Abstract
Astrocytes are proposed to converse with neurons at tripartite synapses, detecting neurotransmitter release and responding with release of gliotransmitters, which in turn modulate synaptic strength and neuronal excitability. However, a paucity of evidence from behavioral studies calls into question the importance of gliotransmission for the operation of the nervous system in healthy animals. Central pattern generator (CPG) networks in the spinal cord and brain stem coordinate the activation of muscles during stereotyped activities such as locomotion, inspiration, and mastication and may therefore provide tractable models in which to assess the contribution of gliotransmission to behaviorally relevant neural activity. We review evidence for gliotransmission within spinal locomotor networks, including studies indicating that adenosine derived from astrocytes regulates the speed of locomotor activity via metamodulation of dopamine signaling.
INTRODUCTION
Locomotor behaviors such as walking, flying, and swimming permit mammals to interact with their environment, thereby satisfying basic requirements of survival. In vertebrates, locomotion arises from the biomechanical properties of the skeletomuscular system and activity within dedicated neural circuitry in the central nervous system (CNS) (Dickinson et al. 2000; Grillner 2006; Kiehn 2006 2016; Orlovsky et al. 1999). Whereas the planning, initiation, and maintenance of movements involve various brain regions, including the cortex, basal ganglia, midbrain, and hindbrain (Orlovsky et al. 1999; Takakusaki et al. 2004), executive control over the timing and coordination of muscle activity is invested in networks of interneurons in the ventral horn of the spinal cord. These networks, like others in the spinal cord and brain stem that produce a stereotyped motor output, are referred to as central pattern generators (CPGs).
Spinal motor networks, including the motoneurons that provide their final common output, are subject to extensive neuromodulation by substances such as the biogenic amines 5-hydroxytryptamine (5-HT/serotonin), dopamine (DA), and norepinephrine, and amino acids including γ-aminobutyric acid (GABA) and glutamate (Miles and Sillar 2011). A given neuromodulator may act at multiple receptor subtypes and in a cell type-specific fashion. In addition, the effects of an individual neuromodulator may be modified by a second-order neuromodulator, a process termed metamodulation (Katz 1999; Miles and Sillar 2011). The resulting multiplicity of signaling mechanisms extends the repertoire of motor patterns that can be generated by a finite population of cells, contributing to the behavioral plasticity necessary for an animal to successfully negotiate its environment.
Although in many cases neuromodulators derive from neurons, they also may be secreted by astrocytes (Araque et al. 1999, 2014), as well as other glial cells (Jackson et al. 2017). Evidence gathered over the past two decades indicates that astrocytes release neuromodulators in response to activity in neighboring neurons, acting as the third partner, along with pre- and postsynaptic neurons, at tripartite synapses throughout the nervous system. So-called gliotransmission represents a form of information processing that extends the canonical role of astrocytes as cellular housekeepers, responsible for tasks including ion homeostasis, metabolic support, and neurotransmitter clearance (Verkhratsky and Butt 2013). However, the importance of gliotransmission to the operation of the nervous system in healthy animals remains controversial, in part because of a paucity of evidence that it contributes to the production of behavior (Agulhon et al. 2012; Bazargani and Attwell 2016; Hamilton and Attwell 2010; Nedergaard and Verkhratsky 2012; Sloan and Barres 2014).
Because the activity of neurons within the spinal cord determines quantifiable behaviors, spinal cord preparations may provide a tractable model for the study of gliotransmission and its role in the operation of neural networks. The lumbar spinal locomotor CPG controls the rhythmic activation of hindlimb muscles during walking and running, and rhythmic, behaviorally relevant patterns of activity can be generated by isolated CPG networks in the absence of descending and peripheral inputs. This has permitted the study of diverse neuromodulatory systems and their roles in networks controlling defined behaviors (Dickinson 2006; Harris-Warrick 2011; Marder and Bucher 2001; Miles and Sillar 2011; Whelan 2010). In the present article, we review recent studies indicating that astrocytes within the spinal cord are a source of neuromodulatory adenosine. We then discuss the modulation of motor networks by adenosine, including recent evidence that it functions principally as a metamodulator of dopamine during motor behaviors. For the most part, we discuss evidence from mice and rats, the models used in nearly all studies of gliotransmission to date. However, a recent report implicates bidirectional signaling between neurons and glia in the regulation of behavior in Drosophila, suggesting the possibility that a greater range of organisms may be investigated in future (Ma et al. 2016).
SPINAL LOCOMOTOR NETWORKS AND NEUROMODULATION
Networks of premotor interneurons selectively excite and inhibit pools of motoneurons with precise timing to ensure appropriate contraction and relaxation within antagonistic pairs of muscles, resulting in smooth, controlled movement (Orlovsky et al. 1999). During rhythmic locomotor behaviors, such as walking, flying, and swimming, spinal networks activate muscles in a cyclically repeated sequence. Like other CPGs within the brain stem and spinal cord, including the networks that coordinate muscle contractions during chewing, swallowing, and respiration (Feldman and Del Negro 2006; Jean 2001; Lund and Kolta 2006; Marder and Bucher 2001), locomotor networks remain capable of generating a basic motor pattern when inputs from descending and peripheral pathways are removed (Brown 1911; Guertin 2009).
The effects of neuromodulators have been studied extensively within spinal cord preparations, most notably from lampreys, Xenopus tadpoles, cats, rats, and mice (Dickinson 2006; Harris-Warrick 2011; Katz 1999; Katz and Frost 1996; Miles and Sillar 2011). Postnatal rodent spinal cords may be isolated and sustained in artificial cerebrospinal fluid in vitro, and rhythmic locomotor-related activity can be evoked in the hindlimb motor circuitry by application of agonists of glutamate and monoamine receptors or by electrical or optogenetic stimulation (Hägglund et al. 2013; Kiehn and Kjaerulff 1996; Kudo and Yamada 1987; Smith and Feldman 1987; Whelan et al. 2000). Isolated networks generate rhythmic activity in hindlimb muscles, if present, or in transected ventral roots containing the motoneurons that innervate them (Hayes et al. 2009; Kudo and Yamada 1987; Smith and Feldman 1987). In preparations in which the ventral roots are transected, field potential (electroneurogram) recordings reveal a pattern of rhythmic bursting in the lumbar ventral roots corresponding to activity observed during treadmill locomotion in adult animals (Cowley and Schmidt 1994; Kiehn and Kjaerulff 1996; Whelan et al. 2000). Bursting in lumbar ventral roots L1–L4 is in phase with hindlimb flexors and out of phase with bursting in roots L5 and L6, which corresponds to bursting in extensors.
The basic output of the locomotor CPG is determined by the intrinsic electrical properties of the neurons that constitute the network and by the synaptic connections between them (Grillner 2006). Neuromodulators adjust both, enabling a circumscribed population of neurons to produce diverse outputs and modes of adaptive behavior (Dickinson 2006; Harris-Warrick 2011; Katz 1999; Katz and Frost 1996; Miles and Sillar 2011). Alterations to motor network output allow animals to adjust their behavior according to developmental stage and physiological state and to meet the challenges posed by different environmental conditions (Grillner 2006). For instance, changes in flexor-extensor coordination within locomotor networks underlie transitions from one gait to another, and gait changes allow animals to efficiently vary the speed of their locomotion (Bellardita and Kiehn 2015; Orlovsky et al. 1999). Multifarious neuromodulatory influences acting in concert give rise to a vast repertoire of network and behavioral outputs and are thus a source of considerable behavioral flexibility (Harris-Warrick 2011; Miles and Sillar 2011). The iterative nature of fictive locomotor activity facilitates comparison of control and treatment conditions, and isolated spinal networks have been used to study the effects of diverse neuromodulators at the network level, by application of pharmacological and genetic tools. Although neuromodulation in locomotor networks has received considerable attention, evidence that neuron-glia interactions play a role has only recently emerged (Acton and Miles 2015; Witts et al. 2012).
GLIOTRANSMISSION
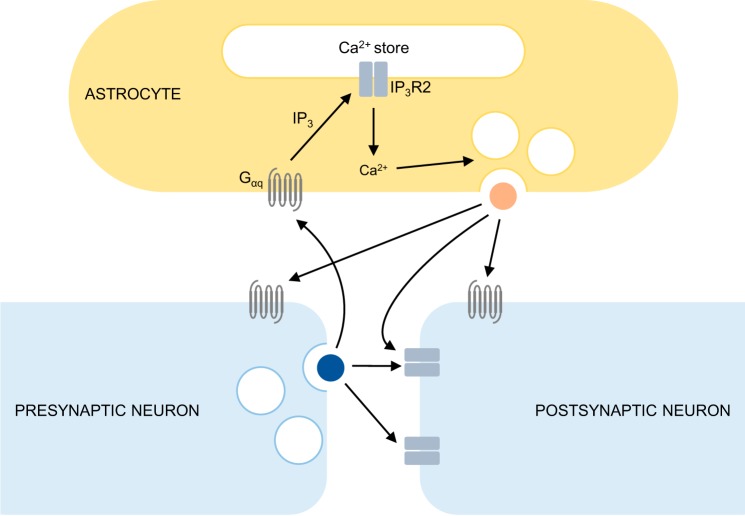
Glia are a class of cell within the central and peripheral nervous systems consisting of macroglia, which include astrocytes, oligodendrocytes and Schwann cells, and microglia (Verkhratsky and Butt 2013). Astrocytes, like the other glial cell types, are traditionally considered to have a merely passive, supportive function within neural networks, with well-established roles in ion homeostasis and the synthesis and clearance of neurotransmitters (Verkhratsky and Butt 2013). However, substantial evidence now indicates that astrocytes are active participants at tripartite synapses, dynamically regulating neuronal excitability and synaptic strength by the release of gliotransmitters (Araque et al. 1999, 2014; Bazargani and Attwell 2016; Haydon and Nedergaard 2015). In its original formulation, the tripartite-synapse model entails 1) the binding of a neurotransmitter released during synaptic activity to an astrocytic Gαq-linked G protein-coupled receptor (GPCR); 2) the production of inositol trisphosphate (IP3) and activation of IP3 receptors, triggering release of Ca2+ from stores within the astrocyte; 3) Ca2+-dependent release of a gliotransmitter such as glutamate (Bezzi et al. 1998; Han et al. 2013; Oh et al. 2012; Parpura et al. 1994; Pasti et al. 1997), adenosine triphosphate (ATP) (Newman 2001; Pryazhnikov and Khiroug 2008), or d-serine (Henneberger et al. 2010; Mothet et al. 2005) by vesicular exocytosis or via ion channels; and 4) the activation of metabotropic or ionotropic receptors on either the pre- (Carlsen and Perrier 2014; Jourdain et al. 2007) or postsynaptic neuron (Angulo et al. 2004; Fellin et al. 2004; Parri et al. 2001) (Fig. 1) of the same (Martín et al. 2015; Navarrete and Araque 2010) or a different (Pascual et al. 2005; Serrano et al. 2006; Zhang et al. 2003) synapse. Although signaling in this manner has been denoted “gliotransmission,” this is arguably a misnomer: modulation of neuronal activity by substances derived from astrocytes occurs on a variable timescale but, because of its dependency on metabotropic receptors, is necessarily slower than acute transmission mediated by ionotropic receptors (Agulhon et al. 2012; Araque et al. 2014). “Gliomodulation” has therefore been proposed as a more appropriate term to distinguish bidirectional signaling between neurons and glia (Agulhon et al. 2012) but is not widely used (“gliotransmission” is used in this review).
Fig. 1.
Established model of bidirectional signaling between neurons and glia at the tripartite synapse. Neurotransmitters activate astrocytic Gαq-linked G protein-coupled receptors (GPCRs), stimulating the production of inositol trisphosphate (IP3), activation of astrocytic IP3 receptors (IP3R2), and release of Ca2+ from internal stores. An increase in intracellular Ca2+ triggers release of gliotransmitters including glutamate, ATP, and d-serine by a vesicular or channel-mediated mechanism; these in turn bind to pre- or postsynaptic GPCRs, or in the case of d-serine, N-methyl-d-aspartate (NMDA) receptors, to modulate synaptic strength or neuronal excitability.
The steps in the pathway described above have been deduced largely from Ca2+ imaging of astrocytes and electrophysiological recordings from neurons and are supported by numerous studies (Haydon and Nedergaard 2015). However, the extent to which gliotransmission is important for the operation of neural networks and in the production of behavior in healthy animals remains controversial (Agulhon et al. 2012; Bazargani and Attwell 2016; Hamilton and Attwell 2010; Nedergaard and Verkhratsky 2012; Sloan and Barres 2014). The coding of information by astrocytes in the form of Ca2+ fluctuations, which vary in kinetics and subcellular localization, is poorly understood, and the physiological relevance of experimental manipulations used to investigate Ca2+ signaling is disputed (Nedergaard and Verkhratsky 2012; Rusakov 2015). Crucially, evidence is lacking that gliotransmission dependent on astrocytic Gαq-linked GPCRs and Ca2+ signaling is important in the generation of behavior.
GLIOTRANSMISSION AND BEHAVIOR
Reports have indicated astrocytic involvement in diverse behaviors (reviewed in Oliveira et al. 2015); these include sleep (Foley et al. 2017; Halassa et al. 2009; however, see Fujita et al. 2014), pain aversion (Foley et al. 2011), recognition memory (Florian et al. 2011; Halassa et al. 2009), motor coordination (Watase et al. 1998), whisker-dependent perception (Han et al. 2014), olfactory responses (Martin et al. 2012), and anxiety-like and depressive behavior (Banasr and Duman 2008; Cao et al. 2013; John et al. 2012). However, despite extensive evidence for bidirectional communication between neurons and astrocytes in in vitro preparations, evidence that gliotransmission is important for the production or modulation of behavior is sparse (for further discussion, see Agulhon et al. 2012; Bazargani and Attwell 2016; Hamilton and Attwell 2010; Nedergaard and Verkhratsky 2012; Sloan and Barres 2014). Uncertainty about the contribution of gliotransmission to the generation of behaviors stems from a lack of acute and selective techniques for the manipulation of astrocytes in vivo and uncertainty about the physiological relevance of the techniques that have been applied to the study of gliotransmission to date (reviewed in Bazargani and Attwell 2016 and Nedergaard and Verkhratsky 2012). Importantly, mice lacking IP3R2, the IP3 receptor isoform that is preferentially expressed in astrocytes, (IP3R2KO mice) have been tested for a range of behaviors, including locomotion, learning, memory, and anxiety, but do not differ from wild-type littermates on most measures (Agulhon et al. 2013; Petravicz et al. 2014); however, the possibility that the importance of IP3R signaling is masked in these studies by compensatory mechanisms during development cannot be excluded. Until further behavioral evidence for gliotransmission in healthy animals is provided, it will remain controversial.
Given the limitations of the techniques currently available for studying gliotransmission in behaving animals, in vitro and in vivo rhythmically active brain stem preparations and in vitro spinal cord preparations may provide insight into the role of bidirectional neuron-glia signaling in behavior. Studies of brain stem networks for respiration and mastication have provided cogent evidence for behaviorally relevant gliotransmission. In the brain stem, astrocytic Ca2+ elevations are evoked by reductions in both pH (Gourine et al. 2010; Kasymov et al. 2013) and the partial pressure of oxygen (Angelova et al. 2015), in both cases causing release of ATP, which stimulates breathing. In intact rats, optogenetic stimulation of astrocytes also triggers release of ATP to stimulate breathing (Gourine et al. 2010), whereas expression of tetanus toxin in astrocytes to inhibit vesicular release prevents increases in respiration normally observed in response to reduced oxygen availability (Angelova et al. 2015). The release of modulators from astrocytes described by these studies departs from the tripartite synapse model, however, because it is stimulated by astrocytic chemoception, rather than detection of a neurotransmitter. Glia are also proposed to modulate neuronal responses to ATP within the pre-Bötzinger complex (pre-BötC), an area of the medulla critical for production of the respiratory rhythm, by releasing glutamate in a Ca2+-dependent manner (Huxtable et al. 2010). A further example of neuron-glia cross talk in a rhythmic network is provided by the brain stem circuity for mastication, where sensory input is proposed to activate astrocytic N-methyl-d-aspartate (NMDA) receptors, eliciting release of the Ca2+-binding protein S100β, with the resulting decrease in extracellular Ca2+ concentration conferring rhythmic bursting properties on neighboring neurons (Morquette et al. 2015). Evidence from networks that generate rhythmic, stereotyped motor behaviors (including evidence from the locomotor CPG, described below) therefore may help to illuminate the extent to which astrocytes participate in information processing within the nervous system of intact animals.
EVIDENCE FOR GLIOTRANSMISSION IN THE SPINAL CORD
Glial Ca2+ Signaling
Although a substantial literature is dedicated to the role of astrocytic information processing in in vitro brain preparations, research into gliotransmission in the spinal cord is in its infancy. Information about the dynamics of Ca2+ signaling in astrocytes has been provided for the most part by studies in the cortex, hippocampus, and cerebellum. Ca2+ signaling in astrocytes has been extensively studied by imaging cells expressing a genetically encoded Ca2+ indicator or loaded with Ca2+-sensitive dyes by means of a patch pipette. Astrocytes are acutely sensitive to neuronal activity, to the extent that they can respond to basal synaptic activity stimulated by a single action potential (Panatier et al. 2011). They display intracellular Ca2+ elevations in response to neuronal activity in both neonatal and adult rodents in vitro and in vivo (Araque et al. 2014; Bazargani and Attwell 2016; Rusakov 2015; Shigetomi et al. 2016). Ca2+ signaling is observed in awake mice in response to sensory stimulation in the brain and dorsal horn of the spinal cord (Sekiguchi et al. 2016; Srinivasan et al. 2015) and during locomotion in the cortex (Dombeck et al. 2007). Given these observations, astrocytes within the ventral horn of the spinal cord may be expected to display rhythmic oscillations corresponding to those of neuronal components of the locomotor CPG during network activity. Although imaging of ventral horn astrocytes in behaving animals may be challenging because of the difficulty of accessing tissue, it is possible to detect Ca2+ signaling in astrocytes in in vitro preparations during network activity. Preliminary studies have reported rhythmic Ca2+ fluctuations in putative astrocytes during pharmacologically induced locomotor-related activity in in vitro hemicord preparations (Chub et al. 2012). Furthermore, Ca2+ fluctuations are selectively blocked during inhibition of metabotropic glutamate receptor 1 (mGluR1) during ventral root stimulation in disinhibited preparations, implying a role for that receptor in neuron-glia signaling in the spinal cord, as reported in the brain (Chub and O’Donovan 2011). Consistent with these results, a subset of astrocytes in the pre-BötC displays rhythmic Ca2+ activity immediately preceding rhythmic respiratory-related neuronal bursts (Okada et al. 2012; Oku et al. 2016). Interestingly, pre-BötC astrocytes also display low-frequency synchronized Ca2+ transients during network activity, indicating a complex relationship between activity in neurons and astrocytes in this network. This finding suggests that astrocytes in locomotor networks may also display patterns of Ca2+ signaling that do not directly correspond to rhythmic locomotor-related activity in neurons. Apart from preliminary evidence from the Ca2+ imaging studies cited above, indirect evidence also supports neuron-to-astrocyte signaling in the spinal cord; this is discussed below.
Stimulation of Spinal Cord Astrocytes
A range of techniques is available for the experimental induction of astrocytic Ca2+ elevations (for further information, see Araque et al. 1999; Bazargani and Attwell 2016; Nedergaard and Verkhratsky 2012), including ultraviolet (UV) photolysis of caged Ca2+ or IP3 introduced via a patch pipette (Hua et al. 2004; Martín et al. 2015; Wang et al. 2013), depolarization of the astrocyte under whole cell patch-clamp conditions (Jourdain et al. 2007; Kang et al. 1998), mechanical stimulation (Hua et al. 2004; Lee et al. 2015), activation of endogenous or transgenically expressed GPCRs, including DREADDs (designer receptors exclusively activated by designer drugs; Agulhon et al. 2010, 2013; Chen et al. 2016; Rae and Irving 2004; Shigetomi et al. 2008), and activation of transgenically expressed channelrhodopsins (Beppu et al. 2014; Gourine et al. 2010; Li et al. 2012). Recent studies of gliotransmission in the spinal cord have exploited protease-activated receptor-1 (PAR1) to trigger or enhance astrocytic Ca2+ signaling (Acton and Miles 2015; Carlsen and Perrier 2014). PAR1 is an endogenous GPCR associated with Gαq proteins, and selective activation by a synthetic ligand results in Ca2+ elevations in astrocytes (Lalo et al. 2014; Lee et al. 2007; Shigetomi et al. 2008). In the spinal cord (Acton and Miles 2015), as in the brain (Junge et al. 2004; Weinstein et al. 1995), PAR-1 is expressed by cells that also express glial fibrillary acidic protein (GFAP), an intermediate filament protein used widely as an astrocyte marker (Wang and Bordey 2008); by contrast, neurons do not appear to express the receptor in the ventral horn (Acton and Miles 2015). Cells expressing PAR1 in the spinal cord are therefore proposed to be astrocytes. However, the possibility that a subset of neural precursor cells of the astrocyte lineage also expresses PAR1 has not been excluded; gliogenesis continues during postnatal stages at which network-level and single-cell recordings are typically made (Tien et al. 2012), and it has been proposed that some neural precursor cells express GFAP in the spinal cord during postnatal development (Chvátal et al. 1995). In addition, the adult spinal cord contains GFAP+ multipotent neural stem cells that are quiescent within the intact spinal cord (Fiorelli et al. 2013). Such populations of nonastrocytic GFAP+ cells must therefore be taken into account when studies using GFAP as a marker of astrocytes in the spinal cord are being interpreted. Whether PAR1 activation results in Ca2+ signaling exclusively in terminally differentiated astrocytes remains to be confirmed, as does the proportion of astrocytes that expresses PAR1. Nevertheless, PAR1 activation offers several advantages as a means of stimulating astrocytes in the spinal cord: activation of PAR1 does not affect activity in locomotor networks following pharmacological ablation of astrocytes, indicating that it does not directly modulate the activity of other cell types (Acton and Miles 2015); similarly, it does not alter the excitability of neurons in the brain (Lalo et al. 2014; Lee et al. 2007; Shigetomi et al. 2008); it is expressed from early postnatal stages in cells across the ventral horn, allowing those cells to be stimulated concurrently during activity of the locomotor CPG by bath application of a selective PAR1 agonist; and finally, because it is an endogenous receptor, experimental PAR1 activation may result in Ca2+ elevations with spatiotemporal properties close to those generated by GPCR activation during neural activity in vivo, which may not be the case for transgenically expressed receptors.
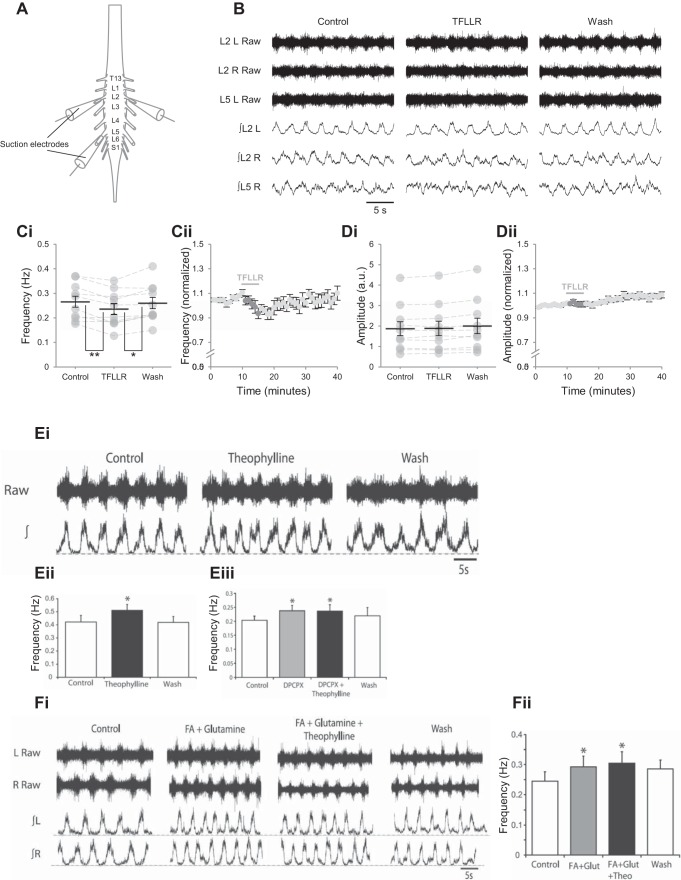
During pharmacologically induced network activity in in vitro spinal cord preparations from postnatal mice, stimulation of glia by PAR1 activation results in a transient reduction in the frequency but not the amplitude of rhythmic bursting (Fig. 2, A–D) (Acton and Miles 2015). This effect is blocked by theophylline, a nonselective adenosine receptor antagonist, and cyclopentyl dipropylxanthine (DPCPX), a selective antagonist of A1-adenosine receptors, indicating that it is mediated by adenosine. Extracellular adenosine is largely formed when ATP is released from cells and then hydrolyzed by ectonucleotidases in the extracellular space, although adenosine may also be secreted from cells directly (Cunha 2001; Klyuch et al. 2012; Wall and Dale 2013). Given that the activity of spinal neurons is unaltered when glia are stimulated in the presence of an ectonucleotidase inhibitor, spinal glia appear to release ATP rather than adenosine itself (Acton and Miles 2015; Carlsen and Perrier 2014).
Fig. 2.
Adenosine derived from glial cells acts via A1 receptors to modulate locomotor-related activity in a murine spinal cord preparation. A: an in vitro spinal cord preparation in which suction electrodes are used to recording compound action potentials from motoneurons in transected ventral roots. B: sample raw (top) and rectified/integrated (bottom) ventral root traces showing rhythmic locomotor-related bursting evoked by bath application of 5-hydroxytryptamine (10 µM), NMDA (5 µM), and dopamine (50 µM) in control conditions, following application of Thr-Phe-Leu-Leu-Arg-NH2 trifluoroacetate salt (TFLLR; 10 µM) to selectively activate glial protease-activated receptor-1 (PAR1) and during washout. Ci: locomotor-burst frequency over 3 min during a control period, immediately following TFLLR application, and following a 20-min washout period. Individual data points are shown in gray, and means are represented by black lines. Error bars indicate ± SE Cii: time course plot of normalized data aggregated into 1-min bins showing a reduction in burst frequency upon application of TFLLR (n = 10 preparations). Di: locomotor-burst amplitude is unaffected by TFLLR application. Dii: time course plot showing no change in burst amplitude upon application of TFLLR. Ei: sample traces showing the effects of the nonselective adenosine receptor antagonist theophylline (20 µM) on locomotor-related activity. Eii: theophylline increases the frequency of locomotor-related bursting, revealing the modulatory role of endogenous adenosine derived from sources within the ventral horn. Values are means ± SE; n = 6. Eiii: the selective A1-adenosine receptor antagonist cyclopentyl dipropylxanthine (DPCPX; 50 µM) also reveals modulation of locomotor-related activity by endogenous adenosine and prevents further frequency increases in the presence of theophylline, demonstrating that adenosine acts via A1 receptors. Values are means ± SE; n = 6. Fi: traces showing the effects of the glial toxin fluoroacetate (FA; 5 mM) when coapplied with glutamine (1.5 mM) and a lack of modulation by endogenous adenosine following glial ablation, as revealed by application of theophylline (20 µM). Fii: theophylline has no effect on the frequency of locomotor activity when applied in the presence of the glial toxins FA and methionine sulfoximine. Values are means ± SE; n = 6. *P < 0.05; **P < 0.01. [B–D are adapted from Acton and Miles (2015). E and F are adapted from Witts et al. (2012).]
It is interesting that inhibition of A1 receptors during fictive locomotion is sufficient to block the effects of PAR1 stimulation, suggesting that ATP-adenosine is the only gliotransmitter produced in these experiments (Acton and Miles 2015). By contrast, stimulation of astrocytes in the dorsal horn has been shown to elicit release of glutamate (Bardoni et al. 2010; Nie et al. 2010), and astrocytes in the brain release substances including glutamate, d-serine, and GABA (Araque et al. 1999, 2014), all of which modulate spinal motor networks (Acton and Miles 2017; Bertrand and Cazalets 1999; Iwagaki and Miles 2011; Miles and Sillar 2011; Wang and Dun 1990). Astrocytes in the ventral horn may not, therefore, match neurons in the diversity of neuromodulatory substances they secrete. However, some techniques fail to elicit gliotransmitter release in preparations where others are effective (Shigetomi et al. 2008; Wang et al. 2013). Activation of either PAR1 or the purinergic receptor P2Y1, both endogenous astrocytic Gαq-linked GPCRs, elicits Ca2+ elevations of similar amplitude in the somas of astrocytes in the CA1 region of the hippocampus, but only PAR1 activation stimulates gliotransmitter release. The cause of this discrepancy is unknown, but could, for instance, be related to differences in the subcellular localization of the receptors in relation to Ca2+ stores and sites of gliotransmitter release (Bazargani and Attwell 2016). For this reason, it is desirable that several techniques for stimulating astrocytes be compared in future studies. In addition, the experiments described above do not fully consider state-dependent differences in gliotransmission. It is conceivable that the influence of astrocyte-derived modulators varies according to the fluctuating neuromodulatory milieu of the spinal cord (Acevedo et al. 2016; Bazargani and Attwell 2016; Gerin et al. 1995; Gerin and Privat 1998). State-dependent modulation of networks by astrocytes may involve changes in the competence of astrocytes to detect neuronal activity or release modulators, or changes in neuronal sensitivity. The possibility that adenosinergic modulation is dependent on the actions of dopamine is discussed below.
In agreement with adenosine release during network activity, PAR1 activation elicits the release of ATP-adenosine from ventral horn glia in slice preparations from postnatal mice, reducing the amplitude of evoked excitatory postsynaptic currents (EPSCs) recorded from interneurons via a presynaptic mechanism mediated by A1 receptors (Carlsen and Perrier 2014). Also, consistent with findings from rhythmically active preparations, this depends on extracellular ectonucleotidase activity, indicating that adenosine but not ATP modulates ventral horn interneurons. However, despite apparent agreement, it remains unclear whether adenosine acts via a common mechanism in spinal cord slices and rhythmically active isolated spinal cord preparations (see below).
Suppression of Gliotransmission in the Spinal Cord
Endogenous release of substances from astrocytes can be suppressed by inhibition of astrocytic Ca2+ signaling by various strategies, including dialysis of astrocytic syncytia by Ca2+ buffers and inhibition of IP3R signaling (for further information, see Bazargani and Attwell 2016; Hamilton and Attwell 2010; Nedergaard and Verkhratsky 2012). In addition, secretion of gliotransmitters can be suppressed by inhibition of G protein signal transduction (Di Castro et al. 2011), disruption of vesicle release mediated by N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins (Araque et al. 2000; Gourine et al. 2010; Jourdain et al. 2007; Lalo et al. 2014; Sultan et al. 2015), or by conditional expression of toxins (Angelova et al. 2015) or dn-SNARE, a dominant-negative cytosolic domain of vesicle-associated membrane protein 2 (VAMP2; a.k.a. synaptobrevin 2) (Fellin et al. 2009; Fujita et al. 2014; Lalo et al. 2014; Pascual et al. 2005; Sultan et al. 2015).
Suppression of astrocytic Ca2+ signaling has not been achieved during locomotor-related network activity in spinal cord preparations, and techniques for selective and acute inhibition of astrocytic Ca2+ signaling are currently unavailable. In acute spinal cord slices from mice, loading astrocytes with a Ca2+ chelator enhances the amplitude of EPSCs in neighboring neurons (Carlsen and Perrier 2014). Although this is proposed to reflect a reduction in Ca2+-dependent release of ATP-adenosine from glia, this has not been tested directly. It has, however, been shown that adenosinergic modulation of network activity is prevented in murine preparations in which astrocytes have been treated with gliotoxins, which comprehensively disrupt astrocytic function (Clarke 1991; Fonnum et al. 1997; Huxtable et al. 2010; Li et al. 2013; Wall and Dale 2013; Witts et al. 2012; Zhang et al. 2003). Network activity persists following application of the gliotoxins methionine sulfoximine or fluoroacetate if glutamine, the astrocyte-derived precursor of glutamate and GABA, is supplemented to support rhythmic activity (Witts et al. 2012). Methionine sulfoximine irreversibly inhibits the astrocytic enzyme glutamine synthetase and causes glycogen accumulation and cell damage in astrocytes, but not other glial cells (Aschner and Kimelberg 1996; De Vellis 2002; Phelps 1975; Ronzio et al. 1969). Fluoroacetate, a precursor of the glial aconitase inhibitor fluorocitrate (Fonnum et al. 1997), is proposed to disrupt astrocyte metabolism by reversible inhibition of the Krebs cycle, resulting in decreased production of ATP (Aschner and Kimelberg 1996; Keyser and Pellmar 1994; Paulsen et al. 1987). Following astrocytic ablation by either substance, blockade of adenosine receptors by DPCPX or theophylline has no effect on network output, revealing that endogenous adenosine no longer modulates network activity (Fig. 2, E and F) (Witts et al. 2012). It should be noted that extensive disruption of astrocytic metabolism is an imprecise technique for suppressing gliotransmission, and off-target effects of gliotransmitters have been reported, including changes in extracellular K+ levels (Largo et al. 1997) and glycogen deposition in cranial motoneurons (Young et al. 2005). However, spinal cord preparations remain sensitive to adenosine following gliotoxin treatment, consistent with disruption of endogenous release of adenosine from astrocytes (Witts et al. 2012). Thus astrocytes are proposed as the source of modulatory adenosine in the mammalian spinal cord during locomotor-related activity, despite reported release of adenosine from neurons and microglia in other systems (Jackson et al. 2017; Wall and Dale 2013).
Adenosine is also released in the spinal cord during hypoxia and hypocapnia, and depresses reflex potentials recorded from the ventral roots (Lloyd et al. 1988; Otsuguro et al. 2006, 2009, 2011). Release of adenosine during hypoxia, although not hypocapnia, is inhibited by fluoroacetate, implying an astrocytic source (Takahashi et al. 2010). However, in this context it is not clear whether adenosine acts on spinal cord neurons or on peripheral afferents. In addition, it should be noted that in primary cultures of brain cells, oxidative stress was found to elicit release of adenosine from microglia and not from astrocytes or neurons (Jackson et al. 2017).
Together, these experiments demonstrate that activation of Gαq-coupled GPCRs expressed by putative astrocytes in the spinal cord results in the production of adenosine, which in turn modulates neuronal activity via A1-adenosine receptors. Production of modulatory adenosine following release of ATP from astrocytes appears to be widespread throughout the CNS, having also been reported in brain preparations (Panatier et al. 2011; Pascual et al. 2005; Serrano et al. 2006).
Indirect Evidence for Neuron-to-Glia Signaling in Motor Networks
The tripartite synapse model implies detection of neuronal activity by astrocytes, which respond with elevations in cytosolic Ca2+. Indirect support for neuron-to-astrocyte signaling during locomotor network activity is provided by the observation that modulation of the frequency of locomotor-related activity by endogenous adenosine scales with network activity (Acton and Miles 2015). This presumably represents activity-dependent release of ATP-adenosine by astrocytes and implies that ventral horn astrocytes detect neuronal activity, for instance, by detection of neurotransmitter release, as predicted by the tripartite synapse model. Astrocytes are therefore proposed to mediate a negative feedback loop, whereby activity-dependent release of adenosine depresses and perhaps stabilizes network output, resulting in smooth, controlled movement (Acton and Miles 2015). However, further Ca2+ imaging is needed to confirm spinal astrocytic responsiveness to neuronal activity, as has been demonstrated in the brain (Araque et al. 2014; Bazargani and Attwell 2016; see above).
Interestingly, it has been observed that astrocytes express receptors for the neurotransmitters released specifically by the neurons they make contact with, implying that astrocytic receptors function to detect synaptic transmission (Verkhratsky and Butt 2013). Thus spinal cord astrocytes are unusual in expressing receptors for glycine, the dominant inhibitory neurotransmitter in the spinal cord (Bowery and Smart 2006; Pastor et al. 1995). Both glycine and GABAA receptors expressed by spinal cord astrocytes carry a Cl− current, as they do in neurons (Pastor et al. 1995). Spinal cord astrocytes also express NMDA receptors and are sensitive to glutamate (Ziak et al. 1998); however, the physiological importance of these receptors remains unknown.
ADENOSINERGIC MODULATION OF SPINAL MOTOR CIRCUITRY
The purines ATP and its derivative adenosine are involved in a myriad of biological processes, most notably energy transfer. In the nervous system, they also function as neuromodulators and are involved in diverse processes in health and disease, including sleep homeostasis, memory, and the regulation of mood (Burnstock 2007; Cunha 2001; Fredholm et al. 2005). Experiments indicating release of modulatory adenosine from astrocytes in the ventral horn are complemented by studies into the functions and mechanisms of purinergic signaling during motor activity. However, some data point to unresolved complexity.
There are four adenosine receptor subtypes, designated A1, A2A, A2B, and A3, which differ in affinity for adenosine, structure, cellular distribution, and G protein coupling (Cunha 2001; Dunwiddie and Masino 2001). A1 and A2A receptors have the highest affinity for adenosine and are the best characterized (Cunha 2001). Both receptors are expressed throughout the spinal cord (Deuchars et al. 2001; Paterniti et al. 2011; Reppert et al. 1991). A1 receptors are tightly coupled to the Gαi pathway, which mediates inhibition of adenylyl cyclase and reduced production of cAMP (Cunha 2001). However, A1 receptors are also coupled to other G proteins and act via adenylyl cyclase-independent pathways to inhibit spontaneous and evoked neurotransmitter release (Cunha 2001). A1 receptors typically mediate presynaptic inhibition via the adenylyl cyclase-independent inhibition of N-type Ca2+ channels (Cunha 2001; Ribeiro 1995). A2A receptors are primarily coupled to Gαs, but also to Gαi and Gα12, and have diverse effects mediated by protein kinase A (PKA), protein kinase C (PKC), and N-type and P-type Ca2+ channels (Cunha 2001). In many systems, the inhibitory actions of A1 receptors are countered by the facilitatory actions of A2A receptors in a concentration-dependent manner (Cunha 2001).
The role of adenosine signaling in the modulation of mammalian locomotor behavior has been addressed by injection of intact animals with receptor antagonists, such as caffeine, which typically have an excitatory effect. This effect has been proposed to be mediated by either A1 (Goldberg et al. 1985; Snyder et al. 1981), A2A (El Yacoubi et al. 2000; Ledent et al. 1997; Lindskog et al. 2002; Svenningsson et al. 1995, 1997) or both A1 and A2A receptors (Antoniou et al. 2005; Karcz-Kubicha et al. 2003; Kuzmin et al. 2006), perhaps indicating a role for both receptor subtypes. However, interpretation of studies in which antagonists are chronically applied is complicated by the reported acquisition of receptor tolerance, to which A1 receptors are particularly susceptible (Karcz-Kubicha et al. 2003). It is also difficult to draw conclusions about the locus or loci of adenosinergic modulation: it is likely that adenosine regulates locomotion in multiple regions of the nervous system involved in motor control, including the basal ganglia (Popoli et al. 1996) and ventral spinal cord (Acevedo et al. 2016; Witts et al. 2012), and the effects of adenosine blockade in one network may mask its effects in another.
Experiments in which in vitro rodent spinal cord preparations were exposed to adenosine-receptor agonists and antagonists have addressed the role of adenosine specifically within spinal locomotor networks. During locomotor-related activity induced by bath application of 5-HT, NMDA, and DA in neonatal mouse preparations, blockade of A1 receptors results in increased burst frequency (Acevedo et al. 2016; Witts et al. 2012), with no effect on amplitude, whereas blockade of A2A receptors has no effect (Acevedo et al. 2016; Witts et al. 2012). Conversely, bath application of adenosine or an A1-selective agonist, but not an A2A agonist, results in reduced burst frequency in a dose-dependent manner, again with no effect on amplitude (Acevedo et al. 2016; Witts et al. 2012). The frequency effects are associated with changes in burst and cycle duration, implying no change in duty cycle (Acevedo et al. 2016). Bath application of ATP has a similar effect to adenosine in reducing burst frequency (Witts et al. 2012); however, both blockade of adenosine receptors and application of ATP have no effect in the presence of an ectonucleotidase inhibitor. This indicates that endogenous adenosine is derived from ATP released into the extracellular space, that ATP itself does not modulate locomotor-related activity, and that both endogenous and exogenous ATP are efficiently degraded to adenosine within the murine spinal cord (Witts et al. 2012). The effects of bath-applied and endogenous adenosine therefore closely resemble those of ATP-adenosine released upon glial stimulation during network activity (Acton and Miles 2015).
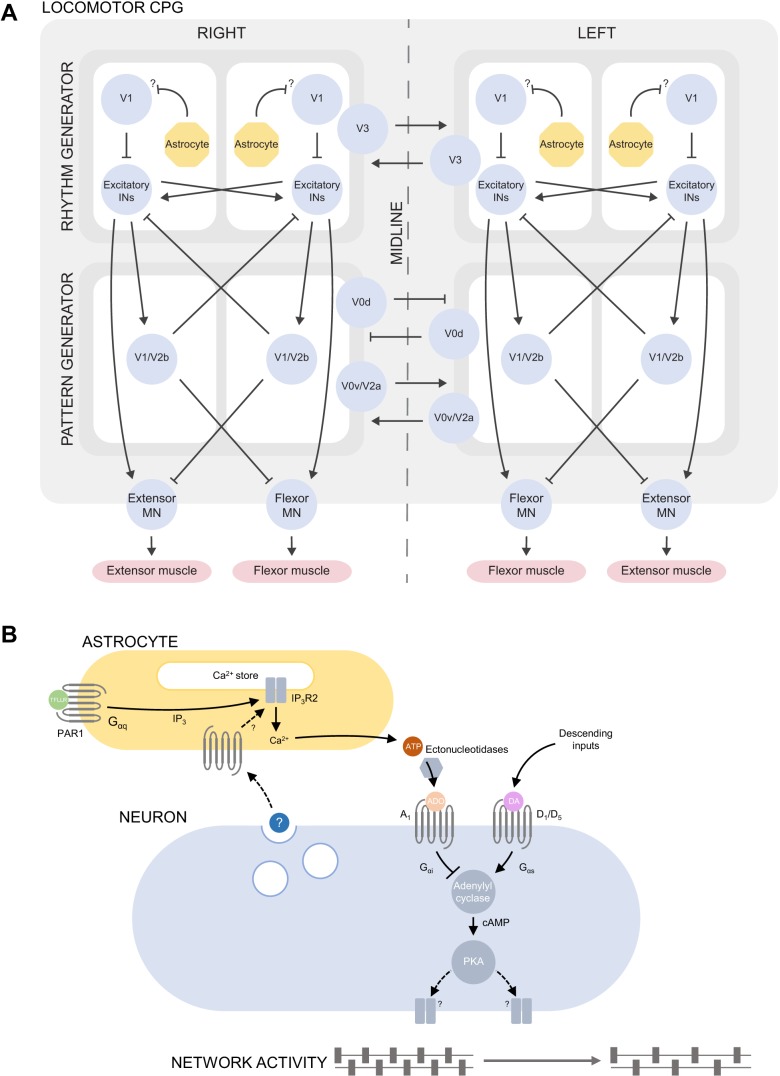
Modulation of the frequency but not the amplitude of locomotor-related activity by adenosine suggests that it acts on the premotor circuitry rather than on motoneurons (Miles and Sillar 2011). Consistent with this hypothesis, neither exogenous adenosine nor adenosine released following PAR1 activation modulates disinhibited activity mediated by motoneurons and excitatory components of the locomotor circuitry alone (Acton and Miles 2015; Witts et al. 2012), implying that adenosine exerts its depressive effects via inhibitory interneurons, which may include the V1 population, for example (Fig. 3A). Ablation or inhibition of V1 interneurons reduces the frequency of locomotor-related activity, similar to the effects of adenosine acting at inhibitory A1 receptors (Gosgnach et al. 2006). However, disinhibited activity, which is generated by spinal cord preparations following application of GABAA and glycine receptor antagonists, is produced by a modified network, and caution must therefore be applied when comparing the effects of adenosine under disinhibited conditions and during fictive locomotion.
Fig. 3.
Schematics illustrating proposed release of ATP-adenosine from astrocytes and inhibition of D1-like receptor signaling within locomotor networks. A: outline of the spinal locomotor CPG as a bilateral network comprising flexor and extensor and rhythm- and pattern-generating modules for the coordination of muscle activation during locomotion. Molecularly defined populations of postmitotic ventral horn neurons (V1, V2a, V2b, V0v, V0d, V3) are represented as blue circles, and some of their proposed interactions are indicated by arrows, signifying excitation, or bars, signifying inhibition. Astrocytes are proposed to modulate the rhythm-generating circuity by exerting an inhibitory effect, via secretion of ATP-adenosine, on an inhibitory population of interneurons that regulates locomotor frequency, possibly the V1 population (see text). For details of the roles of the neuronal populations indicated, see Goulding (2009) and Kiehn (2016). B: ATP is released from putative spinal cord astrocytes during network activity in response to an unidentified neuronal signal and upon experimental activation of the Gαq-linked receptor PAR1 by TFLLR, which is proposed to mimic the endogenous action of neurotransmitters on astrocytic GPCRs. Extracellular ectonucleotidases mediate the hydrolysis of ATP to adenosine, which activates neuronal Gαi-linked A1 receptors to inhibit signaling through Gαs-linked D1-like receptors at the level of adenylyl cyclase. Reduced cAMP production by adenylyl cyclase results in reduced activation of PKA. PKA acts on unidentified targets, perhaps including ion channels and ionotropic receptors, to increase neuronal excitability; PKA inhibition results in a reduced frequency of locomotor-related activity. IN, interneuron; MN, motoneuron.
The effects of bath-applied adenosine on neurons in acute slices from postnatal mice have also been assessed. Adenosine modulates a broad population of ventral horn interneurons, representing both excitatory and inhibitory populations. In these cells, adenosine acts via presynaptic A1 receptors to reduce the frequency of miniature postsynaptic currents (mPSCs; Witts et al. 2015) and the amplitude of evoked excitatory postsynaptic currents (eEPSCs) in a paired simulation protocol (Carlsen and Perrier 2014). Thus adenosine acting at A1 receptors inhibits synaptic transmission onto interneurons. In addition, A1-receptor activation induces a hyperpolarizing current in ventral horn interneurons (Witts et al. 2015). By contrast, adenosine induces a depolarizing current in motoneurons by an unknown mechanism that is sensitive to TTX, although adenosine does not appear to directly modulate synaptic inputs onto motoneurons (Witts et al. 2015). Interestingly, A1-receptor blockade alone has no effect in recordings from neurons within spinal cord slices. It is possible that there is insufficient adenosine present in slices for the tonic activation of these receptors or that adenosine at the concentrations at which it exists in the spinal cord requires concurrent activation of D1-like receptors to exert modulatory effects (Acevedo et al. 2016; see below).
Data suggest differences in purinergic modulation between rats and mice. In isolated rat spinal cord preparations, bath-applied adenosine enhances burst amplitude during pharmacologically induced locomotor-related activity and depresses the frequency of disinhibited bursting in an A1-dependent manner (Taccola et al. 2012), effects not observed in mouse preparations (Acevedo et al. 2016; Witts et al. 2012). In addition, the duration of bouts of locomotor-related activity induced by dorsal root stimulation in rat spinal cord preparations is reduced by exogenous adenosine (Taccola et al. 2012). A1 blockade alone has no effect on drug-induced or electrically stimulated locomotor-related activity in rats (Taccola et al. 2012), whereas in mice, A1 blockade increases the frequency of fictive locomotion (Acevedo et al. 2016; Witts et al. 2012). Differences between mice and rats in purinergic modulation of respiratory networks have been attributed to species-specific ectonucleotidase expression (Huxtable et al. 2009; Zwicker et al. 2011), which results in the mouse network being more strongly modulated by adenosine, and the rat network, by ATP. Further experiments are required to assess whether similar species differences account for the reported differences in the sensitivity of rat and mouse spinal locomotor networks to adenosine. Differential responses to adenosine may also relate to differing roles of coactivation of D1-like receptors (Acevedo et al. 2016), but the potential role of D1/A1 coactivation remains to be investigated in the rat spinal cord.
METAMODULATION OF D1-LIKE DOPAMINE RECEPTORS BY A1 RECEPTORS
Recent data suggest that endogenous adenosine exerts its effects on locomotor-related activity via second-order modulation of dopamine signaling within the mouse spinal cord (Acevedo et al. 2016). Dopamine is a potent modulator of spinal motor circuitry, acting via diverse mechanisms to regulate motor behavior (Miles and Sillar 2011; Sharples et al. 2014). All segments of the mammalian spinal cord receive dopaminergic or l-DOPAergic inputs from descending fibers originating in the brain, most notably from the A11 region of the hypothalamus (Björklund and Skagerberg 1979; Commissiong et al. 1979; Fleetwood-Walker et al. 1988; Hökfelt et al. 1979; Koblinger et al. 2014; Skagerberg and Lindvall 1985), and dopamine levels increase within the ventral horn during locomotion (Gerin et al. 1995; Gerin and Privat 1998).
The five dopamine-receptor subtypes fall into two families: the D1-like receptors are the D1 and D5 subtypes, and the D2-like receptors are the D2, D3, and D4 subtypes (Neve 2010; Pieper et al. 2011). Whereas D1-like receptors signal through Gαq to stimulate phospholipase C and through Gαs to stimulate adenylyl cyclase, D2-like receptors signal through Gαi to inhibit adenylyl cyclase (Abdel-Majid et al. 1998; Pieper et al. 2011). Adenylyl cyclase catalyzes the synthesis of the second messenger cyclic adenosine monophosphate (cAMP), which activates various proteins including PKA. PKA regulates a number of proteins, including Na+-dependent ion transporters, various ion channels, cAMP-responsive element binding protein 1 (CREB1), and dopamine and cyclic AMP-regulated phosphoprotein of 32 kDa (DARPP-32) (Abdel-Majid et al. 1998; Undieh 2010). Dopaminergic modulation of locomotor-related activity in spinal networks is mediated by both D1-like and D2-like receptors (Humphreys and Whelan 2012; Madriaga et al. 2004; Sharples et al. 2015).
A1-adenosine receptors are reported to interact with D1-like dopamine receptors in the spinal locomotor circuitry (Acevedo et al. 2016), as is observed in the basal ganglia (Popoli et al. 1996), where they colocalize (Ferré et al. 1992). Dopamine stabilizes locomotor-related activity (Barrière et al. 2004; Humphreys and Whelan 2012; Jiang et al. 1999; Madriaga et al. 2004; Sharples et al. 2015; Whelan et al. 2000) and is coapplied with 5-HT and NMDA to induce locomotor-related activity in many studies. Unless exogenously applied, it is presumed to be absent at functionally relevant concentrations in isolated lumbar spinal cord preparations because descending dopaminergic inputs are severed in in vitro preparations, and dopamine receptor antagonists do not alter locomotor-related activity induced in the absence of a dopamine receptor agonist (Barrière et al. 2004). When dopamine is absent or when D1-like receptors are selectively blocked, A1 blockade no longer alters the frequency of locomotor-related bursting (Acevedo et al. 2016). A1 blockade is similarly ineffective when the Gαs signaling pathway through which D1-like receptors signal is inhibited at the level of PKA. However, when forskolin is applied to activate adenylyl cyclase in a receptor-independent manner, the effect of A1 blockade on the frequency of locomotor-related bursting is restored.
Collectively, data from isolated spinal cord preparations suggest a model in which astrocytes secrete ATP during locomotor-related activity in an activity-dependent manner. Adenosine produced by hydrolysis of this ATP acts through A1 receptors to regulate the activity of locomotor networks by inhibiting ongoing signaling mediated by D1-like receptors and PKA (Fig. 3B). This may occur through direct inhibition of adenylyl cyclase mediated by the Gαi pathway to which A1 receptors are coupled, and it is possible that A1 and D1-like receptors form heterodimers (Franco et al. 2000); however, colocalization of A1 and D1-like receptors has not yet been demonstrated in the spinal cord.
It is unclear how these results relate to experiments in which adenosine was found to modulate neuronal activity in the absence of dopamine (Carlsen and Perrier 2014; Lloyd et al. 1988; Otsuguro et al. 2006, 2009, 2011; Taccola et al. 2012; Witts et al. 2015). It is possible that high concentrations of adenosine, such as those used for bath applications or produced during hypoxia and hypocapnia, modulate neural activity independently of dopamine, whereas low levels of endogenous adenosine require coactivation of D1-like receptors by dopamine. Consistent with this, A1 blockade has not been shown to modulate neuronal activity in the absence of dopamine (Acevedo et al. 2016; Taccola et al. 2012; Witts et al. 2012), although it does inhibit effects mediated by exogenous adenosine or adenosine released in response to a stimulus (Lloyd et al. 1988; Otsuguro et al. 2006, 2009, 2011; Taccola et al. 2012; Witts et al. 2015); presumably, this indicates low basal levels of adenosine within the spinal cord. Interestingly, all dopamine-dependent and dopamine-independent effects so far described in in vitro spinal cord preparations from healthy animals are mediated by A1 receptors, despite expression of A2A receptors in the spinal cord. It is conceivable that A2A receptors have a functional role only in responses to injury: following spinal cord injury, A2A receptors are upregulated in the spinal cord, and administration of a selective A2A antagonist exerts neuroprotective effects, perhaps by reducing excitotoxicity (Paterniti et al. 2011). Thus A1 receptors may participate in multiple signaling pathways, depending on agonist concentration.
The proposed antagonistic interaction between A1 and D1-like receptors implies that D1-like receptors, which are typically excitatory, enhance the frequency of locomotor-related bursting in neonatal mice. However, this has not been clearly demonstrated. Although D1-like receptors typically have excitatory effects, they have not been reported to increase the frequency of ongoing locomotor-related activity in mice (Humphreys and Whelan 2012; Sharples et al. 2015), but may instead enhance the stability of rhythmic bursting (Sharples et al. 2015). Bath-applied dopamine or a D1-like receptor agonist stimulates locomotor-related activity in neonatal rat preparations (Barrière et al. 2004; Kiehn and Kjaerulff 1996) but not in neonatal mouse preparations (Jiang et al. 1999; Sharples et al. 2015; Whelan et al. 2000); however, the selective D1-like receptor agonist SKF-81927 is able to stimulate locomotor activity in spinalized adult mice (Lapointe et al. 2009). Thus, although D1-like receptors have excitatory effects within the spinal cord, the enhancement of burst frequency that A1 receptors are proposed to counteract in spinal cords from neonatal mice has not been detected.
The effects of dopamine on network activity likely reflect multiple effects mediated by both D1-like and D2-like signaling pathways within diverse cell populations: dopamine has been shown to modulate synaptic strength and passive electrical properties of motoneurons and interneurons (Barrière et al. 2008; Carp and Anderson 1982; Clemens and Hochman 2004; Han et al. 2007; Han and Whelan 2009; Humphreys and Whelan 2012; Maitra et al. 1993; Seth et al. 1993). The extent of second-order modulation of D1-like receptors by adenosine is currently unclear: further experiments are required to reveal whether adenosine acts on all cells expressing D1-like receptors or only on a subset involved in the regulation of speed. Nevertheless, the model by which adenosine is proposed to modulate the effects of D1-like receptor activation during fictive locomotion is consistent with other examples of metamodulation, which is proposed as an efficient mechanism for refining the effects of a first-order neuromodulator; this may be important when the first-order modulator is widespread and/or is capable of acting promiscuously on various cell types to exert diverse effects within a network (Katz 1999). Metamodulation may entail a second-order neuromodulator regulating the availability (release or uptake) of a first-order neuromodulator or, as in the present example, modulation of its effect on a target neuron. Other examples of metamodulation in spinal locomotor networks are provided by Xenopus tadpoles, in which nitric oxide (NO) modulates the release of norepinephrine (McLean and Sillar 2004), and lampreys, in which NO modulates the activity of endocannabinoids (Song et al. 2012).
THE PHYSIOLOGICAL IMPORTANCE OF GLIAL ADENOSINE IN SPINAL LOCOMOTOR NETWORKS
Adenosinergic neuromodulation is proposed to have a homeostatic role in diverse systems, preventing metabolic exhaustion and excitotoxicity (Cunha 2001; Fredholm et al. 2005; Wall and Dale 2008). Activity-dependent production of adenosine has been detected in the mammalian brain and brain stem and in the spinal cord of Xenopus tadpoles (Wall and Dale 2008). In some cases, this may be coupled to the degradation of ATP. ATP is consumed by neurons as a source of energy during activity, and adenosine produced by its hydrolysis may be released directly via neuronal equilibrative nucleoside transporters. Subsequent autocrine inhibition of activity via A1 receptors is proposed as a mechanism to prevent metabolic exhaustion (Cunha 2001; Fredholm et al. 2005). However, this mechanism does not appear to operate in systems, including spinal motor networks in tadpoles and mice, in which the source of adenosine is ATP released into the extracellular space from either neurons or astrocytes (Carlsen and Perrier 2014; Panatier et al. 2011; Pascual et al. 2005; Serrano et al. 2006; Wall and Dale 2008; Witts et al. 2012). Nevertheless, it remains possible that adenosine functions to avert metabolic exhaustion, even if its source is not ATP expended as energy.
Xenopus tadpoles provide an example of purinergic signaling during motor behavior in a nonmammalian vertebrate. ATP released within the spinal cord during episodes of swimming excites the locomotor CPG, extending the duration of swimming bouts (Dale 1998; Dale and Gilday 1996). As swimming progresses, ATP is hydrolyzed to adenosine, which activates A1 receptors to drive down network activity (Brown and Dale 2000). This mechanism may exist to prevent metabolic exhaustion and excitotoxicity (Wall and Dale 2008). The cellular origin of ATP in the tadpole spinal cord is unclear, and given recent evidence from Drosophila larvae that neuron-glia cross talk regulates behavior of nonmammalian species (Ma et al. 2016), glial release of purines should not be ruled out. The temporal dynamics of adenosine release in the mammalian spinal cord have not been investigated; it is possible that gradual increase in adenosinergic tone during motor behaviors could also have a protective function by preventing metabolic exhaustion.
SUMMARY
Recent studies have provided evidence for the release of ATP-adenosine from astrocytes in the spinal cord, as previously demonstrated elsewhere in the CNS. Adenosine operates by multiple pathways within the spinal motor circuitry. During fictive locomotion, it is proposed to function as a second-order modulator of D1-like receptors (Acevedo et al. 2016). This mechanism is likely to be important in refining the influence of dopamine, which has manifold cellular activities during locomotor behaviors. The consequent reduction in locomotor speed may be important in ensuring fluency of movement or have a role in averting cellular fatigue (Acevedo et al. 2016; Acton and Miles 2015; Witts et al. 2012). Adenosine also acts by a dopamine-independent pathway or pathways (Carlsen and Perrier 2014; Lloyd et al. 1988; Otsuguro et al. 2006, 2009, 2011; Taccola et al. 2012; Witts et al. 2012), which may be activated only by high concentrations of adenosine. It will be interesting to compare tissue concentrations of adenosine during inactivity, locomotion, and injurious conditions in which adenosine is released, such as hypoxia and hypercapnia. The agreement between dopamine-dependent effects mediated by A1-adenosine receptors during fictive locomotion and the effects of adenosine released upon glial stimulation suggests that the latter acts via D1-like receptors to modulate rhythmic activity, but this remains to be tested directly.
Although evidence exists for glial release of adenosine (Acton and Miles 2015; Carlsen and Perrier 2014; Witts et al. 2012), detailed Ca2+ imaging of astrocytes is required to confirm a role for Ca2+-dependent neuron-glia signaling in the ventral horn. However, it should also be noted that several studies have provided evidence for cross talk between neurons and astrocytes in a manner that departs from the established tripartite synapse model (Angelova et al. 2015; Gourine et al. 2010; Gourine and Kasparov 2011; McDougal et al. 2013; Torres et al. 2012). Neurotransmitter uptake is as important as release in shaping patterns of synaptic transmission and may be regulated in an activity-dependent manner independently of Ca2+ signaling (Al Awabdh et al. 2016; Attwell et al. 1993; Li et al. 2009; Marcaggi and Attwell 2004; Perego et al. 2000; Roux and Supplisson 2000; Shibasaki et al. 2017). Although PAR1 activation does not appear to elicit release of glutamate or its coagonists at NMDA receptors, glycine and d-serine (Acton and Miles 2015), it has been proposed that coagonist concentrations are regulated by astrocytes in an activity-dependent manner during motor behaviors (Acton and Miles 2017). In addition, activation of astrocytic GPCRs may result in the modulation of neuronal activity by Ca2+-independent pathways. Selective activation of a Gαq-coupled DREADD expressed by astrocytes lacking IP3R2 receptors results in diverse behavioral modifications on a timescale longer than that predicted for Ca2+-dependent responses; these may involve signaling via Gβγ or PKC, for instance (Agulhon et al. 2013). These examples suggest that information processing by astrocytes is underexplored and may be crucial in the generation of behaviors by spinal networks, exceeding their established role as cellular housekeepers.
GRANTS
D. Acton was supported by funds from a Wellcome Trust Institutional Strategic Support Fund grant. G. B. Miles received support from Biotechnology and Biological Science Research Grant BB/M021793/1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.A. prepared figures; D.A. drafted manuscript; D.A. and G.B.M. edited and revised manuscript; D.A. and G.B.M. approved final version of manuscript.
REFERENCES
- Abdel-Majid RM, Leong WL, Schalkwyk LC, Smallman DS, Wong ST, Storm DR, Fine A, Dobson MJ, Guernsey DL, Neumann PE. Loss of adenylyl cyclase I activity disrupts patterning of mouse somatosensory cortex. Nat Genet 19: 289–291, 1998. doi: 10.1038/980. [DOI] [PubMed] [Google Scholar]
- Acevedo J, Santana-Almansa A, Matos-Vergara N, Marrero-Cordero LR, Cabezas-Bou E, Díaz-Ríos M. Caffeine stimulates locomotor activity in the mammalian spinal cord via adenosine A1 receptor-dopamine D1 receptor interaction and PKA-dependent mechanisms. Neuropharmacology 101: 490–505, 2016. doi: 10.1016/j.neuropharm.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acton D, Miles GB. Stimulation of glia reveals modulation of mammalian spinal motor networks by adenosine. PLoS One 10: e0134488, 2015. doi: 10.1371/journal.pone.0134488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acton D, Miles GB. Differential regulation of NMDA receptors by d-serine and glycine in mammalian spinal locomotor networks. J Neurophysiol 117: 1877–1893, 2017. doi: 10.1152/jn.00810.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agulhon C, Boyt KM, Xie AX, Friocourt F, Roth BL, McCarthy KD. Modulation of the autonomic nervous system and behaviour by acute glial cell Gq protein-coupled receptor activation in vivo. J Physiol 591: 5599–5609, 2013. doi: 10.1113/jphysiol.2013.261289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agulhon C, Fiacco TA, McCarthy KD. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science 327: 1250–1254, 2010. doi: 10.1126/science.1184821. [DOI] [PubMed] [Google Scholar]
- Agulhon C, Sun MY, Murphy T, Myers T, Lauderdale K, Fiacco TA. Calcium signaling and gliotransmission in normal vs. Reactive astrocytes. Front Pharmacol 3: 139, 2012. doi: 10.3389/fphar.2012.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Awabdh S, Gupta-Agarwal S, Sheehan DF, Muir J, Norkett R, Twelvetrees AE, Griffin LD, Kittler JT. Neuronal activity mediated regulation of glutamate transporter GLT-1 surface diffusion in rat astrocytes in dissociated and slice cultures. Glia 64: 1252–1264, 2016. doi: 10.1002/glia.22997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelova PR, Kasymov V, Christie I, Sheikhbahaei S, Turovsky E, Marina N, Korsak A, Zwicker J, Teschemacher AG, Ackland GL, Funk GD, Kasparov S, Abramov AY, Gourine AV. Functional oxygen sensitivity of astrocytes. J Neurosci 35: 10460–10473, 2015. doi: 10.1523/JNEUROSCI.0045-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo MC, Kozlov AS, Charpak S, Audinat E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J Neurosci 24: 6920–6927, 2004. doi: 10.1523/JNEUROSCI.0473-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou K, Papadopoulou-Daifoti Z, Hyphantis T, Papathanasiou G, Bekris E, Marselos M, Panlilio L, Müller CE, Goldberg SR, Ferré S. A detailed behavioral analysis of the acute motor effects of caffeine in the rat: involvement of adenosine A1 and A2A receptors. Psychopharmacology (Berl) 183: 154–162, 2005. doi: 10.1007/s00213-005-0173-6. [DOI] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron 81: 728–739, 2014. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Li N, Doyle RT, Haydon PG. SNARE protein-dependent glutamate release from astrocytes. J Neurosci 20: 666–673, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22: 208–215, 1999. doi: 10.1016/S0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Aschner M, Kimelberg HK. The Role of Glia in Neurotoxicity. Boca Raton, FL: CRC, 1996. [Google Scholar]
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron 11: 401–407, 1993. doi: 10.1016/0896-6273(93)90145-H. [DOI] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry 64: 863–870, 2008. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardoni R, Ghirri A, Zonta M, Betelli C, Vitale G, Ruggieri V, Sandrini M, Carmignoto G. Glutamate-mediated astrocyte-to-neuron signalling in the rat dorsal horn. J Physiol 588: 831–846, 2010. doi: 10.1113/jphysiol.2009.180570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrière G, Mellen N, Cazalets JR. Neuromodulation of the locomotor network by dopamine in the isolated spinal cord of newborn rat. Eur J Neurosci 19: 1325–1335, 2004. doi: 10.1111/j.1460-9568.2004.03210.x. [DOI] [PubMed] [Google Scholar]
- Barrière G, Tartas M, Cazalets J-R, Bertrand SS. Interplay between neuromodulator-induced switching of short-term plasticity at sensorimotor synapses in the neonatal rat spinal cord. J Physiol 586: 1903–1920, 2008. doi: 10.1113/jphysiol.2008.150706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazargani N, Attwell D. Astrocyte calcium signaling: the third wave. Nat Neurosci 19: 182–189, 2016. doi: 10.1038/nn.4201. [DOI] [PubMed] [Google Scholar]
- Bellardita C, Kiehn O. Phenotypic characterization of speed-associated gait changes in mice reveals modular organization of locomotor networks. Curr Biol 25: 1426–1436, 2015. doi: 10.1016/j.cub.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beppu K, Sasaki T, Tanaka KF, Yamanaka A, Fukazawa Y, Shigemoto R, Matsui K. Optogenetic countering of glial acidosis suppresses glial glutamate release and ischemic brain damage. Neuron 81: 314–320, 2014. doi: 10.1016/j.neuron.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Bertrand S, Cazalets J-R. Presynaptic GABAergic control of the locomotor drive in the isolated spinal cord of neonatal rats. Eur J Neurosci 11: 583–592, 1999. doi: 10.1046/j.1460-9568.1999.00473.x. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature 391: 281–285, 1998. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Björklund A, Skagerberg G. Evidence for a major spinal cord projection from the diencephalic A11 dopamine cell group in the rat using transmitter-specific fluorescent retrograde tracing. Brain Res 177: 170–175, 1979. doi: 10.1016/0006-8993(79)90927-2. [DOI] [PubMed] [Google Scholar]
- Bowery NG, Smart TG. GABA and glycine as neurotransmitters: a brief history. Br J Pharmacol 147, Suppl 1: S109–S119, 2006. doi: 10.1038/sj.bjp.0706443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Dale N. Adenosine A1 receptors modulate high voltage-activated Ca2+ currents and motor pattern generation in the Xenopus embryo. J Physiol 525: 655–667, 2000. doi: 10.1111/j.1469-7793.2000.00655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TG. The intrinsic factors in the act of progression in the mammal. Proc R Soc Lond B Biol Sci 84: 308–319, 1911. doi: 10.1098/rspb.1911.0077. [DOI] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87: 659–797, 2007. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Cao X, Li LP, Wang Q, Wu Q, Hu HH, Zhang M, Fang YY, Zhang J, Li SJ, Xiong WC, Yan HC, Gao YB, Liu JH, Li XW, Sun LR, Zeng YN, Zhu XH, Gao TM. Astrocyte-derived ATP modulates depressive-like behaviors. Nat Med 19: 773–777, 2013. doi: 10.1038/nm.3162. [DOI] [PubMed] [Google Scholar]
- Carlsen EM, Perrier JF. Purines released from astrocytes inhibit excitatory synaptic transmission in the ventral horn of the spinal cord. Front Neural Circuits 8: 60, 2014. doi: 10.3389/fncir.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp JS, Anderson RJ. Dopamine receptor-mediated depression of spinal monosynaptic transmission. Brain Res 242: 247–254, 1982. doi: 10.1016/0006-8993(82)90307-9. [DOI] [PubMed] [Google Scholar]
- Chen N, Sugihara H, Kim J, Fu Z, Barak B, Sur M, Feng G, Han W. Direct modulation of GFAP-expressing glia in the arcuate nucleus bi-directionally regulates feeding. eLife 5: e18716, 2016. doi: 10.7554/eLife.18716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chub N, Liu W, O’Donovan MJ. A subpopulation of glial cells generate rhythmic calcium transients during locomotor like activity in isolated mouse spinal cord. Program No. 541.21. 2012 Neuroscience Meeting Planner. New Orleans, LA: Society for Neuroscience, 2012. [Google Scholar]
- Chub N, O’Donovan MJ. Mouse spinal cord astrocytes respond with intracellular calcium transients during bursting activity evoked by ventral root stimulation. Program No. 240.18. 2011 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience, 2011. [Google Scholar]
- Chvátal A, Pastor A, Mauch M, Syková E, Kettenmann H. Distinct populations of identified glial cells in the developing rat spinal cord slice: ion channel properties and cell morphology. Eur J Neurosci 7: 129–142, 1995. doi: 10.1111/j.1460-9568.1995.tb01027.x. [DOI] [PubMed] [Google Scholar]
- Clarke DD. Fluoroacetate and fluorocitrate: mechanism of action. Neurochem Res 16: 1055–1058, 1991. doi: 10.1007/BF00965850. [DOI] [PubMed] [Google Scholar]
- Clemens S, Hochman S. Conversion of the modulatory actions of dopamine on spinal reflexes from depression to facilitation in D3 receptor knock-out mice. J Neurosci 24: 11337–11345, 2004. doi: 10.1523/JNEUROSCI.3698-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commissiong JW, Gentleman S, Neff NH. Spinal cord dopaminergic neurons: evidence for an uncrossed nigrospinal pathway. Neuropharmacology 18: 565–568, 1979. doi: 10.1016/0028-3908(79)90102-3. [DOI] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. A comparison of motor patterns induced by N-methyl-d-aspartate, acetylcholine and serotonin in the in vitro neonatal rat spinal cord. Neurosci Lett 171: 147–150, 1994. doi: 10.1016/0304-3940(94)90626-2. [DOI] [PubMed] [Google Scholar]
- Cunha RA. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int 38: 107–125, 2001. doi: 10.1016/S0197-0186(00)00034-6. [DOI] [PubMed] [Google Scholar]
- Dale N. Delayed production of adenosine underlies temporal modulation of swimming in frog embryo. J Physiol 511: 265–272, 1998. doi: 10.1111/j.1469-7793.1998.265bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N, Gilday D. Regulation of rhythmic movements by purinergic neurotransmitters in frog embryos. Nature 383: 259–263, 1996. doi: 10.1038/383259a0. [DOI] [PubMed] [Google Scholar]
- De Vellis J, editor. Neuroglia in the Aging Brain. Totowa, NJ: Humana, 2002. [Google Scholar]
- Deuchars SA, Brooke RE, Deuchars J. Adenosine A1 receptors reduce release from excitatory but not inhibitory synaptic inputs onto lateral horn neurons. J Neurosci 21: 6308–6320, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Castro MA, Chuquet J, Liaudet N, Bhaukaurally K, Santello M, Bouvier D, Tiret P, Volterra A. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat Neurosci 14: 1276–1284, 2011. doi: 10.1038/nn.2929. [DOI] [PubMed] [Google Scholar]
- Dickinson MH, Farley CT, Full RJ, Koehl MA, Kram R, Lehman S. How animals move: an integrative view. Science 288: 100–106, 2000. doi: 10.1126/science.288.5463.100. [DOI] [PubMed] [Google Scholar]
- Dickinson PS. Neuromodulation of central pattern generators in invertebrates and vertebrates. Curr Opin Neurobiol 16: 604–614, 2006. doi: 10.1016/j.conb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron 56: 43–57, 2007. doi: 10.1016/j.neuron.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci 24: 31–55, 2001. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Ménard JF, Parmentier M, Costentin J, Vaugeois JM. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A2A receptors. Br J Pharmacol 129: 1465–1473, 2000. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci 7: 232–242, 2006. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Halassa MM, Terunuma M, Succol F, Takano H, Frank M, Moss SJ, Haydon PG. Endogenous nonneuronal modulators of synaptic transmission control cortical slow oscillations in vivo. Proc Natl Acad Sci USA 106: 15037–15042, 2009. doi: 10.1073/pnas.0906419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 43: 729–743, 2004. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Ferré S, Fuxe K, von Euler G, Johansson B, Fredholm BB. Adenosine-dopamine interactions in the brain. Neuroscience 51: 501–512, 1992. doi: 10.1016/0306-4522(92)90291-9. [DOI] [PubMed] [Google Scholar]
- Fiorelli R, Cebrian-Silla A, Garcia-Verdugo JM, Raineteau O. The adult spinal cord harbors a population of GFAP-positive progenitors with limited self-renewal potential. Glia 61: 2100–2113, 2013. doi: 10.1002/glia.22579. [DOI] [PubMed] [Google Scholar]
- Fleetwood-Walker SM, Hope PJ, Mitchell R. Antinociceptive actions of descending dopaminergic tracts on cat and rat dorsal horn somatosensory neurones. J Physiol 399: 335–348, 1988. doi: 10.1113/jphysiol.1988.sp017084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian C, Vecsey CG, Halassa MM, Haydon PG, Abel T. Astrocyte-derived adenosine and A1 receptor activity contribute to sleep loss-induced deficits in hippocampal synaptic plasticity and memory in mice. J Neurosci 31: 6956–6962, 2011. doi: 10.1523/JNEUROSCI.5761-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J, Blutstein T, Lee S, Erneux C, Halassa MM, Haydon P. Astrocytic IP3/Ca2+ signaling modulates theta rhythm and REM sleep. Front Neural Circuits 11: 3, 2017. doi: 10.3389/fncir.2017.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley JC, McIver SR, Haydon PG. Gliotransmission modulates baseline mechanical nociception. Mol Pain 7: 93, 2011. doi: 10.1186/1744-8069-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F, Johnsen A, Hassel B. Use of fluorocitrate and fluoroacetate in the study of brain metabolism. Glia 21: 106–113, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- Franco R, Ferré S, Agnati L, Torvinen M, Ginés S, Hillion J, Casadó V, Lledó P, Zoli M, Lluis C, Fuxe K. Evidence for adenosine/dopamine receptor interactions indications for heteromerization. Neuropsychopharmacology 23, Suppl 4: S50–S59, 2000. doi: 10.1016/S0893-133X(00)00144-5. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol 63: 191–270, 2005. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- Fujita T, Chen MJ, Li B, Smith NA, Peng W, Sun W, Toner MJ, Kress BT, Wang L, Benraiss A, Takano T, Wang S, Nedergaard M. Neuronal transgene expression in dominant-negative SNARE mice. J Neurosci 34: 16594–16604, 2014. doi: 10.1523/JNEUROSCI.2585-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerin C, Becquet D, Privat A. Direct evidence for the link between monoaminergic descending pathways and motor activity. I. A study with microdialysis probes implanted in the ventral funiculus of the spinal cord. Brain Res 704: 191–201, 1995. doi: 10.1016/0006-8993(95)01111-0. [DOI] [PubMed] [Google Scholar]
- Gerin C, Privat A. Direct evidence for the link between monoaminergic descending pathways and motor activity: II. A study with microdialysis probes implanted in the ventral horn of the spinal cord. Brain Res 794: 169–173, 1998. doi: 10.1016/S0006-8993(98)00278-9. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Prada JA, Katz JL. Stereoselective behavioral effects of N6-phenylisopropyl-adenosine and antagonism by caffeine. Psychopharmacology (Berl) 87: 272–277, 1985. doi: 10.1007/BF00432706. [DOI] [PubMed] [Google Scholar]
- Gosgnach S, Lanuza GM, Butt SJ, Saueressig H, Zhang Y, Velasquez T, Riethmacher D, Callaway EM, Kiehn O, Goulding M. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature 440: 215–219, 2006. doi: 10.1038/nature04545. [DOI] [PubMed] [Google Scholar]
- Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci 10: 507–518, 2009. doi: 10.1038/nrn2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Kasparov S. Astrocytes as brain interoceptors. Exp Physiol 96: 411–416, 2011. doi: 10.1113/expphysiol.2010.053165. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science 329: 571–575, 2010. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron 52: 751–766, 2006. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Guertin PA. The mammalian central pattern generator for locomotion. Brain Res Brain Res Rev 62: 45–56, 2009. doi: 10.1016/j.brainresrev.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Hägglund M, Dougherty KJ, Borgius L, Itohara S, Iwasato T, Kiehn O. Optogenetic dissection reveals multiple rhythmogenic modules underlying locomotion. Proc Natl Acad Sci USA 110: 11589–11594, 2013. doi: 10.1073/pnas.1304365110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 61: 213–219, 2009. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci 11: 227–238, 2010. doi: 10.1038/nrn2803. [DOI] [PubMed] [Google Scholar]
- Han KS, Woo J, Park H, Yoon B-J, Choi S, Lee CJ. Channel-mediated astrocytic glutamate release via Bestrophin-1 targets synaptic NMDARs. Mol Brain 6: 4, 2013. doi: 10.1186/1756-6606-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P, Nakanishi ST, Tran MA, Whelan PJ. Dopaminergic modulation of spinal neuronal excitability. J Neurosci 27: 13192–13204, 2007. doi: 10.1523/JNEUROSCI.1279-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P, Whelan PJ. Modulation of AMPA currents by D1-like but not D2-like receptors in spinal motoneurons. Neuroscience 158: 1699–1707, 2009. doi: 10.1016/j.neuroscience.2008.11.040. [DOI] [PubMed] [Google Scholar]
- Han Y, Yu HX, Sun ML, Wang Y, Xi W, Yu YQ. Astrocyte-restricted disruption of connexin-43 impairs neuronal plasticity in mouse barrel cortex. Eur J Neurosci 39: 35–45, 2014. doi: 10.1111/ejn.12394. [DOI] [PubMed] [Google Scholar]
- Harris-Warrick RM. Neuromodulation and flexibility in central pattern generator networks. Curr Opin Neurobiol 21: 685–692, 2011. doi: 10.1016/j.conb.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG, Nedergaard M. How do astrocytes participate in neural plasticity? Cold Spring Harb Perspect Biol 7: a020438, 2015. doi: 10.1101/cshperspect.a020438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes HB, Chang Y-H, Hochman S. An in vitro spinal cord-hindlimb preparation for studying behaviorally relevant rat locomotor function. J Neurophysiol 101: 1114–1122, 2009. doi: 10.1152/jn.90523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of d-serine from astrocytes. Nature 463: 232–236, 2010. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T, Phillipson O, Goldstein M. Evidence for a dopaminergic pathway in the rat descending from the A11 cell group to the spinal cord. Acta Physiol Scand 107: 393–395, 1979. doi: 10.1111/j.1748-1716.1979.tb06491.x. [DOI] [PubMed] [Google Scholar]
- Hua X, Malarkey EB, Sunjara V, Rosenwald SE, Li WH, Parpura V. Ca2+-dependent glutamate release involves two classes of endoplasmic reticulum Ca2+ stores in astrocytes. J Neurosci Res 76: 86–97, 2004. doi: 10.1002/jnr.20061. [DOI] [PubMed] [Google Scholar]
- Humphreys JM, Whelan PJ. Dopamine exerts activation-dependent modulation of spinal locomotor circuits in the neonatal mouse. J Neurophysiol 108: 3370–3381, 2012. doi: 10.1152/jn.00482.2012. [DOI] [PubMed] [Google Scholar]
- Huxtable AG, Zwicker JD, Alvares TS, Ruangkittisakul A, Fang X, Hahn LB, Posse de Chaves E, Baker GB, Ballanyi K, Funk GD. Glia contribute to the purinergic modulation of inspiratory rhythm-generating networks. J Neurosci 30: 3947–3958, 2010. doi: 10.1523/JNEUROSCI.6027-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable AG, Zwicker JD, Poon BY, Pagliardini S, Vrouwe SQ, Greer JJ, Funk GD. Tripartite purinergic modulation of central respiratory networks during perinatal development: the influence of ATP, ectonucleotidases, and ATP metabolites. J Neurosci 29: 14713–14725, 2009. doi: 10.1523/JNEUROSCI.2660-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwagaki N, Miles GB. Activation of group I metabotropic glutamate receptors modulates locomotor-related motoneuron output in mice. J Neurophysiol 105: 2108–2120, 2011. doi: 10.1152/jn.01037.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EK, Kotermanski SE, Menshikova EV, Dubey RK, Jackson TC, Kochanek PM. Adenosine production by brain cells. J Neurochem 141: 676–693, 2017. doi: 10.1111/jnc.14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev 81: 929–969, 2001. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Carlin KP, Brownstone RM. An in vitro functionally mature mouse spinal cord preparation for the study of spinal motor networks. Brain Res 816: 493–499, 1999. doi: 10.1016/S0006-8993(98)01199-8. [DOI] [PubMed] [Google Scholar]
- John CS, Smith KL, Van’t Veer A, Gompf HS, Carlezon WA, Cohen BM, Öngür D, Bechtholt-Gompf AJ. Blockade of astrocytic glutamate uptake in the prefrontal cortex induces anhedonia. Neuropsychopharmacology 37: 2467–2475, 2012. doi: 10.1038/npp.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci 10: 331–339, 2007. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Junge CE, Lee CJ, Hubbard KB, Zhang Z, Olson JJ, Hepler JR, Brat DJ, Traynelis SF. Protease-activated receptor-1 in human brain: localization and functional expression in astrocytes. Exp Neurol 188: 94–103, 2004. doi: 10.1016/j.expneurol.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci 1: 683–692, 1998. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Karcz-Kubicha M, Antoniou K, Terasmaa A, Quarta D, Solinas M, Justinova Z, Pezzola A, Reggio R, Müller CE, Fuxe K, Goldberg SR, Popoli P, Ferré S. Involvement of adenosine A1 and A2A receptors in the motor effects of caffeine after its acute and chronic administration. Neuropsychopharmacology 28: 1281–1291, 2003. doi: 10.1038/sj.npp.1300167. [DOI] [PubMed] [Google Scholar]
- Kasymov V, Larina O, Castaldo C, Marina N, Patrushev M, Kasparov S, Gourine AV. Differential sensitivity of brainstem versus cortical astrocytes to changes in pH reveals functional regional specialization of astroglia. J Neurosci 33: 435–441, 2013. doi: 10.1523/JNEUROSCI.2813-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz PS, editor. Beyond Neurotransmission: Neuromodulation and Its Importance for Information Processing. New York: Oxford University Press, 1999. [Google Scholar]
- Katz PS, Frost WN. Intrinsic neuromodulation: altering neuronal circuits from within. Trends Neurosci 19: 54–61, 1996. doi: 10.1016/0166-2236(96)89621-4. [DOI] [PubMed] [Google Scholar]
- Keyser DO, Pellmar TC. Synaptic transmission in the hippocampus: critical role for glial cells. Glia 10: 237–243, 1994. doi: 10.1002/glia.440100402. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci 29: 279–306, 2006. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci 17: 224–238, 2016. doi: 10.1038/nrn.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O. Spatiotemporal characteristics of 5-HT and dopamine-induced rhythmic hindlimb activity in the in vitro neonatal rat. J Neurophysiol 75: 1472–1482, 1996. [DOI] [PubMed] [Google Scholar]
- Klyuch BP, Dale N, Wall MJ. Deletion of ecto-5′-nucleotidase (CD73) reveals direct action potential-dependent adenosine release. J Neurosci 32: 3842–3847, 2012. doi: 10.1523/JNEUROSCI.6052-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblinger K, Füzesi T, Ejdrygiewicz J, Krajacic A, Bains JS, Whelan PJ. Characterization of A11 neurons projecting to the spinal cord of mice. PLoS One 9: e109636, 2014. doi: 10.1371/journal.pone.0109636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo N, Yamada T. N-methyl-d,l-aspartate-induced locomotor activity in a spinal cord-hindlimb muscles preparation of the newborn rat studied in vitro. Neurosci Lett 75: 43–48, 1987. doi: 10.1016/0304-3940(87)90072-3. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Johansson B, Gimenez L, Ögren SO, Fredholm BB. Combination of adenosine A1 and A2A receptor blocking agents induces caffeine-like locomotor stimulation in mice. Eur Neuropsychopharmacol 16: 129–136, 2006. doi: 10.1016/j.euroneuro.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Lalo U, Palygin O, Rasooli-Nejad S, Andrew J, Haydon PG, Pankratov Y. Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex. PLoS Biol 12: e1001747, 2014. doi: 10.1371/journal.pbio.1001747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe NP, Rouleau P, Ung RV, Guertin PA. Specific role of D1 receptors in spinal network activation and rhythmic movement induction in vertebrates. J Physiol 5877: 1499–1511, 2009. doi: 10.1113/jphysiol.2008.166314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largo C, Ibarz JM, Herreras O. Effects of the gliotoxin fluorocitrate on spreading depression and glial membrane potential in rat brain in situ. J Neurophysiol 78: 295–307, 1997. [DOI] [PubMed] [Google Scholar]
- Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, Costentin J, Heath JK, Vassart G, Parmentier M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature 388: 674–678, 1997. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- Lee CJ, Mannaioni G, Yuan H, Woo DH, Gingrich MB, Traynelis SF. Astrocytic control of synaptic NMDA receptors. J Physiol 581: 1057–1081, 2007. doi: 10.1113/jphysiol.2007.130377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Chun Y-E, Han K-S, Lee J, Woo DH, Lee CJ. Ca(2+) Entry is required for mechanical stimulation-induced ATP release from astrocyte. Exp Neurobiol 24: 17–23, 2015. doi: 10.5607/en.2015.24.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Hérault K, Isacoff EY, Oheim M, Ropert N. Optogenetic activation of LiGluR-expressing astrocytes evokes anion channel-mediated glutamate release. J Physiol 590: 855–873, 2012. doi: 10.1113/jphysiol.2011.219345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Krupa B, Kang JS, Bolshakov VY, Liu G. Glycine site of NMDA receptor serves as a spatiotemporal detector of synaptic activity patterns. J Neurophysiol 102: 578–589, 2009. doi: 10.1152/jn.91342.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Sacchi S, Pollegioni L, Basu AC, Coyle JT, Bolshakov VY. Identity of endogenous NMDAR glycine site agonist in amygdala is determined by synaptic activity level. Nat Commun 4: 1760, 2013. doi: 10.1038/ncomms2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindskog M, Svenningsson P, Pozzi L, Kim Y, Fienberg AA, Bibb JA, Fredholm BB, Nairn AC, Greengard P, Fisone G. Involvement of DARPP-32 phosphorylation in the stimulant action of caffeine. Nature 418: 774–778, 2002. doi: 10.1038/nature00817. [DOI] [PubMed] [Google Scholar]
- Lloyd HG, Spence I, Johnston GA. Involvement of adenosine in synaptic depression induced by a brief period of hypoxia in isolated spinal cord of neonatal rat. Brain Res 462: 391–395, 1988. doi: 10.1016/0006-8993(88)90571-9. [DOI] [PubMed] [Google Scholar]
- Lund JP, Kolta A. Generation of the central masticatory pattern and its modification by sensory feedback. Dysphagia 21: 167–174, 2006. doi: 10.1007/s00455-006-9027-6. [DOI] [PubMed] [Google Scholar]
- Ma Z, Stork T, Bergles DE, Freeman MR. Neuromodulators signal through astrocytes to alter neural circuit activity and behaviour. Nature 539: 428–432, 2016. doi: 10.1038/nature20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madriaga MA, McPhee LC, Chersa T, Christie KJ, Whelan PJ. Modulation of locomotor activity by multiple 5-HT and dopaminergic receptor subtypes in the neonatal mouse spinal cord. J Neurophysiol 92: 1566–1576, 2004. doi: 10.1152/jn.01181.2003. [DOI] [PubMed] [Google Scholar]
- Maitra KK, Seth P, Thewissen M, Ross HG, Ganguly DK. Dopaminergic influence on the excitability of antidromically activated Renshaw cells in the lumbar spinal cord of the rat. Acta Physiol Scand 148: 101–107, 1993. doi: 10.1111/j.1748-1716.1993.tb09538.x. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Attwell D. Role of glial amino acid transporters in synaptic transmission and brain energetics. Glia 47: 217–225, 2004. doi: 10.1002/glia.20027. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Curr Biol 11: R986–R996, 2001. doi: 10.1016/S0960-9822(01)00581-4. [DOI] [PubMed] [Google Scholar]
- Martin C, Houitte D, Guillermier M, Petit F, Bonvento G, Gurden H. Alteration of sensory-evoked metabolic and oscillatory activities in the olfactory bulb of GLAST-deficient mice. Front Neural Circuits 6: 1, 2012. doi: 10.3389/fncir.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín R, Bajo-Grañeras R, Moratalla R, Perea G, Araque A. Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 349: 730–734, 2015. doi: 10.1126/science.aaa7945. [DOI] [PubMed] [Google Scholar]
- McDougal DH, Viard E, Hermann GE, Rogers RC. Astrocytes in the hindbrain detect glucoprivation and regulate gastric motility. Auton Neurosci 175: 61–69, 2013. doi: 10.1016/j.autneu.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean DL, Sillar KT. Metamodulation of a spinal locomotor network by nitric oxide. J Neurosci 24: 9561–9571, 2004. doi: 10.1523/JNEUROSCI.1817-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Sillar KT. Neuromodulation of vertebrate locomotor control networks. Physiology (Bethesda) 26: 393–411, 2011. doi: 10.1152/physiol.00013.2011. [DOI] [PubMed] [Google Scholar]
- Morquette P, Verdier D, Kadala A, Féthière J, Philippe AG, Robitaille R, Kolta A. An astrocyte-dependent mechanism for neuronal rhythmogenesis. Nat Neurosci 18: 844–854, 2015. doi: 10.1038/nn.4013. [DOI] [PubMed] [Google Scholar]
- Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter d-serine. Proc Natl Acad Sci USA 102: 5606–5611, 2005. doi: 10.1073/pnas.0408483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M, Araque A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 68: 113–126, 2010. doi: 10.1016/j.neuron.2010.08.043. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Verkhratsky A. Artifact versus reality—how astrocytes contribute to synaptic events. Glia 60: 1013–1023, 2012. doi: 10.1002/glia.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve KA, editor. The Dopamine Receptors. Totowa, NJ: Humana, 2010. [Google Scholar]
- Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Müller cells. J Neurosci 21: 2215–2223, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Zhang H, Weng HR. Bidirectional neuron-glia interactions triggered by deficiency of glutamate uptake at spinal sensory synapses. J Neurophysiol 104: 713–725, 2010. doi: 10.1152/jn.00282.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SJ, Han KS, Park H, Woo DH, Kim HY, Traynelis SF, Lee CJ. Protease activated receptor 1-induced glutamate release in cultured astrocytes is mediated by Bestrophin-1 channel but not by vesicular exocytosis. Mol Brain 5: 38, 2012. doi: 10.1186/1756-6606-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Sasaki T, Oku Y, Takahashi N, Seki M, Ujita S, Tanaka KF, Matsuki N, Ikegaya Y. Preinspiratory calcium rise in putative pre-Botzinger complex astrocytes. J Physiol 590: 4933–4944, 2012. doi: 10.1113/jphysiol.2012.231464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oku Y, Fresemann J, Miwakeichi F, Hülsmann S. Respiratory calcium fluctuations in low-frequency oscillating astrocytes in the pre-Bötzinger complex. Respir Physiol Neurobiol 226: 11–17, 2016. doi: 10.1016/j.resp.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Oliveira JF, Sardinha VM, Guerra-Gomes S, Araque A, Sousa N. Do stars govern our actions? Astrocyte involvement in rodent behavior. Trends Neurosci 38: 535–549, 2015. doi: 10.1016/j.tins.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Orlovsky GN, Deliagina TG, Grillner S. Neuronal Control of Locomotion: From Mollusc to Man. New York: Oxford University Press, 1999. [Google Scholar]
- Otsuguro K, Ban M, Ohta T, Ito S. Roles of purines in synaptic modulation evoked by hypercapnia in isolated spinal cord of neonatal rat in vitro. Br J Pharmacol 156: 1167–1177, 2009. doi: 10.1111/j.1476-5381.2009.00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuguro K, Wada M, Ito S. Differential contributions of adenosine to hypoxia-evoked depressions of three neuronal pathways in isolated spinal cord of neonatal rats. Br J Pharmacol 164: 132–144, 2011. doi: 10.1111/j.1476-5381.2011.01333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuguro K, Yamaji Y, Ban M, Ohta T, Ito S. Involvement of adenosine in depression of synaptic transmission during hypercapnia in isolated spinal cord of neonatal rats. J Physiol 574: 835–847, 2006. doi: 10.1113/jphysiol.2006.109660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier A, Vallée J, Haber M, Murai KK, Lacaille JC, Robitaille R. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 146: 785–798, 2011. doi: 10.1016/j.cell.2011.07.022. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature 369: 744–747, 1994. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci 4: 803–812, 2001. doi: 10.1038/90507. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science 310: 113–116, 2005. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci 17: 7817–7830, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor A, Chvátal A, Syková E, Kettenmann H. Glycine- and GABA-activated currents in identified glial cells of the developing rat spinal cord slice. Eur J Neurosci 7: 1188–1198, 1995. doi: 10.1111/j.1460-9568.1995.tb01109.x. [DOI] [PubMed] [Google Scholar]
- Paterniti I, Melani A, Cipriani S, Corti F, Mello T, Mazzon E, Esposito E, Bramanti P, Cuzzocrea S, Pedata F. Selective adenosine A2A receptor agonists and antagonists protect against spinal cord injury through peripheral and central effects. J Neuroinflammation 8: 31, 2011. doi: 10.1186/1742-2094-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen RE, Contestabile A, Villani L, Fonnum F. An in vivo model for studying function of brain tissue temporarily devoid of glial cell metabolism: the use of fluorocitrate. J Neurochem 48: 1377–1385, 1987. doi: 10.1111/j.1471-4159.1987.tb05674.x. [DOI] [PubMed] [Google Scholar]
- Perego C, Vanoni C, Bossi M, Massari S, Basudev H, Longhi R, Pietrini G. The GLT-1 and GLAST glutamate transporters are expressed on morphologically distinct astrocytes and regulated by neuronal activity in primary hippocampal cocultures. J Neurochem 75: 1076–1084, 2000. doi: 10.1046/j.1471-4159.2000.0751076.x. [DOI] [PubMed] [Google Scholar]
- Petravicz J, Boyt KM, McCarthy KD. Astrocyte IP3R2-dependent Ca2+ signaling is not a major modulator of neuronal pathways governing behavior. Front Behav Neurosci 8: 384, 2014. doi: 10.3389/fnbeh.2014.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps CH. An ultrastructural study of methionine sulphoximine-induced glycogen accumulation in astrocytes of the mouse cerebral cortex. J Neurocytol 4: 479–490, 1975. doi: 10.1007/BF01261377. [DOI] [PubMed] [Google Scholar]
- Pieper HO, Clerkin P, MacFarlane A. The impact of direct provision accommodation for asylum seekers on organisation and delivery of local primary care and social care services: a case study. BMC Fam Pract 12: 32, 2011. doi: 10.1186/1471-2296-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli P, Giménez-Llort L, Pezzola A, Reggio R, Martínez E, Fuxe K, Ferré S. Adenosine A1 receptor blockade selectively potentiates the motor effects induced by dopamine D1 receptor stimulation in rodents. Neurosci Lett 218: 209–213, 1996. doi: 10.1016/S0304-3940(96)13143-8. [DOI] [PubMed] [Google Scholar]
- Pryazhnikov E, Khiroug L. Sub-micromolar increase in [Ca2+]i triggers delayed exocytosis of ATP in cultured astrocytes. Glia 56: 38–49, 2008. doi: 10.1002/glia.20590. [DOI] [PubMed] [Google Scholar]
- Rae MG, Irving AJ. Both mGluR1 and mGluR5 mediate Ca2+ release and inward currents in hippocampal CA1 pyramidal neurons. Neuropharmacology 46: 1057–1069, 2004. doi: 10.1016/j.neuropharm.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Stehle JH, Rivkees SA. Molecular cloning and characterization of a rat A1-adenosine receptor that is widely expressed in brain and spinal cord. Mol Endocrinol 5: 1037–1048, 1991. doi: 10.1210/mend-5-8-1037. [DOI] [PubMed] [Google Scholar]
- Ribeiro JA. Purinergic inhibition of neurotransmitter release in the central nervous system. Pharmacol Toxicol 77: 299–305, 1995. doi: 10.1111/j.1600-0773.1995.tb01031.x. [DOI] [PubMed] [Google Scholar]
- Ronzio RA, Rowe WB, Meister A. Mechanism of inhibition of glutamine synthetase by methionine sulfoximine. Biochemistry 8: 1066–1075, 1969. doi: 10.1021/bi00831a038. [DOI] [PubMed] [Google Scholar]
- Roux MJ, Supplisson S. Neuronal and glial glycine transporters have different stoichiometries. Neuron 25: 373–383, 2000. doi: 10.1016/S0896-6273(00)80901-0. [DOI] [PubMed] [Google Scholar]
- Rusakov DA. Disentangling calcium-driven astrocyte physiology. Nat Rev Neurosci 16: 226–233, 2015. doi: 10.1038/nrn3878. [DOI] [PubMed] [Google Scholar]
- Sekiguchi KJ, Shekhtmeyster P, Merten K, Arena A, Cook D, Hoffman E, Ngo A, Nimmerjahn A. Imaging large-scale cellular activity in spinal cord of freely behaving mice. Nat Commun 7: 11450, 2016. doi: 10.1038/ncomms11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano A, Haddjeri N, Lacaille JC, Robitaille R. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J Neurosci 26: 5370–5382, 2006. doi: 10.1523/JNEUROSCI.5255-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P, Gajendiran M, Maitra KK, Ross HG, Ganguly DK. Evidence for D1 dopamine receptor-mediated modulation of the synaptic transmission from motor axon collaterals to Renshaw cells in the rat spinal cord. Neurosci Lett 158: 217–220, 1993. doi: 10.1016/0304-3940(93)90268-P. [DOI] [PubMed] [Google Scholar]
- Sharples SA, Humphreys JM, Jensen AM, Dhoopar S, Delaloye N, Clemens S, Whelan PJ. Dopaminergic modulation of locomotor network activity in the neonatal mouse spinal cord. J Neurophysiol 113: 2500–2510, 2015. doi: 10.1152/jn.00849.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharples SA, Koblinger K, Humphreys JM, Whelan PJ. Dopamine: a parallel pathway for the modulation of spinal locomotor networks. Front Neural Circuits 8: 55, 2014. doi: 10.3389/fncir.2014.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki K, Hosoi N, Kaneko R, Tominaga M, Yamada K. Glycine release from astrocytes via functional reversal of GlyT1. J Neurochem 140: 395–403, 2017. doi: 10.1111/jnc.13741. [DOI] [PubMed] [Google Scholar]
- Shigetomi E, Bowser DN, Sofroniew MV, Khakh BS. Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J Neurosci 28: 6659–6663, 2008. doi: 10.1523/JNEUROSCI.1717-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Patel S, Khakh BS. Probing the complexities of astrocyte calcium signaling. Trends Cell Biol 26: 300–312, 2016. doi: 10.1016/j.tcb.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skagerberg G, Lindvall O. Organization of diencephalic dopamine neurones projecting to the spinal cord in the rat. Brain Res 342: 340–351, 1985. doi: 10.1016/0006-8993(85)91134-5. [DOI] [PubMed] [Google Scholar]
- Sloan SA, Barres BA. Looks can be deceiving: reconsidering the evidence for gliotransmission. Neuron 84: 1112–1115, 2014. doi: 10.1016/j.neuron.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Feldman JL. In vitro brainstem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J Neurosci Methods 21: 321–333, 1987. doi: 10.1016/0165-0270(87)90126-9. [DOI] [PubMed] [Google Scholar]
- Snyder SH, Katims JJ, Annau Z, Bruns RF, Daly JW. Adenosine receptors and behavioral actions of methylxanthines. Proc Natl Acad Sci USA 78: 3260–3264, 1981. doi: 10.1073/pnas.78.5.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Kyriakatos A, El Manira A. Gating the polarity of endocannabinoid-mediated synaptic plasticity by nitric oxide in the spinal locomotor network. J Neurosci 32: 5097–5105, 2012. doi: 10.1523/JNEUROSCI.5850-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Huang BS, Venugopal S, Johnston AD, Chai H, Zeng H, Golshani P, Khakh BS. Ca2+ signaling in astrocytes from Ip3r2−/− mice in brain slices and during startle responses in vivo. Nat Neurosci 18: 708–717, 2015. doi: 10.1038/nn.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan S, Li L, Moss J, Petrelli F, Cassé F, Gebara E, Lopatar J, Pfrieger FW, Bezzi P, Bischofberger J, Toni N. Synaptic integration of adult-born hippocampal neurons is locally controlled by astrocytes. Neuron 88: 957–972, 2015. doi: 10.1016/j.neuron.2015.10.037. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nomikos GG, Fredholm BB. Biphasic changes in locomotor behavior and in expression of mRNA for NGFI-A and NGFI-B in rat striatum following acute caffeine administration. J Neurosci 15: 7612–7624, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nomikos GG, Ongini E, Fredholm BB. Antagonism of adenosine A2A receptors underlies the behavioural activating effect of caffeine and is associated with reduced expression of messenger RNA for NGFI-A and NGFI-B in caudate-putamen and nucleus accumbens. Neuroscience 79: 753–764, 1997. doi: 10.1016/S0306-4522(97)00046-8. [DOI] [PubMed] [Google Scholar]
- Taccola G, Olivieri D, D’Angelo G, Blackburn P, Secchia L, Ballanyi K. A1 adenosine receptor modulation of chemically and electrically evoked lumbar locomotor network activity in isolated newborn rat spinal cords. Neuroscience 222: 191–204, 2012. doi: 10.1016/j.neuroscience.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Otsuguro K, Ohta T, Ito S. Adenosine and inosine release during hypoxia in the isolated spinal cord of neonatal rats. Br J Pharmacol 161: 1806–1816, 2010. doi: 10.1111/j.1476-5381.2010.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakusaki K, Oohinata-Sugimoto J, Saitoh K, Habaguchi T. Role of basal ganglia-brainstem systems in the control of postural muscle tone and locomotion. Prog Brain Res 143: 231–237, 2004. doi: 10.1016/S0079-6123(03)43023-9. [DOI] [PubMed] [Google Scholar]
- Tien AC, Tsai HH, Molofsky AV, McMahon M, Foo LC, Kaul A, Dougherty JD, Heintz N, Gutmann DH, Barres BA, Rowitch DH. Regulated temporal-spatial astrocyte precursor cell proliferation involves BRAF signalling in mammalian spinal cord. Development 139: 2477–2487, 2012. doi: 10.1242/dev.077214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres A, Wang F, Xu Q, Fujita T, Dobrowolski R, Willecke K, Takano T, Nedergaard M. Extracellular Ca2+ acts as a mediator of communication from neurons to glia. Sci Signal 5: ra8, 2012. doi: 10.1126/scisignal.2002160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undieh AS. Pharmacology of signaling induced by dopamine D1-like receptor activation. Pharmacol Ther 128: 37–60, 2010. doi: 10.1016/j.pharmthera.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Butt AM, editors. Glial Physiology and Pathophysiology: A Handbook. Chichester, UK: Wiley-Blackwell, 2013. [Google Scholar]
- Wall M, Dale N. Activity-dependent release of adenosine: a critical re-evaluation of mechanism. Curr Neuropharmacol 6: 329–337, 2008. doi: 10.2174/157015908787386087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MJ, Dale N. Neuronal transporter and astrocytic ATP exocytosis underlie activity-dependent adenosine release in the hippocampus. J Physiol 591: 3853–3871, 2013. doi: 10.1113/jphysiol.2013.253450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, Bordey A. The astrocyte odyssey. Prog Neurobiol 86: 342–367, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Smith NA, Xu Q, Goldman S, Peng W, Huang JH, Takano T, Nedergaard M. Photolysis of caged Ca2+ but not receptor-mediated Ca2+ signaling triggers astrocytic glutamate release. J Neurosci 33: 17404–17412, 2013. doi: 10.1523/JNEUROSCI.2178-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MY, Dun NJ. Phaclofen-insensitive presynaptic inhibitory action of (±)-baclofen in neonatal rat motoneurones in vitro. Br J Pharmacol 99: 413–421, 1990. doi: 10.1111/j.1476-5381.1990.tb14718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watase K, Hashimoto K, Kano M, Yamada K, Watanabe M, Inoue Y, Okuyama S, Sakagawa T, Ogawa S, Kawashima N, Hori S, Takimoto M, Wada K, Tanaka K. Motor discoordination and increased susceptibility to cerebellar injury in GLAST mutant mice. Eur J Neurosci 10: 976–988, 1998. doi: 10.1046/j.1460-9568.1998.00108.x. [DOI] [PubMed] [Google Scholar]
- Weinstein JR, Gold SJ, Cunningham DD, Gall CM. Cellular localization of thrombin receptor mRNA in rat brain: expression by mesencephalic dopaminergic neurons and codistribution with prothrombin mRNA. J Neurosci 15: 2906–2919, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan P, Bonnot A, O’Donovan MJ. Properties of rhythmic activity generated by the isolated spinal cord of the neonatal mouse. J Neurophysiol 84: 2821–2833, 2000. [DOI] [PubMed] [Google Scholar]
- Whelan PJ. Shining light into the black box of spinal locomotor networks. Philos Trans R Soc Lond B Biol Sci 365: 2383–2395, 2010. doi: 10.1098/rstb.2009.0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witts EC, Nascimento F, Miles GB. Adenosine-mediated modulation of ventral horn interneurons and spinal motoneurons in neonatal mice. J Neurophysiol 114: 2305–2315, 2015. doi: 10.1152/jn.00574.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witts EC, Panetta KM, Miles GB. Glial-derived adenosine modulates spinal motor networks in mice. J Neurophysiol 107: 1925–1934, 2012. doi: 10.1152/jn.00513.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JK, Dreshaj IA, Wilson CG, Martin RJ, Zaidi SI, Haxhiu MA. An astrocyte toxin influences the pattern of breathing and the ventilatory response to hypercapnia in neonatal rats. Respir Physiol Neurobiol 147: 19–30, 2005. doi: 10.1016/j.resp.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, Duan S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 40: 971–982, 2003. doi: 10.1016/S0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
- Ziak D, Chvátal A, Syková E. Glutamate-, kainate- and NMDA-evoked membrane currents in identified glial cells in rat spinal cord slice. Physiol Res 47: 365–375, 1998. [PubMed] [Google Scholar]
- Zwicker JD, Rajani V, Hahn LB, Funk GD. Purinergic modulation of preBötzinger complex inspiratory rhythm in rodents: the interaction between ATP and adenosine. J Physiol 589: 4583–4600, 2011. doi: 10.1113/jphysiol.2011.210930. [DOI] [PMC free article] [PubMed] [Google Scholar]