We report on noninvasive optical measurements of skeletal muscle blood flow and oxygen extraction dynamics before/during/after treadmill exercise in peripheral artery disease patients who experience claudication. The measurements tracked the effects of a 3-mo supervised exercise training protocol and revealed that supervised exercise training improved patient ability to increase microvascular calf muscle blood flow and oxygen extraction during physical activity.
Keywords: near-infrared spectroscopy, diffuse correlation spectroscopy, peripheral artery disease, exercise training, claudication
Abstract
We employed near-infrared optical techniques, diffuse correlation spectroscopy (DCS), and frequency-domain near-infrared spectroscopy (FD-NIRS) to test the hypothesis that supervised exercise training increases skeletal muscle microvascular blood flow and oxygen extraction in patients with peripheral artery disease (PAD) who experience claudication. PAD patients (n = 64) were randomly assigned to exercise and control groups. Patients in the exercise group received 3 mo of supervised exercise training. Calf muscle blood flow and oxygen extraction were optically monitored before, during, and after performance of a graded treadmill protocol at baseline and at 3 mo in both groups. Additionally, measurements of the ankle-brachial index (ABI) and peak walking time (PWT) to maximal claudication were made during each patient visit. Supervised exercise training was found to increase the maximal calf muscle blood flow and oxygen extraction levels during treadmill exercise by 29% (13%, 50%) and 8% (1%, 12%), respectively [P < 0.001; median (25th percentile, 75th percentile)]. These improvements across the exercise group population were significantly higher than corresponding changes in the control group (P < 0.004). Exercise training also increased PWT by 49% (18%, 101%) (P = 0.01). However, within statistical error, the ABI, resting calf muscle blood flow and oxygen extraction, and the recovery half-time for hemoglobin\myoglobin desaturation following cessation of maximal exercise were not altered by exercise training. The concurrent monitoring of both blood flow and oxygen extraction with the hybrid DCS/FD-NIRS instrument revealed enhanced muscle oxidative metabolism during physical activity from exercise training, which could be an underlying mechanism for the observed improvement in PWT.
NEW & NOTEWORTHY We report on noninvasive optical measurements of skeletal muscle blood flow and oxygen extraction dynamics before/during/after treadmill exercise in peripheral artery disease patients who experience claudication. The measurements tracked the effects of a 3-mo supervised exercise training protocol and revealed that supervised exercise training improved patient ability to increase microvascular calf muscle blood flow and oxygen extraction during physical activity.
INTRODUCTION
Approximately two million Americans with peripheral artery disease (PAD) experience claudication, a walking-induced muscle pain that is relieved only by rest (55). Claudication discourages ambulation, and patients experiencing claudication generally have sedentary lifestyles and poor health-related quality of life (27, 63, 65). In most patients, the underlying cause of claudication is impaired muscle blood flow caused by systemic atherosclerosis. As a result, these patients experience an exercise-induced imbalance between oxygen supply and demand in active muscles. To date, several studies have reported strong correlations between exercise training and mitigation of claudication symptoms (53, 64).
The mechanisms behind exercise training-induced improvement, however, are not well understood. A useful diagnostic for the presence of PAD is the ankle-brachial index (ABI), the ratio of ankle and brachial (arm) systolic blood pressures measured in the supine position (1, 40). Clinically, a resting ABI < 0.90 indicates the presence of PAD, and lower ABI values correspond to severe arterial stenosis (40, 54). Interestingly, exercise training has not been found to improve ABI in patients with claudication (53); this observation suggests that exercise training does not directly treat systemic atherosclerosis. Instead, exercise training may increase the muscle oxygen supply and demand balance through microvascular alterations such as formation of new collateral blood vessels (i.e., angiogenesis) and improvement of endothelial vessel dilation in existing collateral blood vessels (64). In this study, we employ the optical techniques of diffuse correlation spectroscopy (DCS) and frequency-domain near-infrared spectroscopy (FD-NIRS) to further characterize these responses to exercise and thereby derive a deeper understanding of the underlying mechanisms that produce improvement. We hypothesize that supervised exercise training will increase maximal skeletal muscle microvascular blood flow and oxygen extraction during physical activity in patients with PAD who experience claudication.
DCS and FD-NIRS, respectively, employ near-infrared light to noninvasively measure tissue microvascular blood flow, tissue oxygen saturation, and total hemoglobin/myoglobin concentration (13, 25, 33, 34, 39, 61, 71). In combination, these measurements of blood flow and blood oxygenation provide access to the oxygen metabolic status of muscle (13, 34, 71). Here, we incorporated DCS and FD-NIRS measurements into a randomized clinical study to ascertain the effects of supervised exercise training on microvascular calf muscle blood flow and oxygen extraction in patients with claudication. Through concurrent monitoring of blood flow and blood oxygenation, this work builds upon previous investigations that used continuous-wave near-infrared spectroscopy (CW-NIRS) to study the effects of exercise training on oxygen saturation alone (4, 30). We found that even after a relatively brief 3-mo period of exercise training, maximal calf muscle blood flow, oxygen extraction levels, and oxygen consumption levels increased during physical activity. In addition to quantification and elucidation of these phenomena, the novel measurement approach reported here could help clinicians optimize exercise training protocols for claudication treatment in the future.
METHODS
Study design.
We conducted a randomized clinical trial at the University of Pennsylvania. The study was approved by the institutional review board at the University of Pennsylvania, and all subjects recruited provided written informed consent. Subjects with intermittent claudication and a diagnosis of PAD made two initial visits to the testing center before supervised exercise training. In the first visit, each subject’s medical and surgical history was assessed with a screening questionnaire, and a history of intermittent claudication was confirmed with the San Diego Claudication and Walking Impairment Questionnaires (20). Additionally, each subject’s ABI at rest was measured bilaterally with Doppler sonography (1), and each subject performed a graded treadmill protocol, i.e., 2 mph, 0% initial grade with 2% grade increase every 2 min until maximal claudication (2). The subject’s peak walking time (PWT) was defined as the walking time on the graded treadmill protocol at which ambulation could not continue because of maximal claudication. Reproducibility of each subject’s baseline PWT was confirmed via completion of the graded treadmill protocol at a second visit that took place within 1 mo after the first visit. Subjects for which PWT between the two visits deviated by >25% were excluded.
After the second visit, subjects were randomized to an exercise group or a control group. Subjects in the exercise group performed three 60-min supervised exercise training sessions each week for a period of 3 mo at the vascular laboratory of the University of Pennsylvania. The protocol that was followed during the supervised training sessions is described elsewhere (10). Briefly, subjects walk on a treadmill at an initial speed of 2.0 mph to a mild to moderate pain level, stop and rest until the claudication pain has completely abated, and then resume walking. This pattern repeats for a total of 60 min. If subjects can walk longer than 8 min without rest, the treadmill walking becomes more challenging via grade and speed increases in subsequent training sessions (10).
During a third visit, roughly 3 mo after the second visit, each subject in the exercise and control groups completed the graded treadmill protocol, and each subject’s ABI at rest was measured bilaterally. Furthermore, in 64 subjects (29 in the exercise group, 35 in the control group) calf muscle blood flow and oxygen extraction in the most symptomatic leg were noninvasively monitored before, during, and after the visit 2 and visit 3 graded treadmill tests; these measurements utilized a custom-built hybrid DCS/FD-NIRS instrument described elsewhere (51, 71). The custom instrument interleaved DCS and FD-NIRS measurements to derive muscle blood flow and oxygen extraction fraction every 8 s.
The optical probe (Fig. 1) was secured above the calf flexor (i.e., the probe was centered above the gastrocnemius muscle at the axial position roughly corresponding to the maximum girth of the calf) with double-sided medical tape (no. 1509; 3M Health Care, St. Paul, MN) and a 4-in.-wide elastic bandage (ACE; 3M Health Care). For the 2-min baseline and 4-min recovery monitoring periods immediately before and after the graded treadmill test, respectively, the subjects stood at rest on the treadmill.
Fig. 1.
A: schematic of optical probe head used for monitoring exercise-induced changes in calf muscle blood flow and oxygen extraction. Eight prism-coupled fiber-optic bundles (Fiberoptic Systems, Simi Valley, CA) embedded in urethane rubber enabled probing of the calf muscle with near-infrared light at multiple source-detector distances spanning 2.2–3.8 cm. Near-infrared light was sequentially delivered to the tissue through 5 fused silica multimode source fibers (S1, S2, S3, S4, FS; 400-μm core/0.22 NA). The DCS measurement used a bundle of 8 single-mode fibers (FD; 780 HP/0.13 NA) to detect highly coherent light delivered via fiber FS (2.5 cm source-detector separation), from which a blood flow index is obtained. The FD-NIRS measurement employed two 1-mm diameter bundles of borosilicate fiber (D1, D2; 0.55 NA) to detect the amplitude and phase of multispectral intensity modulated light delivered from fibers S1, S2, S3, and S4. The combination of multispectral amplitude and phase measurements across the 8 source-detector distances provided a single measure of muscle oxygen extraction and total hemoglobin/myoglobin (see text). Orange arrows show the direction of light propagation through an exemplar optical source fiber, which is reflected 90° by the prism to the tissue (the direction is reversed for the detector fibers). B: schematic showing the time line of the randomized clinical study. Sixty-four PAD subjects were monitored with DCS and FD-NIRS before, during, and after a graded treadmill test at 2 visits separated 3 mo apart. After the initial visit, the subjects randomized to the exercise group did 3 mo of supervised exercise training (see text).
Muscle blood flow monitoring with DCS.
DCS estimates blood flow by quantifying rapid speckle intensity fluctuations of multiply scattered coherent near-infrared light induced by red blood cell motion (6, 7, 60). For the DCS measurement, a continuous-wave, long-coherence-length 785-nm laser illuminates a point on the skin above the calf muscle via a multimode prism-coupled source fiber (Fig. 1A). The near-infrared photons propagate diffusively through the muscle along random walk pathways such that the light scatters from moving red blood cells. A bundle of single-mode detection fibers positioned 2.5 cm away from the source couples diffusive light emerging from the calf muscle to two arrays of four single-photon-counting avalanche photodiodes. The detected light fields are sensitive to blood flow at an average depth of roughly 1 cm below the skin surface. Higher blood flow produces comparatively more rapid speckle intensity fluctuations at the detectors.
Specifically, the detected normalized intensity temporal autocorrelation function, , is computed at multiple delay times, τ; here I(t) is the detected light intensity at time t, and the angular brackets, , represent time averages. A DCS blood flow index (F) is derived from the decay rate of g2(τ) with a homogeneous semi-infinite tissue model (6, 7). It also should be noted that the tissue absorption and scattering measurements made with FD-NIRS (see Muscle oxygen extraction fraction monitoring with FD-NIRS) were used as inputs in the semi-infinite tissue model. The DCS method for measuring muscle blood flow has been validated in humans with arterial spin-labeled perfusion MRI (72) and with venous occlusion NIRS (47).
DCS F measurements were made before and after exercise. Since the DCS measurement sampling rate (0.13 Hz) is too low to permit filtering of motion artifacts induced by moving muscle fibers during exercise (62), the DCS-measured index represents a mixture of blood flow and mechanical motion. Consequently, DCS F measurements during treadmill exercise cannot be accurately obtained and were excluded from the analysis.
Muscle oxygen extraction fraction monitoring with FD-NIRS.
Muscle oxygen extraction fraction measurements were made with a custom-built three-wavelength (685, 785, 830 nm) multichannel FD-NIRS spectrophotometer (51, 71). The FD-NIRS device sequentially delivered radio-frequency intensity-modulated light (70 MHz) at each wavelength to four source positions above the calf muscle (Fig. 1A). Each source produced a diffuse photon density wave in the muscle oscillating at the same 70-MHz frequency (25). The amplitude and phase of the diffusing waves were measured at two detector positions over the muscle. Application of the semi-infinite tissue model to the amplitude and phase measurements made at eight source-detector distances spanning 2.2–3.8 cm permitted simultaneous determination of the muscle absorption and reduced scattering coefficients at each wavelength (25). It should be noted that the source and detector fiber coupling coefficients to the tissue were obtained with a self-calibrating approach (3, 71). To reduce cross talk between the muscle absorption and scattering induced by noise, we first temporally averaged the amplitude and phase data across the baseline interval (Fig. 1B); then we computed the baseline absorption and reduced scattering coefficients. We assumed a temporally constant tissue scattering coefficient at this baseline value, and we fit each set of amplitude measurements made at the eight source-detector distances to the semi-infinite tissue model to extract the absorption coefficient.
Muscle absorption in the near-infrared spectral window depends predominantly on oxy-hemoglobin\myoglobin (HbO2\MbO2), deoxy-hemoglobin\myoglobin (Hb\Mb), and water concentrations. Note that since hemoglobin and myoglobin have very similar optical spectra, the HbO2\MbO2 and Hb\Mb notations indicate that the optical measurements of these concentrations should be viewed as a combination of muscle hemoglobin and myoglobin (15). Assuming a muscle water volume fraction of 70% (67), we used measurements of muscle absorption at three near-infrared wavelengths to quantitatively resolve HbO2\MbO2 and Hb\Mb concentrations, from which the muscle total hemoglobin\myoglobin concentration, i.e., THC = HbO2\MbO2 + Hb\Mb, and muscle tissue oxygen saturation, i.e., = (HbO2\MbO2)/THC, were calculated.
FD-NIRS measurements reflect a mixture of arteriole, capillary, and venous blood and do not separate venous from arterial saturations (21). Specifically, = ka + kc + kv, where , , and are the arteriolar, capillary, and venous saturations, respectively, and ka, kc, and kv are the respective weight of each compartment’s contribution to the total blood volume (ka + kc + kv = 1). Here we employ a standard simplification that represents as a weighted average of the arteriolar and venous saturations, i.e., = (1 − kw) + kw. Assuming an arteriolar oxygen saturation of unity, the muscle oxygen extraction fraction [i.e., OEF ≡ ( − )/] is given by OEF = (1 − )/γ, where γ ≡ kv + kckw is a vascular weighting constant (21). For each subject visit, we assumed that γ remained constant through the baseline, graded treadmill, and recovery periods of DCS/FD-NIRS monitoring (Fig. 1B). Consequently, the factor γ divides out in the computation of relative changes in OEF during treadmill exercise. We discuss this “constant-” assumption in discussion.
Data analysis and statistical methods.
Figure 2 shows temporal plots of the visit 2 and visit 3 fractional (relative) changes in calf muscle blood flow (i.e., rF = F/Fo) and OEF (i.e., rOEF = OEF/OEFo) for a PAD subject in the exercise group. Here, Fo and OEFo are the average F and OEF across the baseline monitoring interval. To test the hypothesis that exercise training enhances blood flow, we computed the average rF during the first 30 s of the recovery interval for visit 2 and visit 3 for every subject. These averages are denoted rFv2 and rFv3 (Fig. 2). We further characterized the ability of the vasculature to increase OEF by computing the average rOEF during the last 30 s of treadmill exercise, i.e., rOEFv2 and rOEFv3 (Fig. 2). It should be noted that, since DCS flow (F) measurements during exercise cannot be accurately obtained because of motion artifacts, we assumed that rFv2 and rFv3 are good approximations of maximal end-exercise blood flow levels.
Fig. 2.
Temporal plots of fractional changes in calf muscle blood flow (F) and oxygen extraction fraction (OEF) from treadmill exercise in a PAD patient (see Fig. 1). F measurements during treadmill exercise have motion artifacts and are not used (see text). Relative F and OEF, i.e., rFv2 ≡ Fex,v2/Fo,v2 and rOEFv2 ≡ OEFex,v2/OEFo,v2, are the “maximal” F and OEF changes from treadmill exercise for visit 2; Fex,v2 is the average F during the first 30 s following cessation of treadmill exercise, and OEFex,v2 is the average OEF during the last 30 s of treadmill exercise. Similarly, rFv3 and rOEFv3 are the maximal F and OEF changes for visit 3. TR,v2 and TR,v3 are the visit 2 and visit 3 desaturation recovery times (see text), and PWTv2 and PWTv3 are the peak walking times. Note that PWTv3 > PWTv2, rOEFv3 > rOEFv2, and rFv3 > rFv2.
In combination, measurement of rF and rOEF enables calculation of relative changes in muscle tissue oxidative metabolism (i.e., rVo2 = Vo2/Vo2,o) via Fick’s law (21, 71): rVo2 = rF × rOEF. This expression assumes that arteriolar oxygen concentration (micromole per volume blood) does not change, i.e., arteriolar hematocrit is constant (21, 71). Here, we utilize this expression to compute rVo2 from rOEF and rF measurements.
A third parameter we obtained during visit 2 and visit 3 is the recovery time (TR) for hemoglobin/myoglobin desaturation (15). This is the half-time (i.e., time duration to half-maximum) for the OEF to return to baseline, which is essentially the half-time for to return to baseline (Fig. 2). Additional computed variables at each visit from the calf-muscle DCS/FD-NIRS measurements include THCo, Fo, , ∆THC, and ; here, the subscript “o” denotes the average of the parameter at baseline, and ∆THC and represent average THC and during the last 30 s of treadmill exercise minus THCo and , respectively. Finally, we report baseline reduced scattering coefficients (i.e., ) for the muscle measured at each FD-NIRS wavelength.
For each parameter, we derived a ratio of visit 3 to visit 2 values. A Wilcoxon rank sum test for equal medians implemented in MATLAB R2016a (ranksum; MathWorks, Natick, MA) was used to determine significant differences in the ratios between the exercise and control groups. To test for differences between visit 3 and visit 2 in a single group, a Wilcoxon signed-rank test also implemented in MATLAB R2016a (signrank) was employed. Summary statistics are reported as median (25th percentile, 75th percentile). Finally, to assess the impact of demographic discrepancies between the exercise and control groups, we carried out a least-squares fitting of the rFv3-to-rFv2 ratio to the following multivariate linear regression model (fitlm in MATLAB) of demographic variables: rFv3/rFv2 = β0 + β1Gen + β2HTN + β3Diab + β4Smoke. Here, Gen, HTN, Diab, and Smoke are binary categorical variables (i.e., dummy variables that are either 1 or 0) representing patient sex, hypertensive status, diabetes status, and smoking status. The same fit was carried out for the rOEFv3-to-rOEFv2 ratio.
RESULTS
Demographics of the exercise and control group populations are shown in Table 1. ABI, age, body mass index (BMI), race, and medication use were similar between the two groups. The percentage of men in the exercise group population was larger than in the control group. The exercise group was also modestly more hypertensive, contained a larger percentage of subjects with type 2 diabetes, and contained a lower percentage of current smokers than the control group. Additionally, the thickness of the near-surface tissue layer overlying the muscle (i.e., skin and adipose tissue) was measured from MRI anatomical images and provides rough estimates of the thickness at the site of application of the optical probe (described in Study design). The thickness was slightly lower in the exercise group than in the control group.
Table 1.
Demographics of study population
| Exercise | Control | |
|---|---|---|
| n | 29 | 35 |
| ABI | 0.64 (0.54, 0.76) | 0.65 (0.53, 0.79) |
| Sex | ||
| Male | 24 (83%) | 17 (49%) |
| Female | 5 (17%) | 18 (51%) |
| Age, yr | 66 (58, 69) | 67 (60, 76) |
| BMI, kg/m2 | 28.7 (26, 30.4) | 28.6 (24.5, 30.9) |
| Race | ||
| White | 15 (52%) | 15 (43%) |
| Black | 13 (45%) | 20 (57%) |
| Hispanic | 1 (3%) | |
| Diabetes | ||
| No history | 16 (55%) | 25 (71%) |
| Type 2 | 13 (45%) | 10 (29%) |
| Smoking status | ||
| Nonsmokers | 24 (83%) | 22 (63%) |
| Current smoker | 5 (17%) | 13 (37%) |
| Hypertension | ||
| Hypertensive | 27 (93%) | 26 (74%) |
| Normotensive | 2 (7%) | 9 (26%) |
| Thickness of near-surface layer (skin and adipose tissue), mm | 3.2 (2.4, 4.5) | 4.0 (2.6, 6.1) |
| Medication use | ||
| Statin | 20 (69%) | 27 (77%) |
| Cilostazol | 9 (31%) | 6 (17%) |
Data are n (%) or median (25th percentile, 75th percentile).
Average preexercise training (i.e., visit 2) DCS/FD-NIRS calf muscle measurements, ABI, and PWT across both the exercise and control group populations are reported in Table 2. Both F and OEF increased during exercise, while THC decreased; note that the increase in OEF corresponds to a decrease in . We observed high variability in the Fo measurements. This observation reflects variability in resting muscle blood flow across subjects, and it likely also reflects variability in other factors such as differences in the layer thickness of the adipose tissue located above the muscle and the pressure of the probe against the muscle (47). Variability in the reduced scattering coefficients (i.e., ) of muscle across the subject population was ~20%. We did not observe significant changes in the reduced scattering coefficients due to treadmill exercise (data not shown).
Table 2.
DCS/FD-NIRS preexercise training measurements
| Variables | Mean ± SD | Median (25%, 50%) |
|---|---|---|
| ABI | 0.63 ± 0.14 | 0.63 (0.52, 0.73) |
| PWT, s | 480 ± 283 | 420 (286, 640) |
| , % | 59 ± 14 | 58 (48, 72) |
| THCo, μM | 106 ± 47 | 98 (64, 143) |
| Fo, 10−9 cm2/s | 5.30 ± 5.07 | 3.81 (1.79, 6.17) |
| , % | −14 ± 7 | −13 (−18, −9) |
| ∆THC, μM | −5.6 ± 10.7 | −6.2 (−12.5, 1.8) |
| rOEF ≡ OEFex/OEFo | 1.31 ± 0.16 | 1.30 (1.20, 1.41) |
| rF ≡ Fex/Fo | 2.08 ± 0.79 | 1.97 (1.49, 2.39) |
| rVo2 = rF × rOEF | 2.72 ± 1.02 | 2.55 (1.91, 3.18) |
| TR, s | 109 ± 61 | 101 (55, 153) |
| (685 nm), cm−1 | 5.64 ± 1.16 | 5.82 (4.91, 6.65) |
| (785 nm), cm−1 | 5.56 ± 1.57 | 5.82 (3.82, 6.37) |
| (830 nm), cm−1 | 5.05 ± 1.09 | 4.90 (4.33, 6.00) |
n = 64. See Data analysis and statistical methods for definitions of variables. ABI, PWT, , THC, F, OEF, Vo2, TR, and denote, respectively, the ankle-brachial index, peak walking time, muscle tissue oxygen saturation, muscle total hemoglobin\myoglobin concentration (micromole per volume of tissue), muscle blood flow index, muscle oxygen extraction fraction, muscle oxygen metabolism, half-time (time duration to half maximum) for OEF to recover to baseline after exercise, and muscle tissue reduced scattering coefficient. The subscript “o” denotes the average of the parameter at baseline, ∆THC and represent average THC and during the last 30 s of treadmill exercise minus THCo and , OEFex is the average OEF during the last 30 s of treadmill exercise, and Fex is the average F during the first 30 s of recovery following cessation of treadmill exercise.
Table 3 summarizes the preexercise and postexercise training measurements in both the exercise and control groups. Exercise training improved PWT on average by 49% (18%, 101%) across individuals in the exercise group, but a significant change in the ABI from exercise training was not observed, i.e., 1% (−10%, 10%). In the control group, the changes in PWT and ABI between visit 2 and visit 3 were −3% (−11%, 24%) and 5% (−11%, 13%), respectively; these changes were not significant.
Table 3.
Preexercise training (visit 2) and postexercise training (visit 3) DCS/FD-NIRS measurements in exercise group and control group populations
| Exercise Group (n = 29) |
Control Group (n = 35) |
|||
|---|---|---|---|---|
| Variables | Visit 2 | Visit 3 | Visit 2 | Visit 3 |
| ABI | 0.64 (0.54, 0.76) | 0.65 (0.53, 0.79) | 0.59 (0.52, 0.72) | 0.59 (0.55, 0.67) |
| PWT†, s | 447 (303, 729) | 605 (396, 1153) | 416 (277, 557) | 469 (305, 737) |
| rF* (i.e., Fex/Fo) |
1.94 (1.34, 2.48) | 2.31 (1.94, 2.85) | 1.99 (1.52, 2.21) | 1.74 (1.35, 2.63) |
| rOEF* (i.e., OEFex/OEFo) |
1.27 (1.15, 1.41) | 1.34 (1.21, 1.54) | 1.32 (1.22, 1.41) | 1.21 (1.15, 1.34) |
| rVo2* (i.e., rF × rOEF) |
2.40 (1.82, 3.00) | 3.43 (2.77, 3.83) | 2.59 (1.95, 3.21) | 2.09 (1.69, 3.43) |
| TR, s | 87 (60, 139) | 87 (61, 130) | 114 (50, 162) | 92 (49, 159) |
| , % | 59 (47, 73) | 67 (54, 72) | 56 (42, 68) | 59 (49, 70) |
| *, % | −12 (−18, −7) | −15 (−24, −9) | −14 (−18, −10) | −9 (−15, −7) |
| THCo, μM | 117 (62, 149) | 141 (104, 159) | 91 (64, 137) | 124 (96, 168) |
| ∆THC, μM | −6.0 (−14.2, 2.7) | −5.8 (−16.3, 3.4) | −6.2 (−11.0, 1.4) | −6.6 (−14.1, 0.6) |
| Fo, 10−9 cm2/s | 4.28 (2.66, 8.68) | 5.04 (2.14, 9.65) | 3.41 (1.62, 5.45) | 3.78 (2.05, 6.87) |
Data are median (25th percentile, 75th percentile). ABI, PWT, , THC, F, OEF, Vo2, and TR denote, respectively, the ankle-brachial index, peak walking time, muscle tissue oxygen saturation, muscle total hemoglobin\myoglobin concentration (micromole per volume of tissue), muscle blood flow index, muscle oxygen extraction fraction, muscle oxygen metabolism, and half-time (time duration to half maximum) for OEF to recover to baseline after exercise, respectively. The subscript “o” denotes the average of the parameter at baseline, ∆THC and represent average THC and during the last 30 s of treadmill exercise minus THCo and , OEFex is the average OEF during the last 30 s of treadmill exercise, and Fex is the average F during the first 30 s of recovery following cessation of treadmill exercise.
P < 0.001,
P = 0.001 for the difference in visit 3-to-visit 2 ratios between exercise and control groups. P > 0.05 for other variables.
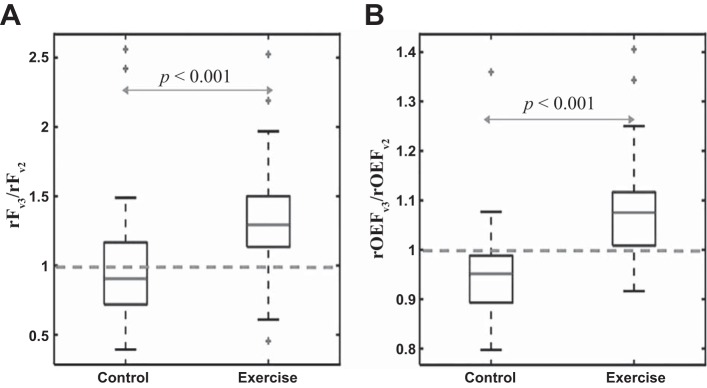
The optical DCS/FD-NIRS results indicate that exercise training enhances both calf muscle oxygen delivery (i.e., via increased blood flow) and oxygen extraction during physical activity (Fig. 3). The ratio rFv3/rFv2 of 1.29 (1.13, 1.50) across individuals in the exercise group was significantly higher than the ratio of 0.90 (0.72, 1.17) in the control group (P < 0.001) (Fig. 3). Similarly, the ratio rOEFv3/rOEFv2 of 1.08 (1.01, 1.12) in the exercise group was significantly higher than the ratio of 0.95 (0.89, 0.99) in the control group (P < 0.001), and the ratio rVo2,v3/rVo2,v2 of 1.46 (1.12, 1.68) in the exercise group was significantly higher than the ratio of 0.83 (0.69, 1.08) in the control group (P < 0.001).
Fig. 3.
Box plots of the ratio rFv3/rFv2 (A) and the ratio rOEFv3/rOEFv2 (B) for the control (n = 35) and exercise (n = 29) PAD population groups (for definitions of the ratios, see Fig. 2). P values in the plots indicate significant differences between the exercise and control groups. Additionally, the ratios for the exercise group of both parameters are significantly higher than unity (P < 0.001).
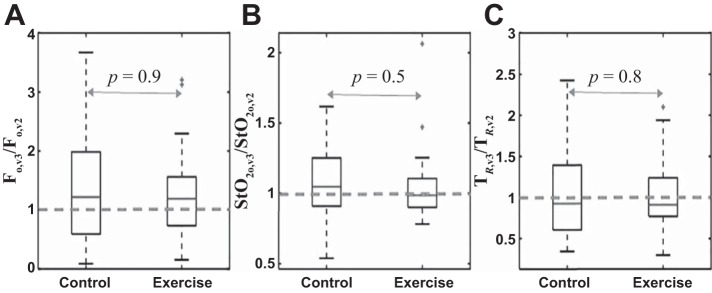
In the exercise group rFv3 was significantly higher than rFv2 (P < 0.001) and rOEFv3 was significantly higher than rOEFv2 (P < 0.001). In the control group there was no significant difference between rFv3 and rFv2 (P = 0.3), but, surprisingly, rOEFv3 was lower than rOEFv2 (P = 0.001). Exercise training did not significantly affect , THCo, Fo, ∆THC, and TR, i.e., no significant differences for these parameters were found in the visit 3-to-visit 2 ratios between the exercise and control groups (Fig. 4 and Table 3). Additionally, exercise training did not significantly affect muscle reduced scattering coefficients (data not shown).
Fig. 4.
Box plots of the ratios Fo,v3/Fo,v2 (A), / (B), and TR,v3/TR,v2 (C) for control (n = 35) and exercise (n = 29) PAD population groups. P values in the plots show no significant differences in these parameters between the exercise and control groups.
Finally, from the multivariate linear regression models (see Data analysis and statistical methods), no demographic variable was significantly related to rFv3/rFv2 and rOEFv3/rOEFv2 (P > 0.2 for all demographic variables).
DISCUSSION
In the clinical study, we used the optical techniques of DCS and FD-NIRS to test the impact of exercise training on calf muscle F and OEF in PAD patients with claudication. Exercise training was found to enhance the vasculature’s ability to increase both oxygen delivery and oxygen extraction during physical activity (Fig. 3). These improvements in oxygen delivery and extraction further demonstrate improved muscle oxidative metabolism (Table 3). Significant effects from exercise training on resting calf-muscle F and were not observed (Fig. 4). The hybrid DCS/FD-NIRS approach enables concurrent monitoring of more physiological variables in PAD patients than NIRS-only approaches (4, 8, 19, 28, 30, 32, 33, 35, 36). Hybrid DCS/FD-NIRS has been demonstrated in a variety of tissues, including skeletal muscle, brain, and tumor (11–13, 17, 25, 26, 34, 39, 61). Importantly, hybrid DCS/FD-NIRS is complementary to venous/arterial occlusion NIRS techniques for measurement of muscle blood flow and oxidative metabolism. Venous/arterial occlusion NIRS techniques can measure absolute muscle blood flow and oxidative metabolism in clinical units (5, 14, 23, 49, 57), but they are not well suited for continuous monitoring since venous/arterial occlusion interrupts blood flow. This limitation can be mitigated by using venous/arterial occlusion NIRS to calibrate hybrid DCS/FD-NIRS for concurrent absolute muscle blood flow and oxidative metabolism monitoring (34).
Regulation of blood flow in tissue under normal conditions is predominantly governed by variations in vasomotor tone and diameter of the terminal small arteries and arterioles (44, 59). During exercise under normal conditions, an increase in blood flow arising from substantial dilation of arterioles is accompanied by an increase in red blood cell number per volume of tissue in the capillary bed. An increased capillary red blood cell density, which arises from capillary blood volume expansion and/or higher capillary hematocrit, enables increased oxygen diffusion to the tissue (i.e., an increased oxygen extraction per unit of blood delivered) (16, 18, 22, 43, 56, 59). Endothelium-mediated vessel dilation is impaired in PAD patients (37, 64, 69), and evidence exists that in animal models (48) and human subjects (9, 50) exercise training improves endothelial dilation of collateral vessels. This mechanism could explain the observed improvement in maximal blood flow reached from treadmill exercise in the PAD exercise training group (Fig. 3).
It is also plausible that improved blood flow from endothelial dilation is associated with improved capillary blood volume expansion. In this case, the resulting enhancement in red blood cell density in the capillaries could explain the observed improvement in maximal oxygen extraction reached because of treadmill exercise (Fig. 3). Potential mechanisms of capillary blood volume expansion include vascular recruitment of additional capillaries (i.e., increased capillary density) (18, 22, 43, 59) as well as elevated blood volume/hematocrit in existing capillaries, which extends the capillary length for oxygen exchange (i.e., longitudinal recruitment) (16, 56). Also, data from animal models show enhanced angiogenesis from exercise training that results in increased capillary density (46, 48). Plausibly, exercise training in PAD patients could increase muscle capillary density by this mechanism; this effect could help account for the improved capillary blood volume expansion and oxygen extraction in the exercise training group.
Although enhanced capillary blood volume expansion raises the expectation that exercise training can significantly affect total hemoglobin/myoglobin concentration (i.e., micromole per volume of tissue), significant changes in THC from exercise training were not observed (Table 3). During exercise, two competing factors can influence THC: muscle contraction and capillary blood volume expansion. Muscle contraction compresses the venous component of the vascular tree, which extrudes venous blood volume from the muscle and decreases THC. Capillary blood volume expansion, on the other hand, increases THC. At maximal exercise, we observed a decrease in THC (Tables 2 and 3), which is consistent with reports from others (52, 71) and suggests that muscle contraction predominantly influences THC via the venous vasculature.
Additionally, the average observed improvement in maximal OEF of 8% from exercise training is fairly small, which suggests that enhancement in capillary blood volume expansion is also a small effect. This expansion effect is likely diluted even further by the presence of tissue “background absorbers” in muscle (e.g., myoglobin, water, lipids, melanin). For example, capillary blood volume expansion affects hemoglobin in blood but not myoglobin in muscle. Thus, the myoglobin contribution to the THC signal, which is unknown but has been roughly estimated at ~20% (28, 71), could reduce the sensitivity of the THC measurement to capillary blood volume increases. Changes in background absorbers during exercise tend to influence THC more than (67), and thus measurement errors are likely larger in ∆THC than in . Thus the enhancement in capillary blood volume expansion from exercise training could be too small to detect with our FD-NIRS instrument. Another possibility is that more effective muscle contraction or muscle hypertrophy in trained subjects leads to greater venous compression that counterbalances improved blood volume expansion in capillaries. Future work is needed to confirm the hypothesis that exercise training does not affect THC.
In two previous clinical studies, venous occlusion strain gauge measurements demonstrated that exercise training in PAD patients increased maximal calf blood flow reached from plantar flexion exercise by 30% (31) and 38% (41). These increases are consistent with our optically measured increase of 29% (13%, 50%) in the maximal calf blood flow from treadmill exercise (Fig. 3). Exercise training has also been shown to decrease short-chain acylcarnitine concentrations (intermediates of oxidative metabolism) in the plasma and muscle of PAD patients, which in turn indicates increased oxidative muscle metabolism (41, 42). This observation is consistent with our results, in which the observed improvement in maximal blood flow and oxygen extraction during physical activity clearly demonstrates improved muscle oxidative metabolism.
We further note that two prior uncontrolled studies (i.e., no randomized control group) of PAD patients examined the effects of exercise training on with CW-NIRS measurements (4, 30). One of these studies (30) found significant changes in from exercise training (consistent with our results in Table 3), while the other study did not (4).
Our measurements did not demonstrate a significant effect from exercise training on the hemoglobin/myoglobin desaturation recovery time TR (Fig. 4). Higher maximal calf blood flow reduces TR, but higher maximal calf oxygen extraction increases TR (15). We speculate that our TR results are explained by these two competing factors roughly canceling each other. The two NIRS studies mentioned above also showed no effects on TR of exercise training (4, 30).
Effects on resting blood flow.
We did not observe significant differences from exercise training in the resting and Fo parameters (Fig. 4 and Table 3). Our resting optical blood flow results are consistent with those reported from a previous clinical study in PAD patients, where venous occlusion strain gauge plethysmography measurements of resting calf muscle blood flow were not affected by exercise training (31). Additionally, and in agreement with our results, a previous CW-NIRS study of PAD patients reported no significant change from exercise training in (4).
Preexercise training results.
Recent reviews have documented previous investigations of muscle hemodynamic measurements with near-infrared light (8, 25, 28, 33, 35, 36, 60, 70). Our average (mean ± SD) visit 2 TR of 109 ± 61 s (Table 2) is comparable to other TR measurements in the literature, e.g., 129 ± 98 s (32) and 104 ± 39 s (19). Our average measurements are also roughly consistent with previously published measurements (4, 19, 32, 51). To our knowledge, Table 2 contains the first reported calf muscle THCo measurement in PAD subjects.
We note that our FD-NIRS measurement of −14 ± 7% for is consistent with previous frequency-domain measurements of in PAD patients (51, 71). On the other hand, our measured drop is lower than other published measurements in the literature made with CW-NIRS devices, e.g., −38 ± 18% (32), −50 ± 30% (19), and −38% (−44%, −33%) (4). In fact, the CW-NIRS systems are low cost and robust, and are sensitive to oxygenation changes, but they do not measure absolute tissue optical properties (absorption and reduced scattering) (28). Typically, CW-NIRS systems have to make assumptions about tissue scattering and mean optical path length of light through tissue in order to derive hemoglobin concentration changes (36, 58). Errors in these assumptions, as well as in the relative changes in optical path lengths that occur during exercise because of variations in tissue blood volume and composition (36), affect the accuracy of absolute estimates based on CW-NIRS. These problems might contribute to the discrepancy between FD-NIRS and CW-NIRS measurements. In the future, it will be useful to directly compare hemodynamic measurements made with CW-NIRS and time-domain/frequency-domain NIRS devices during exercise in PAD subjects.
Our average maximal blood flow increase of 108 ± 79% following treadmill exercise (Table 2) is roughly consistent with previous measurements of blood flow increase from submaximal plantar flexion exercise in healthy control subjects, i.e., 116 ± 96% (39). Another clinical study using venous occlusion strain gauge plethysmography reported close to a 400% maximal hyperemic blood flow increase from plantar flexion exercise in PAD patients (31). In this earlier study, however, subjects performed plantar flexion exercise during arterial cuff occlusion, and consequently the maximal hyperemic blood flow is a result of the combined effects of arterial occlusion and ischemic exercise (31). This “combined effects response” could be substantially higher than the response from ischemic exercise alone.
Constant-γ assumption for OEF calculation with FD-NIRS.
The observed decrease in THC during exercise raises questions about the constant-γ assumption used for calculating OEF changes (see Muscle oxygen extraction fraction monitoring with FD-NIRS). Recall that γ is a vascular weighting constant that depends on the percentages of venous and capillary blood in the blood volume sampled by FD-NIRS (21). If the decrease in THC is reflective of a decrease in venous blood volume, then γ could be smaller during exercise. If γ is not constant, then the maximal oxygen extraction change during exercise is given by OEFex/OEFo = [(1 − )/(1 − ) × (γo/γex)]. Therefore, smaller γ during exercise will produce an underestimate of the OEF change from exercise. Depending on how much γ changes during exercise, our reported results of rOEF in Table 2 and Table 3 may be underestimates.
The magnitude of the γ variation depends on the changes in arteriole, capillary, and venous blood volume fractions in the total blood volume sampled by FD-NIRS. Rough estimates of these blood volume fractions at rest and during exercise have been reported for healthy adults; they are, in part, based on mathematical modeling of oxygen transport in muscle (45). In this work, ka,o, kc,o, and kv,o at rest were roughly 0.1, 0.15, and 0.75, respectively; during exercise, ka,ex, kc,ex, and kv,ex reached approximate steady-state values of 0.1, 0.3, and 0.6, respectively. Using these weights and assuming kw = 0.5, we take the γ coefficients at rest and during exercise to be γo = 0.83 and γex = 0.75. The assumption that γo = γex leads to a 10% underestimate of the actual oxygen extraction fraction change during exercise. In future work, this source of error can be quantified more accurately by using muscle oxidative metabolism measurements made with venous/arterial occlusion NIRS techniques to estimate γo and γex (5, 23, 49, 57).
If the visit 2 and visit 3 γo-to-γex ratios are comparable, then the effects described above normalize out for the ratio rOEFv3/rOEFv2 shown in Fig. 3. We believe that this is a reasonable assumption, since exercise training has not been found to have a significant effect on total hemoglobin (Table 3). If the assumption breaks down, however, then our reported rOEFv3/rOEFv2 still provides a lower bound of the true value. Improved arteriole dilation and capillary blood volume expansion could make ka,ex and kc,ex higher and kv,ex lower in visit 3 compared with visit 2. This phenomenon leads to a larger γo/γex in visit 3 compared with visit 2, since γ is more sensitive to decreasing kv than increasing kc. Therefore, our conclusion that exercise training improves muscle oxygen extraction during physical activity remains intact.
Finally, preliminary evidence of a larger decrease in THC during exercise in PAD patients compared with healthy control subjects has been reported (71). Plausibly then, γo/γex is higher in PAD patients than healthy control subjects. If this is the case, the OEF change during exercise will be more severely underestimated in PAD patients than in healthy control subjects when making the assumption that γo/γex is unity. This confounding factor may help explain why the change in tissue oxygen saturation during exercise has not been consistently found to be an indicator of PAD in the CW-NIRS literature (66).
Limitations.
Demographic differences between the exercise and control groups exist in our study, most noticeably in sex. The exercise group is predominantly male, while the control group is not (Table 1). However, no significant difference in ABI between the exercise and control groups was observed in their preexercise visit (P = 0.3), suggesting that the groups are similar in terms of PAD severity. Also, from a multivariate linear regression analysis, none of the demographic variables was found to be significantly related to rOEFv3/rOEFv2 and rFv3/rFv2. Therefore we do not expect the sex discrepancy between the exercise and control groups to change the conclusions of the study.
In DCS and FD-NIRS muscle measurements, there is always some contribution from overlying tissues above the muscle (e.g., skin and adipose tissues) (71). Given the measured superficial tissue layer thicknesses reported in Table 1, and the source-detector separations of our probe, this effect is expected to be small for changes in F and OEF (71). The superficial tissue contributions could be larger in the control group compared with the exercise group because of the slightly larger superficial tissue thicknesses in the control group. Small sampling depth variations also arise between the DCS and FD-NIRS measurements, since the source-detector geometries for the two measurement techniques are not identical. These sampling depth variations could give rise to small variations in superficial tissue contributions. If the superficial tissue layers contribute similarly to the visit 2 and visit 3 signals, however, we expect that these effects will roughly divide out in rOEFv3/rOEFv2 and rFv3/rFv2. Additionally, since rVo2 = rF × rOEF, these effects would also roughly divide out in rVo2,v3/rVo2,v2.
Tissue heterogeneities that arise from overlying tissues above the muscle can also influence the proportionality coefficient between the DCS blood flow index (F) and absolute muscle blood flow (47, 72). Thus, caution should be exercised per interpretation of Fo as a measure of absolute resting blood flow. Variations in Fo across subjects reflect variations in resting blood flow, but they can also reflect variations in other factors such as adipose/skin thickness (47, 72). Relative blood flow changes (rF), on the other hand, are more robust to these effects (47, 72). The ratio Fo,v3/Fo,v2 (Fig. 4), on which our conclusion about exercise training effects on resting blood flow is based, is also robust to these effects. Nevertheless, future work is desirable to confirm the influence of exercise training on resting muscle blood flow.
In the present study, the DCS sampling rate was too slow to permit filtering of motion artifacts induced by moving muscle fibers during exercise. Because of this limitation, as an approximation of the maximal blood flow reached during exercise, we computed the average relative blood flow during the first 30 s of recovery following cessation of exercise. It should be noted that if the blood flow decreased significantly during this 30-s interval, our computed relative blood flow changes (Tables 2 and 3) will underestimate the relative blood flow change during exercise. In healthy adults, muscle blood flow was previously measured to decay to 63% of maximum after heavy exercise in 40–50 s (29, 38). In our data, the average (mean ± SD) time for the blood flow to decay to half of its maximum value was 72 ± 30 s. It is plausible that blood flow could remain elevated longer in PAD subjects compared with healthy control subjects, because PAD subjects have smaller maximal blood flow levels and it therefore takes longer to clear accumulated metabolites from heavy exercise. In the present study population of PAD subjects, we found no significant difference between the first and last blood flow measurements during the 30 s following cessation of exercise; this observation suggests that blood flow is not decreasing in this interval. However, ultimately the poor DCS sampling rate (i.e., 1 measurement every 8 s) hinders our confidence in conclusions about muscle blood flow kinetics. In future work, this limitation can be overcome by using recent technological improvements that greatly improve the DCS sampling rate (24, 39, 68). We hypothesize that if the reported relative blood flow changes in Table 3 underestimate the relative blood flow changes during treadmill exercise, then the underestimation affects the pre- and postexercise measurements (i.e., rFv2 and rFv3) about equally; in this case our conclusions about the ratio rFv3/rFv2 are not affected.
Conclusions.
In a randomized study of 64 PAD patients with intermittent claudication, we employed optical DCS and FD-NIRS techniques to demonstrate enhanced maximal calf muscle blood flow, oxygen extraction levels, and oxygen metabolism levels from exercise training. No enhancement was observed in resting calf muscle blood flow or oxygen saturation. Additionally, exercise training did not affect the recovery half-time for hemoglobin/myoglobin desaturation.
GRANTS
This work was supported by the National Institutes of Health (Grants R01 HL-075649, R01 NS-060653, P41 EB-015893), the China Postdoctoral Science Foundation (Grant 2016M600884), and the National Natural Science Foundation of China (Grant 81370038).
DISCLOSURES
D. R. Busch, W. B. Baker, and A. G. Yodh have two pending patent applications, and A. G. Yodh has two other patents relevant to this work (US patents 8,082,015 and 6,076,010), but none of these currently generates income. No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.B.B., Z.L., M.C., D.R.B., E.K.E., K.H.S., A.G.Y., T.F.F., and E.R.M. conceived and designed research; W.B.B., Z.L., S.S.S., and M.C. performed experiments; W.B.B. and Z.L. analyzed data; W.B.B., Z.L., A.G.Y., T.F.F., and E.R.M. interpreted results of experiments; W.B.B. and Z.L. prepared figures; W.B.B. drafted manuscript; W.B.B., Z.L., M.C., D.R.B., A.G.Y., T.F.F., and E.R.M. edited and revised manuscript; W.B.B., Z.L., S.S.S., M.C., D.R.B., E.K.E., K.H.S., A.G.Y., T.F.F., and E.R.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge Ken Abramson and Leonid Zubkov for help with optical probe construction/instrumentation; Michael Langham, Elizabeth Medenilla, Erin Buckley, Joel Greenberg, Michael Mullen, Tiffany Ko, Rodrigo Forti, Brian White, and Felix Wehrli for useful discussions about PAD; and Elizabeth Beothy, Scott Welden, and Molly Fanning for assistance with patient recruitment.
REFERENCES
- 1.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jönsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux PM, Stoffers HE, Treat-Jacobson D; American Heart Association Council on Peripheral Vascular Disease; Council on Epidemiology and Prevention; Council on Clinical Cardiology; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia . Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation 126: 2890–2909, 2012. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 2.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Progressive vs single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc 23: 402–408, 1991. doi: 10.1249/00005768-199104000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Baker WB.Optical Cerebral Blood Flow Monitoring of Mice to Men (PhD dissertation). Philadelphia, PA: Univ. of Pennsylvania, 2015. [Google Scholar]
- 4.Beckitt TA, Day J, Morgan M, Lamont PM. Calf muscle oxygen saturation and the effects of supervised exercise training for intermittent claudication. J Vasc Surg 56: 470–475, 2012. doi: 10.1016/j.jvs.2011.11.140. [DOI] [PubMed] [Google Scholar]
- 5.Binzoni T, Cooper CE, Wittekind AL, Beneke R, Elwell CE, Van De Ville D, Leung TS. A new method to measure local oxygen consumption in human skeletal muscle during dynamic exercise using near-infrared spectroscopy. Physiol Meas 31: 1257–1269, 2010. doi: 10.1088/0967-3334/31/9/014. [DOI] [PubMed] [Google Scholar]
- 6.Boas DA, Campbell LE, Yodh AG. Scattering and imaging with diffusing temporal field correlations. Phys Rev Lett 75: 1855–1858, 1995. doi: 10.1103/PhysRevLett.75.1855. [DOI] [PubMed] [Google Scholar]
- 7.Boas DA, Yodh AG. Spatially varying dynamical properties of turbid media probed with diffusing temporal light correlation. J Opt Soc Am A Opt Image Sci Vis 14: 192–215, 1997. doi: 10.1364/JOSAA.14.000192. [DOI] [Google Scholar]
- 8.Boezeman RP, Moll FL, Ünlü Ç, de Vries JP. Systematic review of clinical applications of monitoring muscle tissue oxygenation with near-infrared spectroscopy in vascular disease. Microvasc Res 104: 11–22, 2016. doi: 10.1016/j.mvr.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Brendle DC, Joseph LJ, Corretti MC, Gardner AW, Katzel LI. Effects of exercise rehabilitation on endothelial reactivity in older patients with peripheral arterial disease. Am J Cardiol 87: 324–329, 2001. doi: 10.1016/S0002-9149(00)01367-9. [DOI] [PubMed] [Google Scholar]
- 10.Bronas UG, Hirsch AT, Murphy T, Badenhop D, Collins TC, Ehrman JK, Ershow AG, Lewis B, Treat-Jacobson DJ, Walsh ME, Oldenburg N, Regensteiner JG; CLEVER Research Group . Design of the multicenter standardized supervised exercise training intervention for the claudication: exercise vs endoluminal revascularization (CLEVER) study. Vasc Med 14: 313–321, 2009. doi: 10.1177/1358863X09102295. [DOI] [PubMed] [Google Scholar]
- 11.Buckley EM, Parthasarathy AB, Grant PE, Yodh AG, Franceschini MA. Diffuse correlation spectroscopy for measurement of cerebral blood flow: future prospects. Neurophotonics 1: 011009, 2014. doi: 10.1117/1.NPh.1.1.011009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busch DR, Choe R, Durduran T, Friedman DH, Baker WB, Maidment AD, Rosen MA, Schnall MD, Yodh AG. Blood flow reduction in breast tissue due to mammographic compression. Acad Radiol 21: 151–161, 2014. doi: 10.1016/j.acra.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carp SA, Farzam P, Redes N, Hueber DM, Franceschini MA. Combined multi-distance frequency domain and diffuse correlation spectroscopy system with simultaneous data acquisition and real-time analysis. Biomed Opt Express 8: 3993–4006, 2017. doi: 10.1364/BOE.8.003993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casavola C, Paunescu LA, Fantini S, Gratton E. Blood flow and oxygen consumption with near-infrared spectroscopy and venous occlusion: spatial maps and the effect of time and pressure of inflation. J Biomed Opt 5: 269–276, 2000. doi: 10.1117/1.429995. [DOI] [PubMed] [Google Scholar]
- 15.Chance B, Dait MT, Zhang C, Hamaoka T, Hagerman F. Recovery from exercise-induced desaturation in the quadriceps muscles of elite competitive rowers. Am J Physiol Cell Physiol 262: C766–C775, 1992. [DOI] [PubMed] [Google Scholar]
- 16.Clark M, Rattigan S, Barrett E, Vincent M, Poole DC, Brown MD, Hudlicka O. Point:Counterpoint: There is/is not capillary recruitment in active skeletal muscle during exercise. J Appl Physiol (1985) 104: 889–891, 2008. doi: 10.1152/japplphysiol.00779.2007. [DOI] [PubMed] [Google Scholar]
- 17.Cochran JM, Chung SH, Leproux A, Baker WB, Busch DR, DeMichele AM, Tchou J, Tromberg BJ, Yodh AG. Longitudinal optical monitoring of blood flow in breast tumors during neoadjuvant chemotherapy. Phys Med Biol 62: 4637–4653, 2017. doi: 10.1088/1361-6560/aa6cef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coggins M, Lindner J, Rattigan S, Jahn L, Fasy E, Kaul S, Barrett E. Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes 50: 2682–2690, 2001. doi: 10.2337/diabetes.50.12.2682. [DOI] [PubMed] [Google Scholar]
- 19.Comerota AJ, Throm RC, Kelly P, Jaff M. Tissue (muscle) oxygen saturation (StO2): a new measure of symptomatic lower-extremity arterial disease. J Vasc Surg 38: 724–729, 2003. doi: 10.1016/S0741-5214(03)01032-2. [DOI] [PubMed] [Google Scholar]
- 20.Criqui MH, Denenberg JO, Bird CE, Fronek A, Klauber MR, Langer RD. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med 1: 65–71, 1996. doi: 10.1177/1358863X9600100112. [DOI] [PubMed] [Google Scholar]
- 21.Culver JP, Durduran T, Furuya D, Cheung C, Greenberg JH, Yodh AG. Diffuse optical tomography of cerebral blood flow, oxygenation, and metabolism in rat during focal ischemia. J Cereb Blood Flow Metab 23: 911–924, 2003. doi: 10.1097/01.WCB.0000076703.71231.BB. [DOI] [PubMed] [Google Scholar]
- 22.Dawson D, Vincent MA, Barrett EJ, Kaul S, Clark A, Leong-Poi H, Lindner JR. Vascular recruitment in skeletal muscle during exercise and hyperinsulinemia assessed by contrast ultrasound. Am J Physiol Endocrinol Metab 282: E714–E720, 2002. doi: 10.1152/ajpendo.00373.2001. [DOI] [PubMed] [Google Scholar]
- 23.De Blasi RA, Ferrari M, Natali A, Conti G, Mega A, Gasparetto A. Noninvasive measurement of forearm blood flow and oxygen consumption by near-infrared spectroscopy. J Appl Physiol (1985) 76: 1388–1393, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Dietsche G, Ninck M, Ortolf C, Li J, Jaillon F, Gisler T. Fiber-based multispeckle detection for time-resolved diffusing-wave spectroscopy: characterization and application to blood flow detection in deep tissue. Appl Opt 46: 8506–8514, 2007. doi: 10.1364/AO.46.008506. [DOI] [PubMed] [Google Scholar]
- 25.Durduran T, Choe R, Baker WB, Yodh AG. Diffuse optics for tissue monitoring and tomography. Rep Prog Phys 73: 076701, 2010. doi: 10.1088/0034-4885/73/7/076701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durduran T, Yu G, Burnett MG, Detre JA, Greenberg JH, Wang J, Zhou C, Yodh AG. Diffuse optical measurement of blood flow, blood oxygenation, and metabolism in a human brain during sensorimotor cortex activation. Opt Lett 29: 1766–1768, 2004. doi: 10.1364/OL.29.001766. [DOI] [PubMed] [Google Scholar]
- 27.Feinglass J, McCarthy WJ, Slavensky R, Manheim LM, Martin GJ; The Chicago Claudication Outcomes Research Group . Effect of lower extremity blood pressure on physical functioning in patients who have intermittent claudication. J Vasc Surg 24: 503–511, 1996. doi: 10.1016/S0741-5214(96)70066-6. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari M, Muthalib M, Quaresima V. The use of near-infrared spectroscopy in understanding skeletal muscle physiology: recent developments. Philos Trans A Math Phys Eng Sci 369: 4577–4590, 2011. doi: 10.1098/rsta.2011.0230. [DOI] [PubMed] [Google Scholar]
- 29.Ferreira LF, Harper AJ, Townsend DK, Lutjemeier BJ, Barstow TJ. Kinetics of estimated human muscle capillary blood flow during recovery from exercise. Exp Physiol 90: 715–726, 2005. doi: 10.1113/expphysiol.2005.030189. [DOI] [PubMed] [Google Scholar]
- 30.Figoni SF, Kunkel CF, Scremin AM, Asher A, Banks NL, Rivera A, Tin JK, Cohen B. Effects of exercise training on calf tissue oxygenation in men with intermittent claudication. PM R 1: 932–940, 2009. doi: 10.1016/j.pmrj.2009.08.453. [DOI] [PubMed] [Google Scholar]
- 31.Gardner AW, Katzel LI, Sorkin JD, Bradham DD, Hochberg MC, Flinn WR, Goldberg AP. Exercise rehabilitation improves functional outcomes and peripheral circulation in patients with intermittent claudication: a randomized controlled trial. J Am Geriatr Soc 49: 755–762, 2001. doi: 10.1046/j.1532-5415.2001.49152.x. [DOI] [PubMed] [Google Scholar]
- 32.Gardner AW, Parker DE, Webb N, Montgomery PS, Scott KJ, Blevins SM. Calf muscle hemoglobin oxygen saturation characteristics and exercise performance in patients with intermittent claudication. J Vasc Surg 48: 644–649, 2008. doi: 10.1016/j.jvs.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grassi B, Quaresima V. Near-infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: a review from an exercise physiology perspective. J Biomed Opt 21: 091313, 2016. doi: 10.1117/1.JBO.21.9.091313. [DOI] [PubMed] [Google Scholar]
- 34.Gurley K, Shang Y, Yu G. Noninvasive optical quantification of absolute blood flow, blood oxygenation, and oxygen consumption rate in exercising skeletal muscle. J Biomed Opt 17: 075010, 2012. doi: 10.1117/1.JBO.17.7.075010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamaoka T, McCully KK, Niwayama M, Chance B. The use of muscle near-infrared spectroscopy in sport, health and medical sciences: recent developments. Philos Trans A Math Phys Eng Sci 369: 4591–4604, 2011. doi: 10.1098/rsta.2011.0298. [DOI] [PubMed] [Google Scholar]
- 36.Hamaoka T, McCully KK, Quaresima V, Yamamoto K, Chance B. Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans. J Biomed Opt 12: 062105, 2007. doi: 10.1117/1.2805437. [DOI] [PubMed] [Google Scholar]
- 37.Hamburg NM, Balady GJ. Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation 123: 87–97, 2011. doi: 10.1161/CIRCULATIONAHA.109.881888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harper AJ, Ferreira LF, Lutjemeier BJ, Townsend DK, Barstow TJ. Human femoral artery and estimated muscle capillary blood flow kinetics following the onset of exercise. Exp Physiol 91: 661–671, 2006. doi: 10.1113/expphysiol.2005.032904. [DOI] [PubMed] [Google Scholar]
- 39.Henry B, Zhao M, Shang Y, Uhl T, Thomas DT, Xenos ES, Saha SP, Yu G. Hybrid diffuse optical techniques for continuous hemodynamic measurement in gastrocnemius during plantar flexion exercise. J Biomed Opt 20: 125006, 2015. doi: 10.1117/1.JBO.20.12.125006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med 344: 1608–1621, 2001. doi: 10.1056/NEJM200105243442108. [DOI] [PubMed] [Google Scholar]
- 41.Hiatt WR, Regensteiner JG, Hargarten ME, Wolfel EE, Brass EP. Benefit of exercise conditioning for patients with peripheral arterial disease. Circulation 81: 602–609, 1990. doi: 10.1161/01.CIR.81.2.602. [DOI] [PubMed] [Google Scholar]
- 42.Hiatt WR, Regensteiner JG, Wolfel EE, Carry MR, Brass EP. Effect of exercise training on skeletal muscle histology and metabolism in peripheral arterial disease. J Appl Physiol (1985) 81: 780–788, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Honig CR, Odoroff CL, Frierson JL. Capillary recruitment in exercise: rate, extent, uniformity, and relation to blood flow. Am J Physiol 238: H31–H42, 1980. [DOI] [PubMed] [Google Scholar]
- 44.Kato R, Pinsky MR. Personalizing blood pressure management in septic shock. Ann Intensive Care 5: 41, 2015. doi: 10.1186/s13613-015-0085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai N, Zhou H, Saidel GM, Wolf M, McCully K, Gladden LB, Cabrera ME. Modeling oxygenation in venous blood and skeletal muscle in response to exercise using near-infrared spectroscopy. J Appl Physiol (1985) 106: 1858–1874, 2009. doi: 10.1152/japplphysiol.91102.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laufs U, Werner N, Link A, Endres M, Wassmann S, Jürgens K, Miche E, Böhm M, Nickenig G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation 109: 220–226, 2004. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 47.Li Z, Baker WB, Parthasarathy AB, Ko TS, Wang D, Schenkel S, Durduran T, Li G, Yodh AG. Calibration of diffuse correlation spectroscopy blood flow index with venous-occlusion diffuse optical spectroscopy in skeletal muscle. J Biomed Opt 20: 125005, 2015. doi: 10.1117/1.JBO.20.12.125005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lloyd PG, Yang HT, Terjung RL. Arteriogenesis and angiogenesis in rat ischemic hindlimb: role of nitric oxide. Am J Physiol Heart Circ Physiol 281: H2528–H2538, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Malagoni AM, Felisatti M, Mandini S, Mascoli F, Manfredini R, Basaglia N, Zamboni P, Manfredini F. Resting muscle oxygen consumption by near-infrared spectroscopy in peripheral arterial disease: a parameter to be considered in a clinical setting? Angiology 61: 530–536, 2010. doi: 10.1177/0003319710362975. [DOI] [PubMed] [Google Scholar]
- 50.McDermott MM, Ades P, Guralnik JM, Dyer A, Ferrucci L, Liu K, Nelson M, Lloyd-Jones D, Van Horn L, Garside D, Kibbe M, Domanchuk K, Stein JH, Liao Y, Tao H, Green D, Pearce WH, Schneider JR, McPherson D, Laing ST, McCarthy WJ, Shroff A, Criqui MH. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA 301: 165–174, 2009. doi: 10.1001/jama.2008.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mesquita RC, Putt M, Chandra M, Yu G, Xing X, Han SW, Lech G, Shang Y, Durduran T, Zhou C, Yodh AG, Mohler ER III. Diffuse optical characterization of an exercising patient group with peripheral artery disease. J Biomed Opt 18: 57007, 2013. doi: 10.1117/1.JBO.18.5.057007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mohler ER 3rd, Lech G, Supple GE, Wang H, Chance B. Impaired exercise-induced blood volume in type 2 diabetes with or without peripheral arterial disease measured by continuous-wave near-infrared spectroscopy. Diabetes Care 29: 1856–1859, 2006. doi: 10.2337/dc06-0182. [DOI] [PubMed] [Google Scholar]
- 53.Murphy TP, Cutlip DE, Regensteiner JG, Mohler ER, Cohen DJ, Reynolds MR, Massaro JM, Lewis BA, Cerezo J, Oldenburg NC, Thum CC, Goldberg S, Jaff MR, Steffes MW, Comerota AJ, Ehrman J, Treat-Jacobson D, Walsh ME, Collins T, Badenhop DT, Bronas U, Hirsch AT; CLEVER Study Investigators . Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation 125: 130–139, 2012. doi: 10.1161/CIRCULATIONAHA.111.075770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; TASC II Working Group, Bell K, Caporusso J, Durand-Zaleski I, Komori K, Lammer J, Liapis C, Novo S, Razavi M, Robbs J, Schaper N, Shigematsu H, Sapoval M, White C, White J, Clement D, Creager M, Jaff M, Mohler E 3rd, Rutherford RB, Sheehan P, Sillesen H, Rosenfield K. Inter-society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg 33, Suppl 1: S1–S75, 2007. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 55.Ouriel K. Peripheral arterial disease. Lancet 358: 1257–1264, 2001. doi: 10.1016/S0140-6736(01)06351-6. [DOI] [PubMed] [Google Scholar]
- 56.Poole DC, Copp SW, Ferguson SK, Musch TI. Skeletal muscle capillary function: contemporary observations and novel hypotheses. Exp Physiol 98: 1645–1658, 2013. doi: 10.1113/expphysiol.2013.073874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ryan TE, Erickson ML, Brizendine JT, Young H-J, McCully KK. Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: correcting for blood volume changes. J Appl Physiol (1985) 113: 175–183, 2012. doi: 10.1152/japplphysiol.00319.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saitoh T, Ooue A, Kondo N, Niizeki K, Koga S. Active muscle oxygenation dynamics measured during high-intensity exercise by using two near-infrared spectroscopy methods. Adv Exp Med Biol 662: 225–230, 2010. doi: 10.1007/978-1-4419-1241-1_32. [DOI] [PubMed] [Google Scholar]
- 59.Sarelius I, Pohl U. Control of muscle blood flow during exercise: local factors and integrative mechanisms. Acta Physiol (Oxf) 199: 349–365, 2010. doi: 10.1111/j.1748-1716.2010.02129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shang Y, Gurley K, Yu G. Diffuse correlation spectroscopy (DCS) for assessment of tissue blood flow in skeletal muscle: recent progress. Anat Physiol 3: 128, 2013. doi: 10.4172/2161-0940.1000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shang Y, Li T, Yu G. Clinical applications of near-infrared diffuse correlation spectroscopy and tomography for tissue blood flow monitoring and imaging. Physiol Meas 38: R1–R26, 2017. doi: 10.1088/1361-6579/aa60b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shang Y, Symons TB, Durduran T, Yodh AG, Yu G. Effects of muscle fiber motion on diffuse correlation spectroscopy blood flow measurements during exercise. Biomed Opt Express 1: 500–511, 2010. doi: 10.1364/BOE.1.000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sieminski DJ, Gardner AW. The relationship between free-living daily physical activity and the severity of peripheral arterial occlusive disease. Vasc Med 2: 286–291, 1997. doi: 10.1177/1358863X9700200402. [DOI] [PubMed] [Google Scholar]
- 64.Stewart KJ, Hiatt WR, Regensteiner JG, Hirsch AT. Exercise training for claudication. N Engl J Med 347: 1941–1951, 2002. doi: 10.1056/NEJMra021135. [DOI] [PubMed] [Google Scholar]
- 65.Treat-Jacobson D, Halverson SL, Ratchford A, Regensteiner JG, Lindquist R, Hirsch AT. A patient-derived perspective of health-related quality of life with peripheral arterial disease. J Nurs Scholarsh 34: 55–60, 2002. doi: 10.1111/j.1547-5069.2002.00055.x. [DOI] [PubMed] [Google Scholar]
- 66.Vardi M, Nini A. Near-infrared spectroscopy for evaluation of peripheral vascular disease. A systematic review of literature. Eur J Vasc Endovasc Surg 35: 68–74, 2008. doi: 10.1016/j.ejvs.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 67.Wallace DJ, Michener B, Choudhury D, Levi M, Fennelly P, Hueber DM, Barbieri BB. Results of a 95-subject human clinical trial for the diagnosis of peripheral vascular disease using a near-infrared frequency domain hemoglobin spectrometer. In: BiOS'99 International Biomedical Optics Symposium. Bellingham, WA: International Society for Optics and Photonics, 1999, p. 300–316. [Google Scholar]
- 68.Wang D, Parthasarathy AB, Baker WB, Gannon K, Kavuri V, Ko T, Schenkel S, Li Z, Li Z, Mullen MT, Detre JA, Yodh AG. Fast blood flow monitoring in deep tissues with real-time software correlators. Biomed Opt Express 7: 776–797, 2016. doi: 10.1364/BOE.7.000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yataco AR, Corretti MC, Gardner AW, Womack CJ, Katzel LI. Endothelial reactivity and cardiac risk factors in older patients with peripheral arterial disease. Am J Cardiol 83: 754–758, 1999. doi: 10.1016/S0002-9149(98)00984-9. [DOI] [PubMed] [Google Scholar]
- 70.Yu G. Diffuse correlation spectroscopy (DCS): a diagnostic tool for assessing tissue blood flow in vascular-related diseases and therapies. Curr Med Imaging Rev 8: 194–210, 2012. doi: 10.2174/157340512803759875. [DOI] [Google Scholar]
- 71.Yu G, Durduran T, Lech G, Zhou C, Chance B, Mohler ER 3rd, Yodh AG. Time-dependent blood flow and oxygenation in human skeletal muscles measured with noninvasive near-infrared diffuse optical spectroscopies. J Biomed Opt 10: 024027, 2005. doi: 10.1117/1.1884603. [DOI] [PubMed] [Google Scholar]
- 72.Yu G, Floyd TF, Durduran T, Zhou C, Wang J, Detre JA, Yodh AG. Validation of diffuse correlation spectroscopy for muscle blood flow with concurrent arterial spin labeled perfusion MRI. Opt Express 15: 1064–1075, 2007. doi: 10.1364/OE.15.001064. [DOI] [PubMed] [Google Scholar]