Abstract
Aims
To report results from and explore use of a multicentre, parallel‐group, unblinded, randomized controlled trial testing the effectiveness in terms of well‐being and diabetes management of a person‐centred, web‐based support programme for women with Type 1 diabetes, in pregnancy and postpartum.
Methods
Between 2011 and 2014, 174 pregnant women with Type 1 diabetes were randomly allocated (1:1) to web‐based support and standard care (intervention group, n=83), or standard care (control group, n=91). The web‐based support consisted of evidence‐based information; a self‐care diary for monitoring of daily activities; and peer support in a discussion forum. The primary outcomes (mean difference, measured at 6 months after childbirth) were well‐being and diabetes management.
Results
No differences were found with regard to the primary outcome measure scores for general well‐being [1.04 (95% CI –1.28 to 3.37); P=0.68] and self‐efficacy of diabetes management [0.08 (95% CI –0.12 to 0.28); P= 0.75], after adjustment for baseline differences in the insulin administration method, nor with regard to the secondary outcome measures.
Conclusions
At 6 months after childbirth, the web‐based support plus standard care was not superior to standard care in terms of general well‐being or self‐efficacy of diabetes management. This might be explained by the low number of participants who had a high activity level. Few simultaneously active participants in the web‐based programme and stressors in motherhood and diabetes postpartum were the main barriers to its use. Further intervention studies that offer web‐based support are needed, with lessons learned from the present study.
(Clinicaltrials.gov identification number: NCT015665824)
What's new?
This randomized controlled trial of 174 women with Type 1 diabetes mellitus evaluated a person‐centred, web‐based support programme aimed at improving the well‐being and self‐efficacy of diabetes management during pregnancy and after childbirth, with the primary endpoint being at 6 months after childbirth.
No significant differences were found between the intervention group receiving the web‐based support and standard care, and the control group receiving standard care.
The main barriers to the use of the web‐based support were the low number of simultaneously active participants as a result of the randomization rate, and the high demands in daily life after childbirth in caring for the baby and their diabetes.
What's new?
This randomized controlled trial of 174 women with Type 1 diabetes mellitus evaluated a person‐centred, web‐based support programme aimed at improving the well‐being and self‐efficacy of diabetes management during pregnancy and after childbirth, with the primary endpoint being at 6 months after childbirth.
No significant differences were found between the intervention group receiving the web‐based support and standard care, and the control group receiving standard care.
The main barriers to the use of the web‐based support were the low number of simultaneously active participants as a result of the randomization rate, and the high demands in daily life after childbirth in caring for the baby and their diabetes.
Introduction
Type 1 diabetes mellitus has been shown to affect psychosocial well‐being during pregnancy and in early motherhood 1. The risk of adverse outcomes 2 has been described as a constant worry for pregnant women with Type 1 diabetes 3. To optimize their chances of having a healthy baby and minimize their diabetes‐related risks, women are advised to achieve near‐normoglycaemia before and during pregnancy 4. This often entails monitoring blood glucose around the clock 5. During pregnancy, unfamiliar body cues and fluctuating blood glucose levels with frequent hypoglycaemic episodes create extraordinary challenges with regard to diabetes management 3, 5. New mothers trying to establish breastfeeding have described feelings of uncertainty and unpredictability related to their own unstable glycaemic control and the loss of the professional support that had surrounded them during the pregnancy 6. To achieve the best possible well‐being, women with Type 1 diabetes need support during both pregnancy and the early period following childbirth.
Technology to improve care among people with diabetes is constantly under development. eHealth interventions providing social support have the potential to educate and empower the user 7. In people with Type 2 diabetes, eHealth interventions have proven successful in improving self‐management behaviours, psychosocial outcomes and clinical measures 8. A study investigating Internet use and support needs in women with Type 1 diabetes during pregnancy and the early postpartum period has identified a desire for reliable information regarding diabetes and pregnancy, a technical device for online monitoring of blood glucose, and a way of communicating directly with the healthcare professional as well as a mode of communication with peers 9. With regard to diabetes and pregnancy, the focus of previous documented interventions has mostly been on technical devices, such as continuous subcutaneous insulin therapy and glucose monitoring, and their effect on glycaemic control 10.
The present study tested a specially designed person‐centred, web‐based support intervention to be used during pregnancy and in early motherhood by women with Type 1 diabetes. The web‐based support programme was expected to strengthen autonomy and personal capacity in users, and thereby optimize well‐being and self‐efficacy of diabetes management, with the primary endpoint at 6 months after childbirth. The aim was to report on the effectiveness of this web‐based support in terms of improved self‐efficacy of diabetes management and general well‐being in women with Type 1 diabetes during pregnancy and up to six months after childbirth (primary outcomes), and also with regard to self‐perceived health, sense of coherence, glycaemic control, diabetes distress and fear of hypoglycaemia (secondary outcomes). A further aim was to explore the use of the web‐based support.
Methods
The MODIAB‐Web study (Motherhood and Diabetes Web study), was a two‐armed, parallel‐group, multicentre, unblinded, randomized controlled trial (RCT), conducted in accordance with the CONSORT guidelines 11. Study methods have been reported in detail elsewhere 12.
Sample and participants
Women with Type 1 diabetes were recruited between November 2011 and December 2014 in their first or early second trimester of pregnancy. Data collection was completed in March 2016. The study included literate and Swedish‐speaking pregnant women aged >18 years with a diagnosis of Type 1 diabetes and registered at one of the six participating study centres. In total, 288 pregnant women with Type 1 diabetes were assessed for eligibility by a study midwife at six Swedish hospital‐based antenatal care centres. Of these, 95 declined and 19 were not followed up or dropped out as a result of spontaneous abortion. The remaining 174 women consented to take part and were randomly allocated (1:1) by the study midwife to the intervention group (n=83) or the control group (n=91), using block randomization with prepared closed envelopes. In the period between group allocation and the first follow‐up in late pregnancy, six participants were excluded because of lost baseline data, eight as a result of miscarriage and two because their identity was not registered together with their study identification code. The participants included in the intention‐to‐treat analysis consisted of 158 women (intervention group, n=78; control group, n=80; Fig. 1). Ethical approval was attained from the Ethics Committee of Gothenburg, Sweden (No. 659‐09).
Figure 1.
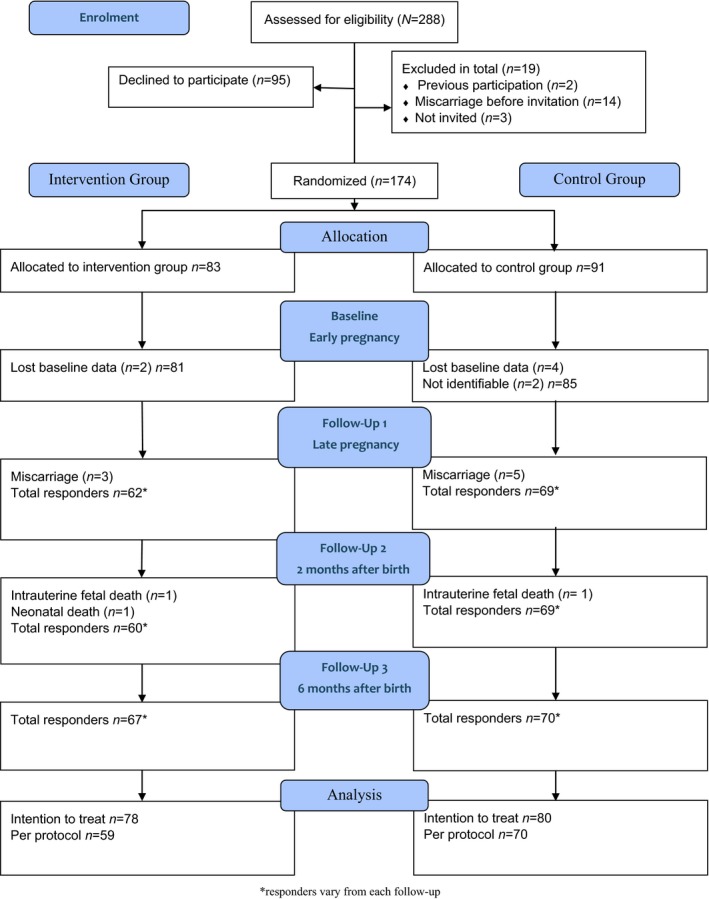
Flow diagram of participation allocation, follow‐up and data analysis in the MODIAB‐Web randomized controlled trial.
Intervention
All participants received standard care during pregnancy, childbirth and immediately after. Standard care varied, although all clinics offered frequent contact with midwives, obstetricians and endocrinologists during pregnancy, and one follow‐up visit after childbirth.
To further support the women's well‐being and diabetes management, the intervention offered complementary web‐based support, inspired by previously identified needs 9, and developed with a participatory design 13 involving experts, patient representatives and researchers 14, 15, 16. The theoretical basis for the intervention was person‐centred care 17, and it was designed to assist in decision‐making, based on the woman's own documentation, to support self‐care and to facilitate contact with peers. The intervention had three components, listed below.
Evidence‐based information on three topics: being pregnant, labour and childbirth, and life as a new mother with diabetes. A large amount of online information regarding pregnancy was available elsewhere in Swedish, but there was very little trustworthy information regarding diabetes and pregnancy.
A self‐care diary for self‐reported monitoring of blood glucose, insulin doses, diet, activities and daily mood—measures that could be viewed and evaluated in tables and diagrams. This was designed as an alternative to the paper diary traditionally used.
A discussion forum for peer support, moderated by the research group. Links to other relevant sites and a section of Frequently Asked Questions were also provided.
Measurements
The primary and secondary outcomes were measured at baseline in early pregnancy (first or early second trimester), late pregnancy (third trimester), 2 months after childbirth, and 6 months after childbirth. The primary endpoints were general well‐being, measured by the 12‐item well‐being questionnaire, the W‐BQ12 18, and self‐efficacy in diabetes management, measured by the Swedish Diabetes Empowerment Scale, short version (SWE‐DES‐10) 19 at 6 months after childbirth.
The secondary outcomes were psychometric scales measuring psychosocial variables and medical outcomes. All psychometric instruments were available in Swedish and have been validated with a good internal consistency 12. The W‐BQ12 consists of 12 items with total scores of 0–36, a high score indicating greater general well‐being 18. The SWE‐DES‐10 uses five‐point Likert scales, ranging from strongly disagree to strongly agree. Higher values indicate stronger empowerment (in the present study this was interpreted as a measure of self‐efficacy in diabetes management), with total scores of 1–5 19. To measure self‐perceived health, a single‐item Likert scale with the values ‘excellent’, ‘very good’, ‘good’, ‘fair’ or ‘poor’ was used. The lower the score, the better self‐perceived health 20. The Swedish Problem Areas in Diabetes Scale (SWE‐PAID‐20) measures diabetes distress, with a total score range of 0–100. The participants rated the degree to which each item was currently a problem for them, ranging from ‘not a problem’ to ‘a serious problem’. A higher score indicates greater emotional distress and the suggested cut‐off for more severe diabetes‐specific emotional problems score is ≥40 21. The 13‐item Sense of Coherence questionnaire (SOC‐13) measures health by investigating to what extent the respondents perceive their life situation as manageable, comprehensible and meaningful. It is scored on a Likert scale from 1 (low) to 7 (high), giving a possible total score range of 13–91 22. A score of ≤60 is considered low, scores of 61–75 as moderate and scores ≥76 as high 23. Fear of hypoglycaemia was measured using the Swedish Hypoglycaemia Fear Survey (SWE‐HFS). Its 20 items are rated on a five‐point Likert scale, ranging from ‘never’ to ‘always’, with a total sum score of 0–80; a high score indicates greater fear of hypoglycaemia 24.
Data on insulin administration, pregnancy and birth‐related data and HbA1c values were collected from electronic medical records. HbA1c values after childbirth and sociodemographic characteristics were self‐reported 12.
The intervention group evaluated the web‐based support using a structured questionnaire also containing a free‐text alternative.
Sample size estimation
Sample size estimation was based on the two primary outcome variables: general well‐being, measured by the W‐BQ12, and self‐efficacy of diabetes management, measured by the SWE‐DES‐10. In order to detect a clinically relevant difference of 1.25 in well‐being between the intervention group and the control group at 6 months after childbirth, 68 participants were needed in each group to reach a statistical power of 80%, assuming an sd of 2.5 in each group, with a significance level of 0.05. Similarly, to detect a difference of 0.2 in self‐efficacy of diabetes management, 68 participants were needed in each group assuming an sd of 0.4 in each group. A total sample size of 160 was chosen to compensate for a probable 10% loss in follow‐up.
Data analysis
Statistical analyses were conducted using spss, version 16.0, and sas, version 9.2 (Cary, NC, USA). Primary analyses were conducted according to the intention‐to‐treat principle. Data missing from the W‐BQ12 were handled according to the guidelines 25, while in the other instruments, a half‐scale approach was taken. At least half of the items included in the total score needed to be answered for the total score to be calculated. Missing values were imputed with the mean of the valid items in order to calculate a total score. Participants were considered to have received the intervention if they had a minimum of two individual logins to the web‐based support and had completed the questionnaire at 6 months after childbirth; these data were included in the per‐protocol analysis.
Descriptive statistics, mean, sd, median, and minimum and maximum values for continuous variables, and number and percentage for categorical variables, were used to characterize the study groups. In addition, the 25th and 75th percentiles were presented for continuous variables in the evaluation of the forum for peer support. To determine differences between groups, Fisher's exact test was used for dichotomous variables, the Mantel–Haenszel chi‐squared test for ordered categorical variables, the chi‐squared test for non‐ordered categorical variables and Fisher's nonparametric permutation test for continuous variables. A complementary analysis of covariance was performed, adjusting for significant differences between baseline characteristics (e.g. insulin delivery). The intention‐to‐treat analysis was performed using last observation carried forward between the 2‐month follow‐up and the 6‐month follow‐up. In seven cases, the responses from 2 months after childbirth were imputed if the 6‐month values were missing. For comparison of changes over time within groups, the Wilcoxon signed‐rank test was used for continuous variables and the sign test for categorical variables. In the dose–response analysis, the Jonckheere–Terpstra test was used. All HbA1c values were collected in mmol/mol and converted to % using the official National Glycohemoglobin Standardization Program converter 26.
Results
The demographics and baseline characteristics of the participants are shown in Table 1. In the intervention group, 74.4% administered insulin via multiple injections vs 53.8% in the control group (P=0.01). Well‐being measured with W‐BQ12 differed at baseline (i.e. in early pregnancy), with the control group scoring lower (P=0.05; Table 2).
Table 1.
Demographics, medical data, pregnancy and childbirth outcomes (intention‐to‐treat analysis)
| Intervention group N=78 | Control group N=80 | P | |
|---|---|---|---|
| Demographics | |||
| Mothers’ age when included, years | 0.10 | ||
| Mean (sd) | 31.4 (4.8) | 30.2 (4.2) | |
| Median (min.; max.) |
31.0 (20.0; 41.0) n=78 |
30.0 (23.0; 42.0) n=80 |
|
| Education, n (%) | 0.65 | ||
| Primary school | 1 (1.3) | 2 (2.5) | |
| Secondary school | 24 (31.2) | 26 (32.5) | |
| University | 52 (67.5) | 52 (65.0) | |
| Marital status, n (%) | 0.98 | ||
| Married or cohabiting | 76 (98.7) | 80 (100.0) | |
| Single | 1 (1.3) | 0 (0.0) | |
| Employment, n (%) | 0.15 | ||
| Employee | 63 (81.8) | 64 (80.0) | |
| Self‐employed | 3 (3.9) | 0 (0.0) | |
| Student | 1 (1.3) | 5 (6.3) | |
| Unemployed | 6 (7.8) | 3 (3.8) | |
| Sick leave | 2 (2.6) | 5 (6.3) | |
| Other | 2 (2.6) | 3 (3.8) | |
| Country of birth, n (%) | 0.30 | ||
| Sweden | 75 (97.4) | 74 (92.5) | |
| Medical data , pregnancy and childbirth outcomes | |||
| Years with diabetes | 0.98 | ||
| Mean (sd) | 16.9 (8.9) | 16.9 (7.5) | |
| Median (min.; max.) |
17.0 (0.3; 35.0) n=77 |
16.0 (2.0; 35.0) n=77 |
|
| HbA1c early pregnancy, mmol/mol | 0.14 | ||
| Mean (sd) | 55 (12) | 58 (14) | |
| Median (min.; max.) | 54 (34; 89) | 55 (38; 112) | |
| HbA1c early pregnancy, % | |||
| Mean (sd) | 7.2 (1.1) | 7.5 (1.3) | |
| Median (min.; max.) |
7.1 (5.3; 10.3) n=78 |
7.2 (5.6; 12.4) n=79 |
|
| Insulin administration, n (%) | 0.01 | ||
| Injection | 58 (74.4) | 43 (53.8) | |
| Pump | 20 (25.6) | 37 (46.3) | |
| Parity | 0.16 | ||
| 0 | 41 (52.6) | 47 (58.8) | |
| Gestational week at delivery | 0.39 | ||
| Mean (sd) | 37.6 (2.4) | 37.3 (2.2) | |
| Median (min.; max.) |
38 (27; 41) n=74 |
38 (25; 40) n=79 |
|
| Childbirth, n (%) | 0.15 | ||
| Normal vaginal birth | 34 (45.9) | 25 (31.6) | |
| Assisted vaginal birth | 5 (6.8) | 9 (11.4) | |
| Elective caesarean | 9 (12.2) | 18 (22.8) | |
| Emergency caesarean | 26 (35.1) | 27 (34.2) | |
| Neonatal care of the infant, n (%) | 27 (36.5) | 32 (40.5) | 0.73 |
For comparison between groups, Fisher's exact test was used for dichotomous variables, the Mantel–Haenszel chi‐squared test was usedfor ordered categorical variables, the chi‐squared test was used for non‐ordered categorical variables and Fisher's nonparametric permutation test was used for continuous variables.
Table 2.
Overview of primary and secondary variables at all times of measure
| Early pregnancy | Late pregnancy | 2 months after childbirth | 6 months after childbirth | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention group N=78) | Control group N=80 | P/adjusted P | Intervention group N=78) | Control group N=80 | P/adjusted P | Intervention group N=78) | Control group N=80 | P/adjusted P | Intervention group N=78) | Control group N=80 | P/adjusted P | |
| Primary outcome variables | ||||||||||||
| W‐BQ12 (score range 0–36) | n=77 | n=80 | 0.048 | n=62 | n=69 | 0.78/0.64 | n=59 | n=68 | 1.00/0.85 | n=69 | n=74 | 0.54/0.68 |
| Mean (sd) | 23.4 (5.4) | 21.6 (6.0) | 22.3 (6.3) | 22.6 (5.1) | 22.6 (6.5) | 22.6 (6.3) | 23.5 (5.9) | 22.8 (6.8) | ||||
| Median (min.; max.) | 24.0 (12.0; 33.0) | 22.0 (5.0; 33.0) | 23.0 (6.0; 36.0) | 23.0 (8.0; 34.0) | 23.0 (8.0; 35.0) | 24.0 (7.0; 32.0) | (10.0; 35.0) 24.0 | 25.0 (5.0; 32.0) | ||||
| SWE‐DES‐10 (1–5) | n=77 | n=80 | 0.79 | n=61 | n=69 | 0.76/0.96 | n=60 | n=68 | 0.24/0.33 | n=69 | n=74 | 0.53/0.75 |
| Mean (sd) | 3.93 (0.47) | 3.90 (0.57) | 3.97 (0.51) | 3.94 (0.56) | 3.95 (0.57) | 3.83 (0.55) | 3.86 (0.56) | 3.80 (0.58) | ||||
| Median (min.; max.) | 4.00 (2.20; 4.90) | 3.90 (2.60; 5.00) | 4.00 (2.60; 5.00) | 4.00 (2.50; 5.00) | 3.90 (2.50; 5.00) | 3.80 (2.00; 5.00) | 4.00 (2.40; 5.00) | 3.80 (1.80; 5.00) | ||||
| Secondary outcome variables | ||||||||||||
| SOC‐13 (score range 13–91) | n=77 | n=80 | 0.62 | n=62 | n=69 | 0.85/0.91 | n=58 | n=69 | 0.32/0.32 | n=69 | n=74 | 0.36/0.31 |
| Mean (sd) | 69.3 (9.1) | 68.6 (9.9) | 68.8 (10.6) | 68.5 (10.7) | 69.6 (10.9) | 67.5 (12.3) | 68.2 (11.1) | 66.2 (14.5) | ||||
| Median (min.; max.) | 70.0 (44.0; 88.0) | 71.3 (41.0; 87.0) | 69.0 (43.0; 88.0) | 71.0 (36.0; 83.0) | 72.0 (45.0; 86.0) | 70.0 (39.0; 84.0) | 68.0 (47.0; 89.0) | 70.0 (23.0; 85.6) | ||||
| SWE‐ PAID‐20 (score range 0–100) | n=67 | n=71 | 0.49 | n=62 | n=66 | 0.39/0.40 | n=59 | n=66 | 0.73/0.70 | n=69 | n=74 | 0.99/0.99 |
| Mean (sd) | 26.2 (16.5) | 28.0 (15.8) | 25.3 (17.8) | 28.0 (18.2) | 27.0 (19.2) | 25.8 (19.0) | 28.2 (19.3) | 28.1 (19.6) | ||||
| Median (min.; max.) | 21.3 (1.3; 68.8) | 26.3 (0.0; 71.3) | 23.7 (0.0; 76.3) | 25.0 (2.5; 83.3) | 22.5 (1.3; 80.0) | 19.4 (0.0; 70.0) | 22.5 (0.0; 86.3) | 26.9 (0.0; 77.5) | ||||
| Swedish Hypoglycaemia Fear Survey (score range 0–80) | n=65 | n=69 | 0.75 | n=61 | n=67 | 0.71/0.86 | n=58 | n=68 | 0.73/0.78 | n=69 | n=73 | 0.17/0.16 |
| Mean (sd) | 25.9 (11.4) | 26.5 (11.4) | 24.2 (11.1) | 24.9 (9.8) | 27.3 (13.8) | 28.1 (12.6) | 25.7 (12.2) | 28.6 (13.3) | ||||
| Median (min.; max.) | 24.0 (4.0; 57.0) | 26.0 (6.0; 56.0) | 24.0 (2.0; 50.0) | 25.0 (5.0; 47.0) | 25.5 (2.0; 61.0) | 27.5 (5.0; 72.0) | 25.0 (2.0; 66.0) | 29.0 (4.0; 59.0) | ||||
| Self‐perceived health, n (%) | ||||||||||||
| Excellent | 6 (7.8) | 4 (5.0) | 8 (12.9) | 4 (5.9) | 9 (15.3) | 8 (11.6) | 9 (13.0) | 5 (6.8) | ||||
| Very good | 34 (44.2) | 35 (43.8) | 26 (41.9) | 30 (44.1) | 23 (39.0) | 27 (39.1) | 21 (30.4) | 33 (44.6) | ||||
| Good | 27 (35.1) | 32 (40.0) | 17 (27.4) | 22 (32.4) | 15 (25.4) | 23 (33.3) | 23 (33.3) | 25 (33.8) | ||||
| Fair | 9 (11.7) | 8 (10.0) | 7 (11.3) | 9 (13.2) | 9 (15.3) | 9 (13.0) | 11 (15.9) | 8 (10.8) | ||||
| Poor | 1 (1.3) | 1 (1.3) | 0.75 | 4 (6.5) | 3 (4.4) | 0.58/0.78 | 3 (5.1) | 2 (2.9) | 0.97/0.84 | 5 (7.2) | 3 (4.1) | 0.44/0.36 |
| HbA1c, mmol/mol | n=78 | n=79 | 0.14 | n=76 | n=77 | 0.02/0.02 | n=41 | n=46 | 0.36/0.42 | n=63 | n=64 | 0.07/0.08 |
| Mean (sd) | 55 (12) | 58 (14) | 44 (8) | 48 (8) | 49 (9) | 51 (11) | 51 (10) | 55 (11) | ||||
| Median (min.; max.) | 54 (34; 89) | 55 (38; 112) | 44 (28; 80) | 46 (33; 84) | 48 (34; 78) | 50 (21; 74) | 52 (30; 80) | 52 (41; 86) | ||||
| HbA1c, % | 7.2 (3.2) | 7.5 (3.4) | 6.2 (2.9) | 6.5 (2.9) | 6.6 (3.0) | 6.8 (3.2) | 6.8 (3.1) | 7.2 (3.2) | ||||
| 7.1 (5.3; 10.3) | 7.2 (5.6; 12.4) | 6.2 (4.7; 9.5) | 6.4 (5.2; 9.8) | 6.5 (5.3; 9.3) | 6.7 (4.1; 8.9) | 6.9 (4.9; 9.5) | 6.9 (5.9; 10.0) | |||||
SOC‐13, 13‐item Sense of Coherence questionnaire; SWE‐DES‐10, Swedish Diabetes Empowerment Scale, short version; SWE‐HFS, SWE‐HFS, Swedish Hypoglycaemia Fear Survey; SWE‐PAID‐20, Swedish Problem Areas in Diabetes Scale; W‐BQ12, 12‐item well‐being questionnaire.
Adjusted P: P value adjusted for insulin administration method.
For comparison between groups, the Mantel–Haenszel chi‐squared test was used for ordered categorical variables and Fisher's
nonparametric permutation test was used for continuous variables.
No statistically significant differences were observed in the primary outcomes, general well‐being and self‐efficacy of diabetes management at 6 months after childbirth, after adjusting for insulin administration. The mean W‐BQ12 score in the intervention group was 23.5 compared with 22.8 in the control group [mean difference 0.45 (95% CI –1.72 to 2.62); P=0.68]. The mean SWE‐DES‐10 score was 3.86 in the intervention group compared with 3.80 in the control group [mean difference 0.03 (95% CI –0.16 to 0.22); P=0.75].The results from the per‐protocol analysis showed no difference between groups; the W‐BQ12 mean scores were 23.9 vs 22.7 in the intervention group vs the control group, respectively [mean difference 1.04 (95% CI –1.28 to 3.37); P=0.39] and the SWE‐DES‐10 mean scores were 3.92 vs 3.82, respectively [mean difference 0.08 (95% CI –0.12 to 0.28); P=0.69]. No effectiveness with regard to self‐perceived health, or SWE‐PAID‐20, SOC‐13 or SWE‐HFS scores was found for the web‐based support (Table 2).
HbA1c levels were significantly lower in the intervention group in late pregnancy; however, when adjusted for their baseline values, this difference did not remain (P=0.07). The change in scores between early and late pregnancy, early pregnancy and 2 months after childbirth and early pregnancy and 6 months after childbirth were calculated. A significant difference was found between groups in terms of change in general well‐being (W‐BQ12) between early and late pregnancy and early pregnancy and 2 months after childbirth, but the difference was not significant after adjustment for general well‐being baseline values (P=0.08 and P=0.16).
Use of the web‐based support
There was a wide variation in use, ranging from no individual logins to the equivalent of 15 logins per day. Of the 78 participants in the intention‐to‐treat analysis of the intervention group, 11 did not fulfil the criteria of two individual logins, leaving usage data from 67 active users to be analysed (Table 3; intervention adherence). To conduct a dose–response analysis, the degree of use was explored in relation to the outcome measures. For this analysis the full intervention group (n=78) was divided into three subgroups: no or low use, medium use, and high use, using the 25th and 75th percentile as the cut‐off. Although not statistically significant, a consistent descriptive difference in the psychosocial measures favouring a higher degree of use at 6 months after childbirth appeared (Table 3; outcome variables divided by level of intervention use).
Table 3.
Intervention adherence and psychosocial outcome measures divided by intervention use
| Intervention adherence | ||
|---|---|---|
| All participants (N=78) | Median (min.; max.) | 25th/75th percentile |
| Total logins to the system (n=67)* | 91 (2; 6413) | 19.75/214.25 |
| Visits to facts page | 8 (0; 508) | 3/26 |
| Number of entries to the self‐care diary | 1 (0; 5850) | 0/25.25 |
| Visits to the forum | 54 (0.0; 703) | 7/125 |
| Outcome variables divided by level of intervention use 6 months after childbirth | ||||
|---|---|---|---|---|
| Participants (N=78) | No/low usage† | Medium usage‡ | High usage§ | P |
| n=17 | n=42 | n=19 | ||
| W‐BQ12 (score range 0–36) | n=14 | n=38 | n=17 | 0.28 |
| Mean (sd) | 21.5 (5.4) | 23.3 (6.4) | 24.7 (5.2) | |
| Median (min.; max.) | 22.5 (15; 31) | 24 (10; 35) | 25 (13; 34) | |
| SWE‐DES‐10 (score range 1–5) | n=14 | n=38 | n=17 | 0.37 |
| Mean (sd) | 3.7 (0.7) | 3.9 (0.5) | 4.0 (0.5) | |
| Median (min.; max.) | 3.9 (2; 5) | 4 (3; 5) | 4 (3; 5) | |
| SOC‐13 (score range 13–91) | n=14 | n=38 | n=17 | 0.37 |
| Mean (sd) | 67.8 (11.5) | 67.0 (11.8) | 71.3 (8.9) | |
| Median (min.; max.) | 65 (48; 84) | 68 (47; 89) | 72 (51; 85) | |
| SWE‐PAID‐20 (score range 0–100) | n=14 | n=38 | n=17 | 0.15 |
| Mean (sd) | 32.1 (20.5) | 29.2 (20.2) | 22.5 (16.0) | |
| Median (min.; max.) | 30 (4; 86) | 26 (0; 70) | 17.5 (5; 58) | |
| SWE‐HFS (score range 0–80) | n=14 | n=38 | n=17 | 0.14 |
| Mean (sd) | 27.8 (10.2) | 26.7 (13.6) | 21.9 (10.2) | |
| Median (min.; max.) | 27.5 (11; 43) | 28.5 (2; 66) | 23 (4; 40) | |
SOC‐13, 13‐item Sense of Coherence questionnaire; SWE‐DES‐10, Swedish Diabetes Empowerment Scale, short version; SWE‐HFS, Swedish Hypoglycaemia Fear Survey; SWE‐PAID‐20, Swedish Problem Areas in Diabetes Scale; W‐BQ12, 12‐item well‐being questionnaire.
For comparison between groups the Jonckheere–Terpstra test was used.
*Eleven participants did not meet the criteria of two individual logins to the system.
Groups for the dose–response analysis were calculated using percentiles as cut‐offs in the following way: †Below the 25th percentile; <9. ‡25th to 75th percentile; 9–191.75. §Above the 75th percentile; ≥ 192.
Brief evaluation of the web‐based support
In written comments, the participants stated foremost that the high demands in daily life in caring for the newborn child and their diabetes contributed to their low level of use of the web‐based support. Elapsed time between posts and responses in the peer support forum was also reported as a barrier that affected engagement. Technical difficulties were infrequent (Table 4), but functional difficulties regarding the mobile phone version of the support and logging of blood glucose levels were reported. Some mentioned that the design of the graph in the self‐care diary was not optimal. In one case, healthcare professionals’ hesitancy in using the web‐based support during clinical visits hindered use. Details of the evaluation questionnaire are presented in Table 4.
Table 4.
Evaluation of the MODIAB‐Web support intervention
| Question | N | Disagree n (%) | Neither nor n (%) | Agree n (%) | Did not use this function n (%) |
|---|---|---|---|---|---|
| Functionality | |||||
| I found it easy to navigate the website | 65 | 1 (2) | 11 (17) | 30 (46) | 23 (35) |
| The site was stable | 64 | 3 (5) | 12 (19) | 28 (44) | 21 (33) |
| The website loaded in a timely manner | 65 | 1 (2) | 17 (26) | 26 (40) | 21 (32) |
| I did not experience any problems navigating the website | 65 | 3 (5) | 13 (20) | 28 (43) | 21 (32) |
| I found the technology to be functioning | 65 | 9 (14) | 11 (17) | 8 (12) | 37 (57) |
| I found the website good | 65 | 5 (8) | 15 (23) | 26 (40) | 19 (29) |
| Information and content | |||||
| I found the information to be up‐to‐date | 65 | 4 (6) | 11 (17) | 26 (40) | 24 (37) |
| The website provided the right information | 65 | 3 (5) | 11 (17) | 28 (43) | 23 (35) |
| The information provided was clear | 65 | 0 | 8 (12) | 34 (52) | 23 (35) |
| The information was easy to find | 65 | 2 (3) | 16 (25) | 24 (37) | 23 (35) |
| The information was good | 65 | 3 (5) | 12 (19) | 29 (45) | 23 (35) |
| Communication | |||||
| The website provided valuable communication between peers | 64 | 7 (11) | 11 (17) | 17 (27) | 29 (45) |
| I was writing actively in the peer forum | 64 | 11 (17) | 10 (16) | 12 (19) | 31 (48) |
| I read the peer forum | 62 | 2 (3) | 8 (13) | 30 (48) | 22 (36) |
Percentages have been rounded to whole numbers to simplify reading; therefore the sum is sometimes not equal to 100%.
Discussion
In this trial of a person‐centred, web‐based support we observed no difference compared with standard care in general well‐being, self‐efficacy of diabetes management at 6 months after childbirth, or in any of the secondary outcome measures. Greater use of the web‐based support appeared to have a positive impact on the psychosocial variables measured. Women have described how professional support disappears after their child is born 6. If used with greater frequency, the web‐based support could form a bridge between maternity services and the ordinary diabetes clinic. Having said this, no such effects were demonstrated in the present study.
Our results concord with those of a recent meta‐analysis of nine studies evaluating the efficacy of Internet‐based self‐monitoring interventions targeted to improve maternal and neonatal outcomes among pregnant women with different types of diabetes. The outcomes were found to be very similar between those with access to an intervention and those receiving the standard care 27. Our psychosocial outcome measures are not directly comparable, but the results highlight the complexity of measuring Internet‐delivered interventions, especially RCTs, as previously discussed by Eysenbach 28. Campbell et al. 29 argue that lack of significant differences between groups in complex health interventions always entails methodological implications. Was the intervention ineffective, meaning that all similar interventions are also ineffective or were the outcome measures and/or design inappropriate?
The web‐based support was intended to facilitate person‐centred care. The woman could share her documentation in the self‐care diary with healthcare professionals during pregnancy and use it as the basis of discussions. After childbirth, the components of the web‐based support were meant to provide an alternative, if professional support was lacking. Person‐centredness is an approach that allows a patient to be viewed as a person with capabilities and an active partner in the care team 17. We did not measure the degree of person‐centredness that occurred in the healthcare interactions. In hindsight, exploring this might have been beneficial.
The relatively low degree of use may have various causes. The frequency or type of prompt might be one; inactive women in the intervention group received a short reminder via text messaging every 2 weeks. The design of the intervention may not have hit the mark, even though it was based on previous research 9, with patient representatives involved during development 14, 15, 16. Key elements may have been missed, such as the possibility of sending direct messages to healthcare professionals (we were unable to provide this at the project start). The long inclusion time contributed to the low use, given the limited number of women able to simultaneously access the peer support forum. In addition, technical advances in blood glucose monitoring and other self‐applications during the study recruitment time made more choices available to the participants, including those in the control group. This could not be foreseen and therefore was not controlled for or taken into account when feasibility was assessed. Barriers to use, such as technical difficulties and healthcare professionals reluctant to adapt clinical care, were identified. The main barrier postpartum appeared to be the stressors of motherhood combined with diabetes, i.e. the problem the intervention was designed to assist in. This needs to be considered when planning future supportive interventions.
This study has some interesting findings that add new knowledge to the field; however, its results should be interpreted with caution considering the number of women who declined participation. Especially interesting was that participants, despite dealing with diabetes‐related challenges, scored well below the cut‐off limit of 40 regarding diabetes distress (high diabetes distress) 21 and fell into the moderate score range of sense of coherence 23. The results could be compared with previous findings regarding the level of distress during pregnancy 1, 3, 5. The total scores obtained from the psychometric instruments (Table 2) could be used as comparison scores for the target group in further studies.
The study was strengthened by its participatory design 13, which was based on previous research 9, including patient representatives 14, 15, 16, and by its principles of person‐centred care 17. Its generalizability was increased by the multicentre design.
A methodological weakness was the lack of analysis of non‐responders. It is possible that the participants in this study experienced better health and had greater control over their diabetes in early pregnancy. The dropout rate was high but not unexpected, and increased with miscarriages, neonatal and infant deaths.
Although the study achieved the required sample size, the per‐protocol requirement of two individual logins was too low to represent anticipated use. The power analysis should possibly have been adjusted to allow for a wider variation in usage. While a larger sample size might have been preferable, it would have prolonged the study inclusion time further. RCTs are viewed as the ‘gold standard’ in evaluating interventions; in the present study, some pitfalls could potentially have been avoided by choosing a different study design.
In conclusion, the combination of web‐based support and standard care was not superior to standard care alone in terms of general well‐being or self‐efficacy of diabetes management in women with Type 1 diabetes at 6 months after childbirth. Future studies, testing different approaches to support, including web‐based support, are needed. Lessons learned from this study, such as the importance of achieving a high activity level in web‐based interventions, should be taken into account when planning future studies.
Funding sources
Funding was received from the Centre for Person‐Centred Care at the University of Gothenburg; the Diabetes Association; the Health and Medical Care Committee of the Regional Executive Board; Region Västra Götaland; and the Institute of Health and Care Sciences, together with the Sahlgrenska Academy at the University of Gothenburg, all in Sweden. The funding sponsors had no role in the study.
Competing interests
None declared.
Acknowledgements
We thank all the participants and healthcare professionals involved in the study.
Diabet. Med. 35, 232–241 (2018)
References
- 1. Rasmussen B, Hendrieckx C, Clarke B, Botti M, Dunning T, Jenkins A et al Psychosocial issues of women with type 1 diabetes transitioning to motherhood: a structured literature review. BMC Pregnancy Childbirth 2013; 13: 218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Colstrup M, Mathiesen ER, Damm P, Jensen DM, Ringholm L. Pregnancy in women with type 1 diabetes: have the goals of St. Vincent declaration been met concerning foetal and neonatal complications? J Matern Fetal Neonatal Med 2013; 26: 1682–1686. [DOI] [PubMed] [Google Scholar]
- 3. Berg M, Honkasalo ML. Pregnancy and diabetes ‐ A hermeneutic phenomenological study of women's experiences. J Psychosom Obstet Gynaecol 2000; 21: 39–48. [DOI] [PubMed] [Google Scholar]
- 4. National Institute for Health and Care Excellence . Diabetes in pregnancy: management from preconception to the postnatal period, Available at https://www.nice.org.uk/guidance/ng3. Last accessed 28 November 2017. [PubMed]
- 5. Berg M. Pregnancy and diabetes: how women handle the challenges. J Perinat Educ 2005; 14: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sparud‐Lundin C, Berg M. Extraordinary exposed in early motherhood ‐ a qualitative study exploring experiences of mothers with type 1 diabetes. BMC Womens Health 2011; 11: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eysenbach G. What is e‐health? J Med Internet Res 2001; 3: E20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vorderstrasse A, Lewinski A, Melkus GD, Johnson C. Social Support for Diabetes Self‐Management via eHealth Interventions. Curr Diab Rep 2016; 16: 56. [DOI] [PubMed] [Google Scholar]
- 9. Sparud‐Lundin C, Ranerup A, Berg M. Internet use, needs and expectations of web‐based information and communication in childbearing women with type 1 diabetes. BMC Med Inform Decis Mak 2011; 11: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Polsky S, Giordano D, Voelmle MK, Garcetti R, Garg SK. Using technology to advance type 1 diabetes care among women during the reproductive years and in pregnancy. Postgrad Med 2016; 128: 418–426. [DOI] [PubMed] [Google Scholar]
- 11. Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010; 8: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adolfsson A, Linden K, Sparud‐Lundin C, Larsson P‐G, Berg M. A web‐based support for pregnant women and new mothers with type 1 diabetes mellitus in Sweden (MODIAB‐Web): study protocol for a randomized controlled trial. Trials 2014; 15: 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spinuzzi C. The methodology of participatory design. Technical Communication 2005; 52: 163–174. [Google Scholar]
- 14. Adolfsson A, Jansson M. Prototype for Internet support of pregnant women and mothers with type 1 diabetes: focus group testing. Psychol Res Behav Manag 2012; 5: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berg M, Adolfsson A, Ranerup A, Sparud‐Lundin C. Person‐centered Web support to women with type 1 diabetes in pregnancy and early motherhood–the development process. Diabetes Technol Ther 2013; 15: 20–25. [DOI] [PubMed] [Google Scholar]
- 16. Linden K, Berg M, Sparud‐Lundin C. Web‐based information for pregnant women and new mothers with type 1 diabetes–a description of the development process. BMC Med Inform Decis Mak 2012; 12: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ekman I, Swedberg K, Taft C, Lindseth A, Norberg A, Brink E et al Person‐centered care–ready for prime time. Eur J Cardiovasc Nurs 2011; 10: 248–251. [DOI] [PubMed] [Google Scholar]
- 18. Pouwer F, van der Ploeg HM, Ader HJ, Heine RJ, Snoek FJ. The 12‐item well‐being questionnaire. An evaluation of its validity and reliability in Dutch people with diabetes. Diabetes Care 1999; 22: 2004–2010. [DOI] [PubMed] [Google Scholar]
- 19. Leksell J, Funnell M, Sandberg G, Smide B, Wiklund G, Wikblad K. Psychometric properties of the Swedish Diabetes Empowerment Scale. Scand J Caring Sci 2007; 21: 247–252. [DOI] [PubMed] [Google Scholar]
- 20. Smith T. The Impact of Alternative Response Scales on Measuring Self‐ratings of Health. Available at http://www.surveypractice.org/index.php/SurveyPractice/rt/printerFriendly/213/html. Last accessed 28 November 2017.
- 21. Amsberg S, Wredling R, Lins PE, Adamson U, Johansson UB. The psychometric properties of the Swedish version of the Problem Areas in Diabetes Scale (Swe‐PAID‐20): scale development. Int J Nurs Stud 2008; 45: 1319–1328. [DOI] [PubMed] [Google Scholar]
- 22. Antonovsky A. The structure and properties of the sense of coherence scale. Soc Sci Med 1993; 36: 725–733. [DOI] [PubMed] [Google Scholar]
- 23. Langius A, Björvell H. The salutogenic model and the use of the sense of coherence scale in nursing research ‐ a methodological report. Vard Nord Utveckl Forsk 1996; 16: 28. [DOI] [PubMed] [Google Scholar]
- 24. Anderbro T, Amsberg S, Wredling R, Lins PE, Adamson U, Lisspers J et al Psychometric evaluation of the Swedish version of the Hypoglycaemia Fear Survey. Patient Educ Couns 2008; 73: 127–131. [DOI] [PubMed] [Google Scholar]
- 25. Bradley C. WB‐Q User Guidelines. In: Health Psychology Research. London: Royal Holloway, University of London, 2003. [Google Scholar]
- 26. NGSP . NGSP converter. Available at http://www.ngsp.org/convert1.asp. Last accessed 4 April 2017.
- 27. Lau Y, Htun TP, Wong SN, Tam WS, Klainin‐Yobas P. Efficacy of Internet‐Based Self‐Monitoring Interventions on Maternal and Neonatal Outcomes in Perinatal Diabetic Women: A Systematic Review and Meta‐Analysis. J Med Internet Res 2016; 18: e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eysenbach G. Issues in evaluating health websites in an Internet‐based randomized controlled trial. J Med Internet Res 2002; 4: E17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Campbell NC, Murray E, Darbyshire J, Emery J, Farmer A, Griffiths F et al Designing and evaluating complex interventions to improve health care. BMJ 2007; 334: 455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]


