Abstract
Schizotypal personality traits may increase proneness to psychosis and likely index familial vulnerability to schizophrenia (SZ), implying shared genetic determinants with SZ. Here, we sought to investigate the contribution of common genetic risk variation for SZ on self-reported schizotypy in 2 ethnically homogeneous cohorts of healthy young males during compulsory military service, enrolled in the Athens Study of Proneness and Incidence of Schizophrenia (ASPIS, N = 875) and the Learning on Genetics of Schizophrenia Spectrum study (LOGOS, N = 690). A follow-up psychometric assessment was performed in a sub-sample of the ASPIS (N = 121), 18 months later at military service completion. Polygenic risk scores (PRS) for SZ were derived based on genome-wide association meta-analysis results from the Psychiatric Genomics Consortium. In the ASPIS, higher PRSSZ significantly associated with lower levels of positive (ie, perceptual distortions), disorganization and paranoid facets of schizotypy, whereas no association with negative (ie, interpersonal) facets was noted. Importantly, longitudinal data analysis in the ASPIS subsample revealed that PRSSZ was inversely associated with positive schizotypy at military induction (stressed condition) but not at follow-up (nonstressed condition), providing evidence for environmental rather than SZ-implicated genetic influences. Moreover, consistent with prior reports, PRSSZ was positively correlated with trait anxiety in the LOGOS and additionally the recruits with higher PRSSZ and trait anxiety exhibited attenuated paranoid ideation. Together, these findings do not support an etiological link between increased polygenic liability for SZ and schizotypy, suggesting that psychosocial stress or trait anxiety may impact schizotypal phenotypic expressions among healthy young adults not genetically predisposed to SZ.
Keywords: anxiety, genetic risk, personality, psychosis, psychosocial stress, schizotypy
Introduction
An etiological continuum between schizotypal personality and the development of psychosis, particularly schizophrenia (SZ)-spectrum disorders, has emerged over the past decades.1 Epidemiological and genetic evidence supports a dimensional relationship between schizotypy and clinical symptomatology reminiscent of SZ,2 demonstrating that schizotypal features cluster in individuals with elevated risk for SZ and are prodromal to the full-blown clinical manifestation of SZ.3–5 Furthermore, the manifestation of schizotypal personality traits has been reported to represent a significant predictor of transition to psychosis later in life.5 Increased levels of schizotypy are often observed among the biological relatives of patients with SZ,6,7 suggesting a connection between schizotypal personality and genetic predisposition to SZ, which is likely attributed to overlapping genetic determinants. Prior candidate gene studies have provided some evidence that common genetic polymorphisms associated with SZ may be linked to schizotypy phenotypic variability among healthy individuals,8–11 further suggesting common genetic underpinnings with SZ. It is also of interest that family-based linkage findings have highlighted a genetic correlation between the diagnosis of SZ and schizotypy in nonpsychotic relatives.12 In addition, substantial heritability estimates have been reported for psychometrically identified schizotypy in twin-based population studies.13–15
Besides a genetic component, several studies have also implicated an environmental impact on schizotypy, which seem to vary depending on the specific schizotypy dimension examined.16–18 Adverse environmental and psychosocial influences, such as stressful life events, childhood trauma and abuse, social maltreatment from others and peer victimization have been considered important risk factors for the exacerbation of schizotypal features and/or subthreshold psychotic experiences.19–25 Of note, a nonpathological role for schizotypal personality has been proposed, which does not reflect a genetic vulnerability with SZ and it is related to psychosocial aspects of everyday life or represents a compensatory mechanism of psychosis risk.26–28 Similarly, positive schizotypy (ie, perceptual distortions) has been associated with a more creative style of living in nonclinical populations,29–31 supporting the notion that certain schizotypal features do not always denote an alarming or imminent sign of psychopathology, instead they might reveal healthy functioning.27 It is well documented that schizotypal traits occur among healthy individuals, outlining a continuity of the schizotypy phenotype within the general population.32–36
In the current study, we aimed to explore the relationship between common genetic risk variation for SZ, defined as increased loading of genetic risk loci associated with SZ (ie, polygenic risk) through large-scale genome-wide association studies (GWAS) and schizotypal traits in 2 independent cohorts of Greek healthy young males undergoing compulsory military training. Military induction is considered to be associated with increases in subjective stress caused by being away from home, exposure to combat scenarios, sleep deprivation and the rules and regime of initiation into the army corps. A number of studies have shown increased stress levels during the first weeks of military service,37–40 followed by a subsequent reduction of stress upon leaving the army.41,42 Therefore, secondary analyses were performed to evaluate the moderating role of environmental stress using a semi-experimental stress exposure paradigm.
Methods
Participants
Athens Study of Psychosis Proneness and Incidence of Schizophrenia.
A detailed description of the Athens Study of Psychosis Proneness and Incidence of Schizophrenia (ASPIS) has been previously reported.19,33,34,43 Briefly, the ASPIS examined randomly selected Caucasian young male conscripts (mean age: 20.8 ± 1.9), during the first 2 weeks of admission to the National Air Force Training Center (Tripolis, Greece). Military service is compulsory in Greece, and all healthy men are recruited and randomly assigned to the different army corps. Within the ASPIS, 8 consecutive separate waves of conscripts underwent psychometric, cognitive, and neurophysiological assessments. A total of N = 1355 conscripts successfully completed self-administered questionnaires measuring schizotypal traits at military induction.34 A follow-up psychometric evaluation was conducted in an ASPIS subsample (N = 145) 18 months later, at the completion of military service.19 We hypothesized that the first 2 weeks of military training represent a period of elevated psychosocial stress (stressed condition), supported by findings of a previous study demonstrating that military service induction resulted in an excess of stress-induced subclinical psychotic experiences in the ASPIS, which were significantly attenuated at military service completion (nonstressed condition).19 In the current study, we included a total of N = 875 eligible individuals who had been genotyped for the purposes of a prior GWAS of neurocognitive functions,44 and had complete psychometric data. Of those, a subsample of N = 121 individuals underwent a follow-up psychometric evaluation. Before participation, all conscripts received a standardized screening interview by a team of military doctors to exclude serious medical conditions, including documented diagnosis of psychotic disorders and substance dependence, and individuals with such conditions were not admitted for military training. Written informed consent was obtained from every individual before enrollment to the study. The research protocol was approved by the Ethics Committee of the University Mental Health Research Institute (Athens, Greece) and the Johns Hopkins University Institutional Review Boards.
Learning on Genetics of Schizophrenia Spectrum.
The Learning on Genetics of Schizophrenia Spectrum (LOGOS) represents an independent cohort of healthy young male army conscripts which has been described in detail previously.45,46 The conscripts participating in the LOGOS did not differ from those of the ASPIS in terms of demographic characteristics (gender, age, years of education, ethnicity), ensuring that the 2 cohorts are highly comparable to each other and suitable for genetic research and behavioral assessment. The LOGOS acquired the same recruitment procedures as the ASPIS, assessing Caucasian healthy male conscripts (N = 690; mean age: 22.3 ± 3.7) on multiple cognitive and psychometric phenotypes, at the Greek Army Training Camp in Heraklion, Crete, Greece. Following presentation of the study’s methods and goals in each consecutive series of new conscripts, every participant willing to volunteer had a detailed information sheet and gave written informed consent. The LOGOS research protocol was approved by the Ethics Committee of the University of Crete, the Executive Army Bureau, and the Bureau for the Protection of Personal Data of the Greek State.
Psychometric Assessment.
In the ASPIS, lifetime schizotypal traits were assessed with the Schizotypal Personality Questionnaire (SPQ),47 and unusual body perceptual experiences with the Perceptual Aberration Scale (PAS),48 both at the time of military induction and at follow-up. The SPQ is a 74-item questionnaire that includes 9 subscales (Ideas of Reference, Social Anxiety, Odd Beliefs/Magical Thinking, Unusual Perceptual Experiences, Eccentric/Odd Behavior and Appearance, No Close Friends, Odd Speech, Constricted Affect, Suspiciousness/Paranoid Ideation) relevant to schizotypal personality disorder as defined in the Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R). The factorial structure of the SPQ as defined by the responses of this sample was assessed through confirmatory factor analysis, which indicated that the best fit to the data was provided by a 4-factor model, namely positive, negative, disorganization, and paranoid factors.34 This 4-factor model of schizotypy has been replicated by other research groups,49 and importantly high SPQ scores in this sample were predictive of an independent diagnosis of Schizotypal Personality Disorder upon SCID-II clinical interview.50 The PAS is a 35-item (yes/no) self-rated scale comprised of items tapping unusual perceptual distortions, mainly related to one’s own body. PAS total scores showed significant skewness in our population, hence a log-transformation was applied to reach a normal distribution before statistical analysis. Schizotypal traits were assessed with the Schizotypal Traits Questionnaire (STQ) in the LOGOS cohort.51 The STQ scale is a 37-item self-report questionnaire derived from the criteria for Schizotypal Personality Disorder in the DSM-III and measures 3 dimensions of positive schizotypy, namely unusual experiences, magical thinking, and paranoid ideation. Each item is scored by using a dichotomous (yes/no) response format. Trait anxiety refers to relatively stable individual differences in proneness to anxiety and was assessed with the Spielberger’s State-Trait Anxiety Inventory-Trait Scale (STAI-T),52 which is a 20-item scale and each item is scored on a 4-point Likert scale, ranging from 1 (very false for me) to 4 (very true for me).
Genome-Wide Genotyping.
Details on the genotyping procedures, single nucleotide polymorphism (SNP) calling and subsequent quality control (QC) filtering steps acquired by the ASPIS and LOGOS have been previously reported.44–53 Appropriate postgenotyping QC data cleaning, as well as multidimensional scaling (MDS) or principal components analysis (PCA) to identify genetic outliers and correct for any residual population substructure were performed using PLINK v1.07 and EIGENSTRAT softwares.54,55 Further information is provided in the supplementary material.
Polygenic Risk Scoring.
Polygenic risk scores (PRS) which encompass the additive effect of multiple common SNPs across the genome, were computed based on the GWAS meta-analysis summary results reported by the Schizophrenia Working Group of the Psychiatric Genomics Consortium (PGC),56 following the procedure originally described by the International Schizophrenia Consortium.57 Different sets of SNPs were filtered by applying increasing P-value thresholds (PT < .001, PT < .01, PT < .05, PT < .1, PT < .3, PT < .5) to PGC GWAS summary statistics (discovery sample). The same sets of SNPs were extracted in the ASPIS and LOGOS (target samples) (supplementary table S2) and PRS was computed using the “--score” command in PLINK, after appropriate linkage disequilibrium-based SNP pruning (r2 < .2 within a 200 kb window), ensuring that only independent association signals are included in PRS. For each individual, a weighted score is derived based on the number of risk alleles that the individual carries at each SNP locus, weighted by the natural logarithm of the reported odds ratio for that particular SNP in the reference PGC GWAS. The sum of single scores across all SNPs denotes the total PRSSZ for each individual.
Statistical Analyses
All statistical analyses were performed using R 3.3.2 (https://www.r-project.org/) and SPSS Statistics 23 (IBM). Linear regression analysis was performed to test for associations between quantitative traits scores and PRSSZ at different PGC GWAS P-value thresholds (PT), including age, years of education and the first two ancestry-based principal components to control for population stratification, as potential confounders. Given the substantial phenotypic correlations between SPQ and PAS scores (Pearson’s r > 50) and the lack of independence between PRSSZ computed at different PT, a conservative Bonferroni correction for multiple comparisons is not preferable, thus statistical significance was determined after a permutation-based resampling procedure (10000 phenotype permutations were tested) to control for possible spurious associations. Following permutation testing, empirical P-values are reported setting the level of statistical significance at P < .05. Separate linear regressions were conducted at baseline (stressed condition) and at follow-up (nonstressed condition), to examine the moderating effect of environmental stress on the association between PRSSZ and schizotypal traits in the ASPIS subsample (N = 121). Linear mixed-effects models with repeated measures were fitted to compare the within-subjects phenotypic differences between the 2 conditions, depending on the computed PRSSZ. Each trait was tested with a different model, entering PRSSZ as a fixed effect term, while including each individual as a random effect term (random intercept). Age, years of education, and ancestry-based principal components were included in the model as both fixed and random effects (random slopes). In this longitudinal analysis, the phenotypic differences observed over time between individuals are compared given a specified reference group. Therefore, a dummy variable coding procedure was applied by stratifying the sample based on the median value of the computed PRSSZ at the PT with the strongest evidence of association. We thus created 2 equally sized groups of individuals (low and high PRSSZ carriers) for further testing, using high PRSSZ carriers as the reference group. For this secondary analysis, uncorrected (nominal) P-values are reported. To examine the moderating effect of trait anxiety (STAI-T) on the relationship between PRSSZ and schizotypal dimensions in the LOGOS, multiple linear regression models were carried out including both main effects and 2-way interaction effect terms (PRSSZ × STAI-T). The Wald χ2-test was used to determine the statistical significance of the examined interactions, reporting 1-sided tests on the basis of prior evidence from the ASPIS that an inverse relationship between PRSSZ and schizotypy emerged upon stress exposure.
Results
PRSSZ Association With Schizotypal Traits in the ASPIS
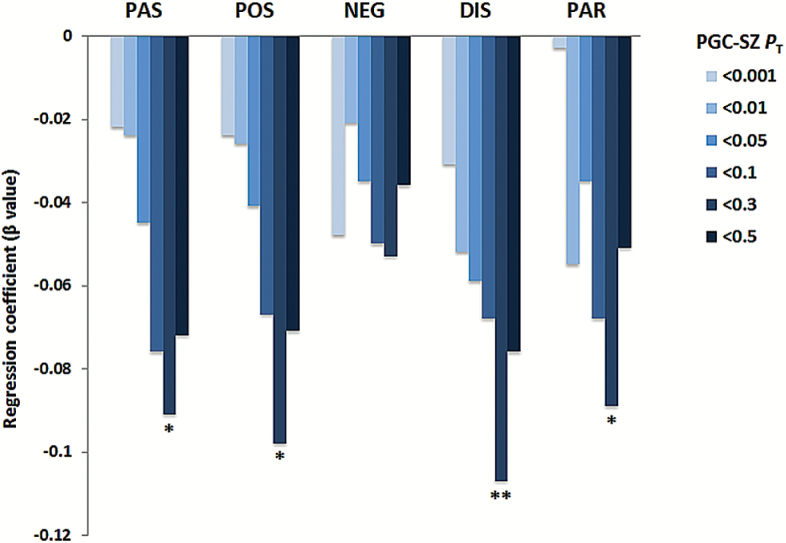
In primary analyses, we examined the relationship between PRSSZ derived from 6 different PT to the PGC GWAS results, the 4 SPQ factor scores (POS: positive, NEG: negative, DIS: disorganization, and PAR: paranoid) and the total PAS score in the ASPIS full sample (N = 875) at military induction. All pairwise phenotypic correlations are reported in supplementary table S2. As shown in figure 1, the computed PRSSZ at PT < .3 showed the strongest inverse correlation with SPQ POS (β = −.10; R2(%) = .96; nominal P = .002; empirical P = .005), DIS (β = −.11; R2(%) = 1.14; nominal P = .0008, empirical P = .003), PAR (β = −.09; R2(%) = .79; nominal P = .009; empirical P = .01) and PAS (β = −.10; R2(%) = .80; nominal P = .004; empirical P = .017). In contrast, no statistically significant correlation was observed for SPQ NEG (β = −.05; R2(%) = .28; nominal P = .12; empirical P = .11).
Fig. 1.
Association between SPQ factor scores and PRSSZ in the ASPIS full sample (N = 875) at military induction (SPQ, Schizotypal Personality Questionnaire; PAS, Perceptual Aberration Scale; POS, SPQ Positive factor; NEG, SPQ Negative factor; DIS, SPQ Disorganization factor; PAR, SPQ Paranoid factor). PT denotes PGC GWAS P-value threshold. *P < .01, **P < .001.
Stress-Related Association Between PRSSZ and Positive Schizotypy.
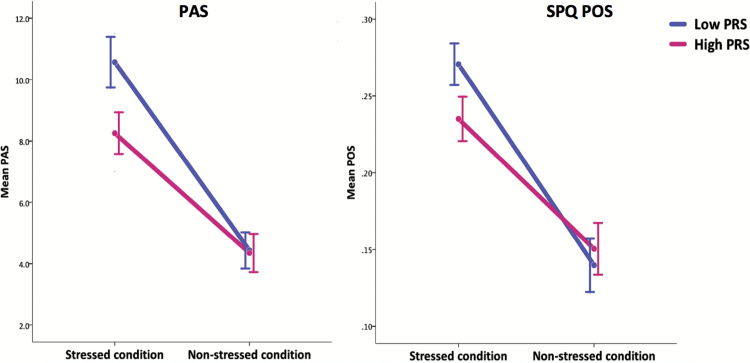
We have previously shown that stress exposure during army induction (stressed condition), evoked increases in all SPQ factor scores in the ASPIS, which were alleviated at the end of military service (nonstressed condition).30 SPQ and PAS mean score reduction in the ASPIS subsample (N = 121) at follow-up is shown in supplementary table S3. We intended to test whether environmental stress represents a significant moderator of the observed relationship between schizotypy and PRSSZ. To test the above hypothesis, we first applied separate regression models in the ASPIS subsample, examining the association of PRSSZ (PGC GWAS PT < .3 was utilized which showed the strongest correlation in primary analysis; figure 1) with PAS and SPQ scores in the 2 time points (stressed vs nonstressed conditions). As shown in table 1, a nominally significant association between PRSSZ and PAS was found at stressed condition (β = −.217; P = .016), while a trend association with SPQ POS was also observed (β = −.164; P = .065). However, not such evidence for association was detected at follow-up and the computed effect sizes (regression coefficients) were markedly decreased (PAS β = −.005; P = .96, SPQ POS β = −.004; P = .97). To further verify the above observation and control for potential random effects and phenotypic correlations over time, linear mixed-effects models with repeated measures were performed to determine whether significant differences in PAS/POS scores occurred between stressed and non-stressed conditions among low vs high PRSSZ carriers. We fitted mixed-effects models including a cross-level interaction between the 2 time point and PRSSZ, allowing for the effect of stress (nonstressed condition is the reference) on schizotypy to vary depending on the computed PRSSZ for each individual. Figure 2 depicts the results of the interaction effects (PRSSZ × condition), illustrating higher PAS scores at stressed condition among low PRSSZ carriers compared to high PRSSZ carriers (low PRSSZ mean difference 58.4% vs high PRSSZ mean difference 46.8%, nominal P = .03). Similarly, we noted a near significant difference for POS scores (low PRSSZ mean difference 48.7% vs high PRSSZ mean difference 38.4%, nominal P = .052). None of the remaining SPQ trait scores showed substantial differences over time (all P > .2, supplementary figure S1). Mean phenotypic values for all traits at both conditions, stratified by PRSSZ status, are shown in supplementary table S4.
Table 1.
Results From Linear Regression Analysis Depicting the Association Between PRSSZ (PGC PT < .3) and Schizotypal Traits in the ASPIS Subsample (N = 121) at Military Induction (Stressed Condition) and at Follow-Up (Nonstressed Condition)
| Schizotypal Trait | Stressed Condition | Nonstressed Condition | ||
|---|---|---|---|---|
| Beta | P value | Beta | P value | |
| PAS | −.217 | .016 | −.005 | .986 |
| POS | −.160 | .065 | −.004 | .998 |
| NEG | .073 | .419 | −.024 | .789 |
| DIS | −.097 | .274 | −.046 | .630 |
| PAR | −.072 | .433 | −.120 | .195 |
Note: Standardized regression coefficients are reported and nominally significant differences at P < .05 (2-sided) are shown in bold. Analyses are controlled for age, years of education, and population stratification principal components. PAS, Perceptual Aberration Scale; POS, SPQ positive factor; NEG, SPQ negative factor; DIS, SPQ disorganization factor; PAR, SPQ paranoid factor.
Fig. 2.
Mean phenotypic differences for positive schizotypy traits (PAS, SPQ POS) at military induction (stressed condition) and at follow-up (nonstressed condition) in the ASPIS subsample (N = 121) stratified by PRSSZ status. Error bars represent the SE of the mean trait scores. PAS Pnominal = .03, SPQ POS Pnominal = .052.
PRSSZ Predicts Trait Anxiety in the LOGOS.
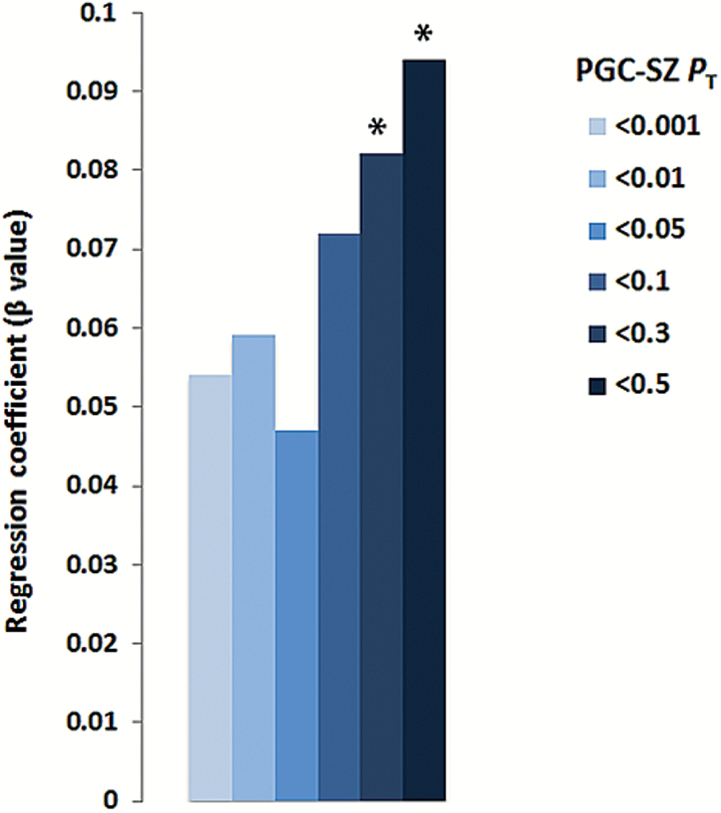
Independent confirmation of the association between PRSSZ and STQ schizotypal traits was attempted in the LOGOS. Additionally, given previous evidence supporting an association between PRSSZ and anxiety symptoms during adolescence,58 we inquired whether trait anxiety (STAI-T) is also associated with PRSSZ in young conscripts. Moderate pairwise phenotypic correlations were observed between the 3 STQ dimensions and STAI-T (supplementary table S5). Linear regressions were carried out to assess the relationship between PRSSZ and the three STQ dimensions (unusual experiences, magical thinking, paranoid ideation), which revealed that none of the STQ dimensions was significantly associated with PRSSZ (P > .25), yet the direction of the correlations was negative in all cases consistent with the ASPIS results. Furthermore, a nominal positive association between PRSSZ and STAI-T was detected at PT < .3 (β = .082; P = .05) and PT < .5 (β = .094; P = .03) (figure 3).
Fig. 3.
Association between trait anxiety (STAI-T) and PRSSZ in the LOGOS (N = 690) (STAI-T; Trait Scale of the State-Trait Anxiety Inventory). PT denotes PGC GWAS P-value threshold. *P < .05.
Joint Effect of PRSSZ and Trait Anxiety on Schizotypy.
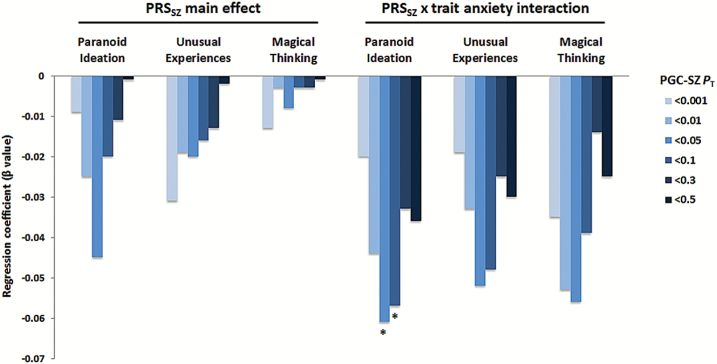
Prompted by the PRSSZ × stress interaction effect observed in the ASPIS, we further explored whether PRSSZ association with STQ dimensions is moderated by trait anxiety in LOGOS. To test the above hypothesis, multiple regression models were fitted for each STQ dimension including appropriate 2-way interactions (PRSSZ × STAI-T) as predictors. We noted that the interaction term predicted lower scores for paranoid ideation compared to PRSSZ alone, with substantially increased effect estimates at PRSSZPT < .05 (β = −.061; 1-sided P = .026) and PT < .1 (β = −.057; 1-sided P = .04). No statistically significant results were obtained for the unusual experiences and magical thinking STQ dimensions, even though increased regression coefficients were also observed for both traits (PRSSZPT < .05 β = −.051; 1-sided P = .064 for unusual experiences, PRSSZPT < .05 β = −.056; 1-sided P = .058 for magical thinking). Figure 4 illustrates the effect estimates for the association between PRSSZ and STQ dimensions before and after the inclusion of interaction effects with STAI-T scores.
Fig. 4.
Associations between STQ dimensions and PRSSZ in the LOGOS before and after accounting for trait anxiety (PRSSZ × STAI-T interaction). STQ; Schizotypy Traits Questionnaire. PT denotes PGC GWAS P-value threshold. *P < .05.
Discussion
We report findings from 2 population-based cohorts, showing that healthy young males carrying a reduced genetic load of risk alleles for SZ exhibit significantly higher schizotypal traits during compulsory military service. Given that individuals characterized by increased schizotypy or subthreshold psychotic experiences (PEs) may be at a higher genetic risk to develop a psychotic disorder, the inverse association between PRSSZ and schizotypy is somewhat counterintuitive and opposes what would be expected.4–7 We found that PRSSZ was negatively associated with unusual perceptual experiences, disorganization, and paranoid behavior in the ASPIS during the first 2 weeks of military admission (stressed condition). Follow-up analysis indicated that the observed relationship between PRSSZ and schizotypy was retained at the beginning but not the end of military service (nonstressed condition), likely revealing an environmental impact and not a SZ-related genetic contribution. This finding also suggests pathways of competing genetic and environmental causes rather than genetic and environmental synergism, consistent with a multifactorial threshold model in which genetic predisposition and environmental risk factors influence schizotypy/PEs independently.59 In addition, a negative correlation between PRSSZ and paranoid behavior was independently observed in the LOGOS and was markedly enhanced when accounting for trait anxiety, further supporting a moderating role for stress/anxiety on the association between PRSSZ and schizotypal personality, at least among young adults.
These findings may offer a tentative explanation for previously reported nonsignificant associations between PRSSZ and PEs in general population samples,58,60 apparently not exposed to the same levels of environmental stress. Seen from an alternative perspective, these earlier studies have observed negative, albeit nonsignificant, correlations between PRSSZ and PEs in children and adolescents, which partially support our findings and imply that the observed associations could not be attributed to trivial phenotype assessment bias (ie, self-ratings of schizotypy) that could have led to spurious results. Indeed, negative associations at trend level between PRSSZ and paranoid behavior as well as cognitive disorganization were previously found among children.60 In addition, the Avon Longitudinal Study of Parents and Children (ALSPAC) found positive associations between PRSSZ and anxiety disorder incidence in a large sample of adolescents,58 which is in agreement with the positive correlation between PRSSZ and trait anxiety in the LOGOS.
We postulate that the phenomenological contradictory relationship between PRSSZ and schizotypy could possibly be interpreted in the light of an innocuous phenotypic expression within healthy individuals (ie, healthy schizotypy), which has been originally described by Jackson as “benign” schizotypy,28 and later by Raine as “pseudo” schizotypy.26 Both the above conceptualizations define a personality trait that mostly corresponds to positive schizotypal features and occasionally might prove beneficial, reflecting a relatively healthy expression or coping style which facilitates individuals’ adaptation to environmental changes and adversities.27 It has been reported that this expression of schizotypy has no common neurodevelopmental or genetic origins with SZ and might be triggered by psychosocial factors.26 The results of this study support the above view, as it may be hypothesized that the occurrence of high genetic risk for SZ among those individuals expressing high schizotypy upon stress exposure, would have possibly caused more detrimental outcomes; for instance the development of a SZ-spectrum psychotic disorder, which defined an exclusion criterion for the current study. Consequently, it is argued that in the nonclinical populations examined in our study, increased schizotypy among low PRSSZ carriers denotes the influence of psychosocial stress and/or anxiety rather than SZ-linked genetic susceptibility, thus supporting the “healthy” schizotypy theoretical framework.26,28 Likewise, it cannot be assumed that high schizotypy scores specify prodromal signs of psychosis, as higher risk for conversion to psychosis also requires treatment-seeking,61–63 and risk for SZ is increased primarily in individuals with very high scores who are probably rare in our cohorts.64,65 It may also be speculated that the lack of polygenic risk for SZ is protective for healthy schizotypes, increasing resilience to psychosis.1 Further, positive schizotypy measured in healthy individuals does not necessarily resemble a pathological condition; instead it may indicate a compensatory mechanism related to healthy functioning, subjective well-being and creative thinking.27,66
Psychosocial stress represents a critical environmental risk factor for psychosis,67,68 and our group has previously reported that elevated stress levels due to military induction predicted subthreshold PEs in the ASPIS.69 We would therefore expect that the military environment induces positive schizotypal expressions in the more genetically predisposed recruits carrying higher PRSSZ, however the opposite pattern was observed. An explanation could be derived from the assumption that positive schizotypy reflects an adaptive emotional response to psychosocial stress, in accordance with recent conceptualizations that disengage schizotypy from a linear and unidimensional link to SZ, emphasizing its relevance to affective and social functioning.70 For example, altered emotional reactivity to stress has been documented in psychotic patients and their first-degree relatives, implying a genetic component to the affective dysregulation observed in psychosis.71–73 Hence, it is plausible that healthy young adults with relatively increased genetic burden for SZ may also be characterized by a blunted affective response to psychosocial stress (a.k.a. stress reactivity), expressed as reduced positive schizotypy.
In summary, this study challenges the view that the emergence of schizotypal traits in healthy young adults reflects higher genetic liability for SZ and supports an important moderating role for psychosocial stress and trait anxiety. Nevertheless, our results should be interpreted with caution due to a number of limitations. Primarily, both ASPIS and LOGOS comprised limited sized cohorts of young conscripts, who define a distinct population subgroup. The noninclusion of community dwellers, female individuals and individuals of a wider age-range does not allow the generalizability of these findings in the general population. Moreover, the lack of a control (ie, unexposed) condition in our quasi-experimental ASPIS study, specifically a psychometric assessment before the initiation of military service, makes it hard to conclude that army-related psychosocial stress drives the reported associations. It is also acknowledged that the assessment of state anxiety in both cohorts, would ideally confirm the moderating role of environmental stress on schizotypy. Lastly, the use of self-administered instruments to assess personality dimensions may have introduced involuntary phenotypic measurement bias. In order to disentangle the above-mentioned concerns, future studies in larger population-based cohorts are required to establish any relationship between SZ genetic liability and schizotypal personality, as well as to clarify the modifying effects of psychosocial stress and trait anxiety.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
This work was supported by grants from the National Institute of Mental Health (RO1-MH-085018 and RO1-MH-092515), an award from the Neurosciences Education and Research Foundation to Dr Avramopoulos and research funding from the Theodor-Theohari Foundation (Athens, Greece) to Dr Stefanis.
Supplementary Material
Acknowledgments
The authors would like to thank Daniel R. Weinberger and Richard E. Straub for their valuable contribution on the conception of this work. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Barrantes-Vidal N, Kwapil TR. The role of schizotypy in the study of the etiology of schizophrenia spectrum disorders. Schizophr Bull. 2015; 41: S408—S416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nelson MT, Seal ML, Pantelis C, Phillips LJ. Evidence of a dimensional relationship between schizotypy and schizophrenia: a systematic review. Neurosci Biobehav Rev. 2013;37:317–327. [DOI] [PubMed] [Google Scholar]
- 3. Maier W, Falkai P, Wagner M. Schizophrenia spectrum disorders: a review. In: Maj M, Sartorius N, eds. WPA Series Evidence and Experience in Psychiatry, Vol. 2, Schizophrenia. Chichester, UK: Wiley and Sons; 1999:311–371. [Google Scholar]
- 4. Poulton R, Caspi A, Moffitt TE, Cannon M, Murray R, Harrington H. Children’s self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch Gen Psychiatry. 2000;57:1053–1058. [DOI] [PubMed] [Google Scholar]
- 5. Kwapil TR, Gross GM, Silvia PJ, Barrantes-Vidal N. Prediction of psychopathology and functional impairment by positive and negative schizotypy in the Chapmans’ ten-year longitudinal study. J Abnorm Psychol. 2013;122:807–815. [DOI] [PubMed] [Google Scholar]
- 6. Kendler KS, McGuire M, Gruenberg AM, Walsh D. Schizotypal symptoms and signs in the Roscommon Family Study. Their factor structure and familial relationship with psychotic and affective disorders. Arch Gen Psychiatry. 1995;52:296–303. [DOI] [PubMed] [Google Scholar]
- 7. Fanous A, Gardner C, Walsh D, Kendler KS. Relationship between positive and negative symptoms of schizophrenia and schizotypal symptoms in nonpsychotic relatives. Arch Gen Psychiatry. 2001;58:669–673. [DOI] [PubMed] [Google Scholar]
- 8. Stefanis NC, Trikalinos TA, Avramopoulos D et al. . Impact of schizophrenia candidate genes on schizotypy and cognitive endophenotypes at the population level. Biol Psychiatry. 2007;62:784–792. [DOI] [PubMed] [Google Scholar]
- 9. Kircher T, Markov V, Krug A et al. . Association of the DTNBP1 genotype with cognition and personality traits in healthy subjects. Psychol Med. 2009;39:1657–1665. [DOI] [PubMed] [Google Scholar]
- 10. Yasuda Y, Hashimoto R, Ohi K et al. . Impact on schizotypal personality trait of a genome-wide supported psychosis variant of the ZNF804A gene. Neurosci Lett. 2011;495:216–220. [DOI] [PubMed] [Google Scholar]
- 11. Stefanis NC, Hatzimanolis A, Avramopoulos D et al. . Variation in psychosis gene ZNF804A is associated with a refined schizotypy phenotype but not neurocognitive performance in a large young male population. Schizophr Bull. 2013;39:1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fanous AH, Neale MC, Gardner CO et al. . Significant correlation in linkage signals from genome-wide scans of schizophrenia and schizotypy. Mol Psychiatry. 2007;12:958–965. [DOI] [PubMed] [Google Scholar]
- 13. Linney YM, Murray RM, Peters ER, MacDonald AM, Rijsdijk F, Sham PC. A quantitative genetic analysis of schizotypal personality traits. Psychol Med. 2003;33:803–816. [DOI] [PubMed] [Google Scholar]
- 14. Lin CC, Su CH, Kuo PH, Hsiao CK, Soong WT, Chen WJ. Genetic and environmental influences on schizotypy among adolescents in Taiwan: a multivariate twin/sibling analysis. Behav Genet. 2007;37:334–344. [DOI] [PubMed] [Google Scholar]
- 15. Ericson M, Tuvblad C, Raine A, Young-Wolff K, Baker LA. Heritability and longitudinal stability of schizotypal traits during adolescence. Behav Genet. 2011;41:499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kendler KS, Hewitt J. The structure of self-report schizotypy in twins. J Person Disord. 1992;6:1–17. [Google Scholar]
- 17. MacDonald AW III, Pogue-Geile MF, Debski TT, Manuck S. Genetic and environmental influences on schizotypy: a community-based twin study. Schizophr Bull. 2001;27:47–58. [DOI] [PubMed] [Google Scholar]
- 18. Hay DA, Martin NG, Foley D, Treloar SA, Kirk KM, Heath AC. Phenotypic and genetic analyses of a short measure of psychosis-proneness in a large-scale Australian twin study. Twin Res. 2001;4:30–40. [DOI] [PubMed] [Google Scholar]
- 19. Stefanis NC, Vitoratou S, Ntzoufras I et al. . Psychometric properties of the Greek version of the Schizotypal Personality Questionnaire (SPQ) in young male obligatory conscripts: a two years test–retest study. Pers Individ Diff. 2006;41:1275–1286. [Google Scholar]
- 20. Steel C, Marzillier S, Fearon P, Ruddle A. Childhood abuse and schizotypal personality. Soc Psychiatry Psychiatr Epidemiol. 2009;44:917–923. [DOI] [PubMed] [Google Scholar]
- 21. Grattan RE, Morton SE, Warhurst ES et al. . Paternal and maternal ages have contrasting associations with self-reported schizophrenia liability. Schizophr Res. 2015;169:308–312. [DOI] [PubMed] [Google Scholar]
- 22. Fung AL, Raine A. Peer victimization as a risk factor for schizotypal personality in childhood and adolescence. J Pers Disord. 2012;26:428–434. [DOI] [PubMed] [Google Scholar]
- 23. Badcock JC, Panton K, Cohen A, Badcock DR. Both harmful and (some) helpful behaviours from others are associated with increased expression of schizotypal traits. Psychiatry Res. 2016;239:308–314. [DOI] [PubMed] [Google Scholar]
- 24. Shakoor S, Zavos HM, Haworth CM et al. . Association between stressful life events and psychotic experiences in adolescence: evidence for gene–environment correlations. Br J Psychiatry. 2016;208:532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rössler W, Ajdacic-Gross V, Rodgers S, Haker H, Müller M. Childhood trauma as a risk factor for the onset of subclinical psychotic experiences: exploring the mediating effect of stress sensitivity in a cross-sectional epidemiological community study. Schizophr Res. 2016;172:46–53. [DOI] [PubMed] [Google Scholar]
- 26. Raine A. Schizotypal personality: neurodevelopmental and psychosocial trajectories. Annu Rev Clin Psychol. 2006;2:291–326. [DOI] [PubMed] [Google Scholar]
- 27. Mohr C, Claridge G. Schizotypy–do not worry, it is not all worrisome. Schizophr Bull. 2015;41(suppl 2):S436–S443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jackson M. Benign schizotypy? The case of religious experience. In: Claridge G, ed. Schizotypy, Implications for Illness and Health. Oxford, UK: Oxford University Press; 1997. [Google Scholar]
- 29. Tsakanikos E, Claridge G. More words, less words: verbal fluency as a function of ‘positive’ and ‘negative’ schizotypy. Pers Indiv Dif. 2005;39:705–713. [Google Scholar]
- 30. Nettle D. Schizotypy and mental health amongst poets, visual artist, and mathematicians. J Res Pers. 2006;40:876–890. [Google Scholar]
- 31. Preti A, Vellante M. Creativity and psychopathology: higher rates of psychosis proneness and nonright-handedness among creative artists compared to same age and gender peers. J Nerv Ment Dis. 2007;195:837–845. [DOI] [PubMed] [Google Scholar]
- 32. Johns LC, van Os J. The continuity of psychotic experiences in the general population. Clin Psychol Rev. 2001;21:1125–1141. [DOI] [PubMed] [Google Scholar]
- 33. Stefanis NC, Hanssen M, Smirnis NK et al. . Evidence that three dimensions of psychosis have a distribution in the general population. Psychol Med. 2002;32:347–358. [DOI] [PubMed] [Google Scholar]
- 34. Stefanis NC, Smyrnis N, Avramopoulos D, Evdokimidis I, Ntzoufras I, Stefanis CN. Factorial composition of self-rated schizotypal traits among young males undergoing military training. Schizophr Bull. 2004;30:335–350. [DOI] [PubMed] [Google Scholar]
- 35. Hanssen M, Krabbendam L, Vollema M, Delespaul P, Van Os J. Evidence for instrument and family-specific variation of subclinical psychosis dimensions in the general population. J Abnorm Psychol. 2006;115:5–14. [DOI] [PubMed] [Google Scholar]
- 36. Rössler W, Ajdacic-Gross V, Müller M, Rodgers S, Haker H, Hengartner MP. Assessing sub-clinical psychosis phenotypes in the general population–a multidimensional approach. Schizophr Res. 2015;161:194–201. [DOI] [PubMed] [Google Scholar]
- 37. Steinberg HR, Durell J. A stressful social situation as a precipitant of schizophrenic symptoms: an epidemiological study. Br J Psychiatry. 1968;114:1097–1105. [DOI] [PubMed] [Google Scholar]
- 38. Hatzitaskos P, Soldatos C, Giouzelis G et al. . Psychotic symptomatology first appeared in the military environment. Psychiatriki. 1997;8:41–48. [Google Scholar]
- 39. Clemons EP. Monitoring anxiety levels and coping skills among military recruits. Mil Med. 1996;161:18–21. [PubMed] [Google Scholar]
- 40. Larson GE, Booth-Kewley S, Merrill LL, Stander VA. Physical symptoms as indicators of depression and anxiety. Mil Med. 2001;166:796–799. [PubMed] [Google Scholar]
- 41. Lerew DR, Schmidt NB, Jackson RJ. Evaluation of psychological risk factors: prospective prediction of psychopathology during basic training. Mil Med. 1999;164:509–513. [PubMed] [Google Scholar]
- 42. Martin PD, Williamson DA, Alfonso AJ, Ryan DH. Psychological adjustment during Army basic training. Mil Med. 2006;171:157–160. [DOI] [PubMed] [Google Scholar]
- 43. Smyrnis N, Avramopoulos D, Evdokimidis I, Stefanis CN, Tsekou H, Stefanis NC. Effect of schizotypy on cognitive performance and its tuning by COMT val158 met genotype variations in a large population of young men. Biol Psychiatry. 2007;61:845–853. [DOI] [PubMed] [Google Scholar]
- 44. Hatzimanolis A, Bhatnagar P, Moes A et al. . Common genetic variation and schizophrenia polygenic risk influence neurocognitive performance in young adulthood. Am J Med Genet B Neuropsychiatr Genet. 2015;168B:392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roussos P, Giakoumaki SG, Georgakopoulos A, Robakis NK, Bitsios P. The CACNA1C and ANK3 risk alleles impact on affective personality traits and startle reactivity but not on cognition or gating in healthy males. Bipolar Disord. 2011;13:250–259. [DOI] [PubMed] [Google Scholar]
- 46. Roussos P, Giakoumaki SG, Adamaki E et al. . The association of schizophrenia risk D-amino acid oxidase polymorphisms with sensorimotor gating, working memory and personality in healthy males. Neuropsychopharmacology. 2011;36:1677–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17:555–564. [DOI] [PubMed] [Google Scholar]
- 48. Chapman LJ, Chapman JP, Raulin ML. Body-image aberration in schizophrenia. J Abnorm Psychol. 1978;87:399–407. [DOI] [PubMed] [Google Scholar]
- 49. Compton MT, Goulding SM, Bakeman R, McClure-Tone EB. Confirmation of a four-factor structure of the Schizotypal Personality Questionnaire among undergraduate students. Schizophr Res. 2009;111:46–52. [DOI] [PubMed] [Google Scholar]
- 50. Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: history, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. [DOI] [PubMed] [Google Scholar]
- 51. Claridge G, Broks P. Schizotypy and hemisphere function–I: theoretical considerations and the measurement of schizotypy. Pers Individ Dif. 1984;5:633–648. [Google Scholar]
- 52. Spielberger C. Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- 53. Roussos P, Giakoumaki SG, Zouraraki C et al. . The relationship of common risk variants and polygenic risk for schizophrenia to sensorimotor gating. Biol Psychiatry. 2016;79:988–996. [DOI] [PubMed] [Google Scholar]
- 54. Purcell S, Neale B, Todd-Brown K et al. . PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. [DOI] [PubMed] [Google Scholar]
- 56. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. International Schizophrenia Consortium , Purcell SM, Wray NR et al. . Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jones HJ, Stergiakouli E, Tansey KE et al. . Phenotypic manifestation of genetic risk for schizophrenia during adolescence in the general population. JAMA Psychiatry. 2016;73:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wigman JT, van Winkel R, Ormel J, Verhulst FC, van Os J, Vollebergh WA. Early trauma and familial risk in the development of the extended psychosis phenotype in adolescence. Acta Psychiatr Scand. 2012;126:266–273. [DOI] [PubMed] [Google Scholar]
- 60. Sieradzka D, Power RA, Freeman D et al. . Are genetic risk factors for psychosis also associated with dimension-specific psychotic experiences in adolescence?PLoS One. 2014;9:e94398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fusar-Poli P, Yung AR, McGorry P, van Os J. Lessons learned from the psychosis high-risk state: towards a general staging model of prodromal intervention. Psychol Med. 2014;44:17–24. [DOI] [PubMed] [Google Scholar]
- 62. Fusar-Poli P, Schultze-Lutter F, Cappucciati M et al. . The dark side of the moon: meta-analytical impact of recruitment strategies on risk enrichment in the clinical high risk state for psychosis. Schizophr Bull. 2016;42:732–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hartmann JA, Yuen HP, McGorry PD et al. . Declining transition rates to psychotic disorder in “ultra-high risk” clients: investigation of a dilution effect. Schizophr Res. 2016;170:130–136. [DOI] [PubMed] [Google Scholar]
- 64. Cannon TD, Yu C, Addington J et al. . An individualized risk calculator for research in prodromal psychosis. Am J Psychiatry. 2016;173:980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Carrión RE, Cornblatt BA, Burton CZ et al. . Personalized prediction of psychosis: external validation of the NAPLS-2 psychosis risk calculator with the EDIPPP Project. Am J Psychiatry. 2016;173:989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nelson B, Rawlings D. Relating schizotypy and personality to the phenomenology of creativity. Schizophr Bull. 2010;36:388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. van Winkel R, Stefanis NC, Myin-Germeys I. Psychosocial stress and psychosis. A review of the neurobiological mechanisms and the evidence for gene-stress interaction. Schizophr Bull. 2008;34:1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. European Network of National Networks studying Gene-Environment Interactions in Schizophrenia (EU-GEI) , van Os J, Rutten BP et al. . Identifying gene–environment interactions in schizophrenia: contemporary challenges for integrated, large-scale investigations. Schizophr Bull. 2014;40:729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stefanis NC, Henquet C, Avramopoulos D et al. . COMT Val158Met moderation of stress-induced psychosis. Psychol Med. 2007;37:1651–1656. [DOI] [PubMed] [Google Scholar]
- 70. Cohen AS, Mohr C, Ettinger U, Chan RC, Park S. Schizotypy as an organizing framework for social and affective sciences. Schizophr Bull. 2015;41(suppl 2):S427–S435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Myin-Germeys I, van Os J, Schwartz JE, Stone AA, Delespaul PA. Emotional reactivity to daily life stress in psychosis. Arch Gen Psychiatry. 2001;58:1137–1144. [DOI] [PubMed] [Google Scholar]
- 72. Myin-Germeys I, van Os J. Stress-reactivity in psychosis: evidence for an affective pathway to psychosis. Clin Psychol Rev. 2007;27:409–424. [DOI] [PubMed] [Google Scholar]
- 73. Horan WP, Blanchard JJ, Clark LA, Green MF. Affective traits in schizophrenia and schizotypy. Schizophr Bull. 2008;34:856–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.