Abstract
Given their established analgesic properties, nonsteroidal anti‐inflammatory drugs (NSAIDs) represent an important postoperative pain management option. This study investigated: (1) the effects of mild or moderate renal insufficiency and mild hepatic impairment on the pharmacokinetics (PK) of diclofenac and hydroxypropyl‐β‐cyclodextrin (HPβCD) following administration of the injectable NSAID HPβCD‐diclofenac; and (2) the PK of HPβCD following administration of HPβCD‐diclofenac and intravenous itraconazole formulated with HPβCD in healthy adults. Diclofenac clearance (CL) and volume of distribution (Vz) tended to increase with decreasing renal function (moderate insufficiency versus mild insufficiency or healthy controls). Regression analysis demonstrated a significant relationship between Vz (but not CL or elimination half‐life, t½) and renal function. HPβCD CL was significantly decreased in subjects with renal insufficiency, with a corresponding increase in t½. There were no significant differences in diclofenac or HPβCD PK in subjects with mild hepatic impairment versus healthy subjects. Exposure to HPβCD in healthy subjects following HPβCD‐diclofenac administration was ∼12% of that with intravenous itraconazole, after adjusting for dosing schedule and predicted accumulation (<5% without adjustment). With respect to PK properties, these results suggest that HPβCD‐diclofenac might be administered to patients with mild or moderate renal insufficiency or mild hepatic impairment without dose adjustment (NCT00805090).
Keywords: hepatic, NSAID, pharmacokinetics, renal, safety
Current approaches to postoperative pain management emphasize the use of multimodal analgesic regimens to provide sufficient analgesia while permitting use of lower doses of individual agents and reducing the risk for adverse events.1, 2 Nonsteroidal anti‐inflammatory drugs (NSAIDs) represent a key aspect of such multimodal approaches.1, 3 Diclofenac is an NSAID that exerts analgesic, antipyretic, and anti‐inflammatory effects via cyclooxygenase (COX)‐1 and COX‐2 inhibition; it has been widely prescribed in multiple formulations since its introduction in the United States in 1988 and has demonstrated efficacy and safety in managing acute and chronic pain.4, 5, 6, 7, 8, 9
HPβCD‐diclofenac is an injectable diclofenac formulation approved for use in the United States, in which diclofenac is solubilized with hydroxypropyl‐β‐cyclodextrin (HPβCD). Solubilization with HPβCD allows diclofenac to be administered as a low‐volume intravenous bolus and makes its preparation and administration less prone to risks associated with parenteral drugs.10, 11 This formulation also allows for immediate release of diclofenac on injection and circumvention of first‐pass metabolic eliminations.12, 13 Solubilization with cyclodextrins allows for rapid drug release via complex dilution, replacement of the drug by another molecule, or transfer of the drug to a lipophilic biological membrane.14 An injectable diclofenac formulation not available in the United States employs propylene glycol and benzyl alcohol (PG‐BA) for solubilization. Unlike HPβCD‐diclofenac, PG‐BA‐diclofenac must be diluted, buffered, and administered over 30–120 minutes.11, 15, 16 Clinical trials have demonstrated the efficacy and safety of HPβCD‐diclofenac when used for acute postsurgical pain,17, 18, 19, 20 as well as lower incidence of thrombophlebitis than with PG‐BA‐diclofenac.21 Further, the pharmacokinetics (PK) of diclofenac following single and multiple doses of HPβCD‐diclofenac have been reported, demonstrating no accumulation following repeat dosing.13
HPβCD‐diclofenac is intended for use in the treatment of acute postsurgical pain. Postsurgical populations typically include patients with renal insufficiency or hepatic impairment, for which analgesic choice can be challenging because of potential efficacy and safety concerns. Side effects of opioids, such as sedation and respiratory depression, for example, may be more serious for patients with renal insufficiency or hepatic impairment because of the accumulation of active metabolites.22, 23 In addition, patients with renal impairment or liver disease may be at risk for NSAID‐related adverse effects, and thus caution is advised when prescribing NSAIDs in these patients.24, 25 Elderly patients also represent an important population in this respect, given that increasing age is associated with reductions in hepatic blood flow and a decline in the activity of hepatic cytochrome P450 enzymes,26, 27 as well as declining renal function,28 which may affect drug metabolism and clearance.
Diclofenac binds extensively to plasma albumin, with substantial concentrations attained in synovial fluid.29 Diclofenac undergoes significant hepatic metabolism and is eliminated following biotransformation to conjugated metabolites (glucuroconjugated and sulfate metabolites), followed by excretion in urine.29 The major primary metabolite of diclofenac is 4‐hydroxy (OH) diclofenac, with 3‐OH and 5‐OH diclofenac minor metabolites undergoing glucuronidation and sulfation.29 In humans, renal excretion predominates, with >60% of each daily dose excreted as a conjugate in urine, and studies have demonstrated a relationship between diclofenac excretion and glomerular filtration rate (GFR).29, 30 Overall, very little drug is eliminated unchanged, with approximately 2% of the dose reported to be excreted unchanged in urine in healthy volunteers.29 HPβCD, as a hydrophilic cyclodextrin, has been shown to be almost exclusively eliminated through the kidneys via glomerular filtration, with plasma hydrolysis showing a brief distribution phase, followed by an elimination phase.14, 31 In light of these metabolic considerations, as well as concerns related to NSAID use in patients with impaired renal or hepatic function, understanding the PK of any NSAID formulation in these populations is critical.
The first objective of this study was to evaluate the PK and safety of diclofenac and HPβCD following administration of a single dose of HPβCD‐diclofenac in subjects with mild or moderate chronic renal insufficiency or mild hepatic impairment compared with in matched healthy adult subjects. The second objective was to evaluate comparative PK and safety of HPβCD following a single dose of HPβCD‐diclofenac and intravenous itraconazole, an approved antifungal drug solubilized with HPβCD, in healthy adult subjects. Intravenous itraconazole (containing 8000 mg HPβCD) was used as a comparator to examine HPβCD exposure following HPβCD‐diclofenac administration (containing 333.3 mg HPβCD), given that it was the only available product using HPβCD as a solubilizing agent that was appropriate for administration to healthy subjects.32, 33, 34
Subjects and Methods
Subjects
There were 40 participants in this study (ClinicalTrials.gov Identifier: NCT00805090; Table 1), which was conducted at 4 sites: Davita Clinical Research (Minneapolis, Minnesota), New Orleans Clinical Center for Research (Knoxville, Tennessee), Orlando Clinical Research Center (Orlando, Florida), and Simbec Research Limited (Mid Glamorgan, UK). The protocol and informed consent form received Independent Ethics Committee and Institutional Review Board (IRB) approval prior to subject enrollment. IRB oversight was obtained from Coast IRB, LLC, (Colorado Springs, Colorado) for the US sites and South East Wales Research Ethics Committee, (Cardiff, UK) for the UK site.
A sufficient number of subjects was screened so that 8 subjects with mild chronic renal insufficiency (14 subjects screened), 5 subjects with moderate chronic renal insufficiency (13 subjects screened). and 8 subjects with mild chronic hepatic impairment completed the study (14 subjects screened). A sample size of 8 subjects with mild chronic renal insufficiency and 8 subjects with mild chronic hepatic impairment was considered typical for a study evaluating the effects of renal or hepatic impairment on PK. A sample size of up to 5 subjects with moderate chronic renal insufficiency was selected to gain clinical experience in this population. A sufficient number of healthy adult subjects were screened, so that 8 healthy adult subjects who were matched by age, sex, and weight to the subjects with mild chronic renal insufficiency and 8 healthy adult subjects who were matched by age, sex, and weight to the subjects with mild chronic hepatic impairment completed the study. One healthy adult subject could be matched to a subject with mild chronic renal insufficiency and to a subject with mild chronic hepatic impairment.
General study inclusion criteria were age ≥18 years, body mass index ≤ 42 kg/m2, ability to stay at the study site for the required number of days and nights and return to the clinic for follow‐up, and if female, nonfertility or use of an accepted method of contraception. Subjects in the renal insufficiency group were required to be 18–75 years old and have stable mild (creatinine clearance [CrCl] ≥ 50 and ≤ 80 mL/min) or moderate (≥ 30 and < 50 mL/min) renal insufficiency for 1 month prior to screening. CrCl was estimated using the Levey relationship of the Modification of Diet in Renal Disease formula: 186 × (serum creatinine)‐1.154 × (age)‐0.203 × (0.742 if female) × (1.212 if African American).35
For inclusion in the mild hepatic impairment group, subjects were required to be 18–75 years old and have a Child‐Pugh Classification A Score of 5–6, serum bilirubin ≤ 2.5 mg/dL, and mild hepatic impairment for at least 3 months prior to screening, with stable disease for at least 30 days. Because diclofenac is largely cleared by hepatic metabolism,36 subjects with fluctuating or rapidly deteriorating hepatic function or a current or past history of hepatic disease were excluded. The PK of HPβCD‐diclofenac were not studied in subjects with moderate to severe hepatic impairment and use in this population is not recommended.11
Healthy subjects were required to be 18–65 years old and have normal renal function (CrCl > 80 mL/min) and normal hepatic function and were matched by age (±10 years), sex, and body weight (±10 kg) with subjects with renal insufficiency or mild hepatic impairment. Subjects with renal insufficiency were permitted to enroll if they had a history of cardiovascular events, diabetes, high blood pressure, and/or hypercholesterolemia, provided that these conditions were stable, were well controlled, and did not pose a significant safety risk. Diabetic subjects with renal insufficiency were required to have been on a stable therapeutic regimen for 4 weeks prior to screening. Comorbidities were permitted in the hepatic impairment groups, provided these were stable and well controlled and did not pose a significant safety risk. Subjects being treated for mild chronic hepatic impairment were required to have been on a stable dose and regimen of standard therapy medication to treat their hepatic disease over the 4 weeks prior to screening.
All participants were required to be nonsmokers, healthy enough for study participation, and able to communicate with study personnel. General exclusion criteria included pregnancy, uncontrolled or poorly controlled diabetes, use of dialysis, fluctuating or rapidly deteriorating hepatic function, hepatic or other cancers, organ transplantation or immunosuppression, acute infections, or asthma. Subjects were excluded if they had a recent serious cardiovascular event, had significant medical history or clinically relevant laboratory test results, were serologically positive for the human immunodeficiency virus, were substance abusers, had donated blood within the past 56 days or plasma within the past 7 days, or had known NSAID or diclofenac hypersensitivity. Subjects were also excluded if they had a history of intestinal disorders or infections, peptic ulcers, gastrointestinal bleeding, or cerebral hemorrhage in the past 2 years. Individuals positive for hepatitis B or hepatitis C were excluded from the healthy and renal insufficiency groups, but were allowed in the mild hepatic impairment group. Use of monoamine oxidase inhibitors or tricyclic antidepressants ≤ 30 days prior to the study, over‐the‐counter medications including aspirin or herbal supplements ≤ 14 days prior to the study or during the study, or short‐acting NSAIDs ≤ 24 hours or long‐acting NSAIDs or COX‐2 inhibitors ≤ 3 days prior to the study also resulted in exclusion. Exposure to drugs that inhibit or induce cytochrome P450 (CYP) 2C9 was not allowed for at least 5 half‐lives prior to dosing with study drug. CYP2C9 inhibitors that were not permitted included azole antifungals, statins used for hypercholesterolemia, fenofibrate, amiodarone, isoniazid, phenylbutazone, probenecid, leukotriene inhibitors, and sertraline. CYP2C9 inducers such as phenobarbital and rifampin were not permitted. Although diclofenac is a substrate of CYP2C9, itraconazole is an inhibitor for CYP3A; however, this was not expected to affect diclofenac PK results. Exclusions for concomitant medication were determined such that a medication would not compromise the outcome or validity of the study (eg, assay interference).
Study Design
This study consisted of (1) an open‐label, single‐dose study of the PK of diclofenac and HPβCD following HPβCD‐diclofenac administration in subjects with renal insufficiency, hepatic impairment, and healthy controls, and (2) a randomized, open‐label, single‐dose, 2‐way crossover study of the PK of HPβCD following HPβCD‐diclofenac and intravenous itraconazole administration in healthy subjects.
Subjects with renal insufficiency or hepatic impairment reported to the study site on study day 0 and remained at the site for 2 nights and 2 days. HPβCD‐diclofenac 37.5 mg (Dyloject, Hospira, Inc., Lake Forest, Illinois) was administered to each subject as an intravenous bolus over 15 seconds on study day 1. Blood samples were obtained via an indwelling intravenous cannula or by direct venipuncture at the following times: time 0 (predose), 5, 10, 20, 30, and 45 minutes and 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, 18, and 24 hours postadministration. The sampling schedule for assessment of PK parameters was deemed appropriate to characterize the profiles of diclofenac and HPβCD in light of the known properties for the compounds evaluated. Subjects were discharged on study day 2 after being assessed by the investigator, and returned 7 ± 3 days after dosing for final safety assessments. If there were no abnormal findings at discharge, follow‐up was completed via telephone.
Healthy subjects reported to the study site on study day 0 and remained at the site for 3 nights and 3 days. HPβCD‐diclofenac 37.5 mg (333.3 mg HPβCD; intravenous bolus) and the comparator intravenous itraconazole 200 mg (Sporanox, Jansen Pharma, Beerse, Belgium; 8000 mg HPβCD; intravenous infusion over 60 minutes), were administered on study day 1 and study day 2 according to randomization codes. Use of intravenous itraconazole as the comparator was based on it being the only available product using HPβCD as a solubilizing agent appropriate for administration to healthy volunteers and that it has extensive postmarketing data.32, 33, 34 Blood samples were obtained from healthy subjects at the same postadministration points described above. When subjects received HPβCD‐diclofenac, 2 blood samples were drawn at each point, one each for diclofenac and HPβCD concentration measurements. Following intravenous itraconazole administration, 1 blood sample was drawn at each time to assay for HPβCD concentrations. Subjects were discharged on study day 3 after being assessed and returned 7 ± 3 days after receiving the last dose of study drug for safety assessments.
Pharmacokinetic and Statistical Analysis
Diclofenac plasma concentrations were measured by a validated liquid chromatography with tandem mass spectrometry (LC‐MS/MS) method performed by CEDRA Clinical Research, LLC (now Worldwide Clinical Trials, Inc., Austin, Texas). Plasma was collected in K2‐ethylenediaminetetraacetic acid (EDTA)–coated vials and then spiked with the internal standard, diclofenac‐D4 (Toronto Research Chemicals, Inc.). Plasma 0.2 mL (subject samples, standards, or quality control [QC] samples) was extracted with organic solvent, which was evaporated, reconstituted, and injected into a Sciex API‐4000 LC‐MS/MS (Applied Biosystems) in positive ion multiple‐reaction monitoring (MRM) mode (calibration curve range, 5–2000 ng/mL). The peak of m/z 296 to 214 diclofenac product ion was measured against the peak area of the m/z 300 to 218 product ion of the internal standard of diclofenac‐D4. Quantitation was performed using weighted (1/x 2) linear least‐squares regression generated from fortified human plasma calibration samples prepared prior to each run. The validated range of the assay was 5–2000 ng/mL. The QC concentrations were 5, 15, 400, 1600, and 10 000 ng/mL, with within‐day precision of 2.5%, 2.0%, 1.0%, and 0.5% (not applicable at QC sample 10 000 ng/mL), respectively, and between‐day precision of 6.3%, 3.2%, 1.9%, 3.4%, and 1.9%, respectively.
Plasma concentrations of HPβCD were determined using a validated LC‐MS/MS assay by Eurofins Medinet (Aurora, Colorado). Plasma was collected in K2‐EDTA‐coated vials. Sample preparation consisted of adding 250 μL of HPβCD‐containing plasma to 750 μL of methanol:acetonitrile:1% formic acid, 5:4:1, v:v:v, in 1.5‐mL microcentrifuge tubes; no internal standard was employed. The precipitated samples were briefly homogenized in a vortex mixer, then moderately agitated on a plate shaker for 5 minutes, and finally spun in a centrifuge at 13 kG × 5 minutes at 4°C to pellet the precipitated proteins and extract HPβCD. A 100‐μL aliquot of supernatant was diluted with 900 μL of 2 mM ammonium acetate (aq, pH 6.8) in a 1.5‐mL amber HPLC vial, capped, briefly vortex‐mixed, and placed in a CTC HTS PAL autosampler (CTC Analytics, AG) kept at 4°C. A 100‐μL aliquot of prepared sample was injected onto an Agilent 1100 HPLC (Agilent Technologies, Inc.) coupled to an ABI API 4000 (Applied Biosystems). The HPβCD population member with 5 degrees of substitution was isolated from interferences via an isocratic step of methanol:2 mM ammonium formate, 35:65, v:v, at 1.2 mL/min at 80°C on a Higgins Analytical Targa C18 column (2.1 × 30 mm, 3 μm; Higgins Analytical, Inc.), blended with 100% acetonitrile postcolumn at 0.8 mL/min to increase the organic content for improved ionization, and then detected via positive turbospray ionization mass spectrometry using the strongest MRM transition for the sodiated adduct of HPβCD DS = 5 of m/z 1447.5 to 447.5. The validated range of the assay was 100 to 10 000 ng/mL. The QC concentrations were 300, 1500, and 7500 ng/mL, with within‐day precision of 2.3%–8.0%, 3.7%–10.9%, and 3.5%–7.0%, respectively, and between‐day precision of 4.8%, 3.6%, and 7.6%, respectively.
PK parameters for diclofenac and HPβCD were calculated using noncompartmental analysis of the plasma concentration–time data. Only plasma concentrations equal to or greater than the lower limit of quantitation (LLOQ) for the respective assays (diclofenac 5 ng/mL and HPβCD 100 ng/mL) were used in the analysis. For both assays, values < LLOQ were set to zero for the calculation of descriptive statistics. For the PK analysis, values < LLOQ before the first value ≥ LLOQ were set to zero, and subsequent values were set to missing. For graphical displays, mean values are presented. Actual sampling times were used in all PK analyses. Per‐protocol times were used to calculate mean plasma concentrations for graphical displays. Overall analysis included calculation of the following PK parameters: maximum plasma concentration (Cmax), time to Cmax (Tmax), area under the curve from zero to final sample (AUC0–t), area under the curve from zero to infinity (AUC∞), elimination rate constant (λz), total plasma clearance (CL), volume of distribution (Vz), and elimination half‐life (t½).
Cmax and Tmax were obtained directly from the data. The elimination rate constant, λz, was calculated as the negative of the slope of the terminal log‐linear segment of the plasma concentration–time curve. The range of data used for each subject and treatment was determined by visual inspection of a semilogarithmic plot of concentration versus time. Elimination half‐life (t½) was calculated according to the equation t½ = 0.693/λz. Area under the curve from zero to the final sample with a concentration ≥ LLOQ (AUC0–t) was calculated using the linear trapezoidal method and extrapolated to infinity using: AUC∞ = AUC0–t + Ctf/λz, where Ctf is the final concentration ≥ LLOQ. CL was calculated as dose/AUC, and Vz was calculated as dose/(λz × AUC).
The effect of renal impairment on the PK parameters Cmax, AUC∞, CL, Vz, and t½ was examined with an analysis of variance (ANOVA) statistical model and subject type as the classification variable, using the natural logarithms of the data. The 3 cohorts were compared using paired t tests. The same model was used to test for the effect of hepatic impairment on the PK of diclofenac and HPβCD but without additional comparisons, as there were only 2 groups. Relationships between the independent PK parameters CL and Vz and the dependent parameter t½ and renal function were examined using linear regression of each PK parameter against the GFR.
Comparing HPβCD‐diclofenac and itraconazole required adjustments for differences in the dosing schedules and the predicted degree of accumulation, taking under consideration the different HPβCD concentration (333.3 mg/mL for diclofenac, 400 mg/mL for itraconazole) and duration (diclofenac, intravenous bolus over 15 seconds; itraconazole, intravenous infusion over 60 minutes). PK comparisons between HPβCD‐diclofenac and itraconazole in healthy subjects were performed with an ANOVA model with sequence, subject within sequence, treatment, and period as the classification variables, using the natural logarithms of the data. Confidence intervals (90%) were constructed for the test‐to‐reference ratio of the 3 parameters using the log‐transformed data and the 2 one‐sided t‐test procedure. Point estimates and confidence limits were exponentiated back to the original scale. PK calculations and individual subject plasma concentration‐versus‐time graphs were prepared using SAS for Windows v.9.1.3.
Safety
All participants who received study medication and had recorded safety data were included in the safety analysis. Safety was assessed via clinical laboratory tests, electrocardiograms, physical examination, vital signs, adverse events (AEs), and concomitant medications. Treatment‐emergent AEs (AEs first occurring or worsening in severity during the course of the study) were monitored for the duration for the study and were coded in accordance with the Medical Dictionary for Regulatory Activities v.11.1. AEs were tabulated by system, organ, class; maximum intensity (mild, moderate, or severe); and relationship to study drug.
Results
Subject Disposition and Demographics
A total of 84 volunteers were screened for this study, and 44 individuals were excluded at screening. The most frequent reasons for screen failure for the overall population were laboratory exclusion (n = 11), enrollment closed (n = 10), concomitant medication exclusion (n = 7), and inability to return during protocol windows (n = 7). All 21 subjects with renal or hepatic impairment who received study medication completed the study and were included in the safety and PK analyses. This included 13 subjects with renal insufficiency (mean CrCl, 56 mL/min) and 8 subjects with hepatic impairment (mean bilirubin, 0.59 mg/dL; mean Child‐Pugh score, 5.5). Nineteen matched healthy subjects were admitted to the study and dosed with study medication. Of these, 14 completed the study successfully, and 13 were included in the PK analysis. The subject who completed the PK portion of the study and was excluded from the PK analysis had a predose plasma diclofenac concentration of 391 ng/mL and 5.7% of Cmax (6860 ng/mL). As this was >5% of Cmax, the subject was excluded from the descriptive statistics and comparative analyses. Four of the 5 withdrawals in the healthy subject group were because of a dosing infusion line error, in which subjects received a lower dose of itraconazole than specified in the protocol. All 19 healthy subjects who received ≥1 dose of the study medication were included in the safety analysis. Demographic characteristics of all 40 enrolled subjects are detailed in Table 1.
Table 1.
Summary of Study Population Demographics
| Subject Group | Treatment Dose and Route | Number of Subjects Enrolleda | Age Range (Years) | Mean Weight, kg (SD) | Mean BMI, kg/m2 (SD) | Female, n (%) | Male, n (%) |
|---|---|---|---|---|---|---|---|
| Renal insufficiency (all) | 37.5 mg HPβCD‐diclofenac IV | 13 | 50–75 | 79.8 (20.2) | 28.3 (5.4) | 8 (61.5) | 5 (38.5) |
| Mild renal insufficiencyb | 37.5 mg HPβCD‐diclofenac IV | 8 | 57–75 | 70.8 (12.6) | 25.9 (3.7) | 5 (62.5) | 3 (37.5) |
| Moderate renal insufficiencyc | 37.5 mg HPβCD‐diclofenac IV | 5 | 50–75 | 94.1 (23.1) | 32.0 (5.9) | 3 (60.0) | 2 (40.0) |
| Hepatic impairmentd | 37.5 mg HPβCD‐diclofenac IV | 8 | 40–61 | 76.4 (12.2) | 25.1 (4.4) | 0 | 8 (100.0) |
| Healthy | 37.5 mg HPβCD‐diclofenac IV | ||||||
| 200 mg itraconazole IV | 19 | 33–65 | 74.9 (10.0) | 25.5 (3.0) | 6 (31.6) | 13 (68.4) |
BMI, body mass index; IV, intravenous.
Number of subjects screened in renal insufficiency, hepatic impairment, and healthy control groups was 27, 14, and 43, respectively.
Mild renal insufficiency: creatinine clearance (CrCl) ≥ 50 and < 80 mL/min.
Moderate renal insufficiency: CrCl ≥ 30 and < 50 mL/min.
Mild hepatic impairment: Child‐Pugh Classification A score 5–6; serum bilirubin ≤ 2.5 mg/dL.
Pharmacokinetics of Diclofenac and HPβCD in Subjects With Renal Impairment
Mean plasma diclofenac concentration curves following administration of HPβCD‐diclofenac were essentially the same for subjects with mild or moderate renal insufficiency and healthy controls (Figure 1A), and overall diclofenac exposure, as measured by AUC∞, did not differ significantly between these groups (P = .13; Table 2). Mean values for all PK parameters were similar in subjects with mild renal insufficiency and matched healthy controls, and there were no significant differences between these 2 cohorts (P ≥ .85 for all parameters; Table 2). In subjects with moderate renal insufficiency, there was a trend toward increased CL and decreased AUC∞ versus in healthy controls; however, there was no statistically significant difference between these groups with respect to either parameter (both P = .068; Table 2). Conversely, Vz was significantly increased and Cmax was significantly decreased in subjects with moderate renal insufficiency versus in healthy controls (P = .019) and subjects with mild renal insufficiency (P < .017). Notably, however, there was overlap of the individual subject values among the 3 cohorts. When the relationships between CL, Vz, and t½ and renal function were examined via regression analysis, a statistically significant relationship was observed for Vz (P = .021) but not for CL or t½ (Table 2).
Figure 1.
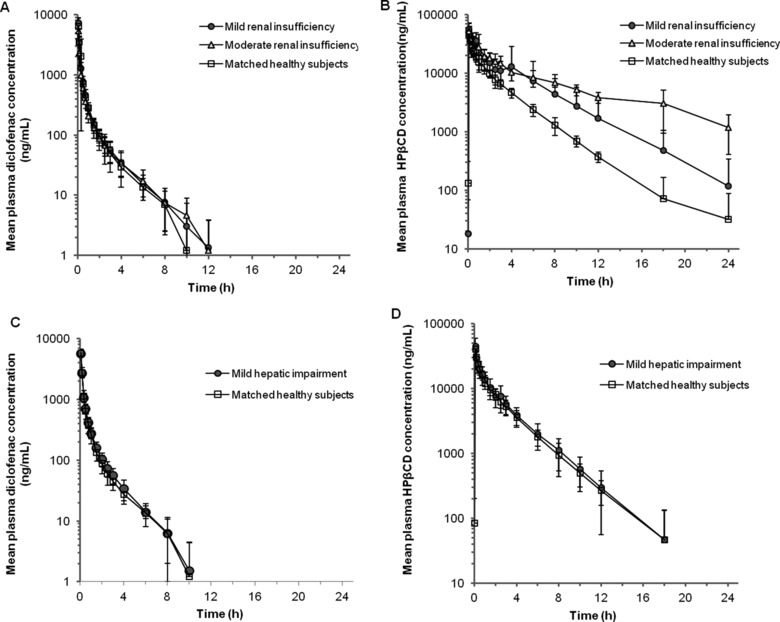
Mean plasma concentrations of diclofenac and hydroxypropyl‐β‐cyclodextrin (HPβCD) following administration of intravenous HPβCD‐diclofenac in subjects with renal insufficiency or hepatic impairment. (A,B) Mean plasma diclofenac (A) and HPβCD (B) concentrations following intravenous administration of a single dose of HPβCD‐diclofenac 37.5 mg in patients with mild or moderate renal insufficiency and healthy subjects. (C,D) Mean plasma diclofenac (C) and HPβCD (D) concentrations following intravenous administration of a single dose of HPβCD‐diclofenac 37.5 mg in patients with mild hepatic impairment and healthy subjects. Data points represent mean values (values below LLOQ were considered zero; thus, some mean values are < LLOQ), and error bars represent the standard deviation (SD) of the mean. Individual patient data are presented in Supplementary Tables 1 and 2.
Table 2.
Pharmacokinetics of Diclofenac and Hydroxypropyl‐β‐Cyclodextrin (HPβCD) by Renal Function Group, Following Administration of Intravenous HPβCD‐Diclofenac
| Subject Group | p Values | |||||||
|---|---|---|---|---|---|---|---|---|
| Parametera | Mild Renal Insufficiencyb (n = 8) | Moderate Renal Insufficiencyc (n = 5) | Healthy Subjects (n = 7) | Mild vs Moderate Renal Insufficiency | Mild Renal Insufficiency vs Healthy | Moderate Renal Insufficiency vs Healthy | Overall Population | Regression vs Renal Function |
| Diclofenac | ||||||||
| t½ (h) | 1.89 ± 0.46 | 2.10 ± 0.44 | 1.90 ± 0.30 | 0.34 | 0.85 | 0.45 | 0.61 | 0.24 |
| CL (mL/min) | 312 ± 73.0 | 401 ± 126 | 303 ± 55.6 | 0.083 | 0.86 | 0.068 | 0.13 | 0.14 |
| Vz (L) | 49.8 ± 12.1 | 69.7 ± 9.22 | 50.2 ± 14.1 | 0.017 | 0.99 | 0.019 | 0.030 | 0.021 |
| Cmax (ng/mL) | 7286 ± 1430 | 5332 ± 1629 | 7163 ± 950 | 0.014 | 0.94 | 0.019 | 0.028 | – |
| AUC∞ (ng·h/mL) | 1943 ± 409 | 1550 ± 422 | 1968 ± 315 | 0.083 | 0.86 | 0.068 | 0.13 | – |
| Tmax (h) | 0.083 | 0.083 | 0.083 | – | – | – | – | – |
| AUC0–t (ng·h/mL) | 1927 ± 409 | 1,531 ± 418 | 1,947 ± 313 | – | – | – | – | – |
| HPβCD | ||||||||
| t½ (h) | 2.87 ± 0.69 | 6.04 ± 1.94 d | 3.29 ±1.66 | 0.003 | 0.17 | 0.007 | 0.009 | 0.018 |
| CL (mL/min) | 59.0 ± 31.3 | 36.2 ± 10.0 d | 85.2 ± 16.5 | 0.17 | 0.044 | 0.005 | 0.015 | 0.002 |
| Vz (L) | 13.6 ± 5.38 | 17.7 ± 1.88d | 23.3 ± 9.84 | 0.18 | 0.019 | 0.43 | 0.054 | 0.26 |
| Cmax (ng/mL) | 60 750 ± 16 275 | 52 700 ± 18 565 | 50 329 ± 7731 | 0.28 | 0.22 | 0.97 | 0.39 | – |
| AUC∞ (ng·h/mL) | 128 349 ± 91 132 | 165 728 ± 60 386 d | 67 316 ± 12 615 | 0.17 | 0.044 | 0.005 | 0.015 | – |
| Tmax (h) | 0.083 | 0.083 | 0.083 | – | – | – | – | – |
| AUC0–t (ng·h/mL) | 127 141 ± 90 489 | 169 042 ± 52 722 | 66 449 ±12 642 | – | – | – | – | – |
Cmax, maximum observed plasma concentration; Tmax, time at which Cmax was observed; AUC0–t, AUC up to the last quantifiable concentration; AUC∞, AUC from time zero to infinity; t½, apparent elimination half‐life; Vz, volume of distribution; CL, clearance; GFR, glomerular filtration rate.
All parameters are presented as arithmetic mean ± standard deviation (SD), except for Tmax, for which the median is reported.
Creatinine clearance (CrCl) ≥ 50 and ≤ 80 mL/min.
Creatinine clearance (CrCl) ≥ 30 and < 50 mL/min.
n = 4.
Mean plasma HPβCD concentration curves revealed greater plasma HPβCD concentrations in subjects with impaired renal function (Figure 1B). There was a statistically significant decrease in HPβCD CL with decreasing renal function (P = .015 for the comparison between all three cohorts), with corresponding significant increases in AUC∞ and t½ (P = .015 and .009, respectively; Table 2). Overall, a 2.4‐fold decrease in CL and a 1.8‐fold increase in t½ were observed in subjects with moderate renal insufficiency when compared with healthy subjects, but there was no statistically significant difference between cohorts with respect to Vz (P = .054; Table 2). Regression analysis revealed significant relationships between CL and t½ and renal function (P = .002 and .018, respectively; Table 2), but not between Vz and renal function (P = .26). PK parameters for individual subjects are provided in Supplementary Tables 1 and 2.
Pharmacokinetics of Diclofenac and HPβCD in Subjects With Hepatic Impairment
There were no differences in the mean diclofenac or HPβCD plasma concentration curves between subjects with mild hepatic impairment and matched healthy controls following HPβCD‐diclofenac administration (Figure 1C,D). There were no statistically significant differences in diclofenac or HPβCD PK parameters between subjects with mild hepatic impairment and healthy subjects (all P ≥ 0.61; Table 3).
Table 3.
Pharmacokinetics of Diclofenac and Hydroxypropyl‐β‐Cyclodextrin (HPβCD) by Hepatic Function, Following Administration of Intravenous HPβCD‐Diclofenac
| Parametera | Mild Hepatic Impairmentb (n = 8) | Healthy Subjects (n = 7) | P |
|---|---|---|---|
| Diclofenac | |||
| Cmax (ng/mL) | 5648 ± 709 | 5884 ± 897 | .61 |
| AUC∞ (ng·h/mL) | 1663 ± 179 | 1640 ± 335 | .76 |
| t½ (h) | 1.97 ± 0.67 | 1.92 ± 0.28 | .97 |
| CL (mL/min) | 353 ± 40.7 | 367 ±74.7 | .76 |
| Vz (L) | 60.1 ± 21.5 | 59.9 ± 9.4 | .81 |
| Tmax (h) | 0.083 | 0.083 | – |
| AUC(0‐t) (ng·h/mL) | 1641 ± 179 | 1618 ± 333 | – |
| HPβCD | |||
| Cmax (ng/mL) | 44 813 ± 14 985 | 40 917 ± 4975 | .74 |
| AUC∞ (ng·h/mL) | 56 802 ±17 412 | 53 651 ± 11 321 | .82 |
| t½ (h) | 2.28 ± 0.60 | 2.28 ± 0.42 | .91 |
| CL (mL/min) | 107 ± 33.8 | 107 ± 21.2 | .82 |
| Vz (L) | 20.0 ± 4.19 | 20.6 ± 2.45 | .62 |
| Tmax (h) | 0.083 | 0.083 | – |
| AUC0–t (ng·h/mL) | 55 946 ± 17 233 | 52 982 ± 11 267 | – |
Cmax, maximum observed plasma concentration; Tmax, time at which Cmax was observed; AUC0–t, AUC up to the last quantifiable concentration; AUC∞, AUC from time zero to infinity; t½, apparent elimination half‐life; Vz, volume of distribution; CL, clearance.
All parameters are presented as arithmetic mean ± standard deviation (SD), except for Tmax, for which the median is reported.
Child‐Pugh Classification A score 5–6; serum bilirubin ≤ 2.5 mg/dL.
Pharmacokinetics of HPβCD in Healthy Subjects
To compare the PK of HPβCD following HPβCD‐diclofenac administration with HPβCD PK following administration of an approved drug containing the same solubilizing agent, healthy subjects received both HPβCD‐diclofenac and intravenous itraconazole. Consistent with the differences in HPβCD dose, exposure to HPβCD following administration of HPβCD‐diclofenac (333.3 mg HPβCD) was markedly lower than with intravenous itraconazole (8000 mg HPβCD) in healthy controls, as were the mean PK parameters related to dose (Cmax, AUC0–t, and AUC∞, all P < .0001; Table 4, Figure 2). Based on the geometric least‐squares mean ratio of AUC∞, HPβCD exposure after a single dose of HPβCD‐diclofenac was 1/20th (4.58%) of that following intravenous itraconazole administration, which was essentially the same as the ratio of HPβCD doses (333.3/8000 mg; 4.17%). Adjusting for differences in the dosing schedules and the predicted degree of accumulation, the steady‐state exposure to HPβCD following HPβCD‐diclofenac administration was estimated to be approximately 1/8th (12.11%) of that following intravenous itraconazole administration. Using the “worst case” of subjects with moderate renal insufficiency, the exposure to HPβCD after administration of HPβCD‐diclofenac 37.5 mg would still be expected to be 7.9‐fold lower based on AUC0–t and 3.9‐fold lower based on the average concentration at steady state (Cav) than in healthy subjects administered intravenous itraconazole. The results of the comparison in healthy subjects do not include adjustments for differences in doses of HPβCD between HPβCD‐diclofenac and intravenous itraconazole; this was done to demonstrate that when compared with intravenous itraconazole, as a clinically relevant and approved standard, HPβCD‐diclofenac had much lower HPβCD exposure.
Table 4.
Pharmacokinetics of Hydroxypropyl‐β‐Cyclodextrin (HPβCD) in Healthy Subjects Following Administration of Intravenous HPβCD‐Diclofenac and Intravenous Itraconazole Formulated With HPβCD
| Parametera | HPβCD‐diclofenac (333.3 mg HPβCD; n = 13) | HPβCD‐Itraconazole (8000 mg HPβCD; n = 13) | P |
|---|---|---|---|
| Cmax (ng/mL) | 44 331 ± 10 004 | 557 538 ± 105 477 | < .0001 |
| AUC(0‐t) (ng·h/mL) | 58 994 ± 14 123 | 1 300 356 ± 264 445 | < .0001 |
| AUC∞ (ng·h/mL) | 59 709 ± 14 217 | 1 301 283 ± 264 630 | < .0001 |
| Tmax (h)b | 0.083 | 1.083 | – |
| t½ (h) | 2.74 ± 1.35 | 2.54 ± 0.25 | – |
| CL (mL/min) | 98.0 ± 22.7 | 106 ± 19.0 | – |
| Vz (L) | 21.8 ± 7.36 | 23.5 ± 5.65 | – |
The results of the comparison in healthy subjects do not include adjustments for differences in doses of HPβCD between HPβCD‐diclofenac and IV itraconazole.
IV, intravenous; Cmax, maximum observed plasma concentration; Tmax, time at which Cmax was observed; AUC0–t, AUC up to the last quantifiable concentration; AUC∞, AUC from time zero to infinite time; t½, apparent elimination half‐life; Vz, volume of distribution; CL, clearance.
All parameters presented as arithmetic mean ± standard deviation (SD), except for Tmax, for which the median is reported.
Difference in Tmax is based on diclofenac group having had an IV bolus (15 seconds), whereas the itraconazole group had an IV infusion (60 minutes).
Figure 2.
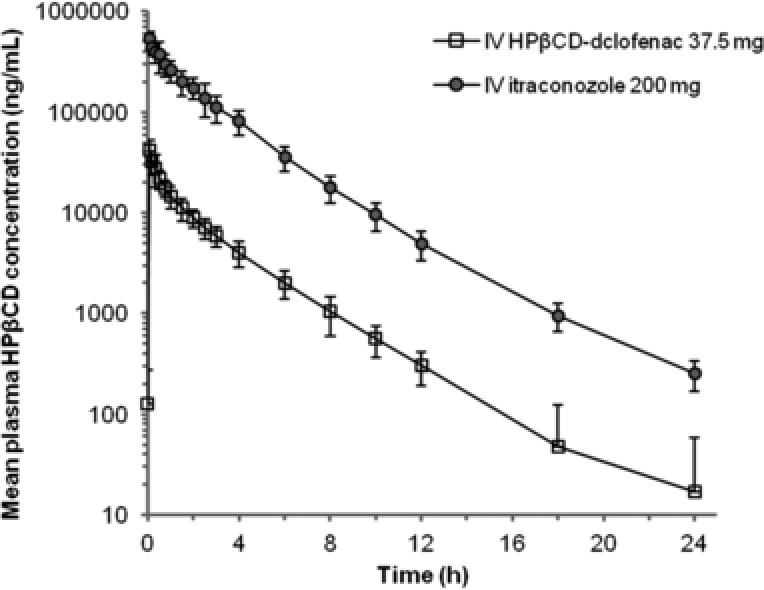
Mean plasma concentrations of hydroxypropyl‐β‐cyclodextrin (HPβCD) in healthy subjects following administration of intravenous HPβCD‐diclofenac and intravenous itraconazole. intravenous HPβCD‐diclofenac 37.5 mg (333.3 mg HPβCD), and intravenous itraconazole 200 mg (8000 mg HPβCD) were both given as a single dose. Data points represent mean values (values below the LLOQ were considered zero), and error bars represent the standard deviation (SD) of the mean. The results of the comparison in healthy subjects do not include adjustments for differences in doses of HPβCD between HPβCD‐diclofenac and intravenous itraconazole.
Safety
There were no deaths, withdrawals because of AEs, or serious adverse events in this study, and all AEs were mild or moderate in severity. The overall incidence of treatment‐emergent AEs was 30.8% (4 of 13) in subjects with mild or moderate renal insufficiency (mild renal insufficiency: dysgeusia [n = 1], wheezing [n = 1]; moderate renal insufficiency: diarrhea [n = 1], dysgeusia [n = 1]), 25.0% (2 of 8) in subjects with mild hepatic impairment (dysgeusia [n = 1], flushing [n = 1]), 6.7% (1 of 15) in healthy subjects following HPβCD‐diclofenac dosing (headache [n = 1]), and 22.2% (4 of 18) in healthy subjects following intravenous itraconazole dosing (vomiting [n = 1], headache [n = 2], thrombophlebitis [n = 1]). Following HPβCD‐diclofenac administration, 2 of 13 subjects with renal insufficiency (15.4%) and 1 of 8 subjects with mild hepatic impairment (12.5%) had a treatment‐related AE (renal insufficiency: dysgeusia [n = 2]; hepatic impairment: dysgeusia [n = 1]). No treatment‐related AEs were reported in healthy subjects. There were no renal or hepatic AEs in individuals with renal or hepatic impairment. No clinically significant study drug effects were evident for clinical chemistry or hematology parameters or for renal or liver function tests, and no clinically significant out‐of‐range vital signs or electrocardiogram results were observed during the study.
Discussion
The results of this study suggest that mild to moderate renal or mild hepatic insufficiency did not significantly affect the exposure to or elimination of diclofenac following administration of a single dose of intravenous HPβCD‐diclofenac. However, renal insufficiency was associated with decreased CL of HPβCD, the compound with which diclofenac is solubilized. Based on these results, HPβCD does not seem to provide any additive or synergistic effect on the PK profile, clearance, or rate of elimination of HPβCD‐diclofenac in individuals with mild or moderate renal insufficiency or mild hepatic impairment. This study also demonstrated that HPβCD exposure was lower following administration of HPβCD‐diclofenac than following a standard dose of the approved drug, intravenous itraconazole, even after adjustment for differences in dosing schedules.
The inclusion of individuals with both mild and moderate renal insufficiency allowed for examination of PK parameters in light of degree of renal insufficiency. There was a trend toward increased CL of diclofenac in subjects with moderate renal insufficiency compared with those with mild renal insufficiency and matched healthy controls; however, there were no statistically significant differences observed between groups. Conversely, Vz was significantly increased in subjects with moderate renal insufficiency, an observation that may be in part because of the small size of the individual cohorts. Importantly, however, regression analysis also demonstrated a significant relationship between Vz and renal function. Although the binding of diclofenac to serum proteins may be lower in subjects with renal failure, which might lead to an increase in Vz,29 protein binding was not examined in the current study. Further, diclofenac is extensively bound in plasma and serum (more than 99.7% bound),29 suggesting that even small changes in binding might affect measurement of PK parameters based on total (free plus bound) plasma concentrations.
The absence of a significant effect of renal insufficiency on diclofenac CL is consistent with previous studies of other diclofenac formulations, in which renal elimination was not found to be a significant pathway for CL,37 and previous investigation in subjects with renal insufficiency (inulin clearance, 60–90, 30–60, and <30 mL/min), revealing comparable AUC and elimination rate of diclofenac compared with healthy subjects.
There was an observed decrease in HPβCD CL, with corresponding increases in AUC∞ and t½, in subjects with decreased renal function. Reduced HPβCD CL in subjects with renal insufficiency is not unexpected, given that, following intravenous injection, HPβCD is almost exclusively eliminated through the kidneys.14 Importantly, however, the observed 2.4‐fold decrease in HPβCD CL following administration of HPβCD‐diclofenac in subjects with moderate renal insufficiency versus healthy controls was such that blood concentrations of HPβCD after therapeutic doses of HPβCD‐diclofenac would remain well below those following intravenous itraconazole, as well as below levels associated with adverse effects.14 Further, HPβCD‐diclofenac was safe in subjects with renal insufficiency, and there was no notable aggravation of underlying disease or marked elevations in serum creatinine or blood urea nitrogen. Still, it is important to note that in an open‐label phase 3 safety study examining repeated‐dose HPβCD‐diclofenac in 971 postsurgical patients, the incidence of acute renal failure/decreased urine output was greater in patients with preexisting renal insufficiency (5 of 57) than in patients with normal baseline renal function (14 of 914) and that acute renal decompensation was observed in 4% of 68 patients with renal insufficiency and treated with HPβCD‐diclofenac in clinical trials in the postoperative period.20 Although the PK of HPβCD‐diclofenac was similar in subjects with renal insufficiency and healthy controls, HPβCD‐diclofenac is contraindicated in patients with moderate to severe renal insufficiency in the postoperative period and who are at risk of volume depletion.11 Use of HPβCD‐diclofenac is to be avoided in patients with advanced renal disease unless benefits are expected to outweigh the risk of worsening renal function.11 Likewise, it is recommended that administration of HPβCD to patients with severe renal insufficiency be avoided.14
Hepatic metabolism accounts for almost 100% of diclofenac elimination, unlike HPβCD, which is not extensively metabolized and of which 80% to 90% of an intravenous dose is excreted unchanged in the urine.14 In the current study, there were no observed differences in the PK profile of diclofenac in subjects with mild hepatic impairment, compared with healthy matched controls, after administration of HPβCD‐diclofenac, a finding in agreement with previous data suggesting no significant changes in diclofenac PK following oral administration in subjects with renal impairment.29 HPβCD‐diclofenac was safe in subjects with mild hepatic impairment in the present study, and there were no notable aggravations of underlying disease or marked elevations in liver function tests. This study provides a first indication that, based on PK parameters, no dose adjustment may be required for patients with mild hepatic impairment; however, the PK and safety of HPβCD‐diclofenac in subjects with moderate or severe hepatic impairment were not investigated, and HPβCD‐diclofenac use is not recommended in patients with moderate to severe hepatic impairment.11
The study findings also demonstrate that when compared with a clinically relevant and approved standard, intravenous itraconazole, HPβCD exposure was much lower following HPβCD‐diclofenac administration, suggesting minimal safety concerns related to HPβCD with HPβCD‐diclofenac. After adjusting for differences in dosing schedules and the predicted degree of accumulation, the steady‐state daily plasma concentration of HPβCD following administration of HPβCD‐diclofenac 37.5 mg was estimated to be approximately 1/8th relative to exposure following administration of intravenous itraconazole. Using moderate renal insufficiency as the worst case, the exposure to HPβCD after administration of HPβCD‐diclofenac was still estimated to be 7.9‐fold lower based on AUC0–t and 3.9‐fold lower based on the average concentration than in healthy subjects administered intravenous itraconazole. Notably, the PK profile of HPβCD following administration of intravenous itraconazole has previously been studied in subjects with mild, moderate, and severe renal insufficiency, with results similar to those observed in the present study — a 2.3‐fold decrease in CL and a 3.7‐fold increase in t½ were observed for subjects with mild or moderate renal insufficiency, whereas a 6‐fold decrease in CL and a 6‐fold increase in t½ were observed for subjects with severe renal insufficiency versus healthy subjects.33, 34
The overall number of subjects could be considered a limitation of this study. Although the results demonstrate the PK properties of diclofenac in the study population, clinical decisions regarding pain management should be based on a range of factors, including PK considerations. A second potential limitation of the study population is that it did not include equal numbers of male and female subjects, a relevant consideration given that the PK parameters of diclofenac may differ in men and women.38 Because of this consideration, subject groups were matched based on sex as well as other relevant factors (age, body weight) for the purpose of comparing mean PK parameters. Thus, the potential bias because of this factor is expected to be limited.
In summary, this study provides key insight into the PK of HPβCD‐diclofenac in patients with renal insufficiency and hepatic impairment, which are important considerations when selecting a patient's postoperative pain management regimen. The study findings are relevant not only because of the presence of patients with preexisting renal insufficiency or hepatic impairment in surgical populations, but also in light of the transient renal insufficiency that can occur as a result of altered hemodynamics (which can affect renal perfusion) during major (eg, intrathoracic, intraperitoneal) surgical procedures.39, 40 The results of this study therefore provide a first indication that HPβCD‐diclofenac may be administered to patients with mild or moderate renal insufficiency or mild hepatic impairment at the usual dose and schedule without a need for dose reduction. Further studies with a larger cohort of patients could further strengthen this conclusion.
Acknowledgments
Editorial/medical writing support was provided by Scott Paluszkiewicz, PhD, Fred Peyerl, PhD, and Jeff Skaar, PhD, of Boston Strategic Partners, Inc., and was funded by Hospira, Inc., which was acquired by Pfizer Inc. in September 2015.
Declaration of Conflicting Interests
D.A. Hamilton was a stockholder and consultant to Javelin Pharmaceuticals, Inc., at the time the study was designed, conducted, and completed and subseqent to the acquisition of Javelin Pharmaceuticals, Inc., by Hospira in 2010 and became a consultant to Hospira. C. Ernst, D. Madden, and E. Liao were employees of the study sponsor at the time of the trial. W.G. Kramer was a paid consultant to the study sponsor during the trial. E. Lang was an employee of the study sponsor at the time of the trial and is currently an employee of Covance, Inc., which has no financial or other interest in this trial. P.G. Lacouture was an employee of Hospira at the time of the study. A. Ramaiya is an employee of Hospira, a Pfizer company. D.B. Carr was the full‐time chief medical officer for the study sponsor during the trial and served as a consultant to Hospira, Inc., following the acquisition of Javelin Pharmaceuticals, Inc., in 2010.
Funding
This study was sponsored by Javelin Pharmaceuticals, Inc. (acquired in 2010 by Hospira, and subsequently acquired by Pfizer in September 2015).
References
- 1. American Society of Anesthesiologists Task Force on Acute Pain Management . Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248–273. [DOI] [PubMed] [Google Scholar]
- 2. Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77(5):1048–1056. [DOI] [PubMed] [Google Scholar]
- 3. Macintyre PE, Schug SA, Scott DA, Visser EJ, Walker SM. Acute Pain Management: Scientific Evidence. 3rd ed Melbourne, Australia: Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine; 2010. [Google Scholar]
- 4. Van Hecken A, Schwartz JI, Depre M, et al. Comparative inhibitory activity of rofecoxib, meloxicam, diclofenac, ibuprofen, and naproxen on COX‐2 versus COX‐1 in healthy volunteers. J Clin Pharmacol. 2000;40(10):1109–1120. [PubMed] [Google Scholar]
- 5. Kato M, Nishida S, Kitasato H, Sakata N, Kawai S. Cyclooxygenase‐1 and cyclooxygenase‐2 selectivity of non‐steroidal anti‐inflammatory drugs: investigation using human peripheral monocytes. J Pharm Pharmacol. 2001;53(12):1679–1685. [DOI] [PubMed] [Google Scholar]
- 6. Barden J, Edwards J, Moore RA, McQuay HJ. Single dose oral diclofenac for postoperative pain. Cochrane Database Syst Rev. 2004(2):CD004768. [DOI] [PubMed] [Google Scholar]
- 7. Catalano MA. Worldwide safety experience with diclofenac. Am J Med. 1986;80(4B):81–87. [DOI] [PubMed] [Google Scholar]
- 8. Gan TJ. Diclofenac: an update on its mechanism of action and safety profile. Curr Med Res Opin. 2010;26(7):1715–1731. [DOI] [PubMed] [Google Scholar]
- 9. Todd PA, Sorkin EM. Diclofenac sodium: a reappraisal of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs. 1988;35(3):244–285. [DOI] [PubMed] [Google Scholar]
- 10. National Patient Safety Agency United Kingdom . Promoting safer use of injectable medicines. 2007. http://www.nrls.npsa.nhs.uk/resources/?EntryId45=59812. Accessed November 9, 2015.
- 11. Hoy SM. Diclofenac sodium bolus injection (Dyloject(TM)): a review in acute pain management. Drugs. 2016;76(12):1213–1220. [DOI] [PubMed] [Google Scholar]
- 12. Loftsson T, Hreinsdottir D, Masson M. Evaluation of cyclodextrin solubilization of drugs. Int J Pharm. 2005;302(1–2):18–28. [DOI] [PubMed] [Google Scholar]
- 13. Mermelstein F, Hamilton DA, Wright C, Lacouture PG, Ramaiya A, Carr DB. Single‐dose and multiple‐dose pharmacokinetics and dose proportionality of intravenous and intramuscular HPβCD‐diclofenac (Dyloject) compared with other diclofenac formulations. Pharmacotherapy. 2013;33(10):1012–1021. [DOI] [PubMed] [Google Scholar]
- 14. Brewster ME, Loftsson T. Cyclodextrins as pharmaceutical solubilizers. Adv Drug Del Rev. 2007;59(7):645–666. [DOI] [PubMed] [Google Scholar]
- 15. Campbell WI, Watters CH. Venous sequelae following i.v. administration of diclofenac. Br J Anaesth. 1989;62(5):545–547. [DOI] [PubMed] [Google Scholar]
- 16. Leeson RM, Harrison S, Ernst CC, et al. Dyloject, a novel injectable diclofenac formulation, offers greater safety and efficacy than voltarol for postoperative dental pain. Reg Anesth Pain Med. 2007;32(4):303–310. [DOI] [PubMed] [Google Scholar]
- 17. Daniels S, Melson T, Hamilton DA, Lang E, Carr DB. Analgesic efficacy and safety of a novel injectable formulation of diclofenac compared with intravenous ketorolac and placebo after orthopedic surgery: a multicenter, randomized, double‐blinded, multiple‐dose trial. Clin J Pain. 2013;29(8):655–663. [DOI] [PubMed] [Google Scholar]
- 18. Christensen K, Daniels S, Bandy D, et al. A double‐blind placebo‐controlled comparison of a novel formulation of intravenous diclofenac and ketorolac for postoperative third molar extraction pain. Anesth Prog. 2011;58(2):73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gan TJ, Daniels SE, Singla N, Hamilton DA, Carr DB. A novel injectable formulation of diclofenac compared with intravenous ketorolac or placebo for acute moderate‐to‐severe pain after abdominal or pelvic surgery: a multicenter, double‐blind, randomized, multiple‐dose study. Anesth Analg. 2012;115(5):1212–1220. [DOI] [PubMed] [Google Scholar]
- 20. Chelly JE, Singla SK, Melson TI, Lacouture PG, Paadre S, Carr DB. Safety of a novel parenteral formulation of diclofenac after major orthopedic or abdominal/pelvic surgery in a population including anticoagulated, elderly or renally insufficient patients: an open‐label, multiday, repeated dose clinical trial. Pain Med. 2013;14(5):749–761. [DOI] [PubMed] [Google Scholar]
- 21. Colucci RD, Wright C, Mermelstein FH, Gawarecki DG, Carr DB. Dyloject®, a novel injectable diclofenac solubilised with cyclodextrin: reduced incidence of thrombophlebitis compared to injectable diclofenac solubilised with polyethylene glycol and benzyl alcohol. Acute Pain. 2009;11(1):15–21. [Google Scholar]
- 22. Dean M. Opioids in renal failure and dialysis patients. J Pain Symptom Manage. 2004;28(5):497–504. [DOI] [PubMed] [Google Scholar]
- 23. Hasselstrom J, Eriksson S, Persson A, Rane A, Svensson JO, Sawe J. The metabolism and bioavailability of morphine in patients with severe liver cirrhosis. Br J Clin Pharmacol. 1990;29(3):289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aubrun F, Marmion F. The elderly patient and postoperative pain treatment. Best Pract Res Clin Anaesthesiol. 2007;21(1):109–127. [DOI] [PubMed] [Google Scholar]
- 25. Risser A, Donovan D, Heintzman J, Page T. NSAID prescribing precautions. Am Fam Physician. 2009;80(12):1371–1378. [PubMed] [Google Scholar]
- 26. Le Couteur DG, McLean AJ. The aging liver. Drug clearance and an oxygen diffusion barrier hypothesis. Clin Pharmacokinet. 1998;34(5):359–373. [DOI] [PubMed] [Google Scholar]
- 27. Schmucker DL. Liver function and phase I drug metabolism in the elderly: a paradoxq. Drugs Aging. 2001;18(11):837–851. [DOI] [PubMed] [Google Scholar]
- 28. Mühlberg W, Platt D. Age‐dependent changes of the kidneys: pharmacological implications. Gerontology. 1999;45(5):243–253. [DOI] [PubMed] [Google Scholar]
- 29. Davies NM, Anderson KE. Clinical pharmacokinetics of diclofenac: therapeutic insights and pitfalls. Clin Pharmacokinet. 1997;33(3):184–213. [DOI] [PubMed] [Google Scholar]
- 30. John VA. The pharmacokinetics and metabolism of diclofenac sodium (Voltarol) in animals and man. Rheumatol Rehabil. 1979;(Suppl 2):22–37. [PubMed] [Google Scholar]
- 31. Loftsson T. Essential Pharmacokinetics: A Primer for Pharmaceutical Scientists. 1st ed Elsevier; 2015. [Google Scholar]
- 32. Lestner J, Hope WW. Itraconazole: an update on pharmacology and clinical use for treatment of invasive and allergic fungal infections. Expert Opin Drug Metab Toxicol. 2013;9(7):911–926. [DOI] [PubMed] [Google Scholar]
- 33. Abdel‐Rahman SM, Jacobs RF, Massarella J, et al. Single‐dose pharmacokinetics of intravenous itraconazole and hydroxypropyl‐beta‐cyclodextrin in infants, children, and adolescents. Antimicrob Agents Chemother. 2007;51(8):2668–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buchanan CM, Buchanan NL, Edgar KJ, et al. Pharmacokinetics of itraconazole after intravenous and oral dosing of itraconazole‐cyclodextrin formulations. J Pharm Sci. 2007;96(11):3100–3116. [DOI] [PubMed] [Google Scholar]
- 35. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. [DOI] [PubMed] [Google Scholar]
- 36. Tang W. The metabolism of diclofenac–enzymology and toxicology perspectives. Curr Drug Metab. 2003;4(4):319–329. [DOI] [PubMed] [Google Scholar]
- 37. Brogden RN, Heel RC, Pakes GE, Speight TM, Avery GS. Diclofenac sodium: a review of its pharmacological properties and therapeutic use in rheumatic diseases and pain of varying origin. Drugs. 1980;20(1):24–48. [DOI] [PubMed] [Google Scholar]
- 38. Mennecozzi M, Landesmann B, Palosaari T, Harris G, Whelan M. Sex differences in liver toxicity‐do female and male human primary hepatocytes react differently to toxicants in vitro? PLoS One. 2015;10(4):e0122786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kheterpal S, Tremper KK, Englesbe MJ, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology. 2007;107(6):892–902. [DOI] [PubMed] [Google Scholar]
- 40. Calvert S, Shaw A. Perioperative acute kidney injury. Perioper Med (Lond). 2012;1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]


