Abstract
Under endoplasmic reticulum (ER)‐stress conditions, the unfolded protein response (UPR) generates a defense mechanism in mammalian cells. The regulation of UPR signaling is important in oocyte maturation, embryo development, and female reproduction of pigs. Recent studies have shown that melatonin plays an important role as an antioxidant to improve pig oocyte maturation. However, there is no report on the role of melatonin in the regulation of UPR signaling and ER‐stress during in vitro maturation (IVM) of porcine oocytes. Therefore, the objective of this study was to investigate the antioxidative effects of melatonin on porcine oocyte maturation through the regulation of ER‐stress and UPR signaling. We investigated the changes in the mRNA/protein expression levels of three UPR signal genes (Bip/Grp78, ATF4, P90/50ATF6, sXbp1, and CHOP) on oocytes, cumulus cells, and cumulus‐oocyte complexes (COCs) during IVM (metaphase I; 22 hours and metaphase II; 44 hours) by Western blot and reverse transcription‐polymerase chain reaction analysis. Treatment with the ER‐stress inducer, tunicamycin (Tm), significantly increased expression of UPR markers. Additionally, cumulus cell expansion and meiotic maturation of oocytes were reduced in COCs of Tm‐treated groups (1, 5, and 10 μg/mL). We confirmed the reducing effects of melatonin (0.1 μmol/L) on ER‐stress after pretreatment with Tm (5 μg/mL; 22 hours) in maturing COCs. Addition of melatonin (0.1 μmol/L) to Tm‐pretreated COCs recovered meiotic maturation rates and expression of most UPR markers. In conclusion, we confirmed a role for melatonin in the modulation of UPR signal pathways and reducing ER‐stress during IVM of porcine oocytes.
Keywords: endoplasmic reticulum‐stress, melatonin, oocyte maturation, pig, unfolding protein response
1. INTRODUCTION
The endoplasmic reticulum (ER) is a key modulator that regulates protein folding, calcium homeostasis, and secretory proteins.1 The accumulation of unfolded or misfolded proteins in the ER lumen disrupts ER functions and induces ER‐stress.2 To reduce ER‐stress, the unfolded protein response (UPR) signal pathways are activated. UPR signals are a cellular defense network of pathways that counteract ER‐stress.3 In addition, the defense function of the UPR signaling pathways is critical for cell survival by minimizing stress caused by hormone or cytokine production of secretory cells as well as cell differentiation and proliferation.4 When ER‐stress occurs, three transmembrane proteins (protein kinase‐like ER kinase; PERK, activating transcription factor 6; ATF6, and inositol‐requiring enzyme 1; IRE1) are separated from Bip/Grp78 in response to the unfolded/misfolded proteins.5 Once activated, PERK phosphorylates eukaryotic initiation factor 2α (eIF‐2α), which leads to an attenuation of translational initiation and blocks new proteins into the ER. To induce transcriptional activation of the P50ATF6 gene, P90ATF6 should be cleaved by site‐1 protease (S1P) and site‐2 protease (S2P) in the Golgi apparatus. Phosphorylation of IRE1 inhibits protein synthesis and induced the splicing x‐box‐binding protein 1 (sXbp1) transcription factors for chaperone functions.6
Although UPR signaling pathway activity regulates the reduction in ER‐stress, an excessive ER‐stress response induces cell death through ER‐stress‐mediated apoptosis. Severe ER‐stress induces the activation of CCAAT‐enhancer‐binding protein homologous protein (CHOP), Jun N‐terminal kinase (JNK), and cleaved caspase 3.7 Regulation of UPR signals and ER‐stress is involved in the female reproductive system through the maintenance of cellular homeostasis and the initiation of apoptosis.7 The ER‐stress response is a key mechanism mediating the developmental potential of oocytes through impaired embryo development and protein secretion in mouse cumulus‐oocyte complexes (COCs).8 Notably, a previous study described that expression of the UPR marker XBP1 may assist porcine oocyte maturation, embryonic genome activation, and early embryonic development in vitro.9 However, there have been no reports on the changes of expression patterns in the three UPR pathways during in vitro maturation (IVM) of porcine oocytes.
Melatonin (N‐Acetyl‐5‐methoxytryptamine) hormone is a synthetic product of the vertebrate pineal gland. Some investigators showed that melatonin is well known for antioxidant properties involved in scavenging of reactive oxygen species (ROS).10, 11 Melatonin also plays an important role in improving female reproductive functions through IVM, in vitro fertilization, and embryo development.12, 13 In addition, melatonin's antioxidant capacity reduces oxidative stress damage ability to directly scavenge ROS in in vitro embryo production of humans.14 Supplementation with melatonin increased oocyte maturation in cattle,15 and it promoted the developmental potential as well as nuclear and cytoplasmic maturation during IVM of porcine oocytes.16, 17 Recently, it has been investigated whether melatonin inhibits the ER‐stress response in mice fibrosis18 and rat neuronal cells.19 Even though many studies have reported the valuable effects of melatonin on the improvement of oocyte maturation and embryo development rate in porcines, the detailed mechanisms related to ER‐stress remain unknown. Therefore, in this study, we investigated the relationship between ER‐stress production from oxidative stress and oocyte maturation rates and quality whether the melatonin as an antioxidant improves oocyte quality. We hypothesized that melatonin has an effect on the reduction in ER‐stress via regulation of three UPR signal genes during IVM. The objective of this study is to confirm the role of melatonin in regulation of UPR signaling or reducing ER‐stress on porcine oocyte maturation and cumulus cells in maturing COCs.
2. MATERIALS AND METHODS
2.1. Chemicals
Unless otherwise indicated, all chemicals in this study were purchased from Sigma‐Aldrich Korea (St. Louis, MO, USA).
2.2. IVM of oocyte
Porcine ovaries were obtained from a local slaughterhouse and transported to the laboratory at 30‐35°C in 0.9% saline (w/v) supplemented with 75 μg/mL potassium penicillin G. Immature COCs were aspirated with an 18‐gauge needle attached to a 10 mL disposable syringe from antral follicles that were 3‐6 mm in diameter. After washing three times with TL‐HEPES medium,20 approximately 50 COCs were matured in 500 mL of IVM medium in a four‐well multidish (Nunc, Roskilde, Denmark) at 38.5°C in 5% CO2 incubator. NCSU‐23 medium supplemented with 10% follicular fluid, 10 IU/mL pregnant mare serum gonadotropin (PMSG), 10 IU/mL human chorionic gonadotropin (hCG), 0.57 mmol/L cysteine, 10 ng/mL epidermal growth factor, and 10 ng/mL β‐mercaptoethanol was used for 22 hours of maturation. After culturing for 22 hours, the same media was used for oocyte maturation without PMSG/hCG for an additional 22 hours.
2.3. Cumulus cell expansion and acetic‐orcein assessment
The degrees of cumulus cell expansion were divided into not expanded, partially expanded, or fully expanded as described by Marei et al.21 After 44 hours of IVM, meiotic maturations were distinguished by nuclear stages. Oocytes denuded by pipetting in TL‐HEPES medium containing 0.1% hyaluronidase were de‐washed in PVA‐PBS and mounted on microscope slides. The samples were fixed for 3 days in acetic acid/ethanol (1:3, v/v) and stained with 1% acetic‐orcein (w/v) for 5 min. The meiotic stage of the samples was evaluated under a microscope (Leica, Solms, Germany).
2.4. Reverse transcription‐PCR
Total RNA was extracted from porcine COCs, denuded oocyte (DO), and cumulus cells (CC) using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Extracted RNA was quantified using a NanoDrop spectrophotometer (ACTgene, Piscataway, NJ, USA). Each cDNA was synthesized from the aliquots (1μg/μL) of total RNA with AccuPower® reverse transcription‐polymerase chain reaction (RT‐PCR) Premix (Bioneer, Daejeon, Korea). PCR was carried out using AccuPower® PCR Premix (Bioneer) containing specific primers (Table 1). Specific primer sequences (Table 1) were designed using the NCBI database.
Table 1.
Primer sequences of porcine UPR marker gene factors for RT‐PCR
| Target | Accession numbers | Primer | Sequence reported 5′‐3′ | T m, °C | Length (bp) |
|---|---|---|---|---|---|
| Bip/Grp78 | XM001927795.5 | Forward: 5′ | GGTGGGCAAACAAAGACATT | 54 | 383 |
| Reverse: 3′ | CGCTGGTCAAAGTCTTCTCC | ||||
| Atf4 | 100144302 | Forward: 5′ | ACTTGATGTCCCCCTTCGAC | 55 | 410 |
| Reverse: 3′ | GGCAACCTGGTCAGTTGTTG | ||||
| sXbp1 | NM001271738.1 | Forward: 5′ | GGCAGAGACCAAGGGGAATG | 58 | 237 |
| Reverse: 3′ | GGGTCGACTTCTGGGAGCTG | ||||
| uXbp1 | NM001142836.1 | Forward: 5′ | GGCAGAGACCAAGGGGAATG | 58 | 263 |
| Reverse: 3′ | TGGAGAAAGCACCTTCCAAAA | ||||
| Chop | 100240743 | Forward: 5′ | AGGCCTGGTATGAGGACCTG | 55 | 339 |
| Reverse: 3′ | GCTGTGCCACTTTCCTTTCA | ||||
| Gapdh | KJ786424.1 | Forward: 5′ | GAAGGTCGGAGTGAACGGAT | 55 | 527 |
| Reverse: 3′ | CATGGACCGTGGTCATGAGT |
Bip/Grp78, binding immunoglobulin protein/78 kDa glucose‐regulated protein; Atf4, activating transcription factor 4; sXbp1, splicing X‐box‐binding protein 1; usXbp1, unsplicing X‐box‐binding protein 1; Chop, CCAAT‐enhancer‐binding protein homologous protein; Gapdh, glyceraldehyde 3‐phosphate dehydrogenase; T m, melting temperature; UPR, unfolded protein response; RT‐PCR, reverse transcription‐polymerase chain reaction.
2.5. Protein extraction and Western blotting
Maturated COC (25 per sample) lysates were extracted in PRO‐PREP protein lysis buffer (iNtRON, Seongnam‐si, Korea). The COC lysates were separated by polyacrylamide gel by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS‐PAGE) in 12% gels. After electrophoresis, the separated proteins were transferred onto nitrocellulose membranes (Pall Life Sciences, Port Washington, NY, USA). The membrane was blocked by incubation with 5% skim milk for 2 hours at room temperature. After blocking, the membranes were incubated with anti‐Bip/Grp78 (1:2000, Catalog number SC‐1050; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti‐ATF4 (1:5000, Catalog number SC‐200; Santa Cruz), anti‐P90/50 ATF6 (1:4000, Catalog number NBP1‐40256; Novus Biologicals, Littleton, CO, USA), anti‐CHOP (1:500, Catalog number SC‐793; Santa Cruz), and anti‐β‐Actin (1:3000, Catalog number SC‐47778; Santa Cruz) antibodies. Membranes were washed three times with TBST buffer. The membranes were then incubated with a secondary HRP‐conjugated anti‐mouse/rabbit IgG (Catalog number 31439/31463; Thermo, Rockford, IL, USA) and an anti‐goat IgG (Catalog number LF‐SA5004; Abfrontier, Seoul, Korea) secondary antibody for 2 hours at room temperature. And then, membranes were washed three times with TBST buffer. Antibody binding was detected using an ECL kit (Advansta, Menlo Park, CA, USA). Band intensities were quantified with Image J software (NIH, MD, USA).
2.6. H&E staining and immunohistochemistry
Pig ovaries were fixed with 10% neutral buffered formalin (Sigma‐Aldrich), embedded in paraffin, and processed into sections 3‐5 μm thick. The sections were then stained with H&E using a procedure described previously.22 For the immunohistochemistry (IHC) assessment, ovaries were fixed in formalin, embedded in paraffin, and cut into sections 1‐3 μm thick. The sections were then deparaffinized and briefly heated before being treated with a protein block solution (Dako, Carpinteria, CA, USA) and incubated with the antibody anti‐P50ATF6 (Abcam, Cambridge, MA, USA). After being washed with 0.1 mol/L TBS containing 0.01% Tween‐20, the sections were incubated with anti‐rabbit polymer (Dako). Peroxidases bound to the antibody complex were visualized by treatment with a 3,3′‐diaminobenzidine (DAB) chromogen substrate solution (Dako). The DAB reaction was monitored under a microscope to determine the optimal incubation time and was stopped by washing several times with 0.1 mol/L TBS. The IHC‐labeled sections were dehydrated in a graded ethanol series, defatted in xylene, and mounted. The sections were examined using a microscope (Leica) under bright field conditions at 200× and 400× magnifications.
2.7. Statistical analysis
All percentage data and data sets were subjected to arcsine transformation and expressed as the mean ± standard deviation. All values of Western blot experiments were presented as the mean ± standard error of the mean. The results were analyzed using a one‐way ANOVA followed by Bonferroni's multiple comparison test and t tests. Histogram values of densitometry analysis were obtained using ImageJ (NIH, Bethesda, MD, USA). All data performed using the GraphPad Prism 5.0 software package (San Diego, CA, USA). Differences were considered significant at *P < .05, **P < .01, and ***P < .001.
3. RESULTS
3.1. Changes in the expression pattern of UPR markers were observed in COCs, DO, and CC during porcine oocytes maturation (22 and 44 hours)
Generally, the mRNA expression level of Bip/Grp78 was significantly higher in COCs at 44 hours compared to 22 hours. However, there was no change in DOs and CC (Figure 1A). Moreover, the protein levels of UPR markers (Bip/Grp78, ATF4, and P50ATF6) significantly increased in COCs at 44 hours (P < .01 compared to COCs at 22 hours). As shown in Figure 1B, the expression patterns of P90ATF6 and P50ATF6 were completely opposite in COCs during M II. The expression of CHOP, an ER‐stress‐mediated apoptotic protein, rapidly increased in COCs of M II compared with M I (Figure 1C). In addition, we observed the direct expression of P50ATF6 in porcine oocytes from ovary follicles using IHC analysis. We separated oocytes into three ovary follicle sizes (1‐2 mm, small; 3‐4 mm, middle; and 5‐6 mm, large) based on the diameter of the follicles. P50ATF6 protein was highly expressed in the oocytes of large follicles. These results indicate that P50ATF6 expression is significantly increased in fully formed COC structures at M II (44 hours). These results demonstrated that most UPR signaling markers increased in COCs during IVM progression. Based on these results, we determined that the porcine COCs at 44 hours would be used for the subsequent experiments.
Figure 1.
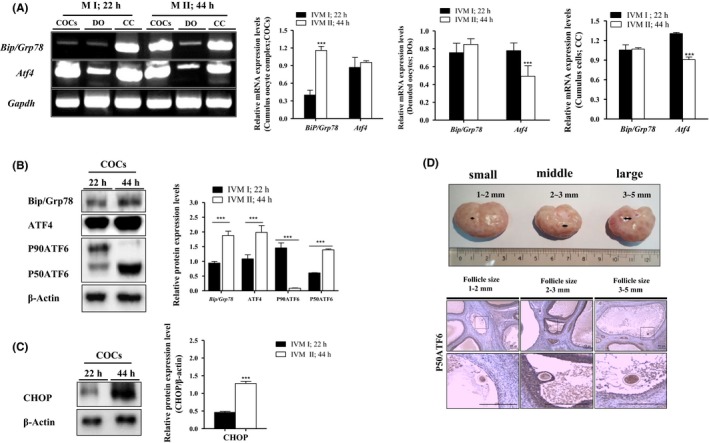
Expression patterns of unfolded protein response (UPR) signal genes in cumulus‐oocyte complexes (COCs), denuded oocyte (DO), and cumulus cells (CC) on porcine oocytes in vitro maturation (IVM) (22 and 44 h). A, The mRNA levels of activated UPR signal transcription factors (Bip/Grp78 and Atf4) on maturing COCs, DO, and CC of porcine IVM process (metaphase, M I; 22 h and metaphase, M II; 44 h) were measured by reverse transcription‐polymerase chain reaction (PCR) (RT‐PCR) analysis. Relative folds of Bip/Grp78 and Atf4 were obtained by normalizing the signals for Gapdh. B‐C, Western blotting results of Bip/Grp78, ATF4, P50ATF6, and CHOP in DO, COCs, and CC were compared at the M I (22 h) and M II (44 h) stages of pig oocyte maturation. Relative folds of UPR marker protein levels were obtained by normalizing the signals for β‐Actin. D, Immunohistochemistry (IHC) staining of P50ATF6 in pig ovaries with different follicle sizes (1‐2 mm, small; 3‐4 mm, middle; and 5‐6 mm, large). IHC staining for P50ATF6 is detected using specific P50ATF6 antibody in in vivo‐maturing oocytes of pig ovary follicles. O = immature oocyte, Scale bar = 200 μm. Histograms represent values of densitometry analysis obtained using ImageJ software. Data in the bar graph are means ± SEM/SD of three independent experiments (per 50 DOs and 30 COCs). ***P < .001
3.2. Effects of ER‐stress on meiotic maturation and cumulus cell expansion of porcine COCs during IVM
To confirm the effects of ER‐stress on porcine IVM, we used the porcine IVM medium supplemented with various concentrations of ER‐stress inducer (Tm 1, 5, and 10μg/mL; Figure 2A). We investigated the meiotic maturation and cumulus cell expansion of COCs by acetic‐orcein staining and partially expanded standard of CC by microscope (Leica), respectively (Figure 2B and C). As expected, the cumulus cell expansion was reduced (P < .05) in COCs of Tm 5 μg/mL‐treated groups compared with control and other treated group (Figure 2B). Under the induction of ER‐stress during porcine oocyte maturation, meiotic maturation significantly decreased (P < .01, Tm 1 μg/mL: 60.1 ± 1.3%; P < .001, 5 μg/mL: 46.5 ± 2.1%, 10 μg/mL: 38.9 ± 5.1% vs control: 76.6 ± 1.9%) in Tm‐treated COCs after 44 hours. Additionally, we confirmed that Tm‐induced ER‐stress through the UPR marker protein/mRNA levels using Western blotting and RT‐PCR analysis. Our results show that mRNA expression of Bip/Grp78, Atf4, and sXbp1 in the Tm 5 μg/mL‐treated COCs was significantly higher (P < .01) than those from the COCs of control or the Tm 1 μg/mL group, and mRNA expression of Chop in the Tm‐treated (1 and 5 μg/mL) COCs was significantly higher (P < .01) than in the control group. In contrast, mRNA expression of uXbp1 in the Tm‐treated (1 and 5 μg/mL) groups was significantly decreased (P < .01) than in the control group. Likewise, the protein levels of Bip/Grp78, ATF4, and P50ATF6 were increased (P < .01) in the COCs after Tm‐treatment (1 and 5 μg/mL). But, no significant differences in protein levels of ATF4 in COCs between control and Tm 5 μg/mL‐treated group were detected. In contrast, the protein levels of P90ATF6 in the Tm‐treated (1 and 5 μg/mL) groups were significantly decreased (P < .01) than in the control group. These results showed that induction of ER‐stress by Tm increased expression of most UPR marker genes and reduced the nuclear maturation and CC expansion of porcine COCs during IVM.
Figure 2.
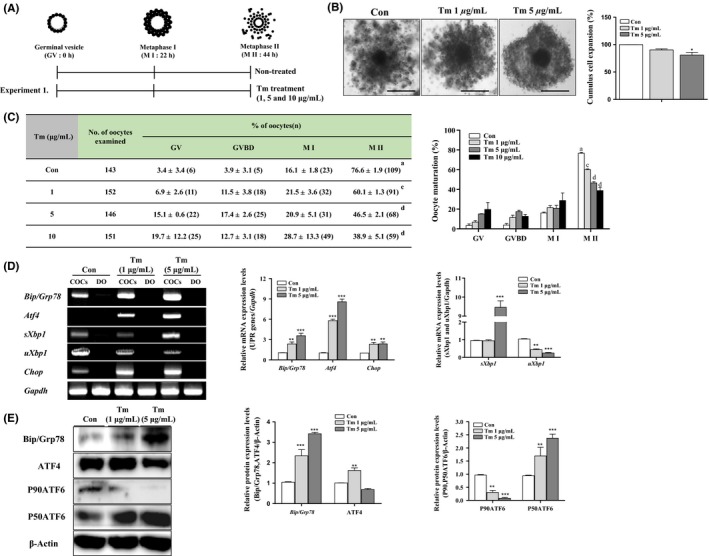
Changes in expression of unfolded protein response (UPR) markers and meiotic maturation rate during in vitro maturation (IVM) of pig oocytes treated with tunicamycin (Tm), endoplasmic reticulum (ER)‐stress inducer. A, Graphical description of Tm, ER‐stress inducer treatment condition in IVM medium. B, Measurement of cumulus cell expansion in cumulus‐oocyte complexes (COCs) after Tm‐treatment (1 and 5 μg/mL). C, Nuclei were classified as germinal vesicle (GV), germinal vesicle breakdown (GVBD), M I, and M II stage. Left panel; percentage oocyte maturation with varying Tm (1 and 5 μg/mL), Right panel; summary of meiotic maturation after IVM II (44 h). Data are means ± SD. Different superscript letters denote a significant difference (cP < 0.01 and dP < 0.001). D, The mRNA levels of activated UPR signal genes (Bip/Grp78, Atf4,sXbp1, and Chop) on Tm‐treated maturing COCs of porcine IVM at the M II (44 h) were measured by reverse transcription‐polymerase chain reaction (RT‐PCR) analysis. Relative folds of the gene mRNA levels were obtained by normalizing the signals for Gapdh. E, Protein expression levels of UPR signal proteins (Bip/Grp78, ATF4 P90ATF6, and P50ATF6) in Tm‐treated maturing COCs were measured by western blotting. Relative folds of these genes were obtained by normalizing the signals to those of β‐Actin. The histogram represents densitometry results that were obtained via ImageJ software. Data in the bar graph represent means ± SEM of three independent experiments (20‐30 COCs per group). *P < .05; ** P < .01; ***P < .001; for analysis of these data, Dunnett's multiple comparison test was applied with comparison to control
3.3. Effects of melatonin on reducing ER‐stress of porcine COCs during IVM
To observe the effects of melatonin‐reducing ER‐stress during IVM of porcine oocytes, we investigated the meiotic maturation and expansion of CC in maturing COCs with the ER‐stress inhibitor tauroursodeoxycholic acid (TUDCA) or melatonin after pretreatment with Tm (5 μg/mL) for M I (0‐22 hours, Figure 3A). As shown in Figure 3B, the percentage of cumulus cell expansion recovered in melatonin‐treated COCs at the M II as compared to COCs of control and TUDCA‐treated groups (Figure 3B). Additionally, nuclear maturation rates were recovered using melatonin (77.2 ± 8.8%) or TUDCA (73.3 ± 0.7%) treatment compared to the proportion of oocyte maturation in control groups (73.0 ± 0.8%). Interestingly, the meiotic maturation rate in melatonin‐treated COCs of M II was higher than that of other treated groups. In sequence, we investigated the changes in the mRNA/protein levels of UPR markers expression on TUDCA or melatonin‐treated COCs of M II (0‐44 hours) after pretreatment with Tm (5 μg/mL) for M I (0‐22 hours). In melatonin supplementary conditions of porcine IVM, the mRNA expression of most of UPR markers (Bip/Grp78, ATF4, sXbp1, and Chop) did not significantly differ in maturing COCs of TUDCA and melatonin‐treatment groups (Figure 3C). As shown in Figure 3D, the protein levels of Bip/Grp78 did not change, while the expression of other UPR markers proteins was reduced (ATF4; P < .05 and P50ATF6; P < .001 vs control group) by the melatonin treatment during M II of porcine oocytes after Tm stimulation. In addition, protein levels of the CHOP gene, an ER‐stress‐mediated apoptosis factor, significantly decreased (P < .001) in melatonin‐treated COCs at 44 hours. These results suggest that melatonin treatment promotes the meiotic maturation and cumulus cell expansion in maturing porcine COCs during IVM, specifically reducing the ER‐stress through the recovered expression of increased UPR marker genes after ER‐stress inducer treatment.
Figure 3.
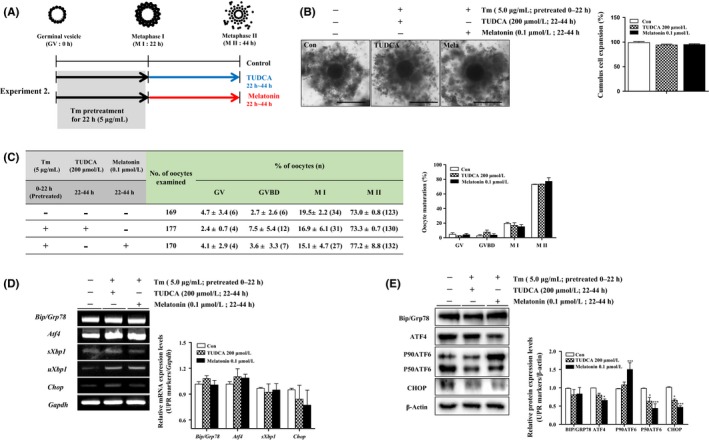
Effects of melatonin‐reducing endoplasmic reticulum (ER)‐stress on porcine cumulus‐oocyte complexes (COCs). A, Graphical description of melatonin (0.1 μmol/L) and tauroursodeoxycholic acid (TUDCA) (ER‐stress inhibitor; 200 μmol/L)‐treatment conditions in in vitro maturation (IVM) medium. B, Measurement of cumulus cell expansion in COCs according to the melatonin and TUDCA‐treatment after tunicamycin (Tm) pretreatment. C, Left panel; percentage oocyte maturation with varying melatonin and TUDCA, Right panel; summary of meiotic maturation after IVM II (44 h). Data are means ± SD. Different superscript letters denote a significant difference (P < .05). D, The mRNA levels of activated unfolded protein response (UPR) signal genes (Bip/Grp78, Atf4,sXbp1, and Chop) on maturing COCs after melatonin and TUDCA‐treatment were measured by reverse transcription‐polymerase chain reaction (RT‐PCR) analysis. Relative folds of the gene mRNA levels were obtained by normalizing the signals for Gapdh. E, Protein expression levels of UPR signal proteins (Bip/Grp78, ATF4 P90ATF6, and P50ATF6) in melatonin and TUDCA‐treated maturing COCs were measured by western blotting. Relative folds of these genes were obtained by normalizing the signals for β‐Actin. The histogram presents densitometry results that were obtained via ImageJ software. Data in the bar graph represent means ± SEM of three independent experiments (20‐30 COCs per group). *P < .05; **P < .01; ***P < .001; for analysis of these data, Dunnett's multiple comparison test was applied in comparison with control
4. DISCUSSION
The present study demonstrated the changes that occur in UPR marker proteins/gene expression pattern in porcine oocytes during DO, CC, and COCs of IVM progression (22 and 44 hours). As a result, ER‐stress generally occurs and induces the increase in UPR signaling genes (Bip/Grp78, ATF4, P50ATF6, and CHOP) under CC and COCs in IVM of porcine. In addition, we first investigated the role of melatonin in reducing of ER‐stress via regulation of UPR signaling protein/gene expression during the IVM process of porcine oocytes.
The UPR signaling is critical for animal development, female reproduction,7 steroid hormone secretion,22 and cellular homeostasis by defending against ER‐stress.23 Zhang et al,24 have reported on the regulation and occurrence of ER‐stress on porcine oocyte maturation and parthenogenetic embryonic development in vitro. Additionally, XBP1 mRNA splicing by activated IRE1 occurred during Tm 5 μg/mL‐treated COCs at 44 hours of IVM. Splicing(s) XBP1 as an IRE1 downstream signal gene is important to embryonic development and mouse oocyte maturation, and cytoplasmic expression of unsplicing(u)XBP1 was detected in the M I, M II, 1‐cell, 2‐cell, and 8‐cell stages.9 Nevertheless, a study of the relationship between ER‐stress marker genes (Bip/Grp78, ATF4, P50ATF6, CHOP) and porcine oocyte maturation in vitro has not been performed. Therefore, we investigated the occurrence of ER‐stress by analysis of the three UPR signaling pathways on COCs, DO, and CC of the porcine IVM process (22 and 44 hours), respectively.
The transcription factor Atf4 for chaperone functions and Chop genes were induced after Bip/Grp78 activation through the PERK‐eIF2a pathways.25 Our results demonstrated that the mRNA levels of Atf4 were induced in COCs after the Bip/Grp78 gene expression increased (Figure 1A). With the IVM progression of porcine oocytes, expression of UPR signaling‐related proteins (Bip/Grp78, ATF4, P50ATF6, and CHOP)/genes (Bip/Grp78, Atf4, and sXbp1) was higher in CC and COCs of 44 hours than 22 hours (Figure 1A‐C). Moreover, we confirmed the direct expression of P50ATF6 in porcine oocytes from ovarian follicles using IHC analysis (Figure 1D). As follicle size increases, P50ATF6 is highly expressed compared to an oocyte of a small follicle. Under normal conditions, ATF6 is constitutively expressed as a 90‐kDa protein (P90ATF6) that is directly converted into a 50‐kDa protein (P50ATF6) in cells experiencing ER‐stress.26, 27 These findings indicated that ER‐stress occurred during IVM of porcine oocytes without any treatment.
To study the link between increased UPR gene expression and occurrence of ER‐stress on the IVM process of porcine, we investigated the changes in oocyte maturation rate and cumulus cell expansion after Tm‐treatment within IVM medium (Figure 2A‐C). Tm, which inhibits N‐linked glycosylation,28 and cell cycle arrest,29 is well known to induce apoptosis. Increased ER‐stress by Tm‐treatment showed negative effects on meiotic maturation and cumulus cell expansion during porcine oocyte IVM (Figure 2B and C). As expected, Western blotting and RT‐PCR analysis of UPR genes revealed significantly increased levels in porcine COCs with Tm‐treatment (1 and 5 μg/mL; Figure 2D and E). Interestingly, protein and mRNA levels of ATF4 were significantly increased in the Tm 1 μg/mL group, whereas levels were significantly decreased in the Tm 5 μg/mL group (Figure 2E). Under continuous or repetitive ER‐stress production, increased levels of ATF4 lead to GADD34‐induced de‐phosphorylation of eIF‐2α and the resumption of protein synthesis to overcome the ER‐stress condition.30 Therefore, we reasoned that the increase in UPR gene expression alleviates ER‐stress during general or an induced ER‐stress condition of porcine oocytes maturation.
Supplementing melatonin to IVM medium for M II (22‐44 hours of IVM) improves the meiotic maturation rate and cumulus cell expansion in maturing COCs by regulating UPR signaling pathways related to the IVM process (Figure 3A and B). These results are similar to those of studies in porcine oocytes by Kang et al.16 Many reports observed the positive effects of melatonin treatment, via regulation of ROS, on the improvement of oocyte maturation rates.12, 25, 31 Especially, oxidative stress causes negative effects on human oocyte maturation and melatonin protects oocytes from oxidative stress such as free radical damage, therefore improves fertilization rate.31 In cattle oocytes, melatonin supplementation resulted in increased rates of oocytes and expanded CC.15 It is known that melatonin improves oocyte maturation in mammals, but the suppressive effects on ER‐stress and changes to the three UPR signaling pathways are unknown.
Recently, other examples of melatonin reducing the ER‐stress condition have been reported. Melatonin attenuated ER‐stress through a modulation of the three arms of UPR signaling in rabbit apoptotic liver damage,32 and this inhibits Tm‐induced ER‐stress in human hepatocellular carcinoma cells,33 Additionally, melatonin suppressed ER‐stress during CCl4‐induced fibrosis in mice.34 Based on the previous studies, we observed the defense effects of melatonin from ER‐stress on porcine oocyte maturation using a comparative analysis of results obtained with the ER‐stress inhibitor TUDCA. TUDCA is chemical chaperone that inhibits ER‐stress35 and can also directly inhibit the production of ROS.36 Moreover, TUDCA attenuates ER‐stress‐induced apoptosis during mouse embryonic development.9 As shown in Figure 3C, the present study also showed that the mRNA expression levels of UPR marker genes (Bip/Grp78, Atf4, sXbp1, and Chop) did not differ in the TUDCA (200 μmol/L) or melatonin (0.1 μmol/L)‐treated group for M II compared to control group. Similarly, the protein level of Bip/Grp78 did not differ, but activated P50ATF6, ATF4, and CHOP protein expression decreased in TUDCA (200 μmol/L) and/or melatonin (0.1 μmol/L)‐treated COCs. In addition, inactivation of p90ATF6 dramatically increased (P < .001) in COCs of the melatonin‐treatment group.
Based on the results of this study, we conclude that the supplementation of IVM medium NSCU with melatonin improves porcine cumulus cell expansion and oocyte maturation by reducing ER‐stress via regulation of UPR signaling (Figure 4). These results suggest that melatonin is likely to improve oocyte quality and maturation rates via regulation of UPR signaling as reduction ER‐stress. Therefore, our study showed that the reduction effects of ER‐stress by melatonin treatment on its ability to regulate the porcine oocytes maturation and CC expansion.
Figure 4.
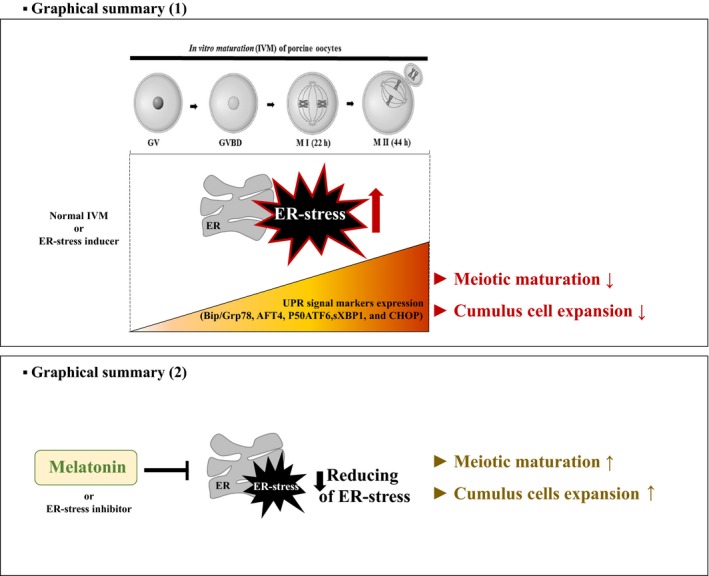
Graphical Summary. (1; top) Our results demonstrated that the occurrence of endoplasmic reticulum (ER)‐stress is continuously maintained during in vitro maturation (IVM) of porcine oocytes without any treatment. Furthermore, protein/mRNA levels of three unfolded protein response (UPR) signaling genes increased with maturation of cumulus‐oocyte complexes (COCs) during IVM of porcine oocytes. (2; bottom) The supplementation of melatonin in IVM medium improves oocyte maturation by reducing ER‐stress via regulation of UPR signaling during the porcine oocyte IVM process
CONFLICT OF INTEREST
None of the authors have any conflict of interest to declare.
AUTHOR CONTRIBUTIONS
H. J. Park, J. Y. Park, J. W. Kim, S. G. Yang, J. M. Jung, and M. J. Kim: performing the in vitro culture of pig oocyte, study conception, data collection, Western analysis, RT‐PCR, IHC analysis, and interpretation. M. J. Kang, Y. H. Cho, G. Wee, H. Y. Yang, and B. S. Song: data collection, analysis, and interpretation. S. U. Kim and D. B. Koo: study conception and design, financial support, data analysis, interpretation, manuscript writing, and final approval of manuscript.
ACKNOWLEDGEMENTS
This work was supported by grants from the Next‐Generation BioGreen 21 Program (PJ01117604), the Bio‐industry Technology Development Program (316037‐04‐2‐HD020), and the Basic Science Research Program (NRF‐2015R1D1A1A01057103) through the Rural Development Administration, the Ministry of Agriculture, Food and Rural Affairs, the Ministry of Education, and the KRIBB Research Initiative Program (KGM4251723), Republic of Korea.
Park H‐J, Park J‐Y, Kim J‐W, et al. Melatonin improves the meiotic maturation of porcine oocytes by reducing endoplasmic reticulum stress during in vitro maturation. J Pineal Res. 2018;64:e12458 https://doi.org/10.1111/jpi.12458
H‐JP and J‐YP are contributed equally to this work.
Contributor Information
Sun‐Uk Kim, Email: sunuk@kribb.re.kr.
Deog‐Bon Koo, Email: dbkoo@daegu.ac.kr.
REFERENCES
- 1. Lin JH, Walter P, Yen TS. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol. 2008;3:399‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gu XW, Yan JQ, Dou HT, et al. Endoplasmic reticulum stress in mouse decidua during early pregnancy. Mol Cell Endocrinol. 2016;434:48‐56. [DOI] [PubMed] [Google Scholar]
- 3. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519‐529. [DOI] [PubMed] [Google Scholar]
- 4. Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89‐102. [DOI] [PubMed] [Google Scholar]
- 5. Latham KE. Stress signaling in mammalian oocytes and embryos: a basis for intervention and improvement of outcomes. Cell Tissue Res. 2016;363:159‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Teske BF, Wek SA, Bunpo P, et al. The eIF2 kinase PERK and the integrated stress response facilitate activation of ATF6 during endoplasmic reticulum stress. Mol Biol Cell. 2011;22:4390‐4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang Y, Pei X, Jin Y, Wang Y, Zhang C. The roles of endoplasmic reticulum stress response in female mammalian reproduction. Cell Tissue Res. 2016;363:589‐597. [DOI] [PubMed] [Google Scholar]
- 8. Wu LL, Russell DL, Norman RJ, Robker RL. Endoplasmic reticulum (ER) stress in cumulus‐oocyte complexes impairs pentraxin‐3 secretion, mitochondrial membrane potential (DeltaPsi m), and embryo development. Mol Endocrinol. 2012;26:562‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang JY, Diao YF, Kim HR, Jin DI. Inhibition of endoplasmic reticulum stress improves mouse embryo development. PLoS ONE. 2012;7:e40433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zang LY, Cosma G, Gardner H, Vallyathan V. Scavenging of reactive oxygen species by melatonin. Biochim Biophys Acta. 1998;1425:469‐477. [DOI] [PubMed] [Google Scholar]
- 11. Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci. 2000;7:444‐458. [DOI] [PubMed] [Google Scholar]
- 12. Shi JM, Tian XZ, Zhou GB, et al. Melatonin exists in porcine follicular fluid and improves in vitro maturation and parthenogenetic development of porcine oocytes. J Pineal Res. 2009;47:318‐323. [DOI] [PubMed] [Google Scholar]
- 13. Gao C, Han HB, Tian XZ, et al. Melatonin promotes embryonic development and reduces reactive oxygen species in vitrified mouse 2‐cell embryos. J Pineal Res. 2012;52:305‐311. [DOI] [PubMed] [Google Scholar]
- 14. Loren P, Sanchez R, Arias ME, Felmer R, Risopatron J, Cheuqueman C. Melatonin scavenger properties against oxidative and nitrosative stress: impact on gamete handling and in vitro embryo production in humans and other mammals. Int J Mol Sci. 2017;18:1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. El‐Raey M, Geshi M, Somfai T, et al. Evidence of melatonin synthesis in the cumulus oocyte complexes and its role in enhancing oocyte maturation in vitro in cattle. Mol Reprod Dev. 2011;78:250‐262. [DOI] [PubMed] [Google Scholar]
- 16. Kang JT, Koo OJ, Kwon DK, et al. Effects of melatonin on in vitro maturation of porcine oocyte and expression of melatonin receptor RNA in cumulus and granulosa cells. J Pineal Res. 2009;46:22‐28. [DOI] [PubMed] [Google Scholar]
- 17. Nakano M, Kato Y, Tsunoda Y. Effect of melatonin treatment on the developmental potential of parthenogenetic and somatic cell nuclear‐transferred porcine oocytes in vitro. Zygote. 2012;20:199‐207. [DOI] [PubMed] [Google Scholar]
- 18. Zhao H, Wu QQ, Cao LF, et al. Melatonin inhibits endoplasmic reticulum stress and epithelial‐mesenchymal transition during bleomycin‐induced pulmonary fibrosis in mice. PLoS ONE. 2014;9:e97266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carloni S, Albertini MC, Galluzzi L, Buonocore G, Proietti F, Balduini W. Melatonin reduces endoplasmic reticulum stress and preserves sirtuin 1 expression in neuronal cells of newborn rats after hypoxia‐ischemia. J Pineal Res. 2014;57:192‐199. [DOI] [PubMed] [Google Scholar]
- 20. Funahashi H, Cantley TC, Stumpf TT, Terlouw SL, Day BN. In vitro development of in vitro‐matured porcine oocytes following chemical activation or in vitro fertilization. Biol Reprod. 1994;50:1072‐1077. [DOI] [PubMed] [Google Scholar]
- 21. Marei WF, Wathes DC, Fouladi‐Nashta AA. The effect of linolenic acid on bovine oocyte maturation and development. Biol Reprod. 2009;81:1064‐1072. [DOI] [PubMed] [Google Scholar]
- 22. Park H‐J, Park S‐J, Koo D‐B, et al. Progesterone production is affected by unfolded protein response (UPR) signaling during the luteal phase in mice. Life Sci. 2014;113:60‐67. [DOI] [PubMed] [Google Scholar]
- 23. Schwarz DS, Blower MD. The endoplasmic reticulum: structure, function and response to cellular signaling. Cell Mol Life Sci. 2016;73:79‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang JY, Diao YF, Oqani RK, Han RX, Jin DI. Effect of endoplasmic reticulum stress on porcine oocyte maturation and parthenogenetic embryonic development in vitro. Biol Reprod. 2012;86:128. [DOI] [PubMed] [Google Scholar]
- 25. Wang F, Tian X, Zhang L, Tan D, Reiter RJ, Liu G. Melatonin promotes the in vitro development of pronuclear embryos and increases the efficiency of blastocyst implantation in murine. J Pineal Res. 2013;55:267‐274. [DOI] [PubMed] [Google Scholar]
- 26. Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10:3787‐3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress‐induced apoptosis. EMBO Rep. 2006;7:880‐885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takatsuki A, Arima K, Tamura G. Tunicamycin, a new antibiotic. I. Isolation and characterization of tunicamycin. J Antibiot (Tokyo). 1971;24:215‐223. [DOI] [PubMed] [Google Scholar]
- 29. Chan SW, Egan PA. Hepatitis C virus envelope proteins regulate CHOP via induction of the unfolded protein response. FASEB J. 2005;19:1510‐1512. [DOI] [PubMed] [Google Scholar]
- 30. Ma Y, Hendershot LM. Delineation of a negative feedback regulatory loop that controls protein translation during endoplasmic reticulum stress. J Biol Chem. 2003;278:34864‐34873. [DOI] [PubMed] [Google Scholar]
- 31. Tamura H, Takasaki A, Miwa I, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008;44:280‐287. [DOI] [PubMed] [Google Scholar]
- 32. Tunon MJ, San‐Miguel B, Crespo I, et al. Melatonin treatment reduces endoplasmic reticulum stress and modulates the unfolded protein response in rabbits with lethal fulminant hepatitis of viral origin. J Pineal Res. 2013;55:221‐228. [DOI] [PubMed] [Google Scholar]
- 33. Fan L, Sun G, Ma T, et al. Melatonin reverses tunicamycin‐induced endoplasmic reticulum stress in human hepatocellular carcinoma cells and improves cytotoxic response to doxorubicin by increasing CHOP and decreasing survivin. J Pineal Res. 2013;55:184‐194. [DOI] [PubMed] [Google Scholar]
- 34. San‐Miguel B, Crespo I, Sanchez DI, et al. Melatonin inhibits autophagy and endoplasmic reticulum stress in mice with carbon tetrachloride‐induced fibrosis. J Pineal Res. 2015;59:151‐162. [DOI] [PubMed] [Google Scholar]
- 35. Hetz C, Chevet E, Harding HP. Targeting the unfolded protein response in disease. Nat Rev Drug Discov. 2013;12:703‐719. [DOI] [PubMed] [Google Scholar]
- 36. Kim JS, Song BS, Lee KS, et al. Tauroursodeoxycholic acid enhances the pre‐implantation embryo development by reducing apoptosis in pigs. Reprod Domest Anim. 2012;47:791‐798. [DOI] [PubMed] [Google Scholar]


