We suggest that Aβ signals do not simply switch modality to signal pain during injury but play a frequency-dependent and dual role in the injured state with both pleasant and unpleasant consequences. These results provide a framework to resolve the apparent paradox of how touch can inhibit pain, as proposed by the Gate Control Theory and the existence of dynamic mechanical allodynia.
Keywords: pain, Gate Control Theory, allodynia, touch
Abstract
In the setting of injury, myelinated primary afferent fibers that normally signal light touch are thought to switch modality and instead signal pain. In the absence of injury, touch is perceived as more intense when firing rates of Aβ afferents increase. However, it is not known if varying the firing rates of Aβ afferents have any consequence to the perception of dynamic mechanical allodynia (DMA). We hypothesized that, in the setting of injury, the unpleasantness of DMA would be intensified as the firing rates of Aβ afferents increase. Using a stimulus-response protocol established in normal skin, where an increase in brush velocity results in an increase of Aβ afferent firing rates, we tested if brush velocity modulated the unpleasantness of capsaicin-induced DMA. We analyzed how changes in estimated low-threshold mechanoreceptor firing activity influenced perception and brain activity (functional MRI) of DMA. Brushing on normal skin was perceived as pleasant, but brushing on sensitized skin produced both painful and pleasant sensations. Surprisingly, there was an inverse relationship between Aβ firing rates and unpleasantness such that brush stimuli that produced low firing rates were most painful and those that elicited high firing rates were rated as pleasant. Concurrently to this, we found increased cortical activity in response to low Aβ firing rates in regions previously implicated in pain processing during brushing of sensitized skin, but not normal skin. We suggest that Aβ signals do not merely switch modality to signal pain during injury. Instead, they exert a high- and low-frequency-dependent dual role in the injured state, with respectively both pleasant and unpleasant consequences.
NEW & NOTEWORTHY We suggest that Aβ signals do not simply switch modality to signal pain during injury but play a frequency-dependent and dual role in the injured state with both pleasant and unpleasant consequences. These results provide a framework to resolve the apparent paradox of how touch can inhibit pain, as proposed by the Gate Control Theory and the existence of dynamic mechanical allodynia.
INTRODUCTION
A major premise of the Gate Control Theory of Pain that Melzack and Wall (1965) proposed was that activation of myelinated low-threshold afferent fibers can inhibit pain. These authors postulated that the myelinated low-threshold mechanoreceptors (LTMs) that signal innocuous touch (Johnson and Hsiao 1992; Vallbo and Johansson 1984) can decrease (by gating) the activity in ascending nociceptive pathways that is generated by small-diameter afferents. The consequence is a reduction of pain.
However, the subsequent identification of Aβ-mediated dynamic mechanical allodynia (DMA) presented a paradox to our understanding of how the Aβ system influences nociceptive signaling (Koltzenburg et al. 1992; Ochoa and Yarnitsky 1993). Dynamic mechanical allodynia is an important feature of neuropathic pain experienced by some patients. Specifically, in addition to reporting spontaneous, often burning, pain, these patients perceive a stroking or brush stimulus as painful. DMA can be experimentally induced after topical application of capsaicin, which via an action on the transient vanilloid receptor (TRPV1) elicits a sensation of burning pain (Caterina et al. 1997). The mechanism through which normally innocuous stroking becomes painful is thought to result from a central sensitization process, in which there is an enhanced excitability of nociceptive dorsal horn neurons that makes them responsive to low-threshold mechanical stimuli (Cook et al. 1987; Woolf 1983). Consistent with this hypothesis, intraneural microstimulation of Aβ fibers in the area sensitized by capsaicin injection can evoke pain (so-called mechanical allodynia) (Torebjörk et al. 1992). Furthermore, pressure block of nerves proximal to skin stimulation, which preferentially blocks Aβ afferents, abolishes brush-evoked allodynia simultaneously with loss of touch sensation (Campbell et al. 1988; Koltzenburg et al. 1992; LaMotte et al. 1991). The fact that DMA cannot be evoked in patients lacking Aβ fibers (Liljencrantz et al. 2013; Treede and Cole 1993) is consistent with a necessary contribution of myelinated LTMs.
LTMs respond vigorously to a brush stroke, which does not activate nociceptors (Vallbo et al. 1999). We also know from microneurography results that all Aβ LTMs (field, hair, SA1, SA2) respond with linearly increasing firing rates as brush velocity increases (Löken et al. 2009). The brush stimulus-response relationship for C-fiber LTMs (CLTMs), on the other hand, is best described as an inverted U-shaped function. Because both Aβ and CLTM afferents lack the capsaicin receptor (TRPV1) (Li et al. 2011; Lumpkin and Caterina 2007; Olausson et al. 2010; Torebjörk et al. 1992), their response to varying brush strokes should be unaltered after capsaicin application.
Although the necessity of Aβ afferents for the sensation of DMA after injury is established, little is known about what discharge rates in these fibers are optimal to signal allodynia. In the absence of injury, the perceived increase in intensity of a tactile stimulus is encoded by an increased firing of Aβ LTMs (Muniak et al. 2007). We therefore hypothesized that, after sensitization, the unpleasantness of DMA would increase by increasing firing rates in Aβ afferents. We decided to vary the firing rates of LTM afferents by changing the velocity of brush strokes on capsaicin-sensitized skin compared with normal skin according to previously established stimulus-response protocols. We investigate the psychophysical stimulus-response curves and the central neural processing related to these expected firing rate changes using functional magnetic resonance imaging (fMRI) in 19 healthy individuals.
METHODS
Participants.
Twenty-two healthy volunteers participated in the experiment. Two participants unable to complete the task were excluded, and one was excluded due to excessive movement. Nineteen participants (10 women; mean age 30 yr, SD 6.7 yr) were included in the study. In addition, six individuals (2 men) participated in a control experiment outside the scanner (see Behavioral experiment 2). The Oxford Research Ethics Committee reviewed and approved the protocol. Informed consent was obtained from all participants before the experiment.
Design.
Using a within-subjects design, we compared perception and central neural processing of soft brush stroking at varying velocity on normal and capsaicin sensitized skin in the 19 healthy individuals while recording behavior and fMRI. We utilized a protocol based on previous microneurography studies that was designed to modulate the firing rates of Aβ and CLTM afferents. Importantly, previous microneurography results have shown that CLTM firing rates in response to varying brush velocity fit an inverted U-shaped function. Aβ LTMs are better described by a linear term, intensifying firing rates as brush velocity increases (Löken et al. 2009). These specific signatures of firing rate functions separating Aβ and CLTM afferents can be correlated with the fMRI data as a means to disambiguate brain activity related to these different fiber classes.
Stimuli and procedure.
On arrival, participants were first given standardized written and spoken instructions about the experimental task. The stimulus was a soft brush (30 mm, oriental hake; Royal Brush Manufacturing, Dudley, UK) swiped over the skin at randomized speeds. The pressure was kept constant by slightly bending the brush at all speeds. Two areas, distal to the epicondyle and proximal to the wrist (width 3 cm, length 6 cm) were marked on the ventral aspect of participants’ left lower forearm (see Fig. 1B). Prior to scan, capsaicin cream (1%) (Propharma, Horsham, UK) was topically applied to sensitize one of the two areas marked on the forearm (location counterbalanced among participants on distal or proximal site). Reactions to capsaicin application can be affected by temperature. Care was taken therefore to make sure that the room was comfortably warm, and the arm was wrapped in plastic with a towel over the area where the capsaicin was applied. The capsaicin was applied and kept on the skin for at least 15 min or until the participant reported a clear burning sensation. Residual capsaicin cream was then wiped off and the participant placed in the scanner.
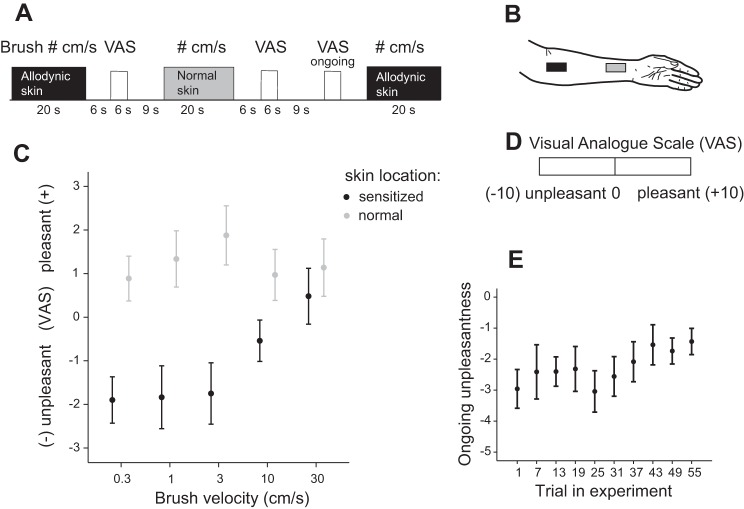
Fig. 1.
Experimental design and behavioral ratings. A: snap shot of stimulus presentation and timings of Visual Analog Scale (VAS) ratings. Stimuli were alternated on sensitized (black) and normal (gray) skin, and brush velocity was randomized within and between experiments. Ongoing unpleasantness as a result of capsaicin was measured at 10 points during the experiment (yellow). B: sketch of stimulated areas. Two areas of 3 × 6 cm were marked on the distal and proximal aspect of the left forearm. One of these areas (randomized between participants) was sensitized with capsaicin before the experiment started. C: group average of VAS ratings as a function of velocity on sensitized skin (black) and normal skin (gray). Note that unpleasantness ratings are denoted by negative values on the Y-axis, and pleasantness by positive values. On sensitized skin, low-frequency Aβ stimuli were rated as unpleasant and brush velocities inducing high discharge rates in Aβ were, on average, pleasant. On normal skin, all stimuli were, on average, pleasant (above 0). D: VAS ratings. After each stimulus, affective ratings were assessed by moving a marker from center (neutral) to unpleasant (left) or pleasant (right). Values ranging from −10 (unpleasant) to 10 (pleasant) were set for analysis to denote range of scale. E: group average of ongoing unpleasantness ratings measured at 10 time points across the experiment. Ongoing unpleasantness decreased across the experiment from an average of −3 to −1.4. All bars denote SE.
Brush stroking was applied manually in proximal to distal direction at velocities of 0.3, 1, 3, 10, or 30 cm/s during blocks of 20 s. Each brush velocity was repeated five times for each skin area. To ensure accurate stimulus velocity, the experimenter (L. S. Löken) was guided by a visual meter not visible to the participant. Stroking stimuli were alternated between sensitized and normal skin, and velocities were presented in randomized order within and between participants. Six seconds after each stimulus, a computerized visual analog scale (VAS) with the end points “unpleasant” and “pleasant” appeared on a screen. Participants moved a marker placed at the center of the scale (neutral) using a button box placed by their right hand. Participants were instructed to rate how pleasant or unpleasant the stimulus was perceived, and on 10 occasions they were cued to rate their ongoing pain using the same scale. They performed ratings within 6 s, and the next stimulus was applied after 9 s of rest. The experimental paradigm lasted ~38 min.
Behavioral experiment 2.
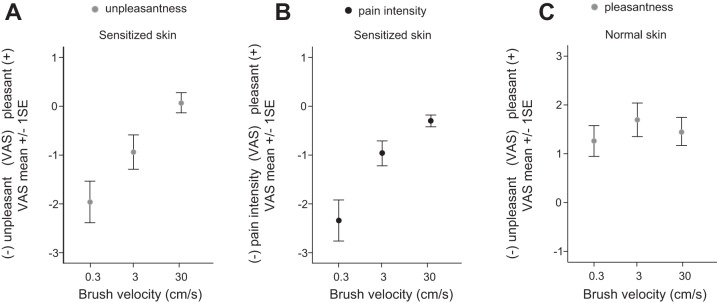
Six volunteers (2 men) participated in the experiment (age 27–42 yr, mean = 31.8 yr, SD = 5.5 yr). Stimuli, capsaicin application (1%), and areas for stimulation (left forearm, width 3 cm, length 6 cm) were the same as in the main experiment (see Fig. 1B). The location of application was counterbalanced between participants on distal or proximal sites. After ~15 min, the participant reported a burning sensation and the capsaicin cream was wiped off. All participants had brush-evoked allodynia outside the area of application that they reported as painful and hyperalgesia outside the area of application that did not extend as far as the normal skin tested. Stimuli were alternated on sensitized and normal skin, and brush velocity was randomized within and between experiments. Brush stroking was applied in proximal to distal direction at velocities of 0.3, 3, or 30 cm/s. Brushing was delivered with single brush strokes, and each brush velocity was repeated three times for each skin area (see Fig. 2). Participants were instructed to rate both the unpleasantness/pleasantness and pain intensity of the brush stroke. After each stimulus, ratings were done by manually putting a mark on a 10-cm line with center (neutral) marked and the end points unpleasant or pain intensity (left) and pleasant (right). For subsequent analysis, marks were measured in millimeters from center with −5 denoting unpleasant and 5 denoting pleasant. The experimental paradigm lasted ~15 min.
Fig. 2.
A and B: visual analog scale (VAS) ratings in response to single brush strokes at 3 velocities across capsaicin-sensitized skin (n = 6). A shows behavioral ratings of unpleasantness (gray) on sensitized skin. B shows pain intensity ratings (black) on sensitized skin. C: VAS ratings in response to single brush strokes at 3 velocities across normal skin. Pleasantness ratings are denoted by positive values and unpleasantness/pain intensity ratings by negative values on the Y-axis (−5 to 5). All bars denote SE.
Methodological considerations.
All included participants had a pronounced red flare and brush-evoked allodynia and hyperalgesia outside the area of application. However, we found no signs of allodynia extending to the normal skin area, because the participants rated the brush stimulus as pleasant in this zone. The method of capsaicin application is similar to the method of Koltzenburg et al. (1992). They too, used 1% capsaicin with similar effects. They suggested that what is commonly termed secondary effects are likely subserved by the same central mechanisms within and outside the area of application. However, the size of the secondary area outside the area of application varies among participants. We wanted to engage sufficient spatial summation in the neurons of interest, and to reliably achieve a clear brush-evoked allodynia along with robust brain activity. Therefore, we applied brush strokes to the primary area but engaged the secondary effects expressed on sensitized skin with the soft brush stroke (for a review of the evidence supporting this method see Devor 2013).
Behavioral analysis.
For statistical analysis of behavioral data, regression analysis was done by transforming velocity to log10 values in concordance with basic laws of the relationship between neural coding and psychophysics (Johnson et al. 2002). To test for significance of a linear compared with a quadratic regression term, the curve fit of a linear regression (reduced regression model) was tested against the fit of a quadratic regression (full regression model), with an F-test for significant reduction of the error sum of squares in the full compared with the reduced model. The t-test and linear regression were performed using SPSS 18.0 (SPSS, Chicago, IL).
fMRI data acquisition.
Functional imaging data were acquired using a 3T Siemens Verio magnetic resonance scanner. We used a head-only 32-channel gradient coil for signal reception. A whole brain T2*-sensitive gradient echo-planar imaging (EPI) sequence with repetition time (TR) = 2.7 s, echo time (TE) = 30 ms, acceleration factor 2 (GRAPPA), 49 contiguous 3-mm-thick slices, field of view (FOV) 192 × 192 mm, and matrix 64 × 64 mm, in 840 volumes. The first four volumes were discarded to permit equilibration of the blood oxygen level-dependent (BOLD) signal. Field maps were obtained using a symmetric-asymmetric spin-echo sequence. A T1-weighted structural (1-mm3 voxel) image was acquired for the registration of statistical activation maps to the standard stereotactic space (Montreal Neurological Institute (MNI; 152 template). T1-weighted structural images were acquired for subject alignment using an MPRAGE sequence with the following parameters: voxel resolution 1 × 1 × 1 mm on a 192 × 174 × 192 grid, TE = 4.7 ms, inversion time (TI) = 900 ms, TR = 2,040 ms.
fMRI data analysis.
The fMRI analysis was conducted with the Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL) using FEAT (FMRI Expert Analysis Tool; version 6.0, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki; Jenkinson et al. 2012). Preprocessing was performed using motion correction (Jenkinson 2003), field map correction of EPI distortion, removal of nonbrain voxels (Smith 2002), and Gaussian spatial smoothing (5 mm) and high-pass filtering with cutoff at 100 s. Physiological recordings were performed using a respiratory bellows and pulse oximeter during the fMRI session. These recordings were used to further de-noise the data by physiological noise modeling (Brooks et al. 2008)
Statistical analysis was performed for each participant’s functional scan using a general linear model (GLM). The relationship between neural firing rates and brain activity was analyzed using Aβ and CLTM firing rate estimates for the different brush velocities, measured previously (Löken et al. 2009). At the subject level, allodynic and normal skin areas were modeled using a separate set of regressors, one for each brush velocity. This resulted in 11 regressors, 5 representing each velocity per skin area and 1 nuisance regressor modeling pressing of the button box at the time of ratings.
For all group-level analysis, images were registered to the high-resolution structural scan using a boundary-based registration (Greve and Fischl 2009). Registration to high-resolution MNI space MNI152 template images was carried out using FLIRT and refined using FNIRT nonlinear registration (Jenkinson et al. 2012). Group-level model estimation was performed using FMRIB’s local analysis of mixed effects (FLAME) with outlier deweighting. Reported cluster-corrected results came from these mixed-effects analyses using Gaussian random-field theory and using the default Z > 2.3 cluster-based thresholding with a corrected cluster significance level of P < 0.05. Statistical images showing a positive correlation for Aβ discharge rates for sensitized and normal skin are displayed separately in Fig. 4, and results were significant at P < 0.001, uncorrected. To illustrate the relationships identified in the spatial image analyses, we extracted the percentage of BOLD signal response for each velocity from the voxels in relevant areas.
Fig. 4.
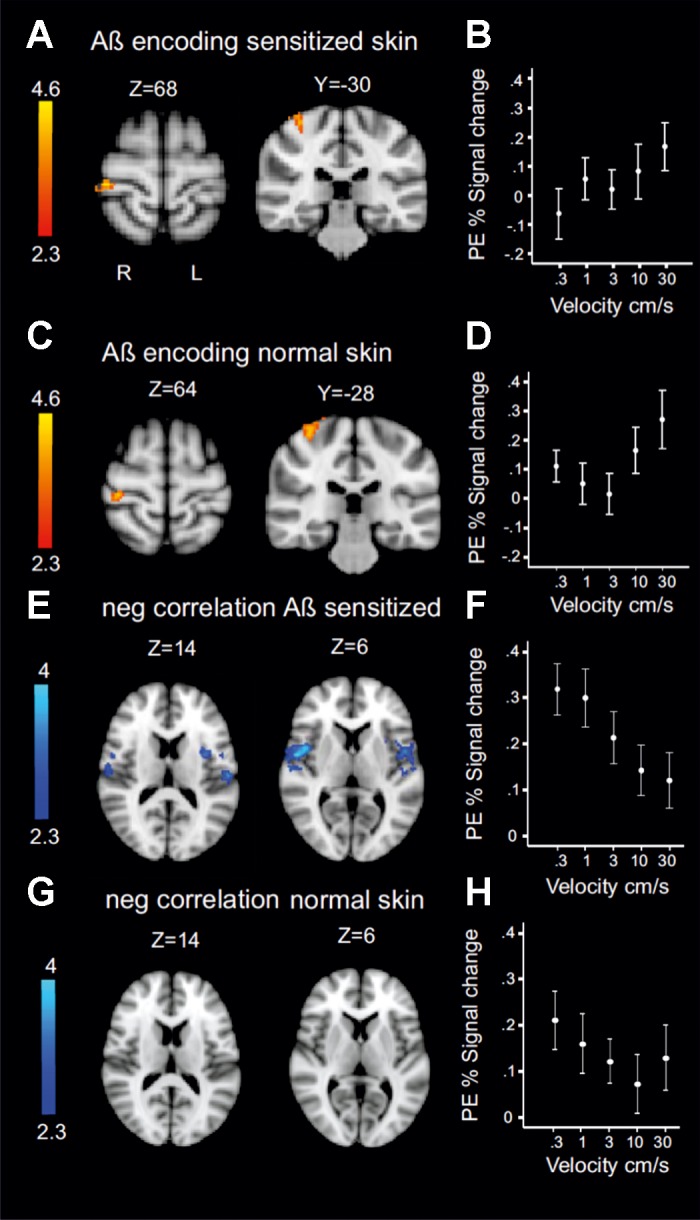
Hemodynamic response as a function of Aβ discharge rate. The function was used for analysis in Fig. 3A. A–D: patterns of estimated discharge rate increase in Aβ afferents on sensitized skin (A and B) and normal skin (C and D). Skin-specific clusters were significant at P < 0.001 (uncorrected). B and D show parameter estimates (PE; BOLD %signal change) on sensitized skin (B) and normal skin (D) across velocities. E: negative correlate to increased Aβ activity on sensitized skin. Clusters were bilateral with peaks located in opercular areas 4 (X = 54, Y = −2, Z = 6) and 1 (X = 56, Y = −16, Z = 10). F: parameter estimates extracted from peak voxels in secondary somatosensory cortex. G: there was no significant activity in SII for the negative correlate of Aβ firing rate during brush on normal skin. H: parameter estimates for secondary somatosensory cortex during normal skin brushing (extracted from the same voxels as in F). See Table 2 for additional cluster peaks. Note use of radiological convention for statistical fMRI maps. Coordinates are in MNI space.
At the group level, we modeled mean effects in normal and sensitized skin across subjects. The group-level analysis also modeled the average ongoing pain rating for each participant across the experiment as a covariate. Ongoing ratings were demeaned across subjects, with higher values reflecting greater pain.
Anatomical areas were defined by the Harvard Oxford Cortical and Subcortical Structural Atlas (probabilistic population based) or Juelich Histological Atlas, both integrated as part of the FSL.
RESULTS
Psychophysical stimulus-response relationship to brushing of sensitized and normal skin.
Previous microneurography recordings during soft brush stroking found that unmyelinated tactile (CLTM) afferents respond with high discharge rates to brush velocities of 1–10 cm/s but respond less to slow (0.3 cm/s) and fast velocities (30 cm/s). At the speeds most effective for CLTMs, the stimulus is also perceived as being most pleasant. In contrast, Aβ LTMs increase their firing rate with increasing brush velocity (Ackerley et al. 2014; Löken et al. 2009). It follows that CLTM firing rates in response to varying brush velocity can be modeled as an inverted U-shaped function but that firing intensity in Aβ fibers increases linearly with brush velocity. We therefore reasoned that if Aβ afferent activity is an important contributor to DMA, then changes in Aβ firing rates, which are linear in relationship to brush velocity, should generate correlated percepts. On the other hand, if CLTMs are the predominant contributor to DMA, then the psychophysical stimulus-response function should best be described statistically by a quadratic term.
Figure 1 illustrates the experimental procedure (Fig. 1, A–D) and behavioral results. Negative and positive values denote unpleasant and pleasant ratings, respectively (−10 to +10), with zero being neutral. We randomized brush velocity within and between participants so that effects of velocity are independent of the linear decrease in capsaicin-induced ongoing pain over time (decrease of ongoing pain as a function of time: R = 0.153, P = 0.035; Fig. 1D). Ratings differed significantly between sensitized (black) and normal (gray) skin [Fig. 1E; F(1, 191) = 38.15, P < 0.001], and the area of stimulation interacted with velocity in significantly different ways [ANCOVA, F(1,191) = 4.43, P = 0.03]. On sensitized skin, we found that a linear term provided a significant fit for the stimulus-response relationship between the logarithm of velocity and unpleasantness (R = 0.298, P = 0.003). The stimulus was most unpleasant at velocities of 0.3–1 cm/s, which were expected to produce low discharge rates in Aβ LTMs. The perception evoked by brushing was rated as neutral/pleasant at high velocity (30 cm/s). The fit of the stimulus-response relationship on sensitized skin was not improved by a quadratic term (F-test). On normal skin, neither a linear nor a quadratic term was a statistically significant descriptor for the stimulus-response relationship.
In a separate cohort outside the scanner, we verified that the psychophysical stimulus-response relationships were reproducible using single brush strokes (Fig. 2). Furthermore, this experiment showed that the stimulus-response patterns for pain intensity and unpleasantness were similar.
The ongoing unpleasantness (VAS 0 to −10) that resulted from capsaicin application only was measured after each brush rating and decreased across the experiment from an average of −6.4 (SD 3.3) to −6 (SD 2.2) (t-test, P > 0.05). On sensitized skin, the slow brush strokes were again rated as most unpleasant and painful. Ratings decreased to pleasant/neutral at high velocities. Unpleasantness ratings were significantly different between a brush velocity of 0.3 cm/s and one at 30 cm/s on sensitized skin (ANOVA, P < 0.001, Dunnet T3 post hoc test, P = 0.001). There was no significant difference between ratings of unpleasantness and pain intensity on sensitized skin (ANOVA, P = 0.23) with the use of single brush strokes (Fig. 2, A and B). The linear decrease in pain and unpleasantness on sensitized skin is more pronounced with the use of single brush strokes compared with the stimulus-response curve shown in the main experiment (Fig. 1C) that used repeated brush strokes over 20 s. This finding compares well with results from neuropathic pain patients where allodynia is also attenuated by repeated brush strokes (Samuelsson et al. 2011). On normal skin, all stimuli were rated as pleasant, and there was no significant difference between velocities (ANOVA, P = 0.63; Fig. 2C).
Central processing of capsaicin-induced mechanical allodynia.
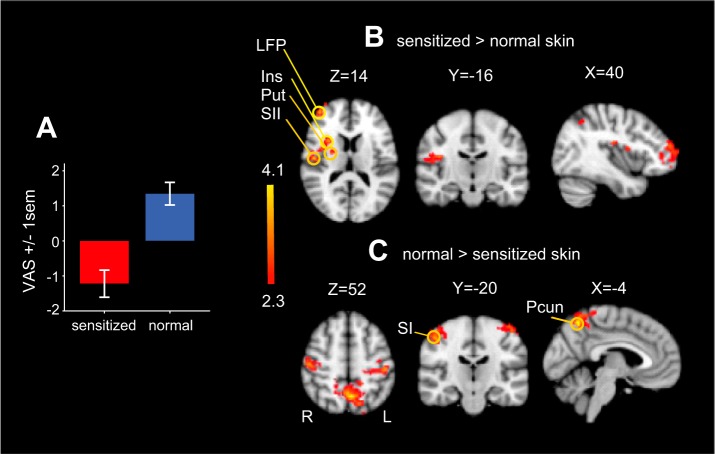
Next, we explored the brain areas that are more active during brushing on sensitized compared with normal skin regardless of brush velocity. Figure 3A shows an average of all behavioral ratings on sensitized skin (red) and normal skin (blue). There was no difference in the magnitude of deviation from zero between the two conditions (sensitized skin: mean = −1.11, SD = 2.9; normal skin: mean = 1.24, SD = 2.7). We used a t-test to identify differences in brain activity between brushing sensitized and normal skin. Brushing on sensitized skin increased activity in areas that likely enable withdrawal from the stimulus (Bingel et al. 2004). For example, clusters of activity were focused contralateral to the brush stimulation location, including contralateral putamen, posterior/medial insular cortex, secondary somatosensory area, and frontal pole, and these regions were significantly more active compared with brushing on normal skin (Fig. 3B). In contrast, regions that were more active during brushing on normal skin compared with sensitized skin (Fig. 3C) included the precuneus and bilaterally in the postcentral gyrus. Both the postcentral gyrus and the precuneus showed a decrease in activity in both conditions, but the decrease was of significantly smaller magnitude for brushing on normal skin compared with brushing on sensitized skin. This is consistent with previous functional neuroimaging pain studies suggesting that reduced activity in the precuneus reflects attention being directed toward a stimulus (Mantini et al. 2009).
Fig. 3.
Central processing of capsaicin-induced mechanical allodynia. A: average of all behavioral ratings on sensitized skin (red) and normal skin (blue). There was no difference in the magnitude of deviation from 0 between the 2 conditions (sensitized skin: mean = −1.11, SD = 2.9; normal skin: mean = 1.24, SD = 2.7). B: unique features in central processing of unpleasant and pleasant touch. A t-test was performed between stimulation-evoked activity on sensitized and normal skin. For sensitized skin, the contralateral putamen (Put; MNI space coordinates: X = 22, Y = 16, Z = 6), insular cortex (Ins; X = 38, Y = 2, Z = 14), secondary somatosensory area (SII; X = 50, Y = −12, Z = 16), lateral frontal pole (LFP; X = 48, Y = 46, Z = 6) were significantly more active compared with normal skin. C: the precuneus (Pcun; X = −4, Y = −60, Z = 54) and postcentral gyrus (SI; X = −30, Y = −28, Z = 74) were less deactivated on normal skin compared with sensitized skin. Note use of radiological convention for statistical fMRI maps (L = left, ipsilateral to stimulation; R = right).
The ongoing burning sensation elicited by capsaicin treatment was rated throughout the scan session. We analyzed how these ratings covaried with the main effects described above. Ongoing burning pain ratings was a significant covariate of activity in a large number of brain areas during brushing on sensitized skin but not on normal skin (Table 1).
Table 1.
Average level of ongoing pain as a covariate to brushing on sensitized skin
| Side | Area | Z-max | X | Y | Z |
|---|---|---|---|---|---|
| Left | Frontal pole | 5.42 | −36 | 44 | 32 |
| Right | Medial frontal gyrus | 5.26 | 30 | 10 | 68 |
| Left | Inferior frontal gyrus | 4.28 | −54 | 12 | 4 |
| Right | Supramarginal gyrus | 4.36 | 54 | −38 | 40 |
| Mid | Paracingulate gyrus | 3.73 | 0 | 14 | 52 |
| Right | Amygdala | 3.65 | 22 | −4 | −22 |
| Right | Cerebellum | 4.82 | 26 | −60 | −56 |
Data are cluster peaks of activity denoting average level of ongoing pain as a covariate to brushing (pooled velocities) on sensitized skin. Ongoing pain was not a significant covariate to brushing on normal skin. Z statistics of peak are denoted as Z-max. Coordinates are in MNI space.
Hemodynamic response as a function of Aβ afferent tuned activity.
We hypothesized that the relationship between activity in Aβ afferents and brush velocity would follow the tuning curve previously described in microneurography studies. We therefore estimated a tuning curve based on the average activity change across velocity changes in slowly adapting type I, slowly adapting type II, hair, and field units. These comprise the myelinated LTMs in hairy skin responding to a brush stroke under normal conditions, and they all respond with low firing rates at slow velocities and increase firing rates as brush velocity increase. Because field units, in particular, show an exponential increase in firing rates to the velocities used, the function used for analysis of brain activity that would fit all Aβ correlated activity was mildly exponential.
We explored if any BOLD activity varied with the Aβ tuning curve across brush velocities on sensitized skin and normal skin. As expected, on the basis of recordings from SI in nonhuman primates (Mountcastle et al. 1969), we found that the contralateral SI BOLD response was positively correlated with Aβ firing rates and produced comparable brain activity when sensitized (Fig. 4, A and B; P < 0.001, uncorrected) and normal skin (Fig. 4, C and D; P < 0.001 uncorrected) were stimulated. We found no significant difference between the activity correlated to Aβ encoding on sensitized and normal skin.
However, as noted above, the behavioral analysis revealed an inverse relationship between allodynia magnitude and brush velocity. That is, stimuli expected to elicit low Aβ firing rates were the most unpleasant (Fig. 1C). We therefore asked whether brain activity also showed a negative correlation with Aβ activity on the two skin areas. On sensitized skin, we found that activity in the secondary somatosensory cortex (SII) best modeled the inverse correlation of Aβ-tuned activity (Fig. 4, E and F). In other words, this analysis showed increased activity at slow stroking, when Aβ discharge rates are relatively low and unpleasantness is high. Clusters of activity were bilateral with peaks located in the opercular areas 4 and 1, ipsilateral insula, and contralateral postcentral gyrus (see Table 2 for additional clusters). There was no corresponding significant response in SII to the inverse correlation of Aβ-tuned activity on normal skin when the brush was perceived as pleasant (Fig. 4G, Table 2). Because the psychophysical stimulus-response curve was not better explained by a quadratic term, which would have indicated that CLTM firing influenced perception, we do not describe fMRI analysis in relation to these afferents.
Table 2.
| Side | Area | Z-max | X | Y | Z |
|---|---|---|---|---|---|
| Negative correlation to Aβ discharge rates on sensitized skin | |||||
| Right | OP4 | 3.94 | 54 | −2 | 6 |
| Left | OP4 | 3.87 | −48 | −4 | 2 |
| Right | Postcentral gyrus | 3.74 | 52 | −16 | 36 |
| Right | OP1 | 3.71 | 56 | −16 | 10 |
| Left | OP1 | 3.55 | −56 | −18 | 12 |
| Left | Insula | 3.21 | −36 | 2 | 14 |
| Negative correlation to Aβ discharge rates on normal skin | |||||
| Right | Supramarginal gyrus | 3.93 | 56 | −20 | 30 |
| Left | Supramarginal gyrus | 3.11 | −44 | −44 | 50 |
| Right | Frontal pole/OFC | 3.75 | 46 | 46 | −14 |
Data are main cluster peaks that showed negative correlation to Aβ contrast on sensitized and normal skin. OP4, opercular area 4; OP1, opercular area 1; OFC, orbital frontal cortex. Coordinates are in MNI space. Areas were defined by the Harvard Oxford Cortical and Subcortical Structural Atlas (probabilistic population based) or the Juelich Histological Atlas, both part of FSL.
DISCUSSION
Our objective was to determine the influence of Aβ firing rates on dynamic mechanical allodynia (DMA) by brushing at varying speeds. We found that the stimulus-behavioral response relationship on sensitized skin is linear and inversely related to the predicted change in neural activity for Aβ afferents. Under normal circumstances, i.e., in the absence of injury, the perceived increase in intensity of a tactile stimulus is encoded by an increased firing of Aβ LTMs (Muniak et al. 2007). We hypothesized that, in the setting of injury, the unpleasantness of DMA would be intensified as the firing rates of Aβ afferents increase. Contrary to our hypothesis, brush-evoked pain on injured skin is greater when Aβ firing is at low frequency, and pain decreases as firing rates increase. Our findings bear directly on a longstanding paradox, namely, how Aβ afferents can both inhibit pain, as proposed by the Gate Control Theory (Melzack and Wall 1965), and facilitate pain, as reported by patients with brush-evoked allodynia. We suggest that Aβ-LTM signals play a dual, frequency-dependent role and can contribute to both unpleasant and pleasant consequences during sensitization.
That brush stimuli inducing high firing rates in Aβ afferents produce neutral/pleasant ratings in the context of sensitization has not, to our knowledge, been shown before. The role of Aβ afferents for inhibiting signals transmitted by nociceptors is not new knowledge (Melzack and Wall, 1965) but has been difficult to reconcile with the phenomenon of allodynia. It was therefore surprising that under the same conditions on sensitized skin, brush stimuli, which under normal circumstances elicit relatively low firing rates in Aβ afferents, were most efficient at evoking unpleasant sensations.
We turned to the brain imaging data in an attempt to disambiguate the central consequences of variation in Aβ firing rate by brushing on sensitized and normal skin. We hypothesized that Aβ afferent firing rates are unaltered after capsaicin application (Torebjörk et al. 1992). Indeed, for both sensitized and normal skin, we found a positive correlation between the predicted brush-evoked Aβ firing rate and activity in the contralateral somatosensory arm area (Penfield and Rasmussen 1950). That Aβ firing is necessary for allodynia is firmly established (Campbell et al. 1988; Koltzenburg et al. 1992; LaMotte et al., 1991; Liljencrantz et al. 2013; Torebjörk et al. 1992; Treede and Cole 1993). However, our results showed that brush-evoked pain on injured skin was greater when Aβ afferents fire at low frequency and that pain decreases when firing frequency increases (Figs. 1C and 2, A and B). We therefore hypothesized that brain activity inversely correlated with the predicted Aβ firing rate, in an area that was more active during brushing on sensitized skin compared with normal skin, would suggest altered processing of a tactile stimulus during allodynia. We identified brain activity that correlated negatively to Aβ firing. There was a large and bilateral pattern of regions negatively correlated to Aβ firing rate during brushing on sensitized skin (Fig. 4). The same analysis during normal skin brushing revealed a different pattern of regions that did not include significant activation of SII and insula (Fig. 4). Of the areas that correlated to the inverse Aβ firing on sensitized skin (Fig. 4E), only SII was significantly more active during brushing on sensitized skin compared with normal skin (Fig. 3B). Regardless of velocity, brushing on sensitized skin increased activation of contralateral insula (Mesulam and Mufson 1982), the putamen (Bingel et al. 2004), and SII (Eickhoff et al. 2006) compared with brushing on normal skin. SII and anterior insula are typically activated during subjective reports of pain in dynamic allodynia (Becerra et al. 2006; Maihöfner et al. 2003; Peyron et al. 2004; Schweinhardt et al. 2006). SII and the concomitantly activated insular cortex are implicated in sensory-motor integration (Disbrow et al. 2003) and pain responses by somatosensory stimulation (Eickhoff et al. 2006; Mazzola et al. 2012; Mesulam and Mufson 1982). SII also encodes complex features of a stimulus, such as cross-modal interactions (Eickhoff et al. 2006). Our imaging results compare well with those described in earlier studies comparing brushing on normal and sensitized skin. Previous imaging studies of capsaicin-induced mechanical allodynia and hyperalgesia have also reported increased activation in SI and thalamus (Maihöfner and Handwerker 2005; Maihöfner et al. 2004; Moulton et al. 2007). There is evidence from single-unit recordings in rodents that thalamocortical processing is altered after capsaicin (Katz et al. 1999), and there are likely complex changes occurring in thalamus and SI that we are unable to capture, because the BOLD response represents the overall neural events taking place across a voxel. Taking a closer look at BOLD responses across velocities during brushing on normal and sensitized skin (Fig. 4), we found that although both conditions showed a correlation with the expected Aβ firing pattern in SI (Fig. 4, A–D), the responses on normal skin showed a slightly exponential shape (Fig. 4D). Deactivation of SI by affiliative touch has been shown previously (Gazzola et al. 2012), and SI also has been shown to be less activated during pleasantness ratings than during intensity ratings (Case et al. 2016). It is possible that the reduced linearity reflects a reduced necessity to discriminate sensation when it is nonpainful and instead assess its (pleasant) affective consequence.
The question remains, how does the brain select and process information from tactile signals to sense allodynia? We analyzed the area of SII that was significantly more active during brushing on sensitized skin and examined the pattern of BOLD response of this area during brushing on sensitized and normal skin (Figs. 3 and 4). Although the activity was significantly stronger during brushing on sensitized skin, the pattern of activity in response to varying brush velocity in SII was similar (Fig. 4, E–H). Electrophysiological recordings from the ventral posterior thalamus in rats suggest that wide dynamic range (WDR) neurons show increased responses to touch after nerve injury than under normal conditions (Patel and Dickenson 2016). The projections of the spinothalamic tract and dorsal column converge to some degree at the same neurons in the ventral posterior thalamus (Ma et al. 1987), which in turn projects to SII (Robinson and Burton 1980; Stevens et al. 1993). These results might explain the stronger activation of SII during brush allodynia. The observation that allodynia produces a difference in quality of pain, rather than adding to the intensity of burning pain (Koltzenburg et al. 1992), suggests that two types of central projecting cell lines are necessary for the detection of a difference in sensation. Much research has tried to unravel how spinal processes induced by nerve damage can link Aβ fibers to pain pathways ascending from lamina I in the dorsal horn (Woolf 2011). The simplest explanation may instead be that the sensation of allodynia depends on supraspinal selection of information from both sensitized lamina I projection cells that receive capsaicin-evoked C-fiber input and tactile information projecting through the dorsal column pathway. Indeed, nerve injury-induced allodynia by spinal nerve ligation in rats can be blocked by an ipsilateral but not contralateral dorsal column lesion (Sun et al. 2001). Furthermore, in the same study, lidocaine microinjected to the ipsilateral but not contralateral nucleus gracilis, where dorsal column neurons carrying Aβ-mediated information are relayed, abolished allodynia (Sun et al. 2001). However, our psychophysical results showing that low-frequency Aβ signals produce pain that decreases as firing increases suggest that the intensity of Aβ signals alone are not enough information for the brain to interpret that the stimulus is painful. If that was the case, one would expect an increase in Aβ firing by fast brush strokes to result in more pain, rather than less pain (Figs. 1C and 2, A and B). We suggest that the combined information from both capsaicin-sensitized spinothalamic nociceptive projection neurons and tactile signals projecting through the dorsal column are necessary for the perception of allodynia. The relative strength of activity between these two pathways dictates whether the perception results in allodynia or relief.
Data from single-unit recordings in monkeys have shown that both nociceptive and WDR neurons (Mendell and Wall 1965) project to overlapping areas in SII (Robinson and Burton 1980; Stevens et al. 1993). LTMs are organized in a partly overlapping, somatotopic fashion and have been suggested to interact over columns across laminae in the dorsal horn (Li et al. 2011). In line with this, it is possible that a high firing rate activity in Aβ LTMs in the context of sensitized neurons within the dorsal horn is possibly attenuated in its activity such that there is less input to SII, and subsequently, it is perceived as less unpleasant/pleasant. Alternatively, inhibition of SII activity by an increasing Aβ LTMs firing rate-driven S1 activity might also explain these results. Certainly, we know that GABAergic interneurons in the superficial dorsal horn, which receives input from Aβ afferents, have been shown to suppress aberrant low-threshold drive onto output projection neurons (Daniele and MacDermott 2009). There are also descending factors influencing the receptive field sensitivity of WDR cells in the spinal cord (Hillman and Wall 1969). Therefore, there are likely interactions between the nociceptive and tactile circuitry occurring at multiple sites at both the spinal and supraspinal level. Further work is needed to disambiguate these possible explanations.
Another group of afferents that have been proposed to contribute to allodynia are CLTM afferents that are coactivated by a brush stimulus. Findings from human microneurography and psychophysics have shown that the hedonic properties of touch on uninjured skin are mediated by unmyelinated low-threshold afferents (CLTMs) (Löken et al. 2009). Because CLTMs respond vigorously to a soft brush stroke, it has been suggested that they contribute to brush-evoked allodynia (Nagi and Mahns 2013; Seal et al. 2009). In neuropathic pain patients, as in our study, mechanical allodynia was most efficiently evoked by slow, repeated brush strokes (Samuelsson et al. 2011). However, consistent with reports showing that Aβ-denervated patients do not display allodynia (Treede and Cole 1993), the stimulus-response function relating brush and pain could not be explained by changes in the predicted CLTM discharge rate. Furthermore, CLTMs fatigue with repeated stimulation (Nordin 1990; Vallbo et al. 1999), even though pain increases with stimulus repetition (Samuelsson et al. 2011). However, it is possible that CLTMs contribute indirectly to the pain sensation. Liljencrantz et al. (2014) suggested that CLTMs have a secondary effect on allodynia by no longer signaling pleasantness. They found that Aβ-denervated subjects did not display capsaicin-induced allodynia but had less clear perception of pleasantness in the area surrounding capsaicin treatment. Our psychophysical results suggest that stimulating Aβ afferents at high frequency is more efficient than stimulating CLTMs for producing analgesia proximal to the focal site of injury. The intermediate brush velocities that are optimal to activate CLTMs will also coactivate Aβ afferents. However, CLTMs may also be a potent target for stimulating analgesia after injury (Delfini et al. 2013), and the conditions under which CLTMs contribute to or regulate allodynia remain to be determined.
We expanded on previous studies that have described pain processing during allodynia by examining the processing of tactile inputs. Importantly, a painful stimulus engages a broad set of brain regions that are not only related to the encoding of ascending inputs, culminating in an extensive network of activity (Baliki and Apkarian 2015; Denk et al. 2014; Wager et al. 2013). Indeed, we found that the level of ongoing pain experienced was a significant predictor of activity in a large number of areas including, but not limited to, amygdala, the frontal pole, and paracingulate gyrus during brushing on sensitized skin but not during brushing on normal skin (Table 1). A detailed understanding of pain percept during allodynia ultimately will be based on a complex integration between these regions and those processing tactile signals.
We suggest that the phenomenon of allodynia and the Gate Control Theory can be reconciled by emphasizing that it is not only an issue of balance between the nonnociceptive and nociceptive afferents but also the frequency of firing of the large-diameter afferents. Moreover, we suggest that the sensation of dynamic mechanical allodynia depends on supraspinal coding of the balance between at least two types of output cells, providing information about the (stimulus independent) ongoing barrage of signals from sensitized dorsal horn nociceptive neurons and firing rate intensity in Aβ afferents. Our results provide significant clarity to the puzzling paradox of how Aβ afferent stimulation can act as a pain facilitator in allodynia yet also as an inhibitor, as proposed by the Gate Control Theory (Braz et al. 2014; Melzack and Wall 1965).
GRANTS
This research was funded by a Sir Henry Wellcome Fellowship, Swedish Society for Medicine and Swedish Research Council (to L. S. Löken), and by the Wellcome Trust and Medical Research Council (Functional Magnetic Resonance Imaging of the Brain Centre and I. Tracey).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.S.L. and I.T. conceived and designed research; L.S.L. and E.D. performed experiments; L.S.L., E.D., and I.T. analyzed data; L.S.L. and I.T. interpreted results of experiments; L.S.L. prepared figures; L.S.L. drafted manuscript; L.S.L., E.D., and I.T. edited and revised manuscript; L.S.L., E.D., and I.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Profs. Stephen McMahon, Allan Basbaum, and Hakan Olausson for kindly reading and commenting on an earlier version of the manuscript.
REFERENCES
- Ackerley R, Backlund Wasling H, Liljencrantz J, Olausson H, Johnson RD, Wessberg J. Human C-tactile afferents are tuned to the temperature of a skin-stroking caress. J Neurosci 34: 2879–2883, 2014. doi: 10.1523/JNEUROSCI.2847-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Apkarian AV. Nociception, pain, negative moods, and behavior selection. Neuron 87: 474–491, 2015. doi: 10.1016/j.neuron.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra L, Morris S, Bazes S, Gostic R, Sherman S, Gostic J, Pendse G, Moulton E, Scrivani S, Keith D, Chizh B, Borsook D. Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J Neurosci 26: 10646–10657, 2006. doi: 10.1523/JNEUROSCI.2305-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingel U, Gläscher J, Weiller C, Büchel C. Somatotopic representation of nociceptive information in the putamen: an event-related fMRI study. Cereb Cortex 14: 1340–1345, 2004. doi: 10.1093/cercor/bhh094. [DOI] [PubMed] [Google Scholar]
- Braz J, Solorzano C, Wang X, Basbaum AI. Transmitting pain and itch messages: a contemporary view of the spinal cord circuits that generate gate control. Neuron 82: 522–536, 2014. doi: 10.1016/j.neuron.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JC, Beckmann CF, Miller KL, Wise RG, Porro CA, Tracey I, Jenkinson M. Physiological noise modelling for spinal functional magnetic resonance imaging studies. Neuroimage 39: 680–692, 2008. doi: 10.1016/j.neuroimage.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Raja SN, Meyer RA, Mackinnon SE. Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain 32: 89–94, 1988. doi: 10.1016/0304-3959(88)90027-9. [DOI] [PubMed] [Google Scholar]
- Case LK, Laubacher CM, Olausson H, Wang B, Spagnolo PA, Bushnell MC. Encoding of touch intensity but not pleasantness in human primary somatosensory cortex. J Neurosci 36: 5850–5860, 2016. doi: 10.1523/JNEUROSCI.1130-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cook AJ, Woolf CJ, Wall PD, McMahon SB. Dynamic receptive field plasticity in rat spinal cord dorsal horn following C-primary afferent input. Nature 325: 151–153, 1987. doi: 10.1038/325151a0. [DOI] [PubMed] [Google Scholar]
- Daniele CA, MacDermott AB. Low-threshold primary afferent drive onto GABAergic interneurons in the superficial dorsal horn of the mouse. J Neurosci 29: 686–695, 2009. doi: 10.1523/JNEUROSCI.5120-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfini MC, Mantilleri A, Gaillard S, Hao J, Reynders A, Malapert P, Alonso S, François A, Barrere C, Seal R, Landry M, Eschallier A, Alloui A, Bourinet E, Delmas P, Le Feuvre Y, Moqrich A. TAFA4, a chemokine-like protein, modulates injury-induced mechanical and chemical pain hypersensitivity in mice. Cell Reports 5: 378–388, 2013. doi: 10.1016/j.celrep.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Denk F, McMahon SB, Tracey I. Pain vulnerability: a neurobiological perspective. Nat Neurosci 17: 192–200, 2014. doi: 10.1038/nn.3628. [DOI] [PubMed] [Google Scholar]
- Devor M. Neuropathic pain: pathophysiological response of nerves to injury. In: Wall and Melzack’s Textbook of Pain (6th ed), edited by McMahon SL, Koltzenburg M, Tracey I, and Turk DC. Philadelphia, PA: Elsevier Saunders, 2013, p. 861–888. [Google Scholar]
- Disbrow E, Litinas E, Recanzone GH, Padberg J, Krubitzer L. Cortical connections of the second somatosensory area and the parietal ventral area in macaque monkeys. J Comp Neurol 462: 382–399, 2003. doi: 10.1002/cne.10731. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Amunts K, Mohlberg H, Zilles K. The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb Cortex 16: 268–279, 2006. doi: 10.1093/cercor/bhi106. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Spezio ML, Etzel JA, Castelli F, Adolphs R, Keysers C. Primary somatosensory cortex discriminates affective significance in social touch. Proc Natl Acad Sci USA 109: E1657–E1666, 2012. doi: 10.1073/pnas.1113211109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 48: 63–72, 2009. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman P, Wall PD. Inhibitory and excitatory factors influencing the receptive fields of lamina 5 spinal cord cells. Exp Brain Res 9: 284–306, 1969. doi: 10.1007/BF00235240. [DOI] [PubMed] [Google Scholar]
- Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med 49: 193–197, 2003. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage 62: 782–790, 2012. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Johnson KO, Hsiao SS. Neural mechanisms of tactual form and texture perception. Annu Rev Neurosci 15: 227–250, 1992. doi: 10.1146/annurev.ne.15.030192.001303. [DOI] [PubMed] [Google Scholar]
- Johnson KO, Hsiao SS, Yoshioka T. Neural coding and the basic law of psychophysics. Neuroscientist 8: 111–121, 2002. doi: 10.1177/107385840200800207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz DB, Simon SA, Moody A, Nicolelis MA. Simultaneous reorganization in thalamocortical ensembles evolves over several hours after perioral capsaicin injections. J Neurophysiol 82: 963–977, 1999. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Lundberg LE, Torebjörk HE. Dynamic and static components of mechanical hyperalgesia in human hairy skin. Pain 51: 207–219, 1992. doi: 10.1016/0304-3959(92)90262-A. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Shain CN, Simone DA, Tsai EF. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol 66: 190–211, 1991. [DOI] [PubMed] [Google Scholar]
- Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, Woodbury CJ, Ginty DD. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell 147: 1615–1627, 2011. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljencrantz J, Björnsdotter M, Morrison I, Bergstrand S, Ceko M, Seminowicz DA, Cole J, Bushnell MC, Olausson H. Altered C-tactile processing in human dynamic tactile allodynia. Pain 154: 227–234, 2013. doi: 10.1016/j.pain.2012.10.024. [DOI] [PubMed] [Google Scholar]
- Liljencrantz J, Marshall A, Ackerley R, Olausson H. Discriminative and affective touch in human experimental tactile allodynia. Neurosci Lett 563: 75–79, 2014. doi: 10.1016/j.neulet.2014.01.041. [DOI] [PubMed] [Google Scholar]
- Löken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat Neurosci 12: 547–548, 2009. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature 445: 858–865, 2007. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- Ma W, Peschanski M, Ralston HJ 3rd. The differential synaptic organization of the spinal and lemniscal projections to the ventrobasal complex of the rat thalamus. Evidence for convergence of the two systems upon single thalamic neurons. Neuroscience 22: 925–934, 1987. doi: 10.1016/0306-4522(87)92970-8. [DOI] [PubMed] [Google Scholar]
- Maihöfner C, Handwerker HO. Differential coding of hyperalgesia in the human brain: a functional MRI study. Neuroimage 28: 996–1006, 2005. doi: 10.1016/j.neuroimage.2005.06.049. [DOI] [PubMed] [Google Scholar]
- Maihöfner C, Neundörfer B, Stefan H, Handwerker HO. Cortical processing of brush-evoked allodynia. Neuroreport 14: 785–789, 2003. doi: 10.1097/00001756-200305060-00002. [DOI] [PubMed] [Google Scholar]
- Maihöfner C, Schmelz M, Forster C, Neundörfer B, Handwerker HO. Neural activation during experimental allodynia: a functional magnetic resonance imaging study. Eur J Neurosci 19: 3211–3218, 2004. doi: 10.1111/j.1460-9568.2004.03437.x. [DOI] [PubMed] [Google Scholar]
- Mantini D, Caulo M, Ferretti A, Romani GL, Tartaro A. Noxious somatosensory stimulation affects the default mode of brain function: evidence from functional MR imaging. Radiology 253: 797–804, 2009. doi: 10.1148/radiol.2533090602. [DOI] [PubMed] [Google Scholar]
- Mazzola L, Faillenot I, Barral FG, Mauguière F, Peyron R. Spatial segregation of somato-sensory and pain activations in the human operculo-insular cortex. Neuroimage 60: 409–418, 2012. doi: 10.1016/j.neuroimage.2011.12.072. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science 150: 971–979, 1965. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Mendell LM, Wall PD. Responses of single dorsal cord cells to peripheral cutaneous unmyelinated fibres. Nature 206: 97–99, 1965. doi: 10.1038/206097a0. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. J Comp Neurol 212: 38–52, 1982. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Moulton EA, Pendse G, Morris S, Strassman A, Aiello-Lammens M, Becerra L, Borsook D. Capsaicin-induced thermal hyperalgesia and sensitization in the human trigeminal nociceptive pathway: an fMRI study. Neuroimage 35: 1586–1600, 2007. doi: 10.1016/j.neuroimage.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB, Talbot WH, Sakata H, Hyvärinen J. Cortical neuronal mechanisms in flutter-vibration studied in unanesthetized monkeys. Neuronal periodicity and frequency discrimination. J Neurophysiol 32: 452–484, 1969. [DOI] [PubMed] [Google Scholar]
- Muniak MA, Ray S, Hsiao SS, Dammann JF, Bensmaia SJ. The neural coding of stimulus intensity: linking the population response of mechanoreceptive afferents with psychophysical behavior. J Neurosci 27: 11687–11699, 2007. doi: 10.1523/JNEUROSCI.1486-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagi SS, Mahns DA. Mechanical allodynia in human glabrous skin mediated by low-threshold cutaneous mechanoreceptors with unmyelinated fibres. Exp Brain Res 231: 139–151, 2013. doi: 10.1007/s00221-013-3677-z. [DOI] [PubMed] [Google Scholar]
- Nordin M. Low-threshold mechanoreceptive and nociceptive units with unmyelinated (C) fibres in the human supraorbital nerve. J Physiol 426: 229–240, 1990. doi: 10.1113/jphysiol.1990.sp018135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa JL, Yarnitsky D. Mechanical hyperalgesias in neuropathic pain patients: dynamic and static subtypes. Ann Neurol 33: 465–472, 1993. doi: 10.1002/ana.410330509. [DOI] [PubMed] [Google Scholar]
- Olausson H, Wessberg J, Morrison I, McGlone F, Vallbo A. The neurophysiology of unmyelinated tactile afferents. Neurosci Biobehav Rev 34: 185–191, 2010. doi: 10.1016/j.neubiorev.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Patel R, Dickenson AH. Neuronal hyperexcitability in the ventral posterior thalamus of neuropathic rats: modality selective effects of pregabalin. J Neurophysiol 116: 159–170, 2016. doi: 10.1152/jn.00237.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, Rasmussen T. The Cerebral Cortex of Man: A Clinical Study of Localization of Function. New York: Macmillan, 1950. [Google Scholar]
- Peyron R, Schneider F, Faillenot I, Convers P, Barral FG, Garcia-Larrea L, Laurent B. An fMRI study of cortical representation of mechanical allodynia in patients with neuropathic pain. Neurology 63: 1838–1846, 2004. doi: 10.1212/01.WNL.0000144177.61125.85. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Burton H. Somatic submodality distribution within the second somatosensory (SII), 7b, retroinsular, postauditory, and granular insular cortical areas of M. fascicularis. J Comp Neurol 192: 93–108, 1980. doi: 10.1002/cne.901920106. [DOI] [PubMed] [Google Scholar]
- Samuelsson M, Leffler AS, Johansson B, Hansson P. The influence of brushing force and stroking velocity on dynamic mechanical allodynia in patients with peripheral neuropathy. Eur J Pain 15: 389–394, 2011. doi: 10.1016/j.ejpain.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Schweinhardt P, Glynn C, Brooks J, McQuay H, Jack T, Chessell I, Bountra C, Tracey I. An fMRI study of cerebral processing of brush-evoked allodynia in neuropathic pain patients. Neuroimage 32: 256–265, 2006. doi: 10.1016/j.neuroimage.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature 462: 651–655, 2009. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155, 2002. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RT, London SM, Apkarian AV. Spinothalamocortical projections to the secondary somatosensory cortex (SII) in squirrel monkey. Brain Res 631: 241–246, 1993. doi: 10.1016/0006-8993(93)91541-Y. [DOI] [PubMed] [Google Scholar]
- Sun H, Ren K, Zhong CM, Ossipov MH, Malan TP Jr, Lai J, Porreca F. Nerve injury-induced tactile allodynia is mediated via ascending spinal dorsal column projections. Pain 90: 105–111, 2001. doi: 10.1016/S0304-3959(00)00392-4. [DOI] [PubMed] [Google Scholar]
- Torebjörk HE, Lundberg LE, LaMotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol 448: 765–780, 1992. doi: 10.1113/jphysiol.1992.sp019069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede RD, Cole JD. Dissociated secondary hyperalgesia in a subject with a large-fibre sensory neuropathy. Pain 53: 169–174, 1993. doi: 10.1016/0304-3959(93)90077-3. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Johansson RS. Properties of cutaneous mechanoreceptors in the human hand related to touch sensation. Hum Neurobiol 3: 3–14, 1984. [PubMed] [Google Scholar]
- Vallbo AB, Olausson H, Wessberg J. Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. J Neurophysiol 81: 2753–2763, 1999. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med 368: 1388–1397, 2013. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature 306: 686–688, 1983. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 152, Suppl: S2–S15, 2011. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]