Abstract
As dysfunction of the vascular system is an early, modifiable step in the progression of many cardiovascular diseases, there is demand for methods to monitor the health of the vascular system noninvasively in clinical and research settings. Validated by very good agreement with more technical assessments of vascular function, like intra-arterial drug infusions and flow-mediated dilation, the passive leg movement (PLM) technique has emerged as a powerful, yet relatively simple, test of peripheral vascular function. In the PLM technique, the change in leg blood flow elicited by the passive movement of the leg through a 90° range of motion is quantified with Doppler ultrasound. This relatively easy-to-learn test has proven to be ≤80% dependent on nitric oxide bioavailability and is especially adept at determining peripheral vascular function across the spectrum of cardiovascular health. Indeed, multiple reports have documented that individuals with decreased cardiovascular health such as the elderly and those with heart failure tend to exhibit a substantially blunted PLM-induced hyperemic response (~50 and ~85% reduction, respectively) compared with populations with good cardiovascular health such as young individuals. As specific guidelines have not yet been put forth, the purpose of this Cores of Reproducibility in Physiology (CORP) article is to provide a comprehensive reference for the assessment and interpretation of vascular function with PLM with the aim to increase reproducibility and consistency among studies and facilitate the use of PLM as a research tool with clinical relevance.
Keywords: endothelial function, nitric oxide, cardiovascular health, guidelines
Significance of Vascular (Dys)Function
A body of evidence indicates that the increase in blood flow (i.e., hyperemia) induced by the passive movement of an individual’s leg (PLM) provides a simple and insightful assessment of peripheral vascular function (45, 71). With up to 80% of PLM-induced hyperemia being dependent on nitric oxide (NO) bioavailability (24, 45, 71), this technique has proven particularly good at discriminating vascular function across the spectrum of cardiovascular health (Fig. 1; Refs. 22, 35, 45, 70, 81). In contrast to other assessments of vascular function, which are often deceptively technically challenging (25), the PLM technique is relatively easy to master. As specific guidelines have not yet been put forth, the purpose of this Cores of Reproducibility in Physiology (CORP) article is to provide a comprehensive reference for the assessment and interpretation of peripheral vascular function with PLM with the aim to increase reproducibility and consistency among studies and facilitate the use of PLM as a research tool with clinical relevance.
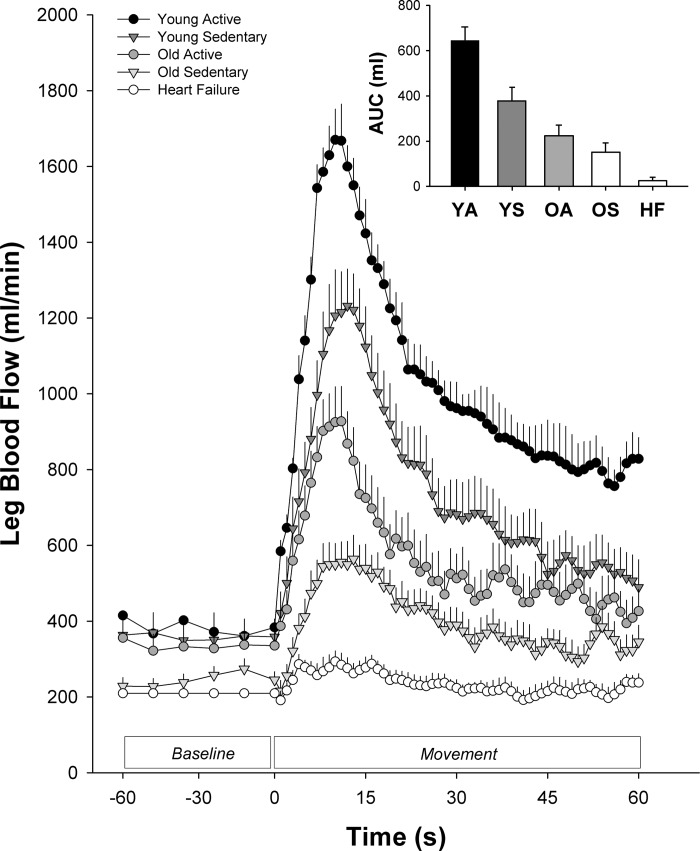
Fig. 1.
Passive leg movement (PLM)-induced hyperemia in groups typified by differing levels of cardiovascular health. YA, Young Physically Active (n = 5; J. R. Gifford, R. Broxterman, and R. S. Richardson, unpublished observations). YS, Young Sedentary (n = 12; Ref. 22). OA, Old Physically Active (n = 10; Ref. 22). OS, Old Sedentary (n = 12; Ref. 22). HF, Heart Failure (n = 14; Ref. 81). AUC, area under the curve.
Many forms of cardiovascular disease (CVD), including atherosclerosis, are preceded by vascular dysfunction, which can broadly be described as subtle impairments in the ability of the vascular system and related systems (e.g., sympathetic nervous system) to adjust vascular tone appropriately in response to a given stimulus (12, 17, 59, 77). In fact, small reductions in endothelium-dependent vasodilation and functional hyperemia are often considered harbingers for the onset of CVD (12, 17). At face value, subtle reductions in peripheral vascular function may seem trivial; however, the ability of the vascular system to control tightly vascular tone actually provides a unique and accessible window into the overall health of the vascular system.
Under normal conditions, the vascular system balances the presence of atherogenic, prothrombotic factors with the production of antiatherogenic, antithrombotic vasodilators like nitric oxide (NO). In conditions such as advancing age and disease, the bioavailability of antiatherogenic agents like NO is attenuated, resulting in a tipping of the balance toward an environment favorable for the development of CVD (59). As vasoactive agents such as NO are also crucial to the control of vascular tone and blood flow (72), this pro-CVD state is also typified by impaired control of vascular tone (59). Thus studying the ability of the vascular system to increase or decrease blood flow in response to a given stimulus (e.g., PLM) serves as a prognosticator for the overall health of the vascular system.
Assessments of Vascular Function
Given the importance of the vascular system in the early development of many cardiovascular diseases, there is demand for implementable screening tests to assess vascular function routinely in both research and clinical settings. However, several tests of vascular function have proven too invasive or too technically challenging for widespread use. For example, quantifying the hyperemia or dilation induced by intra-arterial infusion of the endothelium-dependent vasodilator acetylcholine (ACh) is one the most specific and direct methods for testing vascular function (38, 46, 56). However, although this technique is somewhat of a gold standard (3, 15, 59), the invasive nature of the test (i.e., intra-arterial catheterization) makes it impractical for most settings.
In terms of noninvasive assessments of peripheral vascular function, the flow-mediated dilation technique, which uses Doppler ultrasound to measure very small changes in artery diameter induced by the hyperemia following the release of brief arterial occlusion, has gained the most traction (25). Indeed, peripheral vascular function assessed by flow-mediated dilation (FMD) has been reported to be partially NO-dependent (19, 84), reflect coronary vascular function (3, 62), and predict the incidence of cardiovascular disease (17, 33, 86, 87). Clearly, as members of our group detailed in the past (25), FMD is a very useful assessment of vascular function, but obtaining quality data, which relies on the accurate measurement of very small changes in artery diameter, requires more advanced sonography skills and practice than is often initially appreciated. Moreover, it is not entirely clear how well FMD reflects systemic vascular function, as brachial artery FMD is apparently unrelated to ACh-induced hyperemia in the microcirculation of the arm (14) and FMD of the arteries of the lower limb (64).
Clearly, a more accessible test of vascular function, implemented either independently or in conjunction with other noninvasive tests, is needed to expand the reliable quantification of vascular function in vivo. Here, in the following paragraphs, evidence supporting the use of PLM to assess vascular function and guidelines for performing and interpreting this assessment will be presented. Ultimately, the aim of this article is to promote consistency and reproducibility in the assessment of vascular function utilizing the PLM technique.
Passive Leg Movement: A Simple Assessment of Peripheral Vascular Function
Since the late 1990s, a body of evidence has grown to indicate that the hyperemic response to PLM provides an easily obtained indicator of peripheral vascular function in a wide array of populations. In 1999, when passively moving participants’ legs to overcome the inertia in the flywheel of a knee-extension ergometer, in preparation for exercise, Rådegran and Saltin (54) provided the first indications that blood flow increases with PLM. Successively, in what appears to have been the first purposeful investigation into PLM-induced hyperemia, Wray et al. (82) used PLM to identify the contribution of movement to exercise hyperemia in the absence of increased metabolism. Over the course of this study, Wray et al. (82) detailed the now-familiar PLM-induced rise and fall in leg blood flow that is depicted in Fig. 1.
Subsequent investigations have sought to determine exactly how PLM elicits an increase in leg blood flow. Based on Poiseuille’s law, with vascular tube length and blood viscosity likely remaining relatively constant during PLM, the PLM-induced hyperemia could potentially be driven by two major factors: increased perfusion pressure and increased peripheral vasodilation (i.e., decreased vascular tone). Interestingly, although heart rate and cardiac output tend to increase during PLM, their increase appears to be outweighed be the magnitude of peripheral dilation, as perfusion pressure (i.e., mean arterial pressure minus mean venous pressure) tends to decrease, rather than increase, during PLM (69). Nevertheless, the acute inhibition of the sensory afferents has been reported to result in an attenuated PLM-induced hyperemia by way of a blunted central response (69). Thus, although central hemodynamic factors that affect perfusion pressure, like cardiac output, are certainly necessary components of the response, in most cases, these factors seem to facilitate the response rather than dictate its magnitude.
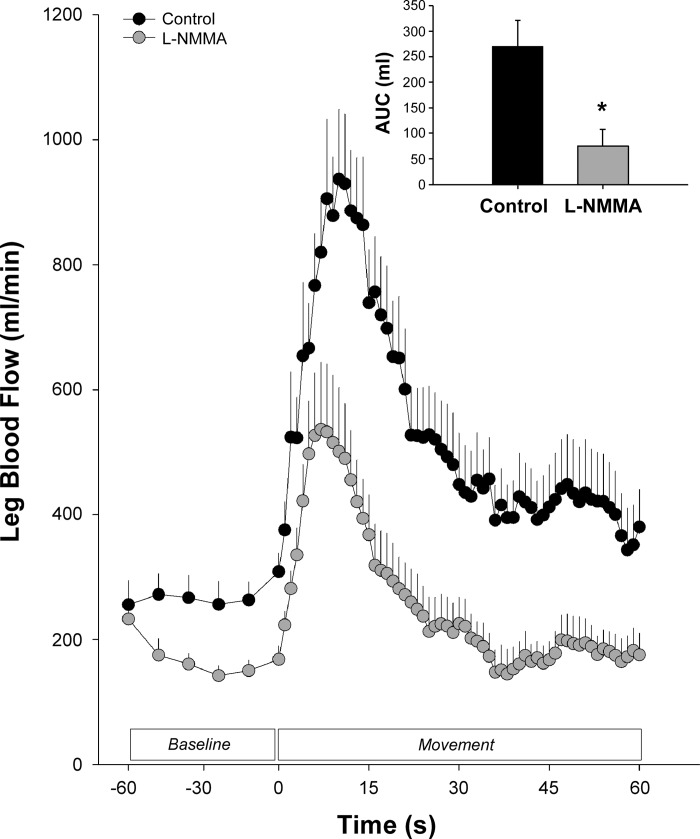
A great deal of evidence indicates that the PLM response is primarily a product of changes in peripheral arterial diameter/tone. For example, when the PLM-induced hyperemia is considered in terms of leg vascular conductance (i.e., blood flow/perfusion pressure), an index of vascular tone, the familiar PLM-induced rise and fall, mirroring the hyperemic response is still clearly evident (45, 70, 81). Subsequent studies, which have sought to elucidate the mechanisms responsible for the PLM-induced change in vascular tone, have revealed an important role for endothelium (10, 24, 45, 70, 71). Most notably, as illustrated in Fig. 2, independent studies by Trinity et al. (71) and Mortensen et al. (45) almost simultaneously reported that up to 80% of the overall increase in leg blood flow during PLM is dependent on NO production. Thus, at this point, it appears that on movement of the leg, NO release (45, 71), and other, unidentified dilator mechanisms, is initiated in response to the mechanical deformation of the moved leg (9, 37), which quickly results in the dilation of the vascular bed. Concomitantly, sensory afferents within the muscle bed signal for a central response that increases heart rate, stroke volume, and cardiac output, thereby facilitating the increased perfusion of the dilated vascular bed (69). Notably, an additional stimulus for further vasodilation likely comes in the form of the increases in perfusion pressure and shear stress that accompany these responses (69). In summary, the coordinated response of multiple mechanisms appears to mediate the PLM-induced hyperemia with the magnitude of the response being primarily dictated by a NO-driven fall in peripheral vascular tone.
Fig. 2.
Effect of nitic oxide (NO) synthase inhibition with l-NMMA on passive leg movement (PLM)-induced hyperemia in young, healthy individuals. These data from Trinity et al. (71) indicate that NO is responsible for up to 80% of the overall PLM-induced hyperemic response. Other studies have verified this observation, supporting the role of NO in the PLM response (24, 45, 70). Note that passive leg movement begins at 0 s. AUC, area under the curve. *Significantly different from control.
Since the initial indications that PLM-induced hyperemia is a highly NO-dependent assessment of vascular function, additional studies have validated the use of the technique in comparison with other, more invasive or technically challenging assessments of vascular function. For example, Mortensen et al. (45) convincingly demonstrated that the magnitude of PLM-induced hyperemia is strongly related (r = 0.84, P < 0.05) to the function of the vascular endothelium, assessed by vasodilation induced by the intra-arterial infusion of ACh. More recently, Rossman et al. (57) reported that PLM hyperemia is also correlated to the well-established, yet more technically challenging, brachial artery FMD in multiple populations and different vessels (r = 0.55–0.88, P < 0.05). Additionally, a relationship between PLM-induced hyperemia and popliteal artery reactive hyperemia (r = 0.68, P < 0.05) has also been reported (75). Thus, in combination, there is validating evidence that PLM-induced hyperemia is reflective of more invasive (24, 45, 71) and more technically challenging (57, 75) assessments of vascular function and NO bioavailability, and it is clear that measuring blood flow while passively moving a person’s leg provides a novel way to assess peripheral vascular function in vivo.
Given the insight that PLM provides regarding the function of the vascular system, multiple studies have utilized PLM to quantify vascular function in several distinct populations. As illustrated in Fig. 1, and as one would expect of a valid assessment of vascular function, populations with greater cardiovascular risk exhibit a reduced PLM-induced hyperemia. For example, aging, a major risk factor for cardiovascular disease, has been associated with a diminished PLM-induced hyperemia (42, 45, 70), largely due to decreased NO bioavailability (24, 70). Importantly, regular physical activity, which is recognized to modulate vascular function and cardiovascular risk, has been documented to attenuate the reduction in PLM-induced hyperemia with age (22). Further reductions, beyond that of typical aging, in PLM hyperemia have also been reported in groups with cardiovascular disease such as heart failure (81) and peripheral arterial disease (45, 75). To date, PLM has been used to quantify vascular function in a wide variety of populations including young (41, 45, 71), old (42, 45, 70), exercise-trained (22), male (41), female (23), heart failure (81), heart transplant (29), spinal cord injury (73), peripheral arterial disease (45), chronic obstructive pulmonary disease (35), and patients with septic shock (47). As clearly illustrated in Fig. 1, PLM has certainly proven to be a relatively simple and insightful way to assess and, therefore, delineate a range of vascular function across multiple populations.
Step-By-Step Instructions for the Passive Leg Movement Test of Vascular Function
Having been validated against intra-arterial infusions of the endothelium-dependent vasodilator ACh (45) and FMD (57) and being up to 80% dependent on NO bioavailability (24, 45, 71), it is clear that PLM-induced hyperemia is a good marker of peripheral vascular function. Nevertheless, it is important to recognize that this validity is not set in stone. Indeed, although the PLM test is conceptually relatively simple, as with any test of vascular function, its validity is dependent on the level of care and control exhibited by the person performing the test, throughout the entire assessment process, a process that needs to start long before the participant arrives at the laboratory and, due to the analyses, ends long after they leave. Therefore, the purpose of this section is to provide a comprehensive, step-by-step guide for the preparation, performance, analysis, and interpretation of the PLM test of vascular function. The specific sequence of requirements and tasks in terms of the necessary equipment, subject preparation, PLM, data acquisition, analysis, and interpretation are illustrated in Fig. 3 and described, individually, in detail here.
Fig. 3.
Schematic illustrating the appropriate steps involved in performing a passive leg movement (PLM) test of vascular function. Note that details about each step are presented in the order indicated in the boxes.
Necessary equipment.
Aside from a Doppler ultrasound system, which many laboratories studying vascular function already have at their disposal, in its most fundamental form, the PLM technique requires very little additional equipment. Descriptions of the necessary equipment to perform the PLM test appropriately are described below.
doppler ultrasound.
Doppler ultrasound is essential to the PLM test and is used to measure both artery diameter and blood velocity, facilitating the calculation of the blood flow response during the test. Consequently, an ultrasound system capable of running in duplex mode (i.e., simultaneous collection of vessel image with B-mode and blood velocity with pulse-wave mode) is required. The ultrasound system should also be capable of acquiring an integrated ECG, reporting velocity as time-averaged, intensity-weighted means, and performing “angle steer” and “insonation angle correction”(see below). As the common femoral artery (CFA) is generally 2–6 cm deep, a transducer probe designed to insonate these depths is recommended (e.g., a 9-MHz probe).
chair with sufficient ground clearance and the potential to recline.
A chair with sufficient clearance to allow the unimpeded movement of the leg from full knee extension (180°) to 90° knee flexion is required (Fig. 4). Although not essential in all subjects, the capacity to recline the chair slightly can be advantageous in terms of facilitating better access to the inguinal region to scan the femoral artery. As too much of a recline may impact the hydrostatic column (24) and, therefore, the perfusion pressure driving the response, a recline >110° from seat to back is not recommended.
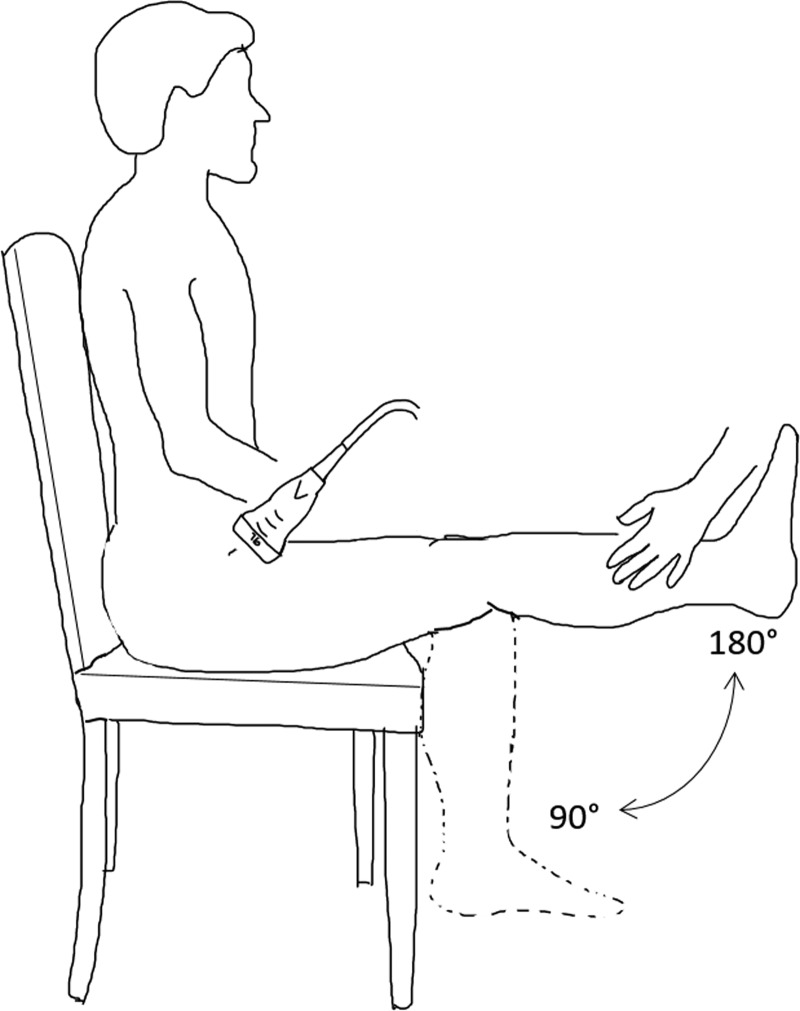
Fig. 4.
Illustration of the passive leg movement (PLM) test of vascular function. In the PLM test, the participant sits in a chair while his or her leg is passively moved through a 90° range of motion at 1 Hz by a member of the research team. Simultaneously, Doppler ultrasound is used to quantify the change in blood flow through the common femoral artery.
knee brace with 90° range of motion or goniometer.
Subtle differences in the magnitude of flexion or extension may introduce unwanted variability between tests. Thus it is recommended that a knee brace with a fixed range of motion (90°) or a standard knee brace with a goniometer providing real-time feedback of knee joint angle is used to standardize the range of motion between PLM tests.
metronome.
The PLM test has traditionally been performed at a cadence of 1 Hz or 60 movements per minute. Moving the leg to the beat of a metronome facilitates greater consistency in the movement rate.
Subject preparation.
For any assessment of vascular function, including PLM, the condition and preparation of the participant may impact the results of the test. The following are factors that should be controlled, as much as possible, when performing a PLM test. Failure to control adequately for these may add variability to the measurement.
familiarization.
It is recommended that participants be familiarized with PLM before data are collected. At the first exposure to this test, some individuals unknowingly assist or resist the passive movement by kicking their leg. In most cases, some coaching and appropriate familiarization with the experience and expectations remedies the situation. Since muscle contractions strongly influence blood flow (34), trials in which the participant kicks or resists the movement should not be considered a successful assessment. Although electromyography, which has previously been used to document negligible activity of the muscle during PLM (31), may be used to screen for active movements, generally, an attentive person moving the leg will be able to detect such contractions.
fasted state.
Vascular function can be affected by meal consumption. For example, the consumption of a high-fat/high-carbohydrate meal a few hours before testing has been documented to attenuate vascular function (25, 49). Therefore, it is recommended that PLM be assessed in a fasted state (4–6 h; Ref. 63). However, when fasting is not possible, the consumption of a standardized low-fat meal (e.g., corn-flake cereal and skim milk), which has been documented to have no effect on vascular function (49), is recommended.
rested state.
Prior exercise results in elevated resting blood flow, which is an important measure in PLM that can last 30–90 min or more (4). Prior exercise has also been reported to alter vascular function temporarily in a biphasic manner (11). Therefore, as with other vascular function assessments (25), it is recommended that subjects avoid exercise for ≥12 h before the PLM test.
medications.
Many medications have direct and/or indirect effects on vascular function. Thus, as with or other vascular assessments (25), if the assessment aims to avoid the impact of medication use, it is recommended that participants refrain from medications, especially medications with known cardiovascular effects, for ≥4 half-lives of the drug before undergoing PLM. With specific reference to common nonsteroidal anti-inflammatory drugs such as ibuprofen or aspirin, which have been documented to affect vascular function (2, 13), a 1- to 3-day washout before vascular assessment is recommended (25). In instances when it is not possible to control for all medications, care should be taken to match medications between repeated visits or, at least, note changes.
supplements.
Vascular function, particularly endothelial function, can be affected by several supplements. For example, common supplements like vitamin C, vitamin E (68, 80, 83), and fish oil (85) have documented effects on vascular function. As circulating levels of common supplements like vitamin C (40) and fish oil (27) have been reported to remain at elevated levels for 12–24 h or more following ingestion, it is recommended that supplements be discontinued ≥72 h before a PLM assessment.
caffeine.
Caffeine, most often in the form of coffee, impacts cardiovascular function (7). Although not all studies agree (60), evidence indicates that, as an inhibitor of a key enzyme in the NO-mediated vasodilation pathway (guanylate cyclase), caffeine likely has an inhibitory effect on vascular function (7, 51). As the reported effects of caffeine typically begin to wane after a few hours (51), it is recommended that caffeine (e.g., coffee, tea, caffeinated soda) be avoided for 12 h before a PLM test.
smoke and ambient air exposure.
Exposure to smoking (cigarette or marijuana), both firsthand and secondhand exposure and, now, even electronic cigarette exposure, has been documented to attenuate vascular function (8, 76). As evidence indicates that the acute effects of smoking on vascular function lasts several hours (30), it is recommended that subjects avoid direct or indirect smoke exposure for ≥12 h before a PLM test. Similarly, exposure to high levels of ambient air pollution (e.g., wood smoke, car exhaust, smog) has also been reported to impair vascular function and should, at least, be documented if ambient levels of pollution are elevated during a PLM assessment (5).
menstrual phase.
The cyclical changes in estrogen and progesterone throughout the menstrual cycle have been documented to impact endothelial NO synthase (NOS) activity (28), NO bioavailability, and vascular function (1, 78). In general, NO bioavailability and vascular function tend to track estrogen levels, exhibiting elevations during the late follicular phase when estrogen is high (1, 26) and potentially exhibiting reductions during the early follicular phase when estrogen levels are lower (78). Consequently, when studying premenopausal women, care should be taken to test the PLM response of each woman at the same point in the menstrual cycle. When comparing sex differences in vascular function, the 1st 1–7 days following menses (early follicular phase) has traditionally been used because it offers the lowest levels of both estrogen and progesterone.
repeated measurements.
When performing repeated assessments of PLM, it is important to allow sufficient time for recovery time. Experience from our group suggests that, for reasons that have yet to be fully elucidated, performing a PLM test within 1–2 min of a previous PLM may result in an attenuated response. Currently, a recovery period of 5 min appears to provide sufficient recovery time. Nevertheless, if an attenuated response is observed with 5-min recovery periods, longer recovery periods should be used.
Passive leg movement.
Although performing the PLM is conceptually straightforward (Fig. 4), several factors during baseline and the movement itself must be well-controlled to increase the consistency of the test.
seated position.
Although a supine position has been used in certain circumstances (24), the participant is usually positioned in a seated posture. This is important because the position of the participant, whether seated or supine, can affect both resting and PLM-induced blood flow. For example, Groot et al. (24) reported that performing the PLM test in a supine posture may decrease the contribution of NO and reduce the overall PLM response, likely by decreasing the perfusion pressure and accompanying shear stress associated with the start of the response. Therefore, to maximize the response as well as the contribution of NO, an upright, seated posture is recommended.
baseline knee joint angle.
McDaniel et al. (43) reported that leg blood flow is dependent on knee joint angle with blood flow decreasing as the knee moves from a fully extended position (180°) to a flexed position (90°). Thus, for the PLM technique, both the baseline blood flow assessments and the cessation of movement are generally performed with the knee joint fully extended (180°; Fig. 4). This can be achieved by having a table placed at chair height, in front of the subject, on which to rest the legs.
baseline acclimatization.
As much of the data from the PLM test is expressed as a change from baseline blood flow, the baseline conditions should be as controlled as possible. Thus, to give the participant a chance to equilibrate adequately to the baseline position and environment, it is recommended that the participants rest in the testing position for ≥15 min before making any measurements. In many cases, this acclimatization period can coincide with subject instrumentation. Additional caution must be taken to ensure that baseline blood flow is stable, as activities as simple as talking or even deep breathing have the potential to alter baseline leg blood flow. As heat stress has the potential to alter blood flow and NO bioavailability (16, 36, 39), care should also be taken to maintain a similar room temperature (e.g., 22–24°C) for each test.
transition from baseline to plm.
When transitioning from the baseline position, small disturbances in leg position immediately before the PLM can alter blood flow and potentially mask the PLM-induced response. Thus it is important for the researcher to disturb the leg as little as possible during this transition. This can be achieved by gently sliding the table/foot stool out from under the leg while manually supporting the leg in the same position. Alternatively, a research team member can hold the leg at 180° throughout the baseline; however, obtaining consistent baseline measures can be more challenging with this approach.
movement range of motion.
The PLM technique typically uses a 90° range of motion (180–90–180°). Moving the leg through greater or lesser range of motion may potentially alter the hyperemic response. As mentioned earlier, the use of a knee brace with a fixed 90° range of motion or a goniometer with real-time joint angle feedback is recommended to ensure consistency of the movement. Care should also be taken to move the leg smoothly at a consistent pace through the entire range of motion. Additionally, particular care should be taken to maintain firm control of the leg when transitioning from flexion to extension or vice versa.
movement frequency.
The frequency of the PLM is generally standardized at 1 Hz, such that the leg goes through one cycle of 180–90–180° every second. As evidenced by McDaniel et al. (43), who reported much smaller levels of hyperemia in response to much slower PLM (0.03 Hz), moving the leg at a different frequency may result in a considerably different response from what has been observed at 1 Hz.
movement duration.
The PLM at 1 Hz is typically performed for ≥1 min. The majority of studies performing PLM for a longer duration have indicated that after the initial peak in blood flow within the 1st 30 s of movement, blood flow gradually returns to baseline values by 3 min (42, 71). As most of the hyperemia occurs within the 1st 60 s of the PLM, the data for the 1st 60 s are what are now most commonly reported.
single-movement alternative.
Recent evidence indicates that the passive movement of the leg through a 90° range of motion a single time (sPLM) is sufficient to elicit a hyperemic response (6, 74, 81). Importantly, although this test merely entails a single movement of the leg, the magnitude of the hyperemic response is strongly related to the hyperemia elicited by the more traditional continuous-movement PLM (r > 0.82, P < 0.05; Ref. 74). Additionally, sPLM also elicits smaller central responses than continuous-movement PLM (e.g., less of an increase in cardiac output; Ref. 74), which may be advantageous when studying peripheral vascular function in groups with impaired central function or at least diminishes concern about the role of central hemodynamics in this assessment. As the simplicity of the sPLM test of vascular function makes it very attractive for clinical applications, further research characterizing the sPLM response is warranted.
Data acquisition with Doppler ultrasound.
When assessing blood flow during a PLM test, the ultrasound system must be functioning in duplex mode, meaning that the system must simultaneously display an image of the femoral artery (B-mode) and blood velocity (pulse-wave mode). Specific considerations for this process are described below.
imaging location.
Blood flow measurements are made in the common femoral artery (CFA), 2–3 cm proximal to the bifurcation. The CFA, which is a continuation of the external iliac artery, is readily insonated in the crease between the thigh and the torso (i.e., inguinal region). The depth of the artery will vary depending on adiposity but is generally between 2 and 6 cm deep. As the CFA bifurcates into deep and superficial components in this region (44), care should be taken to ensure that the CFA is insonated rather than one of its branches.
obtaining an image of the cfa.
Obtaining a clear image of the CFA is important for accurately measuring the diameter as well as for ensuring that the pulse-wave Doppler data are being collected from the appropriate region of the artery. When the CFA is identified, the transducer should be adjusted to be as parallel to the artery as possible, which results in the artery being clearly visible across the entire screen. Care should also be taken to ensure that the superficial and deep walls of the artery are clearly visible (Fig. 5A). Although the nuances of good quality sonography are beyond the scope of this article, briefly, adjustments to B-mode gain, focus, and dynamic range are helpful in this regard.
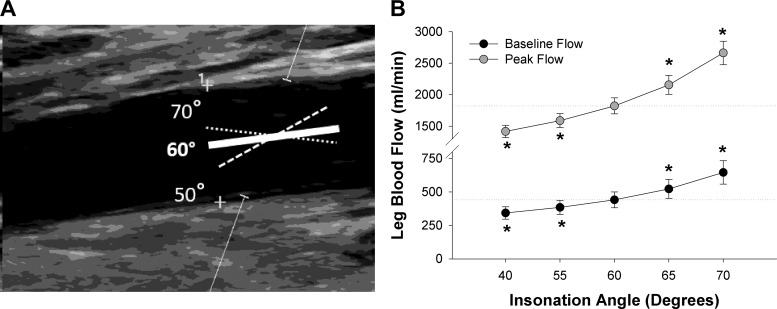
Fig. 5.
Effect of insonation angle on measurements of leg blood flow during passive leg movement (PLM). A: representative image of Doppler ultrasound with the angle correction cursor (solid line) acceptably aligned with the artery at an insonation angle of 60° from the Doppler beam or out of alignment with the artery walls at either 50 or 70° from the Doppler beam. The cross hairs on artery wall represent location of the media layer where diameter measurements were made. B: effect of error in insonation angle (i.e., misalignment of angle correction cursor with the artery wall) on resting blood flow and peak blood flow during PLM (n = 5). Note that the acceptable error condition was taken as an accurately determined insonation angle of 60° with the angle correction cursor parallel to the artery. Dashed lines represent mean value at the acceptable error associated with this 60° insonation angle for each condition. *Significantly different from the acceptable error associated with an insonation angle of 60°.
obtaining blood velocity data.
Obtaining high-quality blood velocity data throughout the entire protocol is critical for the PLM test. Common errors in sonography can yield to erroneous and misleading results from a PLM test.
The maintenance of a constant insonation angle ≤60° is crucial for accurate and reliable Doppler ultrasound measurements. Blood velocity is acquired based on the Doppler shift, which, applied to vascular sonography, indicates that the change in frequency of a soundwave after reflecting off a blood cell is proportional to the velocity of the blood cell (65). One important caveat to the Doppler principle is that the further the angle between the emitted soundwaves (Doppler beam) and the vector of blood flow (i.e., insonation angle) is from 0°, the smaller the signal from the Doppler shift becomes until completely disappearing at 90° (65). Therefore, it is generally accepted that it is important to have the insonation angle set ≤60° on the Doppler ultrasound system, but it is also important to make sure that the insonation angle is actually ≤60° with reference to the path of the artery. In pulse-wave mode, the “angle correction cursor” will appear between the sample volume cursors (Fig. 5A). As the insonation angle is calculated with reference to the angle correction cursor, it is important that the angle correction cursor be aligned to be parallel with the artery walls. Failure to do so can result in substantial error. Indeed, subtle differences in perceived insonation angle (misalignment) can have a large impact on blood flow measurements (55). As illustrated in Fig. 5, a misalignment of just 10° can result in an over- or underestimation of resting blood flow by >100 ml/min and peak flow during PLM by >500 ml/min. Thus it is generally recommended that the insonation angle be set to ≤60°. Therefore, to reiterate, as with all sonographic assessments of blood flow, it is very important always to have an appropriate insonation angle (≤60°), determined by the angle between the Doppler beam and the artery walls.
Sample volume is also very influential in Doppler ultrasound measurements. Blood along the sides of an artery moves slower than blood in the middle of the artery (66). Thus having a sample volume that fails to encompass the blood flow at the sides can result in a ≤80% overestimation of blood flow (25). Consequently, sample volume should encompass the interior of the artery without overlapping the walls of the artery (Fig. 5A).
Data analysis.
The following section will first describe how to calculate blood flow from Doppler ultrasound and then will explain how to calculate important components of the PLM response.
calculation of blood flow.
As described in Eq. 1, blood flow is calculated as the product of the cross-sectional area of the artery (CSA), derived from arterial diameter, multiplied by the time-averaged velocity (TAV) of blood moving through the artery. In general, blood flow is reported in milliliters per minute or liters per minute. By default, many Doppler ultrasound systems report diameter in centimeters and TAV in centimeters per second. In such cases, flow can be multiplied by 60 s/1 min to yield blood flow in milliliters per minute.
| (1) |
Diameter is an important part of the blood flow calculation. As illustrated by the cross hairs in Fig. 5A, artery diameter is measured as the distance from one side of the artery to the other while ensuring that the diameter measurement is perpendicular to the walls of the artery. As the intimal layer of the artery is not always visible, it is common practice to measure the diameter from the superficial medial layer to deep medial layer (25). With the diameter of the artery fluctuating throughout the cardiac cycle, diameter measurements should be made at the same point in the cardiac cycle. End-diastole, represented by the peak of the R wave of the corresponding ECG, is recommended (25, 71). In agreement with previous studies illustrating little to no change in diameter when the CFA is exposed to much higher shear rates than experienced during PLM (18, 53), analysis of CFA diameter by our group with automated edge-detection software before and after 60 s of PLM revealed no effect of PLM on CFA diameter (n = 10; before: 1.036 ± 0.027 cm, after: 1.037 ± 0.023 cm; P = 0.94). Thus a diameter made at baseline, usually the average of ≥5 measurements, can be used to represent the diameter throughout the duration of the test. Importantly, as location of the measurement as well as day-to-day changes and drug-induced changes in CFA diameter are possible, verifying the artery diameter for each visit is highly recommended. Although automated edge-detection software can be used to calculate the CFA diameter, caliper-driven measurements on the ultrasound system are generally accurate enough for this measurement.
As illustrated in Eq. 1, blood velocity (reported as time-averaged velocity, TAV) is an important factor in the calculation of blood flow. Blood velocity should be analyzed on a second-by-second basis to capture the dynamic nature of the response. Analysis of velocity, taking the average of longer time intervals, may not be sensitive enough to discern key characteristics of the response (e.g., peak flow). Furthermore, the intensity-weighted, time-averaged mean velocity, which reflects the time-averaged velocity throughout the entire cross-section of the artery, should be reported for blood velocity. Use of the time-averaged, maximum velocity (i.e., average of the outer envelope of the spectral wave) ignores the heterogeneous distribution of blood velocity throughout the cross-section of the artery, resulting in an overestimation of actual flow. Finally, as the asynchronous superimposition of leg movement on top of the pulse-wave can result in exceptionally high velocity during 1 s followed by exceptionally low velocity the next, smoothing the data with a 3- to 5-s rolling average is recommended (71).
calculation of plm-specific data.
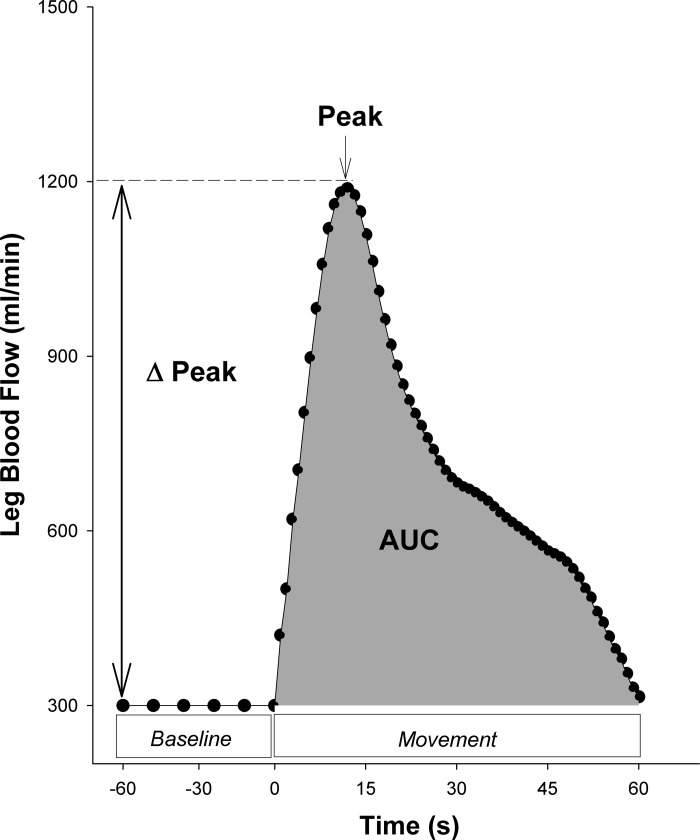
Multiple portions of the PLM response provide insight into the function of the vascular system. Previous studies have examined how each portion of the response, including peak flow (71), total blood flow (i.e., area under the curve, AUC; Ref. 71), antegrade and retrograde flow (41), and the slope of the initial hyperemia (24) relate to vascular function. This section will focus on the three most commonly reported factors for PLM-induced hyperemia: peak flow, change from baseline to peak flow (Δ peak flow), and AUC.
As illustrated in Fig. 6, the peak hyperemic response to PLM is simply the highest point recorded on the PLM blood flow response curve. This typically occurs within the 1st 30 s of PLM. Importantly, the peak hyperemic response tends to differ between populations with individuals with higher risk of cardiovascular disease (e.g., sedentary, elderly), exhibiting lower peak values (Fig. 1). Differences in peak responses may be due, in part, to differing levels of NO bioavailability, as studies have reported that the peak hyperemic response to PLM is reduced by >30% when NO synthesis is inhibited with NG-monomethyl-l-arginine (l-NMMA; Fig. 2; Refs. 45, 70).
Fig. 6.
An illustration of the calculations to assess a passive leg movement (PLM) test of vascular function. Blood flow during the initial baseline period represents the average resting leg blood flow for the ≥60 s immediately before leg movement. Peak blood flow represents the greatest blood flow measured during PLM. Δ Peak blood flow represents the change from baseline to peak blood flow. Area under the curve (AUC) represents the overall PLM-induced hyperemia above baseline.
As illustrated in Fig. 6, the Δ peak of the PLM response is calculated as the change from baseline to peak. The Δ peak offers a way to compare the PLM response across individuals or conditions with differing baseline values such as the infusion of l-NMMA (71). Like the peak response, the Δ peak is also NO-dependent (45, 70, 71).
As illustrated in Fig. 6, the AUC represents overall increase in blood flow above baseline over the course of a minute of PLM. It is typically calculated as the summed second-by-second blood flow values after subtracting the baseline flow (70, 71). The AUC is particularly meaningful when considering endothelial function as this value is up to 80% dependent on NO (10, 24, 45, 71) and is strongly related to endothelial function assessed by ACh-induced hyperemia (r = 0.76, P < 0.01; Ref. 45). Thus differences in PLM AUC are often reflective of differences in vascular endothelial function. Although less common, since NO bioavailability also affects baseline blood flow (24), calculating AUC as the total blood flow >0 ml/min, rather than above baseline, may also be potentially meaningful when investigating NO bioavailability.
Interpretation of PLM data.
With the response to PLM being NO-dependent (10, 24, 45, 71) and related to both FMD (57) and intra-arterial ACh-induced hyperemia (45), the PLM response is clearly reflective of the function of the peripheral vascular system. Nevertheless, as with any bioassay, there are nuances that must be considered when interpreting PLM data.
variation and reproducibility.
One of the first things to bear in mind when interpreting PLM data is that, as with all bioassays of vascular function, there is an appreciable amount of variability in the PLM response. Indeed, evidence indicates that the indices of vascular function assessed by a PLM test exhibit a within-day and day-to-day coefficient of variation of 15–20% (21, 74). Thus an abundance of caution is recommended when interpreting small changes in PLM-induced hyperemia because it may not be clear how much of the change is due to physiology or simply random variation. Thus, as with other assays, taking the average of two or three repeated measurements, separated by at least 5–10 min of rest, may be appropriate at times.
Although this level of random variation is significant, it is important to consider it in the context of PLM. For example, Fig. 7 juxtaposes the day-to-day variability of the PLM response of a young individual with that of an older individual under well-controlled conditions. Although it is easy to see that the PLM response of each individual varies subtly from day to day, when considered in comparison with the PLM response of the other individual, it is clear that this random variation is far outweighed by the difference due, apparently, to age. Indeed, as evidenced by multiple studies reporting large, significant effects of aging (42, 45, 70), physical activity (22), and disease (29, 45, 47, 81), the effect of condition on PLM-induced hyperemia generally outweighs the level of random variation in the response.
Fig. 7.
Evidence of the reproducibility of passive leg movement (PLM)-induced hyperemia. In these examples, PLM-induced hyperemia was measured on 2 individuals (1 young and 1 old) on 3 separate days.
vascular function vs. endothelial function.
With PLM-induced hyperemia being strongly dependent on NO bioavailability, the natural inclination is to define the PLM response as a test of endothelial function or NO bioavailability. Although this is accurate to a certain extent, adopting such specific terminology runs the risk of confining the interpretation of changes in PLM as merely an increase or decrease in NO bioavailability, which, as described below, is not always the case. Thus, in this article, PLM has intentionally been described as a test of vascular function.
It is important to note that, although NO plays a large role in the response, PLM-induced hyperemia is not solely mediated by NO. For example, inhibition of NOS does not completely abolish the PLM response, suggesting other mechanisms are likely involved. Furthermore, old patients with cardiovascular disease exhibit a lower PLM response than old healthy controls despite the fact that the contribution of NO to the PLM response is already relatively minimal in healthy aging (70, 81). Thus the further reduction in patient populations is unlikely to be exclusively due to an additional fall in NO bioavailability, as its role is already minimal.
As the health of the vascular system is not exclusively dictated by NO bioavailability, the conclusion that PLM is not exclusively NO-mediated is not necessarily a detriment. Indeed, despite not being entirely mediated by NO, FMD is still very predictive of cardiovascular health (86, 87). Moreover, with FMD testing, even in conditions where the role of NO is even more diminished, the test is still predictive of cardiovascular outcomes (20). Thus more research regarding what other mechanisms contribute to the PLM-induced hyperemia, and FMD for that matter, are warranted. Considering the literature from other tests of vascular function, it seems possible that important vascular factors such as sympathetic nerve activity (32), endothelin-1 (48), and prostaglandins (10) may also shape the PLM response.
microvascular vs. macrovascular.
Although the PLM-induced blood flow response is measured in a conduit artery (CFA), the PLM test is more reflective of microvascular function than of conduit artery function. Indeed, PLM-induced hyperemia occurs with no apparent change in CFA diameter, and when NOS is inhibited, there is >70% decrease in PLM-induced hyperemia with no change in CFA diameter (71). Moreover, in contrast to FMD, which reportedly bears no relationship with ACh-induced dilation of the microvasculature (14), PLM-induced hyperemia bears a strong relationship with this common marker of microvasculature function (45). Therefore, in contrast to FMD, which, especially when shear rate is taken into account (50, 52), is mostly reflective of conduit artery function (14), PLM-induced hyperemia is predominantly reflective of microvascular function. The observation that PLM and FMD were related (57) despite being in two different parts of the circulation may be due to the influence of reactive hyperemia/shear rate on FMD (50), which is itself largely determined by the microcirculation (52).
role of central and peripheral factors.
At the onset of passive movement, there is a nearly immediate increase in cardiac output, which is partially dependent on functional sensory afferents (69). Simultaneously, there is a decrease in vascular resistance in the leg (i.e., local vasodilation), which, especially in young, healthy subjects, appears to offset the increase in cardiac output such that mean arterial pressure tends to fall. Interestingly, however, during PLM of the right leg, blood flow through the unmoved, left leg increases by approximately 50–125 ml/min (41, 70), which, although only amounting to 10–30% of the magnitude of the hyperemic response in the moved leg, implies the role of a systemic component like cardiac output. However, it must be recognized that based on Poiseuille’s law, cardiac output per se does not actually play a direct role in leg blood flow, which is determined by pressure and resistance across the leg. Therefore, during PLM, the essential but indirect role of cardiac output is the maintenance of systemic blood pressure as the vasculature of the leg dilates in response to the movement.
Clearly, the central component is necessary for the response, but it does not appear to explain differences between many groups. For example, Groot et al. (24) and Trinity et al. (70) reported that healthy, old individuals exhibited a ~58% reduction in PLM hyperemia compared with young controls despite having a similar response in cardiac output. Notably, this similar increase in cardiac output was accompanied by a similar increase in blood flow through the unmoved leg during PLM (70). Moreover, Trinity et al. (71) reported that the effect of peripheral NOS inhibition (70% reduction in the hyperemic response) occurred without any alteration to the cardiac output response. Thus, although the central factors play a role, the changes in peripheral vascular tone clearly dominate the majority of the response.
blood pressure and vascular conductance.
As already described, blood flow through the femoral artery is primarily determined by the resistance or dilation of the vascular bed and the perfusion pressure across the vascular bed (64a). Thus, in the context of PLM, in addition to changes in vascular resistance/dilation, changes in blood pressure can also influence the PLM-induced hyperemia. The role of localized dilation/resistance in the PLM-induced response can be probed by dividing blood flow values by simultaneously recorded beat-by-beat mean arterial pressure measurements (e.g., finger photoplethysmography or an intra-arterial pressure transducer), yielding vascular conductance. Indeed, Mortensen et al. (45) reported that vascular conductance during PLM was even more strongly related to ACh-induced hyperemia than raw blood flow measures (45), indicating that vascular conductance may provide an even more precise perspective on vascular function. Nevertheless, given the inaccessibility of the equipment needed to measure beat-by-beat blood pressure, except in cases when blood pressure is expected to differ markedly between conditions or populations, raw PLM-induced blood flow responses provide a sufficiently clear indication of vascular function (Fig. 1).
muscle mass.
It should be noted that the magnitude of the PLM response can be influenced by the amount of muscle mass in the quadriceps. Whereas small differences in muscle mass are likely to have only a minor effect on the PLM-induced hyperemic response, large differences in muscle mass, like those associated with extreme atrophy (73), may have a significant effect on the magnitude of the response. Thus normalization by muscle mass may be necessary when comparing the PLM response of individuals with substantial differences in leg size.
Summary of Strengths and Limitations of the PLM Assessment of Vascular Function
The PLM technique has many strengths as well as some limitations that should be considered. First of all, as noted throughout the article, the relationship between the PLM-induced hyperemia and NO bioavailability (45, 71) and other assessments of vascular function (45, 57) is certainly a strength. Although PLM is clearly indicative of peripheral vascular function, as it has not yet been compared with the function of other vascular beds (e.g., cerebral or coronary circulation), it is not clear whether the PLM response is indicative of systemic vascular health. As such, the PLM technique should, most appropriately, be considered a test of peripheral vascular function rather than global vascular function.
Another strength of the PLM technique is that, in contrast to tests like FMD, which rely on the accurate quantification of very small changes in artery diameter (approximately 0.1–0.5 mm; Ref. 80), the PLM technique is not greatly impacted by small errors in measurement of vessel diameter and thereby requires less-advanced sonography skills in this regard. For example, as illustrated in Fig. 8, our group has previously documented large decreases in vascular function, assessed by FMD (80) and PLM (81), in patients with heart failure compared with controls. Although the reduction in the FMD of patients with heart failure is impressive and meaningful, it is important to recognize that, as is often the case, the absolute change in diameter only differed by ~0.1 mm between groups, meaning that an error in the measurement of the diameter as small as 0.1 mm would have entirely masked the 33% difference in vascular function between groups. Although FMD has proven valid and reproducible in skilled hands (61), it must be appreciated that 0.1 mm is certainly a small margin of error (61), especially for less-experienced sonographers. In contrast, as the PLM response is primarily mediated by changes in blood velocity, a theoretical 0.1-mm error in the measurement of the CFA diameter would have had a minimal effect on the PLM response (~2% change, Fig. 8, B and C). In fact, to have the controls exhibit a similar PLM response to the patients with heart failure, a 3.0-mm error would be required (30× what was required for FMD). Conversely, the PLM response is highly influenced by errors in the Doppler assessment of blood velocity, which, fortunately, at least in our experience, is far easier to master than obtaining consistent high-resolution images. Therefore, the robustness of the PLM response against errors in diameter measurements is a strong advantage in that it minimizes the learning curve in terms of required sonography skills.
Fig. 8.
Theoretical effect of small errors in the measurement of artery diameter on previously reported values for flow-mediated dilation (FMD) and passive leg movement (PLM) of healthy controls (Con) in relation to patients with heart failure. A: effect of 0.1-mm error (Con – 0.1 mm) in the measurement of brachial artery diameter on the FMD of healthy controls in comparison with the FMD of patients with heart failure (80). Note that the left vertical axis represents FMD as %dilation, whereas the right vertical axis represents the FMD as the absolute change in artery diameter. B: effect of 0.1-mm error (Con – 0.1 mm) in the measurement of common femoral artery diameter on peak hyperemia during PLM of healthy controls in comparison with that of patients with heart failure (81). C: %change in FMD or peak PLM response with a 0.1-mm error in the diameter measurement in healthy controls. *Note that Con and Heart Failure data are derived from previously published studies (80, 81), whereas Con – 0.1 mm and Con – 3.0 mm are the theoretical values that would have been reported if such errors occurred in the measurement of the Con data.
Future Directions
Although much has been described about the determinants of PLM-induced hyperemia and its relationship with vascular health, there is still much to learn. Future investigations should determine the role of factors other than NO in the PLM response and elucidate the prognostic value of PLM-induced hyperemia in terms of cardiovascular health. Additionally, as a proven surrogate of vascular function, clinicians and investigators, especially those previously deterred by the technical nature of other vascular function tests, should consider employing this simple, validated test of vascular function in their studies.
Conclusion
Peripheral vascular function is an important indicator of the overall health of the vascular system. Validated by its good agreement with more invasive and technically challenging assessments of vascular function, the PLM test provides a simple, noninvasive method to measure vascular function. The recommendations in this CORP article are presented to provide researchers with a comprehensive tutorial to assess vascular function reliably with the PLM test and to facilitate the use of PLM as a research tool with clinical relevance.
GRANTS
This work was funded, in part, by the National Heart, Lung, and Blood Institute at the National Institutes of Health (Grant P01-HL-1091830) and the Veterans Health Administration Rehabilitation Research and Development Service (E6910-R, E1697-R, E1433-P, E2323-I, and E9275-L).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.R.G. and R.S.R. conceived and designed research; J.R.G. and R.S.R. performed experiments; J.R.G. and R.S.R. analyzed data; J.R.G. and R.S.R. interpreted results of experiments; J.R.G. and R.S.R. prepared figures; J.R.G. and R.S.R. drafted manuscript; J.R.G. and R.S.R. edited and revised manuscript; J.R.G. and R.S.R. approved final version of manuscript.
REFERENCES
- 1.Adkisson EJ, Casey DP, Beck DT, Gurovich AN, Martin JS, Braith RW. Central, peripheral and resistance arterial reactivity: fluctuates during the phases of the menstrual cycle. Exp Biol Med (Maywood) 235: 111–118, 2010. doi: 10.1258/ebm.2009.009186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmetaj-Shala B, Kirkby NS, Knowles R, Al’Yamani M, Mazi S, Wang Z, Tucker AT, Mackenzie L, Armstrong PC, Nüsing RM, Tomlinson JA, Warner TD, Leiper J, Mitchell JA. Evidence that links loss of cyclooxygenase-2 with increased asymmetric dimethylarginine: novel explanation of cardiovascular side effects associated with anti-inflammatory drugs. Circulation 131: 633–642, 2015. doi: 10.1161/CIRCULATIONAHA.114.011591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, Selwyn AP. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol 26: 1235–1241, 1995. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 4.Bangsbo J, Hellsten Y. Muscle blood flow and oxygen uptake in recovery from exercise. Acta Physiol Scand 162: 305–312, 1998. doi: 10.1046/j.1365-201X.1998.0331e.x. [DOI] [PubMed] [Google Scholar]
- 5.Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, Morishita M, Marsik FJ, Kamal AS, Kaciroti N, Harkema J, Corey P, Silverman F, Gold DR, Wellenius G, Mittleman MA, Rajagopalan S, Brook JR. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension 54: 659–667, 2009. doi: 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broxterman RM, Trinity JD, Gifford JR, Kwon OS, Kithas AC, Hydren JR, Nelson AD, Morgan DE, Jessop JE, Bledsoe AD, Richardson RS. Single passive leg movement assessment of vascular function: the contribution of nitric oxide. J Appl Physiol (1985). First published August 31, 2017; 10.1152/japplphysiol.00533.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buscemi S, Verga S, Batsis JA, Donatelli M, Tranchina MR, Belmonte S, Mattina A, Re A, Cerasola G. Acute effects of coffee on endothelial function in healthy subjects. Eur J Clin Nutr 64: 483–489, 2010. doi: 10.1038/ejcn.2010.9. [DOI] [PubMed] [Google Scholar]
- 8.Carnevale R, Sciarretta S, Violi F, Nocella C, Loffredo L, Perri L, Peruzzi M, Marullo AG, De Falco E, Chimenti I, Valenti V, Biondi-Zoccai G, Frati G. Acute impact of tobacco vs electronic cigarette smoking on oxidative stress and vascular function. Chest 150: 606–612, 2016. doi: 10.1016/j.chest.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Cheng TH, Chen JJ, Shih NL, Lin JW, Liu JC, Chen YL, Chen CH, Chen JJ. Mechanical stretch induces endothelial nitric oxide synthase gene expression in neonatal rat cardiomyocytes. Clin Exp Pharmacol Physiol 36: 559–566, 2009. doi: 10.1111/j.1440-1681.2008.05100.x. [DOI] [PubMed] [Google Scholar]
- 10.Christensen PM, Nyberg M, Mortensen SP, Nielsen JJ, Secher NH, Damsgaard R, Hellsten Y, Bangsbo J. Leg oxygen uptake in the initial phase of intense exercise is slowed by a marked reduction in oxygen delivery. Am J Physiol Regul Integr Comp Physiol 305: R313–R321, 2013. doi: 10.1152/ajpregu.00048.2013. [DOI] [PubMed] [Google Scholar]
- 11.Dawson EA, Green DJ, Timothy Cable N, Thijssen DH. Effects of acute exercise on flow-mediated dilatation in healthy humans. J Appl Physiol (1985) 115: 1589–1598, 2013. doi: 10.1152/japplphysiol.00450.2013. [DOI] [PubMed] [Google Scholar]
- 12.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation 115: 1285–1295, 2007. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 13.Dzeshka MS, Shantsila A, Lip GY. Effects of aspirin on endothelial function and hypertension. Curr Hypertens Rep 18: 83, 2016. doi: 10.1007/s11906-016-0688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eskurza I, Seals DR, DeSouza CA, Tanaka H. Pharmacologic versus flow-mediated assessments of peripheral vascular endothelial vasodilatory function in humans. Am J Cardiol 88: 1067–1069, 2001. doi: 10.1016/S0002-9149(01)01997-X. [DOI] [PubMed] [Google Scholar]
- 15.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 16.Gifford JR, Ives SJ, Park SY, Andtbacka RH, Hyngstrom JR, Mueller MT, Treiman GS, Ward C, Trinity JD, Richardson RS. α1- and α2-Adrenergic responsiveness in human skeletal muscle feed arteries: the role of TRPV ion channels in heat-induced sympatholysis. Am J Physiol Heart Circ Physiol 307: H1288–H1297, 2014. doi: 10.1152/ajpheart.00068.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gokce N, Keaney JF Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol 41: 1769–1775, 2003. doi: 10.1016/S0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 18.Gonzales JU, Miedlar JA, Parker BA, Proctor DN. Relation of femoral diameter, shear rate, and dilatory response to knee extensor exercise. Med Sci Sports Exerc 42: 1870–1875, 2010. doi: 10.1249/MSS.0b013e3181dd1c99. [DOI] [PubMed] [Google Scholar]
- 19.Green D. Flow-mediated dilation does reflect nitric oxide-mediated endothelial function. J Appl Physiol (1985) 99: 1233–1234, 2005. doi: 10.1152/japplphysiol.00601.2005. [DOI] [PubMed] [Google Scholar]
- 20.Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension 57: 363–369, 2011. doi: 10.1161/HYPERTENSIONAHA.110.167015. [DOI] [PubMed] [Google Scholar]
- 21.Groot H, Broxterman R, Garten R, Rossman M, Gifford J, Kwon O, Hydren J, Richardson R. Reliability of the passive leg movement assessment of vascular function. Med Sci Sports Exerc 49: 814, 2017. doi: 10.1249/01.mss.0000519181.00065.5a. [DOI] [Google Scholar]
- 22.Groot HJ, Rossman MJ, Garten RS, Wang E, Hoff J, Helgerud J, Richardson RS. The effect of physical activity on passive leg movement-induced vasodilation with age. Med Sci Sports Exerc 48: 1548–1557, 2016. doi: 10.1249/MSS.0000000000000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groot HJ, Rossman MJ, Trinity JD, Layec G, Ives SJ, Richardson RS. Passive leg movement-induced vasodilation in women: the impact of age. Am J Physiol Heart Circ Physiol 309: H995–H1002, 2015. doi: 10.1152/ajpheart.00422.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groot HJ, Trinity JD, Layec G, Rossman MJ, Ives SJ, Morgan DE, Bledsoe A, Richardson RS. The role of nitric oxide in passive leg movement-induced vasodilatation with age: insight from alterations in femoral perfusion pressure. J Physiol 593: 3917–3928, 2015. doi: 10.1113/JP270195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris RA, Tedjasaputra V, Zhao J, Richardson RS. Premenopausal women exhibit an inherent protection of endothelial function following a high-fat meal. Reprod Sci 19: 221–228, 2012. doi: 10.1177/1933719111418125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris WS, Varvel SA, Pottala JV, Warnick GR, McConnell JP. Comparative effects of an acute dose of fish oil on omega-3 fatty acid levels in red blood cells versus plasma: implications for clinical utility. J Clin Lipidol 7: 433–440, 2013. doi: 10.1016/j.jacl.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi T, Yamada K, Esaki T, Kuzuya M, Satake S, Ishikawa T, Hidaka H, Iguchi A. Estrogen increases endothelial nitric oxide by a receptor-mediated system. Biochem Biophys Res Commun 214: 847–855, 1995. doi: 10.1006/bbrc.1995.2364. [DOI] [PubMed] [Google Scholar]
- 29.Hayman MA, Nativi JN, Stehlik J, McDaniel J, Fjeldstad AS, Ives SJ, Wray DW, Bader F, Gilbert EM, Richardson RS. Understanding exercise-induced hyperemia: central and peripheral hemodynamic responses to passive limb movement in heart transplant recipients. Am J Physiol Heart Circ Physiol 299: H1653–H1659, 2010. doi: 10.1152/ajpheart.00580.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heiss C, Amabile N, Lee AC, Real WM, Schick SF, Lao D, Wong ML, Jahn S, Angeli FS, Minasi P, Springer ML, Hammond SK, Glantz SA, Grossman W, Balmes JR, Yeghiazarians Y. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: sustained vascular injury and blunted nitric oxide production. J Am Coll Cardiol 51: 1760–1771, 2008. doi: 10.1016/j.jacc.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 31.Hellsten Y, Rufener N, Nielsen JJ, Høier B, Krustrup P, Bangsbo J. Passive leg movement enhances interstitial VEGF protein, endothelial cell proliferation, and eNOS mRNA content in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 294: R975–R982, 2008. doi: 10.1152/ajpregu.00677.2007. [DOI] [PubMed] [Google Scholar]
- 32.Hijmering ML, Stroes ES, Olijhoek J, Hutten BA, Blankestijn PJ, Rabelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol 39: 683–688, 2002. doi: 10.1016/S0735-1097(01)01786-7. [DOI] [PubMed] [Google Scholar]
- 33.Hirsch L, Shechter A, Feinberg MS, Koren-Morag N, Shechter M. The impact of early compared to late morning hours on brachial endothelial function and long-term cardiovascular events in healthy subjects with no apparent coronary heart disease. Int J Cardiol 151: 342–347, 2011. doi: 10.1016/j.ijcard.2010.08.069. [DOI] [PubMed] [Google Scholar]
- 34.Hughes WE, Ueda K, Treichler DP, Casey DP. Rapid onset vasodilation with single muscle contractions in the leg: influence of age. Physiol Rep 3: 2499–2504, 2015. doi: 10.14814/phy2.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iepsen UW, Munch GD, Rugbjerg M, Rinnov AR, Zacho M, Mortensen SP, Secher NH, Ringbaek T, Pedersen BK, Hellsten Y, Lange P, Thaning P. Effect of endurance versus resistance training on quadriceps muscle dysfunction in COPD: a pilot study. Int J Chron Obstruct Pulmon Dis 11: 2659–2669, 2016. doi: 10.2147/COPD.S114351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ives SJ, Andtbacka RH, Kwon SH, Shiu YT, Ruan T, Noyes RD, Zhang QJ, Symons JD, Richardson RS. Heat and α1-adrenergic responsiveness in human skeletal muscle feed arteries: the role of nitric oxide. J Appl Physiol (1985) 113: 1690–1698, 2012. doi: 10.1152/japplphysiol.00955.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jufri NF, Mohamedali A, Avolio A, Baker MS. Mechanical stretch: physiological and pathological implications for human vascular endothelial cells. Vasc Cell 7: 8, 2015. doi: 10.1186/s13221-015-0033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamper AM, de Craen AJ, Westendorp RG, Blauw GJ. Endothelium-dependent NO-mediated vasodilation in humans is attenuated by peripheral α1-adrenoceptor activation. Vasc Health Risk Manag 1: 251–256, 2005. [PMC free article] [PubMed] [Google Scholar]
- 39.Keller DM, Sander M, Stallknecht B, Crandall CG. α-Adrenergic vasoconstrictor responsiveness is preserved in the heated human leg. J Physiol 588: 3799–3808, 2010. doi: 10.1113/jphysiol.2010.194506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, Cantilena LR. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci USA 93: 3704–3709, 1996. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDaniel J, Fjeldstad AS, Ives S, Hayman M, Kithas P, Richardson RS. Central and peripheral contributors to skeletal muscle hyperemia: response to passive limb movement. J Appl Physiol (1985) 108: 76–84, 2010. doi: 10.1152/japplphysiol.00895.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McDaniel J, Hayman MA, Ives S, Fjeldstad AS, Trinity JD, Wray DW, Richardson RS. Attenuated exercise induced hyperaemia with age: mechanistic insight from passive limb movement. J Physiol 588: 4507–4517, 2010. doi: 10.1113/jphysiol.2010.198770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDaniel J, Ives SJ, Richardson RS. Human muscle length-dependent changes in blood flow. J Appl Physiol (1985) 112: 560–565, 2012. doi: 10.1152/japplphysiol.01223.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore KL, Dalley AF. Clinically Oriented Anatomy (4th ed.). Philadelphia, PA: Lippincott Williams & Wilkins, 1999. [Google Scholar]
- 45.Mortensen SP, Askew CD, Walker M, Nyberg M, Hellsten Y. The hyperaemic response to passive leg movement is dependent on nitric oxide: a new tool to evaluate endothelial nitric oxide function. J Physiol 590: 4391–4400, 2012. doi: 10.1113/jphysiol.2012.235952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mortensen SP, Nyberg M, Thaning P, Saltin B, Hellsten Y. Adenosine contributes to blood flow regulation in the exercising human leg by increasing prostaglandin and nitric oxide formation. Hypertension 53: 993–999, 2009. doi: 10.1161/HYPERTENSIONAHA.109.130880. [DOI] [PubMed] [Google Scholar]
- 47.Nelson AD, Rossman MJ, Witman MA, Barrett-O’Keefe Z, Groot HJ, Garten RS, Richardson RS. Nitric oxide-mediated vascular function in sepsis using passive leg movement as a novel assessment: a cross-sectional study. J Appl Physiol (1985) 120: 991–999, 2016. doi: 10.1152/japplphysiol.00961.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishiyama SK, Zhao J, Wray DW, Richardson RS. Vascular function and endothelin-1: tipping the balance between vasodilation and vasoconstriction. J Appl Physiol (1985) 122: 354–360, 2017. doi: 10.1152/japplphysiol.00772.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Padilla J, Harris RA, Fly AD, Rink LD, Wallace JP. The effect of acute exercise on endothelial function following a high-fat meal. Eur J Appl Physiol 98: 256–262, 2006. doi: 10.1007/s00421-006-0272-z. [DOI] [PubMed] [Google Scholar]
- 50.Padilla J, Johnson BD, Newcomer SC, Wilhite DP, Mickleborough TD, Fly AD, Mather KJ, Wallace JP. Normalization of flow-mediated dilation to shear stress area under the curve eliminates the impact of variable hyperemic stimulus. Cardiovasc Ultrasound 6: 44, 2008. doi: 10.1186/1476-7120-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papamichael CM, Aznaouridis KA, Karatzis EN, Karatzi KN, Stamatelopoulos KS, Vamvakou G, Lekakis JP, Mavrikakis ME. Effect of coffee on endothelial function in healthy subjects: the role of caffeine. Clin Sci (Lond) 109: 55–60, 2005. doi: 10.1042/CS20040358. [DOI] [PubMed] [Google Scholar]
- 52.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol 568: 357–369, 2005. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rådegran G, Saltin B. Human femoral artery diameter in relation to knee extensor muscle mass, peak blood flow, and oxygen uptake. Am J Physiol Heart Circ Physiol 278: H162–H167, 2000. [DOI] [PubMed] [Google Scholar]
- 54.Rådegran G, Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol Heart Circ Physiol 276: H1951–H1960, 1999. [DOI] [PubMed] [Google Scholar]
- 55.Rizzo RJ, Sandager G, Astleford P, Payne K, Peterson-Kennedy L, Flinn WR, Yao JS. Mesenteric flow velocity variations as a function of angle of insonation. J Vasc Surg 11: 688–694, 1990. doi: 10.1016/0741-5214(90)90215-V. [DOI] [PubMed] [Google Scholar]
- 56.Rosenmeier JB, Dinenno FA, Fritzlar SJ, Joyner MJ. α1- And α2-adrenergic vasoconstriction is blunted in contracting human muscle. J Physiol 547: 971–976, 2003. doi: 10.1113/jphysiol.2002.037937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rossman MJ, Groot HJ, Garten RS, Witman MA, Richardson RS. Vascular function assessed by passive leg movement and flow-mediated dilation: initial evidence of construct validity. Am J Physiol Heart Circ Physiol 311: H1277–H1286, 2016. doi: 10.1152/ajpheart.00421.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 120: 357–375, 2011. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shechter M, Shalmon G, Scheinowitz M, Koren-Morag N, Feinberg MS, Harats D, Sela BA, Sharabi Y, Chouraqui P. Impact of acute caffeine ingestion on endothelial function in subjects with and without coronary artery disease. Am J Cardiol 107: 1255–1261, 2011. doi: 10.1016/j.amjcard.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 61.Sorensen KE, Celermajer DS, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Thomas O, Deanfield JE. Non-invasive measurement of human endothelium dependent arterial responses: accuracy and reproducibility. Br Heart J 74: 247–253, 1995. doi: 10.1136/hrt.74.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takase B, Uehata A, Akima T, Nagai T, Nishioka T, Hamabe A, Satomura K, Ohsuzu F, Kurita A. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am J Cardiol 82: 1535–1539, 1998. doi: 10.1016/S0002-9149(98)00702-4. [DOI] [PubMed] [Google Scholar]
- 63.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thijssen DH, Rowley N, Padilla J, Simmons GH, Laughlin MH, Whyte G, Cable NT, Green DJ. Relationship between upper and lower limb conduit artery vasodilator function in humans. J Appl Physiol (1985) 111: 244–250, 2011. doi: 10.1152/japplphysiol.00290.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64a.Thrush A, Hartshorne T. Blood flow and its appearance on color flow imaging. In: Peripheral Vascular Ultrasound: How, Why, and When. Edinburgh; New York: Churchill Livingstone, 2005, p. 49–62. [Google Scholar]
- 65.Thrush A, Hartshorne T. Doppler ultrasound. In: Peripheral Vascular Ultrasound: How, Why, and When. Edinburgh; New York: Churchill Livingstone, 2005, p. 23–34. [Google Scholar]
- 66.Thrush A, Hartshorne T. Factors that influence the Doppler spectrum. In: Peripheral Vascular Ultrasound: How, Why, and When. Edinburgh; New York: Churchill Livingstone, 2005, p. 63–73. [Google Scholar]
- 68.Tomasian D, Keaney JF, Vita JA. Antioxidants and the bioactivity of endothelium-derived nitric oxide. Cardiovasc Res 47: 426–435, 2000. doi: 10.1016/S0008-6363(00)00103-6. [DOI] [PubMed] [Google Scholar]
- 69.Trinity JD, Amann M, McDaniel J, Fjeldstad AS, Barrett-O’Keefe Z, Runnels S, Morgan DE, Wray DW, Richardson RS. Limb movement-induced hyperemia has a central hemodynamic component: evidence from a neural blockade study. Am J Physiol Heart Circ Physiol 299: H1693–H1700, 2010. doi: 10.1152/ajpheart.00482.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Morgan DE, Gmelch BS, Bledsoe A, Richardson RS. Passive leg movement and nitric oxide-mediated vascular function: the impact of age. Am J Physiol Heart Circ Physiol 308: H672–H679, 2015. doi: 10.1152/ajpheart.00806.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S, Gmelch B, Bledsoe A, Richardson RS. Nitric oxide and passive limb movement: a new approach to assess vascular function. J Physiol 590: 1413–1425, 2012. doi: 10.1113/jphysiol.2011.224741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Umans JG, Levi R. Nitric oxide in the regulation of blood flow and arterial pressure. Annu Rev Physiol 57: 771–790, 1995. doi: 10.1146/annurev.ph.57.030195.004011. [DOI] [PubMed] [Google Scholar]
- 73.Venturelli M, Amann M, Layec G, McDaniel J, Trinity JD, Fjeldstad AS, Ives SJ, Yonnet G, Richardson RS. Passive leg movement-induced hyperaemia with a spinal cord lesion: evidence of preserved vascular function. Acta Physiol (Oxf) 210: 429–439, 2014. doi: 10.1111/apha.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Venturelli M, Layec G, Trinity J, Hart CR, Broxterman RM, Richardson RS. Single passive leg movement-induced hyperemia: a simple vascular function assessment without a chronotropic response. J Appl Physiol (1985) 122: 28–37, 2017. doi: 10.1152/japplphysiol.00806.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walker MA, Hoier B, Walker PJ, Schulze K, Bangsbo J, Hellsten Y, Askew CD. Vasoactive enzymes and blood flow responses to passive and active exercise in peripheral arterial disease. Atherosclerosis 246: 98–105, 2016. doi: 10.1016/j.atherosclerosis.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 76.Wang X, Derakhshandeh R, Liu J, Narayan S, Nabavizadeh P, Le S, Danforth OM, Pinnamaneni K, Rodriguez HJ, Luu E, Sievers RE, Schick SF, Glantz SA, Springer ML. One minute of marijuana secondhand smoke exposure substantially impairs vascular endothelial function. J Am Heart Assoc 5: e003858, 2016. doi: 10.1161/JAHA.116.003858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Widlansky ME, Gokce N, Keaney JF Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol 42: 1149–1160, 2003. doi: 10.1016/S0735-1097(03)00994-X. [DOI] [PubMed] [Google Scholar]
- 78.Williams MR, Westerman RA, Kingwell BA, Paige J, Blombery PA, Sudhir K, Komesaroff PA. Variations in endothelial function and arterial compliance during the menstrual cycle. J Clin Endocrinol Metab 86: 5389–5395, 2001. doi: 10.1210/jcem.86.11.8013. [DOI] [PubMed] [Google Scholar]
- 80.Witman MA, Fjeldstad AS, McDaniel J, Ives SJ, Zhao J, Barrett-O’Keefe Z, Nativi JN, Stehlik J, Wray DW, Richardson RS. Vascular function and the role of oxidative stress in heart failure, heart transplant, and beyond. Hypertension 60: 659–668, 2012. doi: 10.1161/HYPERTENSIONAHA.112.193318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Witman MA, Ives SJ, Trinity JD, Groot HJ, Stehlik J, Richardson RS. Heart failure and movement-induced hemodynamics: partitioning the impact of central and peripheral dysfunction. Int J Cardiol 178: 232–238, 2015. doi: 10.1016/j.ijcard.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wray DW, Donato AJ, Uberoi A, Merlone JP, Richardson RS. Onset exercise hyperaemia in humans: partitioning the contributors. J Physiol 565: 1053–1060, 2005. doi: 10.1113/jphysiol.2005.084327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wray DW, Nishiyama SK, Harris RA, Zhao J, McDaniel J, Fjeldstad AS, Witman MA, Ives SJ, Barrett-O’Keefe Z, Richardson RS. Acute reversal of endothelial dysfunction in the elderly after antioxidant consumption. Hypertension 59: 818–824, 2012. doi: 10.1161/HYPERTENSIONAHA.111.189456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wray DW, Witman MA, Ives SJ, McDaniel J, Trinity JD, Conklin JD, Supiano MA, Richardson RS. Does brachial artery flow-mediated vasodilation provide a bioassay for NO? Hypertension 62: 345–351, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xin W, Wei W, Li X. Effect of fish oil supplementation on fasting vascular endothelial function in humans: a meta-analysis of randomized controlled trials. PLoS One 7: e46028, 2012. doi: 10.1371/journal.pone.0046028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation 115: 2390–2397, 2007. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 87.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 120: 502–509, 2009. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]