Findings suggest that adults with cerebral palsy have less contribution of sensory feedback to ongoing soleus muscle activation during push-off than uninjured individuals. Increased passive stiffness around the ankle joint is likely to diminish sensory feedback during gait, and/or sensory feedback is less integrated with central motor commands in the activation of spinal motor neurons. Consequently, muscle activation must to a larger extent rely on descending drive, which is already decreased because of the cerebral lesion.
Keywords: cerebral palsy, sensory feedback, muscle activation, push-off, gait
Abstract
Exaggerated sensory activity has been assumed to contribute to functional impairment following lesion of the central motor pathway. However, recent studies have suggested that sensory contribution to muscle activity during gait is reduced in stroke patients and children with cerebral palsy (CP). We investigated whether this also occurs in CP adults and whether daily treadmill training is accompanied by alterations in sensory contribution to muscle activity. Seventeen adults with CP and 12 uninjured individuals participated. The participants walked on a treadmill while a robotized ankle-foot orthosis applied unload perturbations at the ankle, thereby removing sensory feedback naturally activated during push-off. Reduction of electromyographic (EMG) activity in the soleus muscle caused by unloads was compared and related to kinematics and ankle joint stiffness measurements. Similar measures were obtained after 6 wk of gait training. We found that sensory contribution to soleus EMG activation was reduced in CP adults compared with uninjured adults. The lowest contribution of sensory feedback was found in participants with lowest maximal gait speed. This was related to increased ankle plantar flexor stiffness. Six weeks of gait training did not alter the contribution of sensory feedback. We conclude that exaggerated sensory activity is unlikely to contribute to impaired gait in CP adults, because sensory contribution to muscle activity during gait was reduced compared with in uninjured individuals. Increased passive stiffness around the ankle joint is likely to diminish sensory feedback during gait so that a larger part of plantar flexor muscle activity must be generated by descending motor commands.
NEW & NOTEWORTHY Findings suggest that adults with cerebral palsy have less contribution of sensory feedback to ongoing soleus muscle activation during push-off than uninjured individuals. Increased passive stiffness around the ankle joint is likely to diminish sensory feedback during gait, and/or sensory feedback is less integrated with central motor commands in the activation of spinal motor neurons. Consequently, muscle activation must to a larger extent rely on descending drive, which is already decreased because of the cerebral lesion.
INTRODUCTION
Cerebral palsy (CP) is caused by a nonprogressive lesion of the developing brain before or at the time of birth (Rosenbaum et al. 2007). Because of the lesion, the development and maturation of the central drive to motor neurons in the spinal cord is impaired with severe consequences for gait ability (Berger et al. 1984; Petersen et al. 2010, 2013; Rose and McGill 2005). Impaired gait ability has been found to have significant impact on quality of life, ability to integrate with peers, and participation in social activities (Parkes et al. 2010; Shikako-Thomas et al. 2012). Deterioration of gait function continues into adulthood, and physical independency is often further reduced with age (Andersson and Mattsson 2001; Jahnsen et al. 2004; Morgan and McGinley 2014).
Reduced plantar flexor activation during push-off in the late stance phase of walking has been shown to contribute to gait impairment in patients with cerebral lesions (Nadeau et al. 1999; Olney et al. 1990; Williams et al. 2013) and to be related to reduced speed, reduced stride length, and increased cadence in adults with CP (Morgan and McGinley 2014; Morgan et al. 2016; Opheim et al. 2013; Roche et al. 2014). The plantar flexors are of particular interest in relation to push-off, because forces produced by these muscles are transferred directly to the ground, supporting the body against gravity and contributing to ensure propulsion during walking (Hof et al. 1992; Honeine et al. 2013; Neptune et al. 2001; Winter 1983).
Exaggerated sensory inputs to the spinal cord have long been assumed to be involved in gait impairments in persons with a central lesion (Sheean 2002; Winters et al. 1987). Therefore, hyperexcitable stretch reflexes have been targeted by antispastic treatments aiming to facilitate more efficient muscle activation (Criswell et al. 2006; Dietz and Sinkjaer 2007). However, several studies have questioned the assumption that exaggerated sensory activity is responsible for gait impairments in persons with spasticity (Dietz et al. 1981; Dietz and Sinkjaer 2007; Sinkjaer and Magnussen 1994). In uninjured individuals, sensory inputs from load sensitive afferents contribute to plantar flexor muscle activation during the late stance phase of walking (Sinkjaer et al. 2000). Sinkjaer et al. (2000) showed this by unloading the triceps surae muscle with a rapid shortening of the muscle. The assumption is that such unload perturbation of the muscle reduces sensory input from Ib and group II afferents to the spinal cord, which is shown as a clear drop in the electromyographic (EMG) activity, a so-called unload response. This indicates that sensory feedback is an integrated part of motor commands in normal voluntary movements, which ultimately helps to drive muscle activation during walking (Dietz and Duysens 2000; Dietz and Sinkjaer 2007; Sinkjaer et al. 2000). This interpretation is consistent with early studies investigating sensory contribution to leg muscle activation in walking cats (Duysens and Pearson 1980; Hiebert and Pearson 1999; Pearson 2004).
Recent studies in adults with stroke and children with CP have shown that the sensory contribution to muscle activity during gait is reduced in the presence of hyperexcitable short-latency stretch reflexes (Mazzaro et al. 2007; Willerslev-Olsen et al. 2014a). Hence, we investigated the contribution of sensory feedback to plantar flexor muscle activation during push-off in adults with CP. The study was a part of a large intervention study investigating effects of progressive gait training on ankle joint stiffness and functional gait parameters in adults with CP (Lorentzen et al. 2016). Thus we additionally investigated whether 6 wk of uphill gait training would improve the contribution of sensory feedback.
METHOD
Participants.
The study was approved by the local ethics committee (H-2-2014-028) in accordance with the Helsinki Declaration. All subjects received written and verbal information. Written consent for participation was obtained before the experiments.
Seventeen adults with CP [age: 37.45 ± 11.45 (SD) yr, range 21–56 yr; 8 men and 9 women] participated in the study and were randomized in either an intervention (n = 9) or control group (n = 8). The participants with CP were recruited through the Danish Cerebral Palsy Organization. Inclusion criteria were spastic CP, a Gross Motor Function Classification Scale score (GMFCS) between I and III, and sufficient cognitive function to be aware and understand the content of the experiments. Exclusion criteria were impaired cognitive function and surgery and/or botulinum toxin treatment within 6 mo before the experiments. An uninjured control group consisted of 12 participants (age: 39.92 ± 12.4 yr, range 28–53 yr; 6 men and 6 women). The uninjured participants were recruited through the University of Copenhagen and the Elsass Institute. This group had no reported history of neurological or musculoskeletal injury that could interfere with the experiment. Descriptive information of the participants is shown in Table 1.
Table 1.
Information about enrolled participants
| CP | Uninjured | |
|---|---|---|
| n | 17 | 12 |
| Sex, men:women | 8:9 | 6:6 |
| Age, yr | 37.35 ± 11.45 | 39.92 ± 12.4 |
| Weight, kg | 66.27 ± 11.96 | 67.09 ± 8.86 |
| Height, cm | 169.1 ± 8.03 | 173.8 ± 8.81 |
| Paresis, diplegic:hemiplegic | 12:5 | |
| GMFCS, I:II:III | 8:7:2 | |
| Antispastic medication, yes:no | 16:1 | |
| No. of tendon-lengthening operations, 0:1:2:3:4:5 | 6:3:4:3:1 | |
| Walking aid, none:2×crutches:rollator | 15:1:1 |
Values are means ± SD; n = no. of participants with cerebral palsy (CP) or with no brain lesion (uninjured). GMFCS, Gross Motor Function Classification System.
Experimental setup.
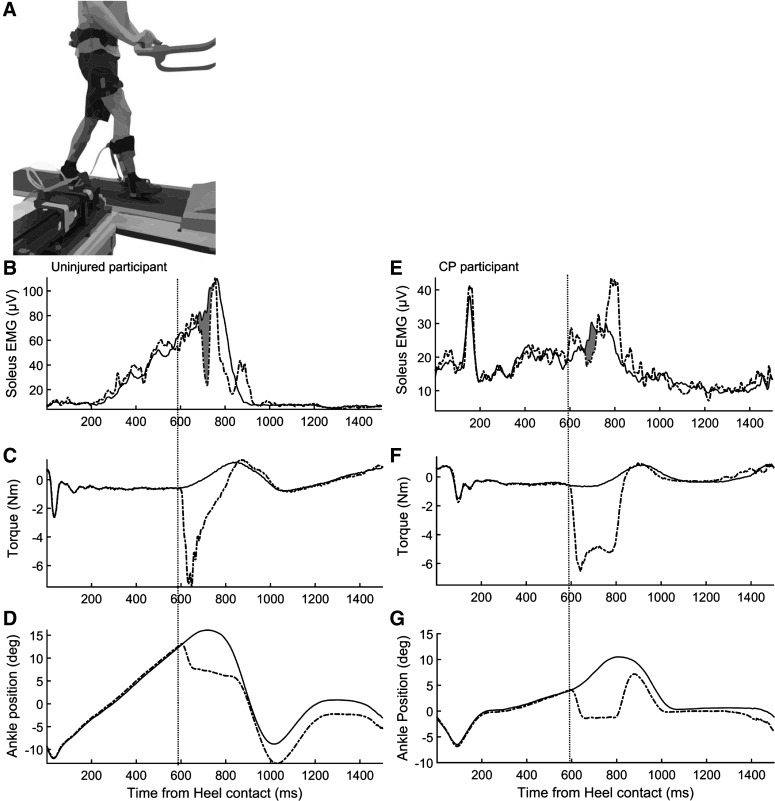
Evaluation of the contribution of sensory feedback to plantar flexor muscle activation was done by suddenly unloading the triceps surae muscle, as previously described by Sinkjaer et al. (2000). Before and after the training period, participants walked on a treadmill without inclination at a gait speed adjusted to their walking ability. The participants walked in a self-selected, comfortable, and not exhausting speed between 1 and 2 km/h while wearing a custom-designed electrohydraulic ankle-foot orthosis (EHO) system (Fig. 1A). Maximal gait velocities of 2 km/h were used in the present study to stay in the torque limits of the EHO. The same gait speed was used for each participant before and after the training. The EHO consisted of a hybrid system that was composed of an electric motor coupled to a hydraulic closed-loop system (Noël et al. 2008). Mechanical power from the electric motor was transferred by hydraulic actuators to the orthosis. To trigger an unload response, the cylinder on the orthosis applied a rapid torque perturbation consisting of 8° plantar flexion with a velocity of 250°/s and a hold duration of 200 ms to the ankle joint. The perturbations were timed individually for each participant based on initial foot contact determined using a footswitch placed under the shoe (Noël et al. 2008). These rapid torque perturbations were applied under position control (ramp and hold command). Interference with normal walking when no perturbation was applied was minimized with the use of an active torque cancellation algorithm (Noël et al. 2008) that used a load cell located at the end of the cylinder attached to the orthosis to measure residual torque applied to the ankle.
Fig. 1.
A: experimental setup for evaluating the contribution of sensory feedback to plantar flexor muscle activation. Participants walked on a treadmill while wearing a custom-designed electrohydraulic ankle-foot orthosis (EHO). In late stance phase, mechanical power from the electric motor was transferred by hydraulic actuators to the EHO, causing rapid plantar flexor perturbations to the ankle joint, which unloaded the plantar flexor muscles during push-off. B–G: examples of average recorded data during control steps (solid lines) and steps with unload perturbations (dashed lines) of the plantar flexors. Perturbation time is indicated by vertical dotted lines. Data in B–D represent an uninjured participant; data in E–G represent a participant with CP. B and E: soleus muscle EMG; shaded area represents the area under the curve used to calculate the reduction in soleus EMG activity caused by unloading the plantar flexors (unload response). C and F: torque deviation produced by the ankle-foot orthosis used to unload the plantar flexors. D and G: ankle position; dashed line shows the amplitude of the ankle deviation toward planter flexion used to unload the plantar flexors.
Five minutes of baseline walking were used for the participants to get familiarized with the EHO. During the familiarization, the perturbations were timed, targeting push-off in late stance phase as close to maximal soleus EMG as possible. The walking period with the perturbations lasted ~10 min depending on the walking speed until ~30 perturbed steps were recorded. The perturbations were induced on average every fifth step, in pseudorandom sequence, so it was not possible for the participants to anticipate the next perturbation and inadvertently adjust their gait pattern.
Data collection.
EMG activity of the soleus and tibialis anterior (TA) muscle was recorded using bipolar electrodes (Blue Sensor NF-00-S/12; Ambu, Ballerup, Denmark; recording area 0.5 cm2, interelectrode distance 2 cm) placed over the respective muscle belly. EMG signals were bandpass filtered (10–1,000 Hz), amplified 1,000 times, and stored on a personal computer for offline analysis. The EMG signals were subsequently digitally filtered (zero-lag Butterworth 4th-order filter; bandpass 10–450 Hz) before rectification.
Custom-made software written in MATLAB 2015b (The MathWorks, Natick, MA) was used for data processing and analysis. The recorded EMG signals were cut into individual gait cycles by using initial foot contact information and were classified as stimulated (STIM) or control (CTRL), depending whether the step was disturbed by the unload perturbation or not. The CTRL cycles were rectified, time normalized to mean CTRL cycle duration, and averaged to represent the mean background EMG activity. The STIM cycles were rectified, synchronized at perturbation onset, and averaged to represent mean EMG during perturbed steps. As described by Sinkjaer et al. (2000), a clear drop in the soleus muscle activity is seen around 70 ms after the plantar flexors are unloaded (Fig. 1B). The magnitude of this reduction in soleus EMG (the unload response) was calculated as the difference between STIM cycles and CTRL cycles, expressed as the percent reduction in EMG activity with respect to CTRL EMG. The unload response was quantified as the area under the curve of the EMG signal in the first 50 ms following the onset of the reduction in soleus EMG, similar to the method used by Mazzaro et al. (2007). This interval was chosen because the reduction in EMG activity following the unload perturbation lasted longer than this in the majority of the participants, and we wanted to minimize involvement of voluntary reactions to the observed measurements. Voluntary reaction time for ankle plantar flexors is usually well above 100 ms, and the unload response must therefore be assumed to be caused predominantly by reflex mechanisms within the initial 50 ms (MacKay and Bonnet 1990). Nevertheless, we also performed separate analysis in which we quantified the area of the whole duration period of the unload response in the individual participants. This did not change significantly any of the results reported in results.
The onset latency of the unload response was determined by visual inspection of the soleus EMG profile, defined as the first large deviation from the CTRL step EMG within 30–80 ms after the perturbation onset. To evaluate the training effect on the contribution of sensory feedback, the latency found at baseline was also used in posttraining analysis. This was done to be confident that the same response of the unload perturbation was included in the analysis.
Stiffness measurements.
Passive and reflex mediated components of stiffness around the ankle joint were obtained in the participants with CP by biomechanical and electrophysiological evaluation according to Lorentzen et al. (2010) and Willerslev-Olsen et al. (2013). Briefly, the CP participants were seated in a reclining armchair with the examined foot attached to a foot plate. The foot plate could be rotated by a motor (model 26; CEM), which was driven by a DC power amplifier (model 2708; Brüel & Kjaer) and could deliver maintained torques up to 80 Nm and peak torques up to 120 Nm. An electrogoniometer connected to the foot plate measured the angle position, and a torque meter measured the torque exerted on the foot plate before and during the stretch perturbations. The hip joint was positioned in 100° flexion, the knee in 55° flexion, and the ankle joint in 20° plantar flexion. The plantar flexors were stretched by perturbations consisted of ramp and hold dorsiflexions with an amplitude of 6° at 17 different velocities between 5° and 220°/s and with a hold time of 460 ms. These stretches were delivered every second in a random order until 10 trials per velocity were collected. The interval between stretches was 1 s.
Passive stiffness was calculated as a mean of torque responses at slow velocities (10°/s) that did not elicited stretch responses in any participants. Reflex-mediated stiffness was calculated as a mean of the torque responses to the fastest velocities (210°/s) minus the passive stiffness (Lorentzen et al. 2010). The fastest velocity was chosen because this elicited the maximal reflex response. To be confident that this was the case, the other 15 velocities were included, although they are not used in the present study. For more detailed description we kindly refer to Lorentzen et al. (2016). Stiffness measurements from the dorsiflexor muscles were not included, because unpublished work from our laboratory (Lorentzen J, personal observation) shows that dorsiflexor stiffness is overruled by the much larger muscle group of the triceps surae during plantar flexor activation.
Training intervention.
Briefly, the gait training consisted of 30 min of daily treadmill walking for 6 wk with increasing speed and inclination. Individual training plans were made for the participants with CP based on the speed they were able to maintain for 30 min with an incline of 3–5% (average initial gait speed: CP training: 3.14 ± 1.45 km/h, CP control: 2.85 ± 0.85 km/h). The aim of training progression was a weekly increase of 0.2 km/h and 1% of incline. Treadmills were brought to each participant’s home, and they received thorough instructions in their use together with the training plan. Participants filled in training logs weekly and sent them to the trainers for evaluation. Based on the evaluations, training intensity was adjusted weekly.
Training effects were evaluated by obtaining three-dimensional gait kinematics using a Qualisys motion capture system (Qualisys, Gothenburg, Sweden) with six synchronous Oqus 1 cameras operating at a sampling frequency of 200 Hz. Data were processed using Qualisys Track Manager and exported into MATLAB for further analysis. Overground walking was evaluated by using shoe-worn inertial Sensors (GaitUp, Lausanne, Switzerland). To evaluate the training effect on daily activities, functional tests were performed: 6-min walk test, 10-m walk test, timed up and go (TUG), and timed up and down stairs (TUDS). A more detailed description can be found in Lorentzen et al. (2016).
Statistics.
The onset latency of the reduction in soleus EMG activity after the ankle perturbations was tested with a nonpaired t-test between the CP group and the uninjured individuals. A nonpaired t-test was also used to compare the size of the reduction in soleus EMG activity elicited by ankle perturbation between the CP group and the group of uninjured participants. Spearman correlation analysis was used to determine a possible relation between the reduction in EMG activity and gait speed/stiffness measurements.
Mixed-model regression analysis was used to evaluate the effect of gait training on the reduction in EMG activity evoked by ankle perturbation, gait kinematics and functional tests. Factors included in the analysis were group (CP training and CP control), time (before training and after training), and the interaction between group and time. Post hoc comparisons were performed for statistically significant comparisons. The results are presented as means ± SD. The level of significance was set at P = 0.05. The statistical tests were carried out using SAS Enterprise Guide 7.1. Illustrations of the EMG activity were made using MATLAB 2015b and other figures using SigmaPlot 13.0.
RESULTS
Gait parameters.
The uninjured participants walked with slightly higher gait speed than the participants with CP (CP: 1.71 ± 0.32 km/h, uninjured: 1.92 ± 0.19 km/h, P = 0.05) and with a similar gait cycle duration (CP: 1.40 ± 0.16 s, uninjured: 1.46 ± 0.17 s, P = 0.35). No statistical differences were observed between the groups regarding duration of stance phase (CP: 64.21 ± 11.47%, uninjured: 60.29 ± 4.21%, P = 0.21) and swing phase (CP: 35.79 ± 11.47%, uninjured: 39.71 ± 4.21%, P = 0.21) in relation to gait cycle duration.
During push-off, the CP group showed a significantly smaller maximal soleus EMG activity than the group of uninjured participants (CP: 50.5 ± 30.2 µV, uninjured: 81.3 ± 31.6 µV, P = 0.01). Peak soleus EMG activity was on average 60 ms earlier in relation to heel strike in the CP group, but this was not a statistically significant difference (CP: 597.5 ± 169.5 ms, uninjured: 657.6 ± 67.09 ms, P = 0.2).
Contribution of sensory feedback.
The torque perturbations occurred at the same time relative to gait cycle duration (CP: 38.1 ± 10.36%, uninjured: 42.9 ± 4.78%, P = 0.15) and to peak soleus EMG (CP: 85.65 ± 12.04%, uninjured: 81.12 ± 8.54%, P = 0.27) in the two groups. The perturbations did not affect the gait duration or stance- and swing-phase durations.
The rapid plantar flexion perturbation (drop in plantar flexor torque in Fig. 1, C and F) elicited a reduction in soleus muscle EMG activity in both uninjured (Fig. 1B) and CP participants (Fig. 1E). In the uninjured participant represented in Fig. 1, B–D, the reduction in the EMG was observed 74 ms following onset of the perturbation and lasted 71 ms. The area of the reduction in EMG was 52.2 µV·s, which corresponded to a reduction of 37.18% of the unperturbed EMG. The reduction in soleus EMG activity had shorter onset latency in the participant with CP (64 ms), shorter duration (63 ms), and a smaller area (6.9 µV·s), which corresponded to a reduction of 25.46%. Additionally, following the reduction of EMG activity a marked increase was observed in the soleus EMG activity in the participant with CP 750–840 ms after heel strike (Fig. 1E). A similar increase in soleus EMG activity following the reduction has also been reported in previous studies and is most likely related to a compensatory response following the unexpected unload perturbation (Christensen et al. 2000; Grey et al. 2007). A similar increase was also observed in other participants in the present study, with no differences in occurrence between CP and uninjured participants.
The observations shown in Fig. 1 were confirmed for the population of participants, except for the difference in onset latency of the EMG reduction (Fig. 2). The average onset latency of the reduction in soleus EMG activity was similar for the two groups (Fig. 2A; CP: 67.41 ± 10.39 ms, uninjured: 67.83 ± 11.35 ms, P = 0.92). The duration of the reduction of EMG was shorter (Fig. 2B; CP: 44.24 ± 18.7 ms, uninjured: 65.42 ± 26.26 ms, P = 0.03), and the area of the reduction expressed as a percentage of the unperturbed EMG activity was significantly smaller in the participants with CP than in the uninjured participants (Fig. 2C; CP: 13.46 ± 9.8%, uninjured: 28.8 ± 12.64%, P = 0.001). Post hoc comparisons between diplegic and hemiplegic CP participants show no significant differences regarding onset latency (diplegic: 67.92 ± 20.25 ms, hemiplegic: 66.20 ± 17.76 ms, P = 0.77), duration (diplegic: 41.25 ± 17.24 ms, hemiplegic: 51.40 ± 22.14 ms, P = 0.32), or size of the unload response (diplegic: 12.96 ± 10.04%, hemiplegic: 14.67 ± 10.25%, P = 0.76).
Fig. 2.
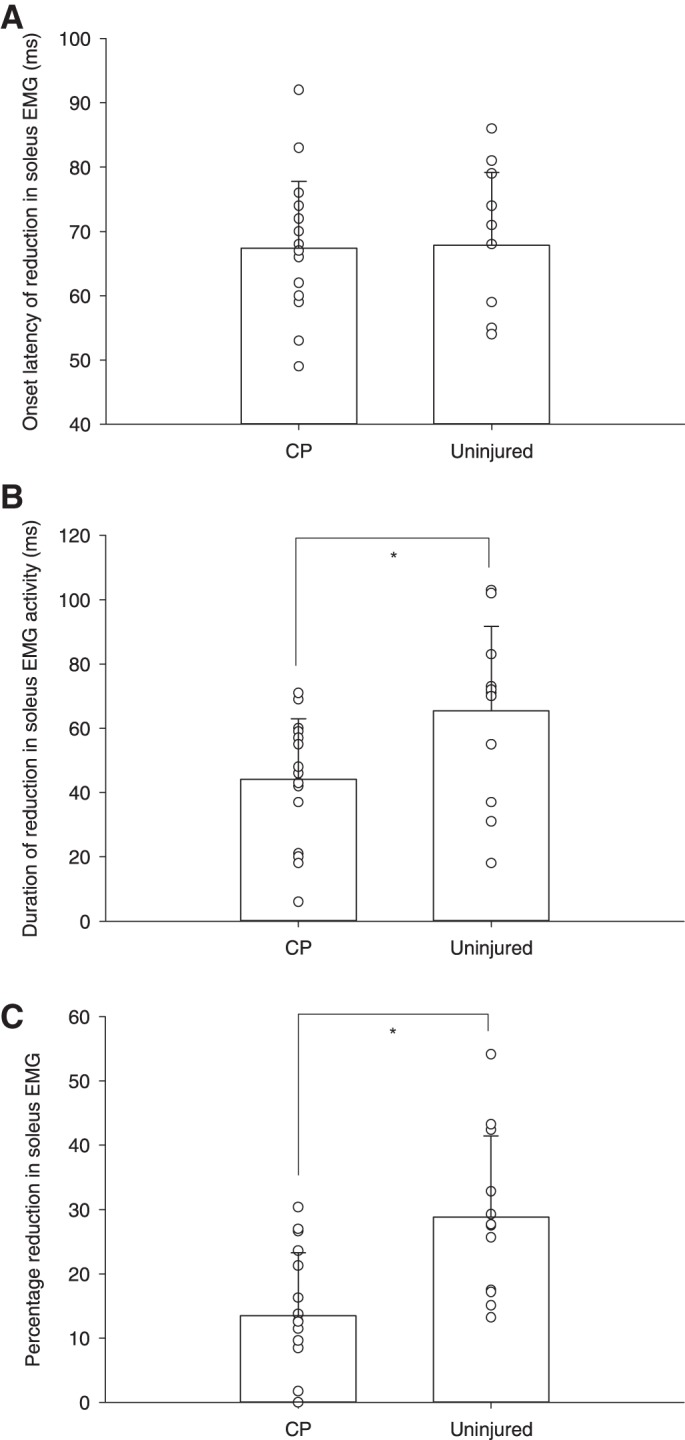
Average group differences between participants with CP and uninjured participants with individual data point for each group. Values are onset latency (A), duration (B), and size (C) of the reduction in soleus EMG activity following unloading the plantar flexors during late stance phase of gait. Error bars indicate the SD. *P < 0.05 indicates statistical significance.
To compare the size of the reduction of EMG induced by the perturbations in the two populations, it is essential that the perturbations elicited a similar reduction in torque. This was also the case (CP: −5.09 ± 1.28 Nm, uninjured: −5.73 ± 1.69 Nm, P = 0.25), but a small difference was observed in the change of ankle joint position during the perturbation (CP: 5.18 ± 1.57°, uninjured: 6.72 ± 0.59° P = 0.004). Most likely, this is related to larger stiffness of the muscle, tendon, joint, or connective tissue in persons with CP, preventing an equal change in ankle position for a given change in torque compared with uninjured individuals (Geertsen et al. 2015). Consistent with previous findings that length changes have little influence on the reduction in soleus EMG activity following unload of plantar flexors in the stance phase during walking (af Klint et al. 2009), we found no correlation between the size of the reduction in soleus EMG activity and the change in ankle joint position induced by the perturbation (CP: r = −0.089, P = 0.74, uninjured: r = 0.38, P = 0.22).
To investigate whether reduced sensory feedback to ankle plantar flexors contributes to gait disability in the participants with CP, we compared the size of the reduction in soleus EMG activity in late stance with gait speed on the treadmill and overground. As shown in Fig. 3, a positive correlation was found between the size of the reduction in soleus EMG activity elicited by unloading the ankle plantar flexors in late stance and the maximal gait speed on the treadmill (Fig. 3A; r = 0.45, P = 0.05) and during overground walking (Fig. 3B; r = 0.54, P = 0.04).
Fig. 3.
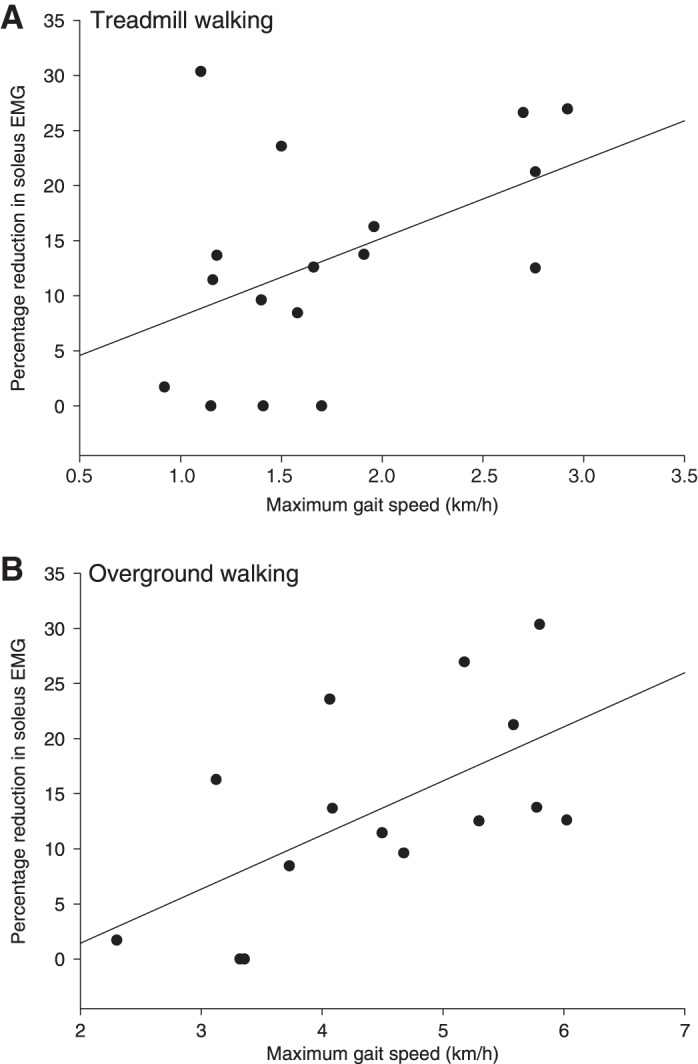
Relationship between the size of the reduction in soleus EMG activity following unloading ankle plantar flexors and maximal gait speed during treadmill walking (A) and overground walking (B) in participants with CP.
The unload perturbation did not elicit short-latency stretch reflexes in TA. In some cases, a long-latency stretch reflex followed the perturbation around 100–120 ms after the perturbation. There was no difference in appearance of long-latency stretch reflexes between the two groups.
Unload response correlated with stiffness measurements.
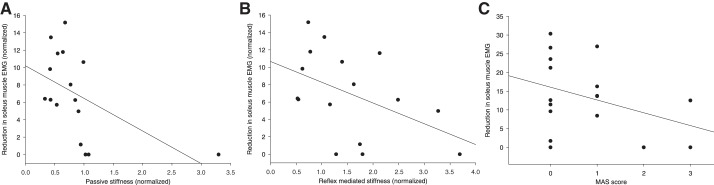
It has been suggested previously that increased passive stiffness of the ankle joint is an important factor for impaired gait ability in persons with CP (Dietz and Berger 1983; Dietz and Sinkjaer 2007; Geertsen et al. 2015). We consequently investigated whether reduced unload response in late stance was related to stiffness of the ankle joint measured by a dynamometer (Lorentzen et al. 2016). Because gait speed is related to the size of unload response (Fig. 3) and stiffness, we normalized the unload response and stiffness measurements with the gait speed used during the perturbation experiments of the individual participant (Fig. 4). As shown in Fig. 4, a significant correlation between the normalized reduction in soleus EMG activity and the normalized passive ankle joint stiffness was observed (Fig. 4A; r = −0.58, P = 0.02). A sensitivity analysis excluding the outlier shown in Fig. 4A did not affect this result (r = −0.54, P = 0.04). Additionally, a negative correlation was also found between the normalized reduction of soleus EMG activity and normalized reflex mediated stiffness (Fig. 4B; r = −0.49), which nearly reached significance (P = 0.054). No correlation was found between the reduction in soleus muscle EMG and the Modified Ashworth Scale (MAS) score (Fig. 4C: r = −0.29, P = 0.3).
Fig. 4.
Relationship between the size of reduction in soleus EMG activity following unloading of ankle plantar flexors and passive stiffness (A), reflex-mediated stiffness (B) and Modified Ashworth Scale (MAS) score (C) in participants with CP.
Training effect on the size of unload response.
To ensure that gait speed did not influence the size of unload response when the effect of gait training was evaluated, all participants walked at the same gait speed before and after training. Because a difference in the size of unload response was observed between the CP training group and the CP control group before the training was initialized (Fig. 5), a mixed-model regression analysis with baseline adjustment was used to evaluate the training effect. The analysis revealed no significant interaction between group (training and control) and time (before and after the training) (P = 0.27), showing no effect of the training intervention on the size of the unload response (CP training group: mean difference 4.02 ± 10.28%, CP control group: mean difference 2.99 ± 8.67%; Fig. 5). The lack of statistical significance does not seems to be due to the large variability, because no training effect was observed in CP participants with either large or small unload responses. No training effect was observed in either the spatiotemporal gait parameters or functional tests in the participants with CP. More information regarding training effects of 6-wk uphill gait training in adults with CP can be found in Lorentzen et al. (2016).
Fig. 5.
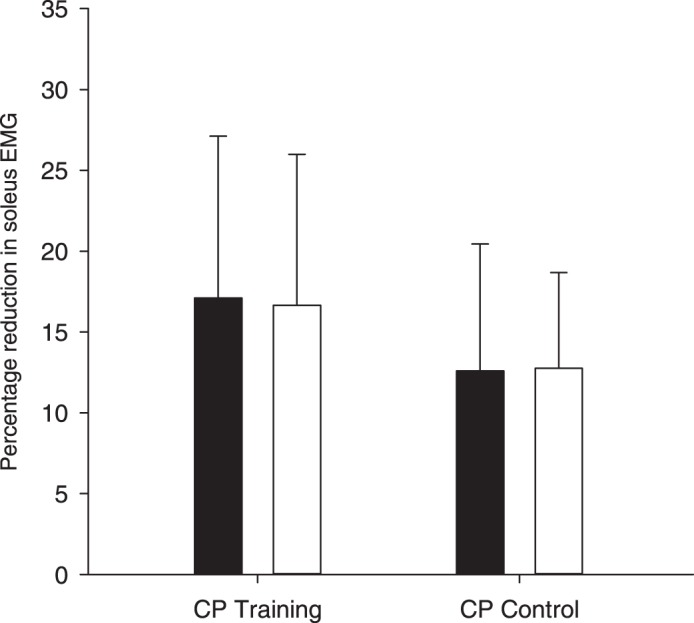
Reduction in soleus EMG activity following unloading of the plantar flexors before (filled bars) and after (open bars) 30 min of daily uphill treadmill gait training for 6 wk in the training group (n = 8) and the control group (n = 9). Error bars indicate SD.
DISCUSSION
Contribution of sensory feedback to ongoing soleus activity during push-off.
The primary finding of the present study is that an unload perturbation in late stance in adults with CP elicits a smaller reduction in soleus EMG activity compared with that in uninjured individuals. This can be interpreted as a reduced contribution of sensory feedback from peripheral afferents to soleus muscle activation during push-off. The implication is that adults with CP are forced to provide a larger part of their muscle activity during gait through descending motor commands in the absence of sufficiently efficient sensory feedback mechanisms. This is a likely explanation why adults with CP quickly experience exhaustion and fatigue during physical activity (Benner et al. 2017; Morgan and McGinley 2014; Neyroud et al. 2017).
The latency, duration, and size of the reduction in soleus EMG activity following ankle joint perturbation in late stance in the uninjured participants are similar to those found in previous studies (Grey et al. 2001, 2007; Mazzaro et al. 2007; Sinkjaer et al. 2000; Willerslev-Olsen et al. 2014a), confirming that we are in all likelihood looking at similar mechanisms. These previous studies have shown that reduced activity in load-sensitive group Ib and group II afferents are most likely responsible for the reduction in muscle activity when the ankle plantar flexors are unloaded in late stance (af Klint et al. 2010a, 2010b; Grey et al. 2007; Mazzaro et al. 2006). The implication of this is that sensory feedback in load-sensitive afferents contributes to the activation of the ankle plantar flexors in late stance and thus likely also contributes to the muscle activity involved in push-off (Grey et al. 2001, 2007). As shown by Sinkjaer et al. (2000) and Grey et al. (2007), length-sensitive afferents, such as group Ia afferents from muscle spindles, appear to make only a small contribution to the plantar flexor EMG activity, if any, at this time of the gait cycle.
The smaller reduction in soleus EMG activity following an unload perturbation in adults with CP then also suggests that these individuals may rely less on the contribution of sensory feedback in load-sensitive afferents at the time of push-off than do uninjured individuals. We found a significantly smaller average unload response in our population of adults with CP (average reduction in soleus EMG activity: 13.46%) compared with that in adults with stroke (average reduction in soleus EMG activity: 30%; Mazzaro et al. 2007), although gait speed and general impairment level, including the MAS score, were similar in the two populations. There may be at least two explanations for this.
One explanation is that adults with CP are unable to integrate sensory feedback with the central motor command during gait to the same extent as uninjured individuals and stroke patients. This may be a consequence of having a lesion of the central motor pathways early during development, which may have impeded the ability of sensory activity to be functionally integrated with central gait commands when gait function was established in the participants with CP. The observation that load-sensitive feedback is generally small in children, and especially in children with CP, is consistent with this (Willerslev-Olsen et al. 2014a). Additionally, the lesion is often diverse, with inconsistent appearance of impairments in persons with CP. Because of the early occurrence of the lesion during development of the brain, adaptive changes may also be more pronounced in this population compared with stroke patients. There are, therefore, several reasons why sensory feedback may be integrated differently in the two populations.
Another possible explanation is that mechanical alterations, such as contractures, may potentially alter how much load-sensitive afferents are stimulated during gait in persons with CP. Load on the muscle may be taken up by passive components of stiffness within the muscle, thereby reducing the load registration of the afferents. This is consistent with our observation of a negative correlation between the size of the unload response and the passive stiffness of the ankle joint (Fig. 4A). Hägglund and Wagner (2011) found that range of motion (ROM) in the ankle joint decreases during development in children with CP and may be a contributing factor for toe walking. Increased passive stiffness as part of contractures has been shown in children and adults with CP and is probably why reduced ROM is more pronounced in persons with CP (Geertsen et al. 2015; Willerslev-Olsen et al. 2014b). Increased stiffness in contracted muscles seems to be related to changes in connective tissue within the muscle (Smith et al. 2011). Mechanisms behind such changes are not clearly known but may be explained by decreased muscle growth during maturation in persons with CP (Gough and Shortland 2012; Shortland et al. 2001).
We also found a negative correlation between the contribution of sensory feedback and reflex-mediated stiffness (Fig. 4B), which is consistent with the idea that reflex hyperexcitability reflects different sensory pathways. The implication, as also pointed out by Mazzaro et al. (2007) and Willerslev-Olsen et al. (2014a), is that stretch reflex hyperexcitability (i.e., spasticity, especially when measured at rest) has little relevance for the functional contribution of sensory feedback to muscle activity around push-off during gait. In addition to this, the unload perturbations of the plantar flexors did not elicit any short-latency stretch reflexes in TA. In some cases, a long-latency stretch reflex appeared in TA following the perturbation. This reflex latency was around 100–120 ms after perturbation, which indicates that this activation is caused by a transcortical reflex or may be of voluntary origin (Christensen et al. 2000). Regardless of origin, reflex responses at such latencies are much later than the unload response (around 70 ms), and this is why these long-latency reflexes are unlikely to influence the unload response.
We cannot exclude the possibilities that plastic changes may have occurred in the participants with CP that affect the integration from Ia and cutaneous afferents. However, Sinkjaer et al. (2000) showed that the unload response in soleus EMG activity was unchanged after the common peroneal nerve innervating the dorsiflexors was blocked in uninjured individuals. As pointed out in their paper, this indicates that peripheral mediated reciprocal inhibition could not be responsible for the unload response in soleus. Additionally, the study also showed that blocking Ia afferents by ischemia did not change the unload response. In line with this, Grey et al. (2004) also showed that when transmission from cutaneous afferents of the foot and ankle was blocked with local anesthesia, the unload response in soleus was unchanged. This also indicates that even though strain on the tissue may be increased due to increased stiffness, this is unlikely to influence sensory activation and thus the unload response in the participants with CP. In summary, these studies provide evidence that group Ia and cutaneous afferents are responsible not for the unload response, but rather Ib and group II load-sensitive afferents (af Klint et al. 2010b; Grey et al. 2004; Sinkjaer et al. 2000).
Neither we nor Mazzaro et al. (2007) found a relation between the size of unload response and the MAS score (Fig. 4C), which may reflect the limitations of the MAS in evaluation of spasticity and muscle stiffness (Lorentzen et al. 2010). Despite these limitations, the MAS was used in the present study because it is one of the few clinical tools currently available to evaluate muscle stiffness.
Regardless of whether the small unload response in adults with CP is explained by altered central integration of sensory feedback or changes in the stiffness of the muscle-tendon-joint complex, our results suggest that adults with CP have to rely more heavily on non-sensory-driven, and hence less automatic, inputs to the spinal motor neurons during gait. Compensation may involve both spinal rhythm-generating networks driven from brain stem centers as well as descending motor tracts (Barthelemy and Nielsen 2010; Barthélemy et al. 2011; Drew et al. 2004; Jordan et al. 2008; Nielsen 2003; Petersen et al. 2012). This impaired utilization of automatic sensory-driven inputs combined with a relative increase in central drive may be assumed to contribute to the higher perceived effort of persons with CP during gait and other functional tasks and possibly also the experience of fatigue, which is a common complaint in adults with CP (Benner et al. 2017; Morgan and McGinley 2014; Neyroud et al. 2017).
The size of the unload response was significantly correlated with maximal gait speed overground and on the treadmill (Fig. 3), suggesting that the amount of sensory feedback and/or its integration with central motor commands to activate the spinal motor neurons is of functional significance for the ability to walk as quickly as possible. This raises the possibility that impaired sensory contribution to ankle plantar flexor muscle activity in late stance may be a factor for reduced push-off and thus reduced forward propulsion in adults with CP. It follows that attempts at reduction of sensory feedback through antispastic therapy may not be expected to facilitate gait function (Dietz and Sinkjaer 2007). Previous studies have even strongly indicated that antispastic medication such as baclofen and diazepam impedes neural plasticity necessary for motor (re)learning (Lapierre et al. 1987; Latash and Penn 1996; Willerslev-Olsen et al. 2011; Ziemann et al. 2001).
No training effect on the contribution of sensory feedback.
Our results also did not show any effect of 6 wk of daily uphill gait training on the contribution of sensory feedback to ankle plantar flexor muscle activity during push-off. As shown in Fig. 5, the unload response remained unaltered in the participants with CP after the training, although this training has been shown to reduce ankle joint stiffness and improve push-off velocity and gait speed (Lorentzen et al. 2016). This may be due to a reduced adaptive capacity of the central neural circuitries involved in controlling gait in adults with CP. Indeed, recent evidence suggests that it is harder to induce plastic changes in the neural drive to spinal motor neurons in children and young adults older than 10 yr of age (Willerslev-Olsen et al. 2015). Mechanical changes in the muscle-tendon-joint complex may also be so rigid that a longer training intervention is needed to induce changes that affect the load-sensitive sensory feedback.
Methodological considerations.
It is possible that the participants with CP walked closer to their maximal gait capacity than the uninjured participants. If the participants with CP had walked at a level of gait performance relative to their maximal capacity similar to that of the uninjured participants, the unload response would in all likelihood be different.
During the experiments the participants held onto the handrail (Fig. 1A). As pointed out by af Klint et al. (2010b), the amount of body weight support alters the loading on the triceps surae muscle and thus the inflow of sensory feedback. However, holding onto the handrails was necessary for safety reasons and to maintain balance in some of the participants with CP. To ensure comparability between the two populations, all participants were consequently instructed to hold on to the handrails. This did not involve weight support but merely helped to make some of the participants more comfortable when walking on the treadmill. Hand rail support is therefore likely to have influenced the data to a limited but equal extent in the two populations.
Conclusion and clinical implications.
The present findings show that adults with CP have less contribution of sensory feedback to ongoing soleus muscle activation during push-off compared with uninjured individuals. These results also suggest that exaggerated sensory activity is unlikely to contribute to impaired gait in adults with CP. Instead, increased passive stiffness around the ankle joint is likely to diminish sensory feedback during gait, and/or sensory feedback is less integrated with central motor commands in the activation of spinal motor neurons during gait, in adults with CP. As a consequence, muscle activation must to a larger extent rely on descending drive to the motor neurons, which is already decreased because of the cerebral lesion. This may explain subjective experienced fatigue in relation to physical activity and should lead to a reevaluation of the balance between functional benefits and adverse effects of antispastic therapy.
GRANTS
The work was supported by a grant from the Elsass foundation and from the Natural Sciences and Engineering Research Council of Canada (NSERC).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.J.B. and J.B.N. conceived and designed research; R.F.F., P.J., H.K., and J.L. performed experiments; R.F.F., P.J., L.J.B., and J.L. analyzed data; R.F.F., L.J.B., J.L., and J.B.N. interpreted results of experiments; R.F.F. and L.J.B. prepared figures; R.F.F. and J.B.N. drafted manuscript; P.J., H.K., L.J.B., and J.L. edited and revised manuscript; J.B.N. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Svend Geertsen and Martin Jorsal for valuable help with the experiments.
REFERENCES
- af Klint R, Cronin NJ, Ishikawa M, Sinkjaer T, Grey MJ. Afferent contribution to locomotor muscle activity during unconstrained overground human walking: an analysis of triceps surae muscle fascicles. J Neurophysiol 103: 1262–1274, 2010a. doi: 10.1152/jn.00852.2009. [DOI] [PubMed] [Google Scholar]
- af Klint R, Mazzaro N, Nielsen JB, Sinkjaer T, Grey MJ. Load rather than length sensitive feedback contributes to soleus muscle activity during human treadmill walking. J Neurophysiol 103: 2747–2756, 2010b. doi: 10.1152/jn.00547.2009. [DOI] [PubMed] [Google Scholar]
- af Klint R, Nielsen JB, Sinkjaer T, Grey MJ. Sudden drop in ground support produces force-related unload response in human overground walking. J Neurophysiol 101: 1705–1712, 2009. doi: 10.1152/jn.91175.2008. [DOI] [PubMed] [Google Scholar]
- Andersson C, Mattsson E. Adults with cerebral palsy: a survey describing problems, needs, and resources, with special emphasis on locomotion. Dev Med Child Neurol 43: 76–82, 2001. doi: 10.1017/S0012162201. [DOI] [PubMed] [Google Scholar]
- Barthélemy D, Grey MJ, Nielsen JB, Bouyer L. Involvement of the corticospinal tract in the control of human gait. Prog Brain Res 192: 181–197, 2011. doi: 10.1016/B978-0-444-53355-5.00012-9. [DOI] [PubMed] [Google Scholar]
- Barthelemy D, Nielsen JB. Corticospinal contribution to arm muscle activity during human walking. J Physiol 588: 967–979, 2010. doi: 10.1113/jphysiol.2009.185520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner JL, Hilberink SR, Veenis T, Stam HJ, van der Slot WM, Roebroeck ME. Long-term deterioration of perceived health and functioning in adults with cerebral palsy. Arch Phys Med Rehabil 98: 2196–2205, 2017. doi: 10.1016/j.apmr.2017.03.013. [DOI] [PubMed] [Google Scholar]
- Berger W, Altenmueller E, Dietz V. Normal and impaired development of children’s gait. Hum Neurobiol 3: 163–170, 1984. [PubMed] [Google Scholar]
- Christensen LO, Petersen N, Andersen JB, Sinkjaer T, Nielsen JB. Evidence for transcortical reflex pathways in the lower limb of man. Prog Neurobiol 62: 251–272, 2000. doi: 10.1016/S0301-0082(00)00007-1. [DOI] [PubMed] [Google Scholar]
- Criswell SR, Crowner BE, Racette BA. The use of botulinum toxin therapy for lower-extremity spasticity in children with cerebral palsy. Neurosurg Focus 21: e1, 2006. doi: 10.3171/foc.2006.21.2.2. [DOI] [PubMed] [Google Scholar]
- Dietz V, Berger W. Normal and impaired regulation of muscle stiffness in gait: a new hypothesis about muscle hypertonia. Exp Neurol 79: 680–687, 1983. doi: 10.1016/0014-4886(83)90032-8. [DOI] [PubMed] [Google Scholar]
- Dietz V, Duysens J. Significance of load receptor input during locomotion: a review. Gait Posture 11: 102–110, 2000. doi: 10.1016/S0966-6362(99)00052-1. [DOI] [PubMed] [Google Scholar]
- Dietz V, Quintern J, Berger W. Electrophysiological studies of gait in spasticity and rigidity. Evidence that altered mechanical properties of muscle contribute to hypertonia. Brain 104: 431–449, 1981. doi: 10.1093/brain/104.3.431. [DOI] [PubMed] [Google Scholar]
- Dietz V, Sinkjaer T. Spastic movement disorder: impaired reflex function and altered muscle mechanics. Lancet Neurol 6: 725–733, 2007. doi: 10.1016/S1474-4422(07)70193-X. [DOI] [PubMed] [Google Scholar]
- Drew T, Prentice S, Schepens B. Cortical and brainstem control of locomotion. Prog Brain Res 143: 251–261, 2004. doi: 10.1016/S0079-6123(03)43025-2. [DOI] [PubMed] [Google Scholar]
- Duysens J, Pearson KG. Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Res 187: 321–332, 1980. doi: 10.1016/0006-8993(80)90206-1. [DOI] [PubMed] [Google Scholar]
- Geertsen SS, Kirk H, Lorentzen J, Jorsal M, Johansson CB, Nielsen JB. Impaired gait function in adults with cerebral palsy is associated with reduced rapid force generation and increased passive stiffness. Clin Neurophysiol 126: 2320–2329, 2015. doi: 10.1016/j.clinph.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Gough M, Shortland AP. Could muscle deformity in children with spastic cerebral palsy be related to an impairment of muscle growth and altered adaptation? Dev Med Child Neurol 54: 495–499, 2012. doi: 10.1111/j.1469-8749.2012.04229.x. [DOI] [PubMed] [Google Scholar]
- Grey MJ, Ladouceur M, Andersen JB, Nielsen JB, Sinkjaer T. Group II muscle afferents probably contribute to the medium latency soleus stretch reflex during walking in humans. J Physiol 534: 925–933, 2001. doi: 10.1111/j.1469-7793.2001.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey MJ, Mazzaro N, Nielsen JB, Sinkjaer T. Ankle extensor proprioceptors contribute to the enhancement of the soleus EMG during the stance phase of human walking. Can J Physiol Pharmacol 82: 610–616, 2004. doi: 10.1139/y04-077. [DOI] [PubMed] [Google Scholar]
- Grey MJ, Nielsen JB, Mazzaro N, Sinkjaer T. Positive force feedback in human walking. J Physiol 581: 99–105, 2007. doi: 10.1113/jphysiol.2007.130088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägglund G, Wagner P. Spasticity of the gastrosoleus muscle is related to the development of reduced passive dorsiflexion of the ankle in children with cerebral palsy: a registry analysis of 2,796 examinations in 355 children. Acta Orthop 82: 744–748, 2011. doi: 10.3109/17453674.2011.618917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert GW, Pearson KG. Contribution of sensory feedback to the generation of extensor activity during walking in the decerebrate Cat. J Neurophysiol 81: 758–770, 1999. [DOI] [PubMed] [Google Scholar]
- Hof AL, Nauta J, van der Knaap ER, Schallig MA, Struwe DP. Calf muscle work and segment energy changes in human treadmill walking. J Electromyogr Kinesiol 2: 203–216, 1992. doi: 10.1016/1050-6411(92)90024-D. [DOI] [PubMed] [Google Scholar]
- Honeine JL, Schieppati M, Gagey O, Do MC. The functional role of the triceps surae muscle during human locomotion. PLoS One 8: e52943, 2013. doi: 10.1371/journal.pone.0052943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen R, Villien L, Egeland T, Stanghelle JK, Holm I. Locomotion skills in adults with cerebral palsy. Clin Rehabil 18: 309–316, 2004. doi: 10.1191/0269215504cr735oa. [DOI] [PubMed] [Google Scholar]
- Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res Brain Res Rev 57: 183–191, 2008. doi: 10.1016/j.brainresrev.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Lapierre Y, Bouchard S, Tansey C, Gendron D, Barkas WJ, Francis GS. Treatment of spasticity with tizanidine in multiple sclerosis. Can J Neurol Sci 14, Suppl: 513–517, 1987. doi: 10.1017/S0317167100038026. [DOI] [PubMed] [Google Scholar]
- Latash ML, Penn RD. Changes in voluntary motor control induced by intrathecal baclofen in patients with spasticity of different etiology. Physiother Res Int 1: 229–246, 1996. doi: 10.1002/pri.67. [DOI] [PubMed] [Google Scholar]
- Lorentzen J, Grey MJ, Crone C, Mazevet D, Biering-Sørensen F, Nielsen JB. Distinguishing active from passive components of ankle plantar flexor stiffness in stroke, spinal cord injury and multiple sclerosis. Clin Neurophysiol 121: 1939–1951, 2010. doi: 10.1016/j.clinph.2010.02.167. [DOI] [PubMed] [Google Scholar]
- Lorentzen J, Kirk H, Fernandez-Lago H, Frisk R, Scharff Nielsen N, Jorsal M, Nielsen JB. Treadmill training with an incline reduces ankle joint stiffness and improves active range of movement during gait in adults with cerebral palsy. Disabil Rehabil 39: 987–993, 2016. doi: 10.1080/09638288.2016.1174745. [DOI] [PubMed] [Google Scholar]
- MacKay WA, Bonnet M. CNV, stretch reflex and reaction time correlates of preparation for movement direction and force. Electroencephalogr Clin Neurophysiol 76: 47–62, 1990. doi: 10.1016/0013-4694(90)90057-Q. [DOI] [PubMed] [Google Scholar]
- Mazzaro N, Grey MJ, do Nascimento OF, Sinkjaer T. Afferent-mediated modulation of the soleus muscle activity during the stance phase of human walking. Exp Brain Res 173: 713–723, 2006. doi: 10.1007/s00221-006-0451-5. [DOI] [PubMed] [Google Scholar]
- Mazzaro N, Nielsen JF, Grey MJ, Sinkjaer T. Decreased contribution from afferent feedback to the soleus muscle during walking in patients with spastic stroke. J Stroke Cerebrovasc Dis 16: 135–144, 2007. doi: 10.1016/j.jstrokecerebrovasdis.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Morgan P, McGinley J. Gait function and decline in adults with cerebral palsy: a systematic review. Disabil Rehabil 36: 1–9, 2014. doi: 10.3109/09638288.2013.775359. [DOI] [PubMed] [Google Scholar]
- Morgan P, Murphy A, Opheim A, McGinley J. Gait characteristics, balance performance and falls in ambulant adults with cerebral palsy: An observational study. Gait Posture 48: 243–248, 2016. doi: 10.1016/j.gaitpost.2016.06.015. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Gravel D, Arsenault AB, Bourbonnais D. Plantarflexor weakness as a limiting factor of gait speed in stroke subjects and the compensating role of hip flexors. Clin Biomech (Bristol, Avon) 14: 125–135, 1999. doi: 10.1016/S0268-0033(98)00062-X. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J Biomech 34: 1387–1398, 2001. doi: 10.1016/S0021-9290(01)00105-1. [DOI] [PubMed] [Google Scholar]
- Neyroud D, Armand S, De Coulon G, Dias Da Silva SR, Maffiuletti NA, Kayser B, Place N. Plantar flexor muscle weakness and fatigue in spastic cerebral palsy patients. Res Dev Disabil 61: 66–76, 2017. doi: 10.1016/j.ridd.2016.12.015. [DOI] [PubMed] [Google Scholar]
- Nielsen JB. How we walk: central control of muscle activity during human walking. Neuroscientist 9: 195–204, 2003. doi: 10.1177/1073858403009003012. [DOI] [PubMed] [Google Scholar]
- Noël M, Cantin B, Lambert S, Gosselin CM, Bouyer LJ. An electrohydraulic actuated ankle foot orthosis to generate force fields and to test proprioceptive reflexes during human walking. IEEE Trans Neural Syst Rehabil Eng 16: 390–399, 2008. doi: 10.1109/TNSRE.2008.926714. [DOI] [PubMed] [Google Scholar]
- Olney SJ, MacPhail HE, Hedden DM, Boyce WF. Work and power in hemiplegic cerebral palsy gait. Phys Ther 70: 431–438, 1990. doi: 10.1093/ptj/70.7.431. [DOI] [PubMed] [Google Scholar]
- Opheim A, McGinley JL, Olsson E, Stanghelle JK, Jahnsen R. Walking deterioration and gait analysis in adults with spastic bilateral cerebral palsy. Gait Posture 37: 165–171, 2013. doi: 10.1016/j.gaitpost.2012.06.032. [DOI] [PubMed] [Google Scholar]
- Parkes J, McCullough N, Madden A. To what extent do children with cerebral palsy participate in everyday life situations? Health Soc Care Community 18: 304–315, 2010. [DOI] [PubMed] [Google Scholar]
- Pearson KG. Generating the walking gait: role of sensory feedback. Prog Brain Res 143: 123–129, 2004. doi: 10.1016/S0079-6123(03)43012-4. [DOI] [PubMed] [Google Scholar]
- Petersen TH, Farmer SF, Kliim-Due M, Nielsen JB. Failure of normal development of central drive to ankle dorsiflexors relates to gait deficits in children with cerebral palsy. J Neurophysiol 109: 625–639, 2013. doi: 10.1152/jn.00218.2012. [DOI] [PubMed] [Google Scholar]
- Petersen TH, Kliim-Due M, Farmer SF, Nielsen JB. Childhood development of common drive to a human leg muscle during ankle dorsiflexion and gait. J Physiol 588: 4387–4400, 2010. doi: 10.1113/jphysiol.2010.195735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen TH, Willerslev-Olsen M, Conway BA, Nielsen JB. The motor cortex drives the muscles during walking in human subjects. J Physiol 590: 2443–2452, 2012. doi: 10.1113/jphysiol.2012.227397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche N, Pradon D, Cosson J, Robertson J, Marchiori C, Zory R. Categorization of gait patterns in adults with cerebral palsy: a clustering approach. Gait Posture 39: 235–240, 2014. doi: 10.1016/j.gaitpost.2013.07.110. [DOI] [PubMed] [Google Scholar]
- Rose J, McGill KC. Neuromuscular activation and motor-unit firing characteristics in cerebral palsy. Dev Med Child Neurol 47: 329–336, 2005. doi: 10.1017/S0012162205000629. [DOI] [PubMed] [Google Scholar]
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, Dan B, Jacobsson B. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl 109: 8–14, 2007. [PubMed] [Google Scholar]
- Sheean G. The pathophysiology of spasticity. Eur J Neurol 9, Suppl 1: 3–9, 2002. doi: 10.1046/j.1468-1331.2002.0090s1003.x. [DOI] [PubMed] [Google Scholar]
- Shikako-Thomas K, Dahan-Oliel N, Shevell M, Law M, Birnbaum R, Rosenbaum P, Poulin C, Majnemer A. Play and be happy? Leisure participation and quality of life in school-aged children with cerebral palsy. Int J Pediatr 2012: 387280, 2012. doi: 10.1155/2012/387280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortland AP, Harris CA, Gough M, Robinson RO. Architecture of the medial gastrocnemius in children with spastic diplegia. Dev Med Child Neurol 43: 796–801, 2001. doi: 10.1017/S001216220100144X. [DOI] [PubMed] [Google Scholar]
- Sinkjaer T, Andersen JB, Ladouceur M, Christensen LO, Nielsen JB. Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. J Physiol 523: 817–827, 2000. doi: 10.1111/j.1469-7793.2000.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkjaer T, Magnussen I. Passive, intrinsic and reflex-mediated stiffness in the ankle extensors of hemiparetic patients. Brain 117: 355–363, 1994. doi: 10.1093/brain/117.2.355. [DOI] [PubMed] [Google Scholar]
- Smith LR, Lee KS, Ward SR, Chambers HG, Lieber RL. Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J Physiol 589: 2625–2639, 2011. doi: 10.1113/jphysiol.2010.203364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerslev-Olsen M, Andersen JB, Sinkjaer T, Nielsen JB. Sensory feedback to ankle plantar flexors is not exaggerated during gait in spastic hemiplegic children with cerebral palsy. J Neurophysiol 111: 746–754, 2014a. doi: 10.1152/jn.00372.2013. [DOI] [PubMed] [Google Scholar]
- Willerslev-Olsen M, Lorentzen J, Nielsen JB. Gait training reduces ankle joint stiffness and facilitates heel strike in children with Cerebral Palsy. NeuroRehabilitation 35: 643–655, 2014b. [DOI] [PubMed] [Google Scholar]
- Willerslev-Olsen M, Lorentzen J, Sinkjaer T, Nielsen JB. Passive muscle properties are altered in children with cerebral palsy before the age of 3 years and are difficult to distinguish clinically from spasticity. Dev Med Child Neurol 55: 617–623, 2013. doi: 10.1111/dmcn.12124. [DOI] [PubMed] [Google Scholar]
- Willerslev-Olsen M, Lundbye-Jensen J, Petersen TH, Nielsen JB. The effect of baclofen and diazepam on motor skill acquisition in healthy subjects. Exp Brain Res 213: 465–474, 2011. doi: 10.1007/s00221-011-2798-5. [DOI] [PubMed] [Google Scholar]
- Willerslev-Olsen M, Petersen TH, Farmer SF, Nielsen JB. Gait training facilitates central drive to ankle dorsiflexors in children with cerebral palsy. Brain 138: 589–603, 2015. doi: 10.1093/brain/awu399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GP, Schache AG, Morris ME. Mobility after traumatic brain injury: relationships with ankle joint power generation and motor skill level. J Head Trauma Rehabil 28: 371–378, 2013. doi: 10.1097/HTR.0b013e31824a1d40. [DOI] [PubMed] [Google Scholar]
- Winter DA. Biomechanical motor patterns in normal walking. J Mot Behav 15: 302–330, 1983. doi: 10.1080/00222895.1983.10735302. [DOI] [PubMed] [Google Scholar]
- Winters TF Jr, Gage JR, Hicks R. Gait patterns in spastic hemiplegia in children and young adults. J Bone Joint Surg Am 69: 437–441, 1987. doi: 10.2106/00004623-198769030-00016. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain 124: 1171–1181, 2001. [DOI] [PubMed] [Google Scholar]