In primates, the neural basis of touch and of our sense of limb posture and movements has been studied in the peripheral nerves and in somatosensory cortex, but coding in the cuneate and external cuneate nuclei, the first processing stage for these signals in the central nervous system, remains an enigma. We have developed a method to record from these nuclei, thereby paving the way to studying how sensory information from the limb is encoded there.
Keywords: touch, proprioception, neural coding, electrode array, brain stem
Abstract
While the response properties of neurons in the somatosensory nerves and anterior parietal cortex have been extensively studied, little is known about the encoding of tactile and proprioceptive information in the cuneate nucleus (CN) or external cuneate nucleus (ECN), the first recipients of upper limb somatosensory afferent signals. The major challenge in characterizing neural coding in CN/ECN has been to record from these tiny, difficult-to-access brain stem structures. Most previous investigations of CN response properties have been carried out in decerebrate or anesthetized animals, thereby eliminating the well-documented top-down signals from cortex, which likely exert a strong influence on CN responses. Seeking to fill this gap in our understanding of somatosensory processing, we describe an approach to chronically implanting arrays of electrodes in the upper limb representation in the brain stem in primates. First, we describe the topography of CN/ECN in rhesus macaques, including its somatotopic organization and the layout of its submodalities (touch and proprioception). Second, we describe the design of electrode arrays and the implantation strategy to obtain stable recordings. Third, we show sample responses of CN/ECN neurons in brain stem obtained from awake, behaving monkeys. With this method, we are in a position to characterize, for the first time, somatosensory representations in CN and ECN of primates.
NEW & NOTEWORTHY In primates, the neural basis of touch and of our sense of limb posture and movements has been studied in the peripheral nerves and in somatosensory cortex, but coding in the cuneate and external cuneate nuclei, the first processing stage for these signals in the central nervous system, remains an enigma. We have developed a method to record from these nuclei, thereby paving the way to studying how sensory information from the limb is encoded there.
a central question in neuroscience is how sensory representations are transformed as they ascend the neuraxis. In primates, the coding of tactile and proprioceptive information has been extensively studied in the nerve and in somatosensory cortex (SC), which encompasses Brodmann’s areas 3a, 3b, 1, and 2. Sensory representations in SC differ from those at the periphery in several important ways. First, while cutaneous and proprioceptive nerve fibers can be classified into a small number of submodalities, each responding to a different aspect of skin or muscle/tendon stimulation, individual SC neurons integrate sensory signals from multiple submodalities (Prud’homme and Kalaska 1994; Saal and Bensmaia 2014). Second, SC neurons tend to respond selectively to behaviorally relevant stimulus features, while afferents are less selective (Bensmaia et al. 2008; Harvey et al. 2013). In the context of proprioceptive responses, cortical neurons convey complex information about limb state compared with peripheral afferents (Costanzo and Gardner 1981; Gardner and Costanzo 1981; London et al. 2011; London and Miller 2013). Little is known about the coding of upper limb tactile and proprioceptive information in brain stem nuclei and the ventroposterior nucleus of the thalamus. Here, we discuss methodological issues associated with recording from the cuneate nucleus (CN) and external cuneate nucleus (ECN) of awake primates with chronically implanted electrode arrays (see also Richardson et al. 2015, 2016) and discuss preliminary results on the response properties of CN/ECN neurons in awake primates.
Recording from the CN/ECN of awake primates may offer key insights into sensorimotor representations of the upper limb. First, while CN responses are modulated by descending cortical input (Andersen et al. 1962, 1964a), the properties of CN neurons have been almost exclusively studied in anesthetized or decerebrate cats (Andersen et al. 1964a, 1964b; Canedo et al. 2000; Hayward et al. 2014; Jabbur and Banna 1968; Jörntell et al. 2014; O’Neal and Westrum 1973), whose descending input is thus abolished. To the extent that the sensory response properties of CN neurons are shaped by this descending input, then, studies of these properties without this input may be misleading.
Second, it is unclear to what extent neural coding in cat CN/ECN will resemble its primate counterpart because cats and primates use their upper limbs in different ways, especially their paws/hands. In fact, the morphological organization and mechanisms of synaptic processing differ between primates and cats (Biedenbach et al. 1971; Harris et al. 1965; Molinari et al. 1996), highlighting the need to repeat in nonhuman primates studies conducted in cats to understand the organization, response properties, and circuitry in primates. Furthermore, while ECN of cats projects solely to the cerebellum, ECN of primates also projects to the ventral posterolateral nucleus of the thalamus (Boivie and Boman 1981). The functional implications of this divergence have yet to be determined.
In the present study, we first established the somatotopic and submodality (cutaneous vs. proprioceptive) topography of CN/ECN in anesthetized rhesus macaques, using a standard electrode microdrive. Although anatomical tracing studies have been carried out in various nonhuman primates (Otolemur garnettii, Aotus trivirgatus, Saimiri sciureus, Macaca radiata, Macaca mulatta) (Florence et al. 1988; Hummelsheim and Wiesendanger 1985; Qi and Kaas 2006), somatotopic electrophysiological mapping has not been reported and the precise location and extent of the nuclei, necessary for a chronic implant, were not provided. Second, we developed and deployed an approach to chronically implant electrode arrays in CN/ECN, which allowed us to record the responses of CN/ECN neurons in awake, behaving macaques. Third, we characterized the stability of the neuronal signals measured through the electrode arrays and the stability of the receptive fields (RFs). These results build a foundation toward exploring, for the first time, tactile and proprioceptive coding in the CN and ECN of intact, awake, and behaving animals.
METHODS
Surgical Approach for Acute Mapping Procedures
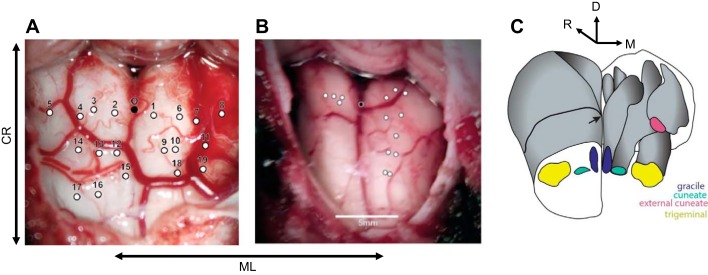
All experimental protocols were reviewed and approved by the University of Chicago Animal Care and Use Committee and the Northwestern University Animal Care and Use Committee and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Surgical anesthesia was induced with ketamine HCl (3 mg/kg im) and dexmedetomidine (75 µg/kg) and maintained with isoflurane (1%). The animal’s head was held in a stereotaxic frame and positioned such that its neck was flexed ~75° relative to the trunk. First, we made a midline incision from the occipital bone to approximately segment C3. Using cautery, we divided the posterior cervical muscles along the midline raphe and removed them from the occipital bone and the posterior ring of segment C1 in a subperiosteal plane. Next, we exposed the foramen magnum and the occipitocervical dura between C1 and the foramen, using a combination of gentle monopolar cautery and sharp dissection. Excess soft tissues were removed to expose clean dura. We enlarged the foramen magnum cranially and laterally with Kerrison rongeurs and excised the dura to provide access to the brain stem both cranially and caudally relative to the obex. We made single-electrode penetrations at various depths within the exposed brain stem (Fig. 1).
Fig. 1.
Surgical exposure for 2 acute procedures. Each white circle represents a penetration site: caudal-rostral (CR), medio-lateral (ML), dorsal (D). A: 1st acute experiment, using low-impedance (0.5 MΩ) electrodes, whose goal was to determine the boundaries of gracile, cuneate, and trigeminal nuclei. B: 2nd acute experiment using higher-impedance electrodes (1–4 MΩ), in which we targeted primarily the right hemisphere to understand submodality organization and somatotopy. Black circle denotes the obex. Cerebellar tonsils are located at top in A and B. C: a reconstructed 3D view of the lower brain stem and relative positioning of the gracile nucleus, cuneate nucleus, external cuneate nucleus, and trigeminal nucleus. Black arrow is pointing toward obex.
Surgical Approach for Chronic Array Implants
We followed a procedure for the chronic array implants similar to that for the acute experiments, with a few exceptions. First, before the skin incision we determined the optimal location for the array pedestal, taking into account skin healing and vulnerability to damage. We also considered the routing of the array lead between pedestal and brain stem, allowing for 1–2 cm of slack for neck movement after the animal woke up. Second, while exposure was similar to that in the acute experiments, the dura over the posterior fossa was opened with a midline linear incision, with the leaves tented back with 6-0 Prolene suture (Ethicon, Somerville, NJ). Third, before the dura was opened, the pedestal was secured to the skull with bone screws, rostral to the occiput.
We implanted Utah Electrode Arrays (UEAs; Blackrock Microsystems, Salt Lake City, UT) and floating microelectrode arrays (FMAs; Microprobes for Life Science, Gaithersburg, MD) into the brain stem of 11 monkeys. Table 1 describes the type of array and design specifications for each implanted array. The FMAs, with electrodes of customized length, allowed us to access neurons with distal cutaneous RFs in the deeper aspects of the nucleus, while UEA electrodes with 1.5-mm-long electrodes were well suited to the location of proprioceptive neurons with RFs on the proximal limb (see below).
Table 1.
Array specifications for each animal
| Monkey ID | Array Type | Size, mm | Electrode Length, mm | Manufacturing Impedance, MΩ | Array Signal Longevity | Likely Failure Mode |
|---|---|---|---|---|---|---|
| WH | FMA HD | 1.6 × 2.95 | 2.0–2.5 | 0.5–0.9 | 6 wk | Wire bundle failure |
| TE | FMA HD | 1.6 × 2.95 | 2.0–2.5 | 0.3–1.1 | 6 mo | Wire bundle failure |
| PI | FMA SD | 1.8 × 4 | 1.5–2.0 | 0.4–0.7 | 6 wk | Wire bundle failure |
| BA | FMA SD | 1.8 × 4 | 2.0–2.5 | 0.7–1.1 | 3 wk | Wire bundle failure |
| CU | FMA HD | 1.6 × 2.95 | 2.0–2.5 | 0.4–1.0 | N/A | Fluid leakage |
| CH2 | FMA SD | 1.6 × 2.8 | 1.5–3.0 | 0.08–1.5 | <1 wk | Array manufacturing defect |
| KR | FMA SD | 1.8 × 4.0 | 1.0–2.0 | 0.3–1 | 2 wk | Array came out |
| MR | FMA HD | 1.6 × 2.8 | 1.25–2.5 | 0.6–1.3 | N/A | Array did not insert |
| HA | FMA SD | 1.8 × 4.0 | 1.0–2.25 | 0.5–1.0 | 6 wk | Wire bundle failure |
| LA1 | FMA HD | 1.6 × 2.8 | 1.0–2.5 | 0.5–1.0 | 2 mo | Wire bundle failure |
| OL | Utah | 4.0 × 4.0 | 1.5 | 0.3–0.8 | 1 day | Meningoencephalitis |
| LA2 | Utah | 4.0 × 4.0 | 1.5 | 0.1–0.8 | >6 mo | N/A |
| CH1 | Utah | 4.0 × 4.0 | 1.3 | 0.2–0.8 | <1 wk | Array came out |
HD, high definition; SD, standard definition; N/A, not applicable.
The insertion technique varied depending on the array: UEAs were implanted with the standard Blackrock pneumatic inserter, and FMAs were inserted slowly with a stereotaxic instrument while being held by a vacuum wand (Musallam et al. 2007; Rousche and Normann 1998). Array insertion was often complicated by brain stem vascularization. Indeed, as a sizable artery often courses along the dorsal brain stem across the desired location of the implant (Fig. 1A), we were occasionally forced to implant the array on the contralateral side to avoid vascular injury.
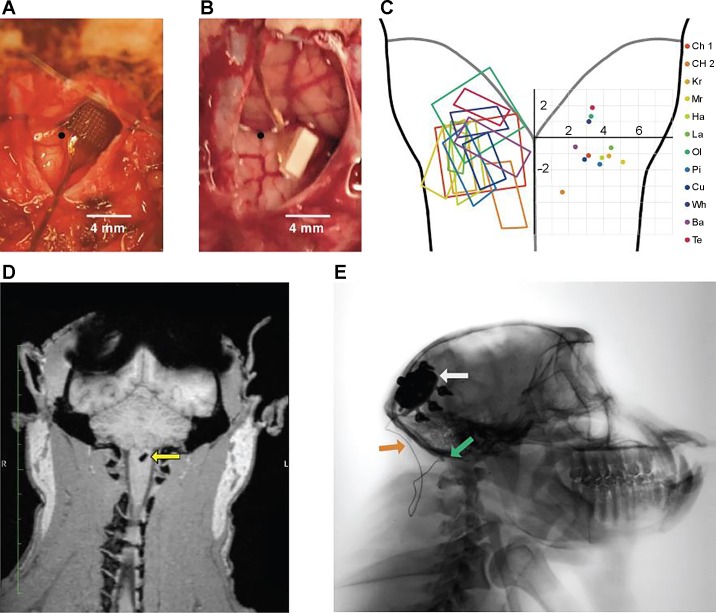
After array insertion, a thin layer of Tisseel fibrin dural sealant (Baxter Healthcare, Deerfield, IL) or Vetbond n-butyl cyanoacrylate (3M, St. Paul, MN) was placed over the array to stabilize it. Indeed, early implants that were not fixed with an adhesive appeared to be expelled from the brain stem shortly after implantation (Table 1; monkeys KR and CH1). The dura was closed with interrupted 6-0 Prolene sutures, with care taken to cinch it around the wire bundle as tightly as possible. Because of the high risk of cerebrospinal fluid leakage with posterior fossa procedures and the fragile nature of the animals’ dura, a layer of DuraGen Plus (Integra, Plainsboro, NJ) followed by a layer of Tisseel was placed over the closed dura to further reduce the chance of a cerebrospinal fluid fistula. Finally, muscle and skin were closed. Figure 2, A and B, show the surgical exposure after insertion of each type of array.
Fig. 2.
Chronic implants. A and B: examples of implanted UEA (A, monkey Ol) and FMA (B, monkey Pi). Obex is indicated by black circle. C, left: approximate location of each array implant shown on a diagram of the brain stem. Right: coordinates of the center of each array. Array locations are all shown on the same hemisphere for visualization purposes. D: 3D MRI scan 5 wk after implantation shows the FMA array (yellow arrow) in the brain stem of monkey Ba. E: sagittal X-ray of monkey Cu shows the pedestal (white arrow), leads (orange arrow), and FMA array (green arrow) 6 wk after implant.
Data Acquisition
In the acute experiments, neural signals were recorded with resin-coated tungsten or glass-coated platinum-iridium electrodes (FHC, Bowdoin, ME) with impedances varying from 0.5 to 4 MΩ. These signals were amplified by a DAM50 amplifier (World Precision Instruments, Sarasota, FL) and simultaneously played through audio speakers and displayed on an oscilloscope. Single-unit and multiunit activity from chronically implanted electrode arrays were recorded with a Cerebus system (Blackrock Microsystems). Spikes were sorted off-line with standard software (Plexon, Dallas, TX).
Receptive Field Mapping
While listening to the neural activity, we manipulated the animals’ joints, squeezed muscles, and gently brushed the surface of the skin to determine whether the neuronal activity was driven by proprioceptive or tactile stimulation. The RFs of cutaneous units were drawn on a body diagram for later analysis. To characterize RF position of both cutaneous and proprioceptive units along the proximal-distal axis of the upper limb, we used a numeric system that defines RF location along this axis: 1, distal digits; 2, proximal digits; 3, wrist; 4, forearm; 5, elbow; 6, upper arm; 7, shoulder.
Vibrotactile Stimulation
We delivered tactile stimuli to the distal pads of the digits with a stainless steel probe with a 1-mm tip diameter, driven by a custom shaker motor (Westling et al. 1976). We delivered sinusoidal stimuli—each 1 s long and separated by a 1-s interstimulus interval—at 5, 10, 20, 30, 40, 50, 100, 200, 250, and 300 Hz. Amplitudes were spaced in 10 equal logarithmic steps spanning the following ranges at each frequency: 13–250 µm at 5–50 Hz, 4–200 µm at 100 Hz, 1–100 µm at 200 Hz, 1.3–75 µm at 250 Hz, and 0.7–50 µm at 300 Hz. The shaker motor was calibrated before each experimental run, and stimuli were presented in pseudorandom order.
Center-Out Task
To study responses to passive perturbations of the arm, the monkey was trained to grasp the handle of a two-dimensional robotic manipulandum that controlled the position of a cursor on a screen. The monkey moved the cursor to a 2-cm target in the center of the screen and held it there for between 1.0 and 1.5 s until a force perturbation was delivered to the handle in a randomly chosen cardinal direction (forward, backward, left, right). The perturbation magnitude was 2.5 N and its duration 125 ms. After the perturbation, the monkey returned the cursor to the center of the screen to receive a liquid reward. Handle kinematics and interface forces were recorded with the Cerebus system at a sampling rate of 100 Hz. Here, we report only CN responses to the perturbation and not to active movements.
Anatomical Imaging
To confirm the position of arrays and leads after weeks of recovery, we performed X-ray on three animals and three-dimensional (3D) magnetic resonance imaging on one animal 5–8 wk after implantation (Fig. 2, D and E). X-ray images in the sagittal plane offer the clearest view of the full implant, including the pedestal, leads, and array.
RESULTS
We characterized the topographical organization of somatosensory brain stem nuclei (CN/ECN, gracile, and trigeminal) in acute experiments and estimated the coordinates for chronic array implantation targeting the cutaneous or proprioceptive representations of the upper limb. Without histological confirmation, we could not distinguish between CN and ECN, as neurons in these nuclei exhibit similar proprioceptive responses, although ECN has been shown to contain a preponderance of proprioceptive neurons (Hummelsheim et al. 1985; Hummelsheim and Wiesendanger 1985; Niu et al. 2013; Witham and Baker 2011). In parallel, we modified the design of electrode arrays and the implantation approach to improve their stability and longevity.
Location and Topographic Organization of Cuneate Nucleus
During acute recordings, we monitored multiunit activity from 90 distinct sites in CN/ECN, 13 in gracile nucleus, and 9 in trigeminal nucleus. Proprioceptive and, especially, tactile responses were most discernible when electrode impedance was >1 MΩ. Our main goal was to characterize the topography of the upper limb representation in CN/ECN. Data used to generate the response maps were pooled across the left and right brain stems of two animals (2 sides from one animal, 1 from the other). While we strived for a consistent coordinate system across experiments, differences in surface curvature, electrode angle, and neck flexion angle may have caused some distortion in the resulting maps of the brain stem.
Borders of observed brain stem nuclei.
Our first goal was to determine the medio-lateral extent of the CN/ECN by finding the medial border with the gracile nucleus and the lateral border with the trigeminal nucleus relative to midline. Figure 3A illustrates the position of upper limb (CN/ECN), lower limb (gracile), and face (trigeminal) units. The gracile nucleus spans the first 1.25–1.5 mm lateral to midline, the CN/ECN span the following 1.5–1.75 mm, and the trigeminal nucleus spans the remaining 1 mm. The medio-lateral extent of these structures remains fairly constant along the rostro-caudal axis (within a range of ±3 mm from obex) as well as in depth. These findings are consistent with previous anatomical studies of primate brain stem nuclei (Fig. 1C illustrates the relative positioning of these nuclei in lower brain stem; see also Kaas 2012).
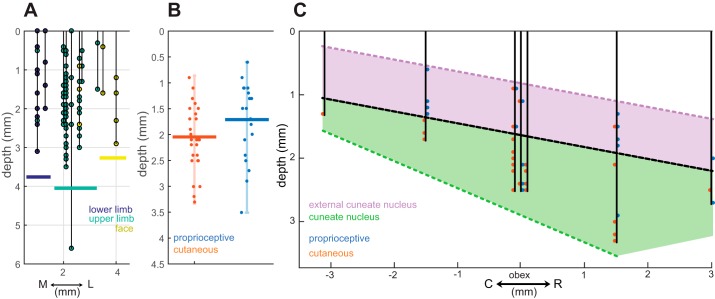
Fig. 3.
Topography of observed brain stem nuclei. A: RF as a function of depth and medio-lateral position of the electrode tip. RFs on the lower limb (gracile) are coded in dark blue, upper limb (cuneate/external cuneate) in light blue, and face (trigeminal) in yellow. The medial and lateral borders of the upper limb units are ~1.5 and 3 mm from the midline, respectively. B: distribution of distances from the neural tissue surface for cutaneous and proprioceptive units of cuneate and external cuneate neurons. Cutaneous units tended to be deeper than proprioceptive units. Vertical bars span the range of observed depths and horizontal bars their mean. C: diagram of several electrode penetrations made along the rostro-caudal and dorsal-ventral (depth) axes. Black dotted line represents estimated boundary between ECN and CN, with ECN shaded in purple (proprioceptive) and CN shaded in green (majority cutaneous).
Response modality.
Next, we aimed to characterize the organization of proprioceptive and cutaneous inputs to CN/ECN and estimate the location of the boundary between the two nuclei. We found that proprioceptive units tended to be more superficial than cutaneous units, and more frequently caudal to obex (Fig. 3, B and C). Additionally, proprioceptive units tended to be more lateral (mean distance from obex: 2.2 mm, range: 1–3.3 mm) than their tactile counterparts (mean: 2.1, range: 1–2.7 mm), in part because the ECN is dominated by proprioceptive responses and is located more laterally.
We estimated the boundary between the ECN (majority proprioceptive) and CN (both cutaneous and proprioceptive) along the dorsal-ventral and rostro-caudal axes (Fig. 3C). We also estimated the lateral boundary between CN and ECN to be located at ~2.7 mm, since no tactile units were observed beyond this point. These findings are consistent with previous studies in macaques and other primates (Fig. 1C) showing that ECN is located dorsal to CN and extends ~2 mm from obex in each direction along the rostro-caudal axis (Florence et al. 1988; Hummelsheim et al. 1985; Hummelsheim and Wiesendanger 1985; Qi and Kaas 2006).
In summary, then, gradients of submodality composition (tactile vs. proprioceptive) were most pronounced along the rostro-caudal axis and the dorsal-ventral axis (depth), as might be expected given that these define the boundary between CN and ECN (Fig. 1C). Tactile units were most frequently observed ~2 mm lateral to obex at a depth of 2 mm or greater. Proprioceptive units were observed relatively uniformly across the rostro-caudal axis (±2 mm to obex) but most frequently observed superficially.
Somatotopic organization of proprioceptive and cuneate units.
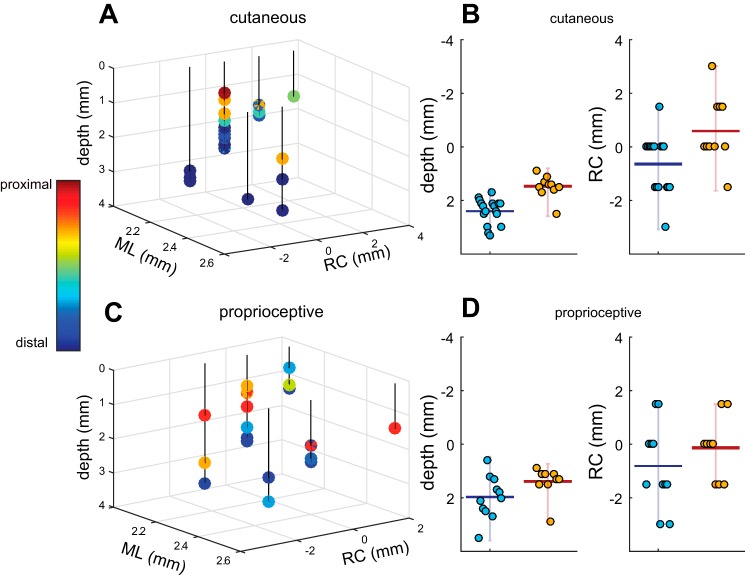
Units with proximal cutaneous RFs tended to be located more superficially than those with distal ones (Fig. 4, A and B). The mean depth of units with RFs proximal to the elbow was ~1.5 mm (range of 0.6–2.9 mm), whereas that of units with RFs distal to the elbow was 2.4 mm (range of 1.2–3.5 mm), consistent with previous findings in other primates (Florence et al. 1988; Qi and Kaas 2006). A topographic progression was also observed along the rostro-caudal axis: units with distal RFs tended to be more rostral to the obex (with a mean location of −0.6 mm and a range of −3.0 to 1.5 mm), while units with proximal RFs tended to be caudal (0.6 mm with a range of −1.5 to 3.0 mm) (Fig. 4, B and D). Somatotopic organization was similar for tactile and proprioceptive units both in depth and along the rostro-caudal axis.
Fig. 4.
Somatotopic organization of CN. A: 3D diagram of penetrations with cutaneous RFs plotted with respect to obex and the surface. Color bar indicates the RF location. ML, medio-lateral; RC, rostro-caudal. B: summary of cutaneous results. Left: distal units tend to be deeper than proximal units. Horizontal bar represents mean values, and vertical bars span the range of values. Right: distal units tend be more cranial (negative along the RC dimension) than proximal units. The forearm served as the boundary between distal and proximal units in the bar plots. C: 3D diagram of penetrations with proprioceptive responses with respect to obex and the surface. Color bar indicates RF location. D: summary of proprioceptive results. Left: distal units tend to be deeper than proximal units. Right: distal units tend to be anterior to their proximal counterparts. Overall, both proprioceptive and cutaneous modalities exhibited similar somatotopic trends: proximal units were located more superficially and more posterior to the obex than distal units.
Array Performance
In total, we placed 13 arrays (4 UEAs and 9 FMAs) into the brain stem nuclei of 11 macaques (2 monkeys were implanted bilaterally in separate surgeries). The design of the arrays and the implantation procedure evolved over time, and the success rate improved progressively. Four arrays (2 UEAs and 2 FMAs) yielded strong single-unit signals, which allowed us to collect information about the stability of RF locations over the course of the arrays’ life spans. Of the remaining arrays, four FMAs and one UEA yielded signals that were limited to a few electrodes, of poor quality, and/or of short life span. We were not able to record any signals from the remaining arrays because of health complications (3/9) or complete array failure (1/9). Figure 2C shows the approximate locations of each array on the brain stem.
FMA Design
An advantage of FMAs is that the electrode length, material, and configuration, as well as the lead length and pedestal shape, can be specified on an array-by-array basis. We found that arrays whose leads were manufactured with steel (rather than the standard gold) and reinforced with a thin silicone coating around the wire bundle exhibited longer life spans. Only monkey TE received an array with steel wires and silicone cable reinforcements, and this array had a signal longevity of 6 mo, while all other FMAs lasted 8 wk or less. Unlike implantation in cortex, for which movement between pedestal and array is relatively limited, brain stem implants move substantially relative to the pedestal, thereby stressing the leads (Buford and Davidson 2004; Fuchs and Luschei 1970; Hoffman et al. 1981). Moreover, the lead traverses neck muscles that are constantly flexing and extending, creating additional stress. Lead breakage was the most likely failure mode for many of our initial FMA implants.
We found that lengths of 7–9 cm were optimal for the leads, in that sufficient slack was provided for movement but not so much excess lead to adhere to muscle and other tissue (assuming that the more foreign material implanted in the tissue, the greater opportunity for adhesion). We began our studies with longer leads (up to 14 cm) and gradually shortened the leads to facilitate implantation of the array. During vacuum insertion, longer cables impose more torque on the array while lowering electrodes into neural tissue, ultimately destabilizing the array during implantation. Shortening the cable not only led to easier insertion but also improved signal longevity (monkeys WH, BA, LA1, HA). After several iterations, we converged onto specifications that yielded long-lasting arrays in brain stem: shorter, silicone-reinforced cables made of steel wire. Monkey TE received an array with all three design features, and signal longevity on this array substantially improved compared with all other FMA implants (Table 1). Although we did not have the same design options available with the UEAs, these arrays seem to be less susceptible to lead breakage, perhaps because of the greater number of individual leads, which results in a stronger aggregate cable.
RF Stability and Array Longevity
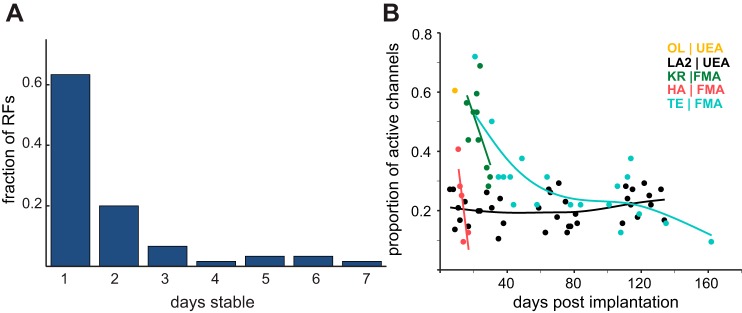
First, we characterized the location of each electrode’s RF in each of two FMAs over the lifetime of the arrays. Specifically, we counted the number of days over which each channel was activated by the same skin location or same joints (Fig. 5A). The majority of upper limb units remained stable for only 1 or 2 days, suggesting significant turnover of neuronal units from day to day. Qualitative assessment of the stability of the longest-lasting UEA suggests that RFs may be more stable in these arrays. It should be noted, however, that proximal RFs are larger than their distal counterparts, so differences in RF location—indicative of different units—may be more difficult to identify for proximal than for distal representations.
Fig. 5.
Electrode and RF stability. A: no. of consecutive days that observed RFs remained stable for each array. Channel RFs tended to remain stable for 1 or 2 days, with a maximum of 7 days. These data were collected over 20 sessions across 2 monkeys (WH and TE), whose distributions were very similar. B: array longevity for 5 electrode arrays. Points represent number of sorted neurons recorded after array implantation. Early implants (KR, HA) degraded rapidly after initial implantation. Longevity in LA2 and TE improved, as measured by no. of units. One subject, OL, developed a fatal case of acute meningoencephalitis.
Second, we characterized the number of channels that yielded discernible units over the lifetime of each array as a gauge of signal stability and array longevity. By this measure, modifications to the arrays and to the insertion procedure led to significantly improved longevity (Fig. 5B). The more recent implants yielded units for up to 6 mo, whereas early arrays failed within days or weeks (Fig. 5B). The improved longevity can be attributed to improvements in FMA design as described above and to modifications to the surgical procedure to include an adhesive that prevented arrays from being expelled from the brain stem. For both UEAs and FMAs, we found that application of Vetbond yielded more stable arrays than Tisseel. Monkeys TE (FMA) and LA1 (UEA) had Vetbond applied to the array after insertion, and these arrays were functional for the longest period of time (Table 1).
Single-Unit Responses
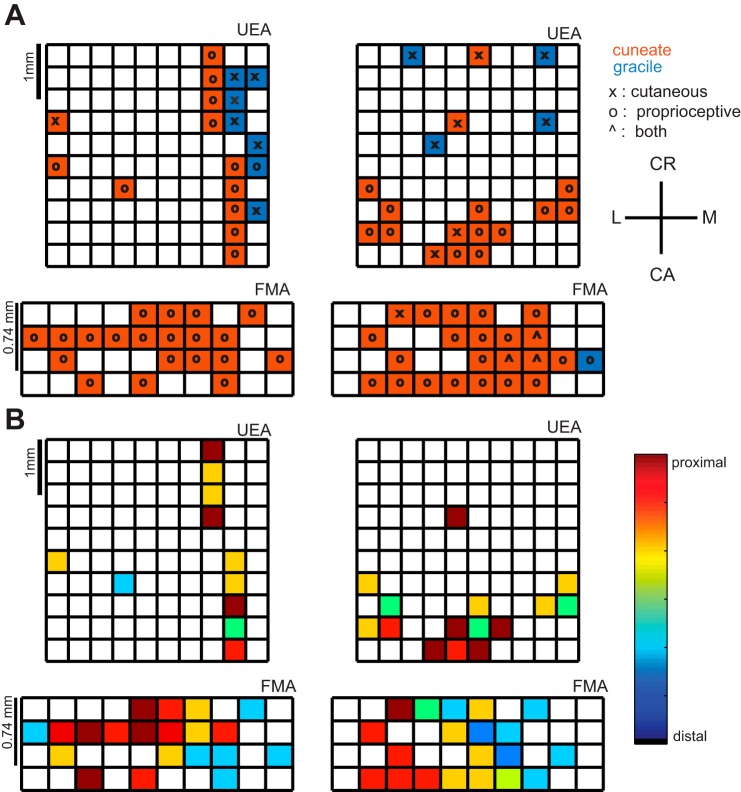
Figure 6 shows the topography of the brain stem inferred from the chronically implanted electrode arrays. Observed topographies were broadly consistent with those in the acute experiments.
Fig. 6.
Topographical organization of brain stem inferred from array recordings. A: RF locations (M, medial; L, lateral; CR, cranial; CA, caudal) of electrodes in 2 UEAs and 2 FMAs, identified by anatomical location and modality. Most units had RFs on the upper limb (as expected, since we targeted CN) and responded to proprioceptive stimulation. B: somatotopy of upper limb proprioceptive units observed on each array. Utah arrays do not show a somatotopic trend, while FMAs show a medio-lateral trend. Distal units are observed medially, while proximal units are observed laterally. Overall, chronic arrays are able to capture proprioceptive cuneate nucleus responses (ECN), but because of the depth and/or insertion of these electrodes, relatively few cutaneous responses (CN) were observed.
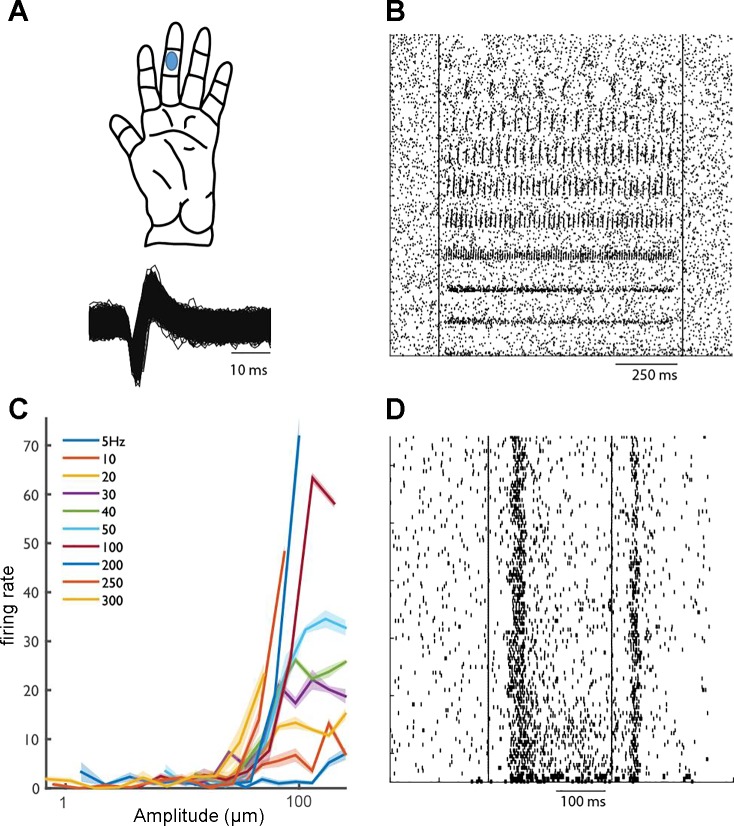
Figure 7 shows the responses of a cutaneous unit in CN to vibratory stimuli that varied in frequency and amplitude. This unit exhibits phase locking—i.e., produces a spike or a burst of spikes within a restricted portion of each stimulus cycle—as has been shown in both tactile nerve fibers (Talbot et al. 1968) and S1 neurons (Harvey et al. 2013). The frequency-response profile of this unit suggests that it receives input from Pacinian afferents. Indeed, it peaks in sensitivity at ∼250–300 Hz, as do Pacinian fibers. Interestingly, however, this unit also exhibits a sustained response to a skin indentation, a property that is only observed in slowly adapting type 1 fibers. This combination of response properties suggests that signals from multiple submodalities converge onto individual neurons in CN (see Saal and Bensmaia 2014 for a review).
Fig. 7.
Responses of a cutaneous unit in CN. A, top: RF center. Bottom: waveforms of identified action potentials. B: responses of a CN neuron to vibrations delivered to the RF. C: rate-intensity relationship demonstrates that this neuron’s responses are frequency dependent and peak in sensitivity at ∼300 Hz, similarly to PC fibers. D: responses of the same neuron to indentations delivered to different locations on the index fingerpad. Responses comprise both a sustained component and a strong off component: the sustained response indicates input from slowly adapting fibers, and the off response indicates input from rapidly adapting fibers.
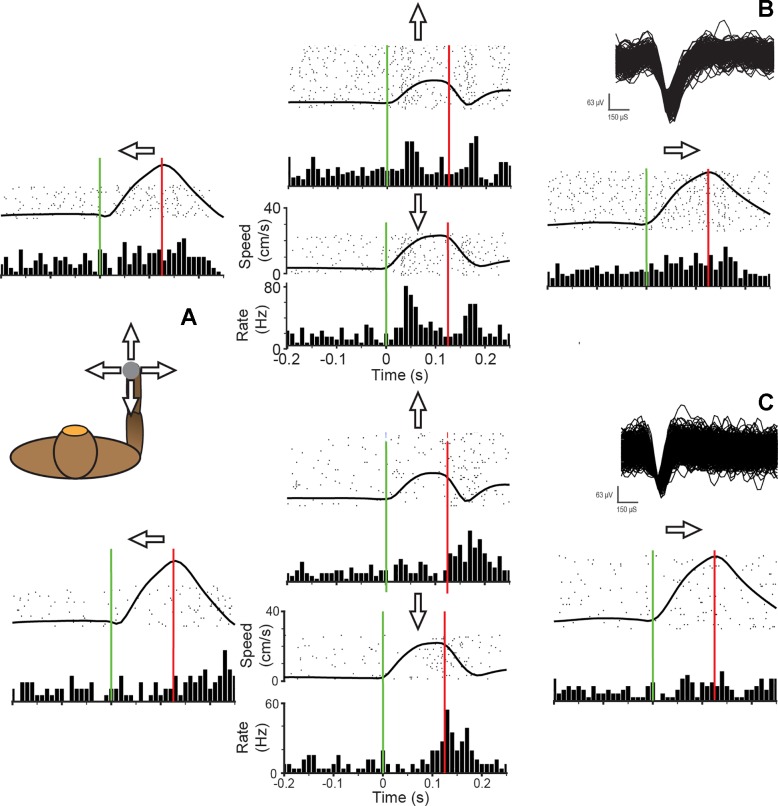
Figure 8 shows the responses of two CN/ECN neurons as we applied perturbations to the monkey’s hand. The first neuron (Fig. 8B) exhibits preferential responses to force pulses in the antero-posterior direction, with little or no response in the medio-lateral direction. Interestingly, the neuron burst at a rate of nearly 80 Hz at both the onset and offset of force pulses either toward or away from the body, with no sustained response. Figure 8C is a simultaneously recorded neuron that exhibited a qualitatively similar response, with weaker tuning directed slightly up and to the right.
Fig. 8.
CN/ECN neuron responses to limb perturbations. A: the monkey was trained to hold onto a powered manipulandum that exerted forces in 4 directions from a central point. A 125-ms bump was applied to the handle to test response of cuneate neurons. B: responses to bumps in each of 4 directions. Plots are arranged with respect to the direction of the bump, with bump onset represented by vertical green bar. Bump offset is represented by vertical red bar. Each row of the raster represents a single trial. A binned average firing rate is plotted below the raster plot. The trial-averaged speed of the handle is superimposed on the raster. Inset: waveforms of isolated action potentials. C: responses to bumps evoked in another CN/ECN neuron. Inset: waveforms of isolated action potentials.
DISCUSSION
Topography of Somatosensory Brain Stem Nuclei
While the organization of the somatosensory brain stem nuclei in primates has been documented in previous studies (Florence et al. 1988; Qi and Kaas 2006), our study is the first to characterize the topographical organization of these nuclei in rhesus macaques. We find that proprioceptive units tend to be more superficial than cutaneous units and that the CN/ECN exhibit a somatotopy both in depth and along the rostro-caudal axis: units with RFs on the proximal limb tend to be more superficial and caudal than those with distal RFs. The dorsal-ventral trends observed in rhesus macaques with regard to somatotopy and submodality distribution match those reported for other macaque species (Florence et al. 1989; Qi and Kaas 2006), with the additional observation of somatotopic trends along the rostro-caudal or medio-lateral axes.
In the FMA recordings, we noticed a distal to proximal trend of RFs along the medio-lateral axis. That is, units with distal RFs tended to be located medially while units with proximal RFs were predominantly located laterally. This somatotopy was observed neither in the anesthetized acute experiments nor in the UEA RF maps and may be attributed to the curvature of the brain stem, which caused medial electrodes to penetrate deeper than others.
Implications of Topography for Chronic Implants
Recording from digit-related units in CN is challenging because of their depth and their location rostral to the obex, which can be partially obstructed by the cerebellum. Electrode arrays must include long (>2 mm) electrodes yet still allow insertion in a far rostral position along the brain stem. Unfortunately, the angle for this insertion is often hindered by the occipital bone, even after a wide suboccipital craniectomy that extends to the transverse sinuses. Proprioceptive units with proximal RFs are more easily accessible given their superficiality and caudal location.
The boundary between CN and ECN is difficult to establish conclusively on the basis of electrophysiological response properties because the nuclei exhibit similar proprioceptive responses (Hummelsheim et al. 1985; Witham and Baker 2011). Any implant is likely to impinge on the two nuclei given their small size, and histology will almost certainly be necessary to distinguish them post hoc.
Array Design Considerations
The manner in which signals recorded by the FMAs decayed over time and subsequently failed suggests either lead or connector breakage. However, the fact that some channels failed while others remained stable seems to exclude connector failure. Impedance spectroscopy indicated open circuits, consistent with lead breakage.
UEAs offer the advantage of greater coverage, more and more closely spaced electrodes, and strong leads. However, targeting cutaneous units requires longer electrodes than are currently commercially available with UEAs. Furthermore, the insertion technique for FMAs, which involves a narrow vacuum probe, facilitates rostral positioning relative to its UEA counterpart, as the pneumatic inserter required for the latter has a wider footprint. Given the rostral position of cutaneous digits, this further favors FMAs over UEAs for studies involving the cutaneous representations of the digits.
Stability of Cutaneous Receptive Fields
That the locations of RFs turn over at a relatively rapid rate for the FMA implants (approximately every other day) suggests movement of the array within the tissue. The great stability of our most recent UEA implants is difficult to explain, unless the much greater number and density of electrodes helped to stabilize the array within the tissue. While cortical implants with either FMAs or UEAs also exhibit some neuronal turnover, its rate is much slower, with some units remaining stable for weeks or even months (Vaidya et al. 2014). The poor stability of CN implants can be attributed to the much greater mobility of the brain stem, which flexes during neck movements, a degree of freedom not present in cranial implants (Buford and Davidson 2004; Fuchs and Luschei 1970; Hoffman et al. 1981).
Conclusions
We have developed a strategy to record stably for single sessions from the somatosensory brain stem nuclei with chronically implanted electrode arrays. We have characterized the somatotopic and functional topography of neurons in the CN/ECN by electrophysiology and illustrated the firing rate characteristics of several neurons in response to passive RF manipulation. Although stereotaxic coordinates cannot offer well-defined boundaries between somatosensory brain stem nuclei because of various confounding factors, the observed topographical trends will inform future array placement and design. Vibratory responses have been extensively characterized in the periphery and cortex of rhesus macaques (Harvey et al. 2013; Johnson and Lamb 1981; Mountcastle et al. 1967; Muniak et al. 2007), as have responses to passive and active limb movements. The ability to collect single-unit data from the CN of awake, behaving primates will provide us with an opportunity to understand how these limb state representations are transformed as they ascend the neuraxis. We may also begin to understand the nature and function of the top-down modulation CN receives from cortex.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS-095162 (S. J. Bensmaia and L. E. Miller) and F31 NS-096952 (A. K. Suresh).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.K.S., J.W., C.V., R.H.C., T.T., L.E.M., and S.J.B. conceived and designed research; A.K.S., J.W., C.V., R.H.C., T.T., and J.M.R. performed experiments; A.K.S., J.W., C.V., R.H.C., and T.T. analyzed data; A.K.S., J.W., C.V., R.H.C., T.T., L.E.M., and S.J.B. interpreted results of experiments; A.K.S., J.W., and C.V. prepared figures; A.K.S. and S.J.B. drafted manuscript; A.K.S., J.W., C.V., R.H.C., T.T., J.M.R., L.E.M., and S.J.B. edited and revised manuscript; A.K.S., J.W., R.H.C., T.T., J.M.R., L.E.M., and S.J.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Philip Troyk for help with the impedance spectroscopy and Molly O’Donnell for help with figure illustration.
REFERENCES
- Andersen P, Eccles JC, Oshima T, Schmidt RF. Mechanisms of synaptic transmission in the cuneate nucleus. J Neurophysiol 27: 1096–1116, 1964a. [DOI] [PubMed] [Google Scholar]
- Andersen P, Eccles JC, Schmidt RF. Presynaptic inhibitory actions: presynaptic inhibition in the cuneate nucleus. Nature 194: 741–743, 1962. doi: 10.1038/194741a0. [DOI] [PubMed] [Google Scholar]
- Andersen P, Eccles JC, Schmidt RF, Yokota T. Identification of relay cells and interneurons in the cuneate nucleus. J Neurophysiol 27: 1080–1095, 1964b. [DOI] [PubMed] [Google Scholar]
- Bensmaia SJ, Denchev PV, Dammann JF 3rd, Craig JC, Hsiao SS. The representation of stimulus orientation in the early stages of somatosensory processing. J Neurosci 28: 776–786, 2008. doi: 10.1523/JNEUROSCI.4162-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedenbach MA, Jabbur SJ, Towe AL. Afferent inhibition in the cuneate nucleus of the rhesus monkey. Brain Res 27: 179–183, 1971. doi: 10.1016/0006-8993(71)90381-7. [DOI] [PubMed] [Google Scholar]
- Boivie J, Boman K. Termination of a separate (proprioceptive?) cuneothalamic tract from external cuneate nucleus in monkey. Brain Res 224: 235–246, 1981. doi: 10.1016/0006-8993(81)90856-8. [DOI] [PubMed] [Google Scholar]
- Buford JA, Davidson AG. Movement-related and preparatory activity in the reticulospinal system of the monkey. Exp Brain Res 159: 284–300, 2004. doi: 10.1007/s00221-004-1956-4. [DOI] [PubMed] [Google Scholar]
- Canedo A, Mariño J, Aguilar J. Lemniscal recurrent and transcortical influences on cuneate neurons. Neuroscience 97: 317–334, 2000. doi: 10.1016/S0306-4522(00)00063-4. [DOI] [PubMed] [Google Scholar]
- Costanzo RM, Gardner EP. Multiple-joint neurons in somatosensory cortex of awake monkeys. Brain Res 214: 321–333, 1981. doi: 10.1016/0006-8993(81)91197-5. [DOI] [PubMed] [Google Scholar]
- Florence SL, Wall JT, Kaas JH. The somatotopic pattern of afferent projections from the digits to the spinal cord and cuneate nucleus in macaque monkeys. Brain Res 452: 388–392, 1988. doi: 10.1016/0006-8993(88)90045-5. [DOI] [PubMed] [Google Scholar]
- Florence SL, Wall JT, Kaas JH. Somatotopic organization of inputs from the hand to the spinal gray and cuneate nucleus of monkeys with observations on the cuneate nucleus of humans. J Comp Neurol 286: 48–70, 1989. doi: 10.1002/cne.902860104. [DOI] [PubMed] [Google Scholar]
- Fuchs A, Luschei E. Firing patterns of abducens neurons of alert monkeys in relationship to horizontal eye movement. J Neurophysiol 33: 382–392, 1970. [DOI] [PubMed] [Google Scholar]
- Gardner EP, Costanzo RM. Properties of kinesthetic neurons in somatosensory cortex of awake monkeys. Brain Res 214: 301–319, 1981. doi: 10.1016/0006-8993(81)91196-3. [DOI] [PubMed] [Google Scholar]
- Harris F, Jabbur SJ, Morse RW, Towe AL. Influence of the cerebral cortex on the cuneate nucleus of the monkey. Nature 208: 1215–1216, 1965. doi: 10.1038/2081215a0. [DOI] [PubMed] [Google Scholar]
- Harvey MA, Saal HP, Dammann JF 3rd, Bensmaia SJ. Multiplexing stimulus information through rate and temporal codes in primate somatosensory cortex. PLoS Biol 11: e1001558, 2013. doi: 10.1371/journal.pbio.1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward V, Terekhov AV, Wong SC, Geborek P, Bengtsson F, Jörntell H. Spatio-temporal skin strain distributions evoke low variability spike responses in cuneate neurons. J R Soc Interface 11: 20131015, 2014. doi: 10.1098/rsif.2013.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman D, Dubner R, Hayes R. Neuronal activity in medullary dorsal horn of awake monkeys trained in a thermal discrimination task. I. Responses to innocuous and noxious thermal. J Neurophysiol 46: 409–427, 1981. [DOI] [PubMed] [Google Scholar]
- Hummelsheim H, Wiesendanger M. Neuronal responses of medullary relay cells to controlled stretches of forearm muscles in the monkey. Neuroscience 16: 989–996, 1985. [DOI] [PubMed] [Google Scholar]
- Hummelsheim H, Wiesendanger R, Wiesendanger M, Bianchetti M. The projection of low-threshold muscle afferents of the forelimb to the main and external cuneate nuclei of the monkey. Neuroscience 16: 979–987, 1985. [DOI] [PubMed] [Google Scholar]
- Jabbur SJ, Banna NR. Presynaptic inhibition of cuneate transmission by widespread cutaneous inputs. Brain Res 10: 273–276, 1968. doi: 10.1016/0006-8993(68)90135-2. [DOI] [PubMed] [Google Scholar]
- Johnson KO, Lamb GD. Neural mechanisms of spatial tactile discrimination: neural patterns evoked by braille-like dot patterns in the monkey. J Physiol 310: 117–144, 1981. doi: 10.1113/jphysiol.1981.sp013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörntell H, Bengtsson F, Geborek P, Spanne A, Terekhov AV, Hayward V. Segregation of tactile input features in neurons of the cuneate nucleus. Neuron 83: 1444–1452, 2014. doi: 10.1016/j.neuron.2014.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH. Somatosensory system. In: The Human Nervous System (3rd ed.), edited by Mai JK, Paxinos G. London: Academic, 2012, p. 1074–1109. [Google Scholar]
- London BM, Miller LE. Responses of somatosensory area 2 neurons to actively and passively generated limb movements. J Neurophysiol 109: 1505–1513, 2013. doi: 10.1152/jn.00372.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London BM, Torres RR, Slutzky MW, Miller LE. Designing stimulation patterns for an afferent BMI: representation of kinetics in somatosensory cortex. Conf Proc IEEE Eng Med Biol Soc 2011: 7521–7524, 2011. doi: 10.1109/IEMBS.2011.6091854. [DOI] [PubMed] [Google Scholar]
- Molinari HH, Schultze KE, Strominger NL. Gracile, cuneate, and spinal trigeminal projections to inferior olive in rat and monkey. J Comp Neurol 375: 467–480, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Talbot WH, Darian-Smith I, Kornhuber HH. Neural basis of the sense of flutter-vibration. Science 155: 597–600, 1967. doi: 10.1126/science.155.3762.597. [DOI] [PubMed] [Google Scholar]
- Muniak MA, Ray S, Hsiao SS, Dammann JF, Bensmaia SJ. The neural coding of stimulus intensity: linking the population response of mechanoreceptive afferents with psychophysical behavior. J Neurosci 27: 11687–11699, 2007. doi: 10.1523/JNEUROSCI.1486-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musallam S, Bak MJ, Troyk PR, Andersen RA. A floating metal microelectrode array for chronic implantation. J Neurosci Methods 160: 122–127, 2007. doi: 10.1016/j.jneumeth.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Niu J, Ding L, Li JJ, Kim H, Liu J, Li H, Moberly A, Badea TC, Duncan ID, Son YJ, Scherer SS, Luo W. Modality-based organization of ascending somatosensory axons in the direct dorsal column pathway. J Neurosci 33: 17691–17709, 2013. doi: 10.1523/JNEUROSCI.3429-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neal JT, Westrum LE. The fine structural synaptic organization of the cat lateral cuneate nucleus. A study of sequential alterations in degeneration. Brain Res 51: 97–124, 1973. doi: 10.1016/0006-8993(73)90367-3. [DOI] [PubMed] [Google Scholar]
- Prud’homme MJ, Kalaska JF. Proprioceptive activity in primate primary somatosensory cortex during active arm reaching movements. J Neurophysiol 72: 2280–2301, 1994. [DOI] [PubMed] [Google Scholar]
- Qi HX, Kaas JH. Organization of primary afferent projections to the gracile nucleus of the dorsal column system of primates. J Comp Neurol 499: 183–217, 2006. doi: 10.1002/cne.21061. [DOI] [PubMed] [Google Scholar]
- Richardson AG, Weigand PK, Sritharan SY, Lucas TH. Somatosensory encoding with cuneate nucleus microstimulation: effects on downstream cortical activity. 7th International IEEE/EMBS Conference on Neural Engineering, 2015, p. 695–698. doi: 10.1109/NER.2015.7146718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AG, Weigand PK, Sritharan SY, Lucas TH. A chronic neural interface to the macaque dorsal column nuclei. J Neurophysiol 115: 2255–2264, 2016. doi: 10.1152/jn.01083.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousche PJ, Normann RA. Chronic recording capability of the Utah Intracortical Electrode Array in cat sensory cortex. J Neurosci Methods 82: 1–15, 1998. doi: 10.1016/S0165-0270(98)00031-4. [DOI] [PubMed] [Google Scholar]
- Saal HP, Bensmaia SJ. Touch is a team effort: interplay of submodalities in cutaneous sensibility. Trends Neurosci 37: 689–697, 2014. doi: 10.1016/j.tins.2014.08.012. [DOI] [PubMed] [Google Scholar]
- Talbot WH, Darian-Smith I, Kornhuber HH, Mountcastle VB. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol 31: 301–334, 1968. [DOI] [PubMed] [Google Scholar]
- Vaidya M, Dickey A, Best MD, Coles J, Balasubramanian K, Suminski AJ, Hatsopoulos NG. Ultra-long term stability of single units using chronically implanted multielectrode arrays. Conf Proc IEEE Eng Med Biol Soc 2014: 4872–4875, 2014. doi: 10.1109/EMBC.2014.6944715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westling G, Johansson RS, Vallbo O. Method for mechanical stimulation of skin receptors. In: Functions of the Skin in Primates, edited by Zotterman Y. Oxford, UK: Pergamon, 1976, p. 151–158. doi: 10.1016/B978-0-08-021208-1.50018-7. [DOI] [Google Scholar]
- Witham CL, Baker SN. Modulation and transmission of peripheral inputs in monkey cuneate and external cuneate nuclei. J Neurophysiol 106: 2764–2775, 2011. doi: 10.1152/jn.00449.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]