Abstract
During the 1918 influenza pandemic, children experienced substantially lower mortality than adults, a striking but unexplained finding. Whether this was due to enhanced resistance (reduced virus load) or better tolerance (reduced impact of infection) has not been defined. We found that prepubertal mice infected with H1N1 influenza virus also showed greater survival than infected pubertal mice, despite similar virus loads. Transcriptome profiling of infected lungs identified estrogen as a regulator of susceptibility in both sexes and also linked better survival to late expression of IL-1β. Blocking puberty with gonadectomy or a gonadotropin-releasing hormone antagonist improved survival. Estrogen or testosterone (which can be converted to estrogen) restored susceptibility of gonadectomized pubertal mice to influenza mortality, but dihydrotestosterone (which cannot be converted to estrogen) did not. Estrogen receptor blockade with fulvestrant in both male and female pubertal mice resulted in improved survival, even when given 3 days after infection. Moreover, late, but not early, IL-1β neutralization after infection was also protective. These findings indicate that pubertal increases in estrogen in both sexes are associated with increased mortality during influenza. This helps explain the reduced mortality of children seen with influenza in 1918 and might also be relevant to childhood tolerance to many other infectious diseases.
Keywords: influenza, age, hormones, immunomodulators
INTRODUCTION
Host response as well as virus strain determine the natural history of influenza (4, 6, 51), but factors that govern the spectrum of disease severity remain incompletely characterized. Transcriptome analysis and “systems biology” have been eagerly embraced (1, 25). They indicate that excessive host inflammatory responses are linked to high mortality (4, 6, 21, 24, 34), and that host-directed immunomodulators could be of benefit (11, 36). However, the hope that an “omics-based, pathway-centered approach” might identify key mediators and promising therapeutic targets has not yet been realized. Comparison of groups that naturally differ in mortality from severe infection might allow progress in this area.
During the 1918 influenza pandemic, children in the age range of 5–14 yr experienced substantially lower mortality than adults, despite similar rates of infection (2). The lower mortality in children compared with adults seen in influenza extends to other infections, such as tuberculosis, measles (2, 8), sepsis (33), and Ebola (40, 53). Milder disease also occurs in weanling vs. adult ferrets with nonlethal influenza (16), but animal models have not been used to directly investigate mortality differences between children and adults. Whether the advantage of infected children is due to enhanced resistance (reduced virus load) or better tolerance (reduced impact of infection) (32) has not been defined. Beyond the truism that it is good to be young, understanding the mechanisms underlying these differences could lead to new insights and therapies for severe influenza and other infections. Epidemiological data in 1918 showed that puberty marked the change to greater mortality, suggesting that onset of sexual maturity could influence susceptibility by changing the host response (2). Hence, we investigated the relative resistance of prepubertal individuals to influenza mortality in a mouse model.
MATERIALS AND METHODS
Animals.
Experiments were approved by the Harvard Medical Area Institutional Animal Care and Use Committee. Male and female C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA) at the prepubertal age of 21 days and were rested in the animal care facility until they reached the desired experimental age, e.g., 25 days (prepubertal) or 28 days (pubertal); in some experiments, older mice were purchased at 35 days of age and were used at 42 days of age.
Reagents.
Agents used to alter hormonal status included the following: leuprolide (Tocris Bioscience), testosterone, dihydrotestosterone, fulvestrant, and anastrazole (Sigma-Aldrich); and estradiol (Cayman Chemical). After being dissolved in ethanol, an emulsion of estradiol and corn oil was created for a final concentration of 10 μg/100 μl, administered intraperitoneally (ip) on days 1–3 or 6–9 of infection. Testosterone and dihydrotestosterone were similarly prepared and administered as 200 μg and 65 μg ip, respectively. Fulvestrant and anastrazole were dissolved in DMSO and delivered in an emulsion of corn oil at 200 μg and 10 μg ip, respectively. Acyline was generously provided by Dr. Min Lee, National Institute of Child Health and Human Development (Rockville, MD). Blocking anti-IL-1β antibody (low endotoxin, azide-free, clone B122) and matching hamster IgG isotype (clone HTK888) were obtained from BioLegend (San Diego, CA).
Influenza model.
A murine-adapted strain of H1N1 influenza virus, A/Puerto Rico/8/1934 (PR8), was obtained initially from Charles River Laboratories [quantified as hemagglutination units (HAU)]; most experiments used the same virus, quantified as plaque-forming units (PFU) from ViraSource (RTP). Mice were anesthetized with 120 mg/kg ketamine plus 16 mg/kg xylazine administered via ip injection. Mice then received an intranasal instillation 50 μl suspension of PBS containing varying amounts of virus (based on HAU or PFU) or vehicle alone. Initial titration identified a dose that led to ~80% mortality in the older (pubertal) mice. The onset of sexual maturation (puberty) in C57Bl/6 mice is approximately postnatal day 27–28 (10). Most experiments were performed with mice at postnatal days 25 (P25, prepubertal) or 28 (P28, pubertal). P25 and P28 female mice were given 87.5 PFUs, and P25 and P28 male mice were give 100 PFUs. For hormonal manipulations to block puberty, leuprolide (25 μg sc every day) or acyline (100 μg sc every 4 days) were given starting at day 21 of age, and this was followed by infection on day 28 of age. The estrogen receptor antagonist fulvestrant was administered (200 μg ip every other day) either starting at age day 21 (pretreatment) followed by infection on day 28 of age, or starting 3 days after infection (posttreatment). In experiments to harvest lung tissue for transcriptome profiling, separate groups of sentinel mice for each age group were followed to monitor the survival rate after infection. In experiments that used older mice (P42), 350 or 250 PFU were administered to male or female mice, respectively. To measure the viral load within infected lung tissue, quantitative PCR assay for influenza M1 mRNA (SA Biosciences, Frederick, MD) was performed using 1 μg total lung RNA per sample with predesigned assays from Applied Biosystems (Foster City, CA) (14). Mice were monitored for morbidity and illness scores daily to determine the need for euthanasia, if found preterminal. Weights were measured on alternate days during the postinfection period. For evaluation of histopathology, a scoring system using the product of a severity score (0–3, based on extent of congestion, edema, infiltration of leukocytes, damage to alveolar architecture) and extent of tissue involvement (0–3, based on involvement of 0, 1–25, 26–50, or >50% of lung tissue sample, respectively).
Transcriptome profiling.
We obtained transcriptome profiles in two tissue types in prepubertal and pubertal mice: blood and lung. Whole blood samples from uninfected prepubertal (P24) or older (P36) mice were analyzed to compare basal gene expression in leukocytes. To evaluate changes during influenza infection, lung tissue was obtained on days 0, 3, 6, and 9 after infection (the onset of mortality occurred around day 8–9). RNA was extracted using Qiagen RNAeasy Micro Kit, following the manufacturer's instructions. For whole blood samples, hemogloblin RNA was depleted using PAXgene Blood RNA system (Qiagen, Valencia, CA). After RNA extraction, all quantitation and microarray experiments were performed at the Dana-Farber Cancer Institute Microarray Core Facility. RNA integrity was analyzed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). RNA purity and concentration were determined using a Nanodrop 8000 (Nanodrop Products, Wilmington, DE). Total RNA from lung homogenates was hybridized to the Affymetrix GeneChip Mouse Gene 2.0 ST array (Affymetrix, Santa Clara, CA), and raw intensity values stored in CEL files were imported into Partek Genomics Suite version 6.6 (PGS) (Partek, St. Louis, MO). Raw values were normalized using the robust multi-array average method with background correction, and the adjusted intensity values were expressed as log2 transformed values. Microarray data sets obtained through this study have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus with accession number GSE99192.
Differentially expressed genes (DEG) and principal component analysis was performed using PGS software, using statistical (P value) and fold-change (FC) settings as reported in results. PGS software also provided the Venn diagram-based analyses of DEGs from different transcriptome profiling studies. For additional analysis to explore biologically significant regulators and networks, we utilized Ingenuity Pathway Analysis (IPA Suite, Ingenuity Systems, Mountain View, CA; https://www.qiagenbioinformatics.com/) as well as the web-based Enrichr suite of analysis tools (http://amp.pharm.mssm.edu/Enrichr/).
Statistics.
Prism software (GraphPad, La Jolla, CA) was used to graph results and for statistical evaluation of data. Group sizes were chosen based on pilot studies. Kaplan-Meier survival curves were compared using the log rank Mantel-Cox test. For comparison of weight loss (or gain), slopes of linear regression curves were evaluated. Other statistical tests used are indicated in the legends of the corresponding figures. P values < 0.05 were considered significant. The number and the type of replicates are indicated in the legends of each figure.
RESULTS
Prepubertal mice experience less influenza morbidity and mortality.
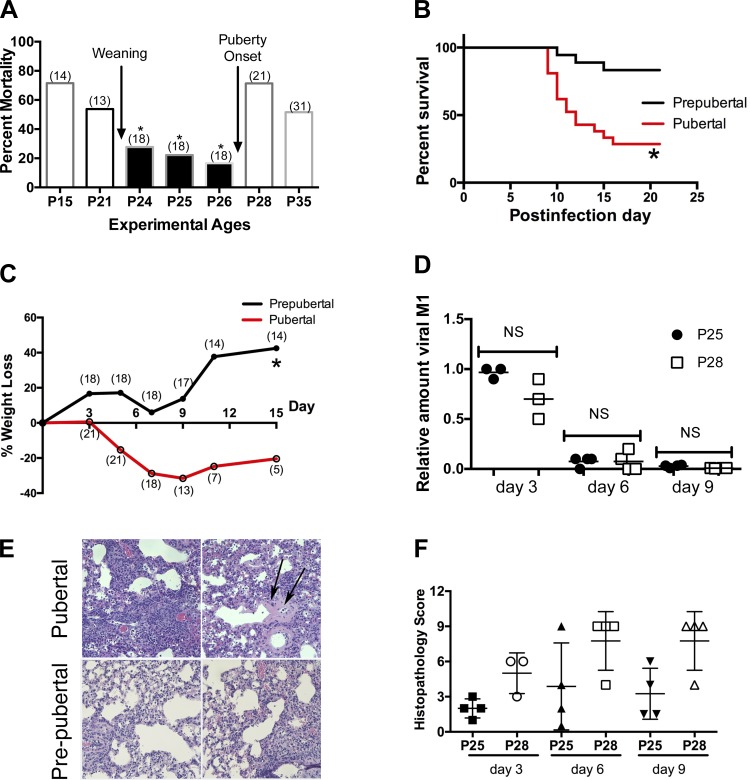
We infected mice with influenza virus at different ages before and after the onset of puberty [~28 days of age, or P28 in C57Bl/6 mice (10)]. Mortality was substantially greater in pubertal mice (infected on P28) (Fig. 1A), with deaths starting at approximately day 9 after infection (Fig. 1B). Mortality was also higher in younger mice before weaning, similar to the higher mortality of infants (ages 0–4 yr) in the 1918 pandemic (2). We focused our investigations on the difference between before and after puberty onset, based on the similarity to the human epidemiology (2). Pubertal mice lost more weight (Fig. 1C), and their lungs showed greater inflammation and damage, including hyaline membranes (Fig. 1, E, top, and F, scoring). Both ages were infected with the same amount of virus (by sex), so the slightly smaller prepubertal animals actually received more virus per body weight, yet virus loads in the lungs of prepubertal and pubertal mice on days 3, 6, and 9 after infection were similar (Fig. 1D).
Fig. 1.
Prepubertal mice experience less influenza morbidity and mortality. A: after intranasal inoculation (i.n.) with PR8 H1N1 virus (1HAU), prepubertal animals (postnatal days P24–P26) experience lower mortality (summary of 3 trials monitored until 21 days after infection; no. of mice/group is shown in parentheses). All survival studies were compared by the Mantel-Cox log-rank test (*P ≤ 0.01 for P24, P25, and P26 vs. P28 compared individually; P ≤ 0.01 when pool of P24–P26 compared with P28 or P35). B and C: survival curve (B) and weight loss (C) comparison between P26 (prepubertal) and P28 (pubertal) mice after i.n. PR8 (1HAU) (summary of 3 trials; n = 25 mice/group; survival differences: *P = 0.006). C: no. of alive mice per group is shown in parentheses (*P = 0.0001, comparison of slopes of linear regression lines). D: viral load in prepubertal and pubertal infected lungs using quantitative PCR for M1 mRNA on postinfection days 3, 6, and 9 (results from duplicate samples from at least 4 mice/time point/group in a single trial; Kruskal-Wallis with Dunn’s multiple-comparisons test). NS, not significant. E: histopathology of lung tissue on day 9 after infection reveal that pubertal (P28) mice have intense interstitial pneumonitis and abundant mononuclear cell inflammation (top left) with areas of diffuse alveolar damage, including hyaline membranes (top right, arrows). The severity and extent of pneumonitis was reduced in samples from prepubertal (P25) mice (bottom right and left). F: histopathology scoring [product of severity score (0–3) and extent of tissue involvement (0–3)] indicates greater injury in pubertal mice (n = 4 per group/time point in a single trial; P = 0.005 for comparison of all P25 vs. all P28 by Kruskal-Wallis test).
Transcriptome profiling.
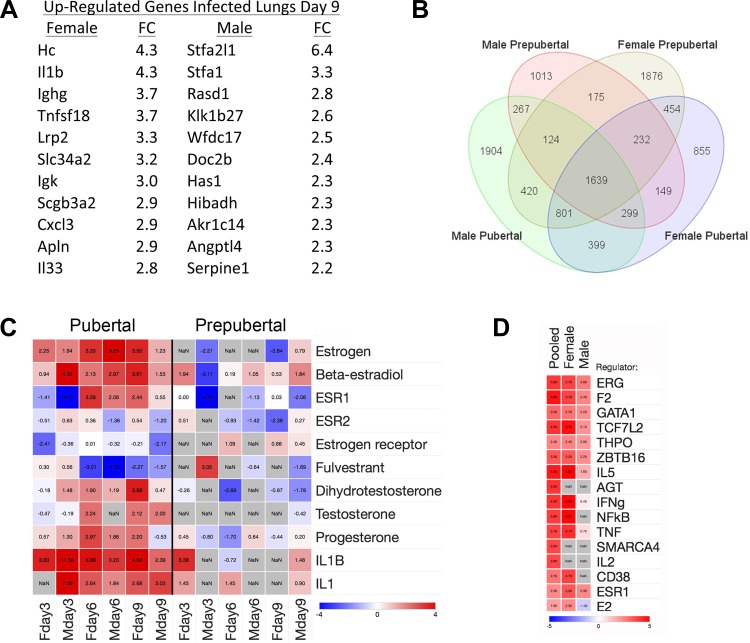
To explore mechanisms for the survival differences, we compared gene expression profiles in lung tissue on days 3, 6, and 9 after infection, and from vehicle-treated control mice for each age group. Direct comparisons of infected samples showed minimal differences at days 3 and 6. In contrast, on day 9, 442 and 323 DEGs were identified in female and male mice, respectively, with FCs of ≥1.5 at a false discovery rate of 0.05 (Supplemental Tables S1 and S2; Supplemental material for this article is available online at the Journal website). Moreover, DEGs in prepubertal vs. pubertal infected lungs on day 9 were substantially different in female vs. male mice, illustrated by comparison of the top 10 DEGs upregulated in pubertal samples (Fig. 2A). These findings agree with prior observations of sex differences in gene expression, including within lungs after influenza (29–31, 39). In female mice, genes showing greater expression in pubertal lungs included Hc (complement component C5), IL-1β, and TNFSF18 (TNF superfamily member 18), all known to contribute to influenza pathophysiology (13, 17, 22, 56).
Fig. 2.
Transcriptome profiling analysis. A: list of top upregulated genes in pubertal lungs illustrates sex differences in differentially expressed genes (DEG) in pubertal vs. prepubertal infected lungs on day 9. B: Venn diagram analysis showing number of shared and distinct DEGs in infected lung samples (day 9) compared with their age-group matched uninfected controls. C: upstream regulator analysis using Ingenuity IPA analysis of DEG lists shows marked enrichment of hormonal regulators, especially estrogen and related molecules in both females and males. This is manifest as increased z-scores for estrogen, β-estradiol, or the estrogen receptor 1 (ESR1) across the time course of infection. The analysis also showed negative z-scores for the estrogen receptor antagonist fulvestrant on days 6 and 9, a finding consistent with enhanced estrogenic signaling. In addition, IL-1 signaling was also prominently identified as activated in both sexes. D: top 10 upstream regulators in normal blood transcriptomes based on an analysis of pubertal vs. prepubertal DEGs from P25 vs. P35 males, females, or pooled samples show considerable overlap; ESR1 is also identified as an upstream regulator in pubertal male and female samples.
We also compared infected lung samples to their age-group matched uninfected controls and identified DEGs unique to prepubertal and pubertal groups for each of the days analyzed (Supplemental Tables S3 and S4). We submitted these DEG lists to Ingenuity IPA analysis to identify upstream regulators and found substantial sex and stage differences, as shown by results from day 9 (Fig. 2B) (full results in Supplemental Tables S5–S8). Notably, pubertal samples showed marked enrichment of hormonal regulators, especially estrogen and related molecules, in both females and males (Fig. 2C). This was shown by increased z-scores for estrogen, β-estradiol, or the estrogen receptor 1 (ESR1) across the time course of infection. The analysis also showed negative z-scores for the estrogen receptor antagonist fulvestrant on days 6 and 9, a finding consistent with enhanced estrogenic signaling. In addition, IL-1 signaling was prominently activated in both sexes.
We also compared gene expression profiles in peripheral blood leukocytes from normal uninfected prepubertal and pubertal mice of both sexes. Total counts and leukocyte differentials did not differ between the experimental groups (data not shown). Our analysis identified 745 DEGs when comparing pubertal vs. prepubertal, as well as sex-based differences (Supplemental Tables S9–S12). In the pubertal age group, we found enrichment of 1) immunoglobulin and B cell genes, and 2) cytokine and chemokine genes, including IL-1β (FC = 2.05, P < 10−4) and related pathways (Supplemental Table S13). The analysis of activated upstream regulators in normal male and female subsets showed substantial overlap (Fig. 2D and Supplemental Table S14), including ESR1. Taken together, the transcriptome profiling data from both lung and blood suggested important roles for estrogen-mediated signaling and IL-1β in the higher mortality seen in pubertal mice.
Role of puberty and sex hormones.
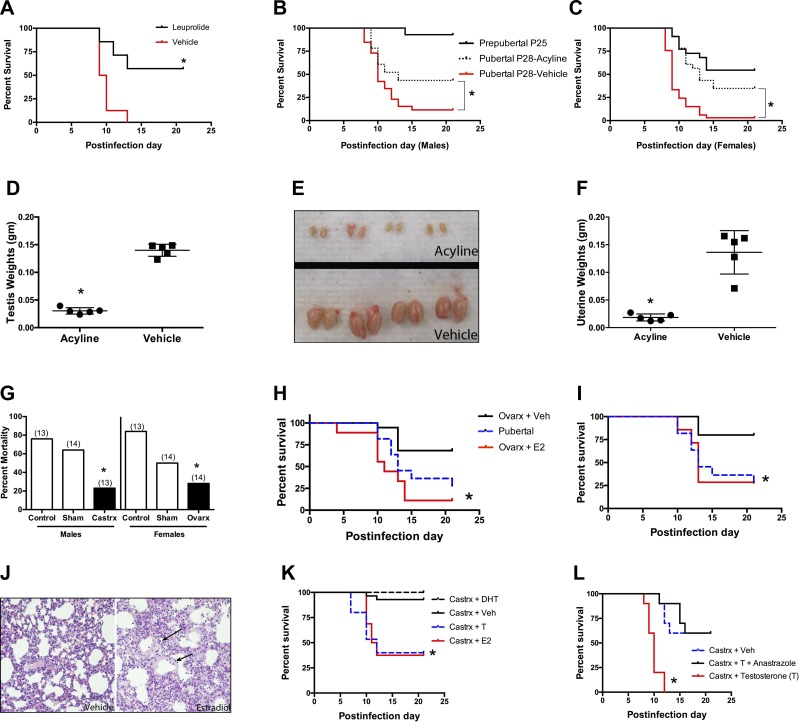
To evaluate the role of puberty and sex hormones more directly, we blocked the onset of puberty with leuprolide, a gonadotropin-releasing hormone (GnRH) analog. Leuprolide desensitizes GnRH receptors and decreases or blocks secretion of gonadotropins, luteinizing hormone, and follicle-stimulating hormone. This blocks normal pubertal increases in estradiol and testosterone levels in humans and rodents (12, 37). Comparison of infected mice of the same chronological age with normal or blocked puberty showed marked protection against mortality in puberty-blocked female mice (Fig. 3A). In contrast, the same leuprolide treatment regimen did not decrease mortality in male mice, but also did not block puberty markers, such as an increase in testicular mass (data not shown). Hence, we also blocked puberty using acyline, a GnRH receptor antagonist (15). Acyline treatment caused substantial protection against influenza mortality in both male and female mice (Figs. 3, B and C) and effectively blocked puberty in both sexes (Figs. 3, D–F).
Fig. 3.
Role of puberty and sex hormones. A: GnRH agonist leuprolide (25 μg sc) delays puberty and improves survival in female mice challenged with influenza virus at age P28 (n > 18 mice/group; summary of 3 trials; *P = 0.0063). B and C: acyline treatment improves survival of influenza in pubertal male (*P = 0.0217 vs. vehicle; B) and pubertal female (*P < 0.0001 vs. vehicle; C) mice (n > 23 for pubertal mice groups; n = 18 for prepubertal mice groups; summary of 3 trials). D and E: acyline blocks puberty-associated increases in testicular weight (D) and size (E) in testes harvested on day 35 of age (*P = 0.008, Mann-Whitney test; n = 5). F: acyline prevent puberty-associated increases in uterus weight (harvested on day 35 of age; n = 5; *P = 0.007, Mann-Whitney test). G: gonadectomized mice (P21) show decreased mortality after influenza virus infection on day 28. Comparison is between gonadectomized [castration (Castrx) or ovary removal (Ovarx)] and control mice, monitored until 21 days after infection (no. of mice/group is shown in parentheses; *P < 0.05, χ2 test; summary of 3 trials). H and I: estrogen replacement decreases survival of gonadectomized female mice when administered early 3 days after infection (n > 9/group; *P = 0.0001 vs. vehicle, summary of 2 trials; H) or late 6 days after infection (n > 7/group, *P = 0.024 vs. vehicle, summary of 2 trials; I). J: histopathology in estrogen-treated gonadectomized mice showing severe lung injury with hyaline membrane deposition (arrows, right side, representative of n = 2). K: gonadectomized male mice that received either estrogen (E2) or testosterone (T) had significantly higher mortality than those receiving vehicle or dihydrotestosterone (DHT), an androgen that cannot be converted to estrogen [*P < 0.004; n = 26 (vehicle), 16 (E2 and T), 15 (DHT); summary of 2 trials]. L: gonadectomized male mice treated with testosterone (200 μg daily) and an aromatase inhibitor (anastrozole, 10 μg daily), which prevents conversion of testosterone to estrogen, showed greater tolerance to influenza virus infection compared with the high mortality seen in mice treated with testosterone alone (n = 10/group; *P < 0.0001; single trial).
The gonads are the primary source of the pubertal increase in sex hormones. Mice that had undergone gonadectomy or sham surgery at P21 were challenged with influenza virus at age P28. Both male and female gonadectomized mice showed much lower mortality compared with both their intact pubertal counterparts and mice that had undergone the P21 sham procedure (Fig. 3G). A partial but unexplained effect of the sham procedure was observed in the female but not male mice. Treatment of ovariectomized female mice with estrogen eliminated the protective effect of ovariectomy and restored higher mortality, whether given early (days 0–2, Fig. 3H) or late (days 6–8, Fig. 3I) in the course of infection. Following ovariectomy (age P21), mice treated with estrogen exhibited greater lung inflammation and damage compared with ovariectomized controls (Fig. 3J). In males, protection by castration was reversed by both estrogen and testosterone. Testosterone can be converted to estrogen by aromatase. In contrast, dihydrotestosterone, which cannot be converted to estrogen, did not reverse the protective effect of castration (Fig. 3K). The role of aromatase conversion of testosterone to estrogen was also shown by the aromatase inhibitor anastrazole, which abrogated the ability of testosterone to reverse the protective effect of castration (Fig. 3L).
Estrogen receptor blockade is protective during influenza infection.
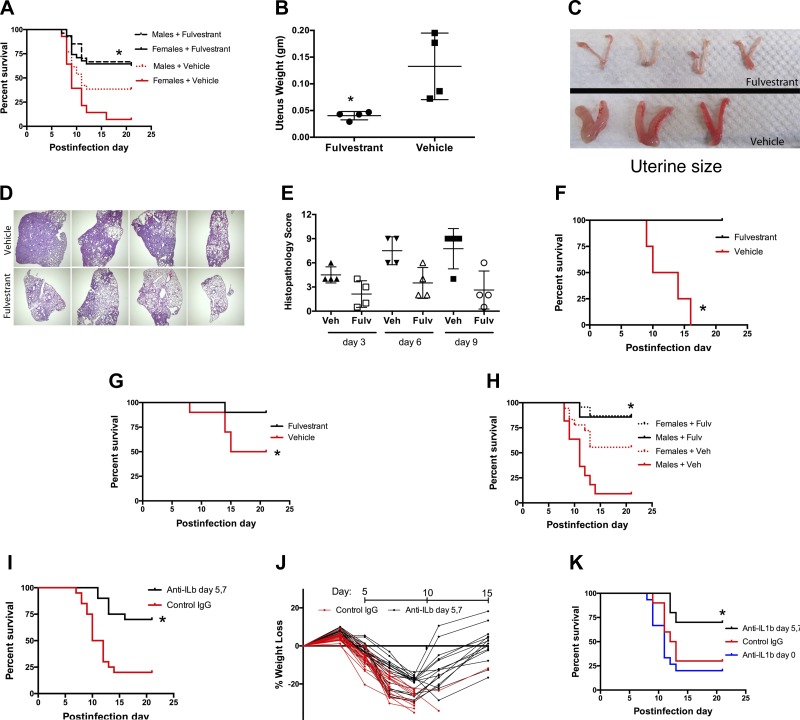
Since both our experimental data and transcriptome profiling indicated a critical role for estrogen in the pubertal susceptibility to influenza mortality in both sexes, we tested the effect of estrogen receptor blockade. Pretreatment of both male and female mice with fulvestrant, a Food and Drug Administration (FDA)-approved estrogen receptor antagonist (19), before influenza virus infection at age P28 (pubertal onset) reduced subsequent mortality (Fig. 4A). In females, fulvestrant blocked puberty, seen as the absence of a normal increase in uterine weight and size (Fig. 4, B and C; similar findings in male mice not shown). Fulvestrant-treated mice also showed reduced histopathological injury (Fig. 4, E and F). Notably, delayed treatment of female pubertal mice (infected on P28) with fulvestrant starting on day 3 after infection also greatly improved survival (Fig. 4D). Pretreatment of older postpubertal female mice (infected on P42) also improved survival (Fig. 4G). Finally, delayed treatment of both male and female postpubertal mice (infected on P42) with fulvestrant also improved survival in both sexes (Fig. 4H). The potential for translational benefit of these findings is enhanced by the efficacy observed with delayed therapy, the established safety profile of fulvestrant in human patients, and the relatively short period of illness when treatment would be needed.
Fig. 4.
Estrogen receptor blockade is protective during influenza infection. A: the estrogen receptor antagonist fulvestrant (200 μg ip every other day, starting day 21 of age) before infection of 28-day-old mice improves survival in both pubertal males and females compared with vehicle-treated mice (*P = 0.049 for male mice, P < 0.001 for female mice, n > 26/group, summary of 3 trials). B and C: fulvestrant prevents the estrogen-dependent pubertal increase in uterine weight (B) and size (C) (*P = 0.02, n = 4, harvest at age day 35, Mann-Whitney test). D and E: lung histopathology shows less consolidation and injury in fulvestrant-treated female mice, illustrated by low-power views of samples taken 9 days after infection (D) and lung injury scores (E) for samples taken 3, 6, and 9 days after infection (*P = 0.0005, Kruskal-Wallis test). F: delayed treatment with fulvestrant, started 3 days after infection of 28-day-old pubertal female mice, improves survival compared with vehicle-treated mice (*P = 0.007, n = 4/group). G: fulvestrant started 7 days before infection in older 42-day-old female mice improves survival compared with vehicle-treated mice (*P = 0.06, n = 10/group, single trial). H: estrogen receptor blockade with fulvestrant started 3 days after infection in 42-day-old female and male adult mice improves survival compared with vehicle-treated mice (*P = 0.019 for female mice, P < 0.0001 for male mice, n > 19/ group, summary of 3 trials). I and J: treatment of P28 female mice on days 5 and 7 after influenza virus infection with 100 μg ip of anti-IL-1β neutralizing antibody improves survival (*P = 0.0009, n = 20/group, summary of 3 trials; I) and allows recovery from the initial weight loss (J). The control group received hamster IgG isotype antibody. K: after influenza virus infection, early treatment (day 0) with anti-IL-1β antibody provides no benefit, in contrast to improved survival seen with delayed treatment 5–7 days after infection (*P < 0.01 vs. control or day 0 treatment; n = 10 for control IgG, delayed treatment groups; n = 15 for early treatment; single trial).
Transcriptome profiling identified increased expression of IL-1β late (day 9) in the lungs of the higher mortality pubertal mice, and increased basal IL-1β expression within normal pubertal blood leukocytes. This prompted testing of the effects of inhibiting this cytokine on influenza mortality. Treatment with neutralizing anti-IL-1β blocking antibody given on days 5 and 7 after infection of pubertal mice at age P28 substantially improved survival and allowed recovery from the initial weight loss seen during the infection (Fig. 4, I and J). Other studies of mice constitutively deficient in IL-1β (27) or IL-1 receptor (17, 43) have shown enhanced mortality, suggesting a beneficial role for IL-1β. To evaluate the effects of IL-1β at different times during the course of influenza, we administered anti-IL-1β antibody early (day 0) or later (days 5 and 7) after infection. Delayed antibody treatment led to improved survival, whereas early treatment did not (Fig. 4K), indicating distinct beneficial and harmful functions of IL-1β at different phases of high-fatality influenza infections.
DISCUSSION
We developed a mouse model of the striking difference in mortality of children vs. adults during the 1918 influenza pandemic. Similar to the human epidemiological data, the pubertal transition in mice was associated with greater influenza mortality. Prevention of puberty by surgical castration or pharmacological blockade preserved the relative greater survival of prepubertal mice. Transcriptome profiling identified estrogen as a regulator of increased susceptibility in both sexes and also linked better survival to late expression of IL-1β. Direct testing of these therapeutic leads showed that fulvestrant (an estrogen receptor antagonist) and late (but not early) anti-IL-1β neutralization both improved survival substantially, even when given days after the onset of infection.
Other studies have compared influenza effects in animals of different age. Similar to our results, younger ferrets have milder disease compared with adult animals (16). The ages of ferrets in this study correspond to postweaning and puberty onset in ferrets, so they roughly match those in our mouse model, but pubertal status was not directly evaluated by the investigators. Other studies have compared mortality in different age groups of mice. The findings vary, possibly reflecting use of different mouse strains, virus strains, or other factors in experimental design. Similar to our own findings, two groups report relatively high mortality in weanling mice (3 wk or 21 days old). Kalter (20) reported that 3-wk-old mice were more susceptible than 6-wk-old mice; similar findings were observed by Yasui et al. (55). Sun et al. (50) found that adult mice (6 mo, 1 yr) fare better than younger mice (4 wk old). In contrast, Vom Steeg et al. (52) compared 2 mo old vs. 18 mo old and found the aged mice did worse. The most consistent finding in this brief overview is that that very young mice (3 wk of age and younger) are highly susceptible to influenza mortality, similar to the high mortality seen in the 0- to 4-yr age group in the 1918 pandemic (2). Other effects of aging on susceptibility of older mice are suggested by the cited work (despite some of the differences noted) and may be interpreted to reflect the well-known increased case fatality rate in elderly individuals (2). However, other than the ferret study, no other experimental data specifically comparing prepubertal vs. pubertal animals, as reported here, are available.
Our findings may seem discordant with reports of beneficial effects of estrogen in injury models (3). However, estrogen effects include proinflammatory effects at low endogenous levels (as seen with puberty onset), but anti-inflammatory actions at pharmacological doses (23, 39, 49). Indeed, influenza causes greater morbidity and mortality in female mice compared with males (which we interpret as reflecting proinflammatory effects of greater endogenous estrogen levels in females), but pharmacological treatment with estrogen improved morbidity scores and survival (39). Differences between our study and this prior work (39) include the age at time of infection (4 wk vs. 6–8 wk), source of mice used (Charles River vs. NCI Frederick), and method and duration of estrogen administration (subcutaneous injection in oil vs. implanted silastic tubing devices. We favor interpreting the differences as primarily reflecting the anti-inflammatory effect of high-dose estrogen treatment, but these and other differences may also contribute. The complexity of estrogen effects is also evident in reported effects on IL-1β, which include findings of increased production or expression (9, 18, 41) or the opposite (42, 46, 47).
The limitations of mouse models are increasingly recognized and debated (35, 44) and merit discussion here. For influenza, relevant limitations include the known differences in susceptibility across different inbred strains (5, 48), as well as imperfect correspondence of developmental stages and function of immune systems in mice to those in people (28, 57). It is possible that results with other mouse strains or other mouse-adapted influenza virus types might differ. The transcriptome profiling was performed on whole lung homogenates rather than purified cell subtypes, a choice that may also introduce limitations. Analysis of the number and subtype of various immune cells did not reveal any consistent differences in pilot studies (data not shown), but a comprehensive investigation over time/age/hormonal status groups could provide additional insights. While cognizant of these limitations, we, nevertheless, consider that our model provides a means to investigate the relative tolerance of prepubertal children and to seek therapeutic interventions to improve outcomes in severe infections.
We observed beneficial effects from two therapeutic leads administered 3 days or later after infection, increasing the potential utility in clinical infections. Additional studies in other animals models (e.g., ferret) and other viral strains are needed to fully evaluate the efficacy of fulvestrant. The potential benefit of the other therapeutic lead identified in this work, i.e., targeting IL-1β, is supported by findings that treatment with IL-1 receptor antagonist (the FDA-approved agent anakinra) from day 2–7 after influenza significantly improved survival (45). We speculate that delaying this intervention (anakinra) might provide even greater benefit, based on the temporal differences we observed with anti-IL-1β neutralization. There is substantial variability in the presentation and course of severe influenza in people. Hence, whether it will be feasible to translate therapies based on specific phases of the illness identified in highly controlled animal models requires further study.
Our results suggest two lines of investigation to further evaluate mechanisms for the greater mortality seen after puberty onset and to identify new drug candidates. First, in addition to estrogen, we identified several upstream regulators (Supplemental Tables S7 and S8) whose modulation might be beneficial. For example, it is noteworthy that one of the upstream regulators identified in the high-survival prepubertal group (Supplemental Table S8) was camptothecin [a topoisomerase inhibitor recently reported to improve survival in mice with influenza (38)]. Second, downstream mediators of estrogen’s effects might provide additional targets. However, a substantial challenge is the great number of genes and proteins whose expression is modulated by estrogen (7, 26). Our laboratory (54) has previously reported a beneficial effect of estrogen on innate immunity to bacterial pneumonia in older mice (8–12 wk). The available epidemiology suggests childhood resistance to severe infections includes those caused by bacterial pathogens. Nevertheless, the impact of pubertal estrogen on resistance to bacterial pneumonia remains to be directly tested experimentally. Future efforts may benefit from focusing on the genes modulated by low levels of estrogen in both sexes, reflecting our findings of effects in both males and females.
We speculate that mechanisms for greater influenza mortality after puberty might also be active and harmful in other severe infections, given the broad tolerance of prepubertal children to many pathogens (2, 8, 33, 40, 53). If this is correct, treatments identified in one model might benefit outcomes in others and eventually lead to the identification of drugs that could be used as syndromic treatment for them all.
GRANTS
This study was supported by National Institute of Environmental Health Sciences Grant ES00002, National Heart, Lung, and Blood Institute Grant T32 HL007118, and Defense Advanced Research Projects Agency Grant W911NF-10–1-0217.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.S. performed experiments; F.S. and L.K. analyzed data; F.S. and L.K. interpreted results of experiments; F.S. and L.K. prepared figures; F.S. and L.K. drafted manuscript; F.S. and L.K. edited and revised manuscript; F.S. and L.K. approved final version of manuscript; L.K. conceived and designed research.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Drs. David Fedson, Kevin Harrod, and Ulrich von Adrian for helpful discussions.
REFERENCES
- 1.Aevermann BD, Pickett BE, Kumar S, Klem EB, Agnihothram S, Askovich PS, Bankhead A III, Bolles M, Carter V, Chang J, Clauss TRW, Dash P, Diercks AH, Eisfeld AJ, Ellis A, Fan S, Ferris MT, Gralinski LE, Green RR, Gritsenko MA, Hatta M, Heegel RA, Jacobs JM, Jeng S, Josset L, Kaiser SM, Kelly S, Law GL, Li C, Li J, Long C, Luna ML, Matzke M, McDermott J, Menachery V, Metz TO, Mitchell H, Monroe ME, Navarro G, Neumann G, Podyminogin RL, Purvine SO, Rosenberger CM, Sanders CJ, Schepmoes AA, Shukla AK, Sims A, Sova P, Tam VC, Tchitchek N, Thomas PG, Tilton SC, Totura A, Wang J, Webb-Robertson B-J, Wen J, Weiss JM, Yang F, Yount B, Zhang Q, McWeeney S, Smith RD, Waters KM, Kawaoka Y, Baric R, Aderem A, Katze MG, Scheuermann RH. A comprehensive collection of systems biology data characterizing the host response to viral infection. Sci Data 1: 140033, 2014. doi: 10.1038/sdata.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed R, Oldstone MBA, Palese P. Protective immunity and susceptibility to infectious diseases: lessons from the 1918 influenza pandemic. Nat Immunol 8: 1188–1193, 2007. doi: 10.1038/ni1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angele MK, Pratschke S, Hubbard WJ, Chaudry IH. Gender differences in sepsis: cardiovascular and immunological aspects. Virulence 5: 12–19, 2014. doi: 10.4161/viru.26982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Askovich PS, Sanders CJ, Rosenberger CM, Diercks AH, Dash P, Navarro G, Vogel P, Doherty PC, Thomas PG, Aderem A. Differential host response, rather than early viral replication efficiency, correlates with pathogenicity caused by influenza viruses. PLoS One 8: e74863, 2013. doi: 10.1371/journal.pone.0074863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boon ACM, deBeauchamp J, Hollmann A, Luke J, Kotb M, Rowe S, Finkelstein D, Neale G, Lu L, Williams RW, Webby RJ. Host genetic variation affects resistance to infection with a highly pathogenic H5N1 influenza A virus in mice. J Virol 83: 10417–10426, 2009. doi: 10.1128/JVI.00514-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boon ACM, Finkelstein D, Zheng M, Liao G, Allard J, Klumpp K, Webster R, Peltz G, Webby RJ. H5N1 influenza virus pathogenesis in genetically diverse mice is mediated at the level of viral load. MBio 2: e00171-11, 2011. doi: 10.1128/mBio.00171-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourdeau V, Deschênes J, Métivier R, Nagai Y, Nguyen D, Bretschneider N, Gannon F, White JH, Mader S. Genome-wide identification of high-affinity estrogen response elements in human and mouse. Mol Endocrinol 18: 1411–1427, 2004. doi: 10.1210/me.2003-0441. [DOI] [PubMed] [Google Scholar]
- 8.Burnet M. The pattern of disease in childhood. Australas Ann Med 1: 93–108, 1952. doi: 10.1111/imj.1952.1.2.93. [DOI] [PubMed] [Google Scholar]
- 9.Calippe B, Douin-Echinard V, Laffargue M, Laurell H, Rana-Poussine V, Pipy B, Guéry J-C, Bayard F, Arnal J-F, Gourdy P. Chronic estradiol administration in vivo promotes the proinflammatory response of macrophages to TLR4 activation: involvement of the phosphatidylinositol 3-kinase pathway. J Immunol 180: 7980–7988, 2008. doi: 10.4049/jimmunol.180.12.7980. [DOI] [PubMed] [Google Scholar]
- 10.DiVall SA, Williams TR, Carver SE, Koch L, Brüning JC, Kahn CR, Wondisford F, Radovick S, Wolfe A. Divergent roles of growth factors in the GnRH regulation of puberty in mice. J Clin Invest 120: 2900–2909, 2010. doi: 10.1172/JCI41069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fedson DS. Treating influenza with statins and other immunomodulatory agents. Antiviral Res 99: 417–435, 2013. doi: 10.1016/j.antiviral.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Fuqua JS. Treatment and outcomes of precocious puberty: an update. J Clin Endocrinol Metab 98: 2198–2207, 2013. doi: 10.1210/jc.2013-1024. [DOI] [PubMed] [Google Scholar]
- 13.Garcia CC, Weston-Davies W, Russo RC, Tavares LP, Rachid MA, Alves-Filho JC, Machado AV, Ryffel B, Nunn MA, Teixeira MM. Complement C5 activation during influenza A infection in mice contributes to neutrophil recruitment and lung injury. PLoS One 8: e64443, 2013. doi: 10.1371/journal.pone.0064443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh S, Gregory D, Smith A, Kobzik L. MARCO regulates early inflammatory responses against influenza: a useful macrophage function with adverse outcome. Am J Respir Cell Mol Biol 45: 1036–1044, 2011. doi: 10.1165/rcmb.2010-0349OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbst KL, Anawalt BD, Amory JK, Bremner WJ. Acyline: the first study in humans of a potent, new gonadotropin-releasing hormone antagonist. J Clin Endocrinol Metab 87: 3215–3220, 2002. doi: 10.1210/jcem.87.7.8675. [DOI] [PubMed] [Google Scholar]
- 16.Huang SSH, Banner D, Degousee N, Leon AJ, Xu L, Paquette SG, Kanagasabai T, Fang Y, Rubino S, Rubin B, Kelvin DJ, Kelvin AA. Differential pathological and immune responses in newly weaned ferrets are associated with a mild clinical outcome of pandemic 2009 H1N1 infection. J Virol 86: 13187–13201, 2012. doi: 10.1128/JVI.01456-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med 206: 79–87, 2009. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itoh Y, Hayashi H, Miyazawa K, Kojima S, Akahoshi T, Onozaki K. 17β-estradiol induces IL-1α gene expression in rheumatoid fibroblast-like synovial cells through estrogen receptor α (ERα) and augmentation of transcriptional activity of Sp1 by dissociating histone deacetylase 2 from ERα. J Immunol 178: 3059–3066, 2007. doi: 10.4049/jimmunol.178.5.3059. [DOI] [PubMed] [Google Scholar]
- 19.Kabos P, Borges VF. Fulvestrant: a unique antiendocrine agent for estrogen-sensitive breast cancer. Expert Opin Pharmacother 11: 807–816, 2010. doi: 10.1517/14656561003641982. [DOI] [PubMed] [Google Scholar]
- 20.Kalter SS. The effect of age upon susceptibility to infection with influenza virus. J Immunol 63: 17–22, 1949. [PubMed] [Google Scholar]
- 21.Kash JC, Taubenberger JK. The role of viral, host, and secondary bacterial factors in influenza pathogenesis. Am J Pathol 185: 1528–1536, 2015. doi: 10.1016/j.ajpath.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim KS, Jung H, Shin IK, Choi B-R, Kim DH. Induction of interleukin-1 beta (IL-1β) is a critical component of lung inflammation during influenza A (H1N1) virus infection. J Med Virol 87: 1104–1112, 2015. doi: 10.1002/jmv.24138. [DOI] [PubMed] [Google Scholar]
- 23.Klein SL, Hodgson A, Robinson DP. Mechanisms of sex disparities in influenza pathogenesis. J Leukoc Biol 92: 67–73, 2012. doi: 10.1189/jlb.0811427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, Hatta Y, Kim JH, Halfmann P, Hatta M, Feldmann F, Alimonti JB, Fernando L, Li Y, Katze MG, Feldmann H, Kawaoka Y. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature 445: 319–323, 2007. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- 25.Kollmus H, Wilk E, Schughart K. Systems biology and systems genetics - novel innovative approaches to study host-pathogen interactions during influenza infection. Curr Opin Virol 6: 47–54, 2014. doi: 10.1016/j.coviro.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol 294: 63–69, 2015. doi: 10.1016/j.cellimm.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozak W, Zheng H, Conn CA, Soszynski D, van der Ploeg LH, Kluger MJ. Thermal and behavioral effects of lipopolysaccharide and influenza in interleukin-1 β-deficient mice. Am J Physiol Regul Integr Comp Physiol 269: R969–R977, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Kuper CF, van Bilsen J, Cnossen H, Houben G, Garthoff J, Wolterbeek A. Development of immune organs and functioning in humans and test animals: Implications for immune intervention studies. Reprod Toxicol 64: 180–190, 2016. doi: 10.1016/j.reprotox.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Lamason R, Zhao P, Rawat R, Davis A, Hall JC, Chae JJ, Agarwal R, Cohen P, Rosen A, Hoffman EP, Nagaraju K. Sexual dimorphism in immune response genes as a function of puberty. BMC Immunol 7: 2, 2006. doi: 10.1186/1471-2172-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larcombe AN, Foong RE, Bozanich EM, Berry LJ, Garratt LW, Gualano RC, Jones JE, Dousha LF, Zosky GR, Sly PD. Sexual dimorphism in lung function responses to acute influenza A infection. Influenza Other Respir Viruses 5: 334–342, 2011. doi: 10.1111/j.1750-2659.2011.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leist SR, Pilzner C, van den Brand JMA, Dengler L, Geffers R, Kuiken T, Balling R, Kollmus H, Schughart K. Influenza H3N2 infection of the collaborative cross founder strains reveals highly divergent host responses and identifies a unique phenotype in CAST/EiJ mice. BMC Genomics 17: 143, 2016. doi: 10.1186/s12864-016-2483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science 335: 936–941, 2012. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melamed A, Sorvillo FJ. The burden of sepsis-associated mortality in the United States from 1999 to 2005: an analysis of multiple-cause-of-death data. Crit Care 13: R28, 2009. doi: 10.1186/cc7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oshansky CM, Gartland AJ, Wong S-S, Jeevan T, Wang D, Roddam PL, Caniza MA, Hertz T, Devincenzo JP, Webby RJ, Thomas PG. Mucosal immune responses predict clinical outcomes during influenza infection independently of age and viral load. Am J Respir Crit Care Med 189: 449–462, 2014. doi: 10.1164/rccm.201309-1616OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osuchowski MF, Remick DG, Lederer JA, Lang CH, Aasen AO, Aibiki M, Azevedo LC, Bahrami S, Boros M, Cooney R, Cuzzocrea S, Jiang Y, Junger WG, Hirasawa H, Hotchkiss RS, Li X-A, Radermacher P, Redl H, Salomao R, Soebandrio A, Thiemermann C, Vincent J-L, Ward P, Yao Y-M, Yu H-P, Zingarelli B, Chaudry IH. Abandon the mouse research ship? Not just yet! Shock 41: 463–475, 2014. doi: 10.1097/SHK.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baillie JK, Digard P. Influenza—time to target the host? N Engl J Med 369: 191–193, 2013. doi: 10.1056/NEJMcibr1304414. [DOI] [PubMed] [Google Scholar]
- 37.Ravivarapu HB, Moyer KL, Dunn RL. Sustained suppression of pituitary-gonadal axis with an injectable, in situ forming implant of leuprolide acetate. J Pharm Sci 89: 732–741, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 38.Rialdi A, Campisi L, Zhao N, Lagda AC, Pietzsch C, Ho JSY, Martinez-Gil L, Fenouil R, Chen X, Edwards M, Metreveli G, Jordan S, Peralta Z, Muñoz-Fontela C, Bouvier N, Merad M, Jin J, Weirauch M, Heinz S, Benner C, van Bakel H, Basler C, García-Sastre A, Bukreyev A, Marazzi I. Topoisomerase 1 inhibition suppresses inflammatory genes and protects from death by inflammation. Science 352: aad7993, 2016. doi: 10.1126/science.aad7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17β-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog 7: e1002149, 2011. doi: 10.1371/journal.ppat.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosello A, Mossoko M, Flasche S, Van Hoek AJ, Mbala P, Camacho A, Funk S, Kucharski A, Ilunga BK, Edmunds WJ, Piot P, Baguelin M, Tamfum JJ. Ebola virus disease in the Democratic Republic of the Congo, 1976-2014. eLife 4: 4, 2015. doi: 10.7554/eLife.09015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruh MF, Bi Y, Cox L, Berk D, Howlett AC, Bellone CJ. Effect of environmental estrogens on IL-1beta promoter activity in a macrophage cell line. Endocrine 9: 207–211, 1998. doi: 10.1385/ENDO:9:2:207. [DOI] [PubMed] [Google Scholar]
- 42.Schaefer TM, Wright JA, Pioli PA, Wira CR. IL-1beta-mediated proinflammatory responses are inhibited by estradiol via down-regulation of IL-1 receptor type I in uterine epithelial cells. J Immunol 175: 6509–6516, 2005. doi: 10.4049/jimmunol.175.10.6509. [DOI] [PubMed] [Google Scholar]
- 43.Schmitz N, Kurrer M, Bachmann MF, Kopf M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol 79: 6441–6448, 2005. doi: 10.1128/JVI.79.10.6441-6448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, López CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG; Inflammation and Host Response to Injury, Large Scale Collaborative Research Program . Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA 110: 3507–3512, 2013. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shirey KA, Lai W, Patel MC, Pletneva LM, Pang C, Kurt-Jones E, Lipsky M, Roger T, Calandra T, Tracey KJ, Al-Abed Y, Bowie AG, Fasano A, Dinarello CA, Gusovsky F, Blanco JC, Vogel SN. Novel strategies for targeting innate immune responses to influenza. Mucosal Immunol 9: 1173–1182, 2016. doi: 10.1038/mi.2015.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith JA, Das A, Butler JT, Ray SK, Banik NL. Estrogen or estrogen receptor agonist inhibits lipopolysaccharide induced microglial activation and death. Neurochem Res 36: 1587–1593, 2011. doi: 10.1007/s11064-010-0336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Speyer CL, Rancilio NJ, McClintock SD, Crawford JD, Gao H, Sarma JV, Ward PA. Regulatory effects of estrogen on acute lung inflammation in mice. Am J Physiol Cell Physiol 288: C881–C890, 2005. doi: 10.1152/ajpcell.00467.2004. [DOI] [PubMed] [Google Scholar]
- 48.Srivastava B, Błazejewska P, Hessmann M, Bruder D, Geffers R, Mauel S, Gruber AD, Schughart K. Host genetic background strongly influences the response to influenza a virus infections. PLoS One 4: e4857, 2009. doi: 10.1371/journal.pone.0004857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Straub RH. The complex role of estrogens in inflammation. Endocr Rev 28: 521–574, 2007. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 50.Sun S, Zhao G, Xiao W, Hu J, Guo Y, Yu H, Wu X, Tan Y, Zhou Y. Age-related sensitivity and pathological differences in infections by 2009 pandemic influenza A (H1N1) virus. Virol J 8: 52, 2011. doi: 10.1186/1743-422X-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.To KK-W, Zhou J, Chan JF-W, Yuen K-Y. Host genes and influenza pathogenesis in humans: an emerging paradigm. Curr Opin Virol 14: 7–15, 2015. doi: 10.1016/j.coviro.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vom Steeg LG, Vermillion MS, Hall OJ, Alam O, McFarland R, Chen H, Zirkin B, Klein SL. Age and testosterone mediate influenza pathogenesis in male mice. Am J Physiol Lung Cell Mol Physiol 311: L1234–L1244, 2016. doi: 10.1152/ajplung.00352.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.WHO Ebola Response Team, Agua-Agum J, Ariyarajah A, Blake IM, Cori A, Donnelly CA, Dorigatti I, Dye C, Eckmanns T, Ferguson NM, Fowler RA, Fraser C, Garske T, Hinsley W, Jombart T, Mills HL, Murthy S, Nedjati Gilani G, Nouvellet P, Pelletier L, Riley S, Schumacher D, Shah A, Van Kerkhove MD. Ebola virus disease among children in West Africa. N Engl J Med 372: 1274–1277, 2015. doi: 10.1056/NEJMc1415318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang Z, Huang YC, Koziel H, de Crom R, Ruetten H, Wohlfart P, Thomsen RW, Kahlert JA, Sørensen HT, Jozefowski S, Colby A, Kobzik L. Female resistance to pneumonia identifies lung macrophage nitric oxide synthase-3 as a therapeutic target. eLife 3: 3, 2014. doi: 10.7554/eLife.03711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yasui H, Kiyoshima J, Hori T. Reduction of influenza virus titer and protection against influenza virus infection in infant mice fed Lactobacillus casei Shirota. Clin Diagn Lab Immunol 11: 675–679, 2004. doi: 10.1128/CDLI.11.4.675-679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zou Q, Wu B, Xue J, Fan X, Feng C, Geng S, Wang M, Wang B. CD8+ Treg cells suppress CD8+ T cell-responses by IL-10-dependent mechanism during H5N1 influenza virus infection. Eur J Immunol 44: 103–114, 2014. doi: 10.1002/eji.201343583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zschaler J, Schlorke D, Arnhold J. Differences in innate immune response between man and mouse. Crit Rev Immunol 34: 433–454, 2014. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.