Abstract
More than 20 years after the clinical high risk syndrome for psychosis (CHR) was first articulated, it remains controversial whether the CHR syndrome predicts onset of psychosis with diagnostic specificity or predicts pluripotential diagnostic outcomes. Recently, analyses of observational studies, however, have suggested that the CHR syndrome is not pluripotential for emergent diagnostic outcomes. The present report conducted additional analyses in previously reported samples to determine (1) whether comorbid disorders were more likely to persist in CHR patients compared to a comparison group of patients who responded to CHR recruitment efforts but did not meet criteria, termed help-seeking comparison subjects (HSC); and (2) whether clinically defined pluripotential CHR subgroups could be identified. All data were derived from 2 multisite studies in which DSM-IV structured diagnostic interviews were conducted at baseline and at 6-month intervals. Across samples we observed persistence of any nonpsychotic disorder in 80/147 CHR cases (54.4%) and in 48/84 HSC cases (57.1%, n.s.). Findings with persistence of anxiety, depressive, and bipolar disorders considered separately were similar. Efforts to discover pluripotential CHR subgroups were unsuccessful. These findings add additional support to the view that the CHR syndrome is not pluripotential for predicting various diagnostic outcomes but rather is specific for predicting emergent psychosis.
Keywords: prodrome, diagnosis, outcome, depression, bipolar, anxiety, pluripotential, clinical high risk
Substantial research effort over the last 2 decades has been directed toward prospectively identifying a group of patients who are at clinical high risk for psychosis (CHR).1 Structured diagnostic instruments for CHR such as the Structured Interview for Psychosis-risk Syndromes (SIPS)2 and Comprehensive Assessment of At-Risk Mental States (CAARMS)3 have achieved excellent levels of diagnostic reliability.4–18 The CHR designation has been used in studies aiming to elucidate the pathophysiology of developing psychosis19–22 and as an indicator of enhanced need for clinical and specialty services.23,24
While evidence has accumulated that the CHR syndrome constitutes a disorder and not merely a state of risk25–27 there has been concern about whether clinical outcomes in CHR patients are specific to psychosis or whether patients so identified are diagnostically pluripotential.28–31 This issue is an important one, because if the CHR syndrome is indeed diagnostically pluripotential, then neurobiologic and biomarker studies of CHR are not investigating the pathophysiology of psychosis but rather the pathophysiology of psychiatric disorder more generally. Moreover, if the CHR syndrome is diagnostically pluripotential its clinical utility might be no more useful than that of a brief general psychopathology screen.
Since the issue is an important one, the field should be careful to describe what we mean when we use the term “pluripotential.” The Oxford English Dictionary defines it as “pluripotent” or “capable of differentiating into more than one type (of mature cell or tissue, current authors” parentheses).32 Merriam-Webster33 also redirects to “pluripotent” and defines it more generally as “not fixed as to developmental potentialities” in addition to the more restrictive sense relating to cell types.
We note that defining “pluripotential” as involving “differentiation” or “development” over time excludes the simple presence of diagnostic comorbidity at single baseline assessment, which does not involve change over time, from qualifying by itself as “pluripotential.” This exclusion pertains even though the proportion of those with CHR that have co-morbid Axis I diagnoses at ascertainment is high.34–41 In this context, it is worth emphasizing that diagnostic comorbidity at baseline is not unique to CHR and that comorbidity rates are high in general when structured interviews are performed.42 Large-scale studies have reported lifetime comorbidity rates of 56%43 and 60%44,45 in epidemiologic samples; 12-month rates were also high at 45%46 and 46%.47 Comorbidity is also common in studies of schizophrenia48–52 and first episode psychosis.53 In addition, baseline comorbidity in CHR studies so far has not predicted emergence of psychosis.34,35,37,54
In keeping with the Oxford and Meriam-Webster definitions, studies investigating the diagnostic pluripotentiality of the CHR syndrome to date have addressed syndromal differentiation and development, as indexed by the emergence of new diagnoses that were not present at baseline assessment. Two studies investigating CHR pluripotentiality as emergence of new disorder have been reported.55,56 The findings of our previous paper55 did not support a view that the CHR syndrome as defined by the SIPS is pluripotential for emergent diagnostic outcomes. In a combined sample from 2 observational studies (n for CHR = 271), psychosis was the only emergent disorder that significantly differed between CHR and a comparison group who answered CHR recruitment efforts but did not meet CHR criteria (help-seeking comparison subjects, HSCs). Nonpsychotic disorders newly emerged in CHR patients at fairly low rates that were no different from those in HSCs. Recently, these findings were replicated in a larger study of 710 CHR and 299 non-CHR patients as defined by the CAARMS,56 where again no evidence of diagnostic pluripotentiality for new emergent disorders was found. Taken together these 2 papers reporting results from 3 samples suggest that the CHR syndrome does not appear pluripotential with regard to emergent diagnoses but rather is specifically associated with an increased risk for emergent psychotic disorders.
Even if the CHR syndrome is not diagnostically pluripotential for emergent disorders, however, it is possible to consider whether it could be diagnostically pluripotential in other ways. If, eg, comorbid nonpsychotic disorders present at baseline were more likely to persist in CHR patients than in a suitable comparison group, such a pattern could be consistent with pluripotential diagnostic outcomes, since the CHR diagnosis would more often “differentiate” or “develop” into a more chronic form of nonpsychotic disorder. Here, we sought to determine if the CHR syndrome is pluripotential for persistence of nonpsychotic disorder by comparing persistence rates of baseline comorbid disorders in CHR patients and in a non-CHR comparison group.
In addition, even if the CHR syndrome as currently defined is not pluripotential either for emergence of new disorder or for persistence of baseline disorder, it is possible that pluripotential subgroups could be identified. Therefore in the present article, we also sought to discover CHR subgroups that could be pluripotential, either for the emergence of new disorder or for the persistence of baseline disorder.
Method
We report data from 2 cohorts of CHR syndrome patients that also featured a comparison group of patients who were identified by the same ascertainment procedures but who did not meet CHR syndrome criteria on evaluation. The second group of patients are termed “help-seeking comparison subjects” (HSCs). The 2 cohorts were the North American Prodromal Longitudinal Study first sample (NAPLS-1) and the PREDICT study. Methods for both studies have been previously reported.13,34 Written informed consent was obtained from all subjects/parents/legal guardians, and the research was approved by institutional review boards at each site, consistent with ethical principles outlined in the Declaration of Helsinki.
Subjects
NAPLS-1 merged data collected at 8 sites on 160 CHR syndrome and 100 HSC patients enrolled between early 1998 and early 2005 who did not overlap with the PREDICT study (supplementary figure S1) and underwent structured diagnostic interviews for DSM-IV Axis I diagnoses at baseline and also at one or more follow-up evaluations.55 Rates of baseline comorbidity have been previously reported.41,55 Rates of follow-up comorbidity have been previously reported in a partial sample, but only within the CHR group and without distinction between emergent and persistent cases.14
PREDICT was conducted at 3 of the NAPLS-1 sites and enrolled 111 CHR syndrome and 71 HSC patients between late 2003 and early 2008 who underwent structured diagnostic interviews for DSM-IV Axis I diagnoses at baseline and at one or more follow-up evaluations.55 Rates of baseline comorbidity have been reported previously.55
Supplementary figure S1 shows the CONSORT diagram for the persistence analyses. For NAPLS-1 a total of 39 CHR and one HSC converted to psychosis and were excluded from the persistence analyses in the present article, leaving 121 CHR and 99 HSC. Exclusion of cases without baseline comorbidity among this group left 97 and 51, and exclusion of those without follow-up diagnostic coding for current disorder left 92 and 47, respectively. For PREDICT, 14 CHR and 2 HSC converted to psychosis and were removed from the persistence analyses, leaving 97 CHR and 69 HSC. Exclusion of cases without baseline comorbidity left 66 and 42, and exclusion of those without follow-up coding for current disorder left 55 and 37, respectively. Our previous report contains the CONSORT diagram for the emergence analyses.55
Data on the recruitment sources in these 2 studies were not collected systematically, but recruitment methods were broadly similar55 to those reported in the later NAPLS-2 sample.57 Inclusion and exclusion criteria were also similar in the 2 studies.55
Assessments
Each site in both studies utilized the SIPS to determine whether psychosis and CHR syndrome criteria were met.2 Reliability of the SIPS was established in these studies for all sites.13 Structured assessment of DSM-IV Axis-I diagnoses in NAPLS-1 varied somewhat within and across site.41,55 PREDICT employed the SCID-NP58 for participants 16 and older and the K-SADS59 for those 15 and under. Follow-up assessments were available at 6-month intervals in both studies, out to 30 months in NAPLS-1 and to 48 months in PREDICT.
Depressive and anxiety comorbidities are the most common in CHR patients,21 and we felt it is important to distinguish between bipolar and nonbipolar disorders. Accordingly, nonpsychotic DSM-IV Axis-I diagnoses were classified into 3 groups: bipolar disorders (DSM-IV nonpsychotic bipolar I disorder, bipolar disorder NOS, bipolar II disorder, and cyclothymic disorder), nonbipolar affective disorders (DSM-IV nonpsychotic major depressive disorder, dysthymic disorder, depressive disorder NOS, and mood disorder NOS), and anxiety disorders (panic disorder with or without agoraphobia, generalized anxiety disorder, agoraphobia without panic disorder, social phobia, specific phobia, obsessive compulsive disorder, post-traumatic stress disorder, separation anxiety disorder, and anxiety disorder NOS). These diagnoses collectively are referred to hereafter as “nonpsychotic disorders.”
We defined persistence of nonpsychotic disorders as follows: presence of a current nonpsychotic disorder at any time point after baseline when a lifetime nonpsychotic disorder from the same class was recorded at baseline. We defined persistence of any nonpsychotic disorder similarly as the presence of any current nonpsychotic disorder at any time point after baseline when a lifetime nonpsychotic disorder from the same class was recorded at baseline. In NAPLS-1, follow-up diagnoses were designated as current if present in the past month. The follow-up protocol in NAPLS-1 did not include assessment of post-traumatic stress disorder, separation anxiety disorder, or anxiety disorder NOS and thus persistence of these disorders could not be evaluated for that study. The PREDICT study designated follow-up diagnoses as current if they were present in the past 6 months. Because of this 6-month time-frame, PREDICT evaluations at 6 months were not considered eligible for persistence evaluation. We defined emergent psychosis and emergent nonpsychotic disorders as previously.55
Data on psychotropic medication use at baseline in the samples have been reported previously.34,55,60–62 Data on psychosocial treatment utilization at baseline has been reported for NAPLS-1.61 The PREDICT study collected similar data were at baseline for individual, family, and group professional psychotherapy. Duration of CHR syndrome data were collected from the SIPS. Both studies used with the Global Assessment of Functioning63 to assess current functioning at baseline.
Statistical Methods
Analyses used SPSS version 24. P values <.05 were considered statistically significant.
Analyses of persistent disorder were restricted to nonconverting subjects with baseline nonpsychotic disorder. The primary analyses employed logistic regression with the dependent variable specified as persistent disorder yes vs no. The models incorporated terms for CHR diagnosis (CHR vs HSC), study (NAPLS-1 vs PREDICT), and their interaction; we dropped the interaction term when its inclusion did not significantly improve the model or when models did not converge. In cases where interaction terms did significantly improve the model, we report study-specific simple main effects and whether the overall main effects are interpretable (supplementary material). To determine whether baseline differences between CHR and HSC could have confounded persistence results, we first tested for differences on baseline measures (from our previous report55) and on follow-up duration (supplementary table S1) using Student’s t-tests and Fisher’s exact tests. Variables on which CHR and HSC groups differed significantly in either study were then evaluated in logistic regression models as potential confounders.
In the search for possibly pluripotential CHR subgroups, our first step was to evaluate predictors of persistence and emergence that could then be used to define subgroups. Details of these prediction analyses are included in the supplement. For variables that significantly predicted persistent or emergent disorder we created CHR subgroups, using for continuous measures the cut-point that maximized Cohen’s kappa.64 Prediction-defined CHR subgroups were considered pluripotential for persistence if nonpsychotic disorders persisted significantly more often than in HSCs or pluripotential for emergence if the ratio of emergent psychosis to emergent nonpsychotic disorder was reduced to the level seen in HSCs.
Results
Persistence of Baseline Nonpsychotic Disorder
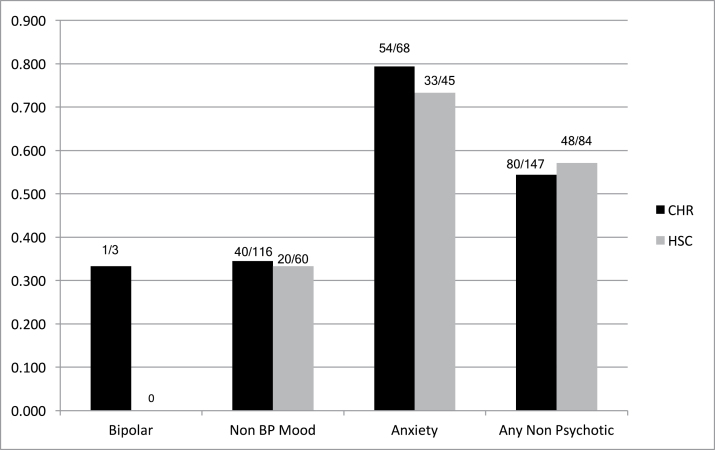
Persistence of any nonpsychotic disorder was observed in 80/147 CHR cases (54.4%) and in 48/84 HSC cases (57.1%, figure 1) across the 2 study samples. Logistic regression revealed a significant main effect of study (higher persistence in NAPLS-1) but no significant main effect of CHR vs HSC status (table 1) or interaction. Findings for the component disorder groups (persistence of bipolar, nonbipolar affective, and anxiety disorders considered separately) were similar (figure 1, table 1), although effects of study were not significant for persistence of nonbipolar affective disorder. The relative persistence of bipolar disorders could not be analyzed statistically due to low frequencies of baseline bipolar disorder.
Fig. 1.
Persistence of DSM-IV baseline nonpsychotic disorder. All comparisons n.s.
Table 1.
Persistence Rates in Patients With Baseline Nonpsychotic Disorders
| NAPLS-1 | PREDICT | NAPLS-1 Vs PREDICT | CHR Vs HSC | |||||
|---|---|---|---|---|---|---|---|---|
| Persistence of Disorder | CHR | HSC | CHR | HSC | OR | 95% CI | OR | 95% CI |
| Any nonpsychotic | 52/92 (56.5%) | 33/47 (70.2%)a | 28/55 (50.9%) | 15/37 (40.5%)a | 1.81 | 1.06–3.10 | 0.86 | 0.50–1.48 |
| Bipolar affective | 1/3 (33.0%) | 0/0 (0.0%) | 0/0 (0.0%) | 0/0 (0.0%) | NA | NA | NA | NA |
| Non-BP affective | 25/74 (33.8%) | 11/36 (30.6%) | 15/42 (37.5%) | 9/24 (37.5%) | 0.85 | 0.45–1.61 | 1.06 | 0.55–2.05 |
| Anxiety | 35/39 (89.7%)b | 25/26 (96.2%)c | 19/29 (65.5%)b | 8/19 (42.1%)c | 9.50 | 3.22–28.0 | 1.53 | 0.57–4.09 |
aGroups with this letter differ P < .01, Fisher’s exact test.
bGroups with this letter differ P < .05, Fisher’s exact test.
cGroups with this letter differ P ≤ .001, Fisher’s exact test.
Supplementary table S1 compares CHR samples to HSC samples on baseline characteristics to search for potential confounders of the persistence analyses. In NAPLS-1, CHR patients were older, had higher SOPS total, positive, and disorganization scores, and were more likely to be on psychotropic medication. In PREDICT, mean follow-up duration was approximately 5 months longer in HSCs. Testing these potential confounders of the CHR vs HSC effect on persistence significantly improved the model in 3 instances: 1 for the prediction of any nonpsychotic disorder and 2 for the prediction of persistence of nonbipolar affective disorder (supplementary table S2); however, although the model, improved inclusion of the potential confounder produced little change in the CHR vs HSC effect on persistence in any of the 3 instances.
Possible Subgroups Pluripotential for Persistence
The initial predictor analyses of persistent disorder found 4 variables to be significantly predictive, but none of them yielded CHR subgroupings that met the pluripotentiality criterion. Higher parental education predicted increased persistence of any nonpsychotic disorder, age and qualifying only for the Attenuated Psychotic Symptoms Syndrome (APSS) CHR subgroup predicted increased persistence of nonbipolar affective disorder, and higher global functioning predicted increased persistence of anxiety disorder (supplementary table S3). SOPS positive symptoms did not predict persistence of nonpsychotic disorder. Predictors of bipolar disorder persistence could not be evaluated due to the small sample sizes. Cohen’s kappa analyses within the combined CHR groups showed that the optimal subgrouping cut-off for parental education was 4-year college degree or higher vs less than a 4-year college degree, for baseline age greater than 19 vs 19 or lower, and for global functioning greater than 45 vs 45 or lower. Although these predictors were statistically significant within the combined CHR group, CHR subgroups based on the optimal cut-offs did not show persistence significantly higher than in HSCs (supplementary material and supplementary table S4). The APSS-only CHR subgroup similarly did not show persistence significantly higher than in HSCs (supplementary material and supplementary table S4).
Possible Subgroups Pluripotential for Emergence
The initial predictor analyses of emergent psychosis vs emergent nonpsychotic disorder found only one variable to be significantly predictive, and it did not yield a subgroup that met the pluripotential criterion. Baseline antipsychotic medication predicted emergent psychosis relative to emergent nonbipolar affective disorder (supplementary table S5). In the no-antipsychotic CHR subgroup the ratio of emergent psychosis relative to emergent nonbipolar affective disorder was lower than in the full CHR group (supplementary material), as expected from supplementary table S5, but was still significantly higher than in HSCs (supplementary table S6). Predictors of psychosis vs bipolar disorder emergence and possible subgroupings could not be evaluated due to the small bipolar sample sizes.
A number of measures predicted emergent nonpsychotic disorder relative to no emergent disorder (supplementary table S7). SOPS total, negative, and general symptoms predicted emergent nonbipolar affective disorder relative to no emergent disorder. Older age and high SOPS general symptoms predicted emergent anxiety disorder, and high SOPS general symptoms also predicted the emergence of any nonpsychotic disorder. SOPS positive symptoms did not predict emergence of nonpsychotic disorders.
Discussion
The principal findings of the present analyses are (1) baseline nonpsychotic disorders persisted at rates that were similar in patients with CHR and a comparison group of patients who responded to CHR recruitment efforts but did not meet CHR criteria (figure 1 and table 1), and (2) no CHR subgroupings could be identified that met criteria for pluripotentiality, either for persistence or emergence of disorder (supplementary tables S3–S6). In addition, since positive symptoms provide the principal basis for the CHR diagnosis,7,26 our results showing that SOPS positive symptoms did not predict persistence or emergence of nonpsychotic disorder (supplementary tables S3, S5, and S7) also support a lack of diagnostic pluripotentiality for the CHR syndrome. Our current findings thus join with previous evidence55,56 suggesting that the CHR syndrome is specific for predicting psychotic diagnostic outcomes rather than predicting a pluripotential variety of psychotic and nonpsychotic outcomes. Our findings also align with those of another article in the current special issue reporting that risk syndromes for nonpsychotic disorder are associated with a roughly 4-fold lower risk of psychosis than the CHR syndrome for psychosis.65
Persistence of Baseline Nonpsychotic Disorder
Early in the development of the CHR field, studies showed high rates of nonpsychotic diagnoses in follow-up of small samples but either did not report on baseline diagnoses or did not relate follow-up to baseline to determine whether follow-up diagnoses were emergent or persistent.66–69 Moreover, early studies did not include a help-seeking comparison group like the current one who responded to CHR recruitment efforts but did not meet CHR criteria and so could not distinguish effects of CHR from more general effects of help-seeking psychopathology. As noted in the introduction, our article from 201555 and a more recent one from a British group56 found that emergent nonpsychotic disorders were relatively uncommon in CHR patients and developed at similar rates in CHR and in non-CHR comparison subjects. The present findings, showing similar persistence of baseline nonpsychotic disorder in CHR patients in relation to non-CHR comparison subjects, appear to be the first in the literature.
A recent study did find that follow-up affective and anxiety disorders were present at similar rates in CHR patients and a non-CHR comparison group but did not distinguish emergent follow-up disorders from persistent ones.70 Also recently, 2 additional studies have reported data in CHR patients (but not compared to a comparison group) from which persistence rates can be calculated in order to compare with the current CHR sample. Lin et al71 reported data on 203 CHR patients as defined by the CAARMS who were followed up after a mean 7 years (range 2–14). At follow-up 53.8% of those with baseline affective disorder (78/145) received affective diagnoses and 37.5% of those with baseline anxiety disorder (33/88) received anxiety diagnoses. Rutigliano et al72 reported on 74 CHR patients as defined by the CAARMS and followed up after a mean 6 years (range 4–10). At follow-up, 40.7% of those with baseline affective disorder (11/27) received affective diagnoses and 50% of those with baseline anxiety disorder (4/8) received anxiety diagnoses. In these studies, the affective disorder persistence rates were somewhat higher than ours (34.5%) and the anxiety disorder persistence rates are somewhat lower than ours (79.4%, figure 1). Inconsistencies across studies may relate to methodologic differences such as the assessment of post-baseline disorder at a single follow-up point relative to at any of several follow-along points. In addition, the second paper included converters in the sample.72 Other possibilities include use at ascertainment of the CAARMS, whose CHR criteria differ somewhat from those of the SIPS,7,73 particularly in their more inclusive criteria for the brief intermittent psychosis CHR subtype, many of whom meet full psychosis criteria on the SIPS.74 The significantly lower rate of persistence of comorbid nonpsychotic disorders in one previously reported CAARMS-defined BLIPS sample,71 however, suggests that their inclusion in the current study would have reduced pluripotentiality for persistence rather than enhancing it. Lastly, possible differences in recruitment strategies75 could also have affected pretest risks.76
Epidemiologic data on persistence of nonpsychotic disorder would be useful as a comparison to ours. Two studies of which we are aware report such data, both in samples of youth, one from the US47 and one from Australia.77 Each study derived persistence data from their cross-sectional designs by dividing 30-day prevalence rates by 12-month prevalence rates, a method that is not directly comparable to ours. In particular, the denominator of prevalence at any point in the previous 12 months differs from ours in assessing disorder over a longer timeframe and in being retrospectively ascertained. Acknowledging these methodological differences, these studies reported persistence rates for nonbipolar depression as 31.8%47 and 24%,77 as opposed to our 34.5% in CHR and 33.3% in HSC (figure 1). Persistence rates for anxiety disorders were 60.1%47 and 49%,77 as opposed to our 79.4% in CHR and 73.3% in HSC (figure 1). The somewhat higher rates of nonpsychotic disorder persistence in both CHRs and HSCs than in either epidemiologic study could well be due to methodologic differences. Even discounting the method variance, however, the higher rates in the CHRs do not suggest that the CHR syndrome is pluripotential for persistent disorder, because our rates were also higher in the HSCs who do not meet CHR criteria. Instead such differences if meaningful would suggest that patients who present for CHR diagnostic evaluation may be at higher risk for nonpsychotic disorder persistence than epidemiologic subjects who meet diagnostic criteria but do not necessarily have need for care.
Search for Subgroups Pluripotential for Persistent or Emergent Disorder
No other studies to our knowledge have used prediction analyses to search for possibly pluripotential CHR subgroups, either for emergence or persistence, so our inability to find pluripotential subgroups is in need of replication.
With regard to the native CHR subtypes, the current data unfortunately shed little light on the present controversy regarding the status of the brief intermittent psychosis (BIPS) CHR subtype,74,78 because, as typical of SIPS-defined samples,41 there were few cases. Of 6 BIPS cases in the prediction of emergent disorder analysis (supplementary tables S5 and S7), 3 developed emergent psychosis, 1 emergent nonpsychotic bipolar disorder, and 2 no emergent disorder. One BIPS case with baseline comorbid anxiety (supplementary table S1) developed persistent anxiety disorder (supplementary table S3). One previous study found a lower rate of emergent nonpsychotic disorder in CAARMS-defined brief intermittent psychosis,74 although this effect was apparently not significant in another study.71 This second study as noted above reported that baseline brief limited psychosis was significantly associated with lower odds of persistent nonpsychotic disorder.71
Strengths and Limitations
The main strengths of the current report are the inclusion of 2 studies, the non-CHR comparison group, and the prospective follow-along at multiple time points after baseline.
The main limitation is sample size, especially for the subgrouping analyses. While the samples of the 2 combined studies are large for some purposes, they become smaller when sorted into groups with and without baseline comorbidity. Then when predictors are uncommon, as for CHR subtypes other than APSS and some of the medication variables, confidence intervals became relatively wide. Sample size did not permit following international guidelines for statistical model development79 and so may have permitted overfitting. Similar analyses should be conducted in additional and larger samples.
Another limitation, also related to sample size, is our description of baseline disorders current at any follow-along time point as “persistent.” The multiple time points available within subject could theoretically permit delineation of more complex patterns such as continuous persistence, recurrence, persistence followed by remission and then relapse, etc, which could possibly differ between CHR and non-CHR groups. The current sample, however, is small for testing differences in multiple complex patterns, a difficulty exacerbated by the variable number of time points available across subject and across study.
A third limitation is our reliance on persistence rates in our help-seeking non-CHR comparison group rather than comparing to epidemiologic, general population samples. It can be argued, however, that the current non-CHR subjects are the optimal ecological comparison group because they are like the CHR subjects in every way except that they did not meet CHR criteria at baseline interview.55 Comparisons with general population samples would be welcome in addition, but unfortunately we were unable to locate such studies with longitudinal methods similar to ours.
Fourthly, neither study permitted longitudinal assessment of personality disorders. Our data therefore cannot speak to the possibility that the CHR syndrome could be pluripotential with regard to personality disorders, either for emergence of new disorder or for persistence of baseline disorder.
Other limitations include the DSM-IV structured interview heterogeneity in these samples as discussed previously.55 Additional studies of the current questions that utilize a homogenous structured interview method are needed. In addition, the timeframes for current disorder differed across study.
Implications
In conclusion, in our samples nonpsychotic disorders did not persist in CHR patients at rates significantly higher than in help-seeking non-CHR comparison subjects, and efforts to find subgroups where nonpsychotic disorders did persist or emerged at higher rates were unsuccessful. Although confirmation is needed in other samples, these findings do not suggest that the CHR syndrome is diagnostically pluripotential, either for emergence of new disorders or for persistence of baseline disorder, but rather that it is specific for risk for emergent psychosis. These findings have led some workers to propose that nomenclature be revised to specify what the clinical high risk syndrome carries risk for, as in “CHR syndrome for psychosis” or “CHR-P.”1
Our findings are restricted to the question of diagnostic pluripotentiality. There is no doubt that the CHR syndrome is pluripotential for psychosis outcomes, with only about a quarter converting to frank psychosis80 and the remainder remitting or persisting with subsyndromal symptoms.81 These nonconversion outcomes are so common that the short-hand term “prodrome for psychosis” is universally considered inappropriate when used prospectively. Coinage of a new short-hand term, such as “pludrome for psychosis,” might be considered.
The implication of psychosis risk specificity for biomarker studies of CHR is that these studies do appear to be investigating a population where candidate markers could potentially relate specifically to psychosis rather than to general psychopathology. That said, such studies should confirm the specificity of predictive markers by applying them to nonpsychotic disorder samples as well and by investigating the impact of baseline comorbidity on results.82
Implications for clinical practice are more nuanced. CHR patients do frequently experience nonpsychotic disorders, especially comorbidly at presentation, and these disorders often persist. CHR clinics therefore must be fully prepared to diagnose and treat them, and future clinical research should determine the degree to which treatment-related changes in nonpsychotic symptoms reduce the chance of conversion to psychosis and overall impairment. However, as a prognostic indicator the CHR diagnosis appears useful specifically for emergence of future psychotic disorders and not for the emergence of new nonpsychotic disorders or for more persistent forms of baseline disorder.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
This work was supported by the National Institute of Mental Health (U01 MH066160 to S.W.W., U01 MH066134 to J.A., R01 MH066069 and P50 MH064065 to D.O.P., R01 MH060720 and K24 MH076191 to K.S.C., R01 MH065079 to T.D.C., R01 MH061523 to B.A.C., U01 MH065562 and P50 MH080272 to L.J.S., R21 MH075027 to M.T.T., R01 MH062066 to E.F.W., K05 MH001654 to T.H.M.); Donaghue Foundation (to S.W.W.); and Eli Lilly & Co (to T.H.M., J.A., and D.O.P.).
Supplementary Material
Acknowledgments
The authors have declared there are no conflicts of interest in relation to the subject of this study.
References
- 1. Fusar-Poli P. The Clinical High-Risk State for Psychosis (CHR-P), Version II. Schizophr Bull. 2017;43:44–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McGlashan TH, Walsh BC, Woods SW.. The Psychosis-Risk Syndrome: Handbook for Diagnosis and Follow-Up. Oxford University Press; 2010. [Google Scholar]
- 3. Yung A, Phillips L, McGorry P.. Treating Schizophrenia in the Prodromal Phase. Taylor & Francis; 2004. [Google Scholar]
- 4. Carol EE, Mittal VA. Resting cortisol level, self-concept, and putative familial environment in adolescents at ultra high-risk for psychotic disorders. Psychoneuroendocrinology. 2015;57:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walker EF, Brennan PA, Esterberg M, Brasfield J, Pearce B, Compton MT. Longitudinal changes in cortisol secretion and conversion to psychosis in at-risk youth. J Abnorm Psychol. 2010;119:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miller TJ, McGlashan TH, Rosen JL et al. . Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159:863–865. [DOI] [PubMed] [Google Scholar]
- 7. Miller TJ, McGlashan TH, Rosen JL et al. . Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703–715. [DOI] [PubMed] [Google Scholar]
- 8. Addington J, Stowkowy J, Cadenhead KS et al. . Early traumatic experiences in those at clinical high risk for psychosis. Early Interv Psychiatry. 2013;7:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meyer SE, Bearden CE, Lux SR et al. . The psychosis prodrome in adolescent patients viewed through the lens of DSM-IV. J Child Adolesc Psychopharmacol. 2005;15:434–451. [DOI] [PubMed] [Google Scholar]
- 10. Comparelli A, Savoja V, Kotzalidis GD et al. . Factor-structure of the Italian version of the Scale of Prodromal Symptoms (SOPS): a comparison with the English version. Epidemiol Psychiatr Sci. 2011;20:45–54. [DOI] [PubMed] [Google Scholar]
- 11. Lindgren M, Manninen M, Kalska H et al. . Predicting psychosis in a general adolescent psychiatric sample. Schizophr Res. 2014;158:1–6. [DOI] [PubMed] [Google Scholar]
- 12. Loewy RL, Therman S, Manninen M, Huttunen MO, Cannon TD. Prodromal psychosis screening in adolescent psychiatry clinics. Early Interv Psychiatry. 2012;6:69–75. [DOI] [PubMed] [Google Scholar]
- 13. Addington J, Cadenhead KS, Cannon TD et al. ; North American Prodrome Longitudinal Study North American Prodrome Longitudinal Study: a collaborative multisite approach to prodromal schizophrenia research. Schizophr Bull. 2007;33:665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Addington J, Cornblatt BA, Cadenhead KS et al. . At clinical high risk for psychosis: outcome for nonconverters. Am J Psychiatry. 2011;168:800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kimhy D, Corcoran C, Harkavy-Friedman JM, Ritzler B, Javitt DC, Malaspina D. Visual form perception: a comparison of individuals at high risk for psychosis, recent onset schizophrenia and chronic schizophrenia. Schizophr Res. 2007;97:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kline E, Thompson E, Demro C, Bussell K, Reeves G, Schiffman J. Longitudinal validation of psychosis risk screening tools. Schizophr Res. 2015;165:116–122. [DOI] [PubMed] [Google Scholar]
- 17. Yung AR, Yuen HP, McGorry PD et al. . Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry. 2005;39:964–971. [DOI] [PubMed] [Google Scholar]
- 18. Fusar-Poli P, Hobson R, Raduelli M, Balottin U. Reliability and validity of the Comprehensive Assessment of the At Risk Mental State, Italian version (CAARMS-I). Curr Pharm Des. 2012;18:386–391. [DOI] [PubMed] [Google Scholar]
- 19. Fusar-Poli P, Borgwardt S, Bechdolf A et al. . The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70:107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brent BK, Thermenos HW, Keshavan MS, Seidman LJ. Gray matter alterations in schizophrenia high-risk youth and early-onset schizophrenia: a review of structural MRI findings. Child Adolesc Psychiatr Clin N Am. 2013;22:689–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dutt A, Tseng HH, Fonville L et al. . Exploring neural dysfunction in ‘clinical high risk’ for psychosis: a quantitative review of fMRI studies. J Psychiatr Res. 2015;61:122–134. [DOI] [PubMed] [Google Scholar]
- 22. Nagai T, Tada M, Kirihara K, Araki T, Jinde S, Kasai K. Mismatch negativity as a “translatable” brain marker toward early intervention for psychosis: a review. Front Psychiatry. 2013; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. NHS England. Achieving Better Access to Mental Health Services by 2020 2014. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/361648/mental-health-access.pdf.
- 24. National Institute for Health and Care Excellence. Psychosis and Schizophrenia in Children and Young People: Recognition and Management. 2016. https://www.nice.org.uk/guidance/cg155/ [PubMed] [Google Scholar]
- 25. Fusar-Poli P, Rocchetti M, Sardella A et al. . Disorder, not just state of risk: meta-analysis of functioning and quality of life in people at high risk of psychosis. Br J Psychiatry. 2015;207:198–206. [DOI] [PubMed] [Google Scholar]
- 26. Woods SW, Miller TJ, McGlashan TH. The “prodromal” patient: both symptomatic and at-risk. CNS Spectr. 2001;6:223–232. [DOI] [PubMed] [Google Scholar]
- 27. Ruhrmann S, Schultze-Lutter F, Klosterkötter J. Probably at-risk, but certainly ill—advocating the introduction of a psychosis spectrum disorder in DSM-V. Schizophr Res. 2010;120:23–37. [DOI] [PubMed] [Google Scholar]
- 28. Fusar-Poli P, Yung AR, McGorry P, van Os J. Lessons learned from the psychosis high-risk state: towards a general staging model of prodromal intervention. Psychol Med. 2014;44:17–24. [DOI] [PubMed] [Google Scholar]
- 29. Fusar-Poli P, Carpenter WT, Woods SW, McGlashan TH. Attenuated psychosis syndrome: ready for DSM-5.1?Annu Rev Clin Psychol. 2014;10:155–192. [DOI] [PubMed] [Google Scholar]
- 30. McGorry P, van Os J. Redeeming diagnosis in psychiatry: timing versus specificity. Lancet. 2013;381:343–345. [DOI] [PubMed] [Google Scholar]
- 31. Nieman DH, McGorry PD. Detection and treatment of at-risk mental state for developing a first psychosis: making up the balance. Lancet Psychiatry. 2015;2:825–834. [DOI] [PubMed] [Google Scholar]
- 32. Oxford English Dictionary. 2017http://www.oed.com/view/Entry/146211?
- 33. Merriam-Webster. 2017. https://www.merriam-webster.com/dictionary/pluripotent
- 34. Addington J, Liu L, Perkins DO, Carrion RE, Keefe RS, Woods SW. The role of cognition and social functioning as predictors in the transition to psychosis for youth with attenuated psychotic symptoms. Schizophr Bull. 2017;43:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Addington J, Piskulic D, Liu L et al. . Comorbid diagnoses for youth at clinical high risk of psychosis. Schizophr Res. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Salokangas RK, Ruhrmann S, von Reventlow HG et al. ; EPOS group Axis I diagnoses and transition to psychosis in clinical high-risk patients EPOS project: prospective follow-up of 245 clinical high-risk outpatients in four countries. Schizophr Res. 2012;138:192–197. [DOI] [PubMed] [Google Scholar]
- 37. Fusar-Poli P, Nelson B, Valmaggia L, Yung AR, McGuire PK. Comorbid depressive and anxiety disorders in 509 individuals with an at-risk mental state: impact on psychopathology and transition to psychosis. Schizophr Bull. 2014;40:120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Svirskis T, Korkeila J, Heinimaa M et al. . Axis-I disorders and vulnerability to psychosis. Schizophr Res. 2005;75:439–446. [DOI] [PubMed] [Google Scholar]
- 39. Rosen JL, Miller TJ, D’Andrea JT, McGlashan TH, Woods SW. Comorbid diagnoses in patients meeting criteria for the schizophrenia prodrome. Schizophr Res. 2006;85:124–131. [DOI] [PubMed] [Google Scholar]
- 40. Lencz T, Smith CW, Auther A, Correll CU, Cornblatt B. Nonspecific and attenuated negative symptoms in patients at clinical high-risk for schizophrenia. Schizophr Res. 2004;68:37–48. [DOI] [PubMed] [Google Scholar]
- 41. Woods SW, Addington J, Cadenhead KS et al. . Validity of the prodromal risk syndrome for first psychosis: findings from the North American Prodrome Longitudinal Study. Schizophr Bull. 2009;35:894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rettew DC, Lynch AD, Achenbach TM, Dumenci L, Ivanova MY. Meta-analyses of agreement between diagnoses made from clinical evaluations and standardized diagnostic interviews. Int J Methods Psychiatr Res. 2009;18:169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kessler RC, McGonagle KA, Zhao S et al. . Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. [DOI] [PubMed] [Google Scholar]
- 44. Robins LN, Locke BZ, Regier DA. An overview of psychiatric disorders in America. In: Robins LN, Regier DA, eds. Psychiatric Disorders in America: The Epidemiologic Catchment Area Study. New York: Maxwell Mcmillan International; 1991. [Google Scholar]
- 45. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. [DOI] [PubMed] [Google Scholar]
- 46. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kessler RC, Avenevoli S, Costello EJ et al. . Prevalence, persistence, and sociodemographic correlates of DSM-IV disorders in the National Comorbidity Survey Replication Adolescent Supplement. Arch Gen Psychiatry. 2012;69:372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Buckley PF, Miller BJ, Lehrer DS, Castle DJ. Psychiatric comorbidities and schizophrenia. Schizophr Bull. 2009;35:383–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Achim AM, Maziade M, Raymond E, Olivier D, Mérette C, Roy MA. How prevalent are anxiety disorders in schizophrenia? A meta-analysis and critical review on a significant association. Schizophr Bull. 2011;37:811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Altamura AC, Serati M, Albano A, Paoli RA, Glick ID, Dell’Osso B. An epidemiologic and clinical overview of medical and psychopathological comorbidities in major psychoses. Eur Arch Psychiatry Clin Neurosci. 2011;261:489–508. [DOI] [PubMed] [Google Scholar]
- 51. Seow LSE, Ong C, Mahesh MV et al. . A systematic review on comorbid post-traumatic stress disorder in schizophrenia. Schizophr Res. 2016;176:441–451. [DOI] [PubMed] [Google Scholar]
- 52. Swets M, Dekker J, van Emmerik-van Oortmerssen K et al. . The obsessive compulsive spectrum in schizophrenia, a meta-analysis and meta-regression exploring prevalence rates. Schizophr Res. 2014;152:458–468. [DOI] [PubMed] [Google Scholar]
- 53. Sim K, Swapna V, Mythily S et al. . Psychiatric comorbidity in first episode psychosis: the Early Psychosis Intervention Program (EPIP) experience. Acta Psychiatr Scand. 2004;109:23–29. [DOI] [PubMed] [Google Scholar]
- 54. Cannon TD, Cadenhead K, Cornblatt B et al. . Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Webb JR, Addington J, Perkins DO et al. . Specificity of incident diagnostic outcomes in patients at clinical high risk for psychosis. Schizophr Bull. 2015;41:1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fusar-Poli P, Rutigliano G, Stahl D et al. . Long-term validity of the At Risk Mental State (ARMS) for predicting psychotic and non-psychotic mental disorders. Eur Psychiatry. 2017;42:49–54. [DOI] [PubMed] [Google Scholar]
- 57. Addington J, Cadenhead KS, Cornblatt BA et al. . North American Prodrome Longitudinal Study (NAPLS 2): overview and recruitment. Schizophr Res. 2012;142:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. First MB, Spitzer RL, Gibbon M, Williams JBW.. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition, (SCID-I/NP Version 2.0).Biometrics Research Department, New York State Psychiatric Institute, 2002. [Google Scholar]
- 59. Kaufman J, Birmaher B, Brent D et al. . Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. [DOI] [PubMed] [Google Scholar]
- 60. Walker EF, Cornblatt BA, Addington J et al. . The relation of antipsychotic and antidepressant medication with baseline symptoms and symptom progression: a naturalistic study of the North American Prodrome Longitudinal Sample. Schizophr Res. 2009;115:50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cadenhead KS, Addington J, Cannon T et al. . Treatment history in the psychosis prodrome: characteristics of the North American Prodrome Longitudinal Study Cohort. Early Interv Psychiatry. 2010;4:220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Woods SW, Addington J, Bearden CE et al. . Psychotropic medication use in youth at high risk for psychosis: comparison of baseline data from two research cohorts 1998–2005 and 2008–2011. Schizophr Res. 2013;148:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hall RC. Global assessment of functioning. A modified scale. Psychosomatics. 1995;36:267–275. [DOI] [PubMed] [Google Scholar]
- 64. Kiernan M, Kraemer HC, Winkleby MA, King AC, Taylor CB. Do logistic regression and signal detection identify different subgroups at risk? Implications for the design of tailored interventions. Psychol Methods. 2001;6:35–48. [DOI] [PubMed] [Google Scholar]
- 65. Lee TY, Lee J, Kim M, Choe E, Kwon JS. Can we predict psychosis outside of the psychosis risk state? A systematic review of risk syndromes for nonpsychotic disorders. Schizophr Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. McGorry PD, Yung AR, Phillips LJ et al. . Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry. 2002;59:921–928. [DOI] [PubMed] [Google Scholar]
- 67. Lam MM, Hung SF, Chen EY. Transition to psychosis: 6-month follow-up of a Chinese high-risk group in Hong Kong. Aust N Z J Psychiatry. 2006;40:414–420. [DOI] [PubMed] [Google Scholar]
- 68. Haroun N, Dunn L, Haroun A, Cadenhead KS. Risk and protection in prodromal schizophrenia: ethical implications for clinical practice and future research. Schizophr Bull. 2006;32:166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Simon AE, Umbricht D. High remission rates from an initial ultra-high risk state for psychosis. Schizophr Res. 2010;116:168–172. [DOI] [PubMed] [Google Scholar]
- 70. Conrad AM, Lewin TJ, Sly KA et al. . Utility of risk-status for predicting psychosis and related outcomes: evaluation of a 10-year cohort of presenters to a specialised early psychosis community mental health service. Psychiatry Res. 2017;247:336–344. [DOI] [PubMed] [Google Scholar]
- 71. Lin A, Wood SJ, Nelson B, Beavan A, McGorry P, Yung AR. Outcomes of nontransitioned cases in a sample at ultra-high risk for psychosis. Am J Psychiatry. 2015;172:249–258. [DOI] [PubMed] [Google Scholar]
- 72. Rutigliano G, Valmaggia L, Landi P et al. . Persistence or recurrence of non-psychotic comorbid mental disorders associated with 6-year poor functional outcomes in patients at ultra high risk for psychosis. J Affect Disord. 2016;203:101–110. [DOI] [PubMed] [Google Scholar]
- 73. Schultze-Lutter F, Schimmelmann BG, Ruhrmann S, Michel C. ‘A rose is a rose is a rose’, but at-risk criteria differ. Psychopathology. 2013;46:75–87. [DOI] [PubMed] [Google Scholar]
- 74. Fusar-Poli P, Cappucciati M, De Micheli A et al. . Diagnostic and prognostic significance of brief limited intermittent psychotic symptoms (BLIPS) in individuals at ultra high risk. Schizophr Bull. 2017;43:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fusar-Poli P, Schultze-Lutter F, Cappucciati M et al. . The dark side of the moon: meta-analytical impact of recruitment strategies on risk enrichment in the clinical high risk state for psychosis. Schizophr Bull. 2016;42:732–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fusar-Poli P, Rutigliano G, Stahl D et al. . Deconstructing pretest risk enrichment to optimize prediction of psychosis in individuals at clinical high risk. JAMA Psychiatry. 2016;73:1260–1267. [DOI] [PubMed] [Google Scholar]
- 77. Lawrence D, Hafekost J, Johnson SE et al. . Key findings from the second Australian Child and Adolescent Survey of Mental Health and Wellbeing. Aust N Z J Psychiatry. 2016;50:876–886. [DOI] [PubMed] [Google Scholar]
- 78. Fusar-Poli P, Cappucciati M, Bonoldi I et al. . Prognosis of brief psychotic episodes: a meta-analysis. JAMA Psychiatry. 2016;73:211–220. [DOI] [PubMed] [Google Scholar]
- 79. Studerus E, Ramyead A, Riecher-Rössler A. Prediction of transition to psychosis in patients with a clinical high risk for psychosis: a systematic review of methodology and reporting. Psychol Med. 2017;47:1163–1178. [DOI] [PubMed] [Google Scholar]
- 80. Fusar-Poli P, Bonoldi I, Yung AR et al. . Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69:220–229. [DOI] [PubMed] [Google Scholar]
- 81. Woods SW, Walsh BC, Addington J et al. . Current status specifiers for patients at clinical high risk for psychosis. Schizophr Res. 2014;158:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Modinos G, Allen P, Frascarelli M et al. . Are we really mapping psychosis risk? Neuroanatomical signature of affective disorders in subjects at ultra high risk. Psychol Med. 2014;44:3491–3501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.