Abstract
Background and purpose
Lithuania is one of the countries with the highest incidence of tick‐borne encephalitis (TBE) in Europe. The aim of this study was to describe the epidemiological patterns of TBE in Lithuania, and characterize clinical features in adults in the light of the high incidence in recent years.
Methods
Surveillance data available on the website of the Centre for Communicable Diseases and AIDS of Lithuania were used to describe the epidemiological patterns of TBE. The retrospective study included 712 patients hospitalized in the Centre for Infectious Diseases and the Centre for Neurology of Vilnius University in the years 2005–2014.
Results
Tick‐borne encephalitis incidence rates have been increasing by 8.5% per year for the 45‐year period from 1970 to 2014. The joinpoint model finds two joinpoints at 1991 and 1994, with a significant decrease of 8.4% per year (P < 0.05) prior to the joinpoint at 1991, and a rise of 195.2% afterwards. TBE presented with meningoencephalitis in 556 cases (81.3%). A total of 129 patients (18%) had a severe case of the disease. The most common neurological signs were ataxia (579, 81.3%), meningeal signs (474, 66.5%) and tremor (338, 47.5%). Limb paresis was observed in 6.3% of patients. Five patients (0.7%) died, and 544 patients (76.7%) were discharged with sequelae.
Conclusions
Intensified efforts in promoting TBE vaccination will be needed in the light of the high incidence and expanded spatial distribution. Significant prognostic factors for severe cases of the disease were age above 61 and delayed immune response of specific immunoglobulin G.
Keywords: clinical forms, epidemiology, prognostic factors, tick‐borne encephalitis
Introduction
Tick‐borne encephalitis (TBE) is the most important flavivirus infection of the nervous system. It is caused by the Far Eastern, Siberian and European subtype of tick‐borne encephalitis virus (TBEV). The European subtype comprises almost all known isolates from Europe. The virus persists in natural foci, where it circulates amongst both vertebrate hosts (mainly rodents) and the arthropod host (tick). In Ixodes ricinus in Europe, TBEV prevalence in unfed ticks varies between 0.1% and 5% 1. The European subtype is associated with milder disease, with mortality rates of 0.5%–2%, and severe neurological sequelae in up to 10% of patients 2. Treatment is based on symptomatic measures. However, TBE can be successfully prevented by a safe and highly effective vaccine 3.
Tick‐borne encephalitis is a growing public health concern over recent decades in Europe 1, 4. The number of human cases of TBE in all endemic regions of Europe has increased by almost 400% in the last 30 years 2. The risk areas have spread, and new foci have been discovered. Lithuania is one of the countries with the highest incidence of TBE in Europe. According to the European Centre for Disease Prevention and Control (ECDC) surveillance data, the notification rate in 2014 was highest in Lithuania (12.0 cases per 100 000 population) 4.
The aim of this study was to describe the epidemiological patterns of TBE in Lithuania, to characterize clinical features in adults in the light of the high incidence in recent years (2005–2014) and also to analyse the prognostic factors for severe cases of the disease.
Methods
Patients and study design
Surveillance data available on the website of the Centre for Communicable Diseases and AIDS of Lithuania (http://www.ulac.lt) were used to describe the epidemiological patterns of TBE in Lithuania. Population data for the calculation of rates were obtained from the Lithuanian Department of Statistics. The population as of 1 January of each year was used. Case rates are given per 100 000 population (the number of reported cases divided by the estimate of the population for that year multiplied by 100 000).
A retrospective study was done to describe the clinical features of TBE in adults. The study included 712 patients diagnosed with TBE and hospitalized in the Centre for Infectious Diseases and the Centre for Neurology of the Medical Faculty of Vilnius University in the years 2005–2014.
Cases were defined on the basis of laboratory results and documented clinical characteristics. The clinical criterion was a person with signs of central nervous system (CNS) involvement. The laboratory criteria were the presence of specific immunoglobulin M (IgM) and IgG in serum, or proven intrathecal synthesis of specific antibodies. The inclusion criterion was all patients with diagnosed TBE, age >18. The exclusion criteria were patients vaccinated against yellow fever or Japanese encephalitis, and patients infected with other flaviviruses. Confirmed cases were included for further analysis. EpiData (v.3.1.; The EpiData Association, Odense, Denmark) was used for data entry and data documentation.
Laboratory diagnosis
Tick‐borne encephalitis was laboratory confirmed by the demonstration of specific IgM and IgG activity by immunological tests with enzyme linked immunosorbent assay (ELISA) using a Virion/Serion (Wurzburg, Germany) kit. Some patients with TBE were diagnosed for possible Borrelia burgdorferi co‐infection. The ELISA kit was used for the demonstration of B. burgdorferi antibodies in sera. A diagnosis of neuroborreliosis was confirmed by the demonstration of specific IgM or IgG antibodies in cerebrospinal fluid (CSF). Virion/Serion kits were used for tick ‐ borne encephalitis virus and B. burgdorferi antibody detection from 2009.
Clinical classification
According to clinical presentation, all TBE cases were classified as meningitis, meningoencephalitis or meningoencephalomyelitis. Patients presenting signs of parenchymatous diseases of the brain such as focal neurological signs, seizures, decreased consciousness and delirium concomitant with CSF leucocytes >5 × 106/l were classified as having meningoencephalitis. Myelitic signs were defined as flaccid paresis of extremities. The clinical presentation of TBE was also classified as mild, moderate and severe. Severe disease was defined as disease with severe diffuse brain dysfunction, severe multifocal CNS symptoms and signs, and myelitic signs.
Statistical methods
Categorical data were analysed using the Pearson chi‐squared test. The Fisher exact test and the Wilcoxon rank sum test were used for continuous variables. P values of <0.05 were considered significant.
Multiple binary logistic regression was used to assess prognostic factors for severe cases of TBE. Predictor variables of gender, age, comorbidities, delayed hospitalization, cytosis, delayed immune response and the presence of the first stage were included in the model.
The GADM database of Global Administrative Areas was used for spatial analysis. Stata/IC 12.1 (StataCorp LP, Stata Statistical Software, College Station, TX, USA) software was used for analysis and to graph data onto maps.
A joinpoint regression model 5 was used to investigate the pattern of TBE incidence rates. The response variable for the analysis of incidence was the natural logarithm of the reported incidence rates, and an independent variable was the reporting year from 1970 to 2014.
The joinpoint regression program (v. 4.5.0.1.; Statistical Research and Applications Branch, National Cancer Institute, Bethesda, MD, USA) was used to analyse the data. A statistically significant joinpoint was P < 0.05.
The Vilnius Regional Bioethics Committee approved this study in 2014 (No. 158200‐14‐742‐259).
Results
General characteristics of TBE in Lithuania
Incidence trends
Tick‐borne encephalitis was first reported in Lithuania in 1968, but serology was introduced in 1970. According to the results of the joinpoint analysis, it was determined that TBE incidence rates have been increasing by 8.5% per year for the 45‐year period 1970–2014. The joinpoint model finds two joinpoints at 1991 and 1994, with a significant decrease of 8.4% per year (P < 0.05) prior to the joinpoint at 1991 and a rise of 195.2% after. There was no significant change in the trend during the high incidence period in 2005–2014. Incidence increased annually by 7.4% (P = 0.1) (Fig. 1).
Figure 1.
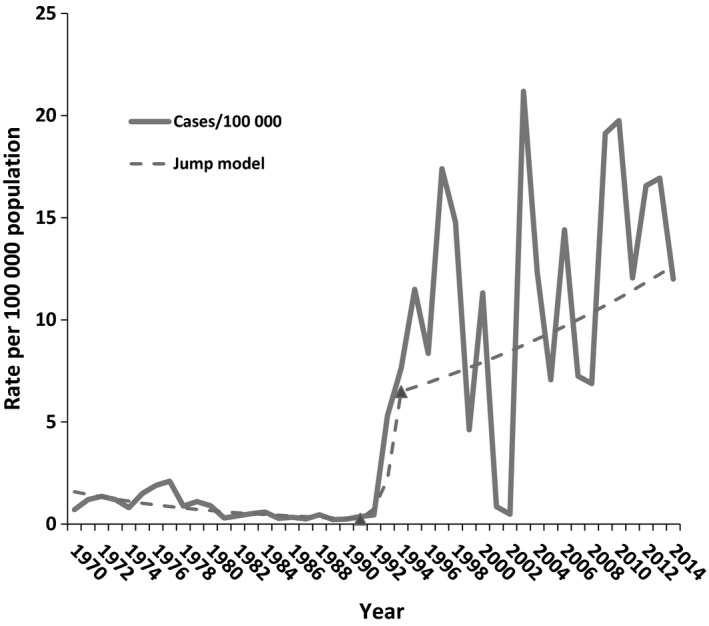
Annual incidence of tick‐borne encephalitis in Lithuania, 1970–2014 (n = 9370).
Spatial distribution
Tick‐borne encephalitis is present throughout Lithuania, but the northeast and central parts of the country are regarded as TBE foci. The incidence of TBE has significantly increased in five out of 10 counties in Lithuania in the last two decades.
Age and gender distribution
The highest notification rate of confirmed TBE was in the 45–54 years group (20.6 cases per 100 000 population), followed by 65–74‐ and 55–64‐year‐olds (19.7 and 19.2 cases per 100 000 population; Fig. 2). 53.3% of all cases were registered in the 45–74 age group during the period 2005–2014. During the period 2005–2014, 54.3% of cases were males, with an average notification rate of 15.5 per 100 000 population in males, and 11.2 per 100 000 population in females; the male‐to‐female ratio was 1.18:1.
Figure 2.
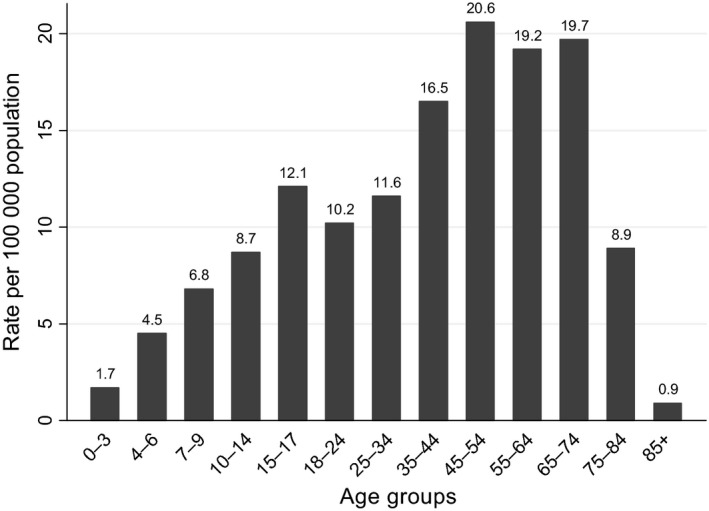
Rates of confirmed reported TBE cases by age, 2005–2014.
Seasonality
Tick‐borne encephalitis is recorded almost throughout the year in Lithuania, but the disease is characterized by clearly expressed seasonality. During 2005–2014, TBE numbers of reported cases started to increase in April–May, peaked in October, and decreased for the rest of the year, with only a small number of cases reported in December and January (Fig. 3). During the 5 months of seasonal increase (June–October), 89.6% cases were recorded with TBE out of all cases.
Figure 3.

Seasonal distribution of confirmed TBE cases in Lithuania, 2005–2014.
A retrospective study of TBE cases hospitalized in the Centre for Infectious Diseases and the Centre for Neurology of the Faculty of Medicine, Vilnius University
Epidemiological and demographic data
A total of 460 (64%) patients noticed one or more tick bites (Table 1), and 92 (13%) patients had more than one tick bite (2–10). The highest number of hospitalized patients was in the years 2012 and 2013.
Table 1.
Epidemiological, demographic data and the clinical course of tick‐borne encephalitis
| Study characteristic | Value, n (%) |
|---|---|
| Inhabitants of cities | 427 (60) |
| Receipt of tick bite/bites | 460 (64) |
| Milk‐borne infection | 49/625 (7.8) |
| Infected in living area | 223/528 (42.2) |
| Job‐related infection | 10/528 (1.9) |
| Incubation period (days) | 11.4 (2–41) |
| Biphasic course | 413 (58.1) |
| Duration of first stage, mean (min–max) (days) | 4.9 (1–19) |
| Interval between first and second stage, mean (min–max) (days) | 14.6 (3–100) |
| Patient's sex | |
| Male | 387 (54) |
| Age, median (min–max) (years) | 51 (18–85) |
| Clinical form of illness | |
| Meningitis | 66 (9.6) |
| Meningoencephalitis | 556 (81.3) |
| Meningoencephalomyelitis | 22 (3.2) |
| Encephalitis | 40 (5.8) |
| Symptoms and signs before hospitalization | |
| Fever | 709 (99.6) |
| Headache | 647 (90.9) |
| Vertigo | 288 (40.4) |
| Nausea/vomiting | 214 (30.1) |
| Muscle pain | 44 (6.2) |
| Diarrhoea | 10 (1.4) |
| Neurological symptoms and signs | 229 (32.2) |
A total of 295 (41%) had pre‐existing conditions, the most common being arterial hypertension (161, 22.6%), coronary heart disease (58, 8.1%) and diabetes mellitus (27, 3.8%). A total of 22 patients (3%) had chronic CNS diseases; two women were pregnant; two women were 3 weeks to 6 months after delivery.
Clinical presentation
The mean duration of the first phase was 4.9 days, according to available data on 322 patients (Table 1). The duration of the asymptomatic period was not related to disease severity (P < 0.05).
The mean time from onset of illness to hospitalization was 5.8 days. The median length of hospital stay was 11.9 days (minimum 1 day, maximum 129 days).
The most frequent clinical form was meningoencephalitis (556/684, 81.3%) (Table 1). A total of 16 patients presented peripheral nervous system involvement (poliradiculitis or polineuritis). Meningitis was more common in the group of young patients (18–30 years); myelitis was more common in older patients (P < 0.001). The majority of patients (489, 69%) had a moderate case of the disease. A total of 129 patients (18%) had a severe case of the disease. A total of 35/88 (39.8%) had severe disease in the age group above 70 years, versus 13/111 (11.7%) in the 18–29 years group (P < 0.01). The highest proportion of mild cases was in young patients aged between 18 and 29 years (P < 0.05). The majority of patients presented general symptoms and signs before hospital; only one‐third had neurological symptoms and signs (Table 1). Neurological symptoms and signs are presented in Table 2. Limb paresis appeared on average on the 10th day of the illness, range eighth to 15th day.
Table 2.
Clinical presentation of central nervous system involvement (n = 712)
| Symptoms and signs | n (%) | Duration of resolved symptoms, mean (max–min) (days) | Not resolved cases (%) |
|---|---|---|---|
| Meningeal signs | |||
| Overall | 474 (66.5) | 4.57 (<1–32) | 2/474 (0.4) |
| Neck stiffness | 442 (62.1) | 4.3 (<1–22) | 2/442 (0.5) |
| Kernig's sign | 331 (46.5) | 4.6 (<1–32) | 2/331 (0.6) |
| Ataxia | 579 (81.3) | 7.4 (<1–37) | 323/579 (55.3) |
| Tremor | 338 (47.5) | 6.2 (<1–42) | 163/338 (47.9) |
| Nystagmus | 219 (30.8) | 4.7 (<1–18) | 5/219 (2.3) |
| Epileptic seizures | 15 (2.1) | 3.25 (<1–15) | 0 |
| Parkinson's syndrome | 11 (1.5) | 7.2 (2–21) | 4/11 (36.4) |
| Pyramidal signs | 88 (8.1) | 5.3 (<1–16) | 2/88 (2.3) |
| Dysphagia | 14 (2) | 3.6 (<1–12) | 1/14 (7.1) |
| Altered consciousness | |||
| Quantitative (overall) | 171 (24) | 2.7 (<1–34) | 3/171 (1.7) |
| Somnolence | 151 (21.2) | 2.1 (<1–16) | 2/151 (1.3) |
| Sopor | 31 (4.4) | 2.5 (<1–8) | 1/31 (3.2) |
| Comatose | 11 (1.6) | 5.2 (1–17) | 3/11 (27.3) |
| Qualitative (overall) | 105 (14.8) | 3/105 (2.9) | |
| Agitation | 36 (5.1) | 1.7 (<1–10) | 1/36 (2.8) |
| Disorientation | 98 (13.8) | 3.3 (<1–40) | 3/98 (3) |
| Hallucinations | 7 (1) | 1 (<1–2) | 1/7 (14.3) |
| Cranial neuropathies (overall) | 159 (22.3) | ||
| Ocular motor paresis (III, IV, VI) | 52 (7.3) | 5.5 (<1–29) | 2/52 (3.8) |
| Facial weakness (VII) | 7 (1) | 4.5 (2–7) | 5/7 (71.4) |
| Hearing disturbance (VIII) | 16 (2.3) | 3 (3–3) | 14/16 (87.5) |
| Bulbar signs | 77 (10.8) | ||
| Dysphonia | 56 (7.9) | 8.1 (2–48) | 18/56 (32.1) |
| Dysarthria | 49 (6.9) | 8.2 (<1–48) | 13/49 (26.5) |
| Dysphagia | 12 (1.6) | 14 (2–48) | 7/12 (58.3) |
| Paresis of elevator muscles of head (XI) | 4 (0.6) | – | 4/4 (100) |
| Visual field defect (II) | 1 (0.1) | – | 1/1 |
| Anosmia (I) | 1 (0.1) | – | 1/1 |
| Masticatory muscle paresis (V) | 1 | – | 1/1 |
| Limb paresis (overall) | 45 (6.3) | – | 31/45 (68.9) |
| Spastic | 21 (3) | 6.5 (<1–20) | 7/21 (33.3) |
| Flaccid (overall) | 24 (3.4) | – | 24/24 (100) |
| Tetraparesis | 10 (1) | – | 10/10 (100) |
Coinfection with Lyme borreliosis was diagnosed in 13 patients (2%).
The results of logistic regression show that significant prognostic factors for a severe case of the disease are age above 61 [adjusted odds ratio (ORa) 2.10, 95% confidence interval 1.04–3.73] and delayed immune response of specific IgG (ORa 2.37, 95% confidence interval 1.05–5.39) (Table 3).
Table 3.
Prognostic factors for severe disease
| Variable | ORc | ORa | 95% CI | P |
|---|---|---|---|---|
| Sex | ||||
| Female | 1.0 | 1.0 | – | 0.27 |
| Male | 1.15 | 1.27 | 0.84–1.92 | |
| Age group (years) | ||||
| <40 | 1.0 | – | – | |
| 41–60 | 1.35 | 1.44 | 0.82–2.55 | 0.20 |
| 61+ | 2.24 | 2.10 | 1.04–3.73 | 0.036 |
| Comorbidities | ||||
| 0 | 1.0 | 1.0 | – | |
| 1 | 1.74 | 1.45 | 0.86–2.43 | 0.16 |
| 2 | 2.22 | 1.91 | 0.97–3.76 | 0.06 |
| 3 | 2.66 | 2.43 | 1.02–5.78 | 0.044 |
| 4+ | 3.15 | 2.05 | 0.72–5.84 | 0.18 |
| Delayed hospitalization | ||||
| 0–5 days | 1.0 | 1.0 | – | |
| 6+ | 0.93 | .71 | 0.45–1.12 | 0.14 |
| Cytosis | ||||
| <500 | 1.00 | 1.0 | 1.0 | |
| 500+ | 2.77 | 2.52 | 0.93–6.81 | 0.07 |
| Delayed immune response | ||||
| (IgM+, IgG−) | 3.27 | 2.37 | 1.05–5.39 | 0.04 |
N = 645. CI, confidence interval; ORc, crude odds ratio; ORa, adjusted odds ratio. Likelihood ratio χ 2 = 42.50, df 11, P < 0.0001. Hosmer–Lemeshow test χ2 = 5.99, df 8, P = 0.65. McFadden's R 2 = 0.068, model correctly classified 81.71%, area under the receiver operating curve 0.67.
Laboratory findings
Cerebrospinal fluid analyses were performed for 684/712 (96.1%) patients. Cytosis above 5 × 106 cells/l was found in 642 (93.8%) patients (mean 137.3, minimum 6, maximum 1381). The mean concentration of protein was 0.83 mg/l (minimum 0.13, maximum 6.23). The mean glucose/blood ratio was 0.57 mmol/l (minimum 0.07, maximum 1.25).
The rate of seroprevalence of B. burgdorferi IgM was 18.5% in 271 patients without any symptoms and signs of borreliosis; B. burgdorferi IgG antibodies were detected in 60 (22.5%) of 267 examined patients.
Computer tomography (CT) abnormalities were found in 16/148 (10.8%) patients. Brain magnetic resonance imaging (MRI) abnormalities were found in 10/63 (15.9%) examined patients. Hyperintensities of white matter and cortex (thalamus, basal ganglia, cerebellum, mesencephalon and pontine) were revealed in T2 sequence (Fig. S1). Spinal CT and MRI did not present any specific findings.
Electroencephalography was done for 13 patients. Abnormalities were found in 10 patients. Non‐specific findings were found.
Complications and outcome
The complication rate was 25/712 (3.5%). The most common complication was ventilation associated pneumonia (7, 1%). Other complications included sepsis caused by Escherichia coli, Gemella morbillorum cases, septic endocarditis caused by Staphylococcus aureus (one case), urinary tract infection (three cases), exacerbation of coronary heart disease (two cases), atrial fibrillation (two cases), gastrointestinal bleeding (one case), methroragia (one case), epistaxis (one case), thrombosis sinus sagittalis (one case).
A total of 11 (1.5%) patients needed mechanical ventilation, which lasted on average 7.4 days. Respiratory failure due to myelitis was presented on discharge for four patients.
A total of 5/712 (0.7%) patients died. A total of 544 patients (76.7%) were discharged with sequelae. The most common persisting general symptoms on discharge were general malaise (333, 47%) and vertigo (232, 32.7%). The most frequent neurological signs were ataxia (320, 45%) and tremor (162, 22.8%). Cognitive disorders were observed in 30 (4.2%) cases. A total of 288 (40.6%) patients needed rehabilitation.
Discussion
Tick‐borne diseases are the most common vector‐borne diseases in Europe, with their infection rate and geographical distribution increasing in the 1980s 6. The apparent increase in the incidence of TBE has been attributed to global warming and various socioeconomic factors 7. The highest incidence (>10 cases per 100 000 population) is registered in the Baltic countries, the Czech Republic, Russia and Slovenia 2. Important changes in TBE were observed in Lithuania in 1991 and 1994, followed by high incidence rates and some fluctuations every year. A complex of factors influenced changes in the TBE situation in Lithuania. The abundance of ticks, climatic and social factors, better laboratory diagnostics and the possible influence of a transitional period were analysed and had an impact on the situation 8.
The observed changes in spatial distribution during the period 2005–2014 showed a significant increase of TBE in areas with previously low incidence in eastern and eastern northern parts of Lithuania. The rise of new foci or the spread of old ones could be reasons for this geographical spread, but a more detailed analysis is needed.
The age and gender distribution of TBE was consistent with the situation in other EU countries 9.
The seasonal distribution in Lithuania is specific and different from the EU. An increase in cases in Lithuania started in April, as in other EU countries. However, a peak was observed in October in Lithuania during 2005–2014, whilst the EU reported a peak in July and a slow decrease for the rest of the year in 2012–2014 9.
The highest proportion of patients with meningoencephalitis (81%) was found compared with previous studies 10, 11. Surprisingly, the rate of patients with meningitis was significantly lower in our study 11, 12. One explanation could be that meningitis forms are undiagnosed, especially if patients present only general symptoms and signs. Another explanation could be related to virus virulence. In Lithuania, the western subtype, which causes milder diseases than the other two subtypes, is prevalent. Research into ticks and TBEV is needed in order to investigate possible changes in TBEV virulence, new foci and new subtypes.
The most common neurological signs were ataxia and tremor, as in previous studies, but the proportions of these signs were higher in our study 10, 11, 13.
Monophasic disease and increasing age were found to correlate with the severity of the illness. These findings were also proved by other authors 10, 12. Contrary to another study 12, it was found that a CSF leukocyte count above 500 is a predictor for severe illness.
In the study by Czupryna et al. 14, MRI abnormalities are present in only approximately 18% of patients. They are non‐specific, and most commonly located in the thalamus, cerebellum, brainstem and in the basal ganglia. Abnormalities in the same localization were found in 16% of examined patients in our study.
Factors such as prolonged hospitalization, long‐lasting rehabilitation and loss of working ability are associated with significant social economic loss. The incidence of TBE is very high in Lithuania, but vaccination coverage is still low, especially in the at‐risk group. Vaccination would significantly reduce the negative socioeconomic impact of TBE. TBE vaccines in general have been proved to be highly immunogenic and safe 3. TBE needs to become an important issue in travel medicine 3, 15.
Conclusions
The incidence of TBE has fluctuated considerably from year to year and has significantly increased since 1992. During the high incidence period of the last two decades, there was no significant change in the incidence trend, but changes in spatial distribution were observed. Intensified efforts in promoting TBE vaccination will be needed in the light of the high incidence and expanded spatial distribution. The most common symptoms and signs of TBE were fever, headache and cerebellar signs. More patients presented only general symptoms and signs than neurological in the first week of the illness. One‐fifth of patients had a life‐threatening severe form of illness. Significant prognostic factors for severe cases of the disease were age above 61 and delayed immune response of specific IgG.
Disclosure of conflicts of interest
The authors declare no financial or other conflicts of interest.
Supporting information
Figure S1. Brain MRI of patient with tick‐borne encephalitis (TBE). Hyperintensities: (a) in cerebellum; (b) in the basal ganglia and thalamus; (c) in subcortical white matter.
References
- 1. Suss J. Tick‐borne encephalitis 2010: epidemiology, risk areas, and virus strains in Europe and Asia – an overview. Ticks Tick Borne Dis 2011; 2: 2–15. [DOI] [PubMed] [Google Scholar]
- 2. European Centre for Disease Prevention and Control . Annual epidemiological report 2014 – emerging and vector‐borne diseases. Stockholm: ECDC, 2014. [Google Scholar]
- 3. Kunze U. Tick‐borne encephalitis: still on the map. Report of the 18th annual meeting of the International Scientific Working Group on Tick‐Borne Encephalitis (ISW‐TBE). Ticks Tick Borne Dis 2016; 7: 911–914. [DOI] [PubMed] [Google Scholar]
- 4. European Centre for Disease Prevention and Control . Annual epidemiological report 2016 – tick‐borne encephalitis. Stockholm: ECDC; https://ecdc.europa.eu/en/tick-bornencephalitis/surveillance-and-disease-data/annual-epidemiological-report (accessed 17/02/17). [Google Scholar]
- 5. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000; 19: 335–351. [DOI] [PubMed] [Google Scholar]
- 6. Amato‐Gauci AJ, Zeller H. Tick‐borne encephalitis joins the diseases under surveillance in the European Union. Euro Surveill 2012; 17: 20299 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20299. [PubMed] [Google Scholar]
- 7. Dorko E, Rimarova K, Kizek P, Stebnicky M, Zakutna L. Increasing incidence of tick‐borne encephalitis and its importance in the Slovak Republic. Cent Eur J Public Health 2014; 22: 277–281. [DOI] [PubMed] [Google Scholar]
- 8. Sumilo D, Asokliene L, Bormane A, Vasilenko V, Golovljova I, Randolph SE. Climate change cannot explain the upsurge of tick‐borne encephalitis in the Baltics. PLoS One 2007; 2: e500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. European Centre for Disease Prevention and Control . Epidemiological situation of tick‐borne encephalitis in the European Union and European Free Trade Association countries. Stockholm: ECDC, 2012. [Google Scholar]
- 10. Czupryna P, Moniuszko A, Pancewicz SA, Grygorczuk S, Kondrusik M, Zajkowska J. Tick‐borne encephalitis in Poland in the years 1993–2008 – epidemiology and clinical presentation. A retrospective study of 687 patients. Eur J Neurol 2011; 18: 673–679. [DOI] [PubMed] [Google Scholar]
- 11. Karelis G, Bormane A, Logina I, et al Tick‐borne encephalitis in Latvia 1973–2009: epidemiology, clinical features and sequelae. Eur J Neurol 2012; 19: 62–68. 12 [DOI] [PubMed] [Google Scholar]
- 12. Mickiene A, Laiskonis A, Guenter G, Vene S, Lundkvist A, Lindquist L. Tick‐borne encephalitis in an area of high endemicity in Lithuania: disease severity and long‐term prognosis. Clin Infect Dis 2002; 35: 650–658. [DOI] [PubMed] [Google Scholar]
- 13. Zielicka‐Hardy A, Rosinska M, Kondrusk M, Hlebowicz M, Konior R, Stefanoff P. Predictors for diagnosis of tick‐borne encephalitis infection in Poland, 2009–2010. Scand J Infect Dis 2015; 47: 604–610. [DOI] [PubMed] [Google Scholar]
- 14. Czupryna P, Tarasow E, Moniuszko‐Malinowska A, et al MRI and planameric CT follow‐up study of patients with severe tick‐borne encephalitis. Scand J Infect Dis 2015; 48: 74–81. [DOI] [PubMed] [Google Scholar]
- 15. Haditsch M, Kunze U. Tick‐ borne encephalitis: a disease neglected by travel medicine. Travel Med Infect Dis 2013; 11: 295–300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Brain MRI of patient with tick‐borne encephalitis (TBE). Hyperintensities: (a) in cerebellum; (b) in the basal ganglia and thalamus; (c) in subcortical white matter.


