These novel findings demonstrate that whole body cooling-induced reductions in mean skin temperature elicited greater increases in blood pressure and muscle sympathetic nerve activity in hypertensive adults. In addition, during moderate cold exposure, sympathetic baroreflex sensitivity increased in hypertensive, but not normotensive, adults.
Keywords: blood pressure, sympathetic baroreflex sensitivity, skin temperature, microneurography
Abstract
During moderate cold exposure, cardiovascular-related morbidity and mortality increase disproportionately in hypertensive adults (HTN); however, the mechanisms underlying this association are not well defined. We hypothesized that whole body cold stress would evoke exaggerated increases in blood pressure (BP) and muscle sympathetic nerve activity (MSNA) in HTN compared with normotensive adults (NTN) and that sympathetic baroreflex function would be altered during cooling in HTN. MSNA (peroneal microneurography) and beat-to-beat BP (Finometer) were measured continuously in 10 NTN (6 men/4 women; age 53 ± 3 yr; resting BP 125 ± 3/79 ± 1 mmHg) and 13 HTN (7 men/6 women; age 58 ± 2 yr; resting BP 146 ± 5/88 ± 2 mmHg) during whole body cooling-induced reductions in mean skin temperature (Tsk; water-perfused suit) from 34.0 to 30.5°C. During cooling, the increase in mean arterial pressure was greater in HTN (NTN: Δ6 ± 2 vs. HTN: Δ11 ± 1 mmHg; P = 0.02) and accompanied by exaggerated increases in MSNA (NTN: Δ8 ± 3 vs. HTN: Δ20 ± 3 bursts/100 heart beats; P < 0.01). The slope of the relation between MSNA and diastolic BP did not change during cooling in NTN (Tsk 34.0°C: −4.4 ± 0.8 vs. Tsk 30.5°C: −5.0 ± 0.3 bursts·100 heart beats−1·mmHg−1; P = 0.47) but increased in HTN (Tsk 34.0°C: −3.6 ± 0.4 vs. Tsk 30.5°C: −5.4 ± 0.4 bursts·100 heart beats)−1·mmHg−1; P = 0.02). These findings demonstrate that the cooling-induced increases in BP and MSNA are exaggerated in HTN. Furthermore, during cooling, sympathetic baroreflex sensitivity increases in HTN, but not NTN, presumably to allow for baroreflex-mediated buffering of excessive cooling-induced increases in BP. Collectively, these findings suggest that sympathetic function is altered during whole body cooling in hypertension.
NEW & NOTEWORTHY These novel findings demonstrate that whole body cooling-induced reductions in mean skin temperature elicited greater increases in blood pressure and muscle sympathetic nerve activity in hypertensive adults. In addition, during moderate cold exposure, sympathetic baroreflex sensitivity increased in hypertensive, but not normotensive, adults.
INTRODUCTION
Epidemiological studies have demonstrated an association between low ambient temperatures during the winter season and the risk of a cardiovascular event (4, 12, 40), with most of the attributable risk occurring on moderately cold days (12). During moderate cold exposure, cardiovascular-related morbidity and mortality increase disproportionately in hypertensive adults (HTN) (29). This increased risk has been attributed to impaired autonomic cardiovascular control in hypertension (14, 36). Given the critical role of the sympathetic nervous system in mediating the physiological responses to cold exposure (16, 30), coupled with the increasing prevalence of hypertension (57), delineating the mechanisms underlying alterations in neural cardiovascular function during moderate cold exposure in hypertension has clinical relevance.
Whole body cold stress elicits reflex activation of the sympathetic nervous system, evoking peripheral vasoconstriction and a robust systemic pressor response (~20 mmHg) (3, 20, 24, 25, 55). In young adults, moderate cold exposure [reductions in mean skin temperature (Tsk) using a water-perfused suit] does not alter muscle sympathetic nervous system activity (MSNA) (3, 20); however, in healthy older adults (~60 yr), this cooling stimulus evokes a significant increase in MSNA, contributing to exaggerated increases in blood pressure (BP) (20). The baroreflex operates as a negative feedback control system that responds to beat-to-beat changes in BP by reflexively adjusting autonomic outflow both to the heart and the peripheral vasculature and is critical for the control of BP during acute cardiovascular stressors (9). In healthy adults (3, 20), whole body cooling shifts the operating point of the baroreflex stimulus-response curve but does not change arterial baroreflex sensitivity within the operating range. Conceivably, hypertension-induced alterations in sympathetic baroreflex function could potentially contribute to aberrant BP and MSNA responsiveness to moderate cold exposure. To date, no studies have directly measured the MSNA response to whole body cooling in human hypertension, nor have any previous studies examined arterial baroreflex control of MSNA during cooling in HTN.
The purpose of the present investigation was to examine sympathetic control of BP during whole body cooling-induced reductions in mean Tsk in otherwise healthy middle-aged adults with primary hypertension. We tested the hypothesis that moderate cold exposure would elicit exaggerated increases in BP and MSNA in HTN compared with normotensive adults (NTN). We further hypothesized that arterial baroreflex control of MSNA would be altered during whole body cooling in HTN.
METHODS
All procedures and protocols were approved by The Pennsylvania State University Institutional Review Board and conformed to the guidelines set forth in the Declaration of Helsinki. Informed verbal and written consent was obtained voluntarily from all subjects before participation.
Subjects.
Ten NTN and 13 HTN participated in this study (Table 1). All participants underwent a complete medical screening, including physical examination, resting 12-lead electrocardiogram, and 12-h fasting blood chemistry (Quest Diagnostics, Pittsburgh, PA). Subjects were nonobese (body mass index <30 kg/m2), did not use tobacco products, and were recreationally active. No subjects had any evidence of overt cardiovascular disease, aside from hypertension, or any evidence or diagnosis of associated comorbidities, including renal, pulmonary, neurological, or dermatological disease. Women taking, or who had recently taken, hormone replacement therapy were excluded.
Table 1.
Subject characteristics
| Baseline Characteristic | NTN | HTN |
|---|---|---|
| n (M/F) | 10 (6/4) | 13 (7/6) |
| Age, yr | 53 ± 3 | 58 ± 2 |
| Height, cm | 174 ± 3 | 170 ± 2 |
| Mass, kg | 81 ± 3 | 79 ± 4 |
| BMI, kg/m2 | 26.9 ± 1.1 | 27.1 ± 0.8 |
| Screening systolic BP, mmHg† | 121 ± 4 | 136 ± 4* |
| Screening diastolic BP, mmHg† | 76 ± 4 | 89 ± 2* |
| 24-h Systolic BP, mmHg† | 110 ± 3 | 129 ± 3* |
| 24-h Diastolic BP, mmHg† | 72 ± 2 | 83 ± 2* |
| Experimental systolic BP, mmHg | 125 ± 3 | 146 ± 5* |
| Experimental diastolic BP, mmHg | 79 ± 1 | 88 ± 2* |
| Blood biochemistry | ||
| Hemoglobin, g/dl | 14.5 ± 0.4 | 14.4 ± 0.4 |
| Hematocrit, % | 43.0 ± 1.0 | 43.8 ± 1.3 |
| Serum sodium, mmol/l | 139.0 ± 0.7 | 138.1 ± 1.1 |
| Serum potassium, mmol/l | 4.4 ± 0.1 | 4.5 ± 0.1 |
| Serum chloride, mmol/l | 105.0 ± 0.7 | 102.7 ± 1.1 |
| Serum creatinine, mg/dl | 1.0 ± 0.1 | 0.9 ± 0.1 |
| HbA1c, % | 5.6 ± 0.1 | 5.5 ± 0.1 |
| Fasting total cholesterol, mg/dl | 185.2 ± 78 | 193.2 ± 9.9 |
| Fasting HDL, mg/dl | 48.1 ± 5.1 | 58.6 ± 5.5 |
| Fasting LDL, mg/dl | 104.0 ± 13.2 | 102.4 ± 12.5 |
| Fasting triglycerides, mg/dl | 117.4 ± 13.0 | 105.5 ± 18.7 |
Values are means ± SE; n, no. of subjects. M, males; F, females; NTN, normotensive; HTN, hypertensive; BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein. Resting seated experimental systolic and diastolic BP values were obtained upon arrival to the laboratory for the protocol visit.
P < 0.05 vs. NTN;
HTN were not withdrawn from antihypertensive medications for these BP measurements.
Consistent with the JNC7 classification criteria and guidelines (2), and assessed in accordance with American Heart Association guidelines (45), HTN had a resting seated systolic BP >140 mmHg or a diastolic BP >90 mmHg or were taking antihypertensive medication. To confirm the diagnosis of hypertension, ambulatory BP monitoring (Ambulo 2400; Mortara Instrument, Milwaukee, WI) was used because of its greater accuracy for describing the BP profile in daily routine and to avoid “white coat” hypertension (42). Ambulatory BP measures were obtained every 30 min while awake and every 60 min while asleep. The average values obtained in 24-h ambulatory monitoring are typically lower than those obtained by office measurements because of nocturnal dipping; thus, the consensus values for the diagnosis of hypertension using 24-h ambulatory measures are systolic BP >130 mmHg or diastolic blood pressure >80 mmHg (37, 42). These diagnostic thresholds for ambulatory BP monitoring yielded 10-yr cardiovascular risks similar to those using diagnostic values obtained on office measurement (33). Those participants taking antihypertensive medication (n = 10; angiotensin receptor blocker, n = 4; angiotensin converting enzyme inhibitor, n = 4; diuretic, n = 3; calcium channel blocker, n = 1) discontinued treatment for two full days before each experimental visit in an effort to reduce the confound of BP-lowering medication on data interpretation (15, 17); medications were not discontinued during 24-h ambulatory BP monitoring. For those participants who discontinued antihypertensive treatment for 48 h before each experimental visit, BP was monitored three times per day with a portable automatic BP monitor (BP742N; Omron Healthcare, Lake Forest, IL) to ensure subject safety during this medication-free period.
Experimental measurements.
Whole body mean Tsk was controlled using a water-perfused suit that covered the entire body except for the head, hands, feet, and lower left leg. Copper-constantan thermocouples were affixed to the surface of the skin at six sites (calf, thigh, abdomen, chest, shoulder, and back), and an unweighted average of these sites was used for continuous measurement of mean Tsk. Heat rate (HR) was continuously monitored using a standard lead II surface electrocardiogram (Cardiocap; GE Healthcare, Milwaukee, WI). Arterial BP was measured on a beat-to-beat basis using servo-controlled finger photoplethysmography (BMEYE; Nexfin, St. Louis, MO) placed on the middle finger of the left hand supported at the level of the heart. The changes in arterial BP measured via photoplethysmography provide an accurate estimate of directly measured intra-arterial BP (21, 48). Additionally, automated brachial artery BPs (Cardiocap; GE Healthcare) were measured on the right arm every 3 min to validate absolute Finometer-derived finger BP measurements. Respiratory movements were monitored using a strain-gauge pneumograph placed in a stable position over the abdomen (Pneumotrace; UFI, Morro Bay, CA) to ensure that subjects did not inadvertently perform Valsalva maneuvers during the protocol and to avoid the influence of any large respiratory excursions on neurocardiovascular variables.
Multiunit postganglionic MSNA was recorded using standard microneurographic techniques, as previously described (17, 20, 51). Briefly, a tungsten microelectrode was placed in muscle fascicles of the peroneal nerve near the left fibular head. A reference electrode was inserted 2–3 cm from the recording electrode. Signals were amplified, filtered (bandwidth: 0.7–2.0 kHz), rectified, and integrated (time constant 0.1 s) to obtain mean voltage neurograms (Nerve Traffic Analyzer 662C-3; University of Iowa Bioengineering, Iowa City, IA). MSNA was identified by the presence of spontaneous bursts with characteristic pulse synchronicity and by its responsiveness to end-expiratory breath holds but not to arousal stimuli or skin stimulation. Mean voltage neurograms were visually displayed and routed to a loudspeaker for continuous monitoring. All neural cardiovascular variables were acquired at a frequency of 1.0 kHz (Powerlab and LabChart; ADInstruments, Bella Vista, NSW, Australia).
Experimental protocol.
On the experimental day, participants were instructed to abstain from eating (4 h), caffeinated and alcoholic beverages (12 h), and strenuous physical activity (24 h). All experiments were conducted in a dimly lit room at an ambient air temperature of 22–24°C. Upon arrival and after thermocouples were affixed to the skin, subjects donned the water-perfused suit and were positioned supine. Next, subjects were instrumented for HR, BP, respiratory movements, and MSNA. Once all signals were acquired, data were collected for at least a 10-min baseline period at thermoneutrality (Tsk ~34°C) to determine resting neural cardiovascular variables. Thereafter, cool water (~16°C) was perfused through the suit to gradually lower mean Tsk from 34°C to 30.5°C (~30 min), where it was clamped for an additional 5 min. This target Tsk is above the threshold for shivering for most adults (5). This cooling stimulus elicits progressive reductions in Tsk without affecting core temperature (15, 19, 20, 50). Acceptable MSNA recordings were maintained in all subjects during whole body cooling.
Data and statistical analysis.
Resting values for HR, BP, and MSNA were calculated as mean values over the last 5 min of the initial thermoneutral baseline period and at each 0.5°C reduction in mean Tsk during whole body cooling (~1–2 min). MSNA was analyzed using a custom-designed LabVIEW program (10, 11), which generated synchronized beat-by-beat data of all recorded variables gated by the R-wave of the electrocardiogram. The MSNA signal was calibrated by assigning the voltage of the three largest bursts during baseline the value of 100 arbitrary units (AU), and all other bursts within the protocol were normalized with respect to this value. MSNA was quantified as burst frequency (bursts/min), burst incidence (bursts/100 heartbeats), total MSNA (AU/beat), and total activity (AU/min). Given that the absolute area of a burst is critically dependent on the location of the microelectrode in relation to the nerve fibers that are being recorded, and because this cannot be determined, our main study conclusions are based on burst frequency, not burst area.
Arterial baroreflex control of MSNA was evaluated during the initial thermoneutral baseline and also at mean Tsk 30.5°C by analyzing the relation between spontaneously occurring variations in diastolic BP and MSNA, as previously described in detail (18, 20, 22, 32, 43, 44, 58). Briefly, the diastolic BP for each cardiac cycle within each data collection period of interest was grouped in 2-mmHg pressure bins. The burst incidence for each pressure bin was calculated by determining the percentage of heart beats that were associated with a sympathetic burst. Total MSNA for each pressure bin was determined by calculating the total area of all MSNA bursts relative to the number of cardiac cycles (i.e., total burst area of all cardiac cycles within a given pressure bin divided by the number of cardiac cycles that occurred within that bin). If a burst did not occur, then a burst area value of zero was entered for that cardiac cycle, such that the average burst area of all cardiac cycles, regardless of whether a burst occurred, was determined. Pressure bins containing zero values for MSNA were included in the linear regression to eliminate subjectivity in the analysis and for consistency with the analysis methodology for the modified Oxford, widely considered the “gold standard” method for the evaluation of sympathetic baroreflex sensitivity (22, 46). Analyses following removal of these zero values yielded similar results; however, for consistency with previously published studies using this methodology, both by our laboratory and others (18, 20, 22, 28, 58), data are only presented based on analyses including pressure bins with zero values for MSNA.
The slope of the relation between MSNA and diastolic BP was identified using weighted linear regression analysis, with a minimum r value of 0.5 used as a criterion for accepting slopes (18, 20, 43, 44, 58). Two NTN and two HTN did not fit this criterion and were excluded from the analysis. The mean r value of the accepted slopes for the initial baseline was −0.76 ± 0.02 and for mean Tsk 30.5°C was −0.81 ± 0.01, indicating adequate fit of the linear regression analysis. Weighted linear regression accounts for the number of cardiac cycles within each pressure bin, removing bias because of bins containing fewer cardiac cycles (18, 20, 43, 44, 58). In NTN, the diastolic BP range used for the weighted linear regression analyses was 11 ± 2 mmHg at mean Tsk 34.0°C and 9 ± 1 mmHg at mean Tsk 30.5°C; in HTN, the range was 11 ± 1 mmHg at mean Tsk 34.0°C and 8 ± 1 mmHg at mean Tsk 30.5°C. Importantly, this technique to examine spontaneous sympathetic baroreflex sensitivity is highly correlated with arterial baroreflex-MSNA gain derived using the more invasive modified Oxford approach (i.e., bolus iv administration of sodium nitroprusside and phenylephrine) (22), thus providing the rationale for using these measures in the current study.
Data were analyzed using two-way (group × temperature) mixed-model repeated-measures ANOVA (version 9.1.3; SAS, Cary, NC). When appropriate, post hoc Bonferroni corrections were applied to correct for multiple comparisons. Subject characteristics were compared using unpaired t-tests. Results are reported as means ± SE, and the α-level was set at P < 0.05.
RESULTS
Participants were well matched for age, anthropometric characteristics, and blood biochemistry (Table 1). By study design, resting systolic and diastolic BP at the screening visit, and 24-h records of systolic and diastolic BP, were significantly elevated in HTN (P < 0.01). Systolic and diastolic BP were also elevated in HTN at the time of the experimental visit (P < 0.01).
Baseline mean Tsk was not different between groups (NTN: 34.2 ± 0.08 vs. HTN: 34.1 ± 0.04°C; P = 0.99). Whole body cooling decreased mean Tsk to 30.5°C in all subjects, with no group differences in the rate of cooling (NTN: 0.11 ± 0.004 vs. HTN: 0.11 ± 0.004°C/min; P = 0.87).
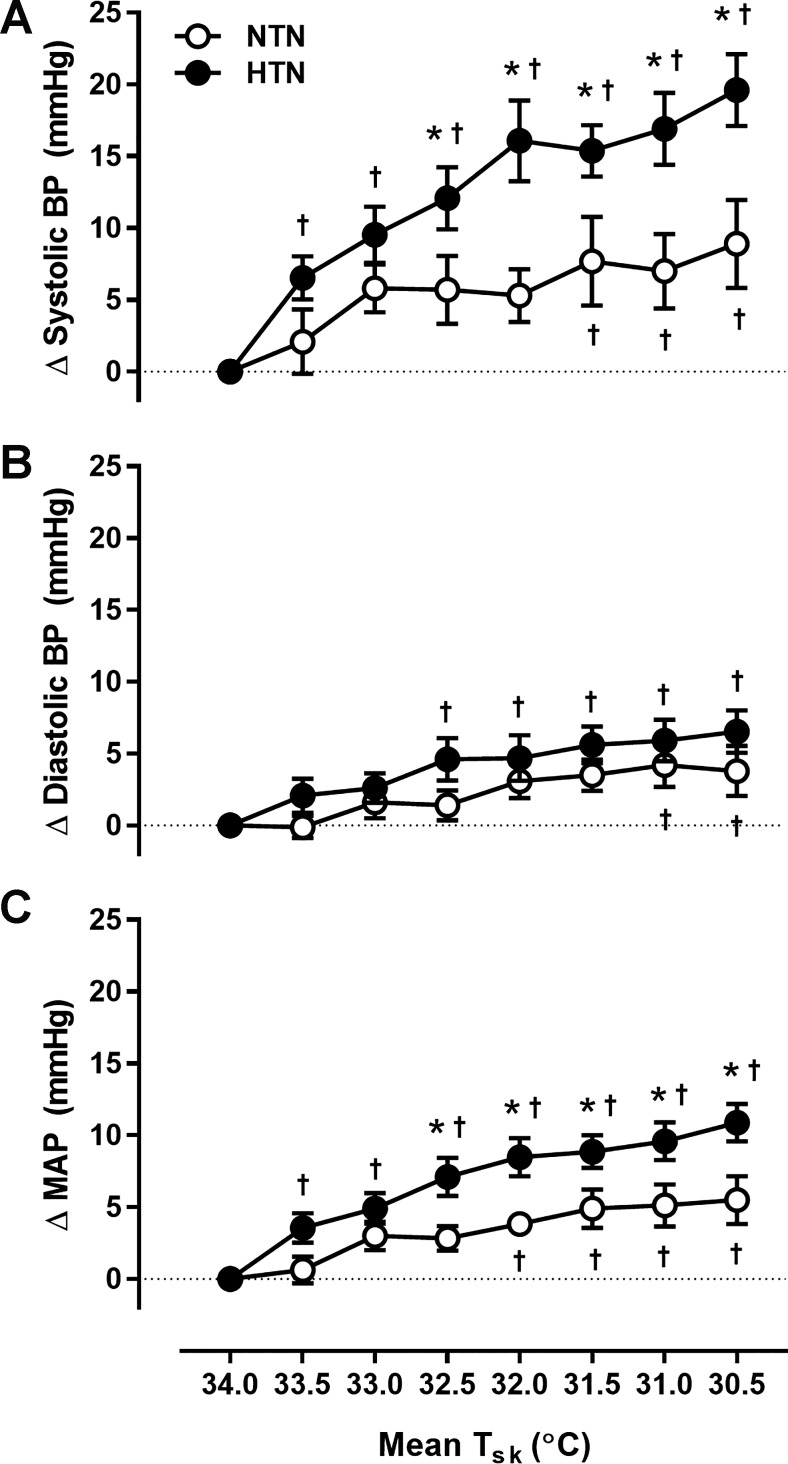
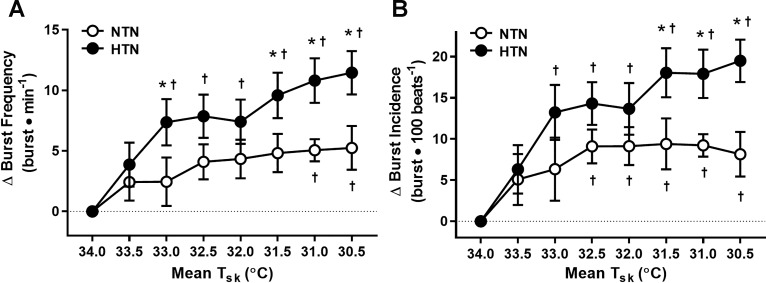
Absolute systolic and diastolic BP remained higher throughout cooling in HTN (Table 2). Cooling elicited increases in blood pressure from baseline in both groups; however, the pressor response to cooling was exaggerated in HTN (Table 2 and Fig. 1). There were no group- or cooling-induced differences in HR (P > 0.05 for all; Table 2). Absolute MSNA at thermoneutrality (Tsk = 34°C) and during whole body cooling (Tsk = 30.5°C) was not different between groups (Table 2). The MSNA response to cooling (i.e., the increase from baseline at Tsk = 34°C) is presented in Fig. 2. The cooling-induced increases in burst frequency and burst incidence were greater in HTN adults (Fig. 2). Similar results were obtained for total MSNA (NTN: Δ6.7 ± 3.0 vs. HTN: Δ15.7 ± 2.1 AU/beat; P = 0.02) and total activity (NTN: Δ421 ± 170 vs. HTN: Δ924 ± 142 AU/min; P = 0.03) responsiveness during cooling (i.e., at mean Tsk = 30.5°C).
Table 2.
Hemodynamic and sympathetic variables at thermoneutrality and during cold stress
| NTN |
HTN |
|||
|---|---|---|---|---|
| Variable | Tsk 34.0°C | Tsk 30.5°C | Tsk 34.0°C | Tsk 30.5°C |
| Systolic BP, mmHg | 121 ± 3 | 130 ± 5 | 130 ± 3 | 149 ± 4*† |
| Diastolic BP, mmHg | 76 ± 2 | 80 ± 3 | 81 ± 2 | 88 ± 2*† |
| MAP, mmHg | 91 ± 2 | 97 ± 3 | 97 ± 2 | 108 ± 2*† |
| HR, beats/min | 57 ± 2 | 57 ± 2 | 61 ± 2 | 59 ± 2 |
| Respiration rate, breaths/min | 11 ± 1 | 12 ± 1 | 14 ± 1 | 14 ± 1 |
| Burst frequency, bursts/min | 29 ± 3 | 34 ± 3 | 28 ± 4 | 40 ± 3 |
| Burst incidence, bursts/100 beats | 51 ± 5 | 59 ± 4 | 47 ± 5 | 66 ± 5 |
| Total MSNA, AU | 32.3 ± 3.4 | 39.1 ± 3.8 | 29.2 ± 3.4 | 45.0 ± 4.0 |
| Total activity, AU/min | 1,823 ± 176 | 2,244 ± 213 | 1,784 ± 249 | 2,707 ± 289 |
Values are means ± SE. Tsk, mean skin temperature; MAP, mean arterial pressure; HR, heart rate; AU, arbitrary units; MSNA, muscle sympathetic nerve activity.
P < 0.05 vs. NTN;
P < 0.05 vs. mean Tsk 34.0°C.
Fig. 1.
Group summary data for the increase (Δ value) in systolic (A) and diastolic (B) blood pressure (BP) and mean arterial pressure (MAP; C) at each 0.5°C decrease in mean skin temperature (Tsk) in normotensive (NTN; open symbols) and hypertensive (HTN; filled symbols) adults. The broken line represents baseline. *P < 0.05 vs. NTN; †P < 0.05 vs. mean Tsk 34°C.
Fig. 2.
Group summary data for the increase (Δ value) in muscle sympathetic nerve activity (MSNA) burst frequency (A) and burst incidence (B) at each 0.5°C decrease in mean Tsk in NTN (open symbols) and HTN (filled symbols) adults. The broken line represents baseline. *P < 0.05 vs. NTN; †P < 0.05 vs. mean Tsk 34°C.
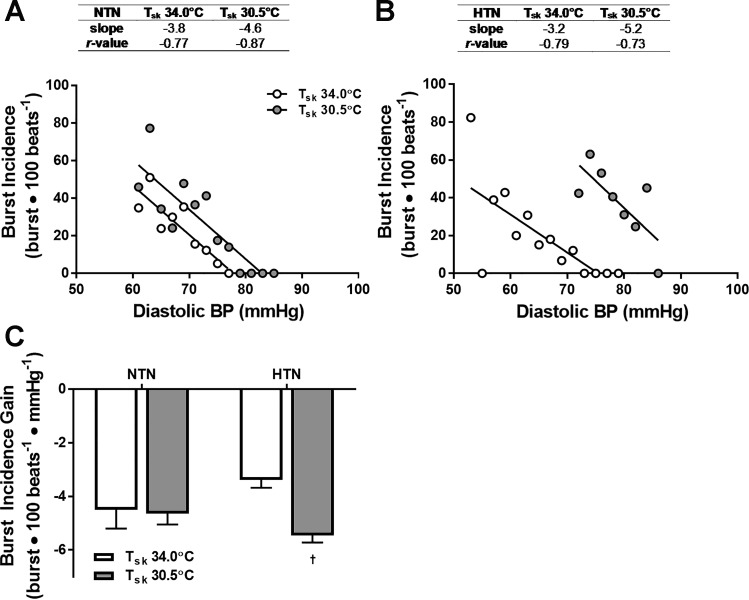
The slopes of the relation between MSNA burst incidence and diastolic BP at mean Tsk of 34°C and 30.5°C are shown in Fig. 3. In NTN, the linear relation did not change during cold stress (Fig. 3, A and C). However, the relation between burst incidence and diastolic BP was shifted rightward and upward with cooling in HTN, indicating a cold-mediated resetting of arterial baroreflex control of MSNA to the higher BP and MSNA. Moreover, the slope of the linear relation between diastolic BP and burst incidence was more negative (i.e., increased) in HTN during whole body cooling (Fig. 3, B and C). Arterial baroreflex gain of total MSNA also increased during cold exposure in HTN (mean Tsk 34.0°C: −2.0 ± 0.3 vs. mean Tsk 30.5°C: −3.8 ± 0.4 AU·beat−1·mmHg−1; P < 0.01) but not NTN (mean Tsk 34.0°C: −2.7 ± 0.6 vs. mean Tsk 30.5°C: −3.7 ± 0.4 AU·beat−1·mmHg−1; P = 0.20).
Fig. 3.
Representative linear relations between burst incidence and diastolic BP in one NTN (A) and one HTN (B) adult during thermoneutral baseline (i.e., mean Tsk 34.0°C; open symbols) and during cooling (i.e., mean Tsk 30.5°C; gray symbols) and group summary data (C). †P < 0.05 vs. mean Tsk 34°C.
DISCUSSION
The primary finding of the current study was that moderate cooling-induced decreases in mean Tsk elicited a greater pressor response, coupled with an exaggerated MSNA response, in HTN. Additionally, sympathetic baroreflex sensitivity increased during whole body cooling in HTN, but not NTN, indicating a greater buffering capacity of the baroreflex during cold stress. These newly documented alterations in sympathetic function in human hypertension during moderate cold exposure are consistent with the increased cold-associated cardiovascular risk in HTN.
Whole body cooling elicits increases in BP (3, 20, 24, 25, 55). In the present study, cooling elicited increases in BP in NTN, consistent with the responses noted in previous studies (15, 20, 24–27). In HTN, BP was elevated at thermoneutrality and remained higher throughout cooling. Moreover, the cooling-induced increase in BP was exaggerated in HTN. Perhaps surprisingly, the limited studies that have measured BP during moderate cold exposure in HTN have reported that the BP response to cooling was not different between HTN and NTN (25–27). However, in those studies, the mode of cold exposure involved a rapid and robust reduction in facial temperature (from 30°C to 15°C in 10 min) with cooling of the remainder of the body prevented by winter clothing. Facial cooling elicits sympathetic activation and vasoconstriction, resulting in increases in peripheral BP (8, 23) as high as 60 mmHg in some individuals (25). Thus, in the aforementioned studies (25–27), although the BP responses to cold exposure tended to be higher in HTN, it is plausible that such large increases in BP in the NTN group explain the lack of statistical difference in BP responsiveness between groups. In a recent study in our laboratory using a whole body cooling protocol identical to that in the present study (15), absolute BP was greater in HTN compared with NTN throughout cooling; however, the increase in BP, although qualitatively larger in HTN, was not statistically different between groups. The precise reason(s) for these disparate findings remains unclear. Nonetheless, an exaggerated cold-induced pressor response against the background of an already elevated baseline BP at thermoneutrality and throughout cooling in HTN (15) likely imparts elevated cardiovascular risk.
Relatively few studies have measured MSNA during precisely controlled whole body cooling-induced reductions in mean Tsk (3, 20). In these studies, cooling elicited pronounced increases in MSNA burst frequency and strength in healthy older, but not young, adults. The increases in sympathetic outflow in older subjects were accompanied by greater increases in BP throughout cooling (20), presumably via increases in peripheral and visceral vascular resistance (55). To date, few studies have examined sympathetic reactivity to cold exposure in HTN (26, 27). In those studies, when HR and BP variability were used to assess autonomic function, perhaps surprisingly, the investigators did not observe sympathetic hyperactivity during cold exposure in HTN. Rather, they reported blunted low-frequency BP variability, considered a potential surrogate measure of vascular sympathetic activity (31), in HTN subjects exposed to environmental cold (26, 27). The authors speculated that blunted sympathetic activity in response to cooling reflects either saturation of sympathetic vascular oscillatory activity or downregulation of sympathetic responsiveness at a central or vascular level. However, these studies are limited in their interpretation by their use of indirect indexes of sympathetic activation (7). Using microneurography to directly record efferent sympathetic outflow, we report significant cooling-induced increases in MSNA in HTN that are nearly double the increases observed in NTN. The exaggerated sympathetic responsiveness to moderate reductions in mean Tsk in HTN in the present study is consistent with our previous findings demonstrating greater increases in skin sympathetic nervous system activity in HTN during a similar cooling paradigm (15). Importantly, in our previous study, the greater increases in neural outflow directed to the cutaneous vasculature were linearly related to greater reductions in skin blood flow in the innervated dermatome, reflective of preserved signal transduction in HTN (15). Collectively, it appears that hypertension is characterized by greater increases in directly measured sympathetic outflow directed to the peripheral vasculature (i.e., both muscle and skin) during moderate whole body cold exposure.
In young healthy adults, the sensitivity of arterial baroreflex control of MSNA (i.e., the slope of the relation between MSNA and diastolic BP) within the operating range, assessed using pharmacological manipulations in BP (3) and during spontaneous fluctuations in BP (20), is not altered during whole body cooling. However, reductions in mean Tsk shift the operating point, defined as the mean MSNA and BP for both thermoneutral and cold conditions, rightward to operate around the cooling-induced increase in BP (3, 20). Preserved sympathetic baroreflex function during cold exposure is also evident in healthy older adults (20). Given the critical role of the baroreflex in modulating the rapid reflex adjustments that accompany acute cardiovascular stressors (9), we reasoned that alterations in sympathetic baroreflex function may contribute to altered BP regulation during cold exposure in HTN adults, resulting in a reduced ability to appropriately buffer increases in BP and MSNA.
To address this possibility, we quantified sympathetic baroreflex sensitivity around the operating point of the arterial baroreflex by analyzing the relation between spontaneously occurring variations in diastolic BP and MSNA. There were no differences in arterial baroreflex control of MSNA between groups at thermoneutrality or during cold stress, which is consistent with some (6, 13), but not all (35, 38), previous reports. In line with the previous report in healthy older adults (20), there was no change in the gain of arterial baroreflex control of MSNA during whole body cooling in NTN subjects tested here. However, cold exposure enhanced sympathetic baroreflex sensitivity in HTN subjects, that is, exaggerated increases in BP and MSNA were observed during cooling in HTN adults despite an increase in the sensitivity of arterial baroreflex control of MSNA. Were it not for an increase in sympathetic baroreflex sensitivity, the BP and MSNA responsiveness to cold exposure may be even greater.
Although we demonstrated an increase in arterial baroreflex gain for the control of both MSNA burst incidence and total MSNA, considerations for the use of one over the other to derive spontaneous baroreflex indexes is warranted. In this regard, gain of arterial baroreflex control of MSNA burst incidence is similar to sympathetic baroreflex sensitivity obtained using more invasive pharmacological manipulations in BP (22, 52). In contrast, the gain of arterial baroreflex control of MSNA burst area calculated using spontaneous techniques is likely influenced to a greater degree by nonbaroreflex inputs (22, 32), potentially limiting interpretation. Moreover, the strength of the relation between MSNA and diastolic BP is much stronger for burst incidence than for burst area (22, 32, 43, 46, 58), and, accordingly, our primary interpretation of sympathetic baroreflex sensitivity is based on burst incidence. Finally, because the methodology to derive spontaneous baroreflex sensitivity does not model the full stimulus-response curve and only provides gain around the operating point, caution should be used in interpreting direct comparisons of arterial baroreflex sensitivity between groups. Although sympathetic baroreflex sensitivity at thermoneutrality is qualitatively lower in HTN, consistent with studies using pharmacologically induced changes in BP (35, 38), this is not a universal finding (6, 13). Thus, future studies designed to include a more robust investigation of arterial baroreflex function are necessary to more mechanistically examine whether alterations in baroreflex function contribute to impaired neurocirculatory modulation during cooling in hypertension.
In the present study, MSNA at rest was not different between groups, nor were there differences in absolute MSNA during whole body cooling. There is significant variability in the human-based literature regarding elevations in basal MSNA in hypertension, even across studies using direct intraneural recordings of sympathetic outflow (1, 6, 17, 41, 53, 54). Furthermore, the influence of chronic antihypertensive treatment on sympathetic activity has been documented (34, 49); thus, it is possible that the inclusion of medicated HTN subjects limited our ability to discern group differences in MSNA at rest. However, previous studies using similar inclusion criteria for HTN patients have demonstrated exaggerated MSNA responsiveness to a variety of laboratory perturbations (6, 15, 17, 47). Consistent with these findings, and in line with our original hypothesis, the HTN adults in the present investigation, including subjects prescribed antihypertensive medication, clearly demonstrated augmented increases in BP and MSNA during moderate cold exposure.
In conclusion, the principal finding of this study was that moderate whole body cooling increased BP and MSNA to a greater extent in HTN compared with NTN. Second, the sensitivity of arterial baroreflex control of MSNA, assessed as the linear relation between spontaneously occurring variations in diastolic BP and MSNA, increased during whole body cold exposure in HTN, but not NTN. Taken together, these findings suggest alterations in sympathetic function during moderate whole body cooling in HTN, providing additional mechanistic insight into increases in cold exposure-related cardiovascular risk in hypertension (12, 39, 56).
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants HL-120471 (J. L. Greaney) and HL-093238 (L. M. Alexander).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.L.G., W.L.K., and L.M.A. conceived and designed research; J.L.G. performed experiments; J.L.G. analyzed data; J.L.G., W.L.K., and L.M.A. interpreted results of experiments; J.L.G. prepared figures; J.L.G. drafted manuscript; J.L.G., W.L.K., and L.M.A. edited and revised manuscript; J.L.G., W.L.K., and L.M.A. approved final version of manuscript.
ACKNOWLEDGMENTS
The time and effort expended by all the volunteer subjects are appreciated. We thank Dr. Paul J. Fadel for the use of the analysis program. We are grateful for the assistance of Susan Slimak and Jane Pierzga.
REFERENCES
- 1.Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension 14: 177–183, 1989. doi: 10.1161/01.HYP.14.2.177. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee . Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42: 1206–1252, 2003. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 3.Cui J, Durand S, Crandall CG. Baroreflex control of muscle sympathetic nerve activity during skin surface cooling. J Appl Physiol (1985) 103: 1284–1289, 2007. doi: 10.1152/japplphysiol.00115.2007. [DOI] [PubMed] [Google Scholar]
- 4.Danet S, Richard F, Montaye M, Beauchant S, Lemaire B, Graux C, Cottel D, Marécaux N, Amouyel P. Unhealthy effects of atmospheric temperature and pressure on the occurrence of myocardial infarction and coronary deaths. A 10-year survey: the Lille-World Health Organization MONICA project (Monitoring trends and determinants in cardiovascular disease). Circulation 100: E1–E7, 1999. doi: 10.1161/01.CIR.100.1.e1. [DOI] [PubMed] [Google Scholar]
- 5.DeGroot DW, Havenith G, Kenney WL. Responses to mild cold stress are predicted by different individual characteristics in young and older subjects. J Appl Physiol (1985) 101: 1607–1615, 2006. doi: 10.1152/japplphysiol.00717.2006. [DOI] [PubMed] [Google Scholar]
- 6.Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ, Farquhar WB. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 299: H1318–H1327, 2010. doi: 10.1152/ajpheart.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Draghici AE, Taylor JA. The physiological basis and measurement of heart rate variability in humans. J Physiol Anthropol 35: 22, 2016. doi: 10.1186/s40101-016-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards DG, Roy MS, Prasad RY. Wave reflection augments central systolic and pulse pressures during facial cooling. Am J Physiol Heart Circ Physiol 294: H2535–H2539, 2008. doi: 10.1152/ajpheart.01369.2007. [DOI] [PubMed] [Google Scholar]
- 9.Fadel PJ, Raven PB. Human investigations into the arterial and cardiopulmonary baroreflexes during exercise. Exp Physiol 97: 39–50, 2012. doi: 10.1113/expphysiol.2011.057554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fairfax ST, Holwerda SW, Credeur DP, Zuidema MY, Medley JH, Dyke PC II, Wray DW, Davis MJ, Fadel PJ. The role of α-adrenergic receptors in mediating beat-by-beat sympathetic vascular transduction in the forearm of resting man. J Physiol 591: 3637–3649, 2013. doi: 10.1113/jphysiol.2013.250894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fairfax ST, Padilla J, Vianna LC, Davis MJ, Fadel PJ. Spontaneous bursts of muscle sympathetic nerve activity decrease leg vascular conductance in resting humans. Am J Physiol Heart Circ Physiol 304: H759–H766, 2013. doi: 10.1152/ajpheart.00842.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gasparrini A, Guo Y, Hashizume M, Lavigne E, Zanobetti A, Schwartz J, Tobias A, Tong S, Rocklöv J, Forsberg B, Leone M, De Sario M, Bell ML, Guo YL, Wu CF, Kan H, Yi SM, de Sousa Zanotti Stagliorio Coelho M, Saldiva PH, Honda Y, Kim H, Armstrong B. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet 386: 369–375, 2015. doi: 10.1016/S0140-6736(14)62114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grassi G, Cattaneo BM, Seravalle G, Lanfranchi A, Mancia G. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension 31: 68–72, 1998. doi: 10.1161/01.HYP.31.1.68. [DOI] [PubMed] [Google Scholar]
- 14.Grassi G, Ram VS. Evidence for a critical role of the sympathetic nervous system in hypertension. J Am Soc Hypertens 10: 457–466, 2016. doi: 10.1016/j.jash.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Greaney JL, Kenney WL, Alexander LM. Neurovascular mechanisms underlying augmented cold-induced reflex cutaneous vasoconstriction in human hypertension. J Physiol 595: 1687–1698, 2017. doi: 10.1113/JP273487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greaney JL, Kenney WL, Alexander LM. Sympathetic regulation during thermal stress in human aging and disease. Auton Neurosci 196: 81–90, 2016. doi: 10.1016/j.autneu.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greaney JL, Matthews EL, Boggs ME, Edwards DG, Duncan RL, Farquhar WB. Exaggerated exercise pressor reflex in adults with moderately elevated systolic blood pressure: role of purinergic receptors. Am J Physiol Heart Circ Physiol 306: H132–H141, 2014. doi: 10.1152/ajpheart.00575.2013. [DOI] [PubMed] [Google Scholar]
- 18.Greaney JL, Schwartz CE, Edwards DG, Fadel PJ, Farquhar WB. The neural interaction between the arterial baroreflex and muscle metaboreflex is preserved in older men. Exp Physiol 98: 1422–1431, 2013. doi: 10.1113/expphysiol.2013.073189. [DOI] [PubMed] [Google Scholar]
- 19.Greaney JL, Stanhewicz AE, Kenney WL, Alexander LM. Impaired increases in skin sympathetic nerve activity contribute to age-related decrements in reflex cutaneous vasoconstriction. J Physiol 593: 2199–2211, 2015. doi: 10.1113/JP270062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greaney JL, Stanhewicz AE, Kenney WL, Alexander LM. Muscle sympathetic nerve activity during cold stress and isometric exercise in healthy older adults. J Appl Physiol (1985) 117: 648–657, 2014. doi: 10.1152/japplphysiol.00516.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harms MP, Wesseling KH, Pott F, Jenstrup M, Van Goudoever J, Secher NH, Van Lieshout JJ. Continuous stroke volume monitoring by modelling flow from non-invasive measurement of arterial pressure in humans under orthostatic stress. Clin Sci (Lond) 97: 291–301, 1999. doi: 10.1042/cs0970291. [DOI] [PubMed] [Google Scholar]
- 22.Hart EC, Joyner MJ, Wallin BG, Karlsson T, Curry TB, Charkoudian N. Baroreflex control of muscle sympathetic nerve activity: a nonpharmacological measure of baroreflex sensitivity. Am J Physiol Heart Circ Physiol 298: H816–H822, 2010. doi: 10.1152/ajpheart.00924.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heath ME, Downey JA. The cold face test (diving reflex) in clinical autonomic assessment: methodological considerations and repeatability of responses. Clin Sci (Lond) 78: 139–147, 1990. doi: 10.1042/cs0780139. [DOI] [PubMed] [Google Scholar]
- 24.Hess KL, Wilson TE, Sauder CL, Gao Z, Ray CA, Monahan KD. Aging affects the cardiovascular responses to cold stress in humans. J Appl Physiol (1985) 107: 1076–1082, 2009. doi: 10.1152/japplphysiol.00605.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hintsala H, Kandelberg A, Herzig KH, Rintamäki H, Mäntysaari M, Rantala A, Antikainen R, Keinänen-Kiukaanniemi S, Jaakkola JJ, Ikäheimo TM. Central aortic blood pressure of hypertensive men during short-term cold exposure. Am J Hypertens 27: 656–664, 2014. doi: 10.1093/ajh/hpt136. [DOI] [PubMed] [Google Scholar]
- 26.Hintsala H, Kenttä TV, Tulppo M, Kiviniemi A, Huikuri HV, Mäntysaari M, Keinänen-Kiukaannemi S, Bloigu R, Herzig KH, Antikainen R, Rintamäki H, Jaakkola JJ, Ikäheimo TM. Cardiac repolarization and autonomic regulation during short-term cold exposure in hypertensive men: an experimental study. PLoS One 9: e99973, 2014. doi: 10.1371/journal.pone.0099973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hintsala HE, Kiviniemi AM, Tulppo MP, Helakari H, Rintamäki H, Mäntysaari M, Herzig KH, Keinänen-Kiukaanniemi S, Jaakkola JJ, Ikäheimo TM. Hypertension Does Not Alter the Increase in Cardiac Baroreflex Sensitivity Caused by Moderate Cold Exposure. Front Physiol 7: 204, 2016. doi: 10.3389/fphys.2016.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holwerda SW, Vianna LC, Restaino RM, Chaudhary K, Young CN, Fadel PJ. Arterial baroreflex control of sympathetic nerve activity and heart rate in patients with type 2 diabetes. Am J Physiol Heart Circ Physiol 311: H1170–H1179, 2016. doi: 10.1152/ajpheart.00384.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikäheimo TM, Lehtinen T, Antikainen R, Jokelainen J, Näyhä S, Hassi J, Keinänen-Kiukaanniemi S, Laatikainen T, Jousilahti P, Jaakkola JJ. Cold-related cardiorespiratory symptoms among subjects with and without hypertension: the National FINRISK Study 2002. Eur J Public Health 24: 237–243, 2014. doi: 10.1093/eurpub/ckt078. [DOI] [PubMed] [Google Scholar]
- 30.Johnson JM, Minson CT, Kellogg DL Jr. Cutaneous vasodilator and vasoconstrictor mechanisms in temperature regulation. Compr Physiol 4: 33–89, 2014. doi: 10.1002/cphy.c130015. [DOI] [PubMed] [Google Scholar]
- 31.Julien C. The enigma of Mayer waves: facts and models. Cardiovasc Res 70: 12–21, 2006. doi: 10.1016/j.cardiores.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol 531: 861–869, 2001. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kikuya M, Hansen TW, Thijs L, Björklund-Bodegård K, Kuznetsova T, Ohkubo T, Richart T, Torp-Pedersen C, Lind L, Ibsen H, Imai Y, Staessen JA; International Database on Ambulatory blood pressure monitoring in relation to Cardiovascular Outcomes Investigators . Diagnostic thresholds for ambulatory blood pressure monitoring based on 10-year cardiovascular risk. Circulation 115: 2145–2152, 2007. doi: 10.1161/CIRCULATIONAHA.106.662254. [DOI] [PubMed] [Google Scholar]
- 34.Klein IH, Ligtenberg G, Oey PL, Koomans HA, Blankestijn PJ. Enalapril and losartan reduce sympathetic hyperactivity in patients with chronic renal failure. J Am Soc Nephrol 14: 425–430, 2003. doi: 10.1097/01.ASN.0000045049.72965.B7. [DOI] [PubMed] [Google Scholar]
- 35.Laterza MC, de Matos LD, Trombetta IC, Braga AM, Roveda F, Alves MJ, Krieger EM, Negrão CE, Rondon MU. Exercise training restores baroreflex sensitivity in never-treated hypertensive patients. Hypertension 49: 1298–1306, 2007. doi: 10.1161/HYPERTENSIONAHA.106.085548. [DOI] [PubMed] [Google Scholar]
- 36.Liu C, Yavar Z, Sun Q. Cardiovascular response to thermoregulatory challenges. Am J Physiol Heart Circ Physiol 309: H1793–H1812, 2015. doi: 10.1152/ajpheart.00199.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F; Task Force Members . 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 31: 1281–1357, 2013. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 38.Matsukawa T, Gotoh E, Hasegawa O, Shionoiri H, Tochikubo O, Ishii M. Reduced baroreflex changes in muscle sympathetic nerve activity during blood pressure elevation in essential hypertension. J Hypertens 9: 537–542, 1991. doi: 10.1097/00004872-199106000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Minami J, Kawano Y, Ishimitsu T, Yoshimi H, Takishita S. Seasonal variations in office, home and 24 h ambulatory blood pressure in patients with essential hypertension. J Hypertens 14: 1421–1425, 1996. doi: 10.1097/00004872-199612000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Morabito M, Modesti PA, Cecchi L, Crisci A, Orlandini S, Maracchi G, Gensini GF. Relationships between weather and myocardial infarction: a biometeorological approach. Int J Cardiol 105: 288–293, 2005. doi: 10.1016/j.ijcard.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 41.Mörlin C, Wallin BG, Eriksson BM. Muscle sympathetic activity and plasma noradrenaline in normotensive and hypertensive man. Acta Physiol Scand 119: 117–121, 1983. doi: 10.1111/j.1748-1716.1983.tb07315.x. [DOI] [PubMed] [Google Scholar]
- 42.O’Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A, Zhang Y; European Society of Hypertension Working Group on Blood Pressure Monitoring . European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens 31: 1731–1768, 2013. doi: 10.1097/HJH.0b013e328363e964. [DOI] [PubMed] [Google Scholar]
- 43.Ogoh S, Fisher JP, Raven PB, Fadel PJ. Arterial baroreflex control of muscle sympathetic nerve activity in the transition from rest to steady-state dynamic exercise in humans. Am J Physiol Heart Circ Physiol 293: H2202–H2209, 2007. doi: 10.1152/ajpheart.00708.2007. [DOI] [PubMed] [Google Scholar]
- 44.Ogoh S, Fisher JP, Young CN, Raven PB, Fadel PJ. Transfer function characteristics of the neural and peripheral arterial baroreflex arcs at rest and during postexercise muscle ischemia in humans. Am J Physiol Heart Circ Physiol 296: H1416–H1424, 2009. doi: 10.1152/ajpheart.01223.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ; Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research . Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 45: 142–161, 2005. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 46.Rudas L, Crossman AA, Morillo CA, Halliwill JR, Tahvanainen KU, Kuusela TA, Eckberg DL. Human sympathetic and vagal baroreflex responses to sequential nitroprusside and phenylephrine. Am J Physiol Heart Circ Physiol 276: H1691–H1698, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Sausen MT, Delaney EP, Stillabower ME, Farquhar WB. Enhanced metaboreflex sensitivity in hypertensive humans. Eur J Appl Physiol 105: 351–356, 2009. doi: 10.1007/s00421-008-0910-8. [DOI] [PubMed] [Google Scholar]
- 48.Shi X, Gallagher KM, SMith SA, Bryant KH, Raven PB. Diminished forearm vasomotor response to central hypervolemic loading in aerobically fit individuals. Med Sci Sports Exerc 28: 1388–1395, 1996. doi: 10.1097/00005768-199611000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Siddiqi L, Oey PL, Blankestijn PJ. Aliskiren reduces sympathetic nerve activity and blood pressure in chronic kidney disease patients. Nephrol Dial Transplant 26: 2930–2934, 2011. doi: 10.1093/ndt/gfq857. [DOI] [PubMed] [Google Scholar]
- 50.Thompson CS, Kenney WL. Altered neurotransmitter control of reflex vasoconstriction in aged human skin. J Physiol 558: 697–704, 2004. doi: 10.1113/jphysiol.2004.065714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vallbo AB, Hagbarth KE, Torebjörk HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979. [DOI] [PubMed] [Google Scholar]
- 52.van Schelven LJ, Karemaker JM, Blankestijn PJ, Oey PL. Short-term sympathetic baroreflex sensitivity increases at lower blood pressures. Clin Neurophysiol 119: 869–879, 2008. doi: 10.1016/j.clinph.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 53.Wallin BG, Delius W, Hagbarth KE. Comparison of sympathetic nerve activity in normotensive and hypertensive subjects. Circ Res 33: 9–21, 1973. doi: 10.1161/01.RES.33.1.9. [DOI] [PubMed] [Google Scholar]
- 54.Wallin BG, Sundlöf G. A quantitative study of muscle nerve sympathetic activity in resting normotensive and hypertensive subjects. Hypertension 1: 67–77, 1979. doi: 10.1161/01.HYP.1.2.67. [DOI] [PubMed] [Google Scholar]
- 55.Wilson TE, Sauder CL, Kearney ML, Kuipers NT, Leuenberger UA, Monahan KD, Ray CA. Skin-surface cooling elicits peripheral and visceral vasoconstriction in humans. J Appl Physiol (1985) 103: 1257–1262, 2007. doi: 10.1152/japplphysiol.00401.2007. [DOI] [PubMed] [Google Scholar]
- 56.Woodhouse PR, Khaw KT, Plummer M. Seasonal variation of blood pressure and its relationship to ambient temperature in an elderly population. J Hypertens 11: 1267–1274, 1993. doi: 10.1097/00004872-199311000-00015. [DOI] [PubMed] [Google Scholar]
- 57.Writing Group Members; Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 133: e38–e360, 2016. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 58.Young CN, Deo SH, Chaudhary K, Thyfault JP, Fadel PJ. Insulin enhances the gain of arterial baroreflex control of muscle sympathetic nerve activity in humans. J Physiol 588: 3593–3603, 2010. doi: 10.1113/jphysiol.2010.191866. [DOI] [PMC free article] [PubMed] [Google Scholar]