitatory and endogenous GABA plays an important role in augmenting peristaltic movements (2, 18, 25, 30, 31, 37, 42, 44). These similarities led us to the hypothesis that ammonia acts through GABAergic signaling to modify enteric neurotransmission. We tested our hypothesis by assessing the ability of the GABAA receptor antagonist bicuculline (1 μM) to block the effects of NH4Cl. We found that blocking GABAA receptors completely abolished the effects of NH4Cl on neurogenic contractions in the mouse ileum and pig jejunum (Fig. 6, A and B) but had no effect on the reduction in neurogenic relaxations driven by NH4Cl (Fig. 6, C and D).
Fig. 6.
NH4Cl promotes the excitability of enteric neurons through GABAergic mechanisms. A and B: the enhancement of neurogenic contractions by NH4 was abolished in the presence of bicuculline and strychnine in the mouse ileum (A) and pig jejunum (B). C and D: the reduction of relaxations by NH4 was not ameliorated in the presence of bicuculline and strychnine in the mouse ileum (C) and pig jejunum (D). Experimental: n = 5 per group; drug control: n = 4 per group. Data are means ± SE and were analyzed by two-way ANOVA with Bonferonni’s post hoc test. **P < 0.01, ***P < 0.005 vs. control. There was no significant effect of drug control alone.
We also considered the possibility that ammonia acts through glycinergic pathways because glycine regulates gut motility through neural pathways that are similar to those of GABA (39). Interestingly, we found that strychnine (10 μM), an antagonist of glycine receptors, also abolished the excitatory effects of NH4Cl on neurogenic contractions in the mouse ileum and pig jejunum (Fig. 6, A and B).
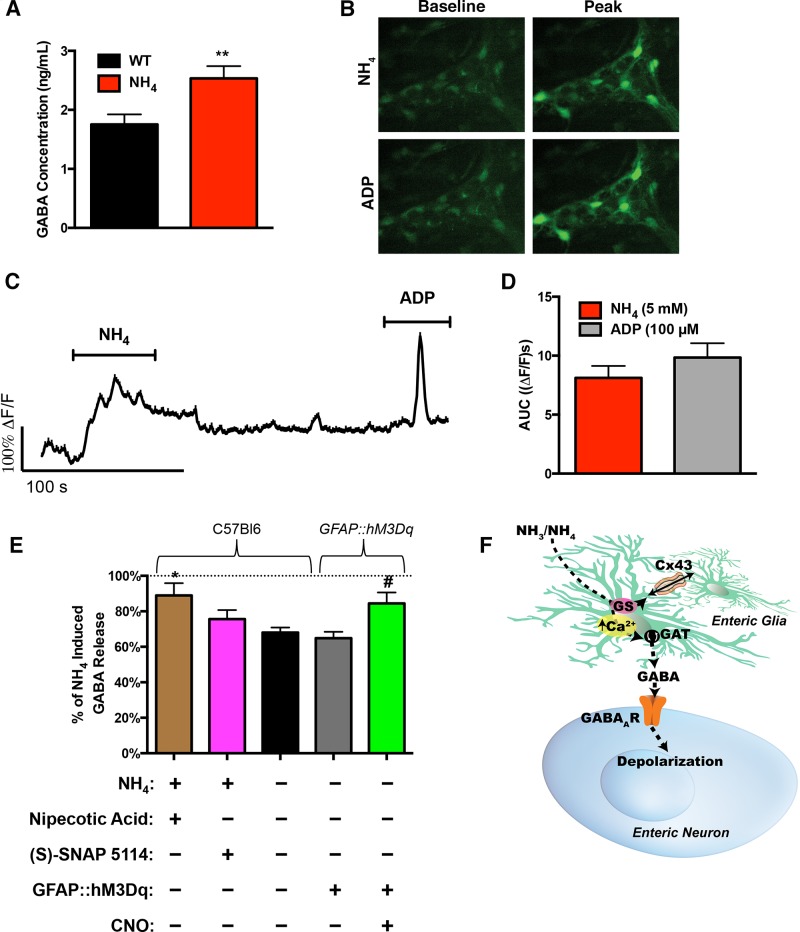
The excitatory effect of glycine in the ENS is not blocked by bicuculline (39), but strychnine can act as an inhibitor of GABA/benzodiazepine receptor complexes (5). Therefore, we concluded that the effects of ammonia on excitatory neuromuscular transmission in the ENS are primarily mediated through actions on GABAergic signaling. In support of this mechanism, we found that ammonia caused a significant increase in the GABA content of supernatants collected from myenteric plexus whole mounts as measured by ELISA (Fig. 7A).
Fig. 7.
Enteric glia contribute to GABA release by reversing GAT activity. A: GABA concentrations measured in supernatants collected from live whole mounts of myenteric plexus from WT mice treated with NH4. **P < 0.01 by Student’s t-test (n = 6 in each group). B–D: Representative Ca2+ imaging data showing images of Fluo-4 fluorescence (B) in a myenteric ganglion from the mouse ileum during stimulation with either 5 mM NH4Cl or 100 μM ADP, a representative glial cell recording (C) and the quantification of responses (D) to NH4Cl and ADP as measured by area under the curve (ΔF/F)s. n = 51 glial cells from 7 ganglia from 4 animals. E: GABA release measured in supernatants collected from live whole mounts of myenteric plexus from GFAP::hM3Dq transgenic mice and WT littermates treated with ammonia. Preparations were additionally incubated with nipecotic acid or (S)-SNAP 5114 to inhibit GAT activity and with clozapine-N-oxide (CNO) in GFAP::hM3Dq mice to activate Ca2+ responses in glial cells. All experiments were performed in the presence of TTX. Data are means ± SE and were analyzed by one-way ANOVA and Bonferonni’s post hoc test. *P < 0.05, compared with WT control; #P < 0.05, compared with TG control (n = 3 in each group). F: proposed mechanisms for the effects of ammonia on neuromuscular function in the gut. Glia are normally capable of detoxifying small amounts of ammonia that leak past the venous blood through the actions of glutamine synthetase and subsequent buffering of metabolites throughout networks of enteric glia connected by gap junctions composed of Cx43. Levels of ammonia that are beyond the glial detoxification and buffering capacity stimulate glial Ca2+ responses and glial excitation reverses glial GATs. Subsequently, glial GABA release activates neuronal GABAA receptors and increases intestinal motility.
Ammonia increases the GABAergic tone in the brain by several mechanisms that include direct actions on GABAA receptors (20, 23, 51) and the stimulation of GABA release (1, 23). Astrocytes are the major cellular target of ammonia in the brain, and hyperammonemia stimulates astrocytic Ca2+ responses (45) and GABA release (1). Based on their similarities to astrocytes, we hypothesized that the effects of ammonia on GABAergic signaling in the gut are mediated by the stimulation of GABA release from enteric glia. In support, NH4Cl elicits intracellular Ca2+ responses within enteric glia that are similar to those reported in astrocytes (45) (Fig. 7, B–D) and enteric glia are capable of concentrating intracellular GABA (13, 27). We directly tested if glial Ca2+ responses stimulate glial GABA release by measuring GABA release from myenteric plexus whole mounts from GFAP::hM3Dq mice (35). Glia in GFAP::hM3Dq mice express designer Gq-coupled receptors that are exclusively activated by the drug CNO. This model allowed us to selectively activate glial cells with CNO (10 μM) and assess GABA release using an ELISA assay. These experiments were performed in the presence of TTX to exclude subsequent neuronal release. The results from these experiments show that the selective activation of enteric glia drove a 23% increase in the amount of GABA present in supernatants within ten minutes (P < 0.01, Fig. 7E).
In astrocytes, GABA release is driven, in part, by the reversal of GABA transporters (GATs) (21, 22). Even relatively modest depolarizations are sufficient to reverse glial GATs because their reversal potential lies near the resting membrane potential (28). Enteric glia express GATs (24), and we previously showed that Ca2+ responses drive the release of ATP, a large negative anion, from enteric glia (6). Therefore, glial depolarization during Ca2+ responses could cause the release of GABA by reversing glial GATs. In support, we found that GABA release measured in the supernatants of whole mount preparations of myenteric plexus exposed to ammonia was abolished by the GAT inhibitor (S)-SNAP 5114 and modestly decreased by the GAT inhibitor nipecotic acid (Fig. 7E). Together, these data indicate that ammonia drives the GABA release through mechanisms that include the reversal of glial GATs.
DISCUSSION
Our findings have uncovered novel enteric mechanisms that regulate intestinal motility in response to fluctuations of ammonia (Fig. 7F). Presumably, the “ammonia reflex” is a protective response that functions to expel potentially hazardous levels of ammonia from the intestine. Defects in this reflex could contribute to systemic hyperammonemia by allowing intestinal ammonia to accumulate. In agreement, ileus and constipation are major contributing factors to hepatic encephalopathy by causing a rapid rise in systemic ammonia levels (38, 49) and lactulose, which, among other effects, increases colonic peristalsis, is a mainstay treatment for systemic hyperammonemia (14).
How local myenteric levels of ammonia correlate with increased luminal levels of ammonia is currently unknown. However, it is clear that luminal elevations in ammonia increase systemic levels of ammonia and subsequently cause increases in even distant tissues such as the brain. Given that there is no blood-brain barrier in the gut, the myenteric plexus would be predicted to be exposed to high local levels of ammonia more rapidly than neural tissue in the brain and this may be beneficial in eliciting mechanisms that eliminate gut ammonia. Ammonia significantly modified both neurogenic contractions and relaxations in humans, pigs, and mice. The consistent effect of ammonia across several species and regions of intestine suggests that this is an important mechanism that has been conserved throughout evolution. The main effect of ammonia was an augmentation of gut motility that was produced by increasing the excitatory component of neuromuscular transmission and decreasing the inhibitory component.
Multiple mechanisms likely contribute to the mean excitatory effect of ammonia on enteric neurotransmission such as altered extracellular ionic concentrations and effects on neuronal ion channels. Our data show that a significant component of the effect involves modifications to GABAergic transmission. GABA is involved in the regulation of both ascending and descending components of the peristaltic reflex (18) and plays an important role in the augmentation of peristaltic movements (25). Most of the effects of GABA on enteric circuits are mediated by GABAA and GABAB receptors on enteric neurons, and gut motility slows and eventually halts in the presence of GABAA and GABAB antagonists (30, 43). However, the specific subtypes of GABA receptors have dichotomous effects on gut motility and subtype specific agonists can both stimulate and inhibit gut motility (15, 16, 29, 30, 37, 43). Our data show that ammonia mainly influences enteric motor reflexes through pathways that utilize GABAA receptors because bicuculline completely blocked the effects of ammonia. The ability of strychnine to antagonize the effects of ammonia also suggests the participation of GABAA receptors because strychnine acts as a potent antagonist of GABAA receptors in addition to blocking glycine receptors (5). In agreement, many of major effects of ammonia on neurotransmission in the brain involve GABAA receptors (8, 20, 51).
Our results show that enteric glia contribute to the regulation of ammonia levels within the ENS. Several distinct populations of enteric glia are present within the gut wall (19), and it is likely that all subtypes contribute to ammonia buffering to some extent. In this study, we specifically focused on motility as an output measurement, and in this case, it is likely that the effects are primarily driven by glia within the myenteric plexus given their proximity to the neural circuits that control motility. Mucosal glia could also contribute to ammonia sensing and detoxification, but we were not able to distinguish the individual contributions of each glial subtype with the current methods. It is likely that mucosal glia play an important role in buffering low levels of ammonia that cross the mucosa en route to the blood stream and that myenteric glia play a more important role in pathophysiological levels of ammonia that might reach the myenteric plexus. However, this is only speculation at this point and additional work will be needed to address this hypothesis.
The major cellular pathway responsible for ammonia detoxification in the brain is by incorporation with glutamate to form glutamine in a reaction catalyzed by glutamine synthetase (9). The expression of glutamine synthetase is restricted to astrocytes in the brain (34, 54), and glutamine synthetase is expressed by enteric glia in the gut (26). The activity of glutamine synthetase effectively traps ammonia metabolites within glial cells. In the brain, the excess astrocytic glutamine contributes to the stereotypical osmotic stress and astrocytic swelling observed in experimental (41) and human hyperammonemia (33). We did not observe any overt evidence of glial cell swelling in our study (data not shown), but this is likely due to the short exposure time that we used in our study compared with the hours of exposure required to induce astrocytic swelling in culture (54). Our data suggest that enteric glia handle the short-term accumulation of glutamine by redistribution throughout gap junction-coupled networks. We observed significantly enhanced effects of ammonia that were equal, but not additive, to the effects of inhibiting glutamine synthetase when we reduced glial gap junction communication by ablating glial Cx43. Together, these data show that glial detoxification and buffering mechanisms are able to effectively control physiological levels of ammonia that might reach the ENS.
Short-term spikes in local ammonia cause glial cells to enact additional emergency mechanisms to remove ammonia by driving gut motility. Potentially pathological concentrations of ammonia stimulated Ca2+ responses in enteric glia and selectively stimulating glial Ca2+ responses drove GABA release. Glial GABA release is likely driven by a reversal of GABA transporters because GABA release was abolished in the presence of GAT inhibitors. We have previously shown that the selective activation of enteric glia drives neural circuits that enhance gut contractility (35). However, the full repertoire of transmitters released by enteric glia and the subsequent neuronal pathways that they act on are not clear. Our data suggest that GABA is a candidate gliotransmitter. The ability of enteric glia to effectively sequester GABA from the extracellular space through a high-affinity GABA uptake system (13, 27), and our data suggest that reversing these mechanisms during glial depolarization could contribute to GABA release. These observations are consistent with prior work showing that similar types of neuroglia such as astrocytes and sympathetic satellite glial cells also use GABA as a transmitter (3, 4, 32), and data showing that ammonia stimulates Ca2+ responses in astrocytes and enhances astrocytic GABA release (1, 45). It is also possible that ammonia has direct modulatory actions on neuronal GABA receptors given the known effects of ammonia in the brain (20, 23, 51). However, our data show that the additional GABA supplied by glia is an early contributing factor to the increased “GABAergic tone” in the presence of ammonia.
In conclusion, our findings provide new insight into mechanisms that may predispose individuals to severe conditions associated with exacerbations in hyperammonemia such as in hepatic encephalopathy and renal failure. We propose that defects in the ammonia sensing mechanisms, as we describe here, may contribute to the development of hepatic encephalopathy given the clear link between intestinal hypo motility as seen with systemic inflammation, opiate usage or other mechanisms and the further exacerbation of hyperammonemia. Therefore, therapeutic manipulation of these paracrine glial-mediated mechanisms may be broadly beneficial for a number of neurological disorders involving toxicity from hyperammonemia.
GRANTS
This work was funded by a Crohn’s and Colitis Foundation of America Senior Research Award (to B. D. Gulbransen), National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-103723 (to B. D. Gulbransen), and start-up funds from the MSU Neuroscience Program (to B. D. Gulbransen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.E.F., R.E.W., S.C.R., and B.D.G. conceived and designed research; D.E.F. performed experiments; D.E.F. analyzed data; D.E.F., R.E.W., S.C.R., and B.D.G. interpreted results of experiments; D.E.F. and B.D.G. prepared figures; D.E.F. drafted manuscript; D.E.F., R.E.W., S.C.R., and B.D.G. edited and revised manuscript; D.E.F., R.E.W., S.C.R., and B.D.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jonathon McClain for assistance with experiments involving transgenic mice and ELISA assays.
REFERENCES
- 1.Albrecht J, Rafałowska U. Enhanced potassium-stimulated gamma-aminobutyric acid release by astrocytes derived from rats with early hepatogenic encephalopathy. J Neurochem 49: 9–11, 1987. doi: 10.1111/j.1471-4159.1987.tb03385.x. [DOI] [PubMed] [Google Scholar]
- 2.Auteri M, Zizzo MG, Serio R. GABA and GABA receptors in the gastrointestinal tract: from motility to inflammation. Pharmacol Res 93: 11–21, 2015. doi: 10.1016/j.phrs.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Bowery NG, Brown DA, Marsh S. gamma-Aminobutyric acid efflux from sympathetic glial cells: effect of ‘depolarizing’ agents. J Physiol 293: 75–101, 1979. doi: 10.1113/jphysiol.1979.sp012879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowery NG, Brown DA, Collins GG, Galvan M, Marsh S, Yamini G. Indirect effects of amino-acids on sympathetic ganglion cells mediated through the release of gamma-aminobutyric acid from glial cells. Br J Pharmacol 57: 73–91, 1976. doi: 10.1111/j.1476-5381.1976.tb07658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braestrup C, Nielsen M. Strychnine as a potent inhibitor of the brain GABA/benzodiazepine receptor complex. Brain Res Bull 5: 681–684, 1980. doi: 10.1016/0361-9230(80)90112-4. [DOI] [Google Scholar]
- 6.Brown IA, McClain JL, Watson RE, Patel BA, Gulbransen BD. Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell Mol Gastroenterol Hepatol 2: 77–91, 2016. doi: 10.1016/j.jcmgh.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butterworth RF, Giguère JF, Michaud J, Lavoie J, Layrargues GP. Ammonia: key factor in the pathogenesis of hepatic encephalopathy. Neurochem Pathol 6: 1–12, 1987. doi: 10.1007/BF02833598. [DOI] [PubMed] [Google Scholar]
- 8.Cauli O, Mansouri MT, Agusti A, Felipo V. Hyperammonemia increases GABAergic tone in the cerebellum but decreases it in the rat cortex. Gastroenterology 136: 1359–1367, 2009. doi: 10.1053/j.gastro.2008.12.057. [DOI] [PubMed] [Google Scholar]
- 9.Cooper AJ, McDonald JM, Gelbard AS, Gledhill RF, Duffy TE. The metabolic fate of 13N-labeled ammonia in rat brain. J Biol Chem 254: 4982–4992, 1979. [PubMed] [Google Scholar]
- 10.Cooper AJ, Plum F. Biochemistry and physiology of brain ammonia. Physiol Rev 67: 440–519, 1987. [DOI] [PubMed] [Google Scholar]
- 11.Ellul MA, Gholkar SA, Cross TJ. Hepatic encephalopathy due to liver cirrhosis. BMJ 351: h4187, 2015. doi: 10.1136/bmj.h4187. [DOI] [PubMed] [Google Scholar]
- 12.Ferenci P, Herneth A, Steindl P. Newer approaches to therapy of hepatic encephalopathy. Semin Liver Dis 16: 329–338, 1996. doi: 10.1055/s-2007-1007245. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher EL, Clark MJ, Furness JB. Neuronal and glial localization of GABA transporter immunoreactivity in the myenteric plexus. Cell Tissue Res 308: 339–346, 2002. doi: 10.1007/s00441-002-0566-3. [DOI] [PubMed] [Google Scholar]
- 14.Fukui H, Saito H, Ueno Y, Uto H, Obara K, Sakaida I, Shibuya A, Seike M, Nagoshi S, Segawa M, Tsubouchi H, Moriwaki H, Kato A, Hashimoto E, Michitaka K, Murawaki T, Sugano K, Watanabe M, Shimosegawa T. Evidence-based clinical practice guidelines for liver cirrhosis 2015. J Gastroenterol 51: 629–650, 2016. doi: 10.1007/s00535-016-1216-y. [DOI] [PubMed] [Google Scholar]
- 15.Giotti A, Luzzi S, Maggi CA, Spagnesi S, Zilletti L. Modulatory activity of GABAB receptors on cholinergic tone in guinea-pig distal colon. Br J Pharmacol 84: 883–895, 1985. doi: 10.1111/j.1476-5381.1985.tb17383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giotti A, Luzzi S, Spagnesi S, Zilletti L. GABAA and GABAB receptor-mediated effects in guinea-pig ileum. Br J Pharmacol 78: 469–478, 1983. doi: 10.1111/j.1476-5381.1983.tb08807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Görg B, Morwinsky A, Keitel V, Qvartskhava N, Schrör K, Häussinger D. Ammonia triggers exocytotic release of L-glutamate from cultured rat astrocytes. Glia 58: 691–705, 2010. doi: 10.1002/glia.20955. [DOI] [PubMed] [Google Scholar]
- 18.Grider JR, Makhlouf GM. Enteric GABA: mode of action and role in the regulation of the peristaltic reflex. Am J Physiol Gastrointest Liver Physiol 262: G690–G694, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 9: 625–632, 2012. doi: 10.1038/nrgastro.2012.138. [DOI] [PubMed] [Google Scholar]
- 20.Ha JH, Basile AS. Modulation of ligand binding to components of the GABAA receptor complex by ammonia: implications for the pathogenesis of hyperammonemic syndromes. Brain Res 720: 35–44, 1996. doi: 10.1016/0006-8993(96)00104-7. [DOI] [PubMed] [Google Scholar]
- 21.Héja L, Barabás P, Nyitrai G, Kékesi KA, Lasztóczi B, Toke O, Tárkányi G, Madsen K, Schousboe A, Dobolyi A, Palkovits M, Kardos J. Glutamate uptake triggers transporter-mediated GABA release from astrocytes. PLoS One 4: e7153, 2009. doi: 10.1371/journal.pone.0007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Héja L, Nyitrai G, Kékesi O, Dobolyi A, Szabó P, Fiáth R, Ulbert I, Pál-Szenthe B, Palkovits M, Kardos J. Astrocytes convert network excitation to tonic inhibition of neurons. BMC Biol 10: 26, 2012. doi: 10.1186/1741-7007-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Itzhak Y, Roig-Cantisano A, Dombro RS, Norenberg MD. Acute liver failure and hyperammonemia increase peripheral-type benzodiazepine receptor binding and pregnenolone synthesis in mouse brain. Brain Res 705: 345–348, 1995. doi: 10.1016/0006-8993(95)01244-3. [DOI] [PubMed] [Google Scholar]
- 24.Jessen KR, Mirsky R, Dennison ME, Burnstock G. GABA may be a neurotransmitter in the vertebrate peripheral nervous system. Nature 281: 71–74, 1979. doi: 10.1038/281071a0. [DOI] [PubMed] [Google Scholar]
- 25.Jessen KR, Mirsky R, Hills JM. GABA as an autonomic neurotransmitter: studies on intrinsic GABAergic neurons in the myenteric plexus of the gut. Trends Neurosci 10: 255–262, 1987. doi: 10.1016/0166-2236(87)90169-X. [DOI] [Google Scholar]
- 26.Jessen KR, Mirsky R. Astrocyte-like glia in the peripheral nervous system: an immunohistochemical study of enteric glia. J Neurosci 3: 2206–2218, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerr DI, Krantis A. Uptake and stimulus-evoked release of [3H]-gamma-aminobutyric acid by myenteric nerves of guinea-pig intestine. Br J Pharmacol 78: 271–276, 1983. doi: 10.1111/j.1476-5381.1983.tb09391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirischuk S, Héja L, Kardos J, Billups B. Astrocyte sodium signaling and the regulation of neurotransmission. Glia 64: 1655–1666, 2016. doi: 10.1002/glia.22943. [DOI] [PubMed] [Google Scholar]
- 29.Krantis A, Harding RK. GABA-related actions in isolated in vitro preparations of the rat small intestine. Eur J Pharmacol 141: 291–298, 1987. doi: 10.1016/0014-2999(87)90274-3. [DOI] [PubMed] [Google Scholar]
- 30.Krantis A, Kerr DI. The effect of GABA antagonism on propulsive activity of the guinea-pig large intestine. Eur J Pharmacol 76: 111–114, 1981. doi: 10.1016/0014-2999(81)90018-2. [DOI] [PubMed] [Google Scholar]
- 31.Krantis A. GABA in the mammalian enteric nervous system. News Physiol Sci 15: 284–290, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Lee M, McGeer EG, McGeer PL. Mechanisms of GABA release from human astrocytes. Glia 59: 1600–1611, 2011. doi: 10.1002/glia.21202. [DOI] [PubMed] [Google Scholar]
- 33.Martinez A. Electron microscopy in human hepatic encephalopathy. Acta Neuropathol 11: 82–86, 1968. doi: 10.1007/BF00692797. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science 195: 1356–1358, 1977. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- 35.McClain JL, Fried DE, Gulbransen BD. Agonist-evoked Ca(2+) signaling in enteric glia drives neural programs that regulate intestinal motility in mice. Cell Mol Gastroenterol Hepatol 1: 631–645, 2015. doi: 10.1016/j.jcmgh.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McClain J, Grubišić V, Fried D, Gomez-Suarez RA, Leinninger GM, Sévigny J, Parpura V, Gulbransen BD. Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology 146: 497–507.e1, 2014. doi: 10.1053/j.gastro.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minocha A, Galligan JJ. Excitatory and inhibitory responses mediated by GABAA and GABAB receptors in guinea pig distal colon. Eur J Pharmacol 230: 187–193, 1993. doi: 10.1016/0014-2999(93)90801-N. [DOI] [PubMed] [Google Scholar]
- 38.Mumtaz K, Ahmed US, Abid S, Baig N, Hamid S, Jafri W. Precipitating factors and the outcome of hepatic encephalopathy in liver cirrhosis. J Coll Physicians Surg Pak 20: 514–518, 2010. . [PubMed] [Google Scholar]
- 39.Neunlist M, Michel K, Reiche D, Dobreva G, Huber K, Schemann M. Glycine activates myenteric neurones in adult guinea-pigs. J Physiol 536: 727–739, 2001. doi: 10.1111/j.1469-7793.2001.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norenberg MD, Huo Z, Neary JT, Roig-Cantesano A. The glial glutamate transporter in hyperammonemia and hepatic encephalopathy: relation to energy metabolism and glutamatergic neurotransmission. Glia 21: 124–133, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 41.Norenberg MD, Lapham LW. The astrocyte response in experimental portal-systemic encephalopathy: an electron microscopic study. J Neuropathol Exp Neurol 33: 422–435, 1974. doi: 10.1097/00005072-197407000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Ong J, Kerr DI. GABAA- and GABAB-receptor-mediated modification of intestinal motility. Eur J Pharmacol 86: 9–17, 1982. doi: 10.1016/0014-2999(82)90390-9. [DOI] [PubMed] [Google Scholar]
- 43.Ong J, Kerr DI. Interactions between GABA and 5-hydroxytryptamine in the guinea-pig ileum. Eur J Pharmacol 94: 305–312, 1983. doi: 10.1016/0014-2999(83)90419-3. [DOI] [PubMed] [Google Scholar]
- 44.Ong J, Kerr DI. Evidence for a physiological role of GABA in the control of guinea-pig intestinal motility. Neurosci Lett 50: 339–343, 1984. doi: 10.1016/0304-3940(84)90509-3. [DOI] [PubMed] [Google Scholar]
- 45.Rangroo Thrane V, Thrane AS, Wang F, Cotrina ML, Smith NA, Chen M, Xu Q, Kang N, Fujita T, Nagelhus EA, Nedergaard M. Ammonia triggers neuronal disinhibition and seizures by impairing astrocyte potassium buffering. Nat Med 19: 1643–1648, 2013. doi: 10.1038/nm.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Record CO. Neurochemistry of hepatic encephalopathy. Gut 32: 1261–1263, 1991. doi: 10.1136/gut.32.11.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riggio O, Varriale M, Testore GP, Di Rosa R, Di Rosa E, Merli M, Romiti A, Candiani C, Capocaccia L. Effect of lactitol and lactulose administration on the fecal flora in cirrhotic patients. J Clin Gastroenterol 12: 433–436, 1990. doi: 10.1097/00004836-199008000-00016. [DOI] [PubMed] [Google Scholar]
- 48.Romero-Gómez M, Jover M, Galán JJ, Ruiz A. Gut ammonia production and its modulation. Metab Brain Dis 24: 147–157, 2009. doi: 10.1007/s11011-008-9124-3. [DOI] [PubMed] [Google Scholar]
- 49.Shashi O, Denis W. Protein metabolism from the standpoint of blood and tissue analysis. Second paper. The orgin and significance of the ammonia in the portal blood. J Biol Chem 11: 161–167, 1912. [Google Scholar]
- 50.Souto PA, Marcotegui AR, Orbea L, Skerl J, Perazzo JC. Hepatic encephalopathy: Ever closer to its big bang. World J Gastroenterol 22: 9251–9256, 2016. doi: 10.3748/wjg.v22.i42.9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi K, Kameda H, Kataoka M, Sanjou K, Harata N, Akaike N. Ammonia potentiates GABAA response in dissociated rat cortical neurons. Neurosci Lett 151: 51–54, 1993. doi: 10.1016/0304-3940(93)90043-K. [DOI] [PubMed] [Google Scholar]
- 52.Vierling JM, Mokhtarani M, Brown RS Jr, Mantry P, Rockey DC, Ghabril M, Rowell R, Jurek M, Coakley DF, Scharschmidt BF. Fasting blood ammonia predicts risk and frequency of hepatic encephalopathy episodes in patients with cirrhosis. Clin Gastroenterol Hepatol 14: 903–906.e1, 2016. doi: 10.1016/j.cgh.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 53.Vince A, Dawson AM, Park N, O’Grady F. Ammonia production by intestinal bacteria. Gut 14: 171–177, 1973. doi: 10.1136/gut.14.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willard-Mack CL, Koehler RC, Hirata T, Cork LC, Takahashi H, Traystman RJ, Brusilow SW. Inhibition of glutamine synthetase reduces ammonia-induced astrocyte swelling in rat. Neuroscience 71: 589–599, 1996. doi: 10.1016/0306-4522(95)00462-9. [DOI] [PubMed] [Google Scholar]
- 55.Wrong O. Nitrogen metabolism in the gut. Am J Clin Nutr 31: 1587–1593, 1978. [DOI] [PubMed] [Google Scholar]