Version Changes
Revised. Amendments from Version 2
The updated version addresses the suggestions that were made by the reviewers. Some of the text and figures have been improved for clarity. Figures 2, S3 and S6 are amended to include the heatmap color scales. We have re-created Figure 2A to show proper clustering of single-cells. A new Panel added to figure 8 showing adjusted mutual information obtained from random networks vs real network. Figures S9 to S14 added to show performance of CIDR comparison to other imputation methods.
Abstract
Single cell RNA-seq (scRNA-seq) experiments suffer from a range of characteristic technical biases, such as dropouts (zero or near zero counts) and high variance. Current analysis methods rely on imputing missing values by various means of local averaging or regression, often amplifying biases inherent in the data. We present netSmooth, a network-diffusion based method that uses priors for the covariance structure of gene expression profiles on scRNA-seq experiments in order to smooth expression values. We demonstrate that netSmooth improves clustering results of scRNA-seq experiments from distinct cell populations, time-course experiments, and cancer genomics. We provide an R package for our method, available at: https://github.com/BIMSBbioinfo/netSmooth.
Keywords: scRNA-seq, single-cell, genomics, imputation, networks
Introduction
Single cell RNA sequencing (scRNA-seq) enables profiling of single cells’ transcriptomes at unprecedented throughput and resolution. It has enabled previously impractical, studies of cell type heterogeneity, differentiation, and developmental trajectories 1. However, the adaptation of RNA sequencing techniques from bulk samples to single cells did not progress without challenges. Typically, only a fraction of a cells transcriptome may be captured by the experiment, leading to so called "drop-out" events where a gene gets a false 0 (or near 0) count in some cell. The dropout rate is related to the population level expression of a gene leading to many false zero counts for lowly expressed genes, and artificially low counts for highly expressed ones 2. Furthermore, the drop-out rate could be related to the biology of the cell type, as some cell types transcribe fewer genes than others, which will appear as drop-out events 2. When summed over many samples, transcript counts from single cells resemble those of bulk experiments 3, but across individual cells there is significant variation. This makes analysis more difficult than in bulk RNA sequencing experiments.
Computational methods designed to deal with these issues treat dropout events as missing data points, whose values may be imputed based on non-missing data points (observed measurements). The proportion of 0 counts per gene, a proxy for its technical dropout rate, is a function of the population-wise mean expression of that gene 2, 4. This observation has led researchers to treat 0 counts as dropout candidates to be imputed.
CIDR 5 attempts to impute missing values based on the predicted mean expression of a gene, given its empirical dropout rate (0-count). scImpute 6 estimates dropout likelihoods per gene and per sample, and assigns each gene in each sample a status as a dropout candidate. Genes might be considered likely dropouts even with nonzero expression, and 0-count genes might not be considered likely dropouts, based on their populationwide expression distributions. It then uses a regularized linear model to predict the expression of dropout genes based on the expression of likely non-dropouts in all other cells. MAGIC 7 performs local averaging after building a topological graph of the data, updating the expression value of all genes in all cells to their local neighborhood average.
All of the methods mentioned above use measured information in the data in order to impute the missing information within the same data. As such, they amplify whatever biases are present in a dataset; similar cells pre-imputation will become more similar after imputation, as expression profiles of non-dropout genes will drive similarities in imputed dropped-out genes. Further, all methods except MAGIC only impute unobserved expression events (0s or near 0s), while the dropout phenomenon actually affects the whole transcriptome. Hence, imputation methods for scRNAseq should also adjust non-0 expression measurements in order to recover the true signal.
We present a method, called netSmooth, that uses prior knowledge to temper noisy experimental data. RNA sequencing experiments produce counts data as a proxy for gene activity, which is not known a-priori, especially for experiments profiling unknown cell types. However, decades of molecular biology research have taught us much about the principles of gene interaction. Interacting genes are likely to be co-expressed in cells 8, 9, and as such, protein-protein interaction (PPI) databases 10, 11 describe genes’ propensity for co-expression. We developed a graph-diffusion method on PPI networks for smoothing of gene expression values. Each node in the graph (a gene) has an associated gene expression value, and the diffusion presents a weighted averaging of gene expression values among adjacent nodes in the graph, within each cell. This is done iteratively until convergence, strengthening co-expression patterns which are expected to be present. Incorporation of prior data from countless experiments in the preprocessing of scRNA-seq experiments improves resistance to noise and dropouts. Similar network based approaches have been used to extract meaningful information from sparse mutational profiles 12, 13, and indirectly on gene expression data by diffusing test statistics on the network to discover regulated gene candidates 14. We propose diffusion of gene expression values directly on the network as a method for data denoising and imputation. Furthermore, the parameters of this proposed method could be optimized using clustering robustness metrics. We applied our method to a variety of single cell experiments and compared its performance to other selected imputation methods scImpute and MAGIC. These methods represent the latest and divergent ways of imputing the scRNA-seq data.
While we mention CIDR in this review, we do not include comparisons to CIDR in the main text, alongside MAGIC and scImpute, because CIDR uses its own clustering procedure as a part of the imputation workflow. scImpute and MAGIC are agnostic about post-imputation analysis, and therefore we were able to compare them to netSmooth using a unified analysis framework (see Methods). For completeness, we include benchmark results of CIDR in the supplement (Figures ?? - ??).
We also made available an R package providing the necessary functionality to use our method on other data. It is available on GitHub: https://github.com/BIMSBbioinfo/netSmooth, or using Bioconductor: https://bioconductor.org/packages/release/bioc/html/netSmooth.html.
Results
Overview of the method
The intuition behind the netSmooth algorithm is that gene networks encoding co-expression patterns can be used to smooth scRNA-seq data, pushing its coexpression patterns in a biologically meaningful direction. We demonstrate this using protein-protein interaction networks, which are predictive of coexpression 9. We produced a PPI graph of high-confidence interactions based on the PPI database STRING 10.
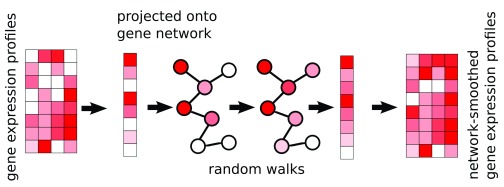
There are 2 inputs to the method: (1) a gene expression matrix, N genes by M cells, and (2) a graph where genes are nodes, and edges indicate genes which are expected to be co-expressed. The edges may be weighed, indicating the strength or direction of a relationship; an edge weight of 2 indicates stronger expected co-expression than an edge weight of 1, and an edge weight of −1 indicates negative expected co-expression, such as one gene being a repressor for another. The expression profile of each cell is then projected onto the graph, and a diffusion process is used to smooth the expression values, within each sample, of adjacent genes in the graph ( Figure 1). In this way, post-smoothing values of genes represent an estimate of activity levels based on reads aligned to that gene, as well as those aligned to its neighbors in the graph. Thus, a gene with a low read count (possible technical drop-out), whose neighbors in the graph are highly expressed, will get a higher value post smoothing. The rate at which expression values of genes diffuse to their neighbors is degree-normalized, so that genes with many edges will affect their neighbors less than genes with more specific interactions. The diffusion is done using a "random walks with restarts" (RWR) process 13, where a conceptual random walker starts in some node in the graph, and at each iteration moves to a neighboring node with a probability determined by the edge weight between the nodes, or, with some probability, restarts the walk from the original node. The network-smoothed value is the stationary distribution of this process. The RWR process has one free parameter, the restart rate. A low value for the restart rate allows diffusion to reach further in the graph; a high restart rate will lead to more local diffusions. For more details see the Methods section.
Figure 1. The netSmooth algorithm takes a gene expression profile, and a gene network.
The expression profile of each sample is projected onto the network, where a diffusion process allows genes’ expression values to be smoothed by their neighbors’. This is done for each cell independently of others. The end result is a network smoothed gene expression matrix.
Network smoothing improves cell type identification from single-cell RNA-seq
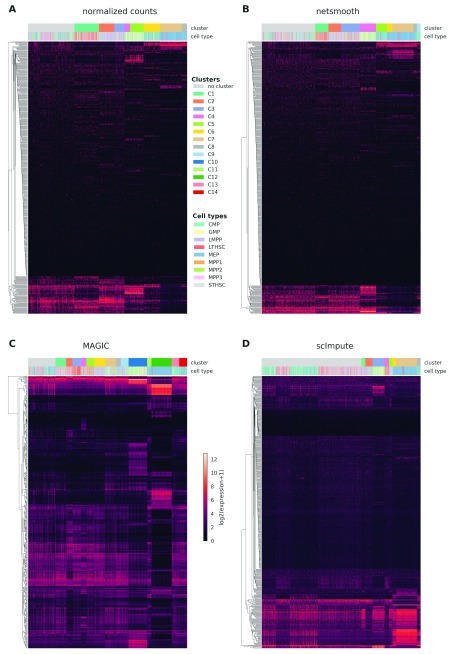
We first assess netSmooth on a dataset of 1645 mouse hematopoietic stem/progenitor cells (HSPCs) assayed using flow cytometry as well as scRNA-seq 15. The cells are FACS-sorted into 12 common HSPC phenotypes. This presents an atlas of the hematopoiesis process at a single cell resolution, showing the differentiation paths taken by E-SLAM HSCs as they differentiate to E, GM, and L progenitors. The authors of this study demonstrate that upon clustering the data, some clusters corresponds to cell types. However, the clusters are not noise free and do not fully recapitulate cell type identity. We obtained clusterings of the cells from the normalized counts, as well as after application of netSmooth, MAGIC 7, and scImpute 6, using a robust clustering procedure based on the clusterExperiment R package 16 (See Methods). After clustering, we used the edgeR-QLF test 17 to identify genes that are differentially expressed in any of the discovered clusters. Figure 2a,b shows the log-transformed expression values of the 500 most differentially expressed genes, before and after application of netSmooth. The column annotations indicate the FACS-sorted cell type of each cell, as well as the cluster assignment obtained from the netSmooth R package. The figure suggests that the network-smoothing effect is subtle on the individual genes, as difference between the heatmaps is negligible visually. Figure 2c,d shows the same for the MAGIC and scImpute-preprocessed data, respectively. MAGIC seems to do the strongest transformation to the data, as is also seen in lower dimension embeddings (Figures ??, ??).
Figure 2. Cells were clustered using the robust clustering procedure, and the log-transformed expression values of the log-transformed expression values of the 500 most differentially expressed genes (by edgeR-QLF test adjusted P value) in any of the discovered clusters are shown in a heatmap, as well as cluster assignments and FACS-sorted cell types.
A) raw (no imputation), B) after application of netSmooth, C) missing values imputed using MAGIC D) missing values imputed using scImpute.
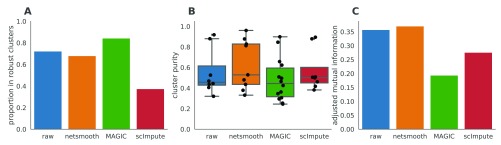
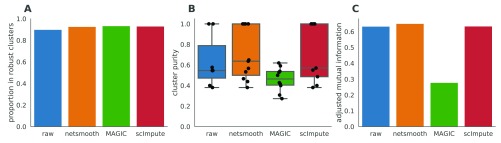
As this dataset has cells with labels independent of the RNAseq (FACS-sorted phenotypes), it presents us with an opportunity to compare the gene expression levels (as measured by RNAseq), to a meaningful phenotypic variable, i.e. the cell type. The cell type discrimination of a clustering result is compared using a cluster purity metric and and the adjusted mutual information (AMI). The cluster purity measures how cell-type specific clusters are by comparing homogeneity of the external labels (FACS-defined cell types), within clusters provided by scRNA-seq data. AMI is a chance-adjusted information theoretic measure of agreement between two labellings. This method accounts for artificially high mutual information between external labels and clusters when there is large number of clusters (See Methods for details on metrics). We also measured number of cells in robust clusters as quantitative metric. The robust clustering procedure allows cells to be omitted (not be assigned to a cluster) if they cannot be placed in a cluster across multiple clustering methods and/or parameters (See Methods). MAGIC-processed data leads to a larger proportion of cells assigned to robust clusters, while netSmooth and scImpute lead to a reduction in the clustering robustness metric ( Figure 3a). All three methods are able to discover some novel clusters in the data with high purity ( Figure 3b). A closer inspection shows that MAGIC achieves this through a proliferation of small clusters, which are not so far as we can judge meaningful, as evidenced by the lower adjusted mutual information score ( Figure 3c). netSmooth-preprocessed clusterslead to a higher AMI score, which, while modest, is biologically relevant.
Figure 3. Hematopoiesis clustering metrics.
A) The proportion of cells which were assigned to robust clusters. B) cluster purity (proportion of dominant cell type) for the robust clusters. netSmooth produces the most pure clusters in terms of cell types. C) AMI of the clustering results obtained after application of each of the methods. Only netSmooth increases the AMI between the clustering and the cell types. The online version of this figure is interactive.
Network smoothing improves capture of developmental expression patterns
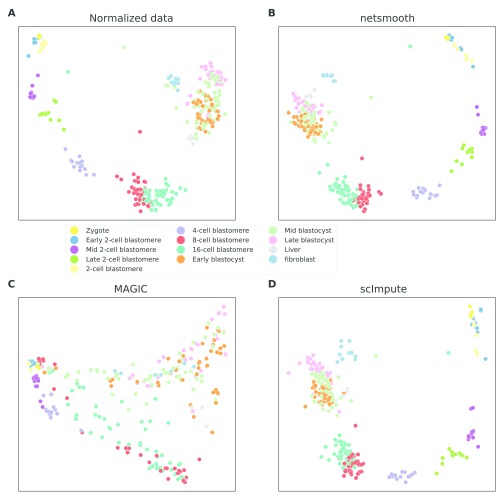
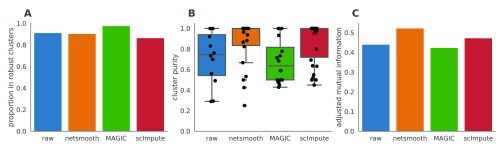
Next, we test netSmooth on 269 isolated cells from mouse embryos at different stages of pre-implantation development between oocyte and blastocyst, as well as 5 liver cells and 10 fibroblast cells 18. The authors of this study demonstrated that lower dimension embeddings capture much of the developmental trajectory ( Figure 4a, Figure ??a, ??a). We then applied netSmooth, MAGIC, and scImpute. Figure 4b shows the principal component analysis of netSmooth-processed data, and Figures 4c and 4d show the PCA plot following application of MAGIC and scImpute, respectively. netSmooth and scImpute preserve most of the variance structure of the data, while MAGIC seems to push the data onto a completely different manifold ( Figure 4, Figure ??). We used the robust clustering procedure to obtain clusters, and computed the cluster purity and AMI metrics. netSmooth enabled the clustering procedure to place more of the samples into robust clusters ( Figure 5a), and as in the hematopoiesis case, netSmooth is able to assist in identifying the developmental stage or tissue that cells belong to better than the other methods, as evidenced by the higher cluster purities ( Figure 5b) and AMI ( Figure 5c). scImpute also improves the cluter purity and AMI metrics ( Figure 5b,c), and is not easily differentiable from netSmooth in the PCA scatter plot ( Figure 4). The netSmooth results are marginally better, which hints at an equivalence in the recovered signal quality between the two methods, netSmooth’s quasiimputation incorporating priors, and scImpute’s linear model-based imputation. scImpute achieves this by reducing the overall 0-count genes significantly more than netSmooth (Figure ??), which suggests that incorporating priors the way netSmooth does can achieve similar results to data-imputation. The smaller change in the proportion of 0-count genes following netSmooth than scImpute (Figure ??) shows that netSmooth works primarily by smoothing values of genes with measured expression, as opposed to imputing suspected missing counts, which suggests a lesser transformation of the data, such as through application of netSmooth, can uncover much of the true signal hidden in the noisy data.
Figure 4.
2D PCA plots of the embryonic development dataset A) no preprocessing, B) after application of netSmooth, C) after imputing missing values with scImpute, and D) after application of MAGIC. The online version of this figure is interactive.
Figure 5. The Embryonic development dataset.
A) The proportion of cells which were assigned to robust clusters. All three methods lead to better clusterability, with MAGIC having the strongest effect. B) cluster purity (proportion of dominant cell type) for the robust clusters. netSmooth produces the most pure clusters in terms of cell types. C) Adjusted mutual information of clusterings and cell types. Only netSmooth increases the AMI over the non-preprocessed data. The online version of this figure is interactive.
Network smoothing improves identification of glioblastoma tumors
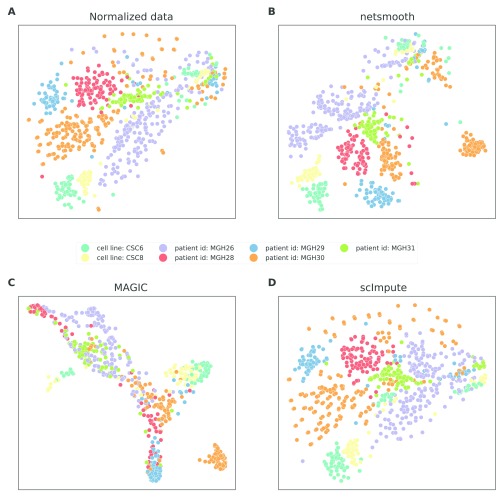
Finally, we demonstrate applicability of netSmooth to cancer research. Patel et al. generated scRNA-seq data of 800 cells from 5 glioblastoma tumors and 2 cell lines 19. Lower dimension embedding plots show that cells from different tumors or cell lines generally group together, but some are not wholly distinguishable from other tumors ( Figure 6a, ??a, ??a). Further, the two cell lines group closer to each other than the other patient samples. After applying netSmooth to the data, tumors become easier to distinguish in a lower dimensional embedding ( Figure 6b), indicating that netSmooth improves assignment of each cell to its tumor, cell line, or clone of origin. Again, scImpute also leads to similar reduced dimension embedding ( Figure 6d), while MAGIC distorted the data more than the other methods ( Figure 6c). We used the robust clustering procedure before and after netSmooth, MAGIC, and scImpute. Only MAGIC increase the clusterabitliy of the data ( Figure 7a), but netSmooth leads to the most pure clusters, in terms of tumor or cell line of origin ( Figure 7b, Figure 7c).
Figure 6.
t-SNE plots of the glioblastoma dataset A) no preprocessing, B) after application of netSmooth, C), using MAGIC, and D) after application of scImpute. The online version of this figure is interactive.
Figure 7. Imputation performance for the glioblastoma dataset.
A) The proportion of cells which were assigned to robust clusters. netSmooth, MAGIC, and scImpute all increased the proportion of cells that are assigned to robust clusters, with MAGIC leading, netSmooth in second place, and scImpute in third. B) cluster purity (proportion of dominant cell type) for the robust clusters. netSmooth produces the most pure clusters in terms of tumor or cell line of origin. C) AMI of the clustering results obtained after application of each of the methods. The online version of this figure is interactive.
Tumor or cell line of origin is an imperfect proxy for phenotypical variation in cancer cells, because some cells cluster by cell type rather than tumor of origin, demonstrating the heterogeneity in these glioblastoma tumors and similarities across origins 19. Nevertheless, we chose to compute cluster purity based on the cell origin rather than other labels which might be assigned to them, as it is the only ground truth variable that is independent of the RNAseq experiment. Further, cells do group by origin ( Figure 6, Figure ??), and identification of origin is an interesting question in its own right in the field of cancer genomics, particularly for heterogeneous tumors such as these.
Sensitivity to the network
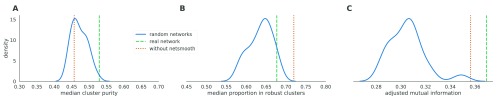
Next, we set out to ensure that the results are not an artifact of the network structure, i.e. that the actual links between genes that we used in the network are important. We expect netSmooth not to perform well when using networks with similar characteristics, but where edges do not represent real interactions. To that effect, we constructed 20 random networks by keeping the same graph structure of the real PPI graph, but shuffling the gene names. Thus, these random networks share all the characteristics of the real network (degree distribution, community structure), except for the true identity of the nodes. We then used those networks as inputs to netSmooth and ran the benchmarks as before on the hematopoiesis dataset. Using random networks as an input to netSmooth gives cluster purities distributed around a mode given by the cluster purities of the raw data, while the cluster purities given from using the real PPI network lie at the extreme edge of the distribution ( Figure 8a). Further, most random networks result in fewer samples belonging to robust clusters ( Figure 8b). Finally, we also calculated the adjusted mutual information of clusterings resulting from the randomized networks ( Figure 8c). Again, most shuffled networks produce worse clusterings, with the real network outperforming all of them, as well as the no-smoothing case. These results demonstrate that it is indeed the information contained in the PPI graph enables netSmooth to transform the gene expression matrix in a more biologically coherent direction, and that the transformation we see can not be explained simply by the network structure.
Figure 8. Performance of netSmooth with randomized networks.
A) The median cluster purity achieved with the random networks. The real network outperforms the random ones, which result in cluster purities distributed around the purity given without using netSmooth. B) The proportion of samples assigned to robust clusters using the random networks as well as the real one. While all networks result in fewer samples robustly clustered (in the hematopoiesis dataset), the real network outperforms most random networks. The online version of this figure is interactive.
Using other networks with netSmooth
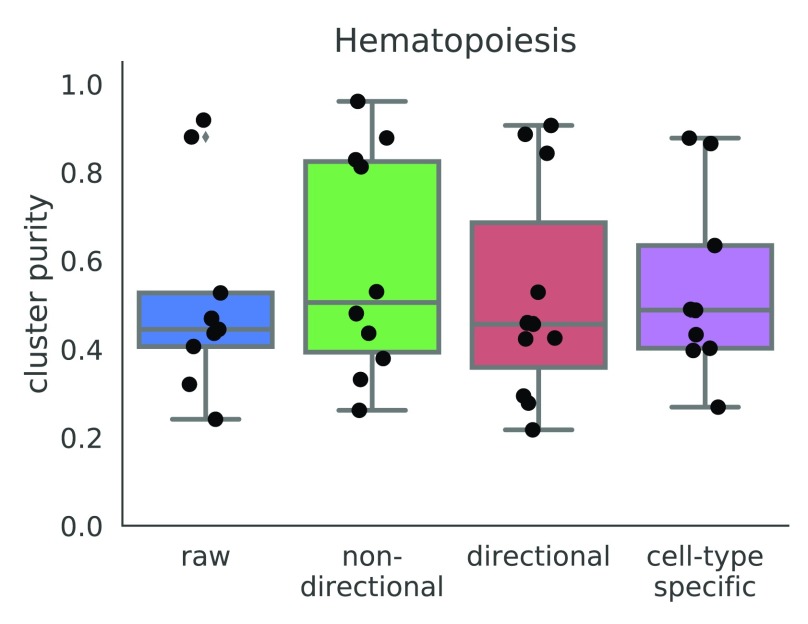
In addition to using an unweighed (where all edge weights are 1), undirected (where all edge weights are positive) network from string-db, we constructed other gene networks and used them as inputs to netSmooth. We created a directed gene network from only those edges in string-db which are marked as activating or inhibiting i. We set the edge weights of the activating interactions to +1, and −1 for the inhibiting interactions, allowing gene expression values to be adjusted downwards for genes whose known antagonists are highly expressed. After smoothing, we set all negative smoothed expression values to 0. We also constructed a gene network from string-db using only genes that are known to demonstrate cell-type specific expression. In order to obtain a list of genes with such cell-type specific expression patterns from the Expression Atlas 20, we used only the genes which show a cell-type specific expression with a mean TPM of at least 1 in some cell type, and used the subset of string-db network containing those genes as an input to netSmooth. Both of those modified graphs perform similarly to the undirected graph from string-db ( Figure 9, Figure ??a, Figure ??b), demonstrating that netSmooth is able to use priors from different types of experiments in order to improve clustering of scRNA-seq.
Figure 9. Cluster purities after applying netSmooth with different input networks.
Raw refers to no smoothing, non-directional is the same as the results shown in previous sections. Directional refers to a gene network where inhibitory relationships have negative edge weights, and cell-type specific refers to a gene network of only genes which are known to have cell-type specific expression patterns. The online version of this figure is interactive.
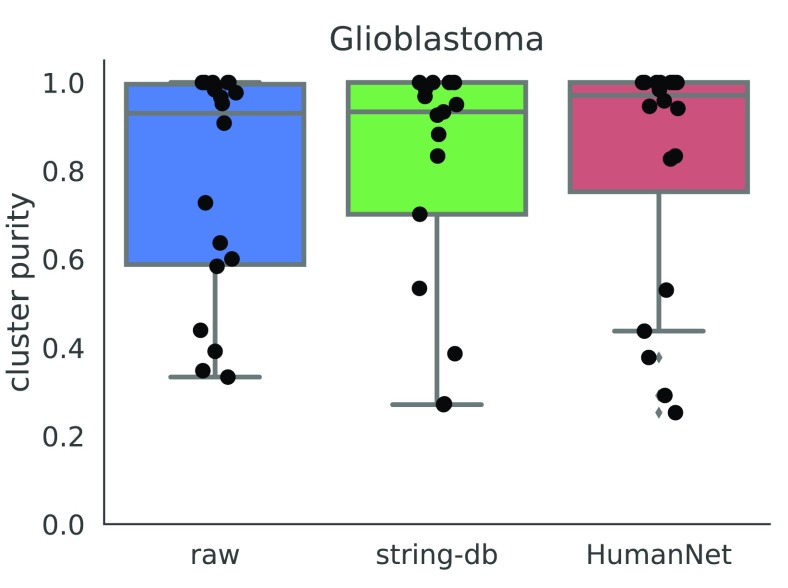
We also considered other sources for the gene network. We constructed a gene network from HumanNet 21, a functional gene network where edges denote interactions between two genes. We constructed a smoothing graph by taking all edges from HumanNet, and producing a graph where all edge weights are set to 1. We then used this graph as an input to netSmooth on the glioblastoma dataset. It performs similarly to the network from string-db ( Figure 10, Figure ??c), demonstrating that other sources for gene interactions may also be used by netSmooth to improve clustering results of scRNA-seq.
Figure 10. Cluster purities after applying netSmooth with different input networks.
Raw refers to no smoothing, string-db is the same as the results shown in previous sections, and HumanNet refers to a gene network constructed from the HumanNet database. The online version of this figure is interactive.
As more scRNAseq experiments are published, context-specific networks will be made possible to create, potentially improving netSmooth’s performance. The networks we have shown above have links between genes which are known in a general context, but scRNAseq experiments might uncover previously unknown cell-type specific gene interactions, which could contribute to the information uncovered by network smoothing. Here, we have demonstrated that even the general context networks we have used are able to assist in identifying specific cell types from noisy scRNAseq datasets.
Optimizing the smoothing parameters by cluster robustness
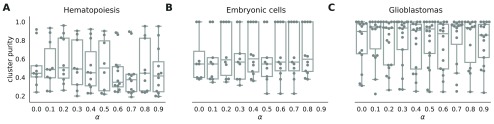
The netSmooth algorithm, given a gene network, has one free parameter - the restart rate of the random walker, (1 − α). Alternatively, α is the complement of the restart rate. An α = 0 indicates a perfect restart rate and consequently no smoothing; an α = 1 corresponds to a random walk without restarts. Intermediate values for α result in increasing levels of smoothing; the value of α determines how far random walks will go on the graph before restarting, or how far along the network a gene’s influence is allowed to reach (See Methods). It is tempting to optimize α with respect to the variable the experiment sets out to measure, e.g. cluster purity. For instance, in the embryonic development dataset, we would choose α = 0.4 as the value that produces the highest cluster purity ( Figure 11b). However, in many experiments the identity of the samples is not known a-priori. Therefore, we propose a data driven workflow to pick a sensible value for α.
Figure 11. Boxplots of cluster purity for clusters obtained by the robust clustering procedure following application of netSmooth with different values of α.
α = 0 is equivalent to not using netSmooth at all. The procedure is robust to alpha, that is, most values of alpha produce more robust clusters. A) HSPCs, B) embryonic cells, C) glioblastomas. The online version of this figure is interactive.
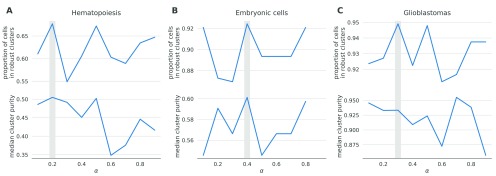
One such data-driven statistic is the proportion of samples assigned to robust clusters; following application of netSmooth, the robust clustering procedure is able to assign more samples to statistically robust clusters. For two of the three datasets, picking the α that gives the highest proportion of cells in robust clusters, also gives the clusters with the highest purity index ( Figure 12). Importantly, this metric is entirely data-driven and does not require external labels, making it feasible for any scRNA-seq study. The results in the previous sections all use the value of α picked to optimize proportion in robust clusters.
Figure 12. The proportion of cells in robust clusters, and cluster purity for those robust clusters, for a range of alpha values, shows that picking the alpha with the highest proportion in robust clusters also picks the alpha with the highest cluster purity.
A) hematopoietic stem/progenitor cells B) embryonic cells, C) glioblastomas. The online version of this figure is interactive.
The netSmooth R package 22 provides an alternative way to optimize α in the absence of true labels, by optimizing the entropy in a 2D embedding of the data. See Methods for details.
Discussion
Single cell RNA sequencing technology provides whole-genome transcriptional profiles at unprecedented throughput and resolution. However, high variance and dropout events that happen in all current scRNA-seq platforms complicate the interpretation of the data. Methods that treat 0 counts as missing values and impute them based on nonzero values in the data may amplify biases in the data.
We presented netSmooth as a preprocessing step for scRNA-seq experiments, overcoming these challenges by the use of prior information derived from protein-protein interactions or other molecular interaction networks. We demonstrated that network smoothing assists in several standard analyses that are common in scRNA-seq studies. This procedure enhances cell type identification in hematopoiesis; it elucidates time series data and assists identification of the developmental stage of single cells. Finally, it is also applicable in cancer, improving identification of tumor of origin for glioblastomas. In addition, we showed that network smoothing parameter can be optimized by cluster robustness metrics, providing a workflow when there are no other external labels to distinguish cells. We demonstrated that netSmooth can use prior information from different sources in order to achieve this. We compared netSmooth with scImpute, a statistical genome-wide imputation method, and MAGIC, a genome-wide data smoothing algorithm, and demonstrated that while scImpute and MAGIC reduce the drop-out phenomenon more than netSmooth does, netSmooth outperforms them in amplifying the biological/technical variability ratio. netSmooth provides clusters that are more homogeneous and have higher adjusted mutual information (AMI) with respect to cell types. Although, in some cases data processed by MAGIC produces more robust clusters, the clusters returned after MAGIC processing do not have higher AMI or cluster purity. Higher robustness achieved by MAGIC processing might be due to the fact that the algorithm reinforces local structures too much in the data and producing artificially similar expression profiles between cells. Comparisons to CIDR (Figures ?? - ??) also show inferior performance to netSmooth.
In most of the benchmarks we ran, scImpute shows similar performance to netSmooth, while the former relies on other data points in order to impute missing data, and the latter performs a quasi-imputation based on priors from other experiments. Our analysis shows that netSmooth affects the drop-out rate less than scImpute, while uncovering slightly more of the biological signal. This happens across the different overall drop-out rates in the 3 experiments we profiled, indicating that netSmooth can achieve better results, with less obtrusive transformations of the data, then the imputation methods, across a range of experimental conditions.
Finally, netSmooth is a versatile algorithm that may be incorporated in any analysis pipeline for any experiment where the organism in question has a high quality PPI network available. Although not shown, the algorithm is applicable to any omics data set that can be constructed as a genes-by-samples matrix, such as proteomics, SNPs and copy number variation. In addition, most of the computational load of network smoothing can be done "off-line". As such it scales well with the number of cells, which is likely to increase in future scRNA-seq experiments. We have made available an R package to that end, which is available on GitHub: https://github.com/BIMSBbioinfo/netSmooth, and Bioconductor: https://bioconductor.org/packages/release/bioc/html/netSmooth.html.
Methods and data
The data sets
The hematopoiesis dataset 15 was obtained from the Gene Expression Omnibus 23. The embryonic 18 and glioblastoma 19 datasets were obtained from conquer 24, a repository of uniformly processed scRNA-seq datasets. We have made the datasets available, see Table 1.
Table 1. Datasets and availability.
| Dataset | URL |
|---|---|
| Hematopoiesis |
https://www.ncbi.nlm.nih.gov/geo/query/acc.
cgi?acc=GSE81682 |
| Embryonic cells |
http://imlspenticton.uzh.ch/robinson_lab/
conquer/data-mae/GSE45719.rds |
| Glioblastoma |
http://imlspenticton.uzh.ch/robinson_lab/
conquer/data-mae/GSE57872.rds |
The random walks with restarts process
The netSmooth algorithm takes a graph G = { V, E} where V = { gene i} is the set of genes, and E = {( i → j)} is the set of edges between genes. The edge weights are degree-normalized, so that each gene’s outgoing edges’ weights sum to 1. We then define a process of random walk with restarts as in 13, on the PPI graph, where a conceptual random walker starts on a node in the graph (a gene/protein) and at each step walks to an adjacent node with the probability determined by the α times the edge weight. Further, at each step, there is a probability of (1 − α) that the walker restarts to its original node.
Mathematically, given a graph defined by an adjacency matrix A [ MxM], where A ij is the edge weight between gene i and gene j (and 0 for unconnected genes), and a vector f [ Mx1], where is the probability that the walker is at node i at step t, the process is defined by
This process is convergent, and the stationary distribution is given by
Hence, the random walk with restarts process is a diffusion process defined on the PPI graph, or through the diffusion kernel (smoothing kernel)
where (1 − α) is the restart probability, and A is the (column normalized) adjacency matrix of the PPI graph. Consequently, we define the network-smoothed expression profile
where E [ MxN] is the normalized count values of the M genes in the N cells.
The clustering procedure
Clustering analysis features prominently in scRNA-seq analyses; whether recapitulating known results or discovering new cell types, clustering cells by their gene expression profiles is commonly used to identify distinct populations. While some approaches directly take into account the zero-inflation of scRNA-seq data 5, other studies use traditional methods 18. There is no standard method for clustering single cell RNAseq data, as different studies produce data with different topologies, which respond differently to the various clustering algorithms.
In order to avoid optimizing different clustering routines for the different datasets we benchmark on, we have implemented a robust clustering routine based on clusterExperiment ii 16, a framework for robust clustering based on consensus clustering of clustering assignments obtained from different clustering algorithms, different parameters for these algorithms, and different views of the data. The different views are different reduced dimensionality projections of the data based on different techniques. Thus, no single clustering result will dominate the data, and only cluster structures which are robust to different analyses will prevail. The procedure we implemented using the framework is as follows:
-
1.
Perform different dimensionality reduction techniques on the data
-
•
PCA on the 500 most variable genes
-
–
with 5 components
-
–
with 15 components
-
–
with 50 components
-
–
-
•
Alternatively to PCA, t-SNE on the 500 most variable genes
-
–
with 2 dimensions
-
–
with 3 dimensions
-
–
-
•
Select the most variable genes
-
–
100 most variable genes
-
–
500 most variable genes
-
–
1000 most variable genes
-
–
-
•
-
2.
On each reduced dimension view of the data, perform PAM clustering with K ranging from 5 to 10
-
3.
Calculate the co-clustering index for each pair of samples (the proportion of times the samples are clustered together, in the different clustering results based on the different reduced dimensions and clustering parameters above)
-
4.
Find a consensus clustering from the co-clustering matrix. This is done by constructing a dendrogram using average linkage, and traversing down the tree until a block with a self-similarity of at least 0.6, and a minimum size of 20 samples emerges. (instead of using cutree).
-
5.
Perform hierarchical clustering of the cluster medioids, with similarities based on expression of the 500 most variable genes
-
6.
Perform a DE analysis between clusters that are adjacent in the hierarchy from (5), and merge them if the proportion of genes that are found to be significantly differentially expressed between them (adjP < .05) is less than than 0.1.
Using only the 500 most variable genes insures the biological variation will dominate the technical variation, and enhances the reproducibility of t-SNE 25.
Importantly, samples that at step (4) don’t have a high enough affinity to any emerging cluster, will not be assigned to any cluster. The clustering is performed using the clusterExperiment::clusterSingle and clusterExperiment::clusterMany functions, the consensus clustering is obtained using the clusterExperiment::combineMany function, and the cluster merging (steps 5 and 6) using the clusterExperiment::makeDendrogram and clusterExperiment::mergeClusters functions. For more details, see 16.
Choice of dimensionality reduction technique in the clustering procedure
In step (1) above, we cluster cells in a lower dimension embedding using either PCA 26 or t-SNE 27, in a dataset-dependent manner. Different single cell datasets respond better to different dimensionality reduction techniques which are better able to tease out the biological cluster structure of the data. In order to pick the right technique algorithmically, we compute the entropy in a 2D embedding. We obtained 2D embeddings from the 500 most variable genes using either PCA or t-SNE, binned them in a 20x20 grid, and computed the entropy using the discretize and entropy functions in the entropy R package iii 28. The entropy in the 2D embedding is a measure for the information captured by it. For the clustering procedure, we pick the embedding with the highest information content. For the hematopoiesis and glioblastoma datasets, this is t-SNE, while for the embryonic development dataset it is PCA ( Table 2). This method may be used to pick any dimensionality reduction technique other than the ones mentioned here, which might be more suitable for other analyses.
Table 2. Entropy in 2D lower dimension embeddings.
| Dataset | PCA Entropy | t-SNE Entropy |
|---|---|---|
| Hematopoiesis | 4.96 | 5.03 |
| Embryonic cells | 4.09 | 3.94 |
| Glioblastoma | 4.87 | 5.06 |
Cluster purity and adjusted mutual information
The cluster purity metric displayed above refers to the proportion of the samples in a cluster which are of the dominant cell type in that cluster. The purity for cluster i is given by
where C i = { j|cell j ∈ cluster i}, label j is the cell type of cell j, n i = | C i| is the number of cells in cluster i, and
is the dominant cell type in cluster C i.
In addition to the cluster purity metric, we computed the Adjusted Mutual Information (AMI) 29, an information theoretic measure of clustering accuracy which accounts for true positives (two cells of the same type in the same cluster) being caused by chance. The AMI between a clustering C and the true labels L is given by
where MI ( a, b) is the mutual information between labellings a and b, H( a) is entropy of clustering a, and E[ ·] denotes the expectation.
We do not compare the clusterings using the Rand index, as that measure penalizes for so-called false negatives (two cells of the same cell type but in different clusters), which is undesirable as cells from the same cell type might be rightly split into several clusters when a novel cell type is identified.
Construction of the smoothing kernel
The PPI graph from which the diffusion kernel was derived was constructed using data from string-db 10. For each pair of proteins, string-db provides a combined interaction score, which is a score indicating how confident we can be in the interaction between the proteins, given the different kinds of evidence string-db collates. We subset the links to only those above the 90th percentile of combined interaction scores, only keeping the 10% most confident interactions. For mouse that is 1,020,816 interactions among 17013 genes. For human, 852,722 interactions among 17467 genes.
MAGIC and scImpute parameters
For all the results presented in this paper, scImpute was run using the default parameters ( drop_thre = 0.5). For MAGIC, we used values for the diffusion time parameter ( T = {1, 2, 4, 8, 16}). Unlike netSmooth, for MAGIC the proportion of samples in robust clusters and the cluster purities were anti-correlated; thus we picked the one that gave the best cluster purities as the best MAGIC parameter. The chosen T values are given in Table 3. We used MAGIC version 0.1 iv and scImpute version 0.0.2 v.
Table 3. Optimal diffusion time values for MAGIC.
| Dataset | Optimal T |
|---|---|
| Hematopoiesis | 1 |
| Embryonic cells | 4 |
| Glioblastoma | 2 |
the netSmooth R package
The analysis for this paper was done using the companion netSmooth R-package 22, which is available online: https://github.com/BIMSBbioinfo/netSmooth.
The netSmooth R package was included in the 3.7 release of Bioconductor: https://bioconductor.org/packages/release/bioc/html/netSmooth.html and was developed and tested under R version 3.5.
Archived code at time of publication: https://doi.org/10.5281/zenodo.1119064.
License: GPLv3.
Acknowledgements
We would like to thank Vedran Franke, Bora Uyar and Brendan Osberg for valuable comments and input for the development of this manuscript.
Funding Statement
AA and JR are funded by core funding from Max Delbrück Center, part of Helmholtz Association.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 3; referees: 2 approved]
Footnotes
Most interactions in string-db do not specify the direction, or nature of the interaction
Version 1.4.0, available from Bioconductor https://bioconductor.org/packages/release/bioc/html/clusterExperiment.html
Version 1.2.1, available from CRAN: https://cran.r-project.org/web/packages/entropy/index.html
Available from GitHub: https://github.com/pkathail/magic.
Available from GitHub: https://github.com/Vivianstats/scImpute.
Supplementary material
Supplementary figures S1–S14.
References
- 1. Wagner A, Regev A, Yosef N: Revealing the vectors of cellular identity with single-cell genomics. Nat Biotechnol. 2016;34(11):1145–1160. 10.1038/nbt.3711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kharchenko PV, Silberstein L, Scadden DT: Bayesian approach to single-cell differential expression analysis. Nat Methods. 2014;11(7):740–742. 10.1038/nmeth.2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu AR, Neff NF, Kalisky T, et al. : Quantitative assessment of single-cell RNA-sequencing methods. Nat Methods. 2014;11(1):41–46. 10.1038/nmeth.2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pierson E, Yau C: ZIFA: Dimensionality reduction for zero-inflated single-cell gene expression analysis. Genome Biol. 2015;16:241. 10.1186/s13059-015-0805-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin P, Troup M, Ho JW: CIDR: Ultrafast and accurate clustering through imputation for single-cell RNA-seq data. Genome Biol. 2017;18(1):59. 10.1186/s13059-017-1188-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li WV, Li JJ: scimpute: Accurate and robust imputation for single cell rna-seq data. bioRxiv. 2017. 10.1101/141598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Dijk D, Nainys J, Sharma R, et al. : Magic: A diffusion-based imputation method reveals gene-gene interactions in single-cell rna-sequencing data. bioRxiv. 2017. 10.1101/111591 [DOI] [Google Scholar]
- 8. Bhardwaj N, Lu H: Correlation between gene expression profiles and protein-protein interactions within and across genomes. Bioinformatics. 2005;21(11):2730–2738. 10.1093/bioinformatics/bti398 [DOI] [PubMed] [Google Scholar]
- 9. Fraser HB, Hirsh AE, Wall DP, et al. : Coevolution of gene expression among interacting proteins. Proc Natl Acad Sci U S A. 2004;101(24):9033–9038. 10.1073/pnas.0402591101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Szklarczyk D, Morris JH, Cook H, et al. : The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45(D1):D362–D368. 10.1093/nar/gkw937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee I, Blom UM, Wang PI, et al. : Prioritizing candidate disease genes by network-based boosting of genome-wide association data. Genome Res. 2011;21(7):1109–1121. 10.1101/gr.118992.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hofree M, Shen JP, Carter H, et al. : Network-based stratification of tumor mutations. Nat Methods. 2013;10(11):1108–1115. 10.1038/nmeth.2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vandin F, Upfal E, Raphael BJ: Algorithms for detecting significantly mutated pathways in cancer. J Comput Biol. 2011;18(3):507–522. 10.1089/cmb.2010.0265 [DOI] [PubMed] [Google Scholar]
- 14. Dørum G, Snipen L, Solheim M, et al. : Smoothing gene expression data with network information improves consistency of regulated genes. Stat Appl Genet Mol Biol. 2011;10(1): pii: /j/sagmb.2011.10.issue-1/sagmb.2011.10.1.1618/sagmb.2011.10.1.1618.xml. 10.2202/1544-6115.1618 [DOI] [PubMed] [Google Scholar]
- 15. Nestorowa S, Hamey FK, Pijuan Sala B, et al. : A single-cell resolution map of mouse hematopoietic stem and progenitor cell differentiation. Blood. 2016;128(8):e20–31. 10.1182/blood-2016-05-716480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Purdom E, Risso D: clusterExperiment: Compare Clusterings for Single-Cell Sequencing. 2017; R package version 1.2.0. [Google Scholar]
- 17. Robinson MD, McCarthy DJ, Smyth GK: edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deng Q, Ramsköld D, Reinius B, et al. : Single-cell RNA-seq reveals dynamic, random monoallelic gene expression in mammalian cells. Science. 2014;343(6167):193–196. 10.1126/science.1245316 [DOI] [PubMed] [Google Scholar]
- 19. Patel AP, Tirosh I, Trombetta JJ, et al. : Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396–1401. 10.1126/science.1254257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Petryszak R, Keays M, Tang YA, et al. : Expression Atlas update--an integrated database of gene and protein expression in humans, animals and plants. Nucleic Acids Research. 2016;44(D1):D746–D752. 10.1093/nar/gkv1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee I, Blom UM, Wang PI, et al. : Prioritizing candidate disease genes by network-based boosting of genome-wide association data. Genome Res. 2011;21(7):1109–1121. 10.1101/gr.118992.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ronen J, Akalin A: netSmooth: Net-work smoothing for scRNAseq. 2018; R package version 1.0. Reference Source [Google Scholar]
- 23. Edgar R, Domrachev M, Lash AE: Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30(1):207–210. 10.1093/nar/30.1.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Soneson C, Robinson MD: Bias, robustness and scalability in differential expression analysis of single-cell rna-seq data. bioRxiv. 2017. 10.1101/143289 [DOI] [PubMed] [Google Scholar]
- 25. McCarthy DJ, Campbell KR, Lun AT, et al. : Scater: pre-processing, quality control, normalization and visualization of single-cell rna-seq data in r. Bioinformatics. 2017;33(8):1179–1186. 10.1093/bioinformatics/btw777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hastie T, Friedman J, Tibshirani R: The Elements of Statistical Learning. Springer Series in Statistics Springer New York Inc., New York, NY, USA,2001. 10.1007/978-0-387-21606-5 [DOI] [Google Scholar]
- 27. van der Maaten LJP, Hinton GE: Visualizing high-dimensional data using t-sne. 2008. Reference Source [Google Scholar]
- 28. Hausser J, Strimmer K: entropy: Estimation of Entropy, Mutual Information and Related Quantities. R package version 1.2.1.2014. Reference Source [Google Scholar]
- 29. Vinh NX, Epps J, Bailey J: Information theoretic measures for clusterings comparison: Variants, properties, normalization and correction for chance. J Mach Learn Res. 2010;11:2837–2854. Reference Source [Google Scholar]