Abstract
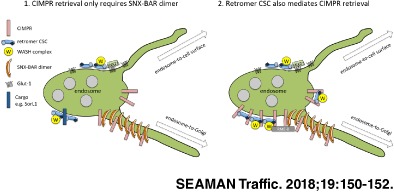
The retromer cargo‐selective complex (CSC) comprising Vps35, Vps29 and Vps26 mediates the endosome‐to‐Golgi retrieval of the cation‐independent mannose 6‐phosphate receptor (CIMPR). Or does it? Recently published data have questioned the validity of this long‐established theory. Here, the evidence for and against a role for the retromer CSC in CIMPR endosome‐to‐Golgi retrieval is examined in the light of the new data that the SNX‐BAR dimer is actually responsible for CIMPR retrieval.
Keywords: cargo‐selection, CIMPR, endosome‐to‐Golgi retrieval, Retromer, SNX‐BAR, sorting motif
1. INTRODUCTION
Since it was first characterized nearly 20 years ago there has been a panoply of published reports detailing the functioning of the retromer complex in trafficking a varied array of membrane proteins from endosomes to either the Golgi complex or the cell surface.1, 2 One of the most widely accepted tenets of retromer function is that, in mammalian cells, the retromer complex mediates the endosome‐to‐Golgi retrieval of the cation‐independent mannose 6‐phosphate receptor (CIMPR) through the association of the cytoplasmic tail of the CIMPR with the cargo‐selective complex (CSC) of retromer comprising the Vps35, Vps29 and Vps26 proteins. Now though, this has been thrown into doubt after studies from Cullen and Steinberg report that the retromer CSC does not associate with the tail of the CIMPR and is not required for the endosome‐to‐Golgi retrieval of the CIMPR. Rather, the SNX‐BAR dimer, comprising SNX1 or SNX2 with either SNX5 or SNX6 associate with the CIMPR to mediate its retrieval.3, 4
2. SOME HISTORY
How and why was the retromer CSC believed to mediate the endosome‐to‐Golgi retrieval of the CIMPR for so long if that theory is wrong? The first reports that the retromer CSC was required to sort the CIMPR for retrieval to the Golgi came from studies independently conducted in the labs of Bonifacino and myself.5, 6 But those studies followed on from the initial work in yeast which favoured a direct association of the retromer CSC with cargo proteins, such as Vps10p.7, 8 As Vps10p in yeast performs the same task as the CIMPR in mammalian cells, it seemed natural that the retromer CSC would associate with the tail of the CIMPR and mediate its endosome‐to‐Golgi retrieval. Indeed the study from the Bonifacino lab reported a direct interaction between Vps35 and the CIMPR tail. Both studies reported that the CIMPR requires the function of the retromer CSC to be retrieved from endosomes to the Golgi.5, 6 Loss of retromer CSC function through knockdown or genetic knockout results in mislocalization of the CIMPR to endosomes and its subsequent degradation in lysosomes. We also reported that mistrafficking of the CIMPR results in a failure to deliver lysosomal hydrolases such as Cathepsin D to the lysosome—a predictable outcome if the retrieval of the CIMPR is impaired.
3. IMPORTANT ADVANCES
Thus, early on in the studies of the retromer complex in mammals, it was established that the retromer CSC associates with the tail of the CIMPR to mediate its endosome‐to‐Golgi retrieval. The motif in the CIMPR tail that is required for retromer‐mediated endosome‐to‐Golgi retrieval was identified through a tried‐and‐tested approach employing CD8 reporters and mutagenesis of the CD8‐CIMPR construct. That study identified a sequence comprising Trp‐Leu‐Met (WLM) in the CIMPR tail as necessary for its retrieval.9 Interestingly, the reports from the Cullen and Steinberg labs reveal that the SNX‐BAR dimer associates with the CIMPR tail via the WLM motif.3, 4
Over time, the functioning of retromer‐mediated endosome‐to‐Golgi retrieval has become better understood. A major advance by the Cullen laboratory was the identification of SNX5 and SNX6 as retromer components and the demonstration that these two SNX‐BAR proteins could associate with dynein by binding to dynactin.10, 11 Subsequently, it was recognized that mammalian retromer, unlike the complex in yeast, is not a stable heteropentamer but a much looser association of the retromer CSC with the SNX‐BAR dimer.12 Thus, the retromer CSC requires the Rab7a and Snx3 proteins for its membrane association, whereas the SNX‐BAR dimer can associate with endosomes through the phox‐homology (PX) domains that bind to phosphotidyl inositol 3‐phosphate.13, 14, 15, 16 The mammalian retromer CSC, unlike the yeast CSC, associates with a collection of accessory proteins, for example, the WASP and Scar Homolog (WASH) complex that is recruited to endosomes through an interaction between the Vps35 protein and the Fam21 subunit of the WASH complex.17, 18, 19, 20 The patho‐physiological importance of the retromer‐WASH complex interaction is underscored by the fact that a Parkinson’s disease (PD)‐causing mutation in Vps35 impairs the binding of the WASH complex to the retromer CSC resulting in less endosomally localized WASH complex leading to trafficking defects.21, 22, 23
4. OUT WITH THE OLD, IN WITH THE NEW?
The recent reports from the Cullen and Steinberg labs have suggested that the role of the retromer CSC in sorting the CIMPR for retrieval to the Golgi is questionable because they could not show any mislocalization of the CIMPR when components of the retromer CSC are silenced by RNAi or deleted by targeted knockout. They cite the studies from my lab and that of Bonifacino5, 6 as the sources for the evidence that the retromer CSC is necessary for the retrieval of the CIMPR but of course there are other published studies that agree with those early papers. There are the data from Bulankina et al that was part of their investigation into the function of the TIP47 protein24 and the study of Hao et al confirmed the requirement for the retromer CSC to mediate the endosome‐to‐Golgi retrieval of the CIMPR in their analysis of WASH complex regulating machinery.25
Could the differences in effects on CIMPR trafficking boil down to different assays—what is the best way to measure the endosome‐to‐Golgi retrieval of the CIMPR? There is no definitive answer to that question and different labs have employed an array of techniques that are centred on the use of microscopy to visualize the CIMPR. The assay used by Hao et al25 measures the dispersal of the CIMPR to peripheral structures and has been used previously in retromer‐focussed studies.26 It was suggested however that the CIMPR dispersal assay employed by Hao et al may be insufficiently robust to properly determine the effect of loss of retromer CSC function on the localization of the CIMPR.4 Curiously, one of the reports to show that the retromer CSC is required for CIMPR retrieval was when the role of SNX5 and SNX6 in CIMPR retrieval was identified using a CIMPR dispersal assay.10 On that occasion knockdowns of Vps35, Vps29 or Vps26 all impaired CIMPR retrieval in a manner similar to a SNX5 or SNX6 knockdown. My own lab generally favours the use of automated microscopy as a means of measuring endosome‐to‐Golgi retrieval to avoid potential bias introduced at the time of imaging. Using such an assay, we have shown that loss of Snx3, Rab7a, Vps26a, Vps35 or SNX1 function all cause a significant endosome‐to‐Golgi retrieval defect of a CD8‐CIMPR reporter protein.17, 27 Another way to measure CIMPR retrieval to the TGN is the application of immunofluorescence with Pearson’s correlation—the technique employed in the paper by Kvainickas et al, where they report no role for the retromer CSC in CIMPR retrieval.4 In a surprising volte‐face, the study by Kvainickas et al stands in stark contrast with a previous report where it was shown that the PD‐causing Vps35 mutant was equivalent to a Vps35 null with respect to impairing CIMPR retrieval to the TGN using immunofluorescence and Pearson’s correlation to measure retrieval.22
5. CONCLUDING REMARKS
The finding that the SNX‐BAR dimer can associate with the tail of the CIMPR is undoubtedly a significant advance in the understanding of how the endosome‐to‐Golgi retrieval of the CIMPR occurs. But does the observation that the SNX‐BAR dimer can bind the CIMPR tail negate the role of the retromer CSC? There is sufficient evidence, accumulated by labs operating independently (including studies authored by Cullen and Steinberg10, 22), that the retromer CSC is required for the retrieval of the CIMPR to argue that it has a major role—possibly by directly associating with the CIMPR tail.5, 6, 9, 10, 14, 17, 22, 24, 25, 28 What then is the truth? Without further experimentation the answer to that question will have to wait. I suspect however that the “truth” will end up being a version of both models where the tail of the CIMPR can associate with the retromer CSC, possibly to ensure it is concentrated, albeit briefly, in endosomal microdomains that may require the presence of WASH‐complex generated F‐actin. Following the concentration of the CIMPR in the endosomal membrane, the SNX‐BAR proteins may bind and direct the CIMPR into a tubule for retrieval to the Golgi. Proteins such as RME‐8 that can associate with both the SNX‐BAR dimer and the WASH complex22, 29, 30, 31 may coordinate the transfer of cargo such as the CIMPR from the retromer CSC to the SNX‐BAR dimer. Both sets of proteins then will have a direct role in the CIMPR retrieval but the relative importance of the retromer CSC and SNX‐BAR dimer may vary from cell‐to‐cell and tissue‐to‐tissue according to the primary function of the cell(s) in question.
ACKNOWLEDGMENTS
M.N.J.S. is funded by the Medical Research Council.
Seaman MNJ. Retromer and the cation‐independent mannose 6‐phosphate receptor—Time for a trial separation?. Traffic. 2018;19:150–152. https://doi.org/10.1111/tra.12542
Funding information Medical Research Council, Grant/Award number: MR/L007533/1
REFERENCES
- 1. Burd C, Cullen PJ. Retromer: a master conductor of endosome sorting. Cold Spring Harb Perspect Biol. 2014;6(2):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seaman MN. The retromer complex—endosomal protein recycling and beyond. J Cell Sci. 2012;125:4693–4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simonetti B, Danson CM, Heesom KJ, Cullen PJ. Sequence‐dependent cargo recognition by SNX‐BARs mediates retromer‐independent transport of CI‐MPR. J Cell Biol. 2017;216:3695–3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kvainickas A, Jimenez‐Orgaz A, Nägele H, Hu Z, Dengjel J, Steinberg F. Cargo‐selective SNX‐BAR proteins mediate retromer trimer independent retrograde transport. J Cell Biol. 2017;216:3677–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arighi CN, Hartnell LM, Aguilar RC, Haft CR, Bonifacino JS. Role of the mammalian retromer in sorting of the cation‐independent mannose 6‐phosphate receptor. J Cell Biol. 2004;165:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seaman MN. Cargo‐selective endosomal sorting for retrieval to the Golgi requires retromer. J Cell Biol. 2004;165:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seaman MN, McCaffery JM, Emr SD. A membrane coat complex essential for endosome‐to‐Golgi retrograde transport in yeast. J Cell Biol. 1998;142:665–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nothwehr SF, Ha SA, Bruinsma P. Sorting of yeast membrane proteins into an endosome‐to‐Golgi pathway involves direct interaction of their cytosolic domains with Vps35p. J Cell Biol. 2000;151:297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seaman MN. Identification of a novel conserved sorting motif required for retromer‐mediated endosome‐to‐TGN retrieval. J Cell Sci. 2007;120:2378–2389. [DOI] [PubMed] [Google Scholar]
- 10. Wassmer T, Attar N, Bujny MV, Oakley J, Traer CJ, Cullen PJ. A loss‐of‐function screen reveals SNX5 and SNX6 as potential components of the mammalian retromer. J Cell Sci. 2007;120:45–54. [DOI] [PubMed] [Google Scholar]
- 11. Wassmer T, Attar N, Harterink M, et al. The retromer coat complex coordinates endosomal sorting and dynein‐mediated transport, with carrier recognition by the trans‐Golgi network. Dev Cell. 2009;17:110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Swarbrick JD, Shaw DJ, Chhabra S, et al. VPS29 is not an active metallo‐phosphatase but is a rigid scaffold required for retromer interaction with accessory proteins. PLoS One. 2011;6(5):e20420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rojas R, van Vlijmen T, Mardones GA, et al. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J Cell Biol. 2008;183:513–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seaman MN, Harbour ME, Tattersall D, Read E, Bright N. Membrane recruitment of the cargo‐selective retromer subcomplex is catalysed by the small GTPase Rab7 and inhibited by the Rab‐GAP TBC1D5. J Cell Sci. 2009;122:2371–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harterink M, Port F, Lorenowicz MJ, et al. A SNX3‐dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion. Nat Cell Biol. 2011;13:914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vardarajan BN, Bruesegem SY, Harbour ME, et al. Identification of Alzheimer disease‐associated variants in genes that regulate retromer function. Neurobiol Aging. 2012;33:2231.e15–2231.e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harbour ME, Breusegem SY, Antrobus R, Freeman C, Reid E, Seaman MN. The cargo‐selective retromer complex is a recruiting hub for protein complexes that regulate endosomal tubule dynamics. J Cell Sci. 2010;123:3703–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harbour ME, Breusegem SY, Seaman MN. Recruitment of the endosomal WASH complex is mediated by the extended 'tail' of Fam21 binding to the retromer protein Vps35. Biochem J. 2012;442:209–220. [DOI] [PubMed] [Google Scholar]
- 19. Jia D, Gomez TS, Billadeau DD, Rosen MK. Multiple repeat elements within the FAM21 tail link the WASH actin regulatory complex to the retromer. Mol Biol Cell. 2012;23:2352–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Helfer E, Harbour ME, Henriot V, et al. Endosomal recruitment of the WASH complex: active sequences and mutations impairing interaction with the retromer. Biol Cell. 2013;105:191–207. [DOI] [PubMed] [Google Scholar]
- 21. Zavodszky E, Seaman MN, Moreau K, et al. Mutation in VPS35 associated with Parkinson's disease impairs WASH complex association and inhibits autophagy. Nat Commun. 2014;5:3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McGough IJ, Steinberg F, Jia D, et al. Retromer binding to FAM21 and the WASH complex is perturbed by the Parkinson disease‐linked VPS35(D620N) mutation. Curr Biol. 2014;24:1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Follett J, Norwood SJ, Hamilton NA, et al. The Vps35 D620N mutation linked to Parkinson's disease disrupts the cargo sorting function of retromer. Traffic. 2014;15:230–244. [DOI] [PubMed] [Google Scholar]
- 24. Bulankina AV, Deggerich A, Wenzel D, et al. TIP47 functions in the biogenesis of lipid droplets. J Cell Biol. 2009;185:641–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hao YH, Doyle JM, Ramanathan S, et al. Regulation of WASH‐dependent actin polymerization and protein trafficking by ubiquitination. Cell. 2013;152:1051–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gomez TS, Billadeau DD. A FAM21‐containing WASH complex regulates retromer‐dependent sorting. Dev Cell. 2009;17:699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Breusegem SY, Seaman MN. (2014). Genome‐wide RNAi screen reveals a role for multipass membrane proteins in endosome‐to‐golgi retrieval. Cell Rep. 2014;9:1931–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fjorback AW, Seaman M, Gustafsen C, et al. Retromer binds the FANSHY sorting motif in SorLA to regulate amyloid precursor protein sorting and processing. J Neurosci. 2012;32:1467–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shi A, Sun L, Banerjee R, Tobin M, Zhang Y, Grant BD. Regulation of endosomal clathrin and retromer‐mediated endosome to Golgi retrograde transport by the J‐domain protein RME‐8. EMBO J. 2009;28:3290–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Popoff V, Mardones GA, Bai SK, et al. Analysis of articulation between clathrin and retromer in retrograde sorting on early endosomes. Traffic. 2009;10:1868–1880. [DOI] [PubMed] [Google Scholar]
- 31. Freeman CL, Hesketh G, Seaman MN. RME‐8 coordinates the activity of the WASH complex with the function of the retromer SNX dimer to control endosomal tubulation. J Cell Sci. 2014;127:2053–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]


