Abstract
Bronchopulmonary dysplasia (BPD) is characterized by impaired alveolar secondary septation and vascular growth. Exposure to high concentrations of oxygen (hyperoxia) contributes to the development of BPD. The male sex is considered an independent risk factor for the development of BPD. The reasons underlying sexually dimorphic outcomes in premature neonates are not known. We hypothesized that sex-specific modulation of biological processes in the lung under hyperoxic conditions contributes to sex-based differences. Neonatal male and female mice (C57BL/6) were exposed to hyperoxia [95% , postnatal day (PND) 1–5: saccular stage of lung development] and euthanized on PND 7 or 21. Pulmonary gene expression was studied using RNA-Seq on the Illumina HiSeq 2500 platform. Analysis of the pulmonary transcriptome revealed differential sex-specific modulation of crucial pathways such as angiogenesis, response to hypoxia, inflammatory response, and p53 pathway. Candidate genes from these pathways were validated at the mRNA level by qPCR. Analysis also revealed sex-specific differences in the modulation of crucial transcription factors. Focusing on the differential modulation of the angiogenesis pathway, we also showed sex-specific differential activation of Hif-1α-regulated genes using ChIP-qPCR and differences in expression of crucial genes (Vegf, VegfR2, and Phd2) modulating angiogenesis. We demonstrate the translational relevance of our findings by showing that our murine sex-specific differences in gene expression correlate with those from a preexisting human BPD data set. In conclusion, we provide novel molecular insights into differential sex-specific modulation of the pulmonary transcriptome in neonatal hyperoxic lung injury and highlight angiogenesis as one of the crucial differentially modulated pathways.
Keywords: hyperoxia, sex, bronchopulmonary dysplasia, RNA-Seq
INTRODUCTION
Bronchopulmonary dysplasia (BPD) is characterized by an arrest in lung development with severe impairment of alveolar septation and vascular development (4). It remains one of the most common and costly morbidities in preterm neonates despite advances in neonatal care and improvement in survival of extremely premature neonates (43). There is an urgent need to develop diagnosis and treatment strategies to decrease the burden of this disease. The etiology of this disease is multifactorial, and exposure to high concentrations of oxygen (hyperoxia) postnatally contributes to its development via generation of reactive oxygen species (41). Exposure of newborn mice to hyperoxia leads to lung pathology with some similarities to BPD (18).
The role of sex as a risk factor for neonatal mortality and morbidities such as BPD has been shown in many clinical studies (44, 54). In a Norwegian study, the female sex was protective against developing BPD in infants with gestational age of <30 wk, with an odds ratio of 0.50 (95% confidence interval: 0.28–0.92) (15). In the same study, 63.3% of the boys vs. 36.6% of the girls developed moderate to severe BPD. Long-term outcomes in survivors such as disability, cognitive delay, and reactive airway disease also show disadvantages for males (37). Although sex-specific differences in the incidence of BPD and impaired lung function in males are well established, the molecular mechanism(s) underlying these phenomena is not completely understood. Physiological, structural, and hormonal differences between male and female premature newborns may affect these outcomes (26).
Lung development is different between male and female fetuses, with females showing earlier surfactant production and more mature lung structure compared with males (7). Histological lung maturity is advanced in female fetuses, especially at gestational ages <32 wk (33). Androgens have been implicated not only in the delay of lung maturation in male fetuses but also in exacerbating hyperoxia-induced lung injury (13, 34). Fetal exposure to androgens may modulate biological pathways and processes that may predispose the males to lung injury. Also, differences in inflammatory responses may dictate distinct injury patterns in male and female mice, as noted in some models (14). Innate sex differences at the cellular level may also explain some of the sex-based proclivity to injury and cell death. Hence, sex-specific modulation of multiple biological processes may lead to the sex-related bias noted in the incidence of BPD in premature neonates.
Neonatal male mice exposed to hyperoxia [postnatal day (PND) 1–5, 95% ] showed more pronounced arrest in alveolarization and vascular development when compared with females (25). Gene expression analyses have been performed to identify the underlying dysregulated pathways in neonatal hyperoxic lung injury models as well as in human patients, but none have attempted to characterize the mechanisms underlying sexual dimorphism in lung injury. There could be molecular pathways that either are protective in females or make the male sex more susceptible to hyperoxia-induced lung injury. It is imperative to understand these pathways as a prerequisite to develop effective therapies (32). Sex-specific differences in the pulmonary transcriptome and the underlying biological pathways and regulatory networks have not been reported. In this study, we employed a nonbiased approach to measure global changes in pulmonary gene expression in male and female neonatal mice following hyperoxia exposure using RNA-Seq. We hypothesized that sex-specific modulation of multiple molecular pathways in the lung under hyperoxic conditions contributes to sex-based differences in hyperoxic lung injury.
MATERIALS AND METHODS
Animals.
Approval for this study was obtained from the Institutional Animal Care and Use Committee (IACUC) of Baylor College of Medicine (protocol no. AN-6474). All experiments were performed in accordance with relevant guidelines and regulations. Care of animals in research met the highest contemporary standards as per the 8th edition of the Guide for the Care and Use of Laboratory Animals and other IACUC protocols. Timed pregnant C57BL/6J wild-type mice were obtained from Charles River Laboratories. The sex in neonatal mouse pups was determined by both the anogenital distance and pigmentation in the anogenital region method (51). We reconfirmed the sex of the mice with PCR analysis for the Sry gene in genomic DNA obtained from mouse tail clips.
Mouse model of BPD.
An arrest of alveolarization was induced in mouse pups by exposure to hyperoxia (95% O2), as described previously (17, 24). This model has previously been described and carefully characterized, in which a pronounced arrest of lung development is seen in response to hyperoxia exposure (2, 5). Mouse pups from multiple litters were pooled before being randomly and equally redistributed to two groups, with one group exposed to normoxia (21% O2) and the other group exposed to hyperoxia (95% O2), within 12 h of birth for 5 days. Animals at this stage of development were chosen because neonatal mice are at the saccular stage of lung development during this period, which is equivalent to 26–36 wk gestation in human neonates. The litter size was limited to six pups to control for the effects of litter size on nutrition and growth. The dams were rotated between air- and hyperoxia-exposed litters every 24 h to prevent oxygen toxicity in the dams and to eliminate maternal effects between the groups. Mice were euthanized on PND7 or PND21 (after recovery in room air for 2 wk), as most of postnatal lung development in mice is completed by this age. In mice, the alveolar stage extends from PND5 to PND28–30 (50). PND7 was an intermediate time point for alveolar development in mice. The control group was kept at room air for the same duration of time (PND7 and PND21).
RNA isolation.
Total RNA from frozen lung samples was isolated using the miRNeasy kit (Qiagen, Valencia, CA). Sample concentration was assayed using Nanodrop-8000 (Thermo Scientific, Wilmington, DE), and quality checks were done using the NanoDrop spectrophotometer and the Agilent Bioanalyzer. RNA quality parameters were as follows: the 260/280 and 260/230 ratios needed to be >1.8. Furthermore, the RNA integrity number (RIN) was analyzed using the Agilent Bioanalzyer. The samples needed to have RIN values of 7–10 and with a range of 1–1.5.
RNA-Seq data analyses, pathway enrichment, and transcription factor analysis.
Pulmonary gene expression was studied using RNA-Seq on an Illumina HiSeq 2500 platform. Data were mapped to the mouse genome using TopHat2 and quantified using Cufflinks2 (22, 49). Gene expression data were quantile normalized and analyzed using the R statistical software (version 3.2.1). A combination of selection criteria comprising nominal P values of <0.05 and linear fold change greater or lesser than 1.25 is used to generate differential expressed gene (DEG) profiles. Data were analyzed both by two-way ANOVA (P < 0.05) and by pairwise parametric t-tests between treatment groups. Because the focus of this study was to look at how the transcriptome is differentially modulated in a sex-specific manner, we further analyzed for transcriptional networks the pairwise comparisons of interest (i.e., male vs. female in room air or hyperoxia). We next employed the gene set enrichment analysis (GSEA) methodology and software against the Molecular Signature database (MSigDB) compendium of gene sets (45). The pathway collections KEGG, Reactome, Hallmark, and GOBP (Gene Ontology Biological Processes) were used to determine enriched pathways. In the gene set enrichment analysis, first an aggregate gene set score [termed enrichment score (ES)] was found. Subsequently, 1,000 permutations were run to establish a background distribution for ES. The ratio between ES and the average ES is termed normalized enrichment score (NES). GSEA essentially determines whether a key component of a pathway or biological process gene set is significantly enriched in upregulated genes (NES > 0, fdr-adjusted q value <0.25) or in downregulated genes (NES < 0, fdr-adjusted q value <0.25). If the NESs for a pathway in comparisons from two different treatments are significant but having opposite signs, then the treatments might be driving the pathways in opposite directions. We also used a compendium of putative transcription factor targets based on the TRANSFAC database to identify enriched transcription factors in the transcriptome footprints analyzed. Overrepresentation (ORA) method was used to identify the key transcription factors modulating gene expression in our experiment (hypergeometric distribution, P < 0.05). The data set has been deposited in NCBI Geo with the identifier GSE97804. In the current study, for genes with a standard deviation within 40% of the mean value, we can detect a fold change of 1.25× at the significance level an α = 0.05 with a detection power of 80%.
Real time qPCR validation.
A subset of genes was validated by quantitative real-time PCR to validate the RNA-Seq results. The genes selected for validation were from the pool of DEGs, which were differentially regulated among male and female mice. RNA (50 ng), isolated as above, was subjected to quantitative TaqMan RT-PCR using 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). 18S was used as the reference gene. Quantitative values were obtained from the threshold PCR cycle number (CT), at which the increase in signal associated with an exponential growth for PCR product becomes detectable. Relative mRNA levels for chosen target genes were normalized to 18S content. Relative expression levels of each target gene were calculated according to the equation, , where ΔCT = CT target gene − CT 18S gene. The primers used are listed in Table 1.
Table 1.
Primer list
| Serial No. | Gene Symbol | Gene Name | Identifier |
|---|---|---|---|
| 1 | Cxcl5 | C-X-C motif chemokine ligand 5 | Mm00436451_g1 |
| 2 | Cpa3 | Carboxypeptidase A3 | Mm00483940_m1 |
| 3 | Fhl5 | Four and a half LIM domains 5 | Mm00480451_m1 |
| 4 | Hif2/Epas1 | Endothelial PAS domain protein 1 | Mm01236112_m1 |
| 5 | Capn9 | Calpain9 | Mm00499260_m1 |
| 6 | Rhox5 | Reproductive homeobox 5 | Mm00476718_m1 |
| 7 | Mpo | Myeloperoxidase | Mm01298424_m1 |
| 8 | Gdf6 | Growth differentiation factor 6 | Mm01222341_m1 |
| 9 | Cxcl3 | C-X-C motif chemokine ligand 3 | Mm01701838_m1 |
| 10 | Vegfa | Vascular endothelial growth factor | Forward: CGGATCAAACCTCACCAAAG |
| Reverse: TCTGGCTTTGTTCTGTCTTTCTT | |||
| 11 | Vegfr2 | Vascular endothelial growth factor receptor 2 | Forward: TTTGGTTTTGGAAGGTTTGC |
| Reverse: CACGTAAGAGTCCGGAAGGA | |||
| 12 | Ang2 | Angiopoietin 2 | Forward: CAACATCAAGGCCATCTGTG |
| Reverse: TTTGTGTGTGCAAGTGGTGA | |||
| 13 | Tie2 | TEK receptor tyrosine kinase | Forward: AGGGCCTAGAGCCAGAGACT |
| Reverse: ATGTGGAAGCGTCCTCAGTT | |||
| 14 | Phd2 | Hypoxia-inducible factor prolyl hydroxylase 2 | Forward: GGAAGATGGAGAACCTGCTG |
| Reverse: GCTTGTGCTTCTTCCAGTCC |
ChIP-qPCR analysis of Hif-1α target genes.
Chromatin immunoprecipitation (ChIP) coupled with quantitative PCR was used to investigate binding of Hif-1α to its known target genes Ca-9 (carbonic anhydrase 9) and Vegf-b (vascular endothelial growth factor-b). The ChIP assay was carried out using a Magna ChIP G tissue kit (catalog no.17-20000; Millipore, Billerica, MA) according to the manufacturer’s instructions. Briefly, fresh mouse lung tissues were stabilized for 3 min using stabilization buffer. The tissues were then incubated in 2% formaldehyde for 20 min at 25°C. After being washed three times with PBS, the lung tissues were incubated in lysis buffer for 15 min on ice. Finally, the lung tissues were sonicated in the dilution buffer using a sonicator with a microprobe at 4°C. Chromatin was pulled down using the following antibodies: IgG (ab124055; Abcam, Cambridge, UK) and Hif1a (ab2185). Real-time PCR was performed on promoter regions of CA9 and VEGFB. The PCR thermal conditions were as follows: one cycle at 95°C for 1 min, 40 cycles at 95°C for 15 s, and one cycle at 60°C for 15 s. The following primers were used: CA9: AAAGGGCACTGTGAGTCAGC (forward), ACACTGTGGACGGGCTGTA (reverse); VEGFB: GCCCCTGAGAGGCTTAACTA (forward), GTGACCTGAAGGACCGAATC (reverse).
Analysis of the preexisting human data set from NCBI GSE32472.
Normalized transcriptome data for a human cohort of human babies with BPD and corresponding controls was downloaded from NCBI GEO. Data were further processed using the R statistical system to generate transcriptome-wide rank file. Gene set enrichment analysis was used to identify enriched pathways and processes against the MSigDB HALLMARK compendium; significance was achieved for an fdr-adjusted q value of <0.25.
RESULTS
Sex-specific differential pulmonary gene expression in hyperoxic lung injury.
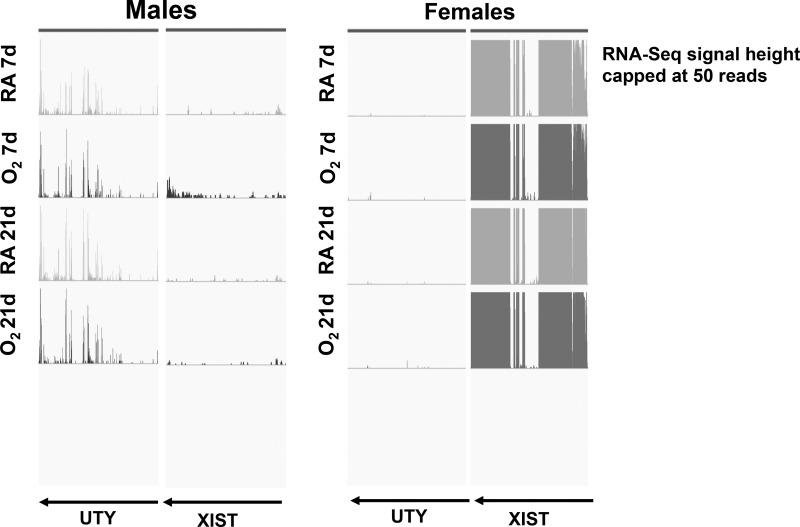
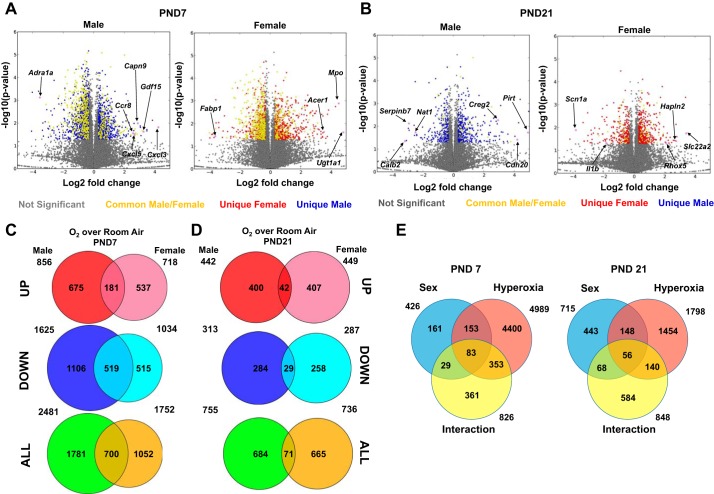
Lung mRNA samples from exposed mice of either sex and room air controls were subjected to RNA-Seq analysis to analyze differences in the pulmonary transcriptome. The sexes of the animals were ascertained using genotyping for the Sry (sex-determining region in Y) gene, as described in materials and methods, but we also analyzed the expression levels of Uty (ubiquitously transcribed tetratricopeptide repeat containing, Y-linked) and Xist (X inactive specific transcript) in the submitted samples. Figure 1 shows the RNA-Seq signals (defined as uniquely mapped read counts) at two genomic loci, Uty (a Y chromosome-specific gene) and Xist (an X chromosome-specific gene), in representative samples for each of the experimental groups submitted for RNA-Seq analysis. As expected, Uty expression was higher in the male samples, and Xist expression was higher in the female samples. Figure 2, A and B, shows the volcano plots of the differentially expressed genes (DEGs; upregulated and downregulated) in male and female neonatal mice in response to hyperoxia exposure compared with room air controls. The genes shaded in yellow are common DEGs between male and female neonatal mice, whereas the genes represented in blue are DEGs exclusive to male, and the genes in red are exclusive to female neonatal mice. On PND7, 1,781 (675 upregulated and 1,106 downregulated) genes were exclusively differentially regulated in hyperoxia exposed neonatal male mice compared with room air controls, whereas 1,052 (537 upregulated and 515 downregulated) DEGs were noted in hyperoxia-exposed female mice. 700 genes (181 upregulated and 519 downregulated) were common to both sexes. The males (1106 genes) had a significantly higher (more than twice as many) number of exclusive downregulated genes compared with females (515 genes) after hyperoxia exposure on PND7. On PND21, 684 exclusive DEGs (400 upregulated and 284 downregulated) were observed in hyperoxia-exposed male mice compared with room air controls, and 665 DEGs (407 upregulated and 258 downregulated) were noted in female mice. Only 71 genes (42 upregulated and 29 downregulated) were common in male and female mice. These results are shown in the Venn diagrams (Fig. 2, C and D. The changes in the pulmonary transcriptome in response to hyperoxia had a robust overlap at PND7 but diverged considerably on PND21. Thus, the sexual dimorphism in pulmonary gene expression was increased on PND21 after recovery in room air from PND6 to PND21. When analyzed by two-way ANOVA (Fig. 2E), on PND7 4,989 genes were differentially regulated by hyperoxia, 426 by sex, and 826 genes showed a significant interaction. On PND21, 1,798 genes were differentially regulated by hyperoxia, 715 by sex, and 848 genes showed a significant interaction between the two variables. Tables 2 (upregulated) and 3 (downregulated) show exclusive genes, with some of the highest fold change in male and female mice on PND7. Tables 4 and 5 show the exclusive up- and downregulated genes in male and female mice on PND21.
Fig. 1.
Expression levels of UTY (ubiquitously transcribed tetratricopeptide repeat containing, Y-linked) and XIST (an X chromosome-specific gene) in male and female neonatal mice lung samples. Lung mRNA samples from mice exposed to hyperoxia [95% , postnatal day (PND)1–5] or room air (RA) were submitted for RNA-Seq analysis. The expression levels of UTY (a Y chromosome-specific gene) and XIST (an X chromosome-specific gene) were determined. RNA-Seq signals at the UTY and XIST loci are shown for representative samples from each treatment group and confirm our determination of the sex.
Fig. 2.
Distinct pulmonary transcriptomic responses were noted in male and female neonatal mice after exposure to hyperoxia. A and B: volcano plots of the differentially expressed genes (DEGs; upregulated and downregulated) in male and female neonatal mice in response to hyperoxia exposure compared with room air controls on PND7 (A) and PND21 (B). The genes shaded in yellow are common DEGs between male and female neonatal mice, whereas the genes represented in blue are DEGs exclusive to male neonatal mice, and those in red are exclusive to female neonatal mice. Select differentially regulated genes are highlighted. C and D: Venn diagrams highlighting the differential gene expression on PND7 (C) and PND21 (D) in male and female neonatal mice exposed to hyperoxia. E: 2-way ANOVA of the pulmonary transcriptome showing effect of hyperoxia, sex, and any interaction between the 2 variables.
Table 2.
Upregulated genes exclusive to a given sex (hyperoxia-exposed mice compared with room air controls) on PND7
| Gene Symbol | Gene Name (PND7) | Fold Change |
|---|---|---|
| Male | ||
| Cxcl3 | C-X-C motif chemokine ligand 3 | 18.9 |
| Rfx4 | Regulatory factor X4 | 11.3 |
| Nxnl1 | Nucleoredoxin-like 1 | 11.3 |
| Gdf15 | Growth differentiation factor 15 | 9.7 |
| Drd1a | Dopamine receptor D1 | 8.6 |
| Slc39a12 | Solute carrier family 39, member 12 | 8.3 |
| Capn9 | Calpain9 | 7.5 |
| CxCl5 | C-X-C motif chemokine ligand 5 | 6.5 |
| Clec5a | C-type lectin domain family 5 member A | 6.2 |
| CCr8 | C-C motif chemokine receptor 8 | 6.01 |
| Female | ||
| Ugt1a1 | UDP-glucuronosyltransferase 1A1 | 28.22 |
| Mpo | Myeloperoxidase | 22.73 |
| Tpsg1 | Tryptase-γ1 | 19.72 |
| Clec4a4 | C-type lectin domain family 4 member A | 14.34 |
| Tfap2c | Transcription factor AP-2γ | 13.66 |
| Acer1 | Alkaline ceramidase 1 | 11.62 |
| Ghrh | Growth hormone-releasing hormone | 11.06 |
| Gabra1 | Gamma-aminobutyric acid type a receptor-α1 subunit | 10.96 |
| Gjd2 | Gap junction protein-δ2 | 10.81 |
| Ceacam19 | Carcinoembryonic antige- related cell adhesion molecule 19 | 10.45 |
PND7, postnatal day 7.
Table 3.
Downregulated genes exclusive to a given sex (hyperoxia-exposed mice compared with room air controls) on PND7
| Gene Symbol | Gene Name (PND7) | Fold Change |
|---|---|---|
| Male | ||
| Mdga2 | MAM domain containing glycosylphosphatidylinositol anchor 2 | 0.2 |
| Samd7 | Sterile-α motif domain containing 7 | 0.2 |
| Myt1 | Myelin transcription factor 1 | 0.2 |
| Sox8 | SRY-box 8 | 0.2 |
| Adra1a | Adrenoceptor-α1A | 0.2 |
| Isx | Intestine-specific homeobox | 0.2 |
| Noto | Notochord homeobox | 0.2 |
| Ptchd1 | Patched domain containing 1 | 0.2 |
| Upb1 | β-Ureidopropionase 1 | 0.2 |
| Female | ||
| Tmem90b | Transmembrane protein 90B | 0.06 |
| Lhcgr | Luteinizing hormone/choriogonadotropin receptor | 0.07 |
| Chrna7 | Cholinergic receptor, nicotinic-α7 | 0.07 |
| Hoxb1 | Homeobox 1 | 0.08 |
| Fabp1 | Fatty acid-binding protein 1 | 0.08 |
| Shc3 | SHC adaptor protein 3 | 0.08 |
| Cyp2e1 | Cytochrome P450 family 2 subfamily E member 1 | 0.18 |
| Pou4f1 | POU class 4 homeobox 1 | 0.08 |
| Tat | Tyrosine aminotransferase | 0.1 |
PND7, postnatal day 7.
Table 4.
Upregulated genes exclusive to a given sex (hyperoxia-exposed mice compared with room air controls) on PND21
| Gene Symbol | Gene Name (PND21) | Fold Change |
|---|---|---|
| Male | ||
| Pirt | Phosphoinositide-interacting regulator of transient receptor potential channels | 30 |
| Gp2 | Glycoprotein 2 | 20.9 |
| Cdh20 | Cadherin 20 | 18.4 |
| Slc12a3 | Solute carrier family 12 member 3 | 16.3 |
| Klkb1 | Kallikrein B1 | 11.7 |
| Mboat4 | Membrane-bound O-acyltransferase domain containing 4 | 9.5 |
| Ern2 | Endoplasmic reticulum to nucleus signaling 2 | 8.9 |
| Creg2 | Cellular repressor of E1A stimulated genes 2 | 7.7 |
| Phgr1 | Proline, histidine, and glycine rich 1 | 5.4 |
| Cdhr1 | Cadherin-related family member 1 | 5.1 |
| Female | ||
| Slc22a2 | Solute carrier family 22 member 2 | 10.7 |
| Gnb3 | G protein subunit-β3 | 7.03 |
| Hapln2 | Hyaluronan and proteoglycan link protein 2 | 6.82 |
| TCerg1l | Transcription elongation regulator 1-like protein | 6.40 |
| Fxyd4 | FXYD domain containing ion transport regulator 4 | 5.18 |
| Rhox5 | Rhox homeobox family member 5 | 4.32 |
| Zbtb16 | Zinc finger and BTB domain containing 6 | 3.86 |
| Col22a1 | Collagen type XXII α1-chain | 3.78 |
| Adra2a | Adrenoceptor-α2A | 3.40 |
| Mas1 | Mas1 oncogene | 3.30 |
PND21, postnatal day 21.
Table 5.
Downregulated genes exclusive to a given sex (hyperoxia-exposed mice compared with room air controls)
| Gene Symbol | Gene Name (PND21) | Fold Change |
|---|---|---|
| Male | ||
| Calb2 | Calbindin 2 | 0.1 |
| Serpinb7 | Serpin family B member 7 | 0.1 |
| Ibsp | Integrin binding sialoprotein | 0.1 |
| Nat1 | N-acetyltransferase 1 | 0.2 |
| Cenpf | Centromere protein F | 0.2 |
| Prss41 | Protease, serine 41 | 0.3 |
| Txndc2 | Thioredoxin domain containing 2 | 0.3 |
| Fam83a | Family with sequence similarity 83 member A | 0.3 |
| Lrr1 | Leucine-rich repeat protein 1 | 0.3 |
| SStr4 | Somatostatin receptor 4 | 0.3 |
| Female | ||
| Otop1 | Otopetrin 1 | 0.05 |
| Scn1a | Sodium voltage-gated channel α-subunit 1 | 0.07 |
| Epgn | Epithelial mitogen | 0.1 |
| Prss57 | Protease, serine 57 | 0.1 |
| Esrrb | Estrogen-related receptor-β | 0.1 |
| Cyp2d26 | Cytochrome P450 family 2 subfamily D member 6 | 0.1 |
| Crybb1 | Crystallin-β B1 | 0.1 |
| Cebpe | CCAAT/enhancer-binding protein-ε | 0.2 |
| Sele | Selectin E | 0.2 |
| Il1b | Interleukin-1β | 0.3 |
PND21, postnatal day 21.
Pathway analysis of DEGs.
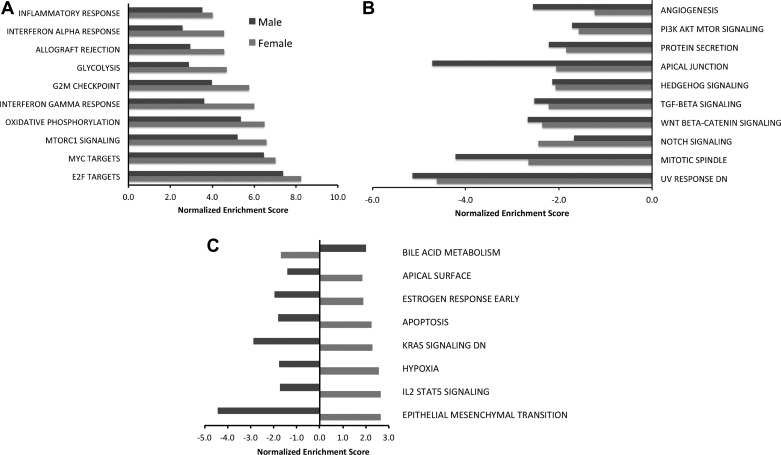
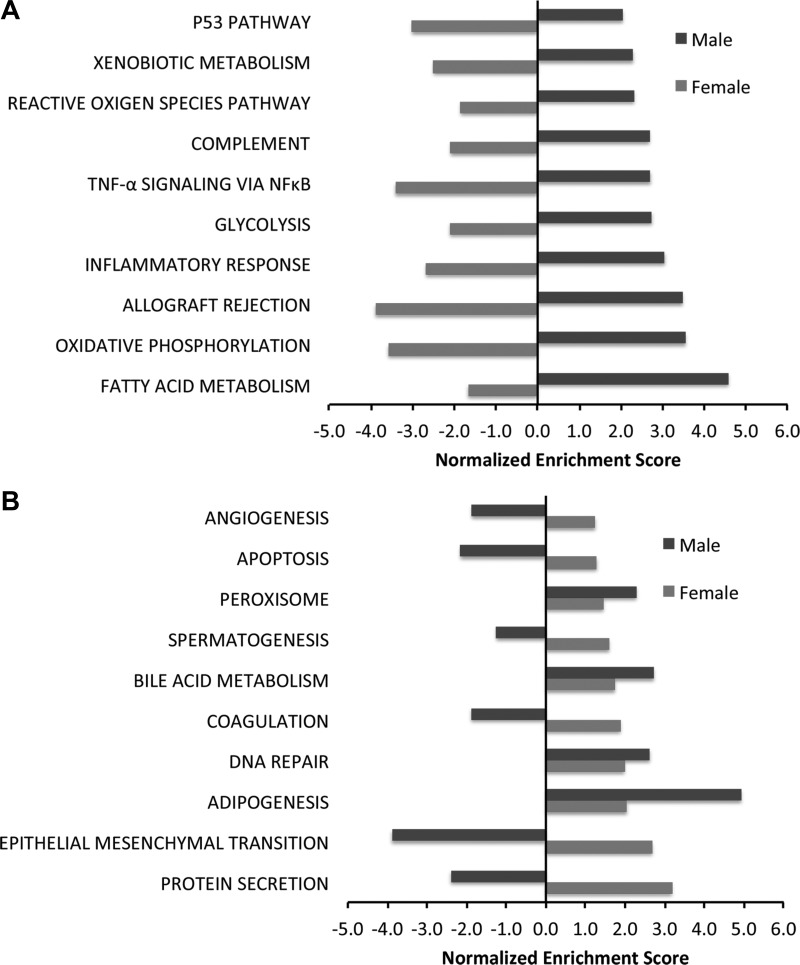
Biological processes that were enriched in neonatal mice after exposure to hyperoxia as well as those that were differentially modulated between male and female mice were identified using gene set enrichment analysis (GSEA) and the Hallmark pathway compendium. Figure 3, A and B, shows the major biological processes that were enriched in the same direction in both male and female mice on PND7. The top five pathways that were upregulated in both male and female animals exposed to hyperoxia on PND7 were E2F targets, Myc targets, MTOR signaling, oxidative phosphorylation, and IFN-Υ response. Angiogenesis, phosphatidylinositol 3-kinase (PI3K)-Akt signaling, and Wnt-β catenin signaling were among the top downregulated pathways. Figure 3C shows the biological pathways that were enriched in opposite directions between males and females on PND7 [q < 0.25; normalized enrichment score (NES) has opposite signs between male and female transcriptome responses]. These included early estrogen response, apoptosis, response to hypoxia, and IL-2 Stat5 signaling pathway, which were upregulated in females and downregulated in males, whereas the pathway of bile acid metabolism was downregulated in females and upregulated in males. On PND21, the sex-specific differences were more marked between male and female mice. Figure 4A shows the top upregulated processes in females on PND21. Protein secretion, epithelial mesenchymal transition, and coagulation were upregulated in females, whereas these were downregulated in similarly exposed male mice on PND21. Adipogenesis, DNA repair, peroxisome, and bile acid metabolism were upregulated in both males and females. In males, fatty acid metabolism, oxidative phosphorylation, allograft rejection and inflammatory response, and p53 pathway were upregulated, whereas these processes were downregulated in females (Fig. 4B). Interestingly, angiogenesis was upregulated in females and downregulated in males, and the biological process of reactive oxygen species was upregulated in males and downregulated in females on PND21.
Fig. 3.
Distinct modulations of pathways are observed between male and female neonatal mice exposed to hyperoxia by gene set enrichment analysis (GSEA) on PND7. Biological processes enriched in the pulmonary transcriptome of hyperoxia-exposed male and female neonatal mice were identified using GSEA. The normalized enrichment score is reported for select enriched pathways (fdr-adjusted q value <0.25). A: top upregulated pathways in both male and female neonatal mice exposed to hyperoxia. B: top downregulated pathways in both male and female neonatal mice. C: pathways that were regulated in opposite directions in male and female mice. PI3K, phosphatidylinositol 3-kinase.
Fig. 4.
Sexually dimorphic modulation of pathways is observed between male and female neonatal mice exposed to hyperoxia by gene set enrichment analysis (GSEA) on PND21. Biological processes enriched in the pulmonary transcriptome of hyperoxia-exposed male and female neonatal mice were identified using GSEA. The normalized enrichment score is reported for select enriched pathways (fdr-adjusted q value <0.25). A: top upregulated pathways in male neonatal mice exposed to hyperoxia on PND21. The graph also shows the direction of enrichment in similarly exposed female mice. B: top upregulated pathways in female mice on PND21 and the direction of enrichment in similarly exposed male mice.
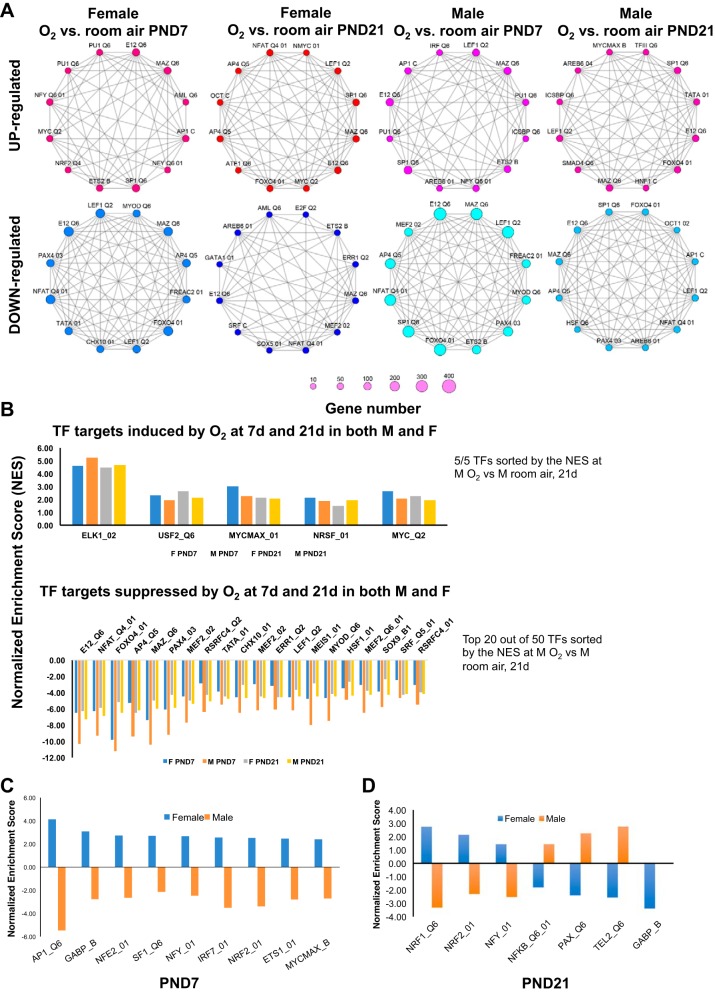
Transcriptional factor analysis.
We identified the network of transcription factors (TFs) that was responsible for modulating the pulmonary gene expression changes after hyperoxia exposure in the lungs of male and female mice. Figure 5A shows networks of the top twelve transcription factors involved in the hyperoxia response via upregulated and downregulated genes in male and female mice on PND7 and PND 21, respectively (hypergeometric distribution, P < 0.05). We then sought to analyze temporal differences in modulation of transcription factors (TFs) between male and female mice using GSEA. In general, there was greater suppression of transcription factor targets in both male and female mice after hyperoxia exposure. As shown in Fig. 5B, five TFs were induced at PND7 and PND21 in both male and female mice, including Elk1 (member of the Ets family of transcription factors and of the ternary complex factor subfamily), Usf2 (upstream transcription factor 2, C-Fos interacting), Myc, and Nrsf. In contrast to the other genes, which act as transcriptional activators, Nrsf, also known as RE1 silencing transcription factor, is a transcriptional repressor that suppresses neuronal genes in nonneuronal tissues. Figure 5, C and D, shows the TFs that were regulated in opposite directions in male and female neonatal mice on PND7 and PND21, respectively, shown in Table 6. Of note is that TFs such as Ap1 and Nrf2 are known to play a role in the modulation of hyperoxic lung injury.
Fig. 5.
Sex-specific differences in transcriptional regulator network modulating the response to hyperoxia. A: analysis of upstream transcriptional factors was performed using overrepresentation analysis to identify the key transcription factors modulating the transcriptomic response to hyperoxia (hypergeometric distribution, P < 0.05). Top transcriptional regulators in up- and downregulated genes are depicted as network nodes; edges indicate common gene targets between transcription factors. B–D: an extensive search was carried out for transcriptional regulators that were enriched after hyperoxia exposure (q < 0.25) using GSEA, and the results were clustered by the relative direction of the gene targets in male and female hyperoxia response. B: transcription factors with gene targets enriched in the same direction, either upregulated in both or downregulated in both male and female hyperoxia-exposed neonatal mice at PND7 and PND21. C: transcriptional regulators with a positive normalized enrichment score in females at PND7 acting primarily as transcriptional activators for female mice hyperoxia while having a negative normalized enrichment score in males at PND7 acting primarily as transcriptional repressors for male mice hyperoxia. D: transcriptional regulators with opposite normalized enrichment score in female vs. male hyperoxia response at PND21.
Table 6.
Transcription factors differentially regulated between male and female mice on PND7 or PND21
| Gene Symbol | Gene Name (Function) |
|---|---|
| PND7 (upregulated in females, downregulated in males) | |
| Ap1 | Activator protein 1 (early stress response factor) |
| Nrf2 | Nuclear factor, erythroid 2 (activated antioxidant genes (30); |
| Sf1 | Splicing factor 1 |
| Nfy | Nuclear transcription factor Y (interacts with p53, especially under conditions of DNA damage,e and plays a crucial role in determining cell survival or death (20); |
| Gabpb | GA-binding protein transcription factor-β |
| Mycmax | Myc-associated factor X |
| Irf7 | Interferon regulatory factor 7 (induced in the lungs of mice exposed to LPS and mechanical ventilation (1); |
| Ets-1 | ETS proto-oncogene 1 |
| PND21 (upregulated in females, downregulated in males) | |
| Nrf1 | Nuclear respiratory factor 1 |
| Nrf2 | Nuclear factor, erythroid 2 |
| Nfy | Nuclear transcription factor Y |
| PND21 (downregulated in females, upregulated in males) | |
| NF-κb | Nuclear factor-κB |
| Gabpb | GA-binding protein transcription factor-β |
| Pax2 | Paired box 2 |
| Tel2/Etv7 | ETS variant 7 (mTOR complex assembly (21) |
PND7, postnatal day 7; PND21, postnatal day 21.
Real time qPCR validation.
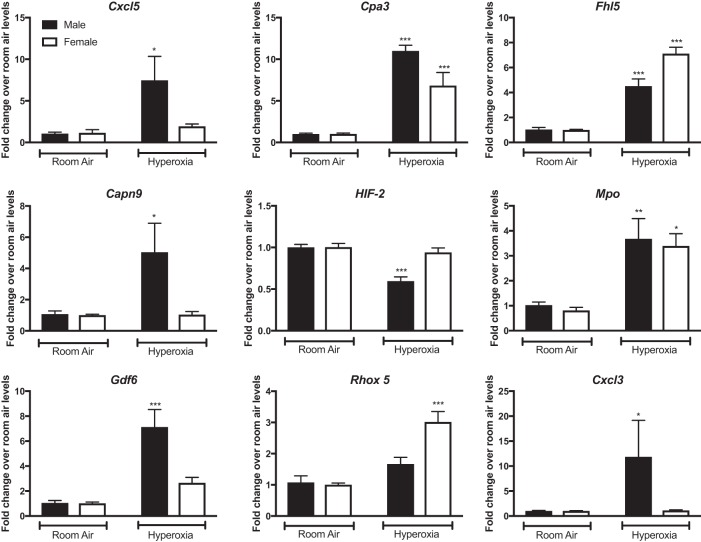
Results from the RNA-Seq analyses were verified by qPCR (Fig. 6). We assessed differences in pulmonary gene expression at PND7. The fold change for each gene was calculated relative to its expression levels in the respective room air controls. Male mice showed significant increases in expression levels of genes such as Cxcl3 (C-X-C motif chemokine ligand 3), Capn9 (calpain 9), Cxcl5 (C-X-C motif chemokine ligand 5), and Gdf6 (growth differentiation factor 6) after exposure to hyperoxia compared with room air controls, whereas female mice did not. There was significant increase in the expression of Cpa3 (carboxypeptidase 3), a mast cell specific marker in both male and female mice, but the increase was greater in hyperoxia-exposed male mice. Female mice showed increased expression of Rhox5 (reproductive homeobox 5) after exposure to hyperoxia, whereas no such increase was noticeable in male mice. There was decreased expression of Hif-2 in male mice at PND7, whereas no decrease in expression was noted in female mice. Both sexes showed increased expression on Fhl5 (four and a half LIM domains 5) and Mpo (myeloperoxidase) after exposure to hyperoxia. There was good correlation in fold changes measured by real-time PCR and by RNA-Seq for the experimentally validated genes, as shown in Table 7. The Pearson correlation coefficient for fold change in gene expression was 0.52 between real-time PCR and RNA-Seq.
Fig. 6.
Quantitative (q)RT-PCR analysis of mRNA from the lungs of male and female neonatal mice (PND7) exposed to room air or hyperoxia shows differential regulation of gene targets. Fold change over room air levels is represented on the y-axis. Two-way ANOVA was used to assess statistical significance in gene expression among sex and the effect of hyperoxia. Significant differences from room air levels are represented as follows: *P < 0.05, **P < 0.01, and ***P < 0.001 (n = 5/group).
Table 7.
Table showing fold change in gene expression for selected genes with real-time PCR and RNA-Seq
| Fold Change From Room Air |
|||||
|---|---|---|---|---|---|
| PCR |
RNA-Seq |
||||
| Gene Name | Gene Symbol | Male | Female | Male | Female |
| C-X-C motif chemokine ligand 5 | Cxcl5 | 7.471 | 1.93 | 6.46 | 2.79 |
| Carboxypeptidase A3 | Cpa3 | 10.988 | 6.827 | 6.74 | 8.01 |
| Four and a half LIM domains 5 | Fhl5 | 4.512 | 7.112 | 2.34 | 4.83 |
| Endothelial PAS domain protein 1 | Hif2/Epas1 | 0.595 | 0.941 | 0.57 | 0.59 |
| Calpain 9 | Capn9 | 5.039 | 1.037 | 7.47 | 2.16 |
| Reproductive homeobox 5 | Rhox5 | 1.668 | 3.015 | 0.97 | 4.56 |
| Myeloperoxidase | Mpo | 3.675 | 3.393 | 7.72 | 22.73 |
| Growth differentiation factor 6 | Gdf6 | 7.126 | 2.652 | 9.13 | 6.25 |
| C-X-C motif chemokine ligand 3 | Cxcl3 | 11.843 | 1.117 | 19.00 | 3.58 |
PAS, Per/Arnt/Sim; LIM, Lin11/ISl1/Mec3.
ChIP-qPCR analysis of Hif-1α target genes in male and female neonatal mice after exposure to hyperoxia.
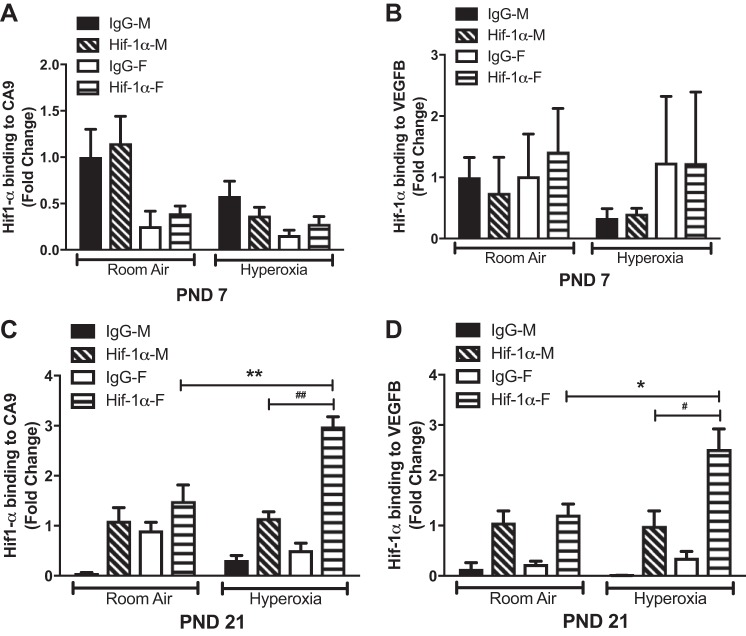
Pathway analysis revealed that the response to hypoxia pathway was upregulated in females and downregulated in males on PND7. Angiogenesis was upregulated in female and downregulated in males at PND21. Since Hif-1α is considered the master transcriptional regulator of cellular and developmental response to hypoxia and also plays a key role in pulmonary vascular development, we analyzed the sex-specific differences in the binding of Hif-1α to its known target genes on PND7 and PND21. We performed ChIP targeted against Hif-1α and performed qPCR with primers for Ca9 and Vegf-b, which are known downstream target genes activated by Hif-1α (27, 48). These results are shown in Fig. 7. On PND7, no significant binding was seen at either the Ca9 or Vegfb site (Fig. 7A); however on PND21, hyperoxia-exposed female mice showed a significant increase in Hif-1α, binding to both the target genes compared with room air controls and hyperoxia-exposed male mice (Fig. 7B).
Fig. 7.
Chromatin immunoprecipitation-qPCR analysis of Hif-1α target genes in male and female neonatal mice after exposure to hyperoxia; Hif-1α binding to CA9 (A and C) and VEGFB (B and D) promoter region in lung tissue in hyperoxia-treated male and female mice at PND7 and -21. Data are shown as means ± SE; n ≥ 3 in each group. *P < 0.05 and **P < 0.01 (room air vs. hyperoxia treated); #P < 0.05 and ##P < 0.01 (male vs. female). Two-way ANOVA was used to assess statistical significance in gene expression among genotypes and the effect of hyperoxia.
Focused angiogenesis pathway analysis and gene expression of key angiogenic mediators in male and female neonatal mice after hyperoxia exposure.
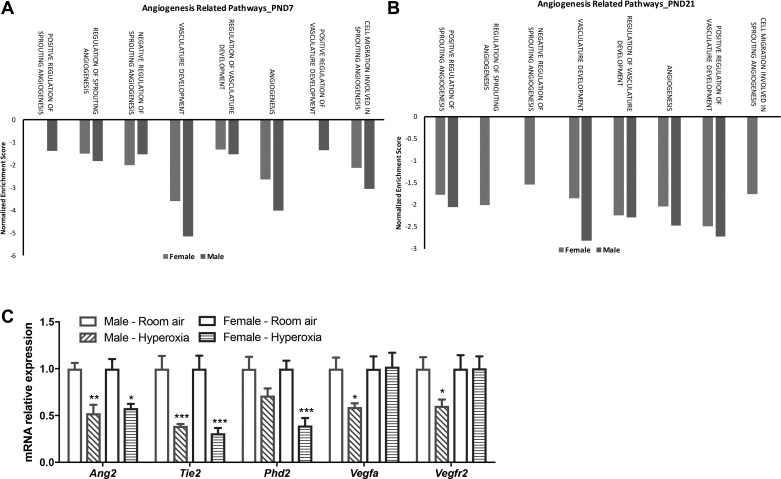
We have shown previously that male neonatal mice have greater impairment in pulmonary vascular development at PND21 after hyperoxia exposure compared with similarly exposed female mice (25). Since the angiogenesis pathway was differentially modulated between male and female mice, we performed further focused pathway analysis for different angiogenesis-related pathways using gene set enrichment analysis against the Gene Ontology (GO) compendium. On PND7, pathways such as positive regulation of vasculature development and sprouting angiogenesis were downregulated in males (Fig. 8A). Most of the other pathways were suppressed to a greater extent in males. A similar pattern was observed at PND21 with equal or greater suppression of angiogenesis-related pathways in male mice, with the exception of cell migration involved in sprouting angiogenesis, which was suppressed only in females. We also measured the expression of key proangiogenic genes on PND21. There was decreased expression of angiopoietin-2 and its receptor Tie-2 in both male and female mice after hyperoxia exposure. Phd2 is the primary regulator of HIF-1α levels in the cell. Interestingly, female mice showed a more significant decrease in Phd2 (HIF prolyl-hydroxylase 2) expression, which may lead to the preservation of Hif-1α levels in the lungs in hyperoxia-exposed female mice. Male mice also showed a greater decrease in Vegf-a and Vegfr2 levels after exposure to hyperoxia compared with similarly exposed female mice. Overall, these results corroborate our previous findings and highlight the differential modulation of crucial proangiogenic mediators in male and female mice exposed to postnatal hyperoxia.
Fig. 8.
Focused angiogenesis pathway analysis and gene expression of key angiogenic mediators in male and female neonatal mice after hyperoxia exposure. A and B: biological processes enriched in the pulmonary transcriptome of hyperoxia-exposed male and female neonatal mice were identified using gene set enrichment analysis (GSEA) on PND7 (A) and PND21 (B). The normalized enrichment score is reported for select enriched pathways (fdr-adjusted q value <0.25). C: qRT-PCR analysis of mRNA from the lungs of male and female neonatal mice (PND21) exposed to room air or hyperoxia shows differential regulation of key proangiogenic gene targets. Fold change over room air levels is represented on the y-axis. Two-way ANOVA was used to assess statistical significance in gene expression among sex and the effect of hyperoxia. Significant differences from room air levels are represented as follows: *P < 0.05, **P < 0.01, and ***P < 0.001 (n = 5/group).
Comparison of sex-specific differential modulation of biological pathways between human preterm neonates and the murine model.
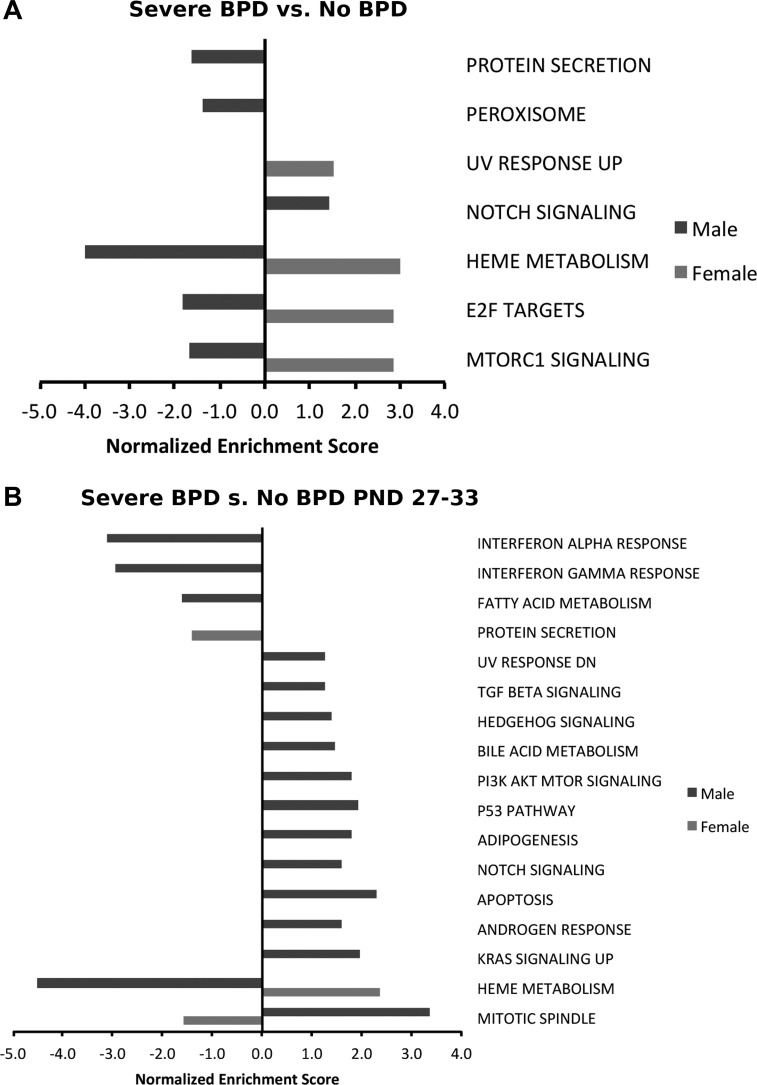
To identify the similarities in differentially modulated pathways between the murine model and human patients, we interrogated the preexisting data set from NCBI GEO with the GEO accession no. GSE32472. This data set has microarray data from RNA samples extracted from peripheral blood leukocytes in preterm neonates (<32 wk, <1,500 g) on 5th, 14th, and 28th days of life. The human cohort contained 68 babies with BPD and 43 controls with no BPD; infants with BPD were further subclassified as having mild, moderate, or severe BPD (38). The babies with severe BPD were younger and smaller than the babies who did not develop BPD. The mean (SD) gestational age of the babies was 29.8 (1.4) wk in the no-BPD group and 25.6 (2.4) wk in the severe-BPD group. The mean birth weight similarly was 1,245 (187) g in the no BPD group and 736 (208) g in the BPD group. Also, more infants in the BPD group were intubated, received surfactant, and received treatment for patent ductus arteriosus. Whereas 9% of the babies with no BPD were small for gestational age (SGA), 33% of the babies with severe BPD were SGA. Also, five of the 43 controls (12%) had a history of maternal fever or infection compared with four of the 15 (27%) in the group with severe BPD. For the purpose of this analysis, we used the gene expression data in babies with severe BPD (n = 15) and controls and identified differentially regulated pathways at PND28 (Fig. 9B) and across all time points (Fig. 9A) in male and female premature neonates. mTOR signaling, E2F targets, and heme metabolism were downregulated in males and increased in females, whereas response to UV radiation was upregulated in female neonates overall. Protein secretion and peroxisome were downregulated in males. Protein secretion was downregulated in male mice at PND21 similarly to the human data, whereas the peroxisomal pathway was upregulated in male mice. Bile acid metabolism, p53 pathway, and adipogenesis were upregulated in male mice at PND21 and premature male newborns with severe BPD at PND28. Although the 28-day time point in human neonates may not be a perfect equivalent to the PND21 time point in mice, and the human transcriptome was generated based on blood samples as opposed to lung in mice, this comparison highlights interesting similarities between the murine model of the disease and human neonates with BPD.
Fig. 9.
Sexually dimorphic modulation of pathways is observed between male and female human neonates with bronchopulmonary dysplasia (BPD) compared with controls with no BPD. Biological processes enriched in the pulmonary transcriptome of hyperoxia-exposed male and female neonatal mice were identified using gene set enrichment analysis. The normalized enrichment score is reported for select enriched pathways (fdr-adjusted q value <0.25). Gene expression data from babies with severe BPD (n = 15) and controls were used to identify differentially regulated pathways across all time points (PND5, -14, and -28; A) and on PND28 (B) and in male and female premature neonates.
DISCUSSION
The key findings of this study are the identification of a comprehensive, sexually dimorphic network of differentially regulated molecular pathways in neonatal hyperoxic lung injury that may explain the susceptibility of male mice to lung injury in this model. Interestingly, the sexual dimorphism in the lung transcriptome was accentuated in the late recovery phase. We have shown previously that after exposure of male and female neonatal mice to hyperoxia (95% , PND1–5), male mice had greater arrest in alveolarization and angiogenesis at PND21 (25). Differences in the lung transcriptome have been described in neonatal hyperoxic lung injury (6, 9), but the sex-specific differences in the pulmonary transcriptome have not been reported previously. In this model, hyperoxia exposure extended from PND1 to PND5, which represents the saccular stage of lung development in mice, corresponding to 24 wk to the late fetal period in human gestation (50). Differences in gene expression were analyzed at PND7 (early), after hyperoxia exposure and PND21 (late), when most of the alveolar development is completed in mice. Apart from the effect of hyperoxia on the pulmonary transcriptome, we also focused on key biological pathways and transcription factors that were regulated in opposite directions between male and female mice. On PND7, compared with females, twice as many differentially regulated genes in male mice were downregulated. This downregulation signature may be in part due to failed alveolarization and vascular development. This phenotype was identified by the focused pathway analysis and validation in crucial domains such as angiogenesis. Our analysis indicated that angiogenesis was suppressed to a greater extent in males on PND7 compared with females and remained suppressed in males at PND21 while recovering in females at PND21.
Effect of hyperoxia exposure on the pulmonary transcriptome.
The E2F target pathway was the top upregulated pathway in both males and females on PND7 (Fig. 3). These transcription factors are required for the regulation of numerous genes involved in DNA replication, cell cycle progression, DNA repair, apoptosis, differentiation, and development. Molecular processes involved in cellular metabolism (such as glycolysis and oxidative phosphorylation), inflammation (inflammatory response, interferon-α and -γ response, and allograft rejection), and cell cycle progression (G2M checkpoint and myc tragets) were also upregulated. These pathways have previously been reported to be associated with BPD in human preterm neonates (11, 38). Angiogenesis was downregulated in hyperoxia-exposed animals, which explains the arrest in pulmonary vascular development in these animals after exposure to hyperoxia. Among the other downregulated pathways are the Wnt/β-catenin and the Notch signaling pathway. Wnt signaling is a critical pathway for normal lung development, and its altered signaling is involved in the pathogenesis of diseases such as bronchopulmonary dysplasia and adult-onset diseases such as COPD and idiopathic pulmonary fibrosis (36). The Notch signaling pathway is involved in branching morphogenesis, alveolarization, and pulmonary vascular development and has also been implicated in other chronic pulmonary diseases such as COPD and pulmonary hypertension (52). Similarly, dysregulation in protein homeostasis and the PI3K/Akt signaling pathway have been described in hyperoxic lung injury (31, 40). Our findings are similar to the study performed in autopsy lung samples of preterm neonates with and without BPD, which also reported cell cycle regulation, immune cell regulation, and sonic hedgehog signaling as differentially regulated in babies with BPD (6).
Sex-specific differential modulation of pathways.
The response to the hypoxia pathway and epithelial-to-mesenchymal transition (EMT) was upregulated in females but downregulated in males on PND7, as shown in Fig. 3C. Although the animals were not subjected to hypoxic conditions in this study, hypoxia at the tissue level cannot be ruled out. Also, there is overlap among the candidate genes included in response to hypoxia pathway and the genes, which play a role in lung injury mediated by hyperoxia exposure. The EMT pathway represents crucial genes that are involved in mesenchymal development, the impairment of which leads to impaired alveologenesis (8). For example, one of the genes in this pathway that was induced (7.2-fold compared with room air controls) in female mice was Spp1 (secreted phosphoprotein 1), which is known to determine pneumocyte growth and lung development in mice (16). On the other hand, in male mice, this pathway was downregulated on both PND7 and -21 and included candidate genes such as PDGFRβ (platelet-derived growth factor receptor-β; 2.4-fold suppression compared with room air controls). PDGF signaling plays an important role in normal lung development. Specifically, PDGFRβ is involved in the activation of the EGFR signaling pathway and angiogenesis (35).
On PND21, the sexual dimorphism in biological pathway modulation was accentuated. This was also evident in the number of DEGs among male and female mice, as shown in Fig. 2. This possibly indicates that recovery from hyperoxic lung injury elicits different genes and pathways in a sex-specific manner, whereas a sex-neutral response with more overlap is seen during the acute phase. The upregulated pathways in male mice, such as p53 pathway, inflammatory response, complement pathway, and reactive oxygen species, were downregulated in females (Fig. 4A). All of the above pathways may represent ongoing apoptosis, inflammation, and oxidative stress in male mice, which may contribute to impaired lung development. Angiogenesis and protein secretion were upregulated in females on PND21 but continued to be suppressed in males (Fig. 4B). Many of the proinflammatory genes validated by PCR showed higher expression in males (Fig. 6). The relevance of the expression of these genes in the setting of hyperoxic lung injury is summarized in Table 8.
Table 8.
Known functions of genes validated by real-time PCR
| Gene Symbol | Known Function |
|---|---|
| Cpa3 (carboxypeptidase A3) | Released by mast cells, and increased expression has been reported in patients with asthma and COPD; increased expression of mast cell markers has also been reported in human BPD patients (6) (28) and in animal models of BPD |
| Cxcl3 (C-X-C motif chemokine ligand 3) | Induced in hyperoxia-induced lung injury as one of the mediators for neutrophil recruitment in the lungs (46) |
| Cxcl5 (C-X-C motif chemokine ligand 5) | Neutrophil recruitment in models of acute lung injury |
| Cap9 (calpain 9) | Class of cysteine proteases that can be activated by calcium influx or under conditions of oxidative stress (39); plays a role in tissue remodeling and fibrosis, and inhibition of calpain has been shown to attenuate fibrosis in a bleomycin-induced lung injury model in mice (47) |
| Gdf6 (growth and differentiation factor 6) | Belongs to the TGFβ superfamily, plays a role in angiogenesis in the retina through its interaction with high-temperature requirement factor A1 (HTRA1) (53) |
| Fhl5 (four and a half limb domains 5) | Expressed with cAMP responsive element modulator and acts as a transcriptional activator (23) |
| Rhox5 (reproductive homeobox 5) | Belongs to a homeobox gene cluster on the mouse X chromosome. Disruption of Rhox5 is known to cause increased germ cell and cancer cell apoptosis (29) |
BPD, bronchopulmonary dysplasia.
Angiogenesis as a key pathway behind the sex-specific differences in neonatal hyperoxic lung injury.
We had previously shown that female mice showed better preservation of alveolarization and angiogenesis following postnatal exposure to hyperoxia. Along with a decreased number of vessels, there was decreased expression of PECAM1/CD31, an endothelial specific marker, and VEGFR2/Flk-1 levels, which is also an important driver of postnatal angiogenesis in the lung in hyperoxia-exposed male mice (25). Hypoxia-inducible factor (HIF) modulates the expression of many oxygen-sensitive genes, including VEGF. HIF-1, HIF-2α, and VEGF expression were significantly decreased in the lungs of neonatal rats exposed to hyperoxia (19). Blockade of prolyl hydroxylase domain-containing proteins preserves the HIF-1α and -2α levels in human microvascular endothelial cells exposed to hyperoxia and leads to increases in VEGF, PECAM-1 levels, and angiogenesis (3). Silencing of Phd2 increased Hif-1α levels and HIF-dependent transcription. Thus, preservation or enhancement of HIF signaling in hyperoxic conditions preserves the proangiogenic environment (10). Unlike HIF-1α, HIF-2α is expressed abundantly even under the normoxic state in both the adult and newborn lung. HIF-2α regulates VEGF expression under normoxic conditions and promotes angiogenesis (12). In our study, there was a significant decrease in Hif-2α expression after hyperoxia exposure in males, but not in females, on PND7. Pathway analysis also revealed that the response to hypoxia and the angiogenesis were upregulated in female and downregulated in males. Because Hif-1α is considered the master transcriptional regulator of cellular and developmental response to hypoxia and also plays a key role in pulmonary vascular development, we hypothesized that the differences in lung vascular development among male and female mice may be explained partly by the gene expression driven by this transcription factor. Our results show that female mice show increased binding of Hif-1α to its target genes, including Vegf-b on PND21, which supports the finding of the upregulation angiogenesis pathway in female mice and not in male mice at PND21. This was also supported by the decreased expression of Phd2 in female mice. Male mice also had decreased expression of Vegf and Vegfr2, which also supports the finding of downregulation of the angiogenesis pathway and the phenotype of impaired pulmonary vascular development that was described previously (25).
We attempted to find similarities between our study and data from human gene expression from male and female premature neonates with BPD. This had a few limitations, as the human gene expression was obtained from peripheral blood leucocytes (compared with murine lung samples) using microarray (as opposed to RNA-Seq for the murine data) and the nonequivalent physiological time points of comparison (day of life 30 in human patients vs. PND7 and -21 in mice). Also, differences between mice and humans in phenotypical features, physiological responses, and regulatory differences affecting gene expression may also limit correlation in the findings. Pathways such as bile acid metabolism, p53, and adipogenesis were similarly upregulated in male mice and premature neonates with BPD.
In conclusion, we present the genome-wide sex-specific changes in gene expression in a model of postnatal hyperoxia exposure. Specifically, differences in the angiogenesis-, inflammation-, and mesenchymal development-related pathways might partially explain the sex-based differences in this model. This increases our understanding of how sex influences the molecular pathways in response to hyperoxia exposure. Since male sex is an independent risk for the development of BPD, knowledge of these sex differences may help devise personalized therapeutic strategies and close this sex-specific disparity in premature neonates.
GRANTS
This work was supported in part by the National Institutes of Health (K08-HL-127103 to K. Lingappan, R01 grants ES-019689, ES-001932, HL-129794, and HL-112516 to B. Moorthy, and grant HL-088343 to X. Couroucli) and American Lung Association Grant RG-418067 to K. Lingappan. The work was further supported by the Alkek Center for Molecular Discovery and CPRIT Award RP170005 (to C. Coarfa, S. Maity, and D. N. Perera). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.C., Y.Z., S.M., W.J., L.W., and K.L. analyzed data; C.C., Y.Z., B.M., and K.L. interpreted results of experiments; C.C., S.M., D.P., and K.L. prepared figures; C.C., Y.Z., and K.L. drafted manuscript; C.C., X.C., B.M., and K.L. edited and revised manuscript; C.C., B.M., and K.L. approved final version of manuscript; Y.Z., W.J., L.W., and K.L. performed experiments; K.L. conceived and designed research.
REFERENCES
- 1.Altemeier WA, Matute-Bello G, Gharib SA, Glenny RW, Martin TR, Liles WC. Modulation of lipopolysaccharide-induced gene transcription and promotion of lung injury by mechanical ventilation. J Immunol 175: 3369–3376, 2005. doi: 10.4049/jimmunol.175.5.3369. [DOI] [PubMed] [Google Scholar]
- 2.Ambalavanan N, Morty RE. Searching for better animal models of BPD: a perspective. Am J Physiol Lung Cell Mol Physiol 311: L924–L927, 2016. doi: 10.1152/ajplung.00355.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asikainen TM, Schneider BK, Waleh NS, Clyman RI, Ho W-B, Flippin LA, Günzler V, White CW. Activation of hypoxia-inducible factors in hyperoxia through prolyl 4-hydroxylase blockade in cells and explants of primate lung. Proc Natl Acad Sci USA 102: 10212–10217, 2005. doi: 10.1073/pnas.0504520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker CD, Abman SH. Impaired pulmonary vascular development in bronchopulmonary dysplasia. Neonatology 107: 344–351, 2015. doi: 10.1159/000381129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger J, Bhandari V. Animal models of bronchopulmonary dysplasia. The term mouse models. Am J Physiol Lung Cell Mol Physiol 307: L936–L947, 2014. doi: 10.1152/ajplung.00159.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharya S, Go D, Krenitsky DLD, Huyck HLH, Solleti SKS, Lunger VAV, Metlay L, Srisuma S, Wert SES, Mariani TJT, Pryhuber GSG. Genome-wide transcriptional profiling reveals connective tissue mast cell accumulation in bronchopulmonary dysplasia. Am J Respir Crit Care Med 186: 349–358, 2012. doi: 10.1164/rccm.201203-0406OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boezen HM, Jansen DF, Postma DS. Sex and gender differences in lung development and their clinical significance. Clin Chest Med 25: 237–245, 2004. doi: 10.1016/j.ccm.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Chao C-M, El Agha E, Tiozzo C, Minoo P, Bellusci S. A breath of fresh air on the mesenchyme: impact of impaired mesenchymal development on the pathogenesis of bronchopulmonary dysplasia. Front Med (Lausanne) 2: 27, 2015. doi: 10.3389/fmed.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho H-Y, van Houten B, Wang X, Miller-DeGraff L, Fostel J, Gladwell W, Perrow L, Panduri V, Kobzik L, Yamamoto M, Bell DA, Kleeberger SR. Targeted deletion of nrf2 impairs lung development and oxidant injury in neonatal mice. Antioxid Redox Signal 17: 1066–1082, 2012. doi: 10.1089/ars.2011.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi CW, Lee J, Lee HJ, Park HS, Chun YS, Kim BI. deferoxamine improves alveolar and pulmonary vascular development by upregulating hypoxia-inducible factor-1α in a rat model of bronchopulmonary dysplasia. J Korean Med Sci 30: 1295–1301, 2015. doi: 10.3346/jkms.2015.30.9.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen J, Van Marter LJ, Sun Y, Allred E, Leviton A, Kohane IS. Perturbation of gene expression of the chromatin remodeling pathway in premature newborns at risk for bronchopulmonary dysplasia. Genome Biol 8: R210, 2007. doi: 10.1186/gb-2007-8-10-r210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, Nemery B, Dewerchin M, Van Veldhoven P, Plate K, Moons L, Collen D, Carmeliet P. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med 8: 702–710, 2002. doi: 10.1038/nm1102-1329b. [DOI] [PubMed] [Google Scholar]
- 13.Dammann CE, Ramadurai SM, McCants DD, Pham LD, Nielsen HC. Androgen regulation of signaling pathways in late fetal mouse lung development. Endocrinology 141: 2923–2929, 2000. doi: 10.1210/endo.141.8.7615. [DOI] [PubMed] [Google Scholar]
- 14.Everhardt Queen A, Moerdyk-Schauwecker M, McKee LM, Leamy LJ, Huet YM. Differential Expression of Inflammatory Cytokines and Stress Genes in Male and Female Mice in Response to a Lipopolysaccharide Challenge. PLoS One 11: e0152289, 2016. doi: 10.1371/journal.pone.0152289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farstad T, Bratlid D, Medbø S, Markestad T; Norwegian Extreme Prematurity Study Group . Bronchopulmonary dysplasia - prevalence, severity and predictive factors in a national cohort of extremely premature infants. Acta Paediatr 100: 53–58, 2011. doi: 10.1111/j.1651-2227.2010.01959.x. [DOI] [PubMed] [Google Scholar]
- 16.Ganguly K, Martin TM, Concel VJ, Upadhyay S, Bein K, Brant KA, George L, Mitra A, Thimraj TA, Fabisiak JP, Vuga LJ, Fattman C, Kaminski N, Schulz H, Leikauf GD. Secreted phosphoprotein 1 is a determinant of lung function development in mice. Am J Respir Cell Mol Biol 51: 637–651, 2014. doi: 10.1165/rcmb.2013-0471OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harijith A, Pendyala S, Reddy NM, Bai T, Usatyuk PV, Berdyshev E, Gorshkova I, Huang LS, Mohan V, Garzon S, Kanteti P, Reddy SP, Raj JU, Natarajan V. Sphingosine kinase 1 deficiency confers protection against hyperoxia-induced bronchopulmonary dysplasia in a murine model: role of S1P signaling and Nox proteins. Am J Pathol 183: 1169–1182, 2013. doi: 10.1016/j.ajpath.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hilgendorff A, Reiss I, Ehrhardt H, Eickelberg O, Alvira CM. Chronic lung disease in the preterm infant. Lessons learned from animal models. Am J Respir Cell Mol Biol 50: 233–245, 2014. doi: 10.1165/rcmb.2013-0014TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosford GE, Olson DM. Effects of hyperoxia on VEGF, its receptors, and HIF-2alpha in the newborn rat lung. Am J Physiol Lung Cell Mol Physiol 285: L161–L168, 2003. doi: 10.1152/ajplung.00285.2002. [DOI] [PubMed] [Google Scholar]
- 20.Imbriano C, Gnesutta N, Mantovani R. The NF-Y/p53 liaison: well beyond repression. Biochim Biophys Acta 1825: 131–139, 2012. doi: 10.1016/j.bbcan.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Kaizuka T, Hara T, Oshiro N, Kikkawa U, Yonezawa K, Takehana K, Iemura S, Natsume T, Mizushima N. Tti1 and Tel2 are critical factors in mammalian target of rapamycin complex assembly. J Biol Chem 285: 20109–20116, 2010. doi: 10.1074/jbc.M110.121699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14: R36, 2013. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lardenois A, Chalmel F, Demougin P, Kotaja N, Sassone-Corsi P, Primig M. Fhl5/Act, a CREM-binding transcriptional activator required for normal sperm maturation and morphology, is not essential for testicular gene expression. Reprod Biol Endocrinol 7: 133, 2009. doi: 10.1186/1477-7827-7-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y-J, Markham NE, Balasubramaniam V, Tang J-R, Maxey A, Kinsella JP, Abman SH. Inhaled nitric oxide enhances distal lung growth after exposure to hyperoxia in neonatal rats. Pediatr Res 58: 22–29, 2005. doi: 10.1203/01.PDR.0000163378.94837.3E. [DOI] [PubMed] [Google Scholar]
- 25.Lingappan K, Jiang W, Wang L, Moorthy B. Sex-specific differences in neonatal hyperoxic lung injury. Am J Physiol Lung Cell Mol Physiol 311: L481–L493, 2016. doi: 10.1152/ajplung.00047.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liptzin DR, Landau LI, Taussig LM. Sex and the lung: Observations, hypotheses, and future directions. Pediatr Pulmonol 50: 1159–1169, 2015. doi: 10.1002/ppul.23178. [DOI] [PubMed] [Google Scholar]
- 27.Liu W, Shen S-M, Zhao X-Y, Chen G-Q. Targeted genes and interacting proteins of hypoxia inducible factor-1. Int J Biochem Mol Biol 3: 165–178, 2012. [PMC free article] [PubMed] [Google Scholar]
- 28.Lyle RE, Tryka AF, Griffin WS, Taylor BJ. Tryptase immunoreactive mast cell hyperplasia in bronchopulmonary dysplasia. Pediatr Pulmonol 19: 336–343, 1995. doi: 10.1002/ppul.1950190605. [DOI] [PubMed] [Google Scholar]
- 29.MacLean JA II, Chen MA, Wayne CM, Bruce SR, Rao M, Meistrich ML, Macleod C, Wilkinson MF. Rhox: a new homeobox gene cluster. Cell 120: 369–382, 2005. doi: 10.1016/j.cell.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 30.McGrath-Morrow SA, Lauer T, Collaco JM, Lopez A, Malhotra D, Alekseyev YO, Neptune E, Wise R, Biswal S. Transcriptional responses of neonatal mouse lung to hyperoxia by Nrf2 status. Cytokine 65: 4–9, 2014. doi: 10.1016/j.cyto.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meiners S, Ballweg K. Proteostasis in pediatric pulmonary pathology. Mol Cell Pediatr 1: 11, 2014. doi: 10.1186/s40348-014-0011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller VM. In pursuit of scientific excellence: sex matters. Am J Physiol Lung Cell Mol Physiol 302: L801–L802, 2012. doi: 10.1152/ajplung.00095.2012. [DOI] [PubMed] [Google Scholar]
- 33.Naeye RL, Freeman RK, Blanc WA. Nutrition, sex, and fetal lung maturation. Pediatr Res 8: 200–204, 1974. doi: 10.1203/00006450-197403000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Neriishi K, Frank L. Castration prolongs tolerance of young male rats to pulmonary O2 toxicity. Am J Physiol 247: R475–R481, 1984. [DOI] [PubMed] [Google Scholar]
- 35.Noskovičová N, Petřek M, Eickelberg O, Heinzelmann K. Platelet-derived growth factor signaling in the lung. From lung development and disease to clinical studies. Am J Respir Cell Mol Biol 52: 263–284, 2015. doi: 10.1165/rcmb.2014-0294TR. [DOI] [PubMed] [Google Scholar]
- 36.Ota C, Baarsma HA, Wagner DE, Hilgendorff A, Königshoff M. Linking bronchopulmonary dysplasia to adult chronic lung diseases: role of WNT signaling. Mol Cell Pediatr 3: 34, 2016. doi: 10.1186/s40348-016-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peacock JL, Marston L, Marlow N, Calvert SA, Greenough A. Neonatal and infant outcome in boys and girls born very prematurely. Pediatr Res 71: 305–310, 2012. doi: 10.1038/pr.2011.50. [DOI] [PubMed] [Google Scholar]
- 38.Pietrzyk JJ, Kwinta P, Wollen EJ, Bik-Multanowski M, Madetko-Talowska A, Günther CC, Jagła M, Tomasik T, Saugstad OD. Gene expression profiling in preterm infants: new aspects of bronchopulmonary dysplasia development. PLoS One 8: e78585, 2013. doi: 10.1371/journal.pone.0078585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Potz BA, Abid MR, Sellke FW. Role of calpain in pathogenesis of human disease processes. J Nat Sci 2: pii:e218, 2016. [PMC free article] [PubMed] [Google Scholar]
- 40.Reddy NM, Potteti HR, Vegiraju S, Chen HJ, Tamatam CM, Reddy SP. PI3K-AKT Signaling via Nrf2 Protects against Hyperoxia-Induced Acute Lung Injury, but Promotes Inflammation Post-Injury Independent of Nrf2 in Mice. PLoS One 10: e0129676, 2015. doi: 10.1371/journal.pone.0129676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saugstad OD. Bronchopulmonary dysplasia-oxidative stress and antioxidants. Semin Neonatol 8: 39–49, 2003. doi: 10.1016/S1084-2756(02)00194-X. [DOI] [PubMed] [Google Scholar]
- 43.Silva DMG, Nardiello C, Pozarska A, Morty RE. Recent advances in the mechanisms of lung alveolarization and the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 309: L1239–L1272, 2015. [DOI] [PubMed] [Google Scholar]
- 44.Stevenson DK, Verter J, Fanaroff AA, Oh W, Ehrenkranz RA, Shankaran S, Donovan EF, Wright LL, Lemons JA, Tyson JE, Korones SB, Bauer CR, Stoll BJ, Papile LA. Sex differences in outcomes of very low birthweight infants: the newborn male disadvantage. Arch Dis Child Fetal Neonatal Ed 83: F182–F185, 2000. doi: 10.1136/fn.83.3.F182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102: 15545–15550, 2005. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sue RD, Belperio JA, Burdick MD, Murray LA, Xue YY, Dy MC, Kwon JJ, Keane MP, Strieter RM. CXCR2 is critical to hyperoxia-induced lung injury. J Immunol 172: 3860–3868, 2004. doi: 10.4049/jimmunol.172.6.3860. [DOI] [PubMed] [Google Scholar]
- 47.Tabata C, Tabata R, Nakano T. The calpain inhibitor calpeptin prevents bleomycin-induced pulmonary fibrosis in mice. Clin Exp Immunol 162: 560–567, 2010. doi: 10.1111/j.1365-2249.2010.04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanimoto K, Tsuchihara K, Kanai A, Arauchi T, Esumi H, Suzuki Y, Sugano S. Genome-wide identification and annotation of HIF-1α binding sites in two cell lines using massively parallel sequencing. HUGO J 4: 35–48, 2010. doi: 10.1007/s11568-011-9150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28: 511–515, 2010. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warburton D, El-Hashash A, Carraro G, Tiozzo C, Sala F, Rogers O, De Langhe S, Kemp PJ, Riccardi D, Torday J, Bellusci S, Shi W, Lubkin SR, Jesudason E. Lung organogenesis. Curr Top Dev Biol 90: 73–158, 2010. doi: 10.1016/S0070-2153(10)90003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolterink-Donselaar IG, Meerding JM, Fernandes C. A method for gender determination in newborn dark pigmented mice. Lab Anim (NY) 38: 35–38, 2009. doi: 10.1038/laban0109-35. [DOI] [PubMed] [Google Scholar]
- 52.Xu K, Moghal N, Egan SE. Notch signaling in lung development and disease. Adv Exp Med Biol 727: 89–98, 2012. doi: 10.1007/978-1-4614-0899-4_7. [DOI] [PubMed] [Google Scholar]
- 53.Zhang L, Lim SL, Du H, Zhang M, Kozak I, Hannum G, Wang X, Ouyang H, Hughes G, Zhao L, Zhu X, Lee C, Su Z, Zhou X, Shaw R, Geum D, Wei X, Zhu J, Ideker T, Oka C, Wang N, Yang Z, Shaw PX, Zhang K. High temperature requirement factor A1 (HTRA1) gene regulates angiogenesis through transforming growth factor-β family member growth differentiation factor 6. J Biol Chem 287: 1520–1526, 2012. doi: 10.1074/jbc.M111.275990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zysman-Colman Z, Tremblay GM, Bandeali S, Landry JS. Bronchopulmonary dysplasia - trends over three decades. Paediatr Child Health 18: 86–90, 2013. doi: 10.1093/pch/18.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]