Abstract
BACKGROUND
Cachexia, described as weight loss (mainly in lean body mass [LBM]) and anorexia, is common in patients with advanced cancer. This study examined the efficacy and safety of anamorelin (ONO‐7643), a novel selective ghrelin receptor agonist, in Japanese cancer patients with cachexia.
METHODS
This double‐blind clinical trial (ONO‐7643‐04) enrolled 174 patients with unresectable stage III/IV non–small cell lung cancer (NSCLC) and cachexia in Japan. Patients were randomized to daily oral anamorelin (100 mg) or a placebo for 12 weeks. The primary endpoint was the change from the baseline LBM (measured with dual‐energy x‐ray absorptiometry) over 12 weeks. The secondary endpoints were changes in appetite, body weight, quality of life, handgrip strength (HGS), and 6‐minute walk test (6MWT) results.
RESULTS
The least squares mean change (plus or minus the standard error) in LBM from the baseline over 12 weeks was 1.38 ± 0.18 and −0.17 ± 0.17 kg in the anamorelin and placebo groups, respectively (P < .0001). Changes from the baseline in LBM, body weight, and anorexia symptoms showed significant differences between the 2 treatment groups at all time points. Anamorelin increased prealbumin at weeks 3 and 9. No changes in HGS or 6MWT were detected between the groups. Twelve weeks' treatment with anamorelin was safe and well tolerated in NSCLC patients.
CONCLUSIONS
Anamorelin significantly increased LBM and improved anorexia symptoms and the nutritional state, but not motor function, in Japanese patients with advanced NSCLC. Because no effective treatment for cancer cachexia is currently available, anamorelin can be a beneficial treatment option. Cancer 2018;124:606‐16. © 2017 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society. This is an open access article under the terms of the Creative Commons Attribution‐NonCommercial‐NoDerivs License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non‐commercial and no modifications or adaptations are made.
Keywords: anamorelin (ONO‐7643), cachexia, lean body mass, non–small cell lung cancer, randomized controlled trial
Short abstract
Anamorelin leads to significant increases in lean body mass and body weight as well as improvements in anorexia symptoms and the nutritional state. Anamorelin can be a beneficial treatment option for cachexia patients.
See also pages 456‐8.
INTRODUCTION
Cachexia is commonly related to many clinically important conditions, such as anorexia, inflammation, and degradation of skeletal muscle protein, in which muscle wasting plays a key role. Cachexia is frequently observed in patients with cancer (50%‐80%) and leads to approximately 20% of deaths among cancer patients.1, 2, 3, 4, 5 Moreover, cancer cachexia not only is associated with higher rates of toxicity from chemotherapeutic drugs6 but also leads to a poor prognosis as well as reduced quality of life (QOL).7
Cancer cachexia cannot be completely reversed with conventional nutritional support,8 and there are limited pharmacological therapies useful for the management of cachexia.
Ghrelin, a peptide hormone produced by ghrelin‐producing endocrine cells in the gut, acts as a regulator of hunger, which is also involved in the regulation of food intake.9, 10, 11 Furthermore, ghrelin induces the secretion of growth hormone and thereby acts as a growth hormone secretagogue.12, 13
Anamorelin (ONO‐7643) is an orally active, high‐affinity, selective agonist of the ghrelin receptor.14, 15 Previous phase 1 and 2 trials have demonstrated the safety and efficacy of anamorelin treatment for increasing body weight, lean body mass (LBM), and food intake.16, 17, 18 Two multinational phase 3 clinical studies in patients with advanced non–small cell lung cancer (NSCLC) and cachexia reported that anamorelin administration for 12 weeks increased LBM and body weight and substantially improved the symptoms of anorexia/cachexia.19
A randomized, double‐blind, phase 2 trial investigated 50 and 100 mg of anamorelin versus a placebo in Japanese patients with NSCLC and cachexia; treatment with 100 mg of anamorelin in that study demonstrated improvements in LBM, body weight, appetite, and QOL with no tolerability issues.20 Therefore, in the current study, 100 mg of anamorelin was selected to confirm its action in increasing LBM in Japanese patients with NSCLC and cachexia.
MATERIALS AND METHODS
Study Design
We conducted a multicenter (43 sites in Japan), randomized, double‐blind, placebo‐controlled trial that comprised an observation/run‐in period of 2 weeks, a treatment period of 12 weeks, and a follow‐up period of 4 weeks. Visits during the treatment period were planned at weeks 0, (baseline/randomization), 1, 3, 6, 9, and 12. All procedures followed during this study were in accordance with the spirit of the Declaration of Helsinki, the study protocol, the standards specified under the Pharmaceutical Affairs Act of Japan (article 80, paragraph 2 and article 14, paragraph 3), and Good Clinical Practice (effective as of April 1, 1997; Japanese Ministry of Health and Welfare Ordinance No. 28). Ethics committee approval for the study was obtained from each center.
Patients
This study included patients with stage III or IV NSCLC who were not to undergo an operation, were 20 years old or older, had involuntary weight loss ≥ 5% within the last 6 months, had anorexia, had 2 or more applicable symptoms (fatigue, malaise, reduced overall muscular strength, and arm muscle circumference [in centimeters] < 10th percentile), and had more than 1 of the following conditions: albumin level < 3.2 g/dL, C‐reactive protein level > 5.0 mg/L, hemoglobin level < 12 g/dL, Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 2, and estimated life expectancy ≥ 4 months. Anorexia, malaise, fatigue, and reduced muscular strength needed to be grade 1 or higher according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (version 4.0). The following formula was used to ascertain the arm muscle circumference:
Arm muscle circumference (cm) = Arm circumference (cm) − 3.14 × Triceps skinfold thickness (mm)/10.
Patients were excluded if they had known symptomatic brain metastases or uncontrolled diabetes. Written informed consent was obtained from each patient. All eligible patients were randomized by a centralized allocation center and were further stratified by the enrollment site and reductions in weight during the last 6 months (5%‐10% and >10%). The randomization methodology used a randomization table and sealed envelopes to randomize the patients.
Interventions and Concomitant Therapies
After enrollment, patients were randomly assigned to either 100 mg of anamorelin or a placebo once daily throughout the therapy period. In this study, patients were enrolled regardless of their treatment history with chemotherapy for NSCLC, but they were prohibited from newly taking epidermal growth factor receptor tyrosine kinase inhibitors during the treatment period because of their possible effect on the QOL assessment.
During the study period, radiotherapy (other than palliative radiation therapy for bone metastases or radiation therapy for metastases in the brain), general corticosteroids, growth hormone formulations, medroxyprogesterone, megestrol acetate, Chinese herbal drugs, antiarrhythmic drugs, antitumor anthracyclines, inhibitors and inducers of cytochrome P450 3A4, and other experimental treatments were not permitted.
Efficacy Assessments
The primary endpoint of the trial was the mean change in LBM (estimated by dual‐energy x‐ray absorptiometry [DEXA]) from the baseline over the 12‐week treatment period. The secondary endpoints of the study were changes in the body weight, body composition (ascertained by DEXA), appetite, Cancer Fatigue Scale (CFS) score, ECOG PS, Karnofsky Performance Scale (KPS) score, handgrip strength (HGS), Quality‐of‐Life Questionnaire for Cancer Patients Treated With Anticancer Drugs (QOL‐ACD) score, 6‐minute walk test (6MWT) results, and serum biomarkers. The LBM and other body composition–related variables, ECOG PS, KPS, HGS, 6MWT, and serum biomarkers were determined at the baseline and in weeks 3, 6, 9, and 12. The body weight, QOL‐ACD score, and CFS score were determined at the baseline and in weeks 1, 3, 6, 9, and 12. In addition, the efficacy parameters were recorded after treatment discontinuation.
Body composition was determined via DEXA with either the GE Lunar system (GE, Wauwatosa, Wisconsin) or the Hologic system (Hologic, Bedford, Massachusetts). DEXA was used to assess the LBM, fat mass, bone mineral content, and overall body mass by means of standardized methods. A grip dynamometer (Tracker Freedom Wireless Grip; JTECH Medical, Midvale, Utah) was used for the measurement of HGS.
The QOL‐ACD (see online supporting information) is a self‐rated measure assessing the condition of a patient during the last few days according to a 1 to 5 scale, and it is composed of 4 domains (functional, physical, mental, and psychosocial) and a global face scale developed as a generic questionnaire for assessing QOL in Japanese cancer patients receiving chemotherapy.21 The CFS is a self‐rated scale evaluating current fatigue in cancer patients, and it has 3 dimensions (physical fatigue, affective fatigue, and cognitive fatigue). The scale is composed of 15 items scored on a 1 to 5 scale for a maximum score of 60, with higher scores indicating more severe fatigue. ECOG PS and KPS were used to quantify the PS of the patients. After a ≥ 12‐hour fast, blood samples were collected for the estimation of insulin‐like growth factor 1 (IGF‐1), insulin‐like growth factor‐binding protein 3 (IGFBP‐3), and prealbumin. Laboratory tests were performed at each study site.
Safety
The safety parameters included the vital signs, electrocardiography (centrally assessed) with all 12 leads, status of the tumor (evaluated by investigators using Response Evaluation Criteria in Solid Tumors [RECIST] guidelines), clinical laboratory tests, and adverse events (AEs). AEs were reported with the National Cancer Institute's Common Terminology Criteria for Adverse Events (version 4.0), and they were classified according to the system organ class/preferred term.
Statistical Analysis
Analyses were performed according to the predetermined study protocol and statistical analysis plan. The full analysis set (FAS) was used for the analysis of all efficacy variables. The FAS comprised all eligible patients who had undergone a minimum of 1 efficacy assessment after the initiation of the study drug. The safety analysis set was used for the analysis of safety data and comprised all patients who had received the study drug at least once.
The findings of a phase 2 trial (ONO‐7643‐03), in which the mean difference in LBM (according to DEXA) between 100 mg of anamorelin and the placebo was 0.89 ± 1.94 kg, were used to determine the sample size for this study.20 At least 76 patients were required in each treatment arm to reject the null hypothesis at P < .05 and a power of 80%. Under the assumption that approximately 10% of the patients would withdraw/drop out of the study, a total of 170 patients (85 patients per group) were to be enrolled. Descriptive statistics were used to summarize the baseline parameters. An analysis of covariance for repeated measurement data, using the study arm, time point, and prior reductions in weight (5%‐10% and >10%) as fixed factors and the baseline value as a covariate, was used to analyze efficacy parameters. The difference in the least squares mean from the initiation of treatment to a specific point of time was determined for both groups. The least squares mean differences between patients who received anamorelin and those who received the placebo were determined with 95% confidence intervals (CIs). A study arm–point of time interaction was incorporated for the assessment of secondary endpoints (differences in the body mass composition, QOL‐ACD score, CFS score, body weight, KPS, serum biomarkers, HGS, and 6MWT results). Descriptive statistics were used to assess safety parameters and are reported as numbers and percentages of patients. The total incidence of AEs and adverse drug reactions (ADRs) was compared between the study groups with the chi‐square test. There was no adjustment for the multiplicity of statistical testing, and an imputation method was not used for missing data.
RESULTS
Patients
A total of 174 patients were enrolled in this study from May 2014 to October 2015. Ninety of these 174 patients were randomized to the placebo group, and 84 were randomized to the 100‐mg anamorelin group (Fig. 1). One patient did not receive treatment. One patient treated with 100 mg of anamorelin failed to meet the inclusion criteria and hence was excluded from the FAS. The FAS comprised 172 patients, with 90 and 82 in the placebo and anamorelin groups, respectively. There were 11 deaths in the placebo group and 5 deaths in the anamorelin group. AEs led to treatment discontinuation in 2 patients from the placebo group and in 5 patients from the anamorelin group. The baseline characteristics of the 2 groups were similar (Table 1).
Figure 1.
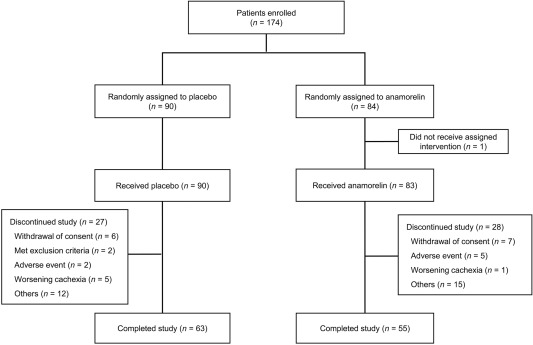
Enrollment and outcomes.
Table 1.
Demographics and Baseline Characteristics of the Patients
| Parameter | Placebo (n = 90) | Anamorelin (n = 84) |
|---|---|---|
| Sex, No. (%) | ||
| Male | 57 (63.3) | 59 (70.2) |
| Female | 33 (36.7) | 25 (29.8) |
| Age, mean ± SD, y | 67.2 ± 7.9 | 67.6 ± 9.9 |
| Weight, mean ± SD, kg | 49.73 ± 8.32 | 52.23 ± 9.43 |
| BMI, mean ± SD, kg/m2 | 19.27 ± 2.31 | 19.81 ± 2.60 |
| Weight loss, No. (%) | ||
| 5‐10 | 52 (57.8) | 50 (60.2) |
| >10 | 38 (42.2) | 33 (39.8) |
| Missing | ― | 1 |
| Body composition (DEXA), mean ± SD, kg | ||
| LBM | 37.06 ± 6.34 | 38.88 ± 7.06 |
| Body fat | 10.68 ± 4.21 | 11.29 ± 5.04 |
| BMC | 1.90 ± 0.56 | 2.06 ± 0.57 |
| Total body mass | 49.63 ± 8.61 | 52.23 ± 9.73 |
| Grip strength, mean ± SD, kg | ||
| Dominant hand | 26.70 ± 8.01 | 27.87 ± 9.35 |
| Nondominant hand | 25.12 ± 7.01 | 26.41 ± 8.30 |
| 6‐min walk distance, mean ± SD, m | 375.7 ± 88.4 | 379.6 ± 89.6 |
| QOL‐ACD, mean ± SD | 70.9 ± 13.0 | 74.9 ± 13.0 |
| Cancer Fatigue Scale, mean ± SD | 23.8 ± 9.7 | 24.4 ± 9.7 |
| ECOG PS, No. (%) | ||
| 0 | 13 (14.4) | 9 (10.8) |
| 1 | 65 (72.2) | 64 (77.1) |
| 2 | 12 (13.3) | 10 (12.0) |
| Missing | ― | 1 |
| NSCLC type per histological criteria, No. (%) | ||
| Adenocarcinoma | 71 (78.9) | 67 (79.8) |
| Squamous cell | 16 (17.8) | 14 (16.7) |
| Other | 1 (1.1) | 2 (2.4) |
| Unknown | 2 (2.2) | 1 (1.2) |
| Disease stage | ||
| IIIA | 1 (1.1) | 3 (3.6) |
| IIIB | 11 (12.2) | 6 (7.1) |
| IV | 60 (66.7) | 49 (58.3) |
| Recurrence | 18 (20.0) | 26 (31.0) |
| Time from diagnosis to starting study drug, mean ± SD, d | 609.4 ± 741.7 | 768.7 ± 698.0 |
| Previous history of chemotherapy (No. of times), No. (%) | ||
| 0 | 2 (2.2) | 2 (2.4) |
| 1 | 31 (34.4) | 20 (23.8) |
| 2 | 18 (20.0) | 19 (22.6) |
| ≥3 | 39 (43.3) | 43 (51.2) |
| Concomitant cancer therapy, No. (%) | ||
| Chemotherapy | 70 (77.8) | 64 (76.2) |
| EGFR TKI | 29 (32.2) | 23 (27.7) |
| Radiation | 6 (6.7) | 7 (8.3) |
| Supportive care | 19 (21.1) | 18 (21.7) |
| Missing | ― | 1 |
Abbreviations: BMC, bone mineral content; BMI, body mass index; DEXA, dual‐energy x‐ray absorptiometry; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; LBM, lean body mass; NSCLC, non–small cell lung cancer; PS, performance status; QOL‐ACD, Quality‐of‐Life Questionnaire for Cancer Patients Treated With Anticancer Drugs (Kurihara Group Questionnaire); SD, standard deviation; TKI, tyrosine kinase inhibitor.
LBM
As shown in Figure 2, the increase in LBM over 12 weeks was found to be significantly larger in the anamorelin‐treated patients versus the placebo‐treated patients, with least squares means and standard errors of 1.38 ± 0.18 and −0.17 ± 0.17 kg, respectively. Overall, the change in the anamorelin‐treated patients versus the placebo‐treated patients was 1.56 kg (95% CI, 1.11‐2.00 kg; P < .0001). At week 3 and thereafter, a significant difference (P < .0001) in the LBM gain in comparison with the baseline was noted between the treatment groups.
Figure 2.
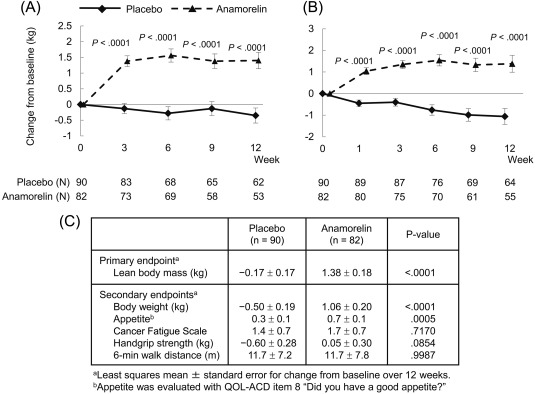
Time‐course changes for the anamorelin and placebo groups in (A) lean body mass and (B) body weight and (C) changes in primary and secondary efficacy measures from the baseline over 12 weeks. QOL‐ACD indicates Quality‐of‐Life Questionnaire for Cancer Patients Treated With Anticancer Drugs.
Body Weight
In comparison with the placebo, anamorelin induced a significant weight gain (Fig. 2), which is in agreement with anamorelin's mechanism of action and the LBM gain. The body weight gain was evident at week 1 of treatment and continued thereafter.
Other Body Composition Parameters
In comparison with the placebo, anamorelin significantly increased other body composition parameters, including the total body mass, fat mass, appendicular LBM (arms and legs), and trunk LBM (Supporting Table 1 [see online supporting information]).
QOL‐ACD
Throughout the study period from week 1, the anamorelin‐treated patients showed significant improvements in comparison with the placebo‐treated patients in the QOL‐ACD scores for items 7 to 11 (“physical condition”), item 8 (“Did you have a good appetite?”), item 9 (“Did you enjoy your meals?”), and item 11 (“Did you lose any weight?”; Fig. 3A‐D). The efficacy of anamorelin was not definite in other domains.
Figure 3.
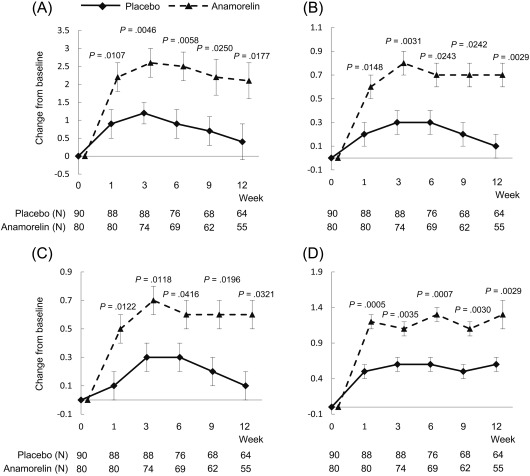
Time‐course changes for the anamorelin and placebo groups in the QOL‐ACD scores for (A) items 7 to 11 (“physical condition”), (B) item 8 (“Did you have a good appetite?”), (C) item 9 (“Did you enjoy your meals?”), and (D) item 11 (“Did you lose any weight?”). QOL‐ACD indicates Quality‐of‐Life Questionnaire for Cancer Patients Treated With Anticancer Drugs.
Other Secondary Endpoints
The effects of 100 mg of anamorelin on CFS, HGS, and 6MWT are shown in Figure 2 and Supporting Table 2 (see online supporting information). There were marginal effects on CFS, HGS, and 6MWT. In comparison with the placebo group, the anamorelin group showed significant increases in the serum IGF‐1, IGFBP‐3, and prealbumin levels (Fig. 4A‐C).
Figure 4.
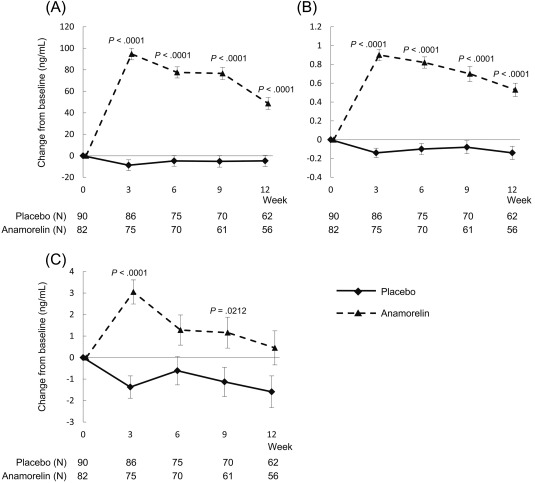
Time‐course changes for the anamorelin and placebo groups in (A) insulin‐like growth factor 1, (B) insulin‐like growth factor‐binding protein 3, and (C) prealbumin.
Safety
On the basis of the RECIST criteria, a complete response and a partial response were observed in 1 (1.5%) and 3 patients (4.5%), respectively, in the anamorelin‐treated group and in 0 (0.0%) and 2 patients (2.7%), respectively, in the placebo group. Stable disease was observed in 21 (31.3%) and 22 patients (29.7%) in the anamorelin‐treated and placebo groups, respectively. Thirty‐eight of the anamorelin‐treated patients (56.4%) showed progressive disease, whereas 47 patients (63.5%) in the placebo group did. Non–complete response/non–progressive disease cases were observed for 4 (6.0%) and 3 patients (4.1%) in the anamorelin‐treated and placebo groups, respectively. The median survival times were found to be similar for the 2 groups (8.08 months [95% CI, 5.98‐11.56 months] for anamorelin and 8.21 months [95% CI, 6.67‐12.39 months] for the placebo; hazard ratio, 1.17 [95% CI, 0.82‐1.67]; P = .3762).
Table 2 summarizes the overall incidences of AEs and ADRs. The frequency of AEs was found to be similar in the anamorelin group and the placebo group. On the other hand, the anamorelin group reported a significantly higher number of ADRs in comparison with the placebo group; however, all the ADRs were grade 3 or lower. The most common ADRs were first‐degree atrioventricular block and rash, which were followed by increased γ‐glutamyltransferase and diabetes mellitus. All these events were grade 1 or 2 except for 1 case of rash.
Table 2.
Safety
| Placebo (n = 90) | Anamorelin (n = 83) | |
|---|---|---|
| AEs, No. (%) | 73 (81.1) | 74 (89.2) |
| AEs | ||
| Difference vs placebo, % (95% CI) | 8.0 (–2.4, 18.5) | |
| P | .1390 | |
| SAEs, No. (%) | 8 (8.9) | 16 (19.3) |
| Discontinuations due to AEs, No. (%) | 2 (2.2) | 3 (3.6) |
| ADRs, No. (%) | 20 (22.2) | 34 (41.0) |
| ADRs | ||
| Difference vs placebo, % (95% CI) | 18.7 (5.1, 32.4) | |
| P | .0079 | |
| Serious ADRs, No. (%) | 0 (0.0) | 2 (2.4) |
| Discontinuations due to ADRs, No. (%) | 1 (1.1) | 2 (2.4) |
| Deaths, No. (%) | 11 (12.2) | 5 (6.0) |
| ADRs by grade, No. (%) | ||
| 1/2 | 18 (20.0) | 28 (33.7) |
| 3 | 2 (2.2) | 6 (7.2) |
| ADRs in > 2% of patients, No. (%) | ||
| First‐degree atrioventricular block | 0 (0.0) | 5 (6.0) |
| Tachycardia | 0 (0.0) | 2 (2.4) |
| Edema | 0 (0.0) | 2 (2.4) |
| Peripheral edema | 0 (0.0) | 2 (2.4) |
| Pyrexia | 0 (0.0) | 2 (2.4) |
| γ‐Glutamyltransferase increase | 1 (1.1) | 3 (3.6) |
| Glycosylated hemoglobin increase | 1 (1.1) | 2 (2.4) |
| Diabetes mellitus | 0 (0.0) | 3 (3.6) |
| Hyperglycemia | 1 (1.1) | 2 (2.4) |
| Headache | 1 (1.1) | 2 (2.4) |
| Rash | 1 (1.1) | 5 (6.0) |
| Hypertension | 0 (0.0) | 2 (2.4) |
| Hot flush | 0 (0.0) | 2 (2.4) |
Abbreviations: ADR, adverse drug reaction; AE, adverse event; CI, confidence interval; SAE, severe adverse event.
DISCUSSION
The current study demonstrated that anamorelin significantly improved LBM and body weight in Japanese patients with NSCLC and cachexia in comparison with a placebo. Significant improvements in LBM and body weight were observed in the anamorelin group at the early time points of week 3 and week 1, respectively, in comparison with the placebo group, and they were sustained thereafter during the 12‐week study period.
The National Comprehensive Cancer Network clinical guidelines for the management of anorexia/cachexia define the primary treatment goals as promoting weight gain/stabilization and relieving symptoms of anorexia.22 Similarly, the clinical practice guidelines on cancer cachexia given by the European Palliative Care Research Collaborative specify that the treatment goals for cachexia should be a reversal of the loss of body weight and muscle mass and that the minimum objective must be the maintenance of body weight and the prevention of further body weight loss.23 The results of the current study satisfy the treatment goals in these guidelines.
As for appetite, a considerable increase was reported in the anamorelin group versus the placebo group as early as week 1, and it was subsequently sustained throughout the study period. The increased level of prealbumin, a nutritional state marker, suggested increased food intake. Decreases in appetite and food intake are considered to be the main underlying causes for the worsening of the physical and psychological status in cancer patients.24 Anorexia leads to QOL deterioration in cancer patients25 and is also a prognostic factor.26 Therefore, oncologists should consider ensuring sufficient energy and protein intake for all cancer patients.27
Under the present conditions, where there are no effective treatment methods for cancer cachexia, anamorelin may have great clinical significance by preventing weight loss and ameliorating anorexia.
In contrast, in the assessment of motor function, including HGS and 6MWT results, no improvement was observed after anamorelin administration. Previous researchers have suggested that patients who are affected by long‐term illness and systemic inflammation may display an unusual association between muscle mass and muscular strength.28, 29, 30 Furthermore, the most suitable measure for muscle strength in advanced cancer patients is unidentified. The 6MWT measurements may have been affected by respiratory insufficiency in patients with NSCLC. The cause of cachexia is multifactorial, and pharmacological treatment alone may not be able to bring about a complete reversal of all features of the syndrome (especially improvements in motor function). Therefore, it is expected that a multimodal treatment combining medicine, exercise, and nutrition may improve the condition and symptoms of cachexia, including motor function.
In general, patients in the 2 treatment groups showed similar overall survival times and tumor responses as assessed by RECIST, and this indicated that the therapy had no effect on the progression of the disease. In addition, patient characteristics such as the disease stage and previous use of chemotherapy and prevailing factors that affect the prognosis were similar between the 2 treatment arms.
In comparison with patients receiving the placebo, the frequency of ADRs was significantly higher in patients receiving anamorelin; however, most deaths and treatment discontinuations were caused by disease progression and not by the study drugs. In comparison with the placebo‐treated patients, first‐degree atrioventricular block and rash occurred at rates ≥ 5%, and they had a higher incidence in the anamorelin‐treated patients. However, all first‐degree atrioventricular block cases were grade 1, and only 1 case of rash was grade 3; this suggests no major risk. The frequencies of these ADRs were observed to be higher in the current study versus the multinational phase 3 studies.19 The frequent electrocardiogram measurements might have caused the higher incidence of first‐degree atrioventricular block. Although no apparent cause of rash has been identified, we think that there is a possibility that this might have been influenced by chemotherapy applied during the study. In agreement with previous studies,19, 20 increases in blood glucose levels were more frequently observed with anamorelin treatment; this was, however, controllable. The changes in glucose homeostasis might have been caused by effects of IGF‐1 and growth hormone on glucose metabolism or by a possible effect of the reversal of cancer anorexia/cachexia syndrome. These findings suggest that anamorelin is safe and well tolerated in Japanese cancer patients with cachexia.
The current research had some shortcomings. First, we could not confirm the efficacy of anamorelin by functional measures of HGS and 6MWT. Second, its efficacy for fatigue was not confirmed. The lack of a treatment effect observed for CFS may have arisen because fatigue associated with cancer is a problematic manifestation to ameliorate in patients with advanced cancer and particularly in patients with more symptomatic disease.31, 32 This is partly due to the multiple and complex causes of fatigue, including chemotherapy, anemia, nutritional issues, and pain.33
The efficacy of anamorelin in the current study for increasing LBM and body weight and improving anorexia symptoms and the nutritional state with no improvement in motor function is consistent with the recent results of 2 multinational phase 3 studies with anamorelin.19 Although anamorelin cannot improve motor function or survival, it may be of great importance for alleviating anorexia, a highly unmet medical need, to help patients with advanced cancer to enjoy their meals and thereby achieve better QOL.
On the basis of our findings, once daily administration of 100 mg of anamorelin showed favorable results for LBM gains in Japanese patients with NSCLC and cachexia; hence 100 mg could be the desired dose for such patients. Anamorelin therapy was associated with augmentation of IGF‐1 and IGFBP‐3 levels, and this suggests an increase in the synthesis of proteins that can have direct growth effects on skeletal muscle. Moreover, anamorelin was associated with favorable improvements in appetite and increases in prealbumin, and this indicates an improved nutritional status. Even though the incidences of ADRs and treatment discontinuations due to AEs were higher in the anamorelin group, most of the treatment discontinuations were associated with the progression of disease and not with anamorelin. The efficacy of anamorelin to improve LBM and anorexia was thus confirmed in Japanese patients with NSCLC and cachexia. Because no effective treatment for cancer cachexia is currently available, anamorelin can be one of the beneficial treatment options.
FUNDING SUPPORT
The study sponsor, Ono Pharmaceutical Co, Ltd (Osaka, Japan), was involved in the study design, provision of study materials, data collection and interpretation, and writing of the report.
CONFLICT OF INTEREST DISCLOSURES
Nobuyuki Katakami reports personal fees from AstraZeneca, Eli Lilly, Pfizer, Boehringer Ingelheim, Ono, Taiho, and Novartis Pharma KK and grants from MSD, Astellas, AstraZeneca, Eisai, Amgen, Shionogi, Daiichi Sankyo, Chugai, Eli Lilly, Boehringer Ingelheim, Bristol‐Myers Squibb, Maruishi, and Merck Serono outside the submitted work. Tateaki Naito reports personal fees from Ono during the conduct of the study. Masashi Kondo reports personal fees from Ono, Chugai, Pfizer, Novartis, Eli Lilly, Taiho, and AstraZeneca and grants from Eli Lilly outside the submitted work. Hiroshi Saito reports personal fees from Ono during the conduct of the study; grants from Taiho and Merck Serono outside the submitted work; and personal fees from Pfizer, AstraZeneca, and Kyowa Hakko Kirin outside the submitted work. Yuichi Takiguchi reports grants and personal fees from Ono, AstraZeneca, Taiho, Nippon Boehringer Ingelheim, and Chugai outside the submitted work. Koichi Takayama reports personal fees from Ono during the conduct of the study and personal fees from AstraZeneca, Eli Lilly, Chugai, and Ono outside the submitted work. Naoyuki Komura and Toru Takiguchi are employees of Ono. Kenji Eguchi reports grants from Ono outside the submitted work.
AUTHOR CONTRIBUTIONS
Nobuyuki Katakami: Conception and design, acquisition of data, data analysis and interpretation, manuscript writing, and final approval of manuscript. Junji Uchino: Acquisition of data, manuscript writing, and final approval of manuscript. Takuma Yokoyama: Acquisition of data, manuscript writing, and final approval of manuscript. Tateaki Naito: Acquisition of data, manuscript writing, and final approval of manuscript. Masashi Kondo: Acquisition of data, manuscript writing, and final approval of manuscript. Kouzo Yamada: Acquisition of data, manuscript writing, and final approval of manuscript. Hiromoto Kitajima: Acquisition of data, manuscript writing, and final approval of manuscript. Kozo Yoshimori: Acquisition of data, manuscript writing, and final approval of manuscript. Kazuhiro Sato: Acquisition of data, manuscript writing, and final approval of manuscript. Hiroshi Saito: Conception and design, acquisition of data, data analysis and interpretation, manuscript writing, and final approval of manuscript. Keisuke Aoe: Conception and design, acquisition of data, data analysis and interpretation, manuscript writing, and final approval of manuscript. Tetsuya Tsuji: Conception and design, data analysis and interpretation, manuscript writing, and final approval of manuscript. Yuichi Takiguchi: Conception and design, acquisition of data, data analysis and interpretation, manuscript writing, and final approval of manuscript. Koichi Takayama: Conception and design, data analysis and interpretation, manuscript writing, and final approval of manuscript. Naoyuki Komura: Conception and design, data analysis and interpretation, manuscript writing, and final approval of manuscript. Toru Takiguchi: Conception and design, data analysis and interpretation, manuscript writing, and final approval of manuscript. Kenji Eguchi: Conception and design, data analysis and interpretation, manuscript writing, and final approval of manuscript.
Supporting information
Additional supporting information may be found in the online version of this article.
Supporting Information 1
Supporting Information Table 1
Supporting Information Table 2
See editorial 456‐8, this issue.
The clinical trial registration was JapicCTI‐142451 (http://www.clinicaltrials.jp/user/search/directCteDetail.jsp?clinicalTrialId=14228).
We thank all the patients as well as the caregivers, investigators, and onsite staff who contributed to this clinical trial.
REFERENCES
- 1. Bruera E. ABC of palliative care. Anorexia, cachexia, and nutrition. BMJ. 1997;315:1219‐1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980;69:491‐497. [DOI] [PubMed] [Google Scholar]
- 3. Reuben DB, Mor V, Hiris J. Clinical symptoms and length of survival in patients with terminal cancer. Arch Intern Med. 1988;148:1586‐1591. [PubMed] [Google Scholar]
- 4. von Haehling S, Anker SD. Treatment of cachexia: an overview of recent developments. J Am Med Dir Assoc. 2014;15:866‐872. [DOI] [PubMed] [Google Scholar]
- 5. von Haehling S, Anker SD. Prevalence, incidence and clinical impact of cachexia: facts and numbers—update 2014. J Cachexia Sarcopenia Muscle. 2014;5:261‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10:90‐99. [DOI] [PubMed] [Google Scholar]
- 7. von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle. 2010;1:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489‐495. [DOI] [PubMed] [Google Scholar]
- 9. Neary NM, Small CJ, Wren AM, et al. Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo‐controlled trial. J Clin Endocrinol Metab. 2004;89:2832‐2836. [DOI] [PubMed] [Google Scholar]
- 10. Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. [DOI] [PubMed] [Google Scholar]
- 11. Akamizu T, Takaya K, Irako T, et al. Pharmacokinetics, safety, and endocrine and appetite effects of ghrelin administration in young healthy subjects. Eur J Endocrinol. 2004;150:447‐455. [DOI] [PubMed] [Google Scholar]
- 12. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth‐hormone–releasing acylated peptide from stomach. Nature. 1999;402:656‐660. [DOI] [PubMed] [Google Scholar]
- 13. Smith RG, Cheng K, Schoen WR, et al. A nonpeptidyl growth hormone secretagogue. Science. 1993;260:1640‐1643. [DOI] [PubMed] [Google Scholar]
- 14. Currow DC, Abernethy AP. Anamorelin hydrochloride in the treatment of cancer anorexia‐cachexia syndrome. Future Oncol. 2014;10:789‐802. [DOI] [PubMed] [Google Scholar]
- 15. Zhang H, Garcia JM. Anamorelin hydrochloride for the treatment of cancer‐anorexia‐cachexia in NSCLC. Expert Opin Pharmacother. 2015;16:1245‐1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia JM, Friend J, Allen S. Therapeutic potential of anamorelin, a novel, oral ghrelin mimetic, in patients with cancer‐related cachexia: a multicenter, randomized, double‐blind, crossover, pilot study. Support Care Cancer. 2013;21:129‐137. [DOI] [PubMed] [Google Scholar]
- 17. Garcia JM, Polvino WJ. Effect on body weight and safety of RC‐1291, a novel, orally available ghrelin mimetic and growth hormone secretagogue: results of a phase I, randomized, placebo‐controlled, multiple‐dose study in healthy volunteers. Oncologist. 2007;12:594‐600. [DOI] [PubMed] [Google Scholar]
- 18. Garcia JM, Polvino WJ. Pharmacodynamic hormonal effects of anamorelin, a novel oral ghrelin mimetic and growth hormone secretagogue in healthy volunteers. Growth Horm IGF Res. 2009;19:267‐273. [DOI] [PubMed] [Google Scholar]
- 19. Temel JS, Abernethy AP, Currow DC, et al. Anamorelin in patients with non–small‐cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double‐blind, phase 3 trials. Lancet Oncol. 2016;17:519‐531. [DOI] [PubMed] [Google Scholar]
- 20. Takayama K, Katakami N, Yokoyama T, et al. Anamorelin (ONO‐7643) in Japanese patients with non–small cell lung cancer and cachexia: results of a randomized phase 2 trial. Support Care Cancer. 2016;24:3495‐3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsumoto T, Ohashi Y, Morita S, et al. The Quality of Life Questionnaire for Cancer Patients Treated With Anticancer Drugs (QOL‐ACD): validity and reliability in Japanese patients with advanced non–small‐cell lung cancer. Qual Life Res. 2002;11:483‐493. [DOI] [PubMed] [Google Scholar]
- 22. National Comprehensive Cancer Network . NCCN clinical practice guidelines in oncology: palliative care. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed December 28, 2016. [DOI] [PubMed]
- 23. Radbruch L, Elsner F, Trottenberg P, Strasser F, Fearon K. Clinical Practice Guidelines on Cancer Cachexia in Advanced Cancer Patients With a Focus on Refractory Cachexia. Aachen, Germany: European Palliative Care Research Collaborative; 2010. [Google Scholar]
- 24. Piil K, Juhler M, Jakobsen J, Jarden M. Controlled rehabilitative and supportive care intervention trials in patients with high‐grade gliomas and their caregivers: a systematic review. BMJ Support Palliat Care. 2016;6:27‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takayama K, Atagi S, Imamura F, et al. Quality of life and survival survey of cancer cachexia in advanced non–small cell lung cancer patients—Japan nutrition and QOL survey in patients with advanced non–small cell lung cancer study. Support Care Cancer. 2016;24:3473‐3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quinten C, Coens C, Mauer M, et al. Baseline quality of life as a prognostic indicator of survival: a meta‐analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009;10:865‐871. [DOI] [PubMed] [Google Scholar]
- 27. Aapro M, Arends J, Bozzetti F, et al. Early recognition of malnutrition and cachexia in the cancer patient: a position paper of a European School of Oncology task force. Ann Oncol. 2014;25:1492‐1499. [DOI] [PubMed] [Google Scholar]
- 28. Chen L, Nelson DR, Zhao Y, Cui Z, Johnston JA. Relationship between muscle mass and muscle strength, and the impact of comorbidities: a population‐based, cross‐sectional study of older adults in the United States. BMC Geriatr. 2013;13:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dobs AS, Boccia RV, Croot CC, et al. Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double‐blind, randomised controlled phase 2 trial. Lancet Oncol. 2013;14:335‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the Health, Aging, and Body Composition Study. Diabetes. 2006;55:1813‐1818. [DOI] [PubMed] [Google Scholar]
- 31. Jean‐Pierre P, Morrow GR, Roscoe JA, et al. A phase 3 randomized, placebo‐controlled, double‐blind, clinical trial of the effect of modafinil on cancer‐related fatigue among 631 patients receiving chemotherapy: a University of Rochester Cancer Center Community Clinical Oncology Program Research Base study. Cancer. 2010;116:3513‐3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Minton O, Berger A, Barsevick A, et al. Cancer‐related fatigue and its impact on functioning. Cancer. 2013;119(suppl 11):2124‐2130. [DOI] [PubMed] [Google Scholar]
- 33. Neefjes EC, van der Vorst MJ, Blauwhoff‐Buskermolen S, Verheul HM. Aiming for a better understanding and management of cancer‐related fatigue. Oncologist. 2013;18:1135‐1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article.
Supporting Information 1
Supporting Information Table 1
Supporting Information Table 2


