Abstract
Diminished motivation is associated with robust impairment in psychosocial functioning in schizophrenia (SZ). Little is known about the reciprocal relationships between motivation and functioning, particularly following a first episode of psychosis. We tested bidirectional associations between motivation and social and occupational functioning in the year following a first episode of SZ spectrum disorder among patients in the Recovery After an Initial Schizophrenia Episode—Early Treatment Program (RAISE-ETP) study. Four hundred four individuals (aged 15–40) who presented with a first episode of SZ spectrum disorder (eg, SZ, schizoaffective or schizophreniform disorder, psychotic disorder not otherwise specified) completed assessments of work and school functioning, social functioning, and motivation at 6- and 12-month follow-up, controlling for assessments at study entry. Controlling for cognition, and psychotic and depressive symptoms measured at each time point, motivation at 6 months was associated with work and school participation at 12 months, but work and school participation at 6 months was not associated with motivation at 12 months. Conversely, social functioning at 6 months was associated with motivation at 12 months, but motivation at 6 months was not associated with social functioning at 12 months. Findings suggest that motivation is associated with later occupational, but not social, functioning in the first year following an initial episode of psychosis. Social functioning, on the other hand, is associated with later motivation. Future intervention trials focused on improving occupational functioning in this population may benefit from targeting patient motivation directly (eg, through motivational interviewing), or indirectly by improving relationships and support networks.
Keywords: motivation, psychosocial functioning, first episode psychosis, schizophrenia, cross-lagged analysis
Introduction
Impairments in motivation and psychosocial functioning (ie, occupational and social functioning) are cardinal features of schizophrenia (SZ) spectrum disorders. These impairments precede the onset of disorder,1,2 persist over the long-term course,3,4 and present a challenge to treatment.5,6 Motivation is a complex construct that has received renewed interest in the context of negative symptoms in SZ.7,8 Negative symptoms have been empirically divided into expressive and experiential domains.7,9 Expressive symptoms consist of affective flattening and alogia, while experiential symptoms are characterized by anhedonia, reduced social drive, and amotivation.7 Amotivation, in particular, shows robust association with psychosocial functioning impairments in SZ.10,11 In general models of motivation, deficits in “liking” (ie, in-the-moment pleasure) appear to be more associated with anhedonia, or diminished interest/pleasure, while deficits in “wanting” (ie, anticipation of pleasure and associated drive) tend to be more associated with deficits in motivated behavior.12,13 Deficits in “wanting,” which are associated with mesolimbic dopaminergic transmission in animal models, have been identified as key contributors to amotivation and associated impairments in goal-directed behavior in SZ.14
When motivation and functioning are measured concurrently, as much as 50%–75% of the variance is shared between them.15–17 Much of this shared variance may be due to item overlap in the measures typically used to assess amotivation within the context of negative symptoms broadly defined. The Scale for the Assessment of Negative Symptoms (SANS18), for example, includes questions covering impersistence at work or school as a key indicator of avolition/apathy. Those not participating in work or school will receive a higher score on this scale, conflating the two constructs. Only more recently has there been a movement in the field to develop negative symptom measures that more specifically assess experiential symptoms, including amotivation.19,20 Thus, most of what we currently know about the connection between motivation and functioning is derived from studies using less sensitive assessments of negative symptoms. In addition, reduced functioning is undoubtedly driven by a multitude of factors in SZ, such as cognitive impairment21,22 and positive,23,24 negative,2,25 and depressive2,26 symptoms. The disruptive effects of psychotic episodes and hospitalizations could also impair functioning through demoralization and, relatedly, reduced motivation.27
While standard measures of negative symptoms often fail to capture amotivation specifically, items from the Quality of Life Scale (QLS28)—motivation, sense of purpose, and curiosity—have shown consistent associations with goal-directed behavior and psychosocial functioning in SZ.25,27 These items also demonstrate convergent validity in their association with avolition as measured by the Schedule for the Deficit Syndrome (SDS29),30 an instrument originally developed to identify patients with primary negative symptom-based deficits, including a reduced sense of purpose and social drive.
Given the lack of examination of the reciprocal associations over time in studies of first episode SZ, it is difficult to infer the relative primacy of these constructs. In addition, the tendency to combine occupational and social functioning into a single outcome precludes evaluation of the potential for differential associations with motivation.
To address these questions, in the current study we examined the prospective, reciprocal associations between motivation and social and occupational functioning in a large sample of patients following a first episode of SZ. Because social and occupational functioning are only moderately correlated in SZ,31,32 we examined these variables separately. We conducted cross-lagged panel analysis using structural equation modeling to examine (a) whether motivation following an initial episode of SZ was associated with subsequent occupational and social functioning, (b) whether psychosocial functioning was associated with later levels of motivation, or (c) both. Because longitudinal evidence suggests motivation can influence functioning and vice versa, we explored the extent to which the two domains of functioning showed reciprocal associations with motivation over time.
Methods
Participants
Participants were part of the longitudinal multisite National Institute of Mental Health’s (NIMH) Recovery After an Initial Schizophrenia Episode—Early Treatment Program (RAISE-ETP).33 RAISE-ETP was a cluster-randomized clinical trial that compared a comprehensive intervention for first episode SZ to usual community care. Four hundred four participants between the ages of 15 and 40 were recruited across 34 community mental health treatment centers in 21 states and were followed up for a minimum of 2 years. All participants received intervention lasting up to 2 years. Individuals were eligible if they had one of the following DSM-IV diagnoses: SZ, schizoaffective disorder, schizophreniform disorder, brief psychotic disorder, or psychotic disorder not otherwise specified. Individuals with clinically significant head trauma or other serious medical conditions were excluded. All participants had experienced only a single episode of psychosis and had ≤6 months of lifetime antipsychotic medication treatment. All participants provided written informed consent (as well as assent and parental/guardian consent for those under 18 years old). A more detailed description of recruitment procedures, clinical site selection and randomization, treatment components and outcomes is provided elsewhere.33–35
Measures
Descriptive statistics of the sample and variables of interest are provided in table 1. Diagnostic and assessment interviews were conducted using 2-way video conferencing completed by remote, centralized personnel (ie, individuals with training to provide research quality interviews who were blind to study design and assignment). One interviewer conducted all the assessments for a given participant at a given time point, but each participant could be assessed by different raters at different time points across the study. The following assessments relevant to the current report were conducted by the raters: the Structured Clinical Interview for DSM-IV (SCID)36 to determine psychiatric diagnoses; the Positive and Negative Syndrome Scale (PANSS)37 to assess psychotic symptoms; the Calgary Depression Scale for Schizophrenia (CDSS)38 to assess symptoms of depression; and the Quality of Life Scale (QLS).37 The QLS is a 21-item scale that assesses qualities such as motivation, social interaction, and engagement in life roles and activities over the past month. Each QLS item is rated on a scale ranging from 0 to 6 with lower scores indicating more severe impairment. The scale is subdivided into the following 4 subscales: Intrapsychic Foundations, Interpersonal Relations, Instrumental Role Functioning, and Common Objects and Activities. The QLS, PANSS, and CDSS were completed every 6 months. Site-based personnel completed the following assessments: Brief Assessment of Cognition in Schizophrenia (BACS),39 that includes tests of verbal memory, digit sequencing, token motor, verbal fluency, symbol coding, and tower of London; the Services Utilization Recording Form (SURF),40 a questionnaire that documents health care services and indices of community participation (eg, work or school) present in the past month. The BACS was completed at baseline, 1-year and 2-year assessments, and the SURF was completed monthly.
Table 1.
Study Sample Characteristics and Variable Descriptive Statistics
| Mean (SD) or % | |
|---|---|
| Demographics | |
| Age | 23.14 (5.07) |
| Gender: male | 72.5% |
| Race | |
| American Indian or Alaskan Native | 5.2% |
| Asian | 3.0% |
| Black or African American | 37.6% |
| Native Hawaiian or Other Pacific Islander | 0.2% |
| White | 54.0% |
| Ethnicity: Hispanic or Latino | 18.1% |
| Outcome variablesa | |
| SURF work or school baseline | 39.8% |
| SURF work or school 6 mo | 71.8% |
| SURF work or school 12 mo | 66.6% |
| QLS motivation baseline | 7.61 (3.52) |
| QLS motivation 6 mo | 8.52 (3.64) |
| QLS motivation 12 mo | 8.31 (3.69) |
| QLS interpersonal relationships baseline | 19.76 (8.70) |
| QLS interpersonal relationships 6 mo | 22.88 (9.76) |
| QLS interpersonal relationships 12 mo | 23.65 (9.51) |
| PANSS positive symptoms baseline | 12.24 (3.83) |
| PANSS positive symptoms 6 mo | 10.04 (4.18) |
| PANSS positive symptoms 12 mo | 10.04 (3.74) |
| CDSS baseline | 4.65 (4.28) |
| CDSS 6 mo | 3.70 (4.33) |
| CDSS 12 mo | 2.61 (3.52) |
| BACS baseline/12 mo | 37.38 (7.02) |
Note: BACS, Brief Assessment of Cognition in Schizophrenia; CDSS, Calgary Depression Scale for Schizophrenia; PANSS, Positive and Negative Syndrome Scale; QLS, Quality of Life Scale; SURF, Service Utilization Recording Form; mo, months.
a Ns range from 257 (12 mo) to 404 (baseline).
Motivation.
Our measure of motivation was based on 3 items of the Intrapsychic Foundations subscale of the QLS, including degree of motivation, sense of purpose, and curiosity. Example questions include “Have you had much enthusiasm, energy and drive?”, “Have you set any goals for yourself?”, and “How often have you seen or heard about something that you wanted to know more about or understand better?” These items have been used as an index of general motivation in various studies of people with SZ.17,27,41 In a recent factor analysis of the QLS, these 3 items loaded most strongly on the subscale.42
Social Functioning.
Social functioning was derived from the Interpersonal Relations subscale of the QLS. We used 7 items from the full scale: intimate interactions, active acquaintances, social activity, involved social network, social initiatives, social withdrawal, and socio-sexual relations. These items capture the multifaceted nature of social relationships, including the quantity (eg, “How many friends do you have”) and quality (eg, “Do you have friends with whom you are especially close other than your immediate family or the people you live with?”) of these relationships.
Occupational Functioning.
Three items from the SURF (ie, Are you currently a student? Have you worked for pay during the past 30 days? Have you done any casual work or day jobs, such as yard work or babysitting for which you got paid?) were combined into a single score to measure work or school functioning at each assessment point (ie, the past month) and aggregated across 6-month time points; each was rated as either 0 = no (ie, no paid work and not a student) or 1 = yes (ie, paid work or a student). The goal of the current study was to examine the extent to which motivation at one point in time predicted involvement in work or school at a later time point (and vice versa), as these are the roles of greatest concern in young people who have recently developed SZ. Therefore, the SURF composite score was used to measure work or school involvement, rather than the more generic Instrumental Role Functioning subscale of the QLS, which focuses on functioning in the “homemaker” role for participants who are not “attempting” the role of student or worker.
Covariates.
Psychotic symptom ratings were based on a Positive Symptom Factor Score of the following PANSS items: delusions, hallucinations, grandiosity, and unusual thought content.43 Depression was calculated based on a total score on the CDSS. A composite score of cognitive functioning was created by computing the average of the six BACS tests; baseline and 12-month BACS scores were averaged in the current study to provide a stable indicator of cognitive ability; this was done because there was some evidence that cognitive functioning varied within participants from baseline to 12-month follow-up. We used subscale averages, as opposed to normed scores, as we were primarily interested in variance in motivation and psychosocial functioning accounted for by cognitive ability.
Although our focus in the current report is on motivation and social functioning, the QLS was originally designed to assess the multifaceted nature of intra- and interpersonal features of SZ. As such, although the PANSS also assesses negative symptoms, we did not include these items in the current analysis given their overlap with the QLS (correlations in the current sample range from .62 to .73) and the inclusion of symptoms in the PANSS that are less robustly related to functioning (eg, blunted affect).11 (Because of the possibility of associations between expressive negative symptoms and psychosocial functioning, we tested the bivariate associations between blunted affect on the PANSS and our measures of psychosocial functioning (SURF work/school and QLS interpersonal relations). Associations between blunted affect and work/school participation were small and nonsignificant at all time points (−0.05 to −0.10). Correlations between blunted affect and social functioning, however, ranged from −0.19 to −0.30. While these are modest associations, we included blunted affect as a covariate at baseline, 6, and 12 months in the primary model testing the association of motivation and social functioning. The inclusion of blunted affect did not change the primary findings.)
Statistical Analyses
Before testing multivariate models, we examined the bivariate correlations among variables of interest across the 3 primary time points (baseline, 6-months, 12-months). We used structural equation modeling (SEM) to evaluate our primary research question, examining occupational and social functioning in separate models. We employed cross-lagged panel analysis to identify the degree to which motivation predicted functioning, and/or functioning predicted motivation, while controlling for previous and concurrent measurements of each variable (and other covariates). Models were tested in sequence, beginning with the autoregressive model (eg, motivation at 6 months predicting motivation at 12 months), then testing each directional model (eg, motivation at 6 months predicting occupational functioning at 12 months). We allowed residuals of the same variables measured across time to covary so as to minimize the impact of multicollinearity. In the case in which both directional associations were significant, fully cross-lagged models were tested, including the autoregressive and directional models simultaneously. Given the known associations among cognition, psychotic symptoms, and depression with motivation and psychosocial functioning, we included these variables as covariates in initial models. Ratings of symptoms of psychosis and depression at study entry (baseline), 6 months, and 12 months, and tests of cognition aggregated across baseline and 12 months, were included as covariates in these models. Path coefficients with P values >.15 were set to 0 in subsequent models.
These data were from a treatment trial of first-episode psychosis in which participants entering the trial had recently experienced an initial psychotic episode. Seventy-eight percent of participants had been hospitalized before study entry (median number of weeks since last hospitalization = 8). In addition, the primary outcomes of RAISE-ETP suggested that the most significant improvements in psychosocial functioning occurred by the 6-month time point.34 Thus, to minimize the influence of symptom variance on the relationships between the variables of interest, we set the 6-month time point as the baseline, using the true baseline time point (ie, Month 0) for motivation and psychosocial functioning as control variables in all models. The treatment group was included as a moderating variable to examine the impact of intervention on the variables of interest. We also accounted for clustering of observations by site. To examine the stability of associations over a longer period of time, we conducted sensitivity analyses aggregating the 12- and 18-month time points of the outcomes of interest among our primary models.
Model fit was assessed using standard criteria, including the χ2 test, root mean square error of approximation (RMSEA), comparative fit index (CFI), and Tucker–Lewis index (TLI). For models with categorical outcomes, we compared fit using robust weighted least squares estimation. For models using continuous outcomes, we utilized χ2 difference tests. Standardized coefficients are presented, and 2-tailed P values <.05 were considered statistically significant. Analyses were performed using Mplus version 7.4.
Results
Missing data due to treatment study dropout ranged from 0% (SURF) to 28% (QLS) at 6 months, and 21% (SURF) to 36% (QLS) at 12 months. Those who did not have follow-up data did not differ in the primary measures of interest at baseline from those who had follow-up data. Missing data were handled in the models using full information maximum likelihood. Bivariate correlations revealed significant associations among our primary variables of interest (supplementary table 1). Cognition (BACS) was unrelated to psychotic (PANSS positive symptoms) and depressive (CDSS) symptoms at all time points.
In preliminary SEM analyses, treatment group, duration of untreated psychosis, gender, age, or ethnicity did not moderate the associations between motivation and psychosocial functioning, so we did not include these variables in the models presented below. As predicted, cognition at baseline, and psychotic and depressive symptoms at corresponding time points were significantly associated with motivation and social and occupational functioning and were thus included as covariates in the models. See supplementary tables for BACS, PANSS, and CDSS coefficients.
Motivation and Occupational Functioning
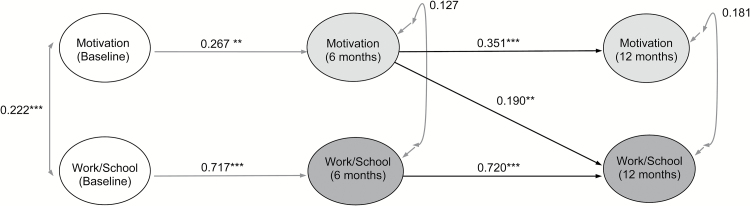
We first tested the autoregressive model, examining the stability of motivation and occupational functioning across baseline, 6, and 12 months. The model provided adequate fit to the data (χ2 (43) = 54.734, P = .108; RMSEA = 0.026; CFI = 0.994; TLI = 0.989). Next, we tested the model including motivation at 6 months as predictor of occupational functioning at 12 months, controlling for baseline assessments. Adding this path resulted in improved model fit (χ2 (41) = 49.979, P = .159; RMSEA = 0.023; CFI = 0.995; TLI = 0.991), though the degree of improvement was at a trend level of significance (χ2 (2) = 5.328, P = .069). Motivation at 6 months was significantly associated with occupational functioning at 12 months (β = 0.190, SE = 0.062, P = .002). We then tested the directional model in which occupational functioning at 6 months was associated with motivation at 12 months. Freeing this parameter resulted in adequate model fit (χ2 (41) = 53.928, P = .085; RMSEA = 0.028; CFI = 0.993; TLI = 0.988), but did not improve fit from the baseline model (χ2 (2) = 0.133, P = .936). Importantly, occupational functioning at 6 months was not associated with motivation at 12 months (β = 0.036, SE = 0.066, P = .578). Because this directional path was not significant, and model fit was not improved, we did not test the fully cross-lagged model with motivation and occupational functioning reciprocally linked. We therefore concluded that motivation at 6 months more likely influenced occupational functioning at 12 months than the reverse (figure 1).
Fig. 1.
Cross-lagged associations between motivation (Quality of Life Scale motivation, sense of purpose, and curiosity) and occupational functioning/work or school (Services Utilization Recording Form). ***P < .001, **P < .01, *P < .05. Covariates include depression (CDSS) and psychotic symptoms (PANSS positive) at each time point, and cognition (BACS)—baseline and 12-month average. Unidirectional arrows indicate standardized regression coefficients; bidirectional arrows indicate covariances. Residuals of the autoregressive associations were allowed to covary.
Motivation and Social Functioning
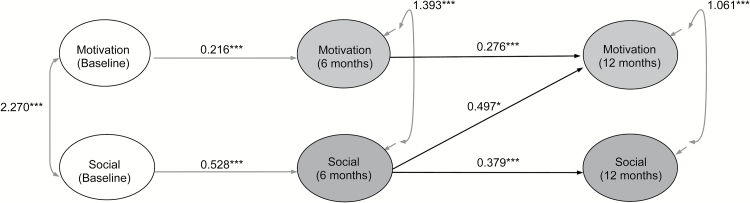
The autoregressive model provided adequate fit to the data (χ2 (41) = 60.000, P = .028; RMSEA = 0.034; CFI = 0.980; TLI = 0.966). The model including motivation at 6 months as a correlate of social functioning at 12 months, controlling for baseline assessments, resulted in adequate fit (χ2 (39) = 59.404, P = .019; RMSEA = 0.036; CFI = 0.978; TLI = 0.962); however, motivation at 6 months was not associated with social functioning at 12 months (β = 0.001, SE = 0.020, P = .994). The directional model in which social functioning at 6 months was associated with motivation at 12 months resulted in adequate model fit (χ2 (39) = 50.823, P = .097; RMSEA = 0.027; CFI = 0.987; TLI = 0.978), and there was a significant improvement in fit from the baseline model (χ2 (2) = 8.508, P < .001). Importantly, social functioning at 6 months was associated with motivation at 12 months (β = 0.497, SE = 0.195, P = .011). Because only this directional path was significant, we did not test the fully cross-lagged model with motivation and social functioning reciprocally linked. We therefore concluded that social functioning at 6 months was more likely to influence 12-month motivation than motivation at 6 months was to influence 12-month social functioning (figure 2).
Fig. 2.
Cross-lagged associations between motivation (Quality of Life Scale [QLS] motivation, sense of purpose, and curiosity) and social functioning (QLS interpersonal relations). ***P < .001, **P < .01, *P < .05. Covariates include depression (CDSS) and psychotic symptoms (PANSS positive) at each time point, and cognition (BACS)—baseline and 12-month average. Unidirectional arrows indicate standardized regression coefficients; bidirectional arrows indicate covariances. Residuals of the autoregressive associations were allowed to covary.
Sensitivity Analysis
To evaluate the stability of these associations over a longer period of time, we tested the above models after aggregating outcome variables across the 12- and 18-month assessments. As opposed to conducting an independent evaluation of the 18-month time point, we aggregated these waves because missing data limited our power to test that model. These tests revealed that the primary relationships held through the 18-month time point: coefficients were comparable in effect size, and model fit indices were good and similar to the more constrained models. Findings from these analyses are presented in supplementary tables 4 and 5.
Discussion
In a large sample of patients with a first episode of psychosis drawn from 34 clinics across 21 states, motivation was associated with later occupational functioning, but occupational functioning was not associated with later motivation. The opposite association was found for the social domain: social functioning was associated with later motivation, but motivation was not associated with later social functioning. The associations were examined in participants after 6 months of treatment, controlling for baseline levels of these variables, and therefore likely reflect stable relationships that are less influenced by the immediate aftermath of a first episode of psychosis. Furthermore, the relationships were replicated in sensitivity analyses aggregating the 12- and 18-month time points, supporting the reliability of the main findings. The findings were also robust to important covariates, including demographic variables such as age, gender, and ethnicity, as well as treatment assignment.
Consistent with previous studies, findings suggest that motivation, as reflected by activation, drive, and goal engagement, is especially critical to occupational functioning in young individuals who have recently developed a psychotic illness.44 Such motivation in the occupational domain may be reflected by individuals identifying work or school goals, independently completing job or school re-enrollment applications, or actively seeking vocational and educational services. That levels of motivation were associated with work and school functioning, but not social functioning, was unexpected. While other factors such as cognition, interpersonal skills, and symptoms may also play a role in success at work or school, their importance may be secondary to the motivation to pursue engagement in those roles. Consistent with this idea, reported desire for work and active efforts to find work are strong predictors of competitive employment up to 2 years later in people with SZ.45
Motivation was not associated with later social functioning; however, other variables not measured in this study may be more predictive of success in this domain, such as social skills/capacity, social support, and social cognition.16,46,47 While several studies have suggested that negative symptoms, including motivation, are associated cross-sectionally with social functioning, there is less evidence that motivation predicts social functioning over time.44,46,48,49 Social functioning may be more dependent on the acquisition of specific skills than is participation in work or school. Therefore, in the relative absence of concerted opportunities to learn social skills, motivation may be more strongly related to work or school participation. In addition, social skills training improves both negative symptoms and social functioning in SZ,50 but has less impact on occupational functioning.51–53 As such, targeting receptive and expressive social skills may be more fruitful than targeting motivation for patients who report a desire to improve social relationships.
So why was social functioning associated with later motivation? Higher levels of social engagement and closer relationships may play a protective role in maintaining motivation in SZ, especially early on in the illness. Regular involvement in the lives of others and reciprocal relationships may provide important social validation and acceptance that facilitates the rebuilding of confidence, self-esteem, and sense of purpose after the devastating effects of a psychotic episode.54 Close and rewarding relationships may, in turn, support the articulation and pursuit of personal goals. Indeed, ample evidence suggests that the presence of relationships and degree of social support have lasting positive effects on the course of SZ.55,56 We are aware of one other prospective study examining reciprocal associations between motivation and psychosocial functioning in patients with SZ.27 Nakagami et al reported that across a 1-year period in patients receiving community-based rehabilitative services, baseline psychosocial functioning predicted changes in motivation, while baseline motivation did not predict changes in functioning. We note, however, that in this study the sample was multi-episode SZ, and social and occupational functioning were combined into one outcome, precluding interpretation of differential outcomes.
While the QLS motivation items are strongly tied to other measures of amotivation/avolition,29,30 and have shown robust associations with psychosocial functioning in several studies,25,27 it is important to note that there are newer rating scales available that provide more comprehensive coverage of the multifaceted nature of experiential negative symptoms in SZ, including the assessment of motivation and pleasure across social, occupational, and recreational domains. The inclusion of scales such as the Clinical Assessment Interview for Negative Symptoms (CAINS19) or Brief Negative Symptom Scale (BNSS20), for example, could improve our understanding of which qualities of experiential negative symptoms might be most critical for understanding psychosocial functioning over time. In addition, the inclusion of behavioral measures translated from animal models, such as those assessing effort-based decision-making (eg, Effort Expenditure for Rewards Task; EEfRT57,58), could improve prediction of important psychosocial functioning outcomes in SZ. Finally, future studies should model relative changes in motivation and psychosocial functioning (eg, using latent growth curve modeling) to improve understanding of the associations of these constructs within people over time.
Our findings suggest that coordinated specialty care programs should target enhancement of motivation to support occupational functioning beyond the effects of evidence-based approaches such as supported education and employment. Conversely, if the goal is to maintain patient motivation, targeting social connection may be fruitful, such as in family-focused interventions. Ultimately, enhancing social relationships in the early phases of recovery may lead to distal improvements in occupational functioning through their impact on patient motivation.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Supplementary Material
Acknowledgments
Dr Fulford had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Conflict of Interest: No sponsor or funder was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. We thank all of our core collaborators and consultants for their invaluable contributions, without whom this study would not have been possible. Executive Committee: John M. Kane, MD, Delbert G. Robinson, MD, Nina R. Schooler, PhD, Kim T. Mueser, PhD, David L. Penn, PhD, Robert A. Rosenheck, MD, Jean Addington, PhD, Mary F. Brunette, MD, Christoph U. Correll, MD, Sue E. Estroff, PhD, Patricia Marcy, BSN, James Robinson, MEd. NIMH Collaborators: Robert K. Heinssen, PhD, ABPP, Joanne B. Severe, MS, Susan T. Azrin, PhD, Amy B. Goldstein, PhD. Additional contributors to design and implementation of NAVIGATE: Susan Gingerich, MSW, Shirley M. Glynn, PhD, Jennifer D. Gottlieb, PhD, Benji T. Kurian, MD, MPH, David W. Lynde, MSW, Piper S. Meyer-Kalos, PhD, LP, Alexander L. Miller, MD, Ronny Pipes, MA, LPC-S. Additional Collaborators: MedAvante for the conduct of the centralized, masked diagnostic interviews and assessments; the team at the Nathan Kline Institute for data management. Thomas Ten Have and Andrew Leon played key roles in the design of the study, particularly for the statistical analysis plan. We mourn the untimely deaths of both. We gratefully acknowledge the contributions of Haiqun Lin and Kyaw (Joe) Sint to statistical analysis planning and conduct. We are indebted to the many clinicians, research assistants, and administrators at the participating sites for their enthusiasm and terrific work on the project as well as the participation of the hundreds of patients and families who made the study possible with their time, trust, and commitment. The participating sites include: Burrell Behavioral Health (Columbia), Burrell Behavioral Health (Springfield), Catholic Social Services of Washtenaw County, Center for Rural and Community Behavior Health New Mexico, Cherry Street Health Services, Clinton-Eaton-Ingham Community Mental Health Authority, Cobb County Community Services Board, Community Alternatives, Community Mental Health Center of Lancaster County, Community Mental Health Center, Inc., Eyerly Ball Iowa, Grady Health Systems, Henderson Mental Health Center, Howard Center, Human Development Center, Lehigh Valley Hospital, Life Management Center of Northwest Florida, Mental Health Center of Denver, Mental Health Center of Greater Manchester, Nashua Mental Health, North Point Health and Wellness, Park Center, PeaceHealth Oregon/Lane County Behavioral Health Services, Pine Belt Mental HC, River Parish Mental Health Center, Providence Center, San Fernando Mental Health Center, Santa Clarita Mental Health Center, South Shore Mental Health Center, St. Clare’s Hospital, Staten Island University Hospital, Terrebonne Mental Health Center, United Services and University of Missouri-Kansas City School of Pharmacy.
References
- 1. Schlosser DA, Fisher M, Gard D, Fulford D, Loewy RL, Vinogradov S. Motivational deficits in individuals at-risk for psychosis and across the course of schizophrenia. Schizophr Res. 2014;158:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fulford D, Niendam TA, Floyd EG et al. Symptom dimensions and functional impairment in early psychosis: more to the story than just negative symptoms. Schizophr Res. 2013;147:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Möller HJ, Bottlender R, Wegner U, Wittmann J, Strauß A. Long‐term course of schizophrenic, affective and schizoaffective psychosis: focus on negative symptoms and their impact on global indicators of outcome. Acta Psychiatr Scand Suppl. 2000;102:54–57. [DOI] [PubMed] [Google Scholar]
- 4. Fenton WS, McGlashan TH. Antecedents, symptom progression, and long-term outcome of the deficit syndrome in schizophrenia. Am J Psychiatry. 1994;151:351–356. [DOI] [PubMed] [Google Scholar]
- 5. Kirkpatrick B, Kopelowicz A, Buchanan RW, Carpenter WT Jr. Assessing the efficacy of treatments for the deficit syndrome of schizophrenia. Neuropsychopharmacology. 2000;22:303–310. [DOI] [PubMed] [Google Scholar]
- 6. Aleman A, Lincoln TM, Bruggeman R et al. Treatment of negative symptoms: where do we stand, and where do we go?Schizophr Res. 2016. doi: 10.1016/j.schres.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 7. Strauss GP, Horan WP, Kirkpatrick B et al. Deconstructing negative symptoms of schizophrenia: avolition-apathy and diminished expression clusters predict clinical presentation and functional outcome. J Psychiatr Res. 2013;47:783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr Bull. 2010;36:919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Strauss GP, Gold JM. A new perspective on anhedonia in schizophrenia. Am J Psychiatry. 2012;169:364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76: 470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Foussias G, Remington G. Negative symptoms in schizophrenia: avolition and Occam’s razor. Schizophr Bull. 2010;36:359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol. 2009;9:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Konstantakopoulos G, Ploumpidis D, Oulis P et al. Apathy, cognitive deficits and functional impairment in schizophrenia. Schizophr Res. 2011;133:193–198. [DOI] [PubMed] [Google Scholar]
- 16. Green MF, Hellemann G, Horan WP, Lee J, Wynn JK. From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Arch Gen Psychiatry. 2012;69:1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Foussias G, Mann S, Zakzanis KK, van Reekum R, Agid O, Remington G. Prediction of longitudinal functional outcomes in schizophrenia: the impact of baseline motivational deficits. Schizophr Res. 2011;132:24–27. [DOI] [PubMed] [Google Scholar]
- 18. Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry. 1982;39:784–788. [DOI] [PubMed] [Google Scholar]
- 19. Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The Clinical Assessment Interview for Negative Symptoms (CAINS): final development and validation. Am J Psychiatry. 2013;170:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kirkpatrick B, Strauss GP, Nguyen L et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. 2011;37:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia?Am J Psychiatry. 1996;153:321–330. [DOI] [PubMed] [Google Scholar]
- 22. Addington J, Addington D. Neurocognitive and social functioning in schizophrenia: a 2.5 year follow-up study. Schizophr Res. 2000;44:47–56. [DOI] [PubMed] [Google Scholar]
- 23. Galderisi S, Rossi A, Rocca P et al. ; Italian Network for Research on Psychoses. The influence of illness-related variables, personal resources and context-related factors on real-life functioning of people with schizophrenia. World Psychiatry. 2014;13:275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cardenas V, Abel S, Bowie CR et al. When functional capacity and real-world functioning converge: the role of self-efficacy. Schizophr Bull. 2013;39:908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fervaha G, Foussias G, Agid O, Remington G. Motivational and neurocognitive deficits are central to the prediction of longitudinal functional outcome in schizophrenia. Acta Psychiatr Scand. 2014;130:290–299. [DOI] [PubMed] [Google Scholar]
- 26. Häfner H, Löffler W, Maurer K, Hambrecht M, an der Heiden W. Depression, negative symptoms, social stagnation and social decline in the early course of schizophrenia. Acta Psychiatr Scand. 1999;100:105–118. [DOI] [PubMed] [Google Scholar]
- 27. Nakagami E, Hoe M, Brekke JS. The prospective relationships among intrinsic motivation, neurocognition, and psychosocial functioning in schizophrenia. Schizophr Bull. 2010;36:935–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heinrichs DW, Hanlon TE, Carpenter WT Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10:388–398. [DOI] [PubMed] [Google Scholar]
- 29. Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT Jr. The Schedule for the Deficit syndrome: an instrument for research in schizophrenia. Psychiatry Res. 1989;30:119–123. [DOI] [PubMed] [Google Scholar]
- 30. Mucci A, Dima D, Soricelli A et al. Is avolition in schizophrenia associated with a deficit of dorsal caudate activity? A functional magnetic resonance imaging study during reward anticipation and feedback. Psychol Med. 2015;45:1765–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Strauss JS, Carpenter WT Jr. The prediction of outcome in schizophrenia. I. Characteristics of outcome. Arch Gen Psychiatry. 1972;27:739–746. [DOI] [PubMed] [Google Scholar]
- 32. Brekke JS, Raine A, Ansel M, Lencz T, Bird L. Neuropsychological and psychophysiological correlates of psychosocial functioning in schizophrenia. Schizophr Bull. 1997;23:19–28. [DOI] [PubMed] [Google Scholar]
- 33. Kane JM, Schooler NR, Marcy P et al. The RAISE early treatment program for first-episode psychosis: background, rationale, and study design. J Clin Psychiatry. 2015;76:240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kane JM, Robinson DG, Schooler NR et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016;173:362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mueser KT, Penn DL, Addington J et al. The NAVIGATE program for first-episode psychosis: rationale, overview, and description of psychosocial components. Psychiatr Serv. 2015;66:680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. First MB, Spitzer RL, Gibbon M, Williams JBW.. Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition (SCID-I/P, Version 2.0). New York: Biometrics Research Department, New York State Psychiatric Institute; 1995:722. [Google Scholar]
- 37. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 38. Addington D, Addington J, Maticka-Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry Suppl. 1993;22:39–44. [PubMed] [Google Scholar]
- 39. Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68:283–297. [DOI] [PubMed] [Google Scholar]
- 40. Rosenheck R, Kasprow W, Frisman L, Liu-Mares W. Cost-effectiveness of supported housing for homeless persons with mental illness. Arch Gen Psychiatry. 2003;60:940–951. [DOI] [PubMed] [Google Scholar]
- 41. Foussias G, Siddiqui I, Fervaha G et al. Motivated to do well: an examination of the relationships between motivation, effort, and cognitive performance in schizophrenia. Schizophr Res. 2015;166:276–282. [DOI] [PubMed] [Google Scholar]
- 42. Mueser KT, Kim M, Addington J, McGurk SR, Pratt SI, Addington DE. Confirmatory factor analysis of the quality of life scale and new proposed factor structure for the quality of life scale-revised. Schizophr Res. 2017;181:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res. 2012;137:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fervaha G, Foussias G, Agid O, Remington G. Motivational deficits in early schizophrenia: prevalent, persistent, and key determinants of functional outcome. Schizophr Res. 2015;166:9–16. [DOI] [PubMed] [Google Scholar]
- 45. Mueser KT, Salyers MP, Mueser PR. A prospective analysis of work in schizophrenia. Schizophr Bull. 2001;27:281–296. [DOI] [PubMed] [Google Scholar]
- 46. Bellack AS, Morrison RL, Wixted JT, Mueser KT. An analysis of social competence in schizophrenia. Br J Psychiatry. 1990;156:809–818. [DOI] [PubMed] [Google Scholar]
- 47. Mueser KT, Bellack AS, Douglas MS, Morrison RL. Prevalence and stability of social skill deficits in schizophrenia. Schizophr Res. 1991;5:167–176. [DOI] [PubMed] [Google Scholar]
- 48. Kalin M, Kaplan S, Gould F, Pinkham AE, Penn DL, Harvey PD. Social cognition, social competence, negative symptoms and social outcomes: inter-relationships in people with schizophrenia. J Psychiatr Res. 2015;68:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Milev P, Ho BC, Arndt S, Andreasen NC. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162:495–506. [DOI] [PubMed] [Google Scholar]
- 50. Kurtz MM, Mueser KT. A meta-analysis of controlled research on social skills training for schizophrenia. J Consult Clin Psychol. 2008;76:491–504. [DOI] [PubMed] [Google Scholar]
- 51. Mueser KT, Aalto S, Becker DR et al. The effectiveness of skills training for improving outcomes in supported employment. Psychiatr Serv. 2005;56:1254–1260. [DOI] [PubMed] [Google Scholar]
- 52. Wallace CJ, Tauber R. Supplementing supported employment with workplace skills training. Psychiatr Serv. 2004;55:513–515. [DOI] [PubMed] [Google Scholar]
- 53. Glynn SM, Marder SR, Noordsy DL et al. An RCT evaluating the effects of skills training and medication type on work outcomes among patients with schizophrenia. Psychiatr Serv. 2017;68:271–277. [DOI] [PubMed] [Google Scholar]
- 54. Schön UK, Denhov A, Topor A. Social relationships as a decisive factor in recovering from severe mental illness. Int J Soc Psychiatry. 2009;55:336–347. [DOI] [PubMed] [Google Scholar]
- 55. Erickson DH, Beiser M, Iacono WG, Fleming JA, Lin TY. The role of social relationships in the course of first-episode schizophrenia and affective psychosis. Am J Psychiatry. 1989;146:1456–1461. [DOI] [PubMed] [Google Scholar]
- 56. Erickson DH, Beiser M, Iacono WG. Social support predicts 5-year outcome in first-episode schizophrenia. J Abnorm Psychol. 1998;107:681–685. [DOI] [PubMed] [Google Scholar]
- 57. Reddy LF, Horan WP, Barch DM et al. Effort-based decision-making paradigms for clinical trials in schizophrenia: part 1—psychometric characteristics of 5 paradigms. Schizophr Bull. 2015;41:1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS One. 2009;4:e6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.