Abstract
We assessed the risk of chronic kidney disease (CKD) in chronic hepatitis C virus (HCV)‐infected patients and the incidence reduction of CKD after receipt of HCV treatment. We also evaluated the risk of membranoproliferative glomerulonephritis (MPGN) and cryoglobulinemia in chronic HCV patients. A retrospective cohort analysis of the Truven Health MarketScan Database (2008‐2015) in the United States was conducted. In a cohort of 56,448 HCV‐infected patients and 169,344 propensity score (1:3)–matched non‐HCV patients, we examined the association of HCV infection with the incidence of CKD. Of 55,818 HCV patients, 6.6 % (n = 3666), 6.3% (n = 3534), and 8.3% (n = 4628) patients received either interferon‐based dual, triple, or all‐oral direct acting antiviral agent therapy, respectively, whereas 79% of patients did not receive any HCV treatment. Cox proportional hazards models were used to compare the risk of developing CKD in HCV patients compared with non‐HCV patients and treated patients compared with untreated HCV patients. In a multivariate time‐varying Cox regression model, HCV‐infected patients had a 27% increased risk of CKD compared with non‐HCV patients (hazard ratio [HR], 1.27; 95% confidence interval [CI], 1.18‐1.37). Among HCV patients, individuals who received the minimally effective HCV treatment for dual, triple, or all‐oral therapy had a 30% decreased risk of developing CKD (HR, 0.70; 95% CI, 0.55‐0.88). In addition, HCV‐infected patients experienced a twofold and a nearly 17‐fold higher risk of MPGN (HR, 2.23; 95% CI, 1.84‐2.71) and cryoglobulinemia (HR, 16.91; 95% CI, 12.00‐23.81) respectively, compared with non‐HCV patients. Conclusion: HCV‐infected individuals in the United States are at greater risk of developing CKD, MPGN, and cryoglobulinemia. Minimally effective treatment of HCV infection can prevent the development of CKD, although the association was not significant for all‐oral therapy. (Hepatology 2018;67:492‐504).
Abbreviations
- ACEI
angiotensin‐converting‐enzyme inhibitor
- ARB
angiotensin II receptor blocker
- CKD
chronic kidney disease
- CI
confidence interval
- DAA
direct acting antiviral agent
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- HR
hazard ratio
- ICD‐9‐CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- MPGN
membranoproliferative glomerulonephritis
- PS
propensity score
The burden of fatal liver disease is increasing in the estimated 3.2 million adults in the United States who are chronically infected with hepatitis C virus (HCV).1 Furthermore, chronic HCV infection is associated with extrahepatic manifestations, reported in up to 74% of patients, which may be present long before advanced liver disease presents itself and responsible for non–liver‐related deaths.2, 3, 4, 5
Chronic kidney disease (CKD) is one of the more common extrahepatic manifestations present in patients with chronic HCV; however, reports on the risk of CKD in the chronically infected HCV population are inconsistent within the United States.6, 7, 8, 9, 10 Two recent studies conducted in US Veteran populations assessed the association of chronic HCV infection with the development/progression of CKD and reported divergent results.6, 9, 10 Molnar et al.6 found that chronic HCV was associated with higher incidence of decreased kidney function, whereas Rogal et al.10 concluded that chronic HCV was associated with decreased incidence of CKD. Two meta‐analyses determined that patients with HCV had a 23%‐43% greater risk of presenting with CKD,11, 12 whereas another meta‐analysis found that HCV was not associated with reduced glomerular filtration rate.8
The most common HCV‐related nephropathy is membranoproliferative glomerulonephritis (MPGN), usually in the context of cryoglobulinemia.8, 13, 14, 15 Mixed cryoglobulinemia represents 60%‐75% of all cryoglobulinemias,16 leading to clinical manifestations ranging from the mixed cryoglobulinemia syndrome to more serious lesions with neurologic and kidney involvement.17 Recently, two studies reported the prevalence of MPGN (0.3%) and cryoglobulinemia (0.4%‐0.9%) in chronically HCV‐infected patients in the United States.18, 19 However, there is limited evidence regarding the incidence of these renal manifestations in HCV patients.20, 21
Until late 2013, interferon and ribavirin were the main components of HCV treatment. Despite the positive effects on slowing the renal disease progression, supported by recent Taiwanese studies,22, 23, 24 interferon and ribavirin treatment carries substantial side effects, leading to very poor adherence and relatively low cure rates.25, 26, 27, 28, 29, 30 In 2014, the US Food and Drug Administration approved the first all‐oral direct acting antiviral agents (DAAs), which have revolutionized the HCV treatment landscape as a result of excellent adherence and very high cure rates (>95%) in as little as 8 weeks even for patients who are very difficult to treat.28, 29, 30 However, it is unclear whether the new DAAs carry an improvement in renal function and or reduce the incidence of CKD among chronically infected HCV patients residing in the United States.
Therefore, the aims of this study were to 1) determine the incidence of CKD among chronically HCV‐infected beneficiaries enrolled in a large health care plan in the United States, 2) determine the impact of treatment on the CKD incident rate in chronically HCV‐ infected patients within United States, and 3) determine the incidence of MPGN and cryoglobulinemia in chronically HCV‐infected patients.
Patients and Methods
DATA SOURCE
We conducted a retrospective cohort study using the Truven Health Analytic MarketScan Commercial and Medicare Supplemental databases (January 2008 through August 2015, prior to the implementation of International Classification of Diseases, Tenth Revision codes). This 8‐year nationwide administrative claims database contains person‐level information of diagnoses, procedures, and prescriptions for over 100 million individuals in the commercial dataset and 10 million individuals in the Medicare Supplement database. This database captures health care utilization and enrollment records across all settings, including physician outpatient office visits, hospital stays, and pharmacy claims. The study population consisted of employees, dependents, and retirees with employer‐sponsored or Medicare Supplemental insurance plans. Institutional review board approval was obtained from the University of Florida.
STUDY POPULATION
Identification of HCV and Non‐HCV Patients (HCV vs. Non‐HCV Cohorts)
Patients with newly diagnosed chronic HCV were identified using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes 070.44, 070.54, 070.70, 070.71, and V02.62. A patient was determined to be infected with chronic HCV if they had one inpatient chronic HCV diagnosis or two outpatient diagnoses of HCV on separate days within 1 year. The first diagnosis was used as the index date. To establish a non‐HCV control group, we selected 20 non‐HCV patients matched for age, sex, and calendar year for each chronic HCV patient. For non–HCV patients, we randomly selected one of their medical service dates as the index date. Patients were included if they were 18 years old and continuously enrolled in the health plan 1 year before and 6 months after the index date. Patients who had a diagnosis of CKD before the index date were excluded. Furthermore, for each chronic HCV patient, three non‐HCV patients were matched using the propensity score (PS) that was calculated to adjust for the baseline differences in risk factors for CKD between HCV and non‐HCV groups. The PS was estimated using logistic regression‐based baseline demographic variables including age and gender, and medical conditions reported in the literature associated with chronic HCV and CKD, including diabetes, hypertension, dyslipidemia, chronic obstructive pulmonary disease, coronary artery disease, peripheral vascular disease, cerebrovascular disease, and heart failure identified by ICD‐9‐CM codes, as well as disease‐modifying medications including angiotensin‐converting‐enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs).
Identification of HCV Treatment Groups According to Antiviral Treatment (Treated vs. Nontreated HCV Patients)
The chronic HCV patients were further categorized based on the receipt, type, and duration of HCV treatment using pharmacy claims for HCV treatment. For this analysis, we excluded patients who had undergone HCV therapy before the index date. HCV treatments included three classes: 1) dual therapy, a combination therapy of interferon and ribavirin (interferon alpha, interferon beta, peg‐ interferon alpha‐2a or peg‐ interferon alpha‐2b with or without ribavirin); 2) triple therapy, a combination of boceprevir, telaprevir, sofosbuvir, or simeprevir plus peg‐interferon and ribavirin; and 3) all‐oral therapy, which included ledipasvir/sofosbuvir, sofosbuvir with ribavirin and ombitasvir/paritaprevir/ritonavir, and dasabuvir with or without ribavirin.31
Based on the receipt and duration of treatment received, we classified patients into three different exposure statuses: 1) no treatment, defined as patients who were not exposed to any HCV treatments; 2) minimum effectively treated, defined as patients who received one of three HCV therapeutic treatment regimens prescribed as at least 16 weeks of dual therapy,24 8‐12 weeks of triple therapy,28, 29 or 8 weeks of all‐oral therapy30; and 3) insufficiently treated, defined as patients who received some treatment but did not meet the criteria for minimum effectively treated yet.
STUDY OUTCOMES
The primary outcome was a diagnosis of CKD stages 3‐5. The ICD‐9‐CM codes of 585.3, 585.4, and 585.5 were used to identify CKD cases.32 CKD was considered to be diagnosed if there was one inpatient or two separate outpatient claims for CKD within 1 year. The earliest date of CKD diagnosis was defined as the date of outcome. Follow‐up started from the index date and continued until study outcome, end of enrollment, or August 31, 2015, whichever came first. The secondary outcomes were the investigations of the renal conditions of nephrotic syndrome or MPGN (ICD‐9‐CM codes 581.0, 581.1, 581.2, 581.81, 581.89, 581.9, or V13.03) and cryoglobulinemia (ICD‐9‐CM code 273.2) within the chronically infected HCV adult population.18, 19
STATISTICAL ANALYSIS
Baseline characteristics were compared between HCV and non‐HCV cohorts using a t test for continuous variables and chi‐square tests for categorical variables. After PS matching, the standardized difference was used to check the balance between two groups, and 0.2 was defined as the threshold to determine statistically significant differences.33, 34 The number of CKD events and person‐years were determined for each group and subsequently used to calculate the incidence rates of CKD (number of events/1000 person‐years). We then stratified CKD by age, sex, diabetes, and cirrhosis status, as previous studies have suggested that there was an effect modification on the rate of CKD among these subpopulations.35, 36 A Cox proportional hazards regression model was used to compare the risk of developing CKD, MPGN, and cryoglobulinemia between HCV and non‐HCV cohorts. A Cox proportional hazards regression model with time‐dependent covariates was also employed in a sensitivity analysis (Model 1) (Supporting Table S1). The covariates were adjusted for alcohol/drug abuse disorders, human immunodeficiency virus (HIV), hepatitis A virus, hepatitis B virus, cirrhosis, decompensated cirrhosis, and hepatocellular carcinoma in addition to covariates adjusted in PS matching. We did not match for presence of liver disease, an effect mediator of HCV rather than a confounder, and variables that were strongly associated with HCV but weakly associated with CKD (e.g., alcohol/drug abuse) and adjusted for regression models, because previous studies found that incorporating these variables can lead to less successful matching and increased variance.37, 38, 39 However, we performed sensitivity analyses with matching/adjustment for all factors including liver disease and other covariates.
To assess the association between HCV treatment and the risk of developing CKD among patients infected with HCV, a time‐dependent exposure analysis was performed. The number of CKD events and person‐years were summarized for each treatment status. Subgroup analyses were performed by type of HCV treatments including dual, triple, and all‐oral therapy. Cox regression models with time‐dependent covariates were used to adjust for all covariates mentioned in the previous analysis, as well as contraindications to pegylated interferon and ribavirin, which included schizophrenia, depression, seizures, pregnancy, transplantation, anemia, and retinopathy (Model 2) (Supporting Table S1). All the analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
PATIENT CHARACTERISTICS
We identified 56,489 HCV patients and 847,113 non‐HCV patients between January 2008 and August 2015 (Fig. 1). Table 1 summarizes the baseline demographic characteristics, comorbid conditions, and medication use between two cohorts before and after PS matching. After PS matching, we identified 56,448 HCV patients and 169,344 non‐HCV patients. In the PS‐matched groups, the patients' demographic characteristics, including age (mean age: 55), sex (60% male), and several comorbid conditions (e.g., hypertension, dyslipidemia, diabetes mellitus) were comparable between two groups. However, heart failure (standardized difference = 0.04) and peripheral vascular disease (standardized difference = 0.04) were slightly more prevalent in the HCV patients compared with the non‐HCV patients, but the differences were still within the threshold of acceptable imbalance.40 The presence of liver disease, not included in PS matching but adjusted in regression models, was more prevalent in HCV patients compared with non‐HCV patients.
Figure 1.
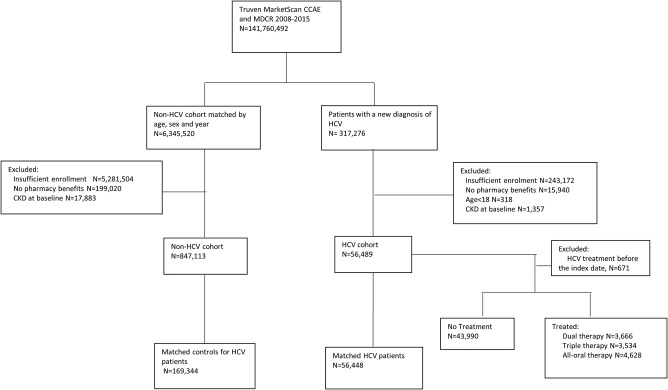
Flow chart of the cohort creation. Abbreviations: CCAE, Commercial Claims and Encounters; CKD, chronic kidney disease; HCV, hepatitis C virus; MDCR, Medicare Supplemental and Coordination of Benefits.
Table 1.
Baseline Characteristics Before and After Propensity Score (PS) Matching
| Before PS Matching | After PS Matchinga | |||||
|---|---|---|---|---|---|---|
| Patient Characteristics | HCV cohort (n = 56,489) | Non‐HCV cohortb (n = 847,113) | Standardized difference, %c | HCV cohort (n = 56,448) | Non‐HCV cohort (n = 169,344) | Standardized difference, %c |
| PS‐adjusted variables | ||||||
| Median age, years (IQR) | 55 (48, 59) | 54 (48, 59) | 0.03 | 55 (48, 59) | 54 (48, 59) | 0.01 |
| Sex, n (%) | 0.03 | ‐0.01 | ||||
| Men | 34,106 (60.37) | 499,756 (59.00) | 34,082 (60.38) | 103,149 (60.91) | ||
| Women | 22,383 (39.63) | 347,357 (41.00) | 22,366 (39.62) | 66,195 (39.09) | ||
| Comorbidities, n (%) | ||||||
| Hypertension | 21,141 (37.42) | 332,581 (39.26) | −0.04 | 21,122 (37.42) | 62,468 (36.89) | 0.01 |
| Dyslipidemia | 140,46 (24.87) | 331,064 (39.08) | −0.31 | 140,34 (24.86) | 42,143 (24.89) | ‐0.00 |
| Diabetes mellitus | 9237 (16.35) | 147,461 (17.41) | −0.03 | 9231 (16.35) | 27,508 (16.24) | 0.00 |
| COPD | 6891 (12.20) | 105,505 (12.45) | −0.01 | 6881 (12.19) | 19,957 (11.78) | 0.01 |
| Heart Failure | 1845 (3.27) | 30,010 (3.54) | −0.02 | 1842 (3.26) | 4412 (2.61) | 0.04 |
| Peripheral vascular disease | 2431 (4.30) | 35,049 (4.14) | 0.01 | 2430 (4.30) | 6048 (3.57) | 0.04 |
| Cerebrovascular disease | 2245 (3.97) | 40,506 (4.78) | −0.04 | 2243 (3.97) | 5681 (3.35) | 0.03 |
| Coronary artery disease | 4097 (7.25) | 83,462 (9.85) | −0.09 | 4092 (7.25) | 10,948 (6.46) | 0.03 |
| Baseline medication use, n (%) | ||||||
| ACEIs | 3003 (5.32) | 58,520 (6.91) | −0.07 | 3001 (5.32) | 8875 (5.24) | 0.00 |
| ARBs | 9969 (17.65) | 155,696 (18.38) | −0.02 | 9955 (17.64) | 29,175 (17.23) | 0.01 |
| PS‐unadjusted variablesb | ||||||
| Comorbidities, n (%) | ||||||
| HIV | 1187 (2.10) | 2749 (0.32) | 0.16 | 1187 (2.10) | 552 (0.33) | 0.16 |
| Hepatitis A | 210 (0.37) | 187 (0.02) | 0.08 | 210 (0.37) | 31 (0.02) | 0.08 |
| Hepatitis B | 1193 (2.11) | 1416 (0.17) | 0.18 | 1190 (2.11) | 297 (0.18) | 0.18 |
| Cirrhosis | 2509 (4.44) | 2440 (0.29) | 0.28 | 2505 (4.44) | 509 (0.30) | 0.27 |
| Decompensated cirrhosis | 1888 (3.34) | 6515 (0.77) | 0.18 | 1886 (3.34) | 1280 (0.76) | 0.18 |
| Hepatocellular carcinoma | 368 (0.65) | 516 (0.06) | 0.10 | 367 (0.65) | 119 (0.07) | 0.10 |
| Alcohol abuse | 3138 (5.56) | 13,288 (1.57) | 0.22 | 3135 (5.55) | 2578 (1.52) | 0.22 |
| Drug abuse | 8260 (14.62) | 45,287 (5.35) | 0.31 | 8257 (14.63) | 8436 (4.98) | 0.33 |
Abbreviations: ACEIs, angiotensin‐converting‐enzyme inhibitors; ARBs, angiotensin II receptor blockers; COPD, chronic obstructive pulmonary disease; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range; PS, propensity score,.
Up to 20 controls without HCV infection were matched for age, sex, and index date with each HCV patient.
PS matching did not include liver‐related comorbidities, alcohol abuse, drug abuse, or medication use; instead, these covariates were adjusted as time‐dependent covariates in the Cox regression model.
Difference in means or proportions divided by standard error; imbalance defined as absolute value >0.20 (small effect size)
RISK OF CKD BETWEEN HCV AND NON‐HCV GROUPS
We identified 1455 new CKD cases in the HCV group (n = 56,448) and 2518 new CKD cases in the non‐HCV group (n = 169,344). The crude incidence rate of CKD was 10.36 per 1000 person‐years in HCV and 5.72 per 1000 person‐years in non‐HCV groups. The Cox proportional hazards regression model indicated that HCV patients had a 57% (hazard ratio [HR], 1.57; 95% confidence interval [CI], 1.47‐1.68) increased risk of developing CKD after adjusting for demographics, baseline covariates, and use of ACEIs and ARBs.
Sensitivity analysis using the Cox regression model with time‐dependent covariates, which took the change of comorbidities and medication use during follow‐up into consideration, revealed that HCV‐infected patients had a 27% increased risk of CKD (HR, 1.27; 95% CI, 1.18‐1.37). In a subgroup analysis, when stratified by different age groups, we found that the association between HCV and CKD was more significant among young adults (age 18‐49 years; HR, 1.47; 95% CI, 1.13‐1.90) compared with the elderly population (age ≥60 years; HR, 1.19; 95% CI, 1.06‐1.33).
This association appeared to be significant among both men and women and patients with and without diabetes, but it was not significant among patients diagnosed with cirrhosis (Table 2). In a sensitivity analysis, when adjusted after PS matching including all covariates associated with HCV and CKD (52,185 HCV patients and 156,567 non‐HCV patients), we found quantitatively similar results (HR, 1.28; 95% CI, 1.19‐1.38) (Supporting Tables S2 and S3).
Table 2.
Incidence Rate and Hazard Ratio (HR) for CKD in the HCV and Non‐HCV Cohorts
| Study Population | HCV Status | No. of Patients | Person‐Years | No. of CKD Events | Mean Time to CKD Event (Months) | Crude Incidence of CKDa | Adjusted HR of CKD (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Baseline Covariates | Baseline and Time‐Varying Covariates | |||||||
| All patients | HCV | 56,448 | 140,468 | 1455 | 20.53 | 10.36 | 1.57 (1.47‐1.68) | 1.27 (1.18‐1.37) |
| Non‐HCV | 169,344 | 440,495 | 2518 | 22.37 | 5.72 | Reference | Reference | |
| Age, years | ||||||||
| 18‐49 | HCV | 15,869 | 37,643 | 139 | 19.53 | 3.69 | 1.87 (1.49‐2.35) | 1.47 (1.13‐1.90) |
| Non‐HCV | 48,044 | 122,666 | 216 | 22.78 | 1.76 | Reference | Reference | |
| 50‐59 | HCV | 27,344 | 72,630 | 685 | 21.95 | 9.43 | 1.75 (1.58‐1.93) | 1.32 (1.18‐1.47) |
| Non‐HCV | 82,304 | 227,193 | 1086 | 22.81 | 4.78 | Reference | Reference | |
| ≥60 | HCV | 13,235 | 30,194 | 631 | 19.22 | 20.90 | 1.38 (1.25‐1.53) | 1.19 (1.06‐1.33) |
| Non‐HCV | 38,996 | 90,636 | 1216 | 21.91 | 13.42 | Reference | Reference | |
| Sex | ||||||||
| Men | HCV | 34,082 | 84,721 | 956 | 20.11 | 11.28 | 1.59 (1.46‐1.73) | 1.26 (1.14‐1.38) |
| Non‐HCV | 103,149 | 268,076 | 1673 | 22.67 | 6.24 | Reference | Reference | |
| Women | HCV | 22,366 | 55,747 | 499 | 21.34 | 8.95 | 1.54 (1.37‐1.74) | 1.26 (1.10‐1.43) |
| Non‐HCV | 66,195 | 172,418 | 845 | 21.77 | 4.90 | Reference | Reference | |
| Cirrhosis | HCV | 2505 | 5561 | 183 | 20.04 | 32.90 | 0.89 (0.63‐1.26) | 0.91 (0.64‐1.29) |
| Non‐HCV | 509 | 1068 | 39 | 15.55 | 36.52 | Reference | Reference | |
| No cirrhosis | HCV | 53,945 | 134,907 | 1272 | 22.48 | 9.43 | 1.59 (1.48‐1.70) | 1.29 (1.20‐1.39) |
| Non‐HCV | 168,835 | 439,427 | 2479 | 20.60 | 5.64 | Reference | Reference | |
| Diabetes | HCV | 9231 | 21,655 | 646 | 19.36 | 29.8 | 1.54 (1.40‐1.71) | 1.23 (1.10‐1.38) |
| Non‐HCV | 27,508 | 69,534 | 1218 | 21.49 | 17.51 | Reference | Reference | |
| Non‐diabetes | HCV | 47,217 | 118,813 | 809 | 21.47 | 6.81 | 1.67 (1.53‐1.83) | 1.32 (1.19‐1.46) |
| Non‐HCV | 141,836 | 370,961 | 1300 | 23.20 | 3.50 | Reference | Reference | |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; HCV, hepatitis C virus; HR, hazard ratio.
Per 1000 person‐years.
RISK OF CKD AMONG HCV PATIENTS WHO RECEIVED MINIMALLY EFFECTIVE TREATMENT, INSUFFICIENT TREATMENT, AND NO TREATMENT
Of 55,818 HCV‐infected patients, 43,990 patients (79%) did not receive any HCV treatment. The remaining 11,828 HCV patients received HCV treatment, including 3666 who received dual therapy, 3534 who received triple therapy, and 4628 who received all‐oral DAA therapy (Table 3). Patients who received the all‐oral DAA regimens were older and had significantly more advanced liver disease (i.e., cirrhosis, decompensated cirrhosis) and comorbidities (e.g., hypertension, diabetes, coronary artery disease, HIV) than the patients in either the dual or triple therapy groups, respectively. However, although the patients in the no treatment group had similar advanced liver disease compared with patients in the all‐oral therapy group, they were more likely to have alcohol/drug abuse and contraindications to pegylated interferon and ribavirin such as schizophrenia, depression, and pregnancy than patients in any of the treatment groups.
Table 3.
Demographics and Clinical Characteristics of HCV‐Infected Patients by Receipt and Type of HCV Treatment
| Patient Characteristics | Dual Therapy (n = 3666) | Triple Therapy (n = 3534) | All‐Oral Therapy (n = 4628) | No Treatment (n = 43,990) | P |
|---|---|---|---|---|---|
| Median age, years (IQR) | 52 (47 ,57) | 54 (49, 58) | 56 (51 ,60) | 55 (48 ,59) | <0.001 |
| Sex, n (%) | |||||
| Men | 2224 (60.67) | 2267 (64.15) | 2934 (63.40) | 26,242 (59.65) | <0.001 |
| Women | 1442 (39.33) | 1267 (35.85) | 1694 (36.60) | 17,748 (40.35) | |
| Comorbidities, n (%) | |||||
| Hypertension | 1187 (32.38) | 1246 (35.26) | 1842 (39.80) | 16679 (37.92) | <0.001 |
| Dyslipidemia | 832 (22.70) | 765 (21.65) | 1112 (24.03) | 11186 (25.43) | <0.001 |
| Diabetes | 457 (12.47) | 514 (14.54) | 794 (17.16) | 7371 (16.76) | <0.001 |
| COPD | 363 (9.90) | 283 (8.01) | 446 (9.64) | 5750 (13.07) | <0.001 |
| Heart failure | 49 (1.34) | 51 (1.44) | 95 (2.05) | 1640 (3.73) | <0.001 |
| Peripheral vascular disease | 99 (2.70) | 79 (2.24) | 190 (4.11) | 2045 (4.65) | <0.001 |
| Cerebrovascular disease | 91 (2.48) | 75 (2.12) | 147 (3.18) | 1914 (4.35) | <0.001 |
| Coronary artery disease | 185 (5.05) | 134 (3.79) | 288 (6.22) | 3453 (7.85) | <0.001 |
| HIV | 76 (2.07) | 45 (1.27) | 106 (2.29) | 960 (2.11) | 0.006 |
| Hepatitis A | 22 (0.60) | 19 (0.54) | 18 (0.39) | 148 (0.34) | 0.025 |
| Hepatitis B | 46 (1.25) | 32 (0.91) | 37 (0.80) | 1063 (2.42) | <0.001 |
| Cirrhosis | 106 (2.89) | 97 (2.74) | 198 (4.28) | 2058 (4.68) | <0.001 |
| Decompensated cirrhosis | 62 (1.69) | 52 (1.47) | 146 (3.15) | 1614 (3.67) | <0.001 |
| Hepatocellular carcinoma | 5 (0.14) | 3 (0.08) | 19 (0.41) | 337 (0.77) | <0.001 |
| Alcohol abuse | 173 (4.72) | 110 (3.11) | 173 (3.74) | 2665 (6.06) | <0.001 |
| Drug abuse | 468 (12.77) | 409 (11.57) | 522 (11.28) | 6797 (15.45) | <0.001 |
| Contraindications, n (%) | |||||
| Schizophrenia | 88 (2.40) | 68 (1.92) | 97 (2.10) | 1404 (3.19) | <0.001 |
| Depression | 455 (12.41) | 399 (11.29) | 515 (11.13) | 6363 (14.46) | <0.001 |
| Seizure | 30 (0.82) | 22 (0.62) | 39 (0.84) | 432 (0.98) | 0.126 |
| Pregnancy | 27 (0.74) | 20 (0.57) | 20 (0.43) | 599 (1.36) | <0.001 |
| Transplant | 22 (0.60) | 9 (0.25) | 49 (1.06) | 514 (1.17) | <0.001 |
| Retinopathy | 1 (0.03) | 0 (0.00) | 3 (0.06) | 26 (0.06) | 0.437 |
| Anemia | 228 (6.22) | 181 (5.12) | 399 (8.62) | 4610 (10.48) | <0.001 |
| Medication use, n (%) | |||||
| ACEIs | 161 (4.39) | 173 (4.90) | 271 (5.86) | 2365 (5.38) | 0.015 |
| ARBs | 569 (15.52) | 593 (16.78) | 881 (19.04) | 7804 (17.74) | 0.002 |
Abbreviations: ACEI, angiotensin‐converting‐enzyme inhibitors; ARB, angiotensin II receptor blockers; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; IQR,interquartile range.
Table 4 shows the risk of developing CKD among the HCV patients who received minimally effective treatment, insufficient treatment, or no treatment. The majority of CKD events (90%) occurred in patients who received no HCV treatment, carrying a CKD incidence rate of 10.79 per 1000 person‐years. The CKD incidence rate decreased among patients who received treatment (10.07 per 1000 person‐years) but decreased greatly when patients were on a minimally effective dose of treatment (6.73 per 1000 person‐years). After adjusting for baseline and time‐dependent covariates, overall, we found HCV‐infected patients who received the minimum effective duration of therapy had a 30% decreased risk of developing CKD compared with those who received no treatment (HR, 0.70; 95% CI, 0.56‐0.88). However, in a subgroup analysis, these associations were only significant for dual (HR, 0.60; 95% CI, 0.43‐0.85) and triple (HR, 0.59; 95% CI, 0.37‐0.94) therapy but not for all‐oral therapy (HR, 1.03; 95% CI, 0.68‐1.55) (Table 4).
Table 4.
HCV Treatment Association with Incidence of CKD Using Time‐Varying Cox‐Proportional Hazards Model
| Treatment Statusa | Person‐Years | No. of CKD Events | Crude Incidence of CKDb | Adjusted HR of CKD (95% CI) |
|---|---|---|---|---|
| All HCV patients (N = 55,818) | ||||
| Minimum Effective TX | 11,737 | 79 | 6.73 | 0.70 (0.55‐0.88) |
| Insufficient TX | 6854 | 69 | 10.07 | 0.85 (0.66‐1.09) |
| No TX | 119,698 | 1291 | 10.79 | Reference |
| Dual therapy (n = 3666) | ||||
| Minimum Effective TX | 6115 | 34 | 5.56 | 0.60 (0.43‐0.85) |
| Insufficient TX | 3245 | 34 | 10.48 | 0.92 (0.65‐1.31) |
| No TX | 108,813 | 1190 | 11.10 | Reference |
| Triple therapy (n = 3534) | ||||
| Minimum Effective TX | 3469 | 19 | 5.48 | 0.59 (0.37‐0.94) |
| Insufficient TX | 3023 | 25 | 8.27 | 0.72 (0.48‐1.07) |
| No TX | 110,623 | 1197 | 10.82 | Reference |
| All‐oral therapy (n = 4628) | ||||
| Minimum Effective TX | 2154 | 26 | 12.07 | 1.03 (0.68‐1.55) |
| Insufficient TX | 585 | 10 | 17.09 | 0.85 (0.39‐1.82) |
| No TX | 114,224 | 1254 | 10.98 | Reference |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; HCV, hepatitis C virus; HR, hazard ratio; TX, treatment.
HCV treatment status was coded as a time‐dependent covariate in the Cox regression model. Therefore, each patient may have a different treatment status during the study follow‐up.
Per 1000 person‐years.
RISK FACTORS RELATED TO INCIDENCE OF CKD IN HCV‐INFECTED PATIENTS
Figure 2 shows risk factors for CKD in HCV‐infected patients. Factors associated with an increased risk of developing CKD included age ≥60 years (HR, 2.12; 95% CI, 1.74‐2.58), diabetes mellitus (HR, 1.79; 95% CI, 1.60‐2.00), congestive heart failure (HR, 2.14; 95% CI, 1.88‐2.45), peripheral vascular disease (HR, 1.20; 95% CI, 1.05‐1.37), cerebrovascular disease (HR, 1.20; 95% CI, 1.04‐1.37), hypertension (HR, 2.43; 95% CI, 2.05‐2.88), HIV (HR, 1.93; 95% CI, 1.44‐2.57), alcohol abuse (HR, 1.27; 95% CI, 1.10‐1.46), decompensated cirrhosis (HR, 1.91; 95% CI, 1.64‐2.24), history of transplantation (HR, 3.19; 95% CI, 2.70‐3.76), and anemia (HR, 2.20; 95% CI, 1.95‐2.47), in addition to receipt of ACEIs (HR, 7.30; 95% CI, 6.12‐8.71) and ARBs (HR, 5.57; 95% CI, 4.86‐6.39).
Figure 2.
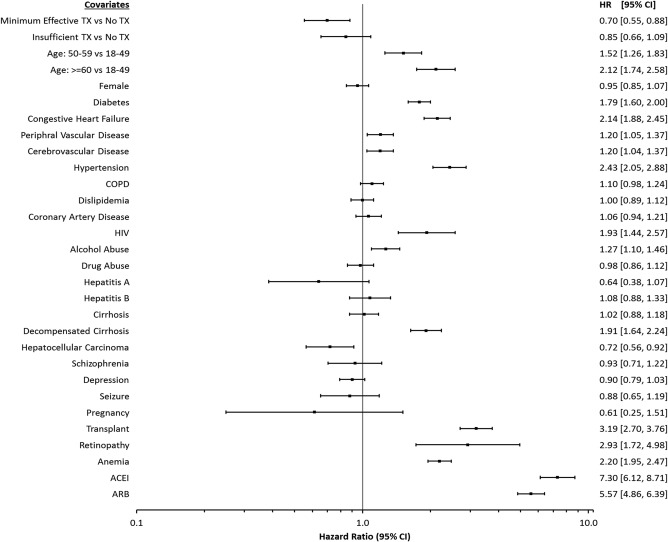
Adjusted hazard ratios for chronic kidney disease in HCV patients using time‐varying Cox‐proportional hazards model. Abbreviations: ACEI, angiotensin‐converting‐enzyme inhibitors; ARB, angiotensin II receptor blockers; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; HR, hazard ratio; TX, treatment.
RISK OF MPGN AND CRYOGLOBULINEMIA BETWEEN HCV AND NON‐HCV GROUPS
For this analysis, we further excluded patients with MPGN and cryoglobulinemia before the index date. Table 5 shows the association between HCV and the development of nephrotic syndrome/MPGN and cryoglobulinemia. The crude incidence rate of MPGN was 0.833 per 1000 person‐years in HCV and 0.221 per 1000 person‐years in non‐HCV groups. The crude incidence rate of cryoglobulinemia was 0.876 per 1000 person‐years in HCV and 0.050 per 1000 person‐years in non‐HCV groups. The Cox proportional hazards regression model indicated that HCV patients had 3.7 and 17 times higher risks of developing nephrotic syndrome/MPGN (HR, 3.74; 95% CI, 2.84‐4.93) and cryoglobulinemia (HR, 17.25; 95% CI, 10.91‐27.26), respectively. Sensitivity analyses using the Cox regression model with time‐dependent covariates, which took the change of comorbidities and medication use during follow‐up into consideration, revealed that HCV‐infected patients had 2 times and 17 times increased risk of MPGN (HR, 2.23; 95% CI, 1.84‐2.71) and cryoglobulinemia (HR, 16.91; 95% CI, 12.00‐23.81). We also found that HCV and MPGN association was stronger among women (HR, 3.78; 95% CI, 2.66‐5.36) compared with men (HR, 1.74; 95% CI, 1.37‐2.19). In contrast, there were no significant differences in the development of cryglobulinemia between HCV‐infected men and women. However, we did not find any effects of the HCV treatments on the risk of developing MPGN and cryoglobulin among the HCV patients who received treatments compared with no treatment (Supporting Tables S4 and S5).
Table 5.
Incidence Rate and Hazard Ratio for MPGN and Cryoglobulinemia in the HCV and Non‐HCV Cohorts, Adjusting for Baseline Characteristics
| Secondary Outcomes | HCV Status | No. of Patients | Person‐Years | No. of Events | Crude Incidencea | Mean Time to Event (Months) | Adjusted HR (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Baseline Covariates | Baseline and Time‐Varying Covariates | |||||||
| MPGN | HCV | 55,618 | 140,408 | 120 | 0.833 | 17.39 | 3.74 (2.84‐4.93) | 2.23 (1.84‐2.71) |
| Non‐HCV | 166,854 | 438,153 | 97 | 0.221 | 19.03 | Reference | Reference | |
| Men | HCV | 33,395 | 84,325 | 83 | 0.984 | 18.40 | 3.50 (2.54‐4.84) | 1.74 (1.37‐2.19) |
| Non‐HCV | 101,422 | 266,423 | 73 | 0.274 | 18.81 | Reference | Reference | |
| Women | HCV | 22,223 | 56,082 | 37 | 0.660 | 15.11 | 4.40 (2.59‐7.47) | 3.78 (2.66‐5.36) |
| Non‐HCV | 65,432 | 171,730 | 24 | 0.140 | 19.72 | Reference | Reference | |
| Cryoglobulinemia | HCV | 55,646 | 140,435 | 123 | 0.876 | 14.17 | 17.25 (10.91‐27.26) | 16.91 (12.00‐23.81) |
| Non‐HCV | 166,938 | 438,946 | 22 | 0.050 | 24.69 | Reference | Reference | |
| Men | HCV | 33,423 | 84,363 | 75 | 0.889 | 14.96 | 21.00 (11.10‐39.73) | 20.03 (12.28‐32.67) |
| Non‐HCV | 100,824 | 265,142 | 11 | 0.041 | 27.25 | Reference | Reference | |
| Women | HCV | 22,223 | 56,072 | 48 | 0.856 | 12.95 | 13.11 (6.76‐25.40) | 14.07 (8.68‐22.81) |
| Non‐HCV | 66,114 | 173,804 | 11 | 0.063 | 22.12 | Reference | Reference | |
Abbreviations: CI, confidence interval; HCV, hepatitis C virus; HR, hazard ratio; MPGN, membranoproliferative glomerulonephritis.
Per 1000 person‐years.
Discussion
This retrospective cohort, PS‐matched study provides United States general population–based evidence to support that HCV infection is linked to an increased risk of CKD. In fact, the crude incidence rate among our cohort of chronically infected HCV patients was 10.36 per 1000 person‐years compared with 5.72 per 1000 person‐years in non‐HCV groups. This significant finding was further confirmed in our Cox proportional hazards regression model, which indicated that persons diagnosed with chronic HCV had a 57% increased risk of developing CKD and in our time‐varying Cox regression model chronic HCV‐infected patients had a 27% increased risk of CKD. The decrease in the risk from 57% to 27% was explained as we controlled for the risk factors known to be associated with the development of CKD after an HCV diagnosis. Nonetheless, our study demonstrates that HCV is significantly associated with increasing the risk for CKD among patients with HCV in the United States, which corroborates other findings that have associated HCV with the incidence and progression of CKD.6, 9, 11, 12, 22, 23, 24
However, a recent study conducted by Rogal et al.10 using the Electronically Retrieved Cohort of HCV Infected Veterans Study Group (ERCHIVES) determined that other factors (older age, female sex, diabetes, hypertension, development of cirrhosis, and substance abuse) rather than HCV were associated with the incidence and progression of CKD. They suggested that the reason that HCV was protective for CKD was probably a result of the amount of time patients were exposed to HCV as their patient population was not newly diagnosed with HCV unlike our population who were newly diagnosed chronic HCV patients. We also suggest that the differences in our findings may be due to the variables controlled for in our time‐varying multivariate analysis. With the use of time‐varying Cox regression modeling, we accounted for the dynamic and complex relationship between variables and time allowing us to identify the top five variables associated with CKD in the chronically infected HCV patient. Specifically these variables were: ACEIs, ARBs, congestive heart failure, hypertension, and transplantation variables similar to the variables Rogal et al. found to be predictors of CKD in their population. Because we accounted for changes that can occur over time, our findings lend more strength of the association of these variables in the development of CKD within patients with chronic HCV infection.
A very promising study finding was that exposure to the minimally effective duration of treatment resulted in chronically HCV‐infected patients experiencing a 30% decreased risk of developing CKD. However, in the subgroup analysis, the association was only observed with the less‐tolerated HCV treatment therapies (dual and triple therapies) and not with the new all‐oral regimens. We believe this discrepancy results from shorter follow‐up for patients with the new DAAs for treatment (person‐years at risk) during the period of study. Although the sofosbuvir plus ribavirin regimen was first used in 2013, US Food and Drug Administration approval for the use of the first all‐oral DAA regimen (ledipasvir/sofosbuvir) did not occur until October 2014, so the patients in our study had less than 1 year to be exposed to the newer all‐oral DAA regimens (the study period ended in August 2015). Nonetheless, we noted a trend toward decreasing the risk of CKD in HCV‐infected patients treated with DAAs—a trend we suspect will become significant as more studies investigate the incidence and progression of CKD in patients with HCV who are treated with the new DAAs.
On the other hand, a disturbing study finding was that the majority of HCV patients (79%) within our study were not treated. Although the no treatment group had similar advanced liver disease (cirrhosis, decompensated cirrhosis) compared with the all‐oral therapy group, the no treatment group was sicker as noted by the increased number of patients with alcohol/drug abuse issues as well as the number of contraindications to pegylated interferon– and ribavirin‐based treatment regimens, which may partially explain why they were not treated. This finding is especially noteworthy because efficacious and safe all‐oral pangenotypic therapies are now available and approved for most people to include patients in whom interferon was contraindicated, difficult‐to‐treat patients, HIV coinfection, and cirrhosis. Nonetheless, the majority of patients not receiving treatment suggests that patients with HCV are still encountering barriers to treatment even within a group with access to health insurance. Therefore, identifying and then overcoming the barriers to identification and treatment remains a significant issue in eradicating HCV as well as eliminating the clinical and economic burden of HCV‐associated extrahepatic manifestations including CKD.3, 41
Another significant and unique finding of this study was the identification of the incidence and risk for developing MPGN and cryoglobunemia among chronically infected HCV patients. To the best of our knowledge, no study has quantified the risk of developing these diseases in the chronically HCV‐infected general population in the United States. The crude incidence rate of MPGN and cryoglobulinemia were 6 times and over 8 times higher compared with non‐HCV patients, respectively. In fact, results from our Cox regression models indicated that chronically infected HCV patients had 2‐3 times higher risk for MPGN and 14‐17 times higher risk of developing cryoglobulinemia after adjusting for covariates. Our results were similar to those in studies of extrahepatic manifestations of HCV in United States veterans.20, 21 A hospital‐based case‐control study performed among hospitalized male United States veterans (1992‐1999) revealed a greater proportion of MPGN (0.36% vs. 0.05%) and cryoglobulinemia (0.57% vs. 0.05%) among patients with HCV infection.20 In another cohort study of veterans (1997‐2004), investigators reported a 4‐fold higher risk of cryoglobulinemia in HCV‐infected US veterans, though the association was notably weaker than our study, perhaps due to differences in the study population and methods.21
Interestingly, in our study, female HCV patients were at a significantly higher risk of developing MPGN compared with male HCV patients (HR, 3.78 vs. 1.74; P > 0.05). Several other studies have revealed a stronger association between female sex and cryoglobulinemia,42, 43, 44 but our study did not reveal any significant sex differences in development of cryglobulinemia. Despite the fact that HCV‐related MPGN and cryoglobulinemia are relatively uncommon in the HCV population, these complications are considered a significant problem as a result of the large number of people infected with HCV in the United States and the potential for serious and life‐threatening complications to include end‐stage renal disease. Unfortunately, we did not find evidence to support a protective effect of HCV treatment in the development of these conditions.
There are several strengths to our study design and the use of a large claims database. First, this study has methodological strength because it employed PS matching, time‐varying Cox proportional hazard models on matched groups, and adjustment for immortal time bias, analyses that are different from other previously published studies and which may help to indicate a stronger relationship of HCV and CKD than that reported elsewhere.22, 23, 24 Second, this study includes the number of strongly associated CKD covariates that were controlled for in the time‐ varying Cox proportional hazard models, including ACEIs, ARBs, congestive heart failure, hypertension, and transplantation; nevertheless, HCV infection was still a positive predictor for developing CKD.6, 10 Third, this study is notable for its large sample size and for it being representative of general populations in the United States. Finally, we conducted numerous sensitivity analyses to assess the robustness of our results and found that none of these analyses produced substantially different results from the main analysis.
Several limitations must also be noted. First, the study lacks laboratory results (e.g., sustained virologic response, glomerular filtration rate) to corroborate ICD coding. We adjusted for as many confounders as available and known to be associated with CKD; however, because we were dependent on administrative data, there may have been some unmeasured confounders that were not reported and thus unavailable. Selection bias was present between treated and untreated groups. Detection bias may be introduced by differential screening frequencies for kidney diseases between HCV and non‐HCV individuals. Finally, we had a relatively short follow‐up, which did not allow us to fully explore the use of DAAs in this population.
In conclusion, individuals infected with chronic HCV in the United States are at a higher risk of developing moderate to severe CKD, MPGN, and cryoglobulinemia. Antiviral treatment for HCV is associated with a decreased incidence of CKD, although the association is yet to be confirmed for the new all‐oral DAA therapy. These findings highlight that treating HCV early helps to change the extrahepatic burden of CKD associated with HCV. Therefore, research must continue to identify barriers to the identification of HCV‐infected patients and improve access to treatment for all HCV patients. Future studies should include a longer study period to investigate the effects of the all‐oral DAA treatment on the development and progression of CKD.
Author names in bold designate shared co‐first authorship.
Supporting information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.29505/suppinfo.
Supporting Information
Potential conflict of interest: David Nelson has received research grant support from AbbVie, Bristol‐Myers Squibb, Gilead, Jassen, and Merck.
REFERENCES
- 1. Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, et al. Chronic hepatitis C virus infection in the United States, national health and nutrition examination survey 2003 to 2010. Ann Intern Med 2014;160:293‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cacoub P, Comarmond C, Domont F, Savey L, Desbois AC, Saadoun D, et al. Extrahepatic manifestations of chronic hepatitis C virus infection. Ther Adv Infect Dis 2016;3:3‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Younossi Z, Park H, Henry L, Adeyemi A, Stepanova M. Extrahepatic manifestations of hepatitis C: a meta‐analysis of prevalence, quality of life, and economic burden. Gastroenterology 2016;150:1599‐1608. [DOI] [PubMed] [Google Scholar]
- 4. Davis GL, Alter MJ, El‐Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)‐infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology 2010;138:513‐521. [DOI] [PubMed] [Google Scholar]
- 5. Jacobson IM, Cacoub P, Dal Maso L, Harrison SA, Younossi ZM. Manifestations of chronic hepatitis C virus infection beyond the liver. Clin Gastroenterol Hepatol 2010;8:1017‐1029. [DOI] [PubMed] [Google Scholar]
- 6. Molnar MZ, Alhourani HM, Wall BM, Lu JL, Streja E, Kalantar‐Zadeh K, et al. Association of hepatitis C viral infection with incidence and progression of chrnonic kidney disease in a large cohort of US veterans. Hepatology 2015;61:1495‐1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fabrizi F, Plaisier E, Saadoun D, Martin P, Messa P, Cacoub P, et al. Hepatitis C virus infection, mixed cryoglobulinemia, and kidney disease. Am J Kidney Dis 2013;61:623‐637. [DOI] [PubMed] [Google Scholar]
- 8. Fabrizi F, Martin P, Dixit V, Messa P. Hepatitis C virus infection and kidney disease: a meta‐analysis. Clin J Am Soc Nephrol 2012;7:549‐557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Butt AA, Wang X, Fried LF. HCV infection and the incidence of CKD. Am J Kidney Dis 2011;57:396‐402. [DOI] [PubMed] [Google Scholar]
- 10. Rogal SS, Yan P, Rimland D, Re VL, Al‐Rowais H, Fried L, et al. Incidence and progression of chronic kidney disease after hepatitis C seroconversion: results from ERCHIVES. Dig Dis Sci 2016;61:930‐936. [DOI] [PubMed] [Google Scholar]
- 11. Park H, Adeyemi A, Henry L, Stepanova M, Younossi Z. A meta‐analytic assessment of the risk of chronic kidney disease in patients with chronic hepatitis C virus infection. J Viral Hepat 2015;22:897‐905. [DOI] [PubMed] [Google Scholar]
- 12. Fabrizi F, Verdesca S, Messa P, Martin P. Hepatitis C virus infection increases the risk of developing chronic kidney disease: a systematic review and meta‐analysis. Dig Dis Sci 2015;60:3801‐3813. [DOI] [PubMed] [Google Scholar]
- 13. Johnson RJ, Gretch DR, Yamabe H, Hart J, Bacchi CE, Hartwell P, et al. Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med 1993;328:465‐470. [DOI] [PubMed] [Google Scholar]
- 14. Perico N, Cattaneo D, Bikbov B, Remuzzi G. Hepatitis C infection and chronic renal diseases. Clin J Am Soc Nephrol 2009;4:207‐220. [DOI] [PubMed] [Google Scholar]
- 15. Latt N, Alachkar N, Gurakar A. Hepatitis C virus and its renal manifestations: a review and update. Gastroenterol Hepatol 2012;8:434. [PMC free article] [PubMed] [Google Scholar]
- 16. Fabrizi F. Hepatitis C virus, cryoglobulinemia, and kidney: novel evidence. Scientifica 2012;2012:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fabrizi F, Dixit V, Messa P. Antiviral therapy of symptomatic HCV‐associated mixed cryoglobulinemia: meta‐analysis of clinical studies. J Med Virol 2013;85:1019‐1027. [DOI] [PubMed] [Google Scholar]
- 18. Moorman AC, Tong X, Spradling PR, Rupp LB, Gordon SC, Lu M, et al. Prevalence of renal impairment and associated conditions among HCV‐infected persons in the chronic hepatitis cohort study (CHeCS). Dig Dis Sci 2016;61:2087‐2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tong X, Spradling P. Increase in nonhepatic diagnoses among persons with hepatitis C hospitalized for any cause, United States, 2004‐2011. J Viral Hepat 2015;22:906‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. El‐Serag HB, Hampel H, Yeh C, Rabeneck L. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology 2002;36:1439‐1445. [DOI] [PubMed] [Google Scholar]
- 21. Giordano TP, Henderson L, Landgren O, Chiao EY, Kramer JR, El‐Serag H, et al. Risk of non‐Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA 2007;297:2010‐2017. [DOI] [PubMed] [Google Scholar]
- 22. Hsu YC, Lin JT, Ho HJ, Kao YH, Huang YT, Hsiao NW, et al. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology 2014;59:1293‐1302. [DOI] [PubMed] [Google Scholar]
- 23. Chen Y‐C, Hwang S‐J, Li C‐Y, Wu C‐P, Lin L‐C. A Taiwanese nationwide cohort study shows interferon‐based therapy for chronic hepatitis C reduces the risk of chronic kidney disease. Medicine 2015;94:e1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hsu Y‐C, Ho HJ, Huang Y‐T, Wang H‐H, Wu M‐S, Lin J‐T, et al. Association between antiviral treatment and extrahepatic outcomes in patients with hepatitis C virus infection. Gut 2015;64:495‐503. [DOI] [PubMed] [Google Scholar]
- 25. Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology 2002;36:237‐244. [DOI] [PubMed] [Google Scholar]
- 26. Manns M, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut 2006;55:1350‐1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McHutchison JG, Manns M, Patel K, Poynard T, Lindsay KL, Trepo C, et al. Adherence to combination therapy enhances sustained response in genotype‐1–infected patients with chronic hepatitis C. Gastroenterology 2002;123:1061‐1069. [DOI] [PubMed] [Google Scholar]
- 28. Cunningham M, Foster GR. Efficacy and safety of telaprevir in patients with genotype 1 hepatitis C infection. Therap Adv Gastroenterol 2012;5:139‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Symonds WT, et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med 2013;368:34‐44. [DOI] [PubMed] [Google Scholar]
- 30. Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014;370:1879‐1888. [DOI] [PubMed] [Google Scholar]
- 31. Younossi ZM, Park H, Dieterich D, Saab S, Ahmed A, Gordon SC, et al. Assessment of cost of innovation versus the value of health gains associated with treatment of chronic hepatitis C in the United States: the quality‐adjusted cost of care. Medicine 2016;95:e5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ronksley PE, Tonelli M, Quan H, Manns BJ, James MT, Clement FM, et al. Validating a case definition for chronic kidney disease using administrative data. Nephrol Dial Transplant 2011;27:1826‐1831. [DOI] [PubMed] [Google Scholar]
- 33. Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS. SAS Global Forum 2012;35:1‐6. [Google Scholar]
- 34. D'Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group. Stat Med 1998;17:2265‐2281. [DOI] [PubMed] [Google Scholar]
- 35. Tsui JI, Vittinghoff E, Shlipak MG, Bertenthal D, Inadomi J, Rodriguez RA, et al. Association of hepatitis C seropositivity with increased risk for developing end‐stage renal disease. Arch Intern Med 2007;167:1271‐1276. [DOI] [PubMed] [Google Scholar]
- 36. Chen Y‐C, Lin H‐Y, Li C‐Y, Lee M‐S, Su Y‐C. A nationwide cohort study suggests that hepatitis C virus infection is associated with increased risk of chronic kidney disease. Kidney Int 2014;85:1200‐1207. [DOI] [PubMed] [Google Scholar]
- 37. Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T, et al. Variable selection for propensity score models. Am J Epidemiol 2006;163:1149‐1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patrick AR, Schneeweiss S, Brookhart MA, Glynn RJ, Rothman KJ, Avorn J, et al. The implications of propensity score variable selection strategies in pharmacoepidemiology: an empirical illustration. Pharmacoepidemiol Drug Saf 2011;20:551‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med 2007;26:734‐753. [DOI] [PubMed] [Google Scholar]
- 40. Normand S‐LT, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol 2001;54:387‐398. [DOI] [PubMed] [Google Scholar]
- 41. Azmi AN, Tan S‐S, Mohamed R. Hepatitis C and kidney disease: an overview and approach to management. World J Hepatol 2015;7:78‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Charles ED, Dustin LB. Hepatitis C virus–induced cryoglobulinemia. Kidney Int 2009;76:818‐824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cicardi M, Cesana B, Del Ninno E, Pappalardo E, Silini E, Agostoni A, et al. Prevalence and risk factors for the presence of serum cryoglobulins in patients with chronic hepatitis C. J Viral Hepat 2000;7:138‐143. [DOI] [PubMed] [Google Scholar]
- 44. Kayali Z, Buckwold VE, Zimmerman B, Schmidt WN. Hepatitis C, cryoglobulinemia, and cirrhosis: A meta‐analysis. Hepatology 2002;36:978‐985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.29505/suppinfo.
Supporting Information


