Abstract
A mucosal oxidative burst is a hallmark response to pollen exposure that promotes allergic inflammatory responses. Reactive species constituents of oxidative stress signal via the modification of cellular molecules including nucleic acids. One of the most abundant forms of oxidative genomic base damage is 8-oxo-7,8-dihydroguanine (8-oxoG), which is removed from DNA by 8-oxoguanine DNA glycosylase 1 (OGG1). OGG1 in complex with 8-oxoG acts as a GDP-GTP exchange factor and induces acute inflammation; however, the mechanism(s) by which OGG1 signaling regulates allergic airway inflammation is not known. Here, we postulate that the OGG1 signaling pathway differentially altered the levels of small regulatory RNAs and increased the expression of T helper 2 (Th2) cytokines in ragweed pollen extract (RWPE)-challenged lungs. To determine this, the lungs of sensitized mice expressing or lacking OGG1 were challenged with RWPE and/or with OGG1’s excision product 8-oxoG. The responses in lungs were assessed by next-generation sequencing, as well as various molecular and histological approaches. The results showed that RWPE challenge induced oxidative burst and damage to DNA and activated OGG1 signaling, resulting in the differential expression of 84 micro-RNAs (miRNAs), which then exacerbated antigen-driven allergic inflammation and histological changes in the lungs. The exogenous administration of the downregulated let-7b-p3 mimetic or inhibitors of upregulated miR-23a or miR-27a decreased eosinophil recruitment and mucus and collagen production via controlling the expression of IL-4, IL-5, and IL-13. Together, these data demonstrate the roles of OGG1 signaling in the regulation of antigen-driven allergic immune responses via differential expression of miRNAs upstream of Th2 cytokines and eosinophils.
Keywords: DNA repair, cell signaling, allergy, micro-RNAs
INTRODUCTION
In organs and tissues affected by environmental allergens, two of the most referenced forms of oxidatively damaged DNA bases are 8-oxo-7,8-dihydroguanine (8-OH-Gua, or 8-oxoG) and its open-ringed form 2,6-diamino-4-hydroxy-5-formamidopyrimidine (FapyGua, or FapyG) from both human patients and experimental animal models (19, 29, 33, 38, 54, 58). Comprehensive studies have also shown that the length of exposure and disease intensity correlate well with increased levels of the genomic 8-oxoG and repair by-product of 8-oxoguanine DNA glycosylase 1 (OGG1) (the free base 8-oxoG) in sputum, serum, urine, and bronchoalveolar lavage fluid (BALF; 38, 58). Despite these close correlations, genomic 8-oxoG and the free 8-oxoG base are traditionally considered as markers of oxidative stress, and whether these lesions contribute to the development, maintenance, and/or exacerbation of allergic disease processes remains unknown (8, 13, 26).
8-OxoG is potentially mutagenic and is preferentially repaired by the DNA base excision repair (BER) pathway (28, 49), which primarily occurs via OGG1. OGG1 excises the lesion by cleaving its N-glycosidic bond to release the 8-oxoG base, followed by endonucleolytic cleavage and filling of nucleoside gaps by DNA polymerases; the DNA strand is then sealed by ligases (28, 49). Deficiency in OGG1-BER leads to the accumulation of 8-oxoG, which is conventionally considered mutagenic and associated with various pathological changes that accelerate aging and age-associated diseases (25). Unexpectedly, the lack of OGG1 activity in Ogg1 knockout mice (Ogg1−/−) and the consequent increases in the level of genomic 8-oxoG did not alter embryogenesis or life span, and mice showed no increased tumor frequency or marked pathological changes (41, 48). Among the phenotypic changes in Ogg1−/− mice best defined thus far are aberrant immune responses to bacterial infections, as well as lipopolysaccharide (LPS) exposures, as shown by lower levels of T helper 1 (Th1) chemokines, cytokines, and interleukin expression (45, 62). In line with these observations, decreased ovalbumin-induced allergic immune responses were documented in Ogg1−/− mice partially due to low levels of phosphorylation of nuclear factor-κB (NF-κB) and STAT6, which decreased the expression of Th1 and Th2 cytokines (44). Silencing Ogg1 expression in the lungs of ragweed pollen extract (RWPE)-sensitized mice resulted in the accumulation of genomic 8-oxoG, but lower allergic inflammatory responses, as documented by the decreased expression of Th2 cytokines (IL-4, IL-5, and IL-13), eosinophilia, and airway hyperresponsiveness (10). Decreased immune responses in OGG1-deficient mice could be explained by both a lack of OGG1 and a lack of free 8-oxoG bases. Follow-up work has documented that the free 8-oxoG base is bound by cytosolic OGG1 and that the formed complex functions as a guanine nucleotide exchange factor to activate small GTPases, including Kirsten-Ras (K-Ras; 1, 15, 32). Downstream from K-Ras, the v-raf murine leukemia viral oncogene homolog 1 (RAF1), mitogen-activated kinases (MEK1/2 and ERK1/2), mitogen stress-related kinase 1 (MSK-1), and phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) pathways are activated, resulting in the phosphorylation and nuclear translocation of transcription factors such as NF-κB (1), which is a key regulator of inflammation in asthma and other chronic pulmonary diseases (16, 60).
Signaling pathways downstream from small GTPases are known to regulate the expression of both coding and noncoding RNAs including miRNAs (3, 18). This study used next-generation sequencing and system-level analysis supported by molecular approaches to demonstrate that OGG1 signaling pathways regulate the expression of miRNAs that contribute to RWPE-induced allergic responses in an experimental mouse model. Modulation of miRNA levels in the lungs by the administration of mimics (or inhibitors) decreased RWPE-induced allergic immune and histological changes in the lungs. These data are the first to show that OGG1 signaling differentially modulates the expression of miRNAs in airways, which leads to the overexpression of Th2 cytokines and the exacerbation of antigen-driven allergic immune and tissue responses. The pharmacological inhibition of DNA damage repair signaling or the topical administration of specific miRNAs could have clinical utility.
MATERIALS AND METHODS
Animals and treatment.
Animal experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Texas Medical Branch (UTMB) Animal Care and Use Committee (approval no. 0807044A). Eight-week-old BALB/c mice (Jackson, Bar Harbor, ME) were used for these studies. Parallel groups of mice were sensitized (on days 0 and 4 via the intraperitoneal route) using LPS-free ragweed (Ambrosia artemisiifolia) pollen extract (RWPE; 100 μg mixed with alum; 14). Allergic immune and tissue responses in OGG1-proficient and OGG1-deficient airways with or without miRNA mimics (or their inhibitors) were induced by LPS-free RWPE (50 μg in 60-μl saline; n = 5 per group; 2, 14). Randomly selected groups of mice were challenged intranasally on day 0 or days 2 and 4 with 60 µl of pH-balanced 8-oxoG (Cayman Chemicals, Ann Arbor, MI) solution (pH 7.4; 0.0005 mg/kg) or saline (3). Using Lipofectamine RNAiMAX (Invitrogen), miRNA mimics or inhibitors were introduced into airways intranasally. Depletion of target gene 8-oxoguanine DNA glycosylase 1 (Ogg1) was performed using stealth RNA interference (RNAi; cat. no. MSS237431; Invitrogen Life Technologies) in RNAi transfection reagent (cat. no. 201-10G; Polyplus-transfection, New York, NY) at −72 and −24 h (1).
Assessment of oxidative stress.
MLE-12 (immortalized mouse lung epithelial) cells were cultured in RPMI 1640 medium per the instructions of American Type Culture Collection. Cells were loaded with 2′-7′-dihydro-dichlorofluorescein diacetate (H2DCF-DA; Molecular Probes) and were mock challenged or challenged with nicotinamide adenine dinucleotide phosphate oxidase (NOX)-active RWPE (RWPENOX) or heat-inactivated (56°C for 30 min) RWPE (RWPEHI). Changes in DCF fluorescence were determined at 480 and 526 nm (excitation and emission, respectively) using a BioTek FLx800 fluorometer (11, 14). Oxidized (carbonylated) protein levels were assessed before and after RWPE challenge. Lung lysates were derivatized with 4-dinitrophenylhydrazine (DNPH; Sigma/Millipore), and carbonylated proteins were detected by anti-DNP antibody (MAB2223, clone 9H8.1) according to manufacturer recommendations and as we published previously (2).
Levels of genomic 8-oxoG in the airways of mock challenged and RWPENOX-challenged OGG1-proficient (OGG1P) and OGG1-deficient (OGG1D) mice were determined by quantitative PCR (qPCR). DNA was isolated at 1-h postchallenge (mock or RWPENOX) using the Qiagen Genomic-tip 20/G kit per the manufacturer’s recommendations. To prevent aerial oxidation during genomic DNA isolation, 100 μM 2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO) was added to the buffers (21). The DNA was quantitated by Pico Green (Molecular Probes), and gene-specific qPCR of DNA was performed after mock or recombinant (r)OGG1 (Novus Biologicals; 5 ng per sample) digestion in a 100-μl reaction buffer [50 mmol/l Tris·HCl (pH 7.4), 50 mM NaCl, 1 mmol/l EDTA, 1 mM dithiothreitol, and 1 mg/ml bovine serum albumin (BSA)] at 37°C for 15 min. After digestion, DNA was extracted and 20 ng were subjected to qPCR. A 3.2-kb strand of the DNA polymerase-β (Polb) gene was amplified using gene-specific primers. The primers were designed using https://www.ncbi.nlm.nih.gov/tools/primer-blast/. The primers were synthesized by Integrated DNA Technologies (Coralville, IA), and the amplified product was validated by sequencing. The base excision activity of rOGG1 was determined using a 40-mer oligonucleotide (100 fmol per reaction) containing an 8-oxoG labeled at the 3′ end with Cy5 (5′-AGAGAAGAAGAAGAAGAA/8oxodG/AGATGGGTTATTCGAACTAGC/3Cy5Sp/-3′), as we previously described (32).
Western blot analysis.
Lungs were homogenized by using a TissueMiser (Fisher Scientific) homogenizer (25,000 rpm, on ice) in lysis buffer containing 25 mM Tris·HCl (pH 7.5), 150 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 1% Nonidet P-40 (NP-40), 1 mM DTT, 5% glycerol, and protein inhibitor cocktail (cat. no. P0044; Sigma-Aldrich). Tissue homogenates were clarified by centrifugation at 12,000 g, the supernatants were collected, 20 µg of protein per lane were separated by 4–20% SDS-polyacrylamide gel electrophoresis (Mini-Protean Gel, cat. no. 546-1094; Bio-Rad), and proteins were subsequently transferred onto nitrocellulose membranes (Amersham Biosciences). The membranes were then blocked with 3% BSA in Tris-buffered saline (TBS) containing 0.1% Tween (TBS-T) for 3 h at room temperature. Primary antibody (rabbit anti-OGG1; cat. no. ab124741; AB Chem) was diluted in 3% BSA in TBS-T (1:3,000) and incubated with the membrane overnight at 4°C as we described previously (9, 52). The membranes were washed, then blocked with 3% BSA as above for 3 h, and incubated with horseradish peroxidase-conjugated secondary antibody (goat anti-rabbit; cat. no. 4050-05; Southern BioTech). The chemiluminescent signals were detected using Western blotting detection reagents (GE Life Sciences) and autoradiography. Membranes were stripped (Restore Plus Western Blot Stripping Buffer; cat. no. 46430; Thermo Fisher Scientific) and reprobed using anti-GAPDH antibody (cat. no. 2118S; Cell Signaling Technology).
RNA isolation, sequencing, and analysis.
Excised lungs (100 mg wet wt) were homogenized and RNAs were extracted using an RNeasy kit per the manufacturer's instructions (Qiagen, Valencia, CA). Ten micrograms of RNA from each sample were provided to UTMB’s Next-Generation Sequencing Core Facility (Dr. Thomas G. Wood, director), and sequencing analysis was performed using an Illumina HiSeq 1000 sequencing system (Illumina, San Diego, CA). Base call conversion to sequence reads was performed using CASAVA-1.8.2. Sequence data were analyzed with the Bowtie2, TopHat, and Cufflinks programs using National Center for Biotechnology Information (NCBI)’s mouse (Mus musculus) genome build reference mm10. RNA-sequencing data have been deposited in the NCBI’s Gene Expression Omnibus (GEO), accession numbers GSE61095 and GSE65031 (3, 4).
Visual exploration of RNA-sequencing data was undertaken using the GENE-E matrix visualization and analysis platform (https://software.broadinstitute.org/GENE-E/). For comparison and visualization of biological lists to create Venn diagrams, Venny online software v2.1 was utilized (http://bioinfogp.cnb.csic.es/tools/venny/). To predict target(s) and functional annotations of miRNAs, the online miRNA database (http://mirdb.org/miRDB/) was utilized. GeneCards (http://www.genecards.org/), an integrated database (an authoritative compendium of annotative information about human and mouse genes), was utilized to identify potential allergy-associated genes.
Quantitative reverse transcription-PCR.
Excised lungs were homogenized, and RNAs were extracted using an RNeasy kit per the manufacturer's instructions (Qiagen). Total RNA (1 μg) was reverse transcribed using a SuperScript III First-Strand Synthesis System (Invitrogen Life Technologies). Levels of mRNA were determined by quantitative reverse transcription-PCR (qRT-PCR). The commercially (Integrated DNA Technologies) validated primers (for the mRNA) were Il4, cat. no. Mm.PT.58.7882098; Il5, cat. no. Mm.PT.58.41498972; Il13, cat. no. Mm.PT.58.31366752; Ogg1, cat. no. MP231384; Hprt, cat. no. Mm.PT.58.32092191; Actb, cat. no. Mm.PT.58.33540333; and Gapdh, cat. no. Mm.PT.39a.1. Collagen I: forward, 5′-TGG CCA AGA AGA CAT CCC TGA AGT-3′; reverse, 5-ACA TCA GGT TTC CAC GTC TCA CCA-3′; qRT-PCR was performed on an ABI7000 thermal cycler, and transcript levels were evaluated using the ΔΔCt method (Ct, cycle threshold; 2).
Evaluation of airway inflammation.
Bronchoalveolar lavage fluid (BALF) was collected at 72-h postchallenge, total cell numbers were determined using a hemocytometer, and cytospin preparations were stained with Modified Wright-Giemsa for Hema-Tek 2000 Slide Stainer (protocol) for differential cell counts as previously described (2, 14). For histology, lungs were fixed in 10% neutral buffered formalin overnight and embedded in optimum cutting temperature compound for frozen sectioning. Four-micrometer lung sections were stained with hematoxylin and eosin (for general morphology and to visualize inflammatory cell infiltrates), periodic acid-Schiff stain [to visualize epithelial cell differentiation to the neutral glycoprotein (mucus)-producing cells], and Masson's trichrome stain (to observe collagen depositions) at the Research Histopathology Core at UTMB (Dr. G. Valbuena, director).
Collagen levels were determined by assessing the hydroxyproline content of the mouse lungs using a colorimetric detection assay (cat. no. MAK008-1KT) as recommended by the manufacturer (Sigma-Aldrich). In brief, lung parenchyma (free extrapulmonary airways and major blood vessels) was homogenized in 1.0 ml of PBS and acid hydrolyzed at 110°C for 24 h (34). Hydroxyproline concentrations were determined by the reaction of hydroxyproline with 4-(dimethylamino)benzaldehyde at 560 nm using the BioTek FLx800 fluorometer. Standard curves were generated for each experiment using hydroxyproline reagent as a standard. The results were expressed as micrograms of hydroxyproline contained in total lung tissue.
Mucin-5 subtype AC (MUC5AC) levels were determined by enzyme-linked immunosorbent assay (ELISA) as we previously described (2). In brief, BALF was serially diluted in coating buffer (50 mM Na2CO3 and 50 mM NaHCO3), and 100-μl aliquots were added to 96-well plates for “coating.” Primary mouse MUC5AC antibody was used (1:16,000; cat. no. MA5-12178; Invitrogen; Thermo Fisher Scientific). Bound primary antibody was assessed by peroxidase substrate (tetramethylbenzidine) and peroxidase-conjugated goat anti-mouse secondary antibody (1:5,000; Amersham Biosciences). Incubations were performed at 37°C. Changes in absorbance were determined at 450 nm using a SpectraMax 190 ELISA plate reader (Molecular Devices, Sunnyvale, CA).
Statistical analysis.
Statistical analysis was performed using Student’s t-test or ANOVA, followed by post hoc tests: Bonferroni’s and Dunnett’s T3 with SPSS 14.0 software. The data are presented as means ± SE; *P < 0.05, **P < 0.01, ***P < 0.001.
RESULTS
Pollen antigen-induced allergic responses are exacerbated by OGG1 signaling.
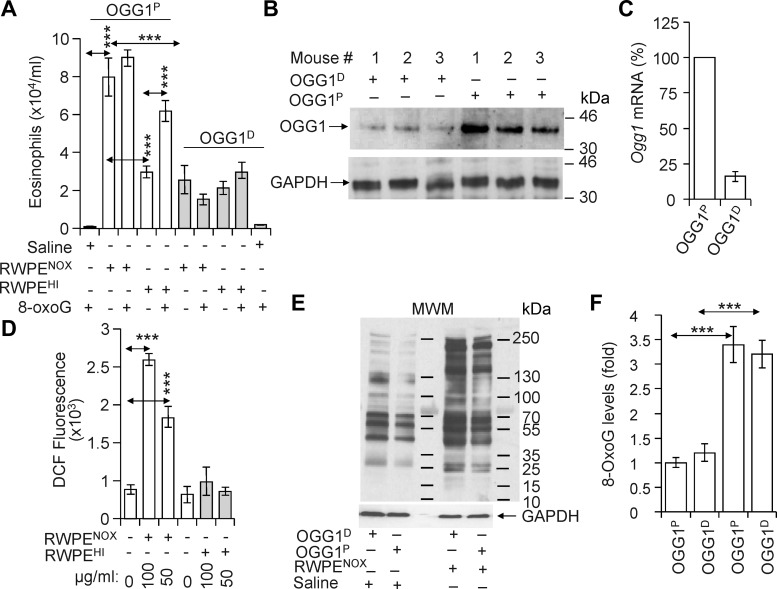
Ragweed pollen extract containing intrinsic NADPH oxidases (NOX; RWPENOX) that were documented to generate reactive oxygen species (ROS; 11, 14) induced robust recruitment of eosinophils into the airways after challenge of sensitized OGG1-expressing (OGG1-proficient; OGG1P) mice (Fig. 1A). In controls, OGG1P mice were challenged with heat-inactivated (HI) RWPE (RWPEHI; 56°C for 30 min), a treatment that decreases NOX activity (14). RWPEHI caused a significantly lower eosinophil recruitment into lungs, as assessed by numerating cells in BALF (Fig. 1A). RWPENOX challenge of lungs oxidatively modifies macromolecules including DNA (10). To determine whether repair of oxidatively damaged DNA and consequent activation of the OGG1 signaling pathway plays a role in eosinophilic inflammation, OGG1 expression was silenced in the lungs (OGG1D; Fig. 1, B and C; see materials and methods). OGG1 silencing resulted in a ~90% (Fig. 1B, lanes 1–3) decrease in protein levels compared with OGG1P mice (Fig. 1B, lanes 4–6). Parallel groups of OGG1P and OGG1D mice were then challenged with RWPENOX or RWPEHI, and the inflammatory response was determined. Compared with OGG1P mice, those lacking OGG1 showed significantly decreased numbers of eosinophils (Fig. 1A) after challenge with RWPENOX. Interestingly, numbers of eosinophils in RWPEHI-challenged groups of OGG1P and OGG1D mice were similar (Fig. 1A) implying a profound role of RWPENOX-ROS in allergen-induced inflammatory response. To document differences in the capacities of RWPENOX and RWPEHI to alter levels of intracellular ROS, we showed that it was increased by RWPENOX but not by RWPEHI in cultured airway epithelial cells (Fig. 1D). To exclude the possibility that decreased inflammatory responses in lungs of OGG1D mice were due to an increased antioxidant capacity, we show that changes in the levels of oxidative stress markers (carbonylated protein, Fig. 1E) and fold changes in genomic 8-oxoG (Fig. 1F) are similar after RWPENOX challenge. We note that in nonsensitized OGG1P mice, RWPENOX challenge induced a robust transient innate immune response, whereas in challenge of OGG1D mice, a poor innate immune response was observed, which is consistent with previous findings (11, 14).
Fig. 1.
OGG1 expression exacerbates pollen-induced allergic inflammation. A: OGG1 silencing in lungs decreases the accumulation of eosinophils (gray bars) compared with those expressing OGG1 (open bars). Sensitized OGG1-expressing (OGG1P) and OGG1-deficient (OGG1D) mice were challenged with intrinsic NADPH oxidase of RWPE (RWPENOX), heat-inactivated RWPE (RWPEHI), RWPENOX+8-oxoG, or RWPEHI+8-oxoG. Seventy-two hours later, lungs were lavaged, and the numbers of BALF eosinophils were determined. B and C: expression of OGG1 at protein (B) and RNA (C) levels in the lungs of sensitized mice prior to and after OGG1 silencing. OGG1 silencing in airways is described in materials and methods. Protein and RNA levels in lungs of OGG1D and OGG1P mice were determined 72 h after RWPE challenge. D: RWPENOX increases cellular ROS levels in cultured airway epithelial (AML-12) cells (open bars). RWPEHI had no effect on cellular reactive ROS levels (gray bars). Changes in ROS levels were determined by oxidation of H2DCF (see materials and methods). E and F: RWPENOX challenge increased the protein carbonyl (E) and genomic 8-oxoG (F) levels in lungs of OGG1P and OGG1D. MWM, molecular weight marker. Data are presented as means ± SE of six individual mice. ***P < 0.001.
To further test the potential role(s) of the OGG1 pathway in exacerbation of allergic inflammatory responses, we administered 8-oxoG base [0.00005 mg/kg, a dose that may be generated by OGG1-BER upon oxidative stress (1)] along with either RWPENOX or RWPEHI to OGG1P mice. The coadministration of the 8-oxoG base with RWPENOX was insignificant but consistently enhanced eosinophil recruitment into the lungs. In contrast, cochallenge of OGG1P mice with RWPEHI and 8-oxoG significantly increased the allergic immune responses (Fig. 1A). In controls, cochallenge of OGG1D mice with 8-oxoG and RWPENOX or RWPEHI did not significantly alter eosinophil counts in BALF (Fig. 1A). These results suggest that both OGG1 and free base 8-oxoG (the product of OGG1-BER) are required for robust antigen-driven allergic immune responses.
OGG1 signaling induces differential expression of miRNAs.
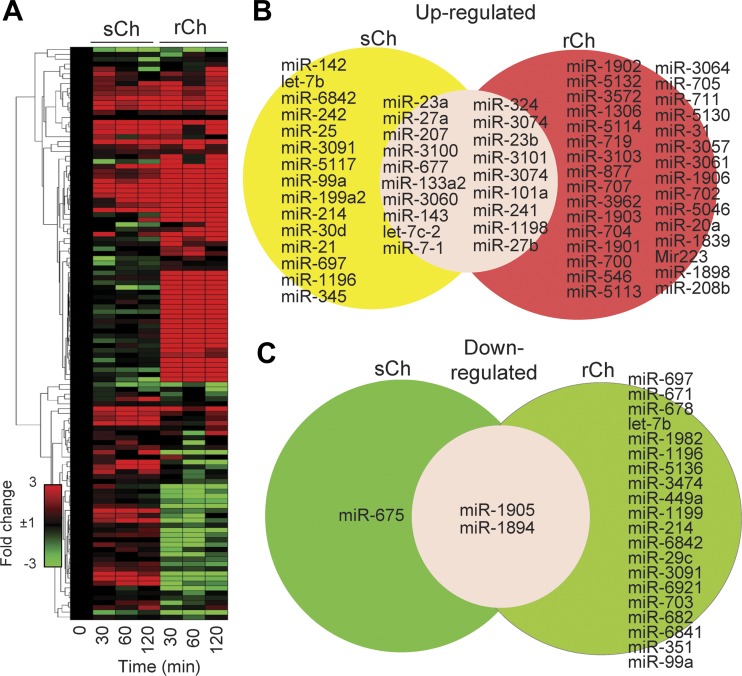
To obtain overall insight into the mechanism by which the activation of the OGG1 pathway could be implicated in allergic inflammatory responses, we mimicked OGG1-BER (the by-product of which is free 8-oxoG base) by directly instilling 8-oxoG into the lungs of OGG1P mice. We administered 0.00005 mg/kg 8-oxoG once or repeatedly [3 times; 48-h intervals (3)]. Single 8-oxoG challenge (sCh; serving as a control) was used to mimic the impact of the OGG1 pathway immediately after RWPENOX challenge (10, 11). Repeated challenges (rCh) were intended to mimic prolonged OGG1 signaling, which may occur under chronic oxidative stress induced by pathophysiological immune and tissue responses upon allergen exposures (10, 37). The 8-oxoG base is rapidly taken up by cells and bound by cytosolic OGG1. The formed complex then activates small GTPases and downstream signaling (1, 15, 32). To assess cellular responses to 8-oxoG challenge, lungs were harvested and total RNAs were isolated at 0, 30, 60, and 120 min in sCh and after the last challenge in an rCh model. Changes at RNA levels were determined by unbiased RNA sequencing (see materials and methods). Analysis of RNA-sequencing data showed differential expression of 1,592 and 2,080 coding mRNAs after sCh and rCh, respectively (1, 3, 8). Among noncoding transcripts, 118 differentially expressed miRNAs were identified. Fold changes in the levels of each miRNA as a function of time and their database ID number are summarized in Supplemental Material, Supplemental Table S1 (Supplemental Material for this article is available online from the Journal website). Levels/expression of 84 of 118 miRNAs were significantly (>3-fold) changed (Fig. 2A). rCh challenges modulated the levels of 73 miRNAs, of which 51 were upregulated (Fig. 2B) and 22 were downregulated (Fig. 2C). After sCh, the levels of most miRNAs were increased (22 miRNAs, of which 10 overlapped with those enhanced by rCh), while 3 were decreased (Fig. 2, A–C). These data suggest that activation of OGG1 signaling in lungs modulates the levels of miRNAs and that robust allergic inflammation in OGG1P mice may be due to the changes in miRNA expression/levels.
Fig. 2.
Differential expression of miRNAs in lungs induced by 8-oxoG, the product of OGG1-BER. A: hierarchical clustering of miRNAs. The heat map was generated using the GENE-E matrix visualization and analysis platform. B: list of miRNAs upregulated after single and repeated challenges of airways. C: list of downregulated (>50%) miRNAs after single (sCh) and repeated challenges (rCh) of lungs.
Replenishment of miRNAs downregulated by OGG1 signaling decreases allergic inflammation.
Taking into account the roles of miRNAs in gene expression at both posttranscriptional and translational levels (7), we used the miRNA database and GeneCards (see materials and methods) to select potential allergic inflammation-relevant miRNAs among those most significantly altered by OGG1 signaling. These included let-7b, which was upregulated (6.4-fold) by sCh but downregulated (8.5-fold, nearly 90%) compared with unchallenged controls after rCh. In silico analysis showed that let-7b has 56 allergy-relevant targets including mRNAs of Il4, Il5, Il13, and KRas. After challenge, miR-23a and miR-27a were upregulated in lungs by both sCh (21.7-fold) and rCh (9.9-fold) and had 29 and 34 targets, respectively (Supplemental Table S2). miR-23a and miR-27a are potentially implicated in the modulation of allergic immune responses via the regulation of T cell functions (22). To provide evidence that the OGG1 signaling-induced miRNAs (Fig. 2) play a role, we determined whether their replenishment by synthetic mimics (and inhibitors) modulated RWPENOX-induced recruitment of eosinophils into the lungs of OGG1P mice.
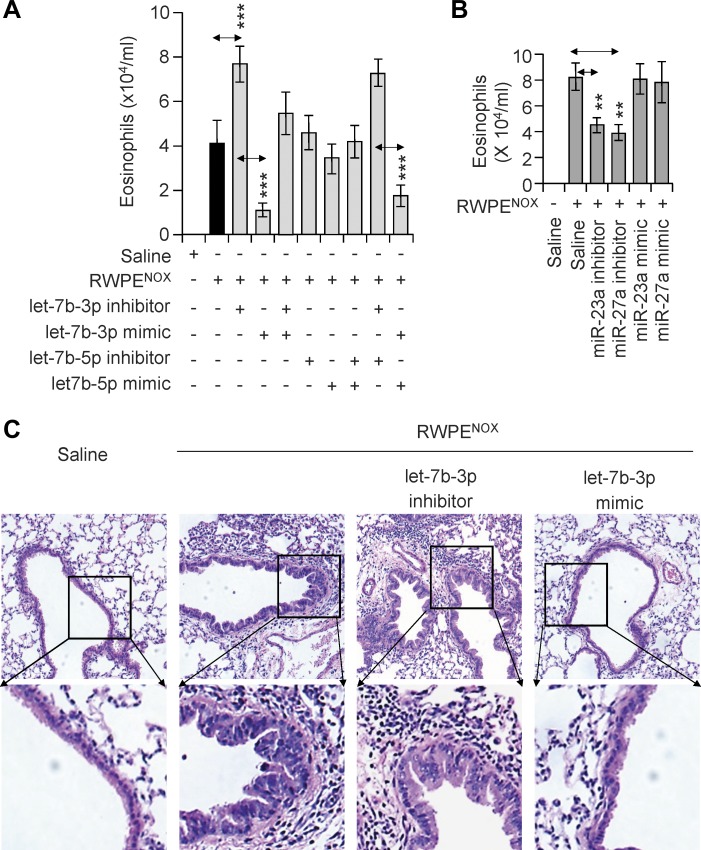
One hour before RWPENOX challenge, miRNA mimics and inhibitors of let7b-3p, let7b-5p, miR-23a, and miR-27a were individually introduced into the lungs of parallel groups of sensitized OGG1P mice. An additional dose was applied at 35-h postchallenge (see materials and methods). These time points were selected on the basis of our previous studies that examined kinetics changes in the levels of IL-4, IL-5, and IL-13 in BALF after RWPENOX exposure (10). Control groups of mice received RWPENOX or solvent (saline). At 72-h postexposure, the lungs were lavaged, and the numbers of eosinophils were determined. The let-7b-3p mimic significantly (P < 0.001) decreased the number of eosinophils in BALF (Fig. 3A), while its inhibitor increased (P < 0.01) their counts. When the let-7b-3p mimic and its inhibitor were administered together, there was no significant effect. Interestingly, neither the let-7b-5p mimic nor its inhibitor significantly changed the number of eosinophils in BALF induced by RWPENOX (Fig. 3A). Mimics of miR-23a and miR-27a had no effect; however, their inhibitors decreased (P < 0.01) the RWPENOX-induced accumulation of eosinophils (Fig. 3B). The addition of miRNA mimics or inhibitors alone to sensitized mice induced insignificant levels of neutrophilia (determined at 16-h postchallenge; data not shown). In support of the impact of let-7b-3p on RWPENOX-induced BALF eosinophil counts, we show that administration of the let-7b-3p inhibitor increased, while its mimetic decreased, RWPENOX-induced tissue infiltration of eosinophils compared with RWPENOX challenge alone (Fig. 3C). Although miR-23a and miR-27a inhibitors decreased the RWPENOX-induced accumulation of eosinophils in BALF, there were no significant observable differences in the levels of tissue infiltration (data not shown). These data together suggest that the recruitment of eosinophils into BALF and lung tissues of OGG1P mice was due to a significant degree to the downregulation of let-7b by OGG1 signaling.
Fig. 3.
Modulation of RWPE-induced accumulation of eosinophils by miRNA mimics and inhibitors in sensitized OGG1P mice. A and B: effect of miRNA mimics or inhibitors on RWPENOX-induced eosinophilia. Individual miRNAs (let-7b-3p, let-7b-5p, miR-23a, or miR-27a) were introduced into lungs 1 h before and 35 h after RWPENOX challenge in sensitized mice. Differential cell counts in BALF were determined at 72-h postchallenge with RWPENOX. C: let-7b-3p mimic decreased RWPENOX-induced eosinophil accumulation. Accumulation of inflammatory cells (eosinophils) in peribronchial regions of RWPENOX-challenged lungs (hematoxylin and eosin staining of lung sections) is shown. The let-7b-3p inhibitor increased, while its mimic decreased, the density of eosinophils in peribronchial regions of the lungs. Panels at top, magnification ×20; panels at bottom, magnification ×60. Histological images show representative staining of six sections per mice for each treatment. Data are presented as means ± SE of six mice. **P < 0.01, ***P < 0.001.
OGG1 signaling increases Th2 cytokine levels via the downregulation of let-7b-3p.
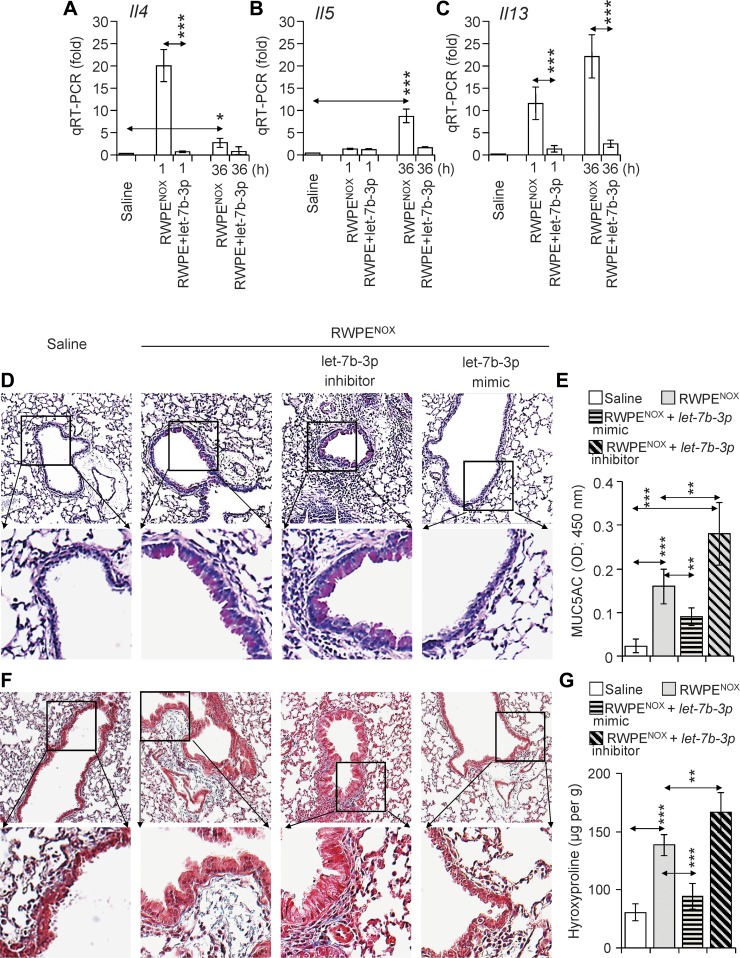
Integrated signals generated by IL-13, IL-4, and IL-5 are primarily responsible for immune and histopathological changes documented in airways exposed to allergens (14, 64). The let-7b-3p mimic significantly decreased the number of eosinophils in the lungs (Fig. 3), which may be due to decreased levels of its target mRNAs including Il4, Il5, and Il13. Notably, let-7b-3p also targeted mRNA of KRas, which encodes for a small GTPase and is directly activated by the OGG1-8-oxoG complex (1). To determine whether the let7b-3p mimic alters the mRNA levels of Il4, Il5, and Il13, mice were RWPENOX challenged with and without instillation of the let-7b-3p mimic, and total RNAs were isolated. The transcript levels of Il4, Il5, and Il13 were determined by qRT-PCR. RWPENOX challenge alone induced a rapid (1 h) increase in the transcript levels of Il4 and Il13. At 36-h postchallenge with RWPENOX, the expression levels of Il4 showed only a modest increase (>3-fold, Fig. 4A), while Il5 (9.8 ± 2.5-fold) and Il13 (>22 ± 9.5-fold) were significantly elevated (Fig. 4, B and C). The addition of let-7b-3p mimic significantly (P < 0.001) decreased the RWPENOX-induced transcript levels of Il4, Il5, and Il13, in line with its predicted targets (Supplemental Table S2 and Figs. 3 and 4). These results suggest that OGG1 signaling increased the inflammatory response via the downregulation of miRNA let-7b expression in the lungs of RWPENOX-challenged sensitized mice.
Fig. 4.
Replenishment of miRNA let-7b-3p decreases the RWPENOX-induced expression of Th2 cytokines and histological changes. A: let-7b-3p mimic decreases mRNA levels of Il4, Il5, and Il13. The let-7b-3p mimic or its inhibitor were instilled 1 h before and 35 h after RWPENOX challenge. Mice were euthanized 1 h after miRNA addition, and total RNAs were isolated. One microgram of reverse-transcribed RNA from individual lungs was subjected to qRT-PCR analysis (see materials and methods) for the expression of Il4 (A), Il5 (B), and Il13 (C). D and E: let-7b-3p mimic decreased the mucus levels induced by RWPENOX challenge. The let-7b-3p inhibitor increased, while its mimic decreased, the density of magenta-colored lung epithelial cells (D). Changes in periodic acid-Schiff staining are consistent with MUC5A levels in BALF (E). F and G: let-7b-3p mimic decreased the density of collagen deposition induced by RWPENOX in OGG1P mice (F). Lungs were subjected to acid hydrolysis, and the resulting hydroxyproline contents (G) were determined as described in materials and methods. A–C, E, and G: data are presented as means ± SE of five mice. *P < 0.05, **P < 0.01, ***P < 0.001. D and F: histological images show representative staining of six sections per mouse for each treatment.
IL-4 and IL-13 are documented to be pleiotropic cytokines playing a central role in the pathogenesis of asthma including increases in goblet cell differentiation (23). IL-4 and IL-13 also induce the expression of transforming growth factor-α (TGF-α) by mucosal epithelial cells and via autocrine signaling, resulting in mucous metaplasia (36); the latter is a characteristic of RWPE-induced allergic tissue responses (14). To investigate the effects of the let-7b-3p mimic on RWPENOX-induced mucus production, lung sections were examined for neutral glycoprotein (mucus)-producing cells using periodic acid-Schiff (PAS) stain, and MUC5A was also determined in BALF. The results summarized in Fig. 4D show excessive PAS staining in RWPENOX and let-7b-3p inhibitor+RWPENOX-challenged lungs (Fig. 4D, panels at left middle and right middle), while let-7b-3p mimic plus RWPENOX challenge significantly decreased PAS staining (Fig. 4D, panels at far right) and the levels of MUC5A in BALF (Fig. 4E). These results together imply that the activation of OGG1 signaling via the downregulation of let-7b-3p exacerbates mucous cell metaplasia and the consequent oversecretion of mucus, which are key features of both acute and chronic allergic lung inflammation in asthma (35).
Collagen deposition is a hallmark of asthma in animal models and in asthmatic patients (30, 36, 39, 50). Our in silico analysis revealed that let-7b-3p targets both transforming growth factor-β receptor 1 (Tgfbr1) and transforming growth factor-β (Tgfb), which are documented to be involved in collagen expression (61). To determine whether OGG1 signaling upregulates TGF-β-driven collagen production, lung sections were Masson's trichrome stained. Compared with control (saline challenge), RWPENOX challenge induced extensive collagen depositions in subepithelial regions and alveolar parenchyma (Fig. 4F, panels at far left and left middle). The inhibitor of let-7b-3p further increased collagen deposition, while let-7b-3p mimic administration decreased the levels of collagen deposition induced by RWPENOX nearly to those observed in saline-challenged lungs (Fig. 4F, panels at far left and far right). To support the histological observations, hydroxyproline levels were determined in the total lung extracts. The lungs of the saline-treated mice had 61 ± 14.4 μg/g collagen content as assessed by levels of hydroxyproline. RWPENOX challenge of lungs resulted in 177 ± 17.9 μg/g hydroxyproline (collagen) content. The instillation of let-7b-3p inhibitor to RWPENOX-challenged lungs further increased (233 ± 17.9 μg/g) hydroxyproline levels (Fig. 4G). Lung extracts from let-7b-3p mimic plus RWPENOX challenge showed significantly decreased levels of hydroxyproline (89 ± 22 μg/g) compared with those challenged only with RWPENOX. Changes in hydroxyproline (collagen) levels support the histological observations and suggest that OGG1 signaling increases collagen levels via let-7b depletion in RWPE-induced allergic airway inflammation.
DISCUSSION
Allergic immune and tissue responses are manifested via a highly complex interplay between dysregulated airway epithelial cells, mast cells, basophils, dendritic cells, B and T cells, neutrophils, and eosinophils, involving a multitude of soluble mediators and ROS (19, 55). Upon exposure to allergen-containing entities, e.g., pollens, molds, pet dander, and others, ROS levels are increased in all cell types, where they function as signaling entities and oxidatively modify various macromolecules (24). Most ROS-modified molecules are degraded; however, oxidatively damaged DNA is repaired to maintain genomic integrity (28, 49). Here, for the first time, we document that repair of the most abundant DNA base lesion, 8-oxoG, by OGG1 and downstream signaling exacerbate RWPENOX-induced allergic inflammation via modulating levels of miRNAs. To prove this hypothesis, the administration of miRNA mimics or inhibitors modulated by OGG1 signaling significantly modulated RWPENOX-induced allergic immune and histological changes. These results suggest that the pharmacological modulation of OGG1 signaling or the topical administration of specific miRNAs could have clinical utility.
ROS generated immediately after RWPENOX challenge have been shown to be key in the exacerbation of allergic immune and tissue responses (11–14). Accordingly, RWPEHI (lacking NADPH oxidase activity) induced significantly less inflammation compared with RWPENOX. Intriguingly, the addition of a physiologically relevant dose of 8-oxoG base together with RWPEHI increased inflammation to the level induced after RWPENOX challenge and allows us to propose that OGG1-BER plays a key role in allergic immune responses. Although the exact mechanism is yet to be determined, these observations may mean that downstream signaling from OGG1-BER, including the activation of small GTPases (1, 15), exacerbates antigen-induced allergic inflammation. These results also raise the possibility that increased 8-oxoG base levels in the serum, sputum, and BALF of allergic human subjects could have pathological relevance, although it is traditionally considered a biomarker of oxidative stress and diseases processes (8, 57, 58).
To obtain insight into the etiological role of OGG1-8-oxoG signaling in allergic immune and tissue responses and to identify the potential mechanism, we utilized sensitized mice lacking OGG1 expression in lungs (1, 10). The rationale for topical OGG1 silencing is that Ogg1−/− mice cannot be efficiently sensitized: they generate significantly less (~10-fold) allergen-specific IgE antibodies compared with those of their wild-type counterparts. These observations are in line with OGG1’s role in regulating both innate and adaptive immune responses (1, 4, 8, 10). To test the potential role of OGG1 signaling, mouse lungs were challenged repeatedly with 8-oxoG to mimic reoccurring oxidative DNA damage repair under chronic inflammatory conditions such as those that occur in asthma (51). A system-level analysis of the entire transcriptome identified differentially modulated coding RNAs of which protein products are potentially involved in atopic, nonatopic asthma, mucus production, and airway hyperresponsiveness (3, 4, 8, 13). RNA-sequencing analysis also identified a large number of noncoding RNAs including miRNAs. The latter observation implies that complex events induced by allergens in RWPE are exacerbated by the OGG1 pathway, which may involve gene regulation at both the transcriptional and posttranscriptional levels.
Originally discovered in Caenorhabditis elegans, miRNAs are highly conserved classes of small RNAs that regulate gene expression via RNA silencing and decreasing translation efficiency (7). Signaling downstream from OGG1-BER modulated levels of over 80 differentially expressed miRNAs. Several of these target mRNAs encode for proteins implicated in allergic immune and tissue responses (27, 53). On the basis of their potential targets, we selected let-7b (upregulated by sCh but downregulated by rCh), miR-23a, and miR-27a (upregulated by both sCh and rCh). Administration of let-7a-3p significantly decreased RWPENOX-induced allergic airway inflammation and histological changes. These data are in line with those found using the ovalbumin model of allergic airway inflammation (43, 53). Also, let-7b-3p decreased collagen deposition in the subepithelial region and reticular basement membrane, which was documented to occur in asthma (30, 39). Decreased RWPENOX-induced immune and tissue responses by let-7b-3p were due to the downregulation of Il4, Il5, and Il13 expression at the posttranscriptional level. As expected, an inhibitor of let-7b-3p exacerbated RWPENOX-induced allergic responses. We note that let-7b contributes to the regulation of numerous biological processes including innate and adaptive immune responses. For instance, let-7b regulates the expression of the Il6 and Il10 genes upon bacterial and protozoan infection of macrophages or exposure to LPS (5, 27). No significant effect was had by let-7b-5p, which is in line with previous studies showing that when let-7b-5p interacts with its target elements (e.g., 5′ untranslated region of mRNA), it stimulates, rather than inhibits, gene transcription and protein translation (40).
Belonging to the miR-23-27-24 miRNA cluster, miR-23a and miR-27a are involved in a variety of biological processes (22, 63, 65). Although their levels are increased by OGG1 signaling, miR-23a and miR-27a mimics did not alter RWPENOX-induced eosinophilia. Interestingly, these inhibitors decreased the number of eosinophils in BALF. Their role in RWPENOX-induced inflammatory responses warrants future study. We note that miR-27a is an important regulator of inflammatory response in sepsis (63), whereas miR-23a has diverse functions including the regulation of TGF-β-induced epithelial-mesenchymal transition by targeting E-cadherin in lung cells (20). Of note, our primary goal was to prove the role of OGG1 signaling in modulating miRNA levels relevant to allergic airway inflammation; therefore we tested few of these. We note that among the 84 miRNAs altered by OGG1 signaling, there are several that are considered to be disease markers (6), while others are etiologically involved in disease pathogenesis (27, 47, 56).
Questions remain regarding how downstream signaling from OGG1-BER regulates the expression of various miRNA families. miRNA promoters are under positive and negative control of transcription factors (TFs), enhancers, and silencing elements, which are also affected by chromatin modifications, as well as DNA methylation (17, 46). DNA encoding for miRNA is located in intergenic regions, exons, or introns of genes and is primarily transcribed by RNA polymerase II and processed by multiprotein complexes in the nucleus (e.g., Drosha microprocessor complex). It is then exported to the cytoplasm by the exportin-5/Ran-GTP complex, where it is further processed to generate miRNAs [review by Breving and Esquela-Kerscher (17)]. TFs that regulate miRNA transcription largely overlap with those that control protein-coding genes, e.g., NF-κB, tumor protein-53 (TP53), v-Myc avian myelocytomatosis viral oncogene homolog (MYC), specificity protein-1 (SP1), early growth response protein-1 (EGR1), Kruppel-like factor 4 (KLF4), and repressor element-1 (RE1)-silencing transcription factor (a KLF family protein that is associated with promoter regions), controlling miRNAs (42, 59). Intronic promoters also contain binding sites for TFs, such as forkhead box protein-A1, -A2, and -A3, GATA-binding protein-1, and forkhead-related transcription factor 2 (17, 42, 59). Although we did not perform mechanistic studies, our previous Ingenuity Pathways Knowledge Base analysis of microarray (Affymetrix GeneChip) data showed that in airway epithelial cells, (A549) OGG1-Ras signaling modulated networks regulated by TFs, including NF-κB, TP53, KLF4, MYC, and others (31). Thus we speculate that OGG1-BER-small GTPases {[Harvey-Ras (H-Ras), K-Ras, and neuroblastoma-Ras (N-Ras)] RAF1-MEK1/2, ERK1/2, and phospho-PI3K (p85)} and the consequent activation of TFs and/or OGG1-mediated facilitation of TF binding (1, 9, 15, 32, 52) could be involved in modulating miRNA expression.
In conclusion, allergic asthma is a complex disorder of the airways involving chronic inflammation, decline in airway function, and tissue remodeling. Disease severity is linked to genetic susceptibility and coexposures to environmental agents including gases, particles, or infection. Although many pathways are identified, the overarching mechanism common to all asthma phenotypes is not well understood. Oxidative stress and oxidatively modified DNA bases and strands are common and represent the earliest signs of allergen exposure and immune reactions; their levels and quality are biomarkers of allergic diseases and are well documented. The most referenced biomarker of allergic immune responses is genomic 8-oxoG, which is primarily repaired by OGG1 and, in complex with its repair by-product, activates small GTPases. Here, we are the first to demonstrate that OGG1-driven cell signaling exacerbates pollen antigen-induced allergic inflammation via the downregulation of specific miRNAs’ expression/levels, which is targeted against mRNA coding for Th2 cytokines as well as signal generators, such as the small GTPase K-Ras. These results and those from previous studies support the notion that cell signaling downstream from OGG1-BER leads to multilayered transcriptional and posttranscriptional control over immune and tissue responses. The results are in line with decreased allergic responses in Ogg1 knockout mice, which lack both OGG1 activity and the BER product 8-oxoG. Thus one may propose that the topical modulation of OGG1’s activity by small-molecule inhibitors or the application of a miRNA mimetic could have clinical utility in the treatment of pollen allergen-driven diseases.
GRANTS
This work was supported by National Institute of Environmental Health Sciences (NIEHS) Grant 18948 (I. Balogh), National Institute of Allergy and Infectious Diseases Grant 62885 (I. Balogh and A. R. Brasier), Hungarian Scientific Research Fund Grant K-109595 (A. Bacsi); National Natural Science Foundation of China Grants 31000575 (X. Li) and 31571339 (X. Ba), NIEHS Center Grant 6676 (I. Balogh and A. R. Brasier), and NIEHS Grant 7254-22 (L. Aguilera-Aguirre).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.A.-A., X.B., and I.B. conceived and designed research; L.A.-A., W.H., L.P., and A.B. performed experiments; L.A.-A., W.H., L.P., and A.B. analyzed data; L.A.-A., W.H., L.P., X.L., A.S.-M., A.B., Z.R., S.S., A.R.B., X.B., and I.B. interpreted results of experiments; L.A.-A., W.H., and L.P. prepared figures; L.A.-A. and I.B. drafted manuscript; L.A.-A., W.H., L.P., X.L., A.S.-M., A.B., Z.R., S.S., A.R.B., X.B., and I.B. edited and revised manuscript; L.A.-A., W.H., L.P., X.L., A.S.-M., A.B., Z.R., S.S., A.R.B., X.B., and I.B. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Thomas C. Hardy (editor in chief) and editors at English Editing Services, Medical Journal Editors (http://www.medicaljournaleditors.com/), for their scientific input and for critically editing the manuscript.
Present addresses: X. Ba, W. Hao, X. Li, and L. Pan, The Key Laboratory of Molecular Epigenetics, Institute of Genetics and Cytology, Northeast Normal Univ., 5268 Renmin St., Changchun 130024, Jilin, China; A. Bacsi, Dept. of Immunology, School of Medicine, Univ. of Debrecen, Egyetem tér 1, Debrecen, Hungary H-4032; Z. Radak, Research Institute of Sport Science, Univ. of Physical Education, Alkotás u. 44, Budapest, Hungary H-1123; and A. Saavedra-Molina, Instituto de Investigaciones Químico-Biológicas, Univ. Michoacana de San Nicolás de Hidalgo, Av. Universidad s/n, Edif. B-3, Ciudad Universitaria, Morelia, Michoacan, Mexico.
REFERENCES
- 1.Aguilera-Aguirre L, Bacsi A, Radak Z, Hazra TK, Mitra S, Sur S, Brasier AR, Ba X, Boldogh I. Innate inflammation induced by the 8-oxoguanine DNA glycosylase-1-KRAS-NF-κB pathway. J Immunol 193: 4643–4653, 2014. doi: 10.4049/jimmunol.1401625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilera-Aguirre L, Bacsi A, Saavedra-Molina A, Kurosky A, Sur S, Boldogh I. Mitochondrial dysfunction increases allergic airway inflammation. J Immunol 183: 5379–5387, 2009. doi: 10.4049/jimmunol.0900228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguilera-Aguirre L, Hosoki K, Bacsi A, Radák Z, Sur S, Hegde ML, Tian B, Saavedra-Molina A, Brasier AR, Ba X, Boldogh I. Whole transcriptome analysis reveals a role for OGG1-initiated DNA repair signaling in airway remodeling. Free Radic Biol Med 89: 20–33, 2015. doi: 10.1016/j.freeradbiomed.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aguilera-Aguirre L, Hosoki K, Bacsi A, Radák Z, Wood TG, Widen SG, Sur S, Ameredes BT, Saavedra-Molina A, Brasier AR, Ba X, Boldogh I. Whole transcriptome analysis reveals an 8-oxoguanine DNA glycosylase-1-driven DNA repair-dependent gene expression linked to essential biological processes. Free Radic Biol Med 81: 107–118, 2015. doi: 10.1016/j.freeradbiomed.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed W, Zheng K, Liu ZF. Small non-coding RNAs: new insights in modulation of host immune response by intracellular bacterial pathogens. Front Immunol 7: 431, 2016. doi: 10.3389/fimmu.2016.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alipoor SD, Adcock IM, Garssen J, Mortaz E, Varahram M, Mirsaeidi M, Velayati A. The roles of miRNAs as potential biomarkers in lung diseases. Eur J Pharmacol 791: 395–404, 2016. doi: 10.1016/j.ejphar.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson SD, Kippelen P. Airway injury as a mechanism for exercise-induced bronchoconstriction in elite athletes. J Allergy Clin Immunol 122: 225–235, 2008. doi: 10.1016/j.jaci.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Ba X, Aguilera-Aguirre L, Sur S, Boldogh I. 8-Oxoguanine DNA glycosylase-1-driven DNA base excision repair: role in asthma pathogenesis. Curr Opin Allergy Clin Immunol 15: 89–97, 2015. doi: 10.1097/ACI.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ba X, Bacsi A, Luo J, Aguilera-Aguirre L, Zeng X, Radak Z, Brasier AR, Boldogh I. 8-Oxoguanine DNA glycosylase-1 augments proinflammatory gene expression by facilitating the recruitment of site-specific transcription factors. J Immunol 192: 2384–2394, 2014. doi: 10.4049/jimmunol.1302472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bacsi A, Aguilera-Aguirre L, Szczesny B, Radak Z, Hazra TK, Sur S, Ba X, Boldogh I. Down-regulation of 8-oxoguanine DNA glycosylase 1 expression in the airway epithelium ameliorates allergic lung inflammation. DNA Repair (Amst) 12: 18–26, 2013. doi: 10.1016/j.dnarep.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacsi A, Choudhury BK, Dharajiya N, Sur S, Boldogh I. Subpollen particles: carriers of allergenic proteins and oxidases. J Allergy Clin Immunol 118: 844–850, 2006. doi: 10.1016/j.jaci.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bacsi A, Dharajiya N, Choudhury BK, Sur S, Boldogh I. Effect of pollen-mediated oxidative stress on immediate hypersensitivity reactions and late-phase inflammation in allergic conjunctivitis. J Allergy Clin Immunol 116: 836–843, 2005. doi: 10.1016/j.jaci.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bacsi A, Pan L, Ba X, Boldogh I. Pathophysiology of bronchoconstriction: role of oxidatively damaged DNA repair. Curr Opin Allergy Clin Immunol 16: 59–67, 2016. doi: 10.1097/ACI.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boldogh I, Bacsi A, Choudhury BK, Dharajiya N, Alam R, Hazra TK, Mitra S, Goldblum RM, Sur S. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J Clin Invest 115: 2169–2179, 2005. doi: 10.1172/JCI24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boldogh I, Hajas G, Aguilera-Aguirre L, Hegde ML, Radak Z, Bacsi A, Sur S, Hazra TK, Mitra S. Activation of ras signaling pathway by 8-oxoguanine DNA glycosylase bound to its excision product, 8-oxoguanine. J Biol Chem 287: 20769–20773, 2012. doi: 10.1074/jbc.C112.364620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brasier AR. The NF-κB regulatory network. Cardiovasc Toxicol 6: 111–130, 2006. doi: 10.1385/CT:6:2:111. [DOI] [PubMed] [Google Scholar]
- 17.Breving K, Esquela-Kerscher A. The complexities of microRNA regulation: mirandering around the rules. Int J Biochem Cell Biol 42: 1316–1329, 2010. doi: 10.1016/j.biocel.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Bruyand M, Ryom L, Shepherd L, Fatkenheuer G, Grulich A, Reiss P, de Wit S, D Arminio Monforte A, Furrer H, Pradier C, Lundgren J, Sabin C; D:A:D Study Group . Cancer risk and use of protease inhibitor or nonnucleoside reverse transcriptase inhibitor-based combination antiretroviral therapy: the D:A:D study. J Acquir Immune Defic Syndr 68: 568–577, 2015. doi: 10.1097/QAI.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 19.Calhoun WJ, Reed HE, Moest DR, Stevens CA. Enhanced superoxide production by alveolar macrophages and air-space cells, airway inflammation, and alveolar macrophage density changes after segmental antigen bronchoprovocation in allergic subjects. Am Rev Respir Dis 145: 317–325, 1992. doi: 10.1164/ajrccm/145.2_Pt_1.317. [DOI] [PubMed] [Google Scholar]
- 20.Cao M, Seike M, Soeno C, Mizutani H, Kitamura K, Minegishi Y, Noro R, Yoshimura A, Cai L, Gemma A. miR-23a regulates TGF-β-induced epithelial-mesenchymal transition by targeting E-cadherin in lung cancer cells. Int J Oncol 41: 869–875, 2012. doi: 10.3892/ijo.2012.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chatterjee A, Saha S, Chakraborty A, Silva-Fernandes A, Mandal SM, Neves-Carvalho A, Liu Y, Pandita RK, Hegde ML, Hegde PM, Boldogh I, Ashizawa T, Koeppen AH, Pandita TK, Maciel P, Sarkar PS, Hazra TK. The role of the mammalian DNA end-processing enzyme polynucleotide kinase 3′-phosphatase in spinocerebellar ataxia type 3 pathogenesis. PLoS Genet 11: e1004749, 2015. doi: 10.1371/journal.pgen.1004749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho S, Wu CJ, Yasuda T, Cruz LO, Khan AA, Lin LL, Nguyen DT, Miller M, Lee HM, Kuo ML, Broide DH, Rajewsky K, Rudensky AY, Lu LF. miR-23~27~24 clusters control effector T cell differentiation and function. J Exp Med 213: 235–249, 2016. doi: 10.1084/jem.20150990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corren J. Role of interleukin-13 in asthma. Curr Allergy Asthma Rep 13: 415–420, 2013. doi: 10.1007/s11882-013-0373-9. [DOI] [PubMed] [Google Scholar]
- 24.D’Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 8: 813–824, 2007. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 25.David SS, O’Shea VL, Kundu S. Base-excision repair of oxidative DNA damage. Nature 447: 941–950, 2007. doi: 10.1038/nature05978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dedon PC, DeMott MS, Elmquist CE, Prestwich EG, McFaline JL, Pang B. Challenges in developing DNA and RNA biomarkers of inflammation. Biomarkers Med 1: 293–312, 2007. doi: 10.2217/17520363.1.2.293. [DOI] [PubMed] [Google Scholar]
- 27.Dissanayake E, Inoue Y. MicroRNAs in allergic disease. Curr Allergy Asthma Rep 16: 67, 2016. doi: 10.1007/s11882-016-0648-z. [DOI] [PubMed] [Google Scholar]
- 28.Dizdaroglu M. Base-excision repair of oxidative DNA damage by DNA glycosylases. Mutat Res 591: 45–59, 2005. doi: 10.1016/j.mrfmmm.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 29.Dizdaroglu M. Chemical determination of free radical-induced damage to DNA. Free Radic Biol Med 10: 225–242, 1991. doi: 10.1016/0891-5849(91)90080-M. [DOI] [PubMed] [Google Scholar]
- 30.Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J Clin Invest 104: 1001–1006, 1999. doi: 10.1172/JCI8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.German P, Saenz D, Szaniszlo P, Aguilera-Aguirre L, Pan L, Hegde ML, Bacsi A, Hajas G, Radak Z, Ba X, Mitra S, Papaconstantinou J, Boldogh I. 8-Oxoguanine DNA glycosylase1-driven DNA repair: a paradoxical role in lung aging. Mech Ageing Dev 161, Part A: 51–65, 2017. doi: 10.1016/j.mad.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.German P, Szaniszlo P, Hajas G, Radak Z, Bacsi A, Hazra TK, Hegde ML, Ba X, Boldogh I. Activation of cellular signaling by 8-oxoguanine DNA glycosylase-1-initiated DNA base excision repair. DNA Repair (Amst) 12: 856–863, 2013. doi: 10.1016/j.dnarep.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasbal C, Aksu BY, Himmetoglu S, Dincer Y, Koc EE, Hatipoglu S, Akcay T. DNA damage and glutathione level in children with asthma bronchiale: effect of antiasthmatic therapy. Pediatr Allergy Immunol 21, Part 2: e674–e678, 2010. doi: 10.1111/j.1399-3038.2009.00959.x. [DOI] [PubMed] [Google Scholar]
- 34.Hattori N, Degen JL, Sisson TH, Liu H, Moore BB, Pandrangi RG, Simon RH, Drew AF. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest 106: 1341–1350, 2000. doi: 10.1172/JCI10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holgate ST. Epithelium dysfunction in asthma. J Allergy Clin Immunol 120: 1233–1244, 2007. doi: 10.1016/j.jaci.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 36.Holgate ST. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol Rev 242: 205–219, 2011. doi: 10.1111/j.1600-065X.2011.01030.x. [DOI] [PubMed] [Google Scholar]
- 37.Holgate ST, Roberts G, Arshad HS, Howarth PH, Davies DE. The role of the airway epithelium and its interaction with environmental factors in asthma pathogenesis. Proc Am Thorac Soc 6: 655–659, 2009. doi: 10.1513/pats.200907-072DP. [DOI] [PubMed] [Google Scholar]
- 38.Igishi T, Hitsuda Y, Kato K, Sako T, Burioka N, Yasuda K, Sano H, Shigeoka Y, Nakanishi H, Shimizu E. Elevated urinary 8-hydroxydeoxyguanosine, a biomarker of oxidative stress, and lack of association with antioxidant vitamins in chronic obstructive pulmonary disease. Respirology 8: 455–460, 2003. doi: 10.1046/j.1440-1843.2003.00490.x. [DOI] [PubMed] [Google Scholar]
- 39.Ijaz T, Pazdrak K, Kalita M, Konig R, Choudhary S, Tian B, Boldogh I, Brasier AR. Systems biology approaches to understanding epithelial mesenchymal transition (EMT) in mucosal remodeling and signaling in asthma. World Allergy Organ J 7: 13, 2014. doi: 10.1186/1939-4551-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janowski BA, Younger ST, Hardy DB, Ram R, Huffman KE, Corey DR. Activating gene expression in mammalian cells with promoter-targeted duplex RNAs. Nat Chem Biol 3: 166–173, 2007. doi: 10.1038/nchembio860. [DOI] [PubMed] [Google Scholar]
- 41.Klungland A, Rosewell I, Hollenbach S, Larsen E, Daly G, Epe B, Seeberg E, Lindahl T, Barnes DE. Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc Natl Acad Sci USA 96: 13300–13305, 1999. doi: 10.1073/pnas.96.23.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 11: 597–610, 2010. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 43.Kumar M, Ahmad T, Sharma A, Mabalirajan U, Kulshreshtha A, Agrawal A, Ghosh B. let-7 microRNA-mediated regulation of IL-13 and allergic airway inflammation. J Allergy Clin Immunol 128: 1077–1085.e10, 2011. doi: 10.1016/j.jaci.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 44.Li G, Yuan K, Yan C, Fox J III, Gaid M, Breitwieser W, Bansal AK, Zeng H, Gao H, Wu M. 8-Oxoguanine-DNA glycosylase 1 deficiency modifies allergic airway inflammation by regulating STAT6 and IL-4 in cells and in mice. Free Radic Biol Med 52: 392–401, 2012. doi: 10.1016/j.freeradbiomed.2011.10.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mabley JG, Pacher P, Deb A, Wallace R, Elder RH, Szabó C. Potential role for 8-oxoguanine DNA glycosylase in regulating inflammation. FASEB J 19: 290–292, 2005. doi: 10.1096/fj.04-2278fje. [DOI] [PubMed] [Google Scholar]
- 46.Mansfield JH, Harfe BD, Nissen R, Obenauer J, Srineel J, Chaudhuri A, Farzan-Kashani R, Zuker M, Pasquinelli AE, Ruvkun G, Sharp PA, Tabin CJ, McManus MT. MicroRNA-responsive ‘sensor’ transgenes uncover Hox-like and other developmentally regulated patterns of vertebrate microRNA expression. Nat Genet 36: 1079–1083, 2004. doi: 10.1038/ng1421. [DOI] [PubMed] [Google Scholar]
- 47.Mattes J, Collison A, Plank M, Phipps S, Foster PS. Antagonism of microRNA-126 suppresses the effector function of TH2 cells and the development of allergic airways disease. Proc Natl Acad Sci USA 106: 18704–18709, 2009. doi: 10.1073/pnas.0905063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minowa O, Arai T, Hirano M, Monden Y, Nakai S, Fukuda M, Itoh M, Takano H, Hippou Y, Aburatani H, Masumura K, Nohmi T, Nishimura S, Noda T. Mmh/Ogg1 gene inactivation results in accumulation of 8-hydroxyguanine in mice. Proc Natl Acad Sci USA 97: 4156–4161, 2000. doi: 10.1073/pnas.050404497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitra S, Hazra TK, Roy R, Ikeda S, Biswas T, Lock J, Boldogh I, Izumi T. Complexities of DNA base excision repair in mammalian cells. Mol Cells 7: 305–312, 1997. [PubMed] [Google Scholar]
- 50.Mookerjee I, Solly NR, Royce SG, Tregear GW, Samuel CS, Tang ML. Endogenous relaxin regulates collagen deposition in an animal model of allergic airway disease. Endocrinology 147: 754–761, 2006. doi: 10.1210/en.2005-1006. [DOI] [PubMed] [Google Scholar]
- 51.Murdoch JR, Lloyd CM. Chronic inflammation and asthma. Mutat Res 690: 24–39, 2010. doi: 10.1016/j.mrfmmm.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan L, Zhu B, Hao W, Zeng X, Vlahopoulos SA, Hazra TK, Hegde ML, Radak Z, Bacsi A, Brasier AR, Ba X, Boldogh I. Oxidized guanine base lesions function in 8-oxoguanine DNA glycosylase-1-mediated epigenetic regulation of nuclear factor κB-driven gene expression. J Biol Chem 291: 25553–25566, 2016. doi: 10.1074/jbc.M116.751453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polikepahad S, Knight JM, Naghavi AO, Oplt T, Creighton CJ, Shaw C, Benham AL, Kim J, Soibam B, Harris RA, Coarfa C, Zariff A, Milosavljevic A, Batts LM, Kheradmand F, Gunaratne PH, Corry DB. Proinflammatory role for let-7 microRNAs in experimental asthma. J Biol Chem 285: 30139–30149, 2010. doi: 10.1074/jbc.M110.145698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Proklou A, Soulitzis N, Neofytou E, Rovina N, Zervas E, Gaga M, Siafakas NM, Tzortzaki EG. Granule cytotoxic activity and oxidative DNA damage in smoking and nonsmoking patients with asthma. Chest 144: 1230–1237, 2013. doi: 10.1378/chest.13-0367. [DOI] [PubMed] [Google Scholar]
- 55.Sahiner UM, Birben E, Erzurum S, Sackesen C, Kalayci O. Oxidative stress in asthma. World Allergy Organ J 4: 151–158, 2011. doi: 10.1097/WOX.0b013e318232389e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shaoqing Y, Ruxin Z, Guojun L, Zhiqiang Y, Hua H, Shudong Y, Jie Z. Microarray analysis of differentially expressed microRNAs in allergic rhinitis. Am J Rhinol Allergy 25: e242–e246, 2011. doi: 10.2500/ajra.2011.25.3682. [DOI] [PubMed] [Google Scholar]
- 57.Son J, Pang B, McFaline JL, Taghizadeh K, Dedon PC. Surveying the damage: the challenges of developing nucleic acid biomarkers of inflammation. Mol Biosyst 4: 902–908, 2008. doi: 10.1039/b719411k. [DOI] [PubMed] [Google Scholar]
- 58.Suzuki J, Inoue Y, Suzuki S. Changes in the urinary excretion level of 8-hydroxyguanine by exposure to reactive oxygen-generating substances. Free Radic Biol Med 18: 431–436, 1995. doi: 10.1016/0891-5849(94)00152-A. [DOI] [PubMed] [Google Scholar]
- 59.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA 103: 12481–12486, 2006. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tian B, Brasier AR. Identification of a nuclear factor kappa B-dependent gene network. Recent Prog Horm Res 58: 95–130, 2003. doi: 10.1210/rp.58.1.95. [DOI] [PubMed] [Google Scholar]
- 61.Tian B, Patrikeev I, Ochoa L, Vargas G, Belanger KK, Litvinov J, Boldogh I, Ameredes BT, Motamedi M, Brasier AR. NF-κB mediates mesenchymal transition, remodeling, and pulmonary fibrosis in response to chronic inflammation by viral RNA patterns. Am J Respir Cell Mol Biol 56: 506–520, 2017. doi: 10.1165/rcmb.2016-0259OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Touati E, Michel V, Thiberge JM, Avé P, Huerre M, Bourgade F, Klungland A, Labigne A. Deficiency in OGG1 protects against inflammation and mutagenic effects associated with H. pylori infection in mouse. Helicobacter 11: 494–505, 2006. doi: 10.1111/j.1523-5378.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 63.Wang Z, Ruan Z, Mao Y, Dong W, Zhang Y, Yin N, Jiang L. miR-27a is up regulated and promotes inflammatory response in sepsis. Cell Immunol 290: 190–195, 2014. doi: 10.1016/j.cellimm.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 64.Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol 15: 271–282, 2015. doi: 10.1038/nri3831. [DOI] [PubMed] [Google Scholar]
- 65.Yao L, Liu Z, Zhu J, Li B, Chai C, Tian Y. Clinical evaluation of circulating microRNA-25 level change in sepsis and its potential relationship with oxidative stress. Int J Clin Exp Pathol 8: 7675–7684, 2015. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.