Abstract
Introduction
Patient registries serve an important role in rare disease research, particularly for the recruitment and planning of clinical trials. The Canadian Neuromuscular Disease Registry was established with the primary objective of improving the future for neuromuscular (NM) patients through the enablement and support of research into potential treatments.
Methods
In this report, we discuss design and utilization of the Canadian Neuromuscular Disease Registry with special reference to the paediatric cohort currently enrolled in the registry.
Results
As of July 25, 2017, there are 658 paediatric participants enrolled in the registry, 249 are dystrophinopathies (229 are Duchenne muscular dystrophy), 57 are myotonic dystrophy participants, 98 spinal muscular atrophy participants and 65 are limb girdle muscular dystrophy. A total of 175 patients have another NM diagnosis. The registry has facilitated 20 clinical trial inquiries, 5 mail-out survey studies and 5 other studies in the paediatric population.
Discussion
The strengths of the registry are discussed. The registry has proven to be an invaluable tool to NM disease research and has increased Canada’s visibility as a competitive location for the conduct of clinical trials for NM therapies.
Keywords: Clinical trials, Neuromuscular, Rare disease, Registry, Surveys
There are over 150 neuromuscular (NM) disorders, as delineated by the World Federation of Neurology (1). The most common childhood NM diseases are: Duchenne muscular dystrophy (DMD), with an incidence of between 1 in 3500 and 1 in 5000 newborn males; myotonic dystrophy (DM), with a prevalence of between 0.5 and 18.1 in 100,000 worldwide (2,3); and spinal muscular atrophy (SMA), which has an incidence of 1 in 11,000 live births (4).
Patient registries serve as important tools for organizing patient clinical and demographic information, as well as accelerating and supporting translational research. Registries are especially important for rare disease communities in the recruitment and facilitation of clinical trials (5,6). Patient registries such as the Canadian Neuromuscular Disease Registry (CNDR) provide a platform whereby the target populations can be quickly and easily identified across a national complement of clinics facilitating research activities and investigator collaboration.
The CNDR was established in 2011 as a tool that connects both adult and paediatric Canadians living with a NM disease with national and international research opportunities. Apart from planning and recruitment for clinical trials, which has been the principal activity, survey, epidemiological and natural history studies have been conducted using participants and patient information obtained from the CNDR (7).
The CNDR is part of a multinational effort to coordinate and consolidate NM disease registry data to enable broader high-impact research efforts. In this regard, the Translational Research in Europe for the Assessment and Treatment of Neuromuscular Disease (TREAT-NMD) Alliance is a global effort configured to harmonize translational research in NM disease. One of TREAT-NMD’s main goals is the development of a set of standardized principles and a consistent framework for NM patient registries. With this framework in place to foster a collaborative environment, the TREAT-NMD Global registry group has been able to publish large studies addressing important issues for the NM community (5,8). The CNDR is a participating member of the TREAT-NMD Global registry alliance and contributes data on behalf of Canadian patients to this important international collaboration.
The CNDR collects detailed medical data on four rare paediatric NM diseases collectively known as ‘indexed’ diseases (DMD, DM, SMA and limb girdle muscular dystrophy [LGMD]). Data are updated at a minimum interval of once in every 12 months. The registry also enrols other childhood-onset NM disorders, collecting only contact details, basic demographics (e.g., age, gender) and a diagnosis. These diseases are termed ‘non-indexed diseases’. Depending on resource and funding availability to support active recruitment and tracking, a non-indexed disease may become an indexed disease. In this report, we discuss the design and utilization of the registry and describe the paediatric population currently enrolled. The objective of this paper is to raise awareness among Canadian paediatricians and paediatric subspecialists about the registry and highlight the valuable nature of this tool for collaborative research in rare childhood disorders.
METHODS
Objectives
The CNDR aims to improve the future for Canadians with NM disorders through the enablement and support of research that enhances the understanding of epidemiology of NM diseases in Canada, attracts international trial opportunities and fosters collaboration among Canadian investigators.
Registry design
The CNDR operates as a network of university-affiliated NM clinics and local principal investigators. Adult and paediatric clinics function as separate sites even if they are in the same city or even institution. Although the focus of this paper is on the paediatric component of the registry, the organization and processes between the adult and paediatric components are identical. Patient enrollment from clinics is supported and overseen centrally by the CNDR National Office at the University of Calgary. Each clinic obtains local research ethics board approval using a standardized ethics package that is common across all centres.
Patient eligibility and consent
All children and adults living in Canada with a NM condition are eligible for enrollment into the registry. The majority of participants enrolled in the registry are enrolled during routine clinic visits by a research coordinator or clinician. In addition, patients not attending an affiliated clinic or otherwise without specialist care may self-register by contacting the CNDR National Office. Any physician in Canada may also refer their patients to the CNDR by directing them to the CNDR National Office.
Data collection and management
Clinical data are collected retrospectively by trained research personnel accessing clinic charting or by the attending physician or a trained research coordinator during a clinic visit. Identified and de-identified data are directly entered into a secure web portal. De-identified medical data quality and compliance audited by CNDR National Office staff.
Access to CNDR data
Access to de-identified aggregated registry data is facilitated through a formal application process. There are two types of data requests: research proposal requests and statistical data requests. Research proposals involve the export of de-identified CNDR data for research use or use of identified data by CNDR National Office staff to contact CNDR subjects for research study participation on behalf of the study team and/or sponsor. Proposals may be submitted by an investigator or sponsor and must include approval from a relevant ethics board. These studies are then reviewed by the CNDR Advisory Committee.
Statistical data requests involve an application for aggregated statistics such as information on the distribution of patients in Canada by location or by disease group. These requests do not involve the export of records from the CNDR and are of a data granularity that does not risk individual identification. These requests are typically used to inform study feasibility or to provide information for government analysis, charitable inquiries or pharmaceutical company clinical trial planning. Typically, the request is driven by the need to examine aggregate data to better understand patient numbers and characteristics across Canada. All data inquiries are reviewed by the CNDR Advisory Committee. In addition to exporting data and providing statistical information, the CNDR also facilitates notification through mail-outs to eligible study participants and can provide custom study data collection or facilitate the collection of anonymized study data through survey mail-outs.
RESULTS
The CNDR began paediatric enrollment in June of 2011 and as of July 25, 2017 totals 658 participants (19% of registry overall).
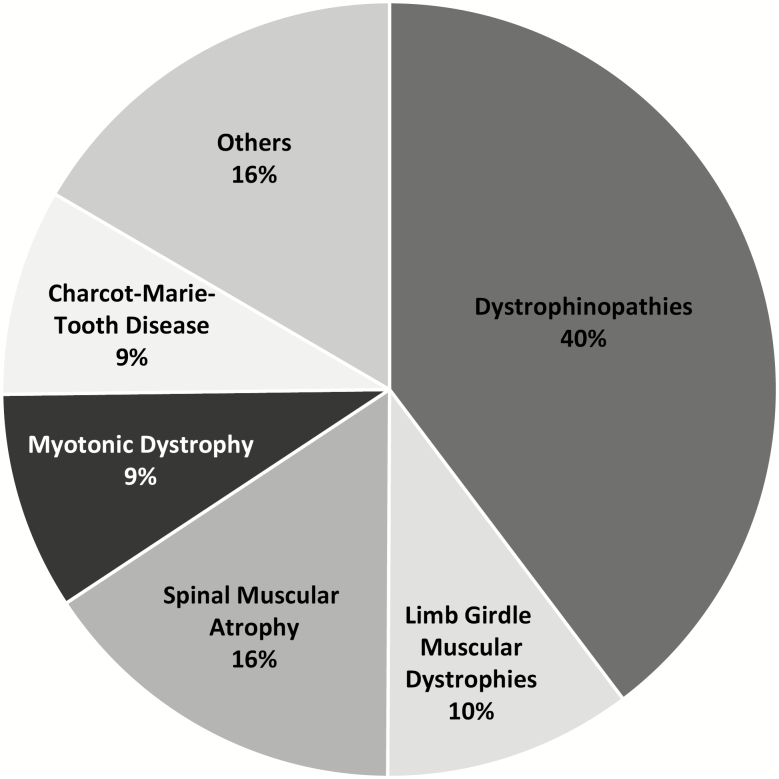
Ten of the 24 CNDR clinics recruit paediatric participants (Table 1). Figure 1 shows the breakdown of all paediatric NM diseases. Dystrophinopathies account for the largest proportion of participants, followed by SMA, LGMD and DM, together making up 71.3% of the entire paediatric population. Table 2 summarizes the genetic characteristics of these index disease participants, and Table 3 summarizes other clinical information such as ventilation status.
Table 1.
Current and former paediatric neuromuscular clinics participating in the Canadian Neuromuscular Disease Registry
| City | Clinical site | Academic institution | Site principal investigators |
|---|---|---|---|
| Current | |||
| Vancouver, BC | BC Children’s Hospital | University of British Columbia | Dr. Kathryn Selby |
| Calgary, AB | Alberta Children’s Hospital | University of Calgary | Dr. Jean Mah |
| Toronto, ON | Holland Bloorview Kids Rehabilitation Hospital | University of Toronto | Dr. Laura McAdam and Dr. Doug Biggar |
| London, ON | Thames Valley Children’s Centre | Western University | Dr. Craig Campbell |
| Ottawa, ON | Children’s Hospital of Eastern Ontario | University of Ottawa | Dr. Anna McCormick and Dr. Hugh McMillan |
| Kingston, ON | Child Development Centre, Hotel Dieu Hospital | Queen’s University | Dr. Garth Smith |
| Montreal, QC | Montreal Children’s Hospital | McGill University | Dr. Maryam Oskoui |
| Fredericton, NB | Stan Cassidy Rehabilitation Centre | University of New Brunwick/Dalhousie University | Dr. Colleen O’Connell and Dr. Scott Worley |
| Halifax, NS | IWK Health Centre | Dalhousie University | Dr. Joseph Dooley |
| Winnipeg, MB | Winnipeg Health Sciences Centre | University of Manitoba | Dr. Edward Leung |
| Past | |||
| Mississauga, ON | Erin Oak Kids Centre for Treatment and Development | None | Dr. Gillian Hogan |
Figure 1.
Breakdown of paediatric diseases in the Canadian Neuromuscular Disease Registry. Dystrophinopathies include Duchenne, Becker and intermediate muscular dystrophies. A detailed breakdown of other neuromuscular disorders can be found in Table 4
Table 2.
Diagnostic and genetic characteristics of paediatric patients with dystrophinopathies, SMA and DM
| Diagnosis (N) | Breakdown by disease subtype | Genetic information | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dystrophinopathies (245) | Duchenne | Becker | Intermediate | Mutation types | |||||||||||
| 224 | 18 | 3 | Deletions | Duplications | Point mutations | Negative | Unknown | ||||||||
| 162 | 25 | 28 | 5 | 29 | |||||||||||
| SMA (98) | Type 1 | Type II | Type III | Distal SMA | Unknown* | SMN2 copy numbers | |||||||||
| 28 | 46 | 19 | 3 | 2 | Type I (N = 23) | Type II (N = 27) | Type III (N = 7) | ||||||||
| 2.3 | 3.0 | 3.7 | |||||||||||||
| DM (58) | Congenital DM | DM type I | Average number of CTG repeats | ||||||||||||
| 30 | 22 | Congenital (N = 24) | DM1 (N = 18) | ||||||||||||
| 1469 | 650 | ||||||||||||||
DM Myotonic dystrophy; SMA Spinal muscular atrophy; SMN2 Survivor motor neuron 2. *SMA was originally a pending disease and disease subtype information was not collected
Table 3.
Clinical characteristics of paediatric patients with dystrophinopathies, spinal muscular atrophy and myotonic dystrophy
| Diagnosis (N) | Average age (years) | Clinical trial participation | Ambulation (N) | Non-invasive ventilation (N) | Invasive ventilation (N) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes (no, unknown) | Yes | No | Unknown | Yes | No | Unknown | Yes | No | Unknown | ||
| Dystrophinopathies (249) | 12 | 60 (148, 41) | 178 | 45 | 25 | 6 | 216 | 25 | 0 | 224 | 25 |
| Spinal muscular atrophy (85)* | 9 | 3 (49, 33) | 7 | 34 | 6 | 24 | 46 | 15 | 2 | 68 | 15 |
| Myotonic dystrophy (58) | 10 | No data | 39 | 12 | 7 | 6 | 43 | 9 | 3 | 46 | 9 |
*Only includes spinal muscular atrophy patients for whom medical information is available (i.e., indexed patients)
Table 4.
Non-index diseases included in the CNDR
| Muscle (N=40) | Nerve (N=18) | Neuromuscular junction (N=13) | Other (N=11) |
|---|---|---|---|
| Congenital muscular dystrophy (20) | Friedreich’s ataxia (8) | Myasthenia gravis (7) | Arthrogryposis (5) |
| Myasthenia gravis—ocular (1) | Amyoplasia (5) | ||
| Congenital muscular dystrophy— Merosin deficiency (7) | Hereditary sensory autonomic neuropathy (4) | Myasthenic syndrome/congenital myasthenic syndrome (5) | Motor neuron disease (1) |
| Mitochondrial myopathy (2) Facioscapulohumeral muscular dystrophy (7) | Chronic inflammatory demyelinating polyneuropathy (1) | Poland syndrome (1) | |
| Dejerine-Sottas disease (1) | |||
| Myotonia congenita—Thomsen disease (2) | Hereditary neuralgia amyotrophy (1) | ||
| Paramyotonia congenital (4)Dermatomyositis (1) | Hereditary neuropathy with liabilitiy to pressure palsies (2) | ||
| King-Denborough disease (1) | Peripheral neuropathy (1) | ||
| X-linked myopathy with excessive autophagy (1) | |||
CNDR Canadian Neuromuscular Disease Registry; PTEN Phosphatase and tensin homolog
Since its launch, the CNDR has approved 30 studies that involve the paediatric population: 20 were clinical trial inquiries, 5 were mail-out surveys and 5 were other studies. The five mail-out survey studies included two that examined the quality of life in boys with DMD (9), parent-reported disease burden of congenital and childhood-onset DM (10); an access to technology survey for individuals with DM, and a respiratory needs survey in boys with DMD and SMA. Five other studies included a study of congenital DM; a study of a respiratory intervention in children; an inquiry regarding ventilation support use in paediatric patients with type I DM; an inquiry regarding steroid use, growth and walking ability in DMD patients; and a study on genetics of dystrophinopathies and disease severity.
DISCUSSION
The CNDR has grown to be a valuable national tool in the facilitation of research in children with NM disorders. From an international perspective, the CNDR is one of 49 registries that enroll DMD patients, 37 registries that enroll SMA patients, 14 registries that enroll DM patients and 1 of 4 registries that enroll LGMD patients worldwide (11). The CNDR is unique among many NM registries in that it is a multi-disease registry with participants enrolled and consented directly in NM clinics with clinic verified data. The strengths of the design and organization of the CNDR have led to a number of successes for the paediatric NM community in Canada.
Success in clinical trial facilitation
Canada is currently without a defined orphan disease strategy, or even an orphan disease designation within the Health Canada pharmaceutical review process, which limits the incentive for drug development in rare diseases (12). Despite this disadvantage of working in Canada, the launch of the CNDR has nonetheless encouraged more industry partners to consider establishing clinical trial sites in Canada. In our view, the development of the CNDR has therefore allowed Canada to be viewed more favourably by biopharmaceutical companies involved in drug development leading to clinical trial opportunities for Canadian NM patients. The CNDR is now exploring models for post-marketing surveillance for new therapies to further enhance the attractiveness of Canada as a market for novel therapeutics.
Registry information can also benefit research at the pre-clinical stage prior to human study. For example, a theoretical bioinformatics study using the DMD registry in France has allowed for the prediction that development of a multi-exon skipping antisense oligonucleotide could help 63% of patients with dystrophin deletions, in contrast to single exon skipping of even the most frequently deleted exons which would help only 16% of affected boys (13).
Although randomized clinical trials (RCTs) are the gold standard for providing clinical evidence to guide treatment decisions, the costs of RCTs in the rare disease population are prohibitive and participants are not always representative of the patient population at large. One proposed solution is registry-based RCTs (14,15). This type of trial design has been used an open-label method to evaluate treatments, strategies and devices. The baseline and end points are already included in the database. Registry-based clinical trials are relatively simple to design, far less costly and more applicable to real-world patients compared with RCTs. The CNDR is an ideal registry for identifying patients eligible for enrollment in registry-based randomized trials.
Success in facilitating other clinical research
Although the planning, facilitation and implementation of clinical trials are paramount objectives for the CNDR, it also enables many other important research initiatives. The CNDR has facilitated a variety of clinical and survey-based studies including the ongoing longitudinal study examining health-related quality of life (HRQOL) in boys with DMD. This is one of the largest and most representative HRQOL studies in this population and has already yielded intriguing results. Notably, subjective levels of fatigue were found to be highly associated with HRQOL, suggesting reducing levels of fatigue could lead to improvement of HRQOL in the paediatric DMD population (9). The CNDR plays a crucial role in the ongoing success of this project, from assisting with patient enrollment through study notifications and providing accurate clinical data.
The CNDR can also foster collaborations between specialists in the NM field and paediatricians and paediatric health care professionals from other disciplines, studying a wide range of issues, such as those pertaining to chronic disease, genetic disorders, transition to adult care, end-o-life care, quality of life, health service delivery and health maintenance.
Multi-disease, clinic-based model optimizes efficiency and accuracy of data collection
The CNDR is a comprehensive registry that has the capacity to enroll all patients with a NM disease. While DMD, DM, LGMD and SMA are the diseases of focus, there is value in collecting basic demographic information on other NM diseases. These diseases are even more rare, thus recruitment for clinical trial and other research studies is even more challenging. A centrally managed database that harmonizes participant information collection is the most efficient way of achieving this goal, ensuring that participants with even the rarest NM diseases are not overlooked for research.
The decision to run a clinic-based enrollment and data entry model for the CNDR was based on several goals: retaining participants through regular clinic contact, increasing the accuracy of data through data collection from clinic charts by trained personnel and assuring full and ongoing informed consent. The inadequacy of follow-up in self-report registries has been outlined in a paper contrasting an active surveillance registry with a patient-driven registry for DM; these include several clinically relevant pieces of information being missing and concerns regarding accuracy of reported data due to recall bias (16).
Potential as a clinic-management tool and epidemiologic tool
Although NM diseases are known to comprise a sizeable component of the neurological burden of disease globally, the epidemiologic data available for specific NM diseases in Canada or globally remain very limited. In this regard, CNDR data have already contributed to global collection of epidemiological data, ranging from mutation types and frequencies (17) to monitoring of disease trends and care practices (18). However, at present, there are gaps in patient recruitment as not all Canadian paediatric NM clinics are enrolling in the CNDR, and so the CNDR numbers should not be considered a comprehensive epidemiological tool. A registry that is embedded within the clinic setting can have a beneficial quality assurance impact, ensuring that patients are not ‘falling through the cracks’ of a busy practice, flagging that a participant is overdue for a visit or has not had a certain test at the indicated time. Further, national practice differences can be examined using registry data as has been done for cystic fibrosis resulting in advanced and more codified standards of care (19). The CNDR aims to increase this potential function of the registry over time.
Funding model
The CNDR has a unique funding structure: it is funded by a mix of charitable and industry sponsors and entirely unfunded by government or research grant agencies. Each sponsor assumes a portion of the registry’s central operating costs and covers disease-specific costs for their disease of choice. In this way, each sponsoring organization is able to minimize individual costs while maximizing collective benefit and multiplying the value of every dollar invested.
CONCLUSIONS
The CNDR is a clinic-based registry of national scope that has been successful in connecting Canadian NM patients to national and international clinical trials and other research initiatives. With the recent launch of a national rare disease strategy, the advent of next-generation sequencing diagnoses, the burgeoning interest of pharmaceutical and biotechnology industries in rare disease and the resulting rare disease therapeutic pipeline, there will be increasing focus on, and opportunities for, rare disease clinical trials for NM diseases. The CNDR will act as a valuable resource enabling capture of national data and facilitating a network of geographically disparate teams to help to rectify this gap. The registry will continue to engage stakeholders to enable and support research into potential treatments, with specific measures of success being the number of studies and publications with high impact that are realized due to the CNDR. Paediatric clinicians and researchers are encouraged to submit research proposals or data requests that will engage the Canadian paediatric NM community and ultimately deliver benefits to NM patients.
Acknowledgements
The authors would like to thank Jane Terhaerdt and Dr. Laura McAdam for providing valuable feedback on the manuscript. The authors would also like to thank national and regional CNDR coordinators for maintaining the registry at its highest quality. The CNDR has been supported by the ALS Society of Canada, Biogen, Families of SMA Canada, Jesse’s Journey, the Marigold Foundation and the Starratt Family Foundation. S.V. reports CME Honoria Speaker’s fees from Genzyme Canada. M.O. is part of a Ionis clinical trial, part of the Biogen SMA expert panel and has received funding from the Sick Kids Foundation for a study on cerebral palsy. L.K. reports grants from Jesse’s Journey, ALS Canada, Families of SMA, Marigold Foundation, Biogen Idec and Starratt Family Foundation during the conduct of the study. C.C. is the Chair of the Canadian Advisory Board, a site investigator for clinical trials and received support for scientific meetings from PTC Therapeutics. C.C. also reports to be part of Biomarin and Ionis/Biogen clinical trials and received funding from Biomarin and Ionis/Biogen to travel to consultant meetings and Eli Lily, Acceleron and GSK to travel to investigator meetings, and grants from Valerion Pharmaceuticals and Jesse’s journey Foundation. The Canadian Neuromuscular Disease Registry is housed at the University of Calgary and ethics approval was obtained from the Conjoint Health Research Ethics Board (CHREB) at the University of Calgary.
References
- 1. Rowland LP, McLeod JG. Classification of neuromuscular disorders. J Neurol Sci 1994;124(Suppl.):109–30. [DOI] [PubMed] [Google Scholar]
- 2. Campbell C, Levin S, Siu VM, Venance S, Jacob P. Congenital myotonic dystrophy: Canadian population-based surveillance study. J Pediatr 2013;163(1):120–5, e1–3. [DOI] [PubMed] [Google Scholar]
- 3. Theadom A, Rodrigues M, Roxburgh R et al. . Prevalence of muscular dystrophies: A systematic literature review. Neuroepidemiology 2014;43(3–4):259–68. [DOI] [PubMed] [Google Scholar]
- 4. D’Amico A, Mercuri E, Tiziano FD, Bertini E. Spinal muscular atrophy. Orphanet J Rare Dis 2011;6:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bushby K, Lynn S, Straub T, TREAT-NMD Network Collaborating to bring new therapies to the patient—the TREAT-NMD model. Acta Myol Myopathies Cardiomyopathies 2009;28(1):12–5. [PMC free article] [PubMed] [Google Scholar]
- 6. Sárközy A, Bushby K, Béroud C, Lochmüller H. 157th ENMC International Workshop: Patient registries for rare, inherited muscular disorders, 25–27 January 2008, Naarden, the Netherlands. Neuromuscul Disord 2008;18(12):997–1001. [DOI] [PubMed] [Google Scholar]
- 7. Korngut L, Campbell C, Johnston M et al. . The CNDR: Collaborating to translate new therapies for Canadians. Can J Neurol Sci. 2013;40(5):698–704. [DOI] [PubMed] [Google Scholar]
- 8. Bladen CL, Rafferty K, Straub V et al. . The TREAT-NMD Duchenne muscular dystrophy registries: Conception, design, and utilization by industry and academia. Hum Mutat 2013;34(11):1449–57. [DOI] [PubMed] [Google Scholar]
- 9. Wei Y, Speechley KN, Zou G, Campbell C. Factors associated with health-related quality of life in children with Duchenne muscular dystrophy. J Child Neurol 2016;31(7):879–86. [DOI] [PubMed] [Google Scholar]
- 10. Johnson NE, Ekstrom A-B, Campbell C et al. . Parent-reported multi-national study of the impact of congenital and childhood onset myotonic dystrophy. Dev Med Child Neurol 2016;58(7):698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. TREAT-NMD: List of Registries by Disease [Internet]. <www.treat-nmd.eu/resources/patient-registries/list/> (Accessed January 26, 2016).
- 12. Our Work | Canadian Organization for Rare Disorders [Internet]. <www.raredisorders.ca/our-work/> (Accessed January 26, 2016).
- 13. Béroud C, Tuffery-Giraud S, Matsuo M et al. . Multiexon skipping leading to an artificial DMD protein lacking amino acids from exons 45 through 55 could rescue up to 63% of patients with Duchenne muscular dystrophy. Hum Mutat 2007;28(2):196–202. [DOI] [PubMed] [Google Scholar]
- 14. Lauer M, D’Agostino R. The randomized registry trial—the next disruptive technology in clinical research?N Engl J Med 2013;369(17):1579–81. [DOI] [PubMed] [Google Scholar]
- 15. Harrington RA. Appropriate use criteria for coronary revascularization and the learning health system: A good start. JAMA 2015;314(19):2029–31. [DOI] [PubMed] [Google Scholar]
- 16. Prendergast P, Magalhaes S, Campbell C. Congenital myotonic dystrophy in a national registry. Paediatr Child Health 2010;15(8):514–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bladen CL, Salgado D, Monges S et al. . The TREAT-NMD DMD Global Database: Analysis of more than 7,000 Duchenne muscular dystrophy mutations. Hum Mutat 2015;36(4):395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bladen CL, Thompson R, Jackson JM et al. . Mapping the differences in care for 5,000 spinal muscular atrophy patients, a survey of 24 national registries in North America, Australasia and Europe. J Neurol 2014;261(1):152–63. [DOI] [PubMed] [Google Scholar]
- 19. The Canadian CF Registry Data—Cystic Fibrosis Canada [Internet]. <www.cysticfibrosis.ca/cf-care/cf-registry/the- canadian-cf-registry-data/> (Accessed January 26, 2016).