Abstract
The late gestation fetal ECG (fECG) has traditionally been difficult to characterize due to the low fECG signal relative to high maternal noise. Although new technologies have improved the feasibility of its acquisition and separation, little is known about its development in late gestation, a period in which the fetal heart undergoes extensive maturational changes. Here, we describe a method for the chronic implantation of radiotelemetry devices into late gestation ovine fetuses to characterize parameters of the fECG following surgery, throughout late gestation, and in the perinatal period. We found no significant changes in mean aortic pressure (MAP), heart rate (HR), or ECG in the 5 days following implantation; however, HR decreased in the first 24 h following the end of surgery, with associated increases in RR, PR, and QRS intervals. Over the last 14 days of fetal life, fetal MAP significantly increased, and HR significantly decreased, as expected. MAP and HR increased as labor progressed. Although there were no significant changes over time in the ECG during late gestation, the duration of the PR interval initially decreased and then increased as birth approached. These results indicate that although critical maturational changes occur in the late gestation fetal myocardium, the mechanisms that control the cardiac conduction are relatively mature in late gestation. The study demonstrates that radiotelemetry can be successfully used to assess fetal cardiac function, in particular conduction, through the process of labor and delivery, and may therefore be a useful tool for study of peripartum cardiac events.
Keywords: pregnancy, fetus, ECG, telemetry
the ovine fetus has long been used as a model of fetal development, particularly because of the similar developmental trajectory to the human fetus and the relative quiescence of the ovine uterus, reducing the risks inherent with open fetal surgery. These properties have made the chronically instrumented ovine fetus invaluable in the characterization and manipulation of physiological processes. The ovine fetus has been particularly useful as a model for study of fetal organ maturation, as the ovine fetus has similarities in maturational trajectory to the human, owing in part to the similar surge in fetal cortisol production in the days preceding birth (8, 19). Cortisol contributes to maturation of many fetal tissues including the heart, lung, gastrointestinal tract, liver, and brain (4, 29, 42, 48, 52).
Our laboratory has been interested in the study fetal cardiac maturation and in particular the adverse effects of elevated maternal cortisol on the fetal heart (13, 23, 44, 47) As we have found an increase in peripartum stillbirth in our model of late gestation maternal stress, with transcriptomic effects indicative of altered cardiac metabolism, we wanted to develop a refinement in the methods for assessing fetal cardiac function, and in particular ECG, during labor, delivery, and the immediate perinatal period. Many previous studies have documented the late gestation changes in fetal blood pressure and heart rate (HR), as well as responses of fetal HR and ECG to manipulations such as hypoxia, cord occlusion, or chronic growth restriction (14, 27, 30, 36, 62, 64). Fetal ECG (fECG) is suggested as a good measure for assessing fetal brain ischemia, and this has been widely studied in that context as an index of acute fetal stress (38, 51). However, very chronic study of fECG and study during labor and delivery are complicated by the need to limit maternal movements during signal acquisition. Telemetric devices have been successfully used in the ovine fetus to measure renal sympathetic nerve activity (5). The feasibility of chronic implantation of radiotelemetry devices for measurement of fetal blood pressure or ECG in the late gestation ovine fetus is supported by short-term limited studies by others (1, 17) but has not been rigorously tested. In the current study, we use surgically implanted radiotelemetry devices in late gestation ovine fetuses and assess the use of this method to characterize changes in the fECG occurring after surgery, throughout late gestation, and throughout labor and delivery.
METHODS
Ewes of known gestational age and their lambs were studied. Ewes were of either Rambouillet or Dorset breed, averaging 71.0 ± 7.1 kg at the time of surgery; lambs were 4.18 ± 0.33 kg at birth (143 ± 1; days 139–148 gestation). All animals were housed in a facility with temperature- and light-controlled (lights on 0700 to 1900) rooms throughout the study period; the University of Florida Institutional Animal Care and Use Committee approved all animal use for this study. Ewes were housed in individual pens of ~2.25 m2 each throughout the study. Husbandry, including cleaning of the pens and feeding, typically lasted ~30 min and was completed between 0700 and 0800. Ewes were fed a diet of pelleted feed (Nutrena Goat and Sheep Feed: Cargill) according to National Research Council guidelines based on weight of the ewe and the gestational age. This diet was fed each morning and was supplemented with loose alfalfa hay and/or alfalfa cubes in morning and evening. An entry sheet was used to record times for husbandry and feeding as well as postoperative care; these times were excluded from the analysis.
At approximately day 118 (±1) of gestation, survival surgery was performed. Ewes were induced with isoflurane by mask, intubated, and maintained on isoflurane anesthesia with controlled ventilation at 8–12 breaths/min with 2–3 l/min of oxygen. The total period of anesthesia, including preperation and surgery, was 4.5 ± 0.3 h. Maternal end-expiratory Pco2, O2 saturation, blood pressure, and HR were monitored throughout the procedure. A flow probe (6-mm 6PSS; Transonics, Ithaca, NY) was placed on the main uterine artery for assessment of labor. To study changes in fetal aortic pressure and ECG, a radiotelemetry device (DSI PA- D70 PCTP; Data Sciences International, Minneapolis, MN) was placed within the fetus to allow continuous measurement of fetal aortic pressure, amniotic pressure, fECG, and temperature. Briefly, the head and neck of the fetus were located in the uterus and exposed. A midline incision was made in the fetal neck and the telemetry device was placed subcutaneously at the level of the clavicle and sutured in place. The grounding lead for the ECG was tunneled subcutaneously using a forcep and attached to the inner surface of the skin of the thorax using a sterilized fishing hook (size 6; Baitholder; Eagle Claw, Denver, CO). The solid tip ECG probe was placed into the right jugular vein of the fetus, advanced into the superior vena cava until a P wave was visualized and optimized in the acquisition software (Ponemah 5.00), and secured at the entry point in the jugular with sterile tissue adhesive (Vetbond; 3M, St. Paul, MN). The two telemetry device catheters were then placed for measurement of aortic pressure and amniotic pressure. For placement of the aortic pressure catheter, purse-string sutures (5–0 Prolene Suture, Ethicon; Somerville, NJ) were placed in the left carotid artery of the fetus, and the catheter was inserted in the carotid and advanced into the aorta, using the acquisition software to assure placement in the aorta outside of the left ventricle. The catheter was secured in place in the carotid using the purse string sutures and a drop of tissue adhesive. The other pressure catheter was tunneled underneath the fetal skin and sutured to the skin of the fetal neck with the tip exposed. Therefore, all parts of the telemetry device were implanted within the fetus. All aortic pressures were corrected by subtraction of amniotic fluid pressure. Catheters for blood sample collection were also placed in the fetal saphenous arteries and veins and advanced to the fetal femoral arteries and veins (24) and in the maternal femoral arteries and veins. At the end of surgery, elastic surgical dressing was placed around the abdomen of the ewe (size 9 and 10 Surgilast; Derma Sciences, Princeton, NJ) and these fetal and maternal catheters were placed in a pocket made from sterilization wrap (Kimguard; Kimberly Clark, Roswell, GA) which was placed under the Surgilast on the flank of the ewes. A repeater device for the telemetry device was secured in place on the flank of the ewe after wrapping the unit in bandage tape (Vetwrap; 3M) and fastened with a cable tie to the Surgilast over the maternal abdomen.
Six fetuses died because of surgical complications; all of these deaths appeared to be attributable to cord occlusion. Subsequently, we altered the surgery to minimize the extent to which the fetus was displaced during surgery; only the fetal head and neck was exposed and ~500 ml of sterile saline were infused back into the amniotic cavity.
After surgery, ewes were returned to their housing pens (24 ft2 each) in which they were allowed to move freely. The ECG and aortic and amniotic pressure signals were continuously acquired from the repeater device by a radioreciever fastened to the front of the pen, which was connected to the DSI exchange matrix and computer. The system used in these studies used a repeater device to assure that animals in adjacent pens could have data collected at unique frequencies; the current systems available from DSI do not require use of the repeater and instead use multiple receivers placed around the pen. Although the signal strength of the telemetry device is sufficient to allow complete implantation into the fetus, the repeater device assured that the rebroadcast signal allowed for uninterrupted communication with the receiver without interference with the signal from adjacent pens. This arrangement allows for the acquisition of data during labor, delivery, and thereafter from multiple animals simultaneously. Each signal (aortic pressure, amniotic pressure, and ECG) was sampled at 500 Hz by the Dataquest ART software. The catheters were used to collect samples for maternal and fetal cortisol (EA65; Oxford Biomedical, Rochester Hills, MI), glucose and lactate (YSI Model 2700 glucose/lactate analyzer, Yellow Springs, OH), measurements, and for fetal blood gases (iSTAT Handheld; Abbott Point of Care, Princeton, NJ). The cortisol ELISA is run after extraction of plasma with 100% ethanol; the assay has a minimal detectable concentration of 0.5 ng/ml and the coefficient of variation for the pools of nonpregnant, pregnant, and fetal plasma were 12.2, 8.7, and 5.2%, respectively. These samples were collected without restraint of the ewe and were collected in the morning at least 1 h after completion of the daily husbandry activities. Measurements for maternal and fetal cortisol concentrations as well as fetal blood gases are presented in Table 1.
Table 1.
Maternal and fetal plasma cortisol and fetal blood gases
| Plasma Cortisol, ng/ml |
Fetal |
||||
|---|---|---|---|---|---|
| Maternal | Fetal* | Po2 | Pco2 | pH* | |
| 125 days | 10.4 ± 3.3 (8) | 4.5 ± 1.5 (8) | 18 ± 1 | 49.5 ± 3.0 | 7.36 ± 0.02 |
| 130 days | 5.6 ± 1.4 (8) | 6.5 ± 2.2 (8) | 17 ± 1 | 54.5 ± 1.5 | 7.37 ± 0.02 |
| 135 days | 4.9 ± 0.9 (7) | 16.4 ± 4.9 (7) | 19 ± 1 | 54.0 ± 1.6 | 7.35 ± 0.01 |
| 138 days | 7.4 ± 1.9 (8) | 24.6 ± 6.4 (7) | 18 ± 1 | 52.4 ± 1.6 | 7.37 ± 0.01 |
| 140 days | 10.3 ± 3.1 (7) | 33.6 ± 13.3 (7) | 18 ± 2 | 54.4 ± 1.4 | 7.35 ± 0.02 |
| Day of birth | 17.4 ± 3.8 (7) | 72.3 ± 12.6 (7) | 17 ± 2 | 59.2 ± 2.8 | 7.32 ± 0.02 |
Data are shown as means ± SE. Number in parenthese indicates number of animals; N for fetal cortisol and fetal blood gases is the same.
Significant change over time.
Ewes were treated at the end of surgery and for 2 days postoperatively with analgesic (flunixin meglamine;1 mg/kg sid; Merck Animal Health) and for 5 days postoperatively with antibiotic (Polyflex; 12–15 mg/kg bid; Boehringer Ingelheim Vetmedica, St. Joseph, MO); rectal temperature was measured twice a day for 5 days. Ewes were fed a diet of pelleted feed per National Research Council standards adjusted for the ewe’s body weight and fetal gestational age.
One ewe was euthanized in labor due to dystocia, one delivered a live lamb that died shortly following birth after suspected dystocia, and one fetus was hypoxic throughout the pregnancy and died. The chronically hypoxic fetus (Po2 of 10–15 mmHg) was not included in the analysis. In this study, a total of four male and four female lambs were born to eight ewes at 143 ± 1 days (range 139–148 days) of gestation.
Analysis of the acquired aortic pressure, HR, and fECG was performed using analysis modules in DSI Dataquest Open A.R.T 4.31 and Ponemah 5.00 Software Blood Pressure Analysis Module and ECG PRO, respectively. For calculation of MAP, amniotic pressure was used as the reference pressure. Hourly means of aortic and amniotic pressures and HR were calculated; HR calculation used the peak to peak intervals in the aortic pressure waveform. For the fECG analysis, data were imported into the Ponemah Analysis modules, and the software was assigned elements of the ECG (i.e., start and end of P wave, QRS, and T wave peak and end); these template cycles were then finely adjusted by the operator, and added to a library for each animal that was used to match the remaining cycles. Unmatched cycles were excluded from the analysis. About 80% of cycles in each period chosen for analysis could be matched.
Mean aortic pressure, HR, and fECG characteristics after surgery.
One hour means of mean aortic pressure (MAP), HR, and fECG parameters [P duration (atrial depolarization), PR interval from the start of atrial depolarization to the start of ventricular depolarization), QRS duration (ventricular depolarization), corrected QT interval (depolarization and repolarization of the ventricles, QT interval corrected for RR interval, QTc), and ST interval (isoelectric period between ventricular depolarization and repolarization)] were collected and analyzed over the first 24 h following the end of surgery. After the first postoperative day, 6-h means were calculated over the next four postoperative days for statistical analysis.
MAP, HR, and fECG characteristics in late gestation.
MAP and HR were calculated as 24-h means over the 14 days before birth; this period was chosen to allow inclusion of data from all fetuses following the 5 days of recovery from surgery. The parameters of the fECG (P and QRS duration; PR, QR, QRS, QTc, and ST intervals) were calculated for the 1-h interval between 0600 and 0700 for the 14 days before birth.
MAP, HR, and fECG characteristics 24 h before birth.
In the 24 h before birth, MAP, HR, and fECG parameters were calculated as hourly mean values; in the last hour before delivery and, when possible, in the first 10 min after birth data were calculated as 1-min means. In most cases, the signal was disrupted by the final process of delivery, although intermittent reading over a number of beats was still possible between maternal “pushes.” Because the repeater was affixed to the ewes abdomen and needs to be within ~40 cm of the fetal transmitter, postnatal signals were not reliably collected unless someone was present at the time of birth to move the repeater to the neck of the lamb. However, in many cases the newborn stayed close enough to the ewe to collect data in the immediate postpartum period. In all animals, the time of birth was confirmed using the telemetry record for fetal/neonatal temperature, which in all cases showed a decrease in temperature measured by the telemetry device at the time of birth.
Analysis of diurnal rhythms for MAP, HR, and fECG characteristics.
On days 11, 10, and 9 before birth, hourly means for MAP, HR, and fECG were calculated across the 72 h to test for diurnal rhythms. The cosinor [Michael Sachs. (2014) cosinor: Tools for estimating and predicting the cosinor model. https://cran.r-project.org/web/packages/psych/index.html; version 1.6.9.) and psych [Revelle, W. (2016) psych: Procedures for Personality and Psychological Research, Northwestern University, Evanston, IL; https://cran.r-project.org/web/packages/cosinor/index.html] packages were used in R software (http://www.R-project.org/) to fit hourly mean data to a cosine function, and depict the 24 h variation graphically. The Metacycle package was used in R software to test for significance of rhythmicity in individual fetuses, and to determine acrophase (time of the peak of the rhythm), amplitude (the difference between the peak and the mean value), and MESOR (estimate of the average value of the oscillating variable) describing the rhythms (67).
Statistical analysis.
Analyses of fetal MAP, HR, and fECG parameters following surgery and in late gestation were performed using one-way ANOVA corrected for repeated measures across time in IBM SPSS version 23.0 (IBM, Armonk, NY). For all analyses, statistical significance was set for P < 0.05. Values represent means ± SE unless otherwise stated.
RESULTS
MAP, HR, and fECG characteristics after surgery.
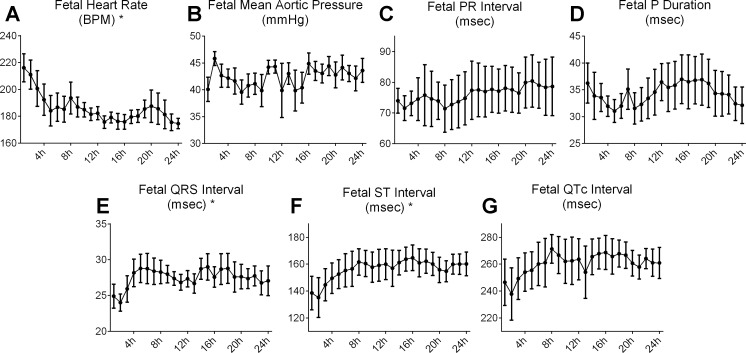
In the 24 h following the end of surgery, fetal MAP did not significantly differ over time (Fig. 1B). The change in HR significantly declined over the first 24 h following surgery (from 216 ± 10 to 176 ± 5 beats/min in the first 13 h) (Fig. 1A); likewise, the duration of the RR interval calculated from the fECG significantly increased (281 ± 14 to 335 ± 18 ms in the first 5 h). The duration of the QRS and ST intervals significantly increased in the hours after the end of surgery (QRS: 24.9 ± 1.8 to 27.1 ± 2.1 ms in the first 4 h, and ST: 139 ± 13 to 160 ± 9 ms in the first 9 h, respectively) (Fig. 1, C–G). After end of the first day, there were no significant changes over time in MAP and HR nor in any parameter of the ECG over the next 4 days (data not shown).
Fig. 1.
Fetal heart rate (HR; A), aortic blood pressure (BP; B), and fetal ECG (fECG) parameters (C–G) in the 24 h following the end of surgery (n = 6). BPM, beats/min. Data are means ± SE. *Significant change over time.
MAP, HR, and fECG characteristics in late gestation.
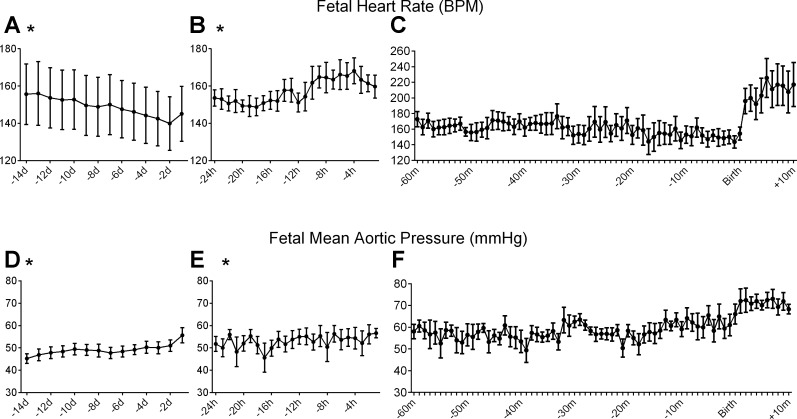
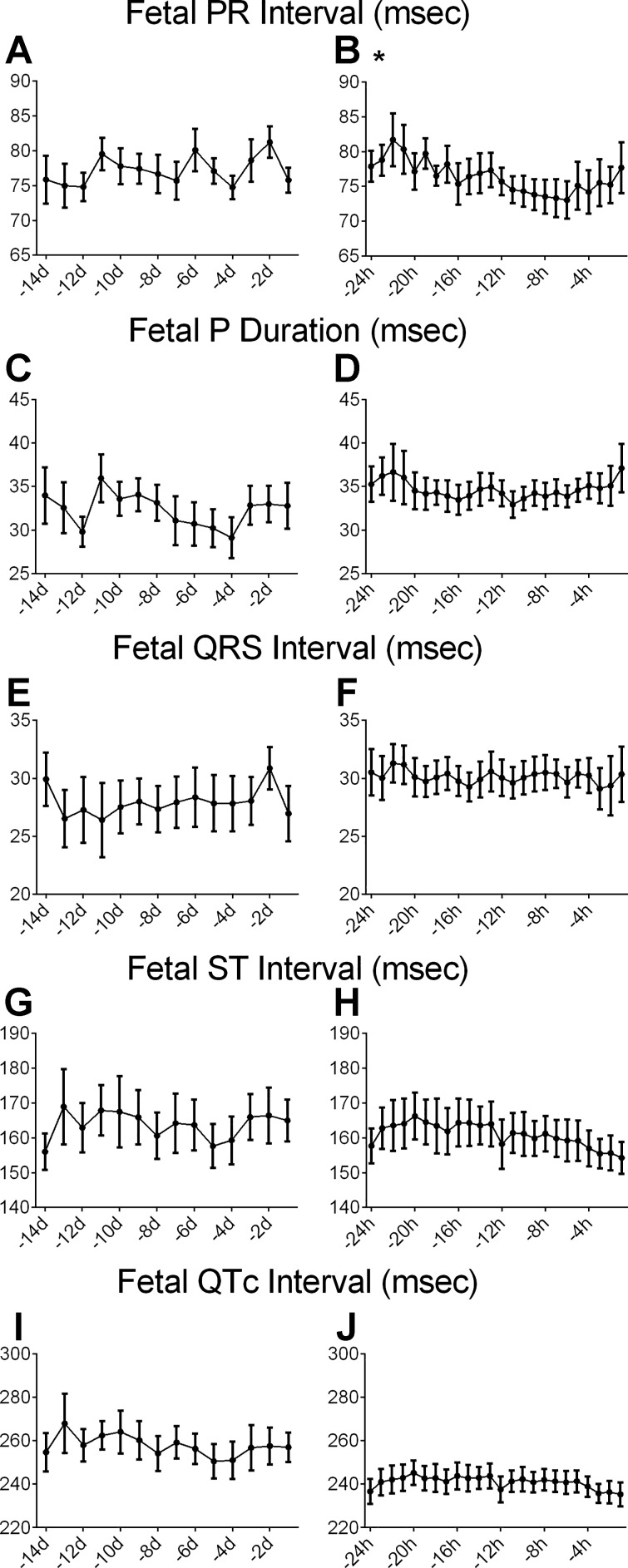
Over the last 14 days of fetal life, fetal MAP significantly increased (from 45.2 ± 2.2 to 55.6 ± 3.4 mmHg) and HR significantly decreased (from 156 ± 16 to 145 ± 15 beats/min) (Fig. 2, A and D). There were no significant changes over time in in the measured parameters of the fECG except for the RR interval (Fig. 3, A, C, E, G, and I).
Fig. 2.
Fetal heart rate (A–C) and aortic blood pressure (D–F) in the final 14 days before birth (A and D), in the final 24 h (B and E), and from 60 min before birth to 10 min after birth (C and F). Data are means ± SE of 1 h of data in the morning in A and D (n = 8): 1-h data over 24 h in B and E (n = 8) and 1 min of data in C and F (n = 6). Note that the y-axis range in C is different than A and B. *Significant change over time.
Fig. 3.
fECG parameters in the final 14 days before birth (A, C, E, G, and I) and 24 h before birth shown as hourly means (B, D, F, H, and J). Data are means ± SE (n = 8). *Significant change over time.
MAP, HR, and fECG characteristics before and after birth.
In the final 24 h before birth, the hourly mean in aortic pressure significantly increased (from 51.8 ± 3.3 to 56.6 ± 2.1 mmHg) and the hourly mean HR significantly increased (from 154 ± 4 to 160 ± 6 beats/min) as labor progressed (Fig. 2, B and E). The PR and RR intervals were the only parameters of the ECG to significantly change over this period (Fig. 3, B, D, F, H, and J). The PR interval initially decreased as labor progressed, with a small increase shortly before birth (Fig. 3B). In the immediate perinatal period, the fetal HR decreased in the final hour before birth from 173 to 144 beats/min at birth and averaged 217 beats/min over the first 10 min of ex utero life (Fig. 2C). Similarly, the fetal blood pressure rose from 58 to 66 mmHg in the final hour and averaged 68 mmHg in the early postnatal period (Fig. 2F).
Analysis of diurnal rhythms for MAP, HR, and fECG characteristics.
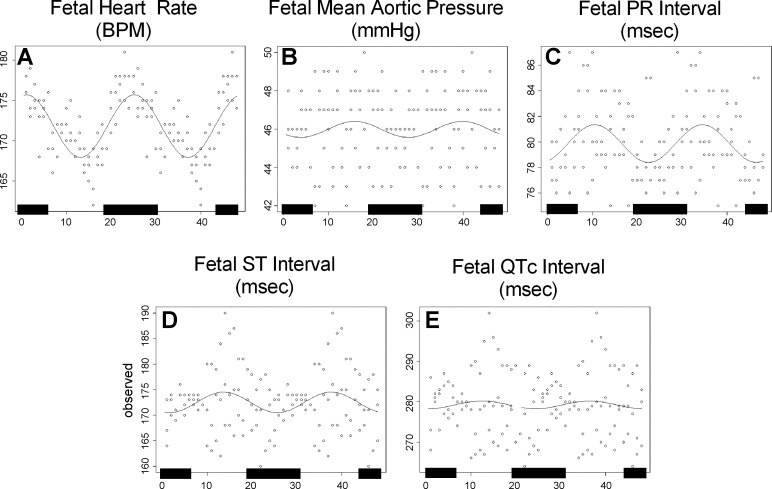
Analysis for 24 h patterns over 3 consecutive days in late gestation indicated significant patterns in HR and the RR interval in four out of seven fetuses; in the duration of the ST interval in three out of seven fetuses; in the duration of the QRS, PR, and QTc intervals in two out of seven fetuses; but in MAP in only one out of seven fetuses (Fig. 4, A–F; acrophases, amplitudes, and MESOR for each parameter are presented in Table 2).
Fig. 4.
Twenty-four hour diurnal patterns in fetal HR, BP, and fECG parameters in fetuses (n = 7). Circles indicate the hourly mean values on the 3 consecutive days (days 11, 10, and 9 before birth); lines indicate the cosine fit of these mean values; black bars on x-axis indicate period of lights off.
Table 2.
Values of parameters of the 24-h patterns in the late gestation ovine fetus
| Baseline | Amplitude | Acrophase | No. of Fetuses With Significant Rhythm | |
|---|---|---|---|---|
| HR, beats/min | 172 | 3.9 | 0159 | 4/7 |
| MAP, mmHg | 46 | 0.8 | 2241 | 1/7 |
| RR interval, ms | 356 | 6.7 | 2236 | 4/7 |
| ST interval, ms | 173 | 3.4 | 1909 | 3/7 |
| QRS interval, ms | 28.4 | 0.9 | 0057 | 2/7 |
| PR interval, ms | 79.8 | 2.1 | 0239 | 2/7 |
| QTc interval, ms | 279 | 3.9 | 1831 | 2/7 |
| QR interval, ms | 12.8 | 3.6 | 0255 | 0/7 |
| P interval, ms | 36.3 | 1.6 | 2324 | 0/7 |
Data are mean values for 7 fetuses calculated using the Metacycle Package for R. HR, heart rate; MAP, mean aortic pressure.
DISCUSSION
In this study, we described a method for the successful implantation of radiotelemetry devices to chronically measure fetal MAP, HR, and fECG. We used this method to characterize changes over time in MAP, HR, and parameters of the fECG, following surgery, throughout late gestation, and in the immediate perinatal period. Furthermore, we examined these for 24-h patterns of rhythmicity in late gestation ovine fetus. However, the appreciable advantage of the use of telemetry in this animal is its ability to be used throughout labor and delivery and after birth to measure acute responses that may be affected by various treatments or interventions. Although other methods that require some maternal restraint or housing in metabolic cages can be used to effectively determine fetal cardiovascular responses over shorter periods of study, these present difficulties during labor and delivery.
Our results using telemetry agree with already published data on MAP and HR using other methods in “control” fetuses. We found that fetal MAP and HR increased over the final day as birth approached, with a concomitant decrease in the PR as well as in the RR interval, although no other parameters of the fECG were changed. In the ovine fetus, a gradual rise in fetal blood pressure normally occurs in the hour before parturition, which is associated with the occurrence of uterine contractions (10). In these control fetuses, aortic pressure steadily rose over the last hour of life, including the immediate postpartum period. These findings reflect the cardiovascular adaptations associated with birth and the transition to ex utero life. In the sheep, cardiac output approximately doubles following birth, as the ventricular circulation transitions from a parallel (fetal) to in-series circuit (infant/adult) (18). Systolic blood pressure also rises at birth in part due to the removal of the low resistance placental unit, increased systemic vascular resistance, and circulating vasoactive hormones including cortisol and catecholamines (19, 60). These adaptations are thought to provide for an increased metabolic demand and processes regulating breathing and thermogenesis, which is reflected by changes in blood flow to certain organs during this transition (2). Comline and Silver (10) also showed that fetal HR during labor varied, but decreased overall in the last hour before birth. Our data also reveal a gradual increase in HR; we observed a variable increase in HR at the time of birth that was not significant overall. Others have shown that HR increases immediately following birth in humans (12) and in mice is accompanied by a decrease in the duration of the QRS and QTc intervals (37).
This methodology also allowed us to examine the recovery of the fetus from our surgical manipulation, which involved fairly extensive manipulation of the fetus. Although there was no significant change in MAP in the hours following surgery, ST interval and QRS duration increased, while the HR (and RR interval) decreased after surgery and stabilized after several hours. These findings indicate that ventricular activation and relaxation times are affected by surgery; however, these changes were transient and there was a rapid recovery during the first postoperative day. These postoperative changes likely reflect the fetuses intraoperative exposure to isoflurane, which is known to decrease blood pressure and HR in the fetus, and to alter the ECG in adolescents and adults (22, 40, 50, 56).
We confirmed the increase in arterial pressure and decrease in HR during late gestation that was reported by Unno et al. (62); Unno et al. showed in their study that the fetal HR increases between 140 and 143 days of gestation, corresponding to the exponential rise in fetal cortisol concentrations. However, we found little change in the parameters of the fECG occurring over the same period. This indicates that while the cardiac tissue undergoes maturational changes throughout late gestation, the dynamics of the fetal cardiac action potentials and the conduction system are mature by the last 0.10 of gestation, suggesting that cardiac ion channels are present and mature by this time. This is consistent with our previous observation in transcriptomic analyses that genes associated with Purkinje fiber and ion channel maturation are not differentially expressed between 0.90 and 0.97 gestation in the ovine fetus (46). We found only 11 genes that were related to voltage-gated ion channels, ligand-gated ion channels, or other ion channels and significantly changed between 0.90 of gestation and postnatal day 14 (55); however, the change in expression of these genes occurred postnatally. Similar studies in the mouse have shown that although expression of various cardiac ion channel genes changes considerably from embryonic day (E)17.5 to adulthood, less change occurs from E17.5 to 1 day postnatal, as a relatively mature conduction system is likely necessary for survival at birth (16). Although studies using fECG and magnetocardiography indicate that considerable change in some parameters occur between mid and late gestation in the human fetus (particularly increasing durations of the P and T waves, PR, QRS, and QTc intervals), less change occurs during late gestation (9). It has been proposed that this is due to the growing myocardial mass during development, which creates a larger surface area that the electrical signal must traverse (9). In premature human neonates (26–37 wk of gestation), the duration of the P wave, QRS and QT intervals are shorter compared with the full-term neonate, and this is consistent with an association of the size of the chambers to the conduction times through the heart (58, 59). However, estimates of the duration of the P wave and QRS and PR intervals in the human fetus at ≥37 wk of gestation were considerably longer than measured in the sheep fetus from our study (P: 53 vs. 32 ms; PR:110 vs. 78 ms; QRS duration: 53 vs. 28 ms; Ref. 9). These discrepancies are likely not related to differences in heart size since it is similar in both species at birth (this study: 25.5 vs. 24.5 g; Refs. 15, 32). Instead, this might reflect differences in methodology, proteins involved in cardiac conduction, or perhaps, most likely, differences in autonomic activity between the human and sheep fetus. Measurements of the PR interval in our study are similar to those made invasively in other studies in the sheep fetus (65).
Significant diurnal rhythms in HR were observed in the majority of the fetuses in the current study. Studies in several species, including the late gestation fetal baboon, sheep, and humans have shown significant 24-h rhythms in HR, HR variability, and MAP (6, 7, 41, 62). These variations can be influenced by behavioral state, rest and activity cycles, and body/breathing movements (31, 33, 34, 43, 57). The autonomic nervous system of the fetus also considerably influences HR. Sympathetic tone predominates in the fetus, but the relative contribution declines as gestation advances and parasympathetic tone increases; however, neither sympathetic nor parasympathetic input alone are essential for the diurnal rhythms of HR (11, 21, 63). Unno et al. (62) previously found an acrophase in HR occurring ~2330, using 14-h light and 10-h dark periods, compared with ~0200 in our study that used 12-h light and 12-h dark periods. In our study, there was no observed rhythm in MAP. Others have found a significant rhythm in MAP, with peak times occurring around the time of the HR peak (62). Maternal factors including daylight, feeding, and melatonin are known to contribute to the entrainment of fetal circadian rhythms, which could contribute to differences between studies (39, 45, 61). The absence of significant variation in mean pressure could reflect an absence of rhythm in fetal cortisol. In previous studies from our laboratory (3), we found no diurnal rhythm in fetuses in which ewes were fed ad libitum; in contrast, others have found a rhythm in animals when fed for a limited time in the morning (35, 53, 54).
We also found there to be significant 24-h rhythms for several parameters of the fECG occurring in some of the fetuses, including the duration of the PR, RR, ST, QTc, and QRS intervals. These are likely to be driven by the autonomic nervous system as it contributes to the regulation of the ECG through innervation of the sinoatrial and atrioventricular nodes and the ventricular myocardium. However, intrinsic factors that might influence the rhythms of the fECG include circadian expression of cardiac ion channels, as has been shown for adult human and rodent hearts (28, 49, 68) and in association with circadian changes in HR (20, 69) and metabolism (26).
There are some pitfalls that we encountered while implementing this method that should be considered, particularly instances of unexpected and sudden fetal death occurring in the first 48 h of implantation. These are easily detected post hoc as acute increases in aortic pressure and HR, followed by severe bradycardia and progressive hypotension until death. Several of these instances could be attributed to umbilical cord occlusion resulting from manipulation during surgery or insufficient replacement of the amniotic fluid with sterile saline. Because of these issues, we adjusted our protocol to minimize fetal manipulation during surgery and to infuse sterile saline into the amniotic cavity. Implementing these strategies greatly improved the likelihood of a successful preparation.
Perspectives and Significance
Our study demonstrates that the use of implanted radiotelemetry devices is a powerful tool that can be used to allow analysis of fECG and aortic pressures in the chronically instrumented late gestation ovine fetus. The method offers the ability to continuously measure fetal cardiac function over a relatively long period in gestation, in our study from day 118 to delivery at 140–147 days. Notably, we were able to measure ECG as well as fetal pressure during labor and delivery and in the first minutes after birth, even in instances when the investigator was not present. This methodology also allows study of the ovine fetus without maternal restraint and therefore with minimal stress to the ewe. This therefore offers an opportunity for refinement of the animal model. The results from this study also help establish baseline measures of ECG parameters in the late gestation ovine fetus and characterize fetal HR and MAP changes in the fetus in unrestrained and freely moving ewes.
GRANTS
This work was funded by National Institute of Child Health and Human Development Grant HD-057871 and the American Heart Association Grant 14GRNT20420048.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.A., C.E.W., and M.K.-W. conceived and designed research; A.A., C.E.W., and M.K.-W. performed experiments; A.A. and M.K.-W. analyzed data; A.A., C.E.W., and M.K.-W. interpreted results of experiments; A.A. prepared figures; A.A. and M.K.-W. drafted manuscript; A.A., C.E.W., and M.K.-W. edited and revised manuscript; A.A., C.E.W., and M.K.-W. approved final version of manuscript.
REFERENCES
- 1.Abi-Nader KN, Mehta V, Shaw SW, Bellamy T, Smith N, Millross L, Laverick B, Filippi E, Boyd M, Peebles DM, David AL. Telemetric monitoring of fetal blood pressure and heart rate in the freely moving pregnant sheep: a feasibility study. Lab Anim 45: 50–54, 2011. doi: 10.1258/la.2010.010059. [DOI] [PubMed] [Google Scholar]
- 2.Behrman RE, Lees MH. Organ blood flows of the fetal, newborn and adult rhesus monkey: a comparative study. Biol Neonate 18: 330–340, 1971. doi: 10.1159/000240374. [DOI] [PubMed] [Google Scholar]
- 3.Bell ME, Wood CE, Keller-Wood M, Kane C, Kluwe C, Manlove E, Taranovich C, Johnson J. Influence of reproductive state on pituitary-adrenal activity in the ewe. Domest Anim Endocrinol 8: 245–254, 1991. doi: 10.1016/0739-7240(91)90060-W. [DOI] [PubMed] [Google Scholar]
- 4.Bolt RJ, van Weissenbruch MM, Lafeber HN, Delemarre-van de Waal HA. Glucocorticoids and lung development in the fetus and preterm infant. Pediatr Pulmonol 32: 76–91, 2001. doi: 10.1002/ppul.1092. [DOI] [PubMed] [Google Scholar]
- 5.Booth LC, Malpas SC, Barrett CJ, Guild SJ, Gunn AJ, Bennet L. Renal sympathetic nerve activity during asphyxia in fetal sheep. Am J Physiol Regul Integr Comp Physiol 303: R30–R38, 2012. doi: 10.1152/ajpregu.00063.2012. [DOI] [PubMed] [Google Scholar]
- 6.Braaksma MA, Poortinga FM, Aarnoudse JG. Daily rhythms in renal blood flow and urine production rate in the near-term sheep fetus. Pediatr Res 47: 773–777, 2000. doi: 10.1203/00006450-200006000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Brace RA, Moore TR. Diurnal rhythms in fetal urine flow, vascular pressures, and heart rate in sheep. Am J Physiol Regul Integr Comp Physiol 261: R1015–R1021, 1991. [DOI] [PubMed] [Google Scholar]
- 8.Challis JR, Brooks AN. Maturation and activation of hypothalamic-pituitary adrenal function in fetal sheep. Endocr Rev 10: 182–204, 1989. doi: 10.1210/edrv-10-2-182. [DOI] [PubMed] [Google Scholar]
- 9.Chia EL, Ho TF, Rauff M, Yip WC. Cardiac time intervals of normal fetuses using noninvasive fetal electrocardiography. Prenat Diagn 25: 546–552, 2005. doi: 10.1002/pd.1184. [DOI] [PubMed] [Google Scholar]
- 10.Comline RS, Silver M. The composition of foetal and maternal blood during parturition in the ewe. J Physiol 222: 233–256, 1972. doi: 10.1113/jphysiol.1972.sp009795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalton KJ, Dawes GS, Patrick JE. The autonomic nervous system and fetal heart rate variability. Am J Obstet Gynecol 146: 456–462, 1983. doi: 10.1016/0002-9378(83)90828-1. [DOI] [PubMed] [Google Scholar]
- 12.Dawson JA, Kamlin CO, Wong C, te Pas AB, Vento M, Cole TJ, Donath SM, Hooper SB, Davis PG, Morley CJ. Changes in heart rate in the first minutes after birth. Arch Dis Child Fetal Neonatal Ed 95: F177–F181, 2010. doi: 10.1136/adc.2009.169102. [DOI] [PubMed] [Google Scholar]
- 13.Feng X, Reini SA, Richards E, Wood CE, Keller-Wood M. Cortisol stimulates proliferation and apoptosis in the late gestation fetal heart: differential effects of mineralocorticoid and glucocorticoid receptors. Am J Physiol Regul Integr Comp Physiol 305: R343–R350, 2013. doi: 10.1152/ajpregu.00112.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fletcher AJ, Gardner DS, Edwards CM, Fowden AL, Giussani DA. Development of the ovine fetal cardiovascular defense to hypoxemia towards full term. Am J Physiol Heart Circ Physiol 291: H3023–H3034, 2006. doi: 10.1152/ajpheart.00504.2006. [DOI] [PubMed] [Google Scholar]
- 15.Guihard-Costa AM, Ménez F, Delezoide AL. Organ weights in human fetuses after formalin fixation: standards by gestational age and body weight. Pediatr Dev Pathol 5: 559–578, 2002. doi: 10.1007/s10024-002-0036-7. [DOI] [PubMed] [Google Scholar]
- 16.Harrell MD, Harbi S, Hoffman JF, Zavadil J, Coetzee WA. Large-scale analysis of ion channel gene expression in the mouse heart during perinatal development. Physiol Genomics 28: 273–283, 2007. doi: 10.1152/physiolgenomics.00163.2006. [DOI] [PubMed] [Google Scholar]
- 17.Hermans B, Lewi L, Jani J, De Buck F, Deprest J, Puers R. Feasibility of in utero telemetric fetal ECG monitoring in a lamb model. Fetal Diagn Ther 24: 81–85, 2008. doi: 10.1159/000142132. [DOI] [PubMed] [Google Scholar]
- 18.Heymann MA, Iwamoto HS, Rudolph AM. Factors affecting changes in the neonatal systemic circulation. Annu Rev Physiol 43: 371–383, 1981. doi: 10.1146/annurev.ph.43.030181.002103. [DOI] [PubMed] [Google Scholar]
- 19.Hillman NH, Kallapur SG, Jobe AH. Physiology of transition from intrauterine to extrauterine life. Clin Perinatol 39: 769–783, 2012. doi: 10.1016/j.clp.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu K, Ivanov PC, Hilton MF, Chen Z, Ayers RT, Stanley HE, Shea SA. Endogenous circadian rhythm in an index of cardiac vulnerability independent of changes in behavior. Proc Natl Acad Sci USA 101: 18223–18227, 2004. doi: 10.1073/pnas.0408243101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen EC, Bennet L, Guild SJ, Booth LC, Stewart J, Gunn AJ. The role of the neural sympathetic and parasympathetic systems in diurnal and sleep state-related cardiovascular rhythms in the late-gestation ovine fetus. Am J Physiol Regul Integr Comp Physiol 297: R998–R1008, 2009. doi: 10.1152/ajpregu.90979.2008. [DOI] [PubMed] [Google Scholar]
- 22.Kato K, Wakai J, Ozawa K, Sekiguchi M, Katahira K. Different sensitivity to the suppressive effects of isoflurane anesthesia on cardiorespiratory function in SHR/Izm, WKY/Izm, and Crl:CD (SD) rats. Exp Anim 65: 393–402, 2016. doi: 10.1538/expanim.16-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller-Wood M, Feng X, Wood CE, Richards E, Anthony RV, Dahl GE, Tao S. Elevated maternal cortisol leads to relative maternal hyperglycemia and increased stillbirth in ovine pregnancy. Am J Physiol Regul Integr Comp Physiol 307: R405–R413, 2014. doi: 10.1152/ajpregu.00530.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keller-Wood M, Wood CE, McCartney J, Jesse NM, Perrone D. A role for mineralocorticoid receptors in the physiology of the ovine fetus: effects on ACTH and lung liquid composition. Pediatr Res 69: 491–496, 2011. doi: 10.1203/PDR.0b013e318217f4cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimura Y, Okamura K, Watanabe T, Murotsuki J, Suzuki T, Yano M, Yajima A. Power spectral analysis for autonomic influences in heart rate and blood pressure variability in fetal lambs. Am J Physiol Heart Circ Physiol 271: H1333–H1339, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Kohsaka A, Das P, Hashimoto I, Nakao T, Deguchi Y, Gouraud SS, Waki H, Muragaki Y, Maeda M. The circadian clock maintains cardiac function by regulating mitochondrial metabolism in mice. PLoS One 9: e112811, 2014. doi: 10.1371/journal.pone.0112811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koome ME, Bennet L, Booth LC, Davidson JO, Wassink G, Gunn AJ. Ontogeny and control of the heart rate power spectrum in the last third of gestation in fetal sheep. Exp Physiol 99: 80–88, 2014. doi: 10.1113/expphysiol.2013.074567. [DOI] [PubMed] [Google Scholar]
- 28.Leibetseder V, Humpeler S, Svoboda M, Schmid D, Thalhammer T, Zuckermann A, Marktl W, Ekmekcioglu C. Clock genes display rhythmic expression in human hearts. Chronobiol Int 26: 621–636, 2009. doi: 10.1080/07420520902924939. [DOI] [PubMed] [Google Scholar]
- 29.Liggins GC. The role of cortisol in preparing the fetus for birth. Reprod Fertil Dev 6: 141–150, 1994. doi: 10.1071/RD9940141. [DOI] [PubMed] [Google Scholar]
- 30.Lilja H, Karlsson K, Kjellmer I, Lindecrantz K, Olsson T, Rosen KG. Heart rate variability and electrocardiogram changes in the fetal lamb during hypoxia and beta-adrenoceptor stimulation. J Perinat Med 12: 115–125, 1984. doi: 10.1515/jpme.1984.12.3.115. [DOI] [PubMed] [Google Scholar]
- 31.Lunshof S, Boer K, van Hoffen G, Wolf H, Mirmiran M. The diurnal rhythm in fetal heart rate in a twin pregnancy with discordant anencephaly: comparison with three normal twin pregnancies. Early Hum Dev 48: 47–57, 1997. doi: 10.1016/S0378-3782(96)01802-6. [DOI] [PubMed] [Google Scholar]
- 32.Maroun LL, Graem N. Autopsy standards of body parameters and fresh organ weights in nonmacerated and macerated human fetuses. Pediatr Dev Pathol 8: 204–216, 2005. doi: 10.1007/s10024-004-7084-0. [DOI] [PubMed] [Google Scholar]
- 33.Marzbanrad F, Kimura Y, Palaniswami M, Khandoker AH. Quantifying the interactions between maternal and fetal heart rates by transfer entropy. PLoS One 10: e0145672, 2015. doi: 10.1371/journal.pone.0145672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massin MM, Maeyns K, Withofs N, Ravet F, Gérard P. Circadian rhythm of heart rate and heart rate variability. Arch Dis Child 83: 179–182, 2000. doi: 10.1136/adc.83.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMillen IC, Thorburn GD, Walker DW. Diurnal variations in plasma concentrations of cortisol, prolactin, growth hormone and glucose in the fetal sheep and pregnant ewe during late gestation. J Endocrinol 114: 65–72, 1987. doi: 10.1677/joe.0.1140065. [DOI] [PubMed] [Google Scholar]
- 36.Murotsuki J, Bocking AD, Gagnon R. Fetal heart rate patterns in growth-restricted fetal sheep induced by chronic fetal placental embolization. Am J Obstet Gynecol 176: 282–290, 1997. doi: 10.1016/S0002-9378(97)70486-1. [DOI] [PubMed] [Google Scholar]
- 37.Neary MT, Mohun TJ, Breckenridge RA. A mouse model to study the link between hypoxia, long QT interval and sudden infant death syndrome. Dis Model Mech 6: 503–507, 2013. doi: 10.1242/dmm.010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neilson JP. Fetal electrocardiogram (ECG) for fetal monitoring during labour. Cochrane Database Syst Rev 2: CD000116, 2003. doi: 10.1002/14651858.CD000116. [DOI] [PubMed] [Google Scholar]
- 39.Ohta H, Xu S, Moriya T, Iigo M, Watanabe T, Nakahata N, Chisaka H, Hanita T, Matsuda T, Ohura T, Kimura Y, Yaegashi N, Tsuchiya S, Tei H, Okamura K. Maternal feeding controls fetal biological clock. PLoS One 3: e2601, 2008. doi: 10.1371/journal.pone.0002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okutomi T, Whittington RA, Stein DJ, Morishima HO. Comparison of the effects of sevoflurane and isoflurane anesthesia on the maternal-fetal unit in sheep. J Anesth 23: 392–398, 2009. doi: 10.1007/s00540-009-0763-2. [DOI] [PubMed] [Google Scholar]
- 41.Patrick J, Campbell K, Carmichael L, Natale R, Richardson B. Daily relationships between fetal and maternal heart rates at 38 to 40 weeks of pregnancy. Can Med Assoc J 124: 1177–1178, 1981. [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips ID, Anthony RV, Butler TG, Ross JT, McMillen IC. Hepatic prolactin receptor gene expression increases in the sheep fetus before birth and after cortisol infusion. Endocrinology 138: 1351–1354, 1997. doi: 10.1210/endo.138.3.5102. [DOI] [PubMed] [Google Scholar]
- 43.Pillai M, James D. The development of fetal heart rate patterns during normal pregnancy. Obstet Gynecol 76: 812–816, 1990. doi: 10.1097/00006250-199011000-00017. [DOI] [PubMed] [Google Scholar]
- 44.Reini SA, Dutta G, Wood CE, Keller-Wood M. Cardiac corticosteroid receptors mediate the enlargement of the ovine fetal heart induced by chronic increases in maternal cortisol. J Endocrinol 198: 419–427, 2008. doi: 10.1677/JOE-08-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reppert SM, Schwartz WJ. Maternal coordination of the fetal biological clock in utero. Science 220: 969–971, 1983. doi: 10.1126/science.6844923. [DOI] [PubMed] [Google Scholar]
- 46.Richards EM, Rabaglino MB, Antolic A, Wood CE, Keller-Wood M. Patterns of gene expression in the sheep heart during the perinatal period revealed by transcriptomic modeling. Physiol Genomics 47: 407–419, 2015. doi: 10.1152/physiolgenomics.00027.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richards EM, Wood CE, Rabaglino MB, Antolic A, Keller-Wood M. Mechanisms for the adverse effects of late gestational increases in maternal cortisol on the heart revealed by transcriptomic analyses of the fetal septum. Physiol Genomics 46: 547–559, 2014. doi: 10.1152/physiolgenomics.00009.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rog-Zielinska EA, Richardson RV, Denvir MA, Chapman KE. Glucocorticoids and foetal heart maturation; implications for prematurity and foetal programming. J Mol Endocrinol 52: R125–R135, 2014. doi: 10.1530/JME-13-0204. [DOI] [PubMed] [Google Scholar]
- 49.Sakamoto K, Nagase T, Fukui H, Horikawa K, Okada T, Tanaka H, Sato K, Miyake Y, Ohara O, Kako K, Ishida N. Multitissue circadian expression of rat period homolog (rPer2) mRNA is governed by the mammalian circadian clock, the suprachiasmatic nucleus in the brain. J Biol Chem 273: 27039–27042, 1998. doi: 10.1074/jbc.273.42.27039. [DOI] [PubMed] [Google Scholar]
- 50.Shintaku T, Ohba T, Niwa H, Kushikata T, Hirota K, Ono K, Matsuzaki Y, Sawamura D, Murakami M. Effects of isoflurane inhalation anesthesia on mouse ECG. Hirosaki Med J 66: 1–7, 2015. doi: 10.1254/jphs.14181FP. [DOI] [PubMed] [Google Scholar]
- 51.Signorini MG, Fanelli A, Magenes G. Monitoring fetal heart rate during pregnancy: contributions from advanced signal processing and wearable technology. Comput Math Methods Med 2014: 707581, 2014. doi: 10.1155/2014/707581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silver M. Prenatal maturation, the timing of birth and how it may be regulated in domestic animals. Exp Physiol 75: 285–307, 1990. doi: 10.1113/expphysiol.1990.sp003405. [DOI] [PubMed] [Google Scholar]
- 53.Simonetta G, Walker DW, McMillen IC. Effect of feeding on the diurnal rhythm of plasma cortisol and adrenocorticotrophic hormone concentrations in the pregnant ewe and sheep fetus. Exp Physiol 76: 219–229, 1991. doi: 10.1113/expphysiol.1991.sp003488. [DOI] [PubMed] [Google Scholar]
- 54.Slater JS, Mellor DJ. Within-day variations in the composition of maternal and fetal plasma from catheterised ewes fed once daily or at hourly intervals during late pregnancy. Res Vet Sci 31: 224–230, 1981. [PubMed] [Google Scholar]
- 55.Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, Buneman OP, Davenport AP, McGrath JC, Peters JA, Spedding M, Catterall WA, Fabbro D, Davies JA; NC-IUPHAR . The IUPHAR/BPS Guide to Pharmacology in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44, D1: D1054–D1068, 2016. doi: 10.1093/nar/gkv1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Staikou C, Stamelos M, Stavroulakis E. Impact of anaesthetic drugs and adjuvants on ECG markers of torsadogenicity. Br J Anaesth 112: 217–230, 2014. doi: 10.1093/bja/aet412. [DOI] [PubMed] [Google Scholar]
- 57.Swartjes JM, van Geijn HP, Mantel R, Schoemaker HC. Quantitated fetal heart rhythm at 20, 32 and 38 weeks of gestation and dependence on rest-activity patterns. Early Hum Dev 28: 27–36, 1992. doi: 10.1016/0378-3782(92)90005-2. [DOI] [PubMed] [Google Scholar]
- 58.Thomaidis C, Varlamis G, Karamperis S. Comparative study of the electrocardiograms of healthy fullterm and premature newborns. Acta Paediatr Scand 77: 653–657, 1988. doi: 10.1111/j.1651-2227.1988.tb10725.x. [DOI] [PubMed] [Google Scholar]
- 59.Tipple M. Interpretation of electrocardiograms in infants and children. Images Paediatr Cardiol 1: 3–13, 1999. [PMC free article] [PubMed] [Google Scholar]
- 60.Top AP, Tasker RC, Ince C. The microcirculation of the critically ill pediatric patient. Crit Care 15: 213, 2011. doi: 10.1186/cc9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Torres-Farfan C, Rocco V, Monsó C, Valenzuela FJ, Campino C, Germain A, Torrealba F, Valenzuela GJ, Seron-Ferre M. Maternal melatonin effects on clock gene expression in a nonhuman primate fetus. Endocrinology 147: 4618–4626, 2006. doi: 10.1210/en.2006-0628. [DOI] [PubMed] [Google Scholar]
- 62.Unno N, Wong CH, Jenkins SL, Wentworth RA, Ding XY, Li C, Robertson SS, Smotherman WP, Nathanielsz PW. Blood pressure and heart rate in the ovine fetus: ontogenic changes and effects of fetal adrenalectomy. Am J Physiol Heart Circ Physiol 276: H248–H256, 1999. [DOI] [PubMed] [Google Scholar]
- 63.Walker AM, Cannata J, Dowling MH, Ritchie B, Maloney JE. Sympathetic and parasympathetic control of heart rate in unanaesthetized fetal and newborn lambs. Biol Neonate 33: 135–143, 1978. doi: 10.1159/000241063. [DOI] [PubMed] [Google Scholar]
- 64.Wassink G, Bennet L, Booth LC, Jensen EC, Wibbens B, Dean JM, Gunn AJ. The ontogeny of hemodynamic responses to prolonged umbilical cord occlusion in fetal sheep. J Appl Physiol (1985) 103: 1311–1317, 2007. doi: 10.1152/japplphysiol.00396.2007. [DOI] [PubMed] [Google Scholar]
- 65.Westgate JA, Gunn AJ, Bennet L, Gunning MI, de Haan HH, Gluckman PD. Do fetal electrocardiogram PR-RR changes reflect progressive asphyxia after repeated umbilical cord occlusion in fetal sheep? Pediatr Res 44: 297–303, 1998. doi: 10.1203/00006450-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 66.Wood CE, Powers Fraites M, Keller-Wood M. Blockade of PGHS-2 inhibits the hypothalamus-pituitary-adrenal axis response to cerebral hypoperfusion in the sheep fetus. Am J Physiol Regul Integr Comp Physiol 296: R1813–R1819, 2009. doi: 10.1152/ajpregu.90917.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu G, Anafi RC, Hughes ME, Kornacker K, Hogenesch JB. MetaCycle: an integrated R package to evaluate periodicity in large scale data. Bioinformatics 32: 3351–3353, 2016. doi: 10.1093/bioinformatics/btw405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamashita T, Sekiguchi A, Iwasaki YK, Sagara K, Iinuma H, Hatano S, Fu LT, Watanabe H. Circadian variation of cardiac K+ channel gene expression. Circulation 107: 1917–1922, 2003. doi: 10.1161/01.CIR.0000058752.79734.F0. [DOI] [PubMed] [Google Scholar]
- 69.Young ME. The circadian clock within the heart: potential influence on myocardial gene expression, metabolism, and function. Am J Physiol Heart Circ Physiol 290: H1–H16, 2006. doi: 10.1152/ajpheart.00582.2005. [DOI] [PubMed] [Google Scholar]